Perturbations of metabolic pathways are an overarching theme spanning numerous pathological conditions and diseases, including pulmonary hypertension (PH). The majority of metabolic disturbances in PH can be linked with underlying mitochondrial dysfunction (1). Pulmonary artery cells from patients with PH have higher glucose uptake but lower oxidative glucose metabolism in the mitochondria (2–4). Patients with PH display higher lipid accumulation in the heart (5) and lower mitochondrial fatty acid oxidation in the pulmonary artery cells (4), similar to altered glucose handling. Although metabolic disturbances are unlikely to be the causative factor in the development of PH, they give diseased cells a competitive growth and survival advantage, such as the reported increase in glutaminolysis (6) and one-carbon or glycine metabolism in patients with PH (7). Ultimately, the mitochondrial dysfunction leads to more inefficient ATP production through glycolysis. This potential energy deficit is, in part, compensated through higher glucose uptake and supported by increased glutaminolysis. Additionally, increased glutaminolysis and one-carbon metabolism support increased demand for biochemical building blocks such as purine nucleosides in proliferating pulmonary artery cells. Finally, the shifting energy demands and metabolic pathways also affect cellular redox balance, with downregulation and diminished activity of mitochondrial superoxide dismutase (SOD2) being a hallmark of PH-associated oxidative stress (1). This vicious cycle centered on dysfunctional mitochondria can thus coordinately sustain an altered cellular phenotype associated with disease progression. It is interesting that these disturbances are preserved in cultured cells (8, 9) and cannot be reversed by fuel shunting back into the mitochondrial tricyclic acid cycle (10).
It is becoming clear that maintaining such a persistent disease phenotype requires more profound and deeper mechanisms that go beyond transcription factor–controlled gene expression changes. Epigenetic reprogramming was recently described as an underlying mechanism that orchestrates expression changes in human adventitial fibroblasts from patients with PH (11). In this issue of the Journal, Li and colleagues (pp. 570–583) link diminished mitochondrial function and increased proliferation of adventitial fibroblasts with increased acetylation of mitochondrial proteins and lower SIRT3 protein levels in PH samples (12). SIRT3 is a NAD+-dependent mitochondrial protein deacetylase that acts as a crucial regulator of mitochondrial function (13). Loss of SIRT3 is associated with decreased mitochondrial respiratory capacity and higher oxidative stress (13). SIRT3 regulates the activity of mitochondrial enzymes involved in the formation of the epigenetic modulators acetyl-coenzyme A and β-hydroxybutyrate (14). SIRT3 is, therefore, a central regulator, linking mitochondrial metabolic processes with cellular health, survival, and phenotypic characteristics. Li and colleagues show lower SIRT3 expression in bovine PH lung samples and correspondingly higher acetylation of mitochondrial proteins, including SOD2. They further show lower SIRT3 expression in human and bovine PH-adventitial fibroblasts. Quantifying the acetylation of select mitochondrial proteins such as MPC1 and SOD2 as a surrogate measure of SIRT3 activity, the authors provide further evidence of diminished SIRT3 expression and activity levels in PH fibroblasts. Taking advantage of the distinctive fluorescent properties of endogenous metabolites, the authors applied a novel fluorescence lifetime imaging microscopy technique to subcellularly resolve metabolic activities and show diminished NAD+ levels in PH fibroblasts. They go on to show that genetic or pharmacological approaches that have been used to restore SIRT3 levels or boost its activity improve mitochondrial health and normalize the adventitial fibroblast phenotype. In particular, a combination of SIRT3 overexpression and NAD+ supplementation was the most effective in normalizing mitochondrial protein acetylation, increasing oxidative phosphorylation, and normalizing fibroblast proliferation and apoptosis rates.
This study puts another piece of the puzzle in place and establishes SIRT3 as a crucial novel therapeutic target for the treatment of PH. Studies by Paulin and colleagues have shown the beneficial effects of SIRT3 restoration in human pulmonary artery smooth muscle cells and animal models of PH (15). Li and colleagues extend the concept that modulation of SIRT3 levels and activity could be beneficial for patients with PH by investigations using adventitial fibroblasts. However, a study by Waypa and colleagues showed that the loss of SIRT3 does not exacerbate chronic hypoxia-induced PH (16). Nevertheless, on closer inspection of the underlying data, the authors show a clear trend in shortening of the normalized pulmonary artery acceleration time and increased arterial wall thickness in the hypoxic SIRT3-knockout mice compared with hypoxic wild-type mice, indicating a potential PH augmentation (16). It is interesting that the discordance was attributed possibly to mouse strain–specific differences in underlying metabolism. Although, in their current work, Li and colleagues did not address potential metabolic differences between different cell types and their effect on SIRT3-controlled processes, their study shows that reduced SIRT3 levels or diminished activity is a recurring feature in PH pathogenesis that is shared across multiple cell types.
Restoration of SIRT3 activity by boosting NAD+ levels was shown as a promising therapeutic approach in a cardiovascular disease setting. Oral supplementation with nicotinamide, an NAD+ precursor, normalized myocardial metabolism and function, lowered blood pressure, and improved mortality in animal models of heart failure (17). It is intriguing that certain exercise regimes were associated with tissue-specific increases in NAD+ levels (18). A well-balanced and controlled level of physical activity through regular exercise was also shown to be beneficial for patients with PH (19).
The combined use of over-the-counter dietary supplements with personalized physical activity modalities could represent an innovative approach in the management of PH (Figure 1). This opens a window into potential cost-effective novel treatment strategies centered on SIRT3-NAD+. However, before wider use or uncontrolled self-medication, more research such as the study by Li and colleagues is needed to carefully and systematically investigate all aspects and long-term changes associated with mechanisms that target epigenetic reprogramming.
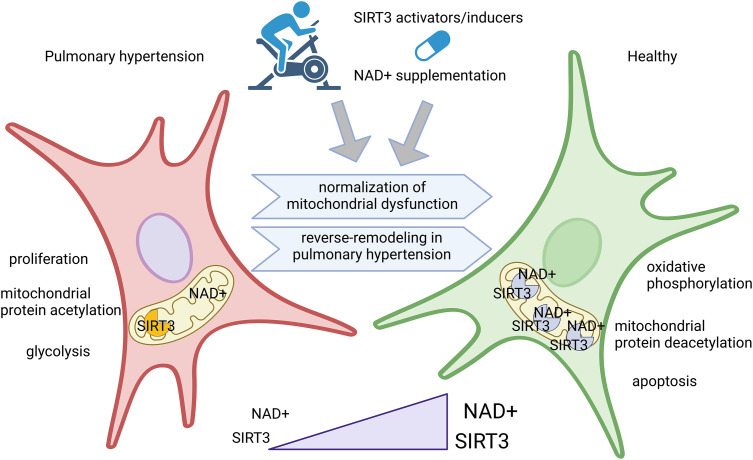
Figure 1.
SIRT3-centered approach of rebalancing mitochondrial health as a novel reverse-remodeling strategy for pulmonary hypertension treatment. Nutritional supplementation with SIRT3 cofactor NAD+, or with its precursor nicotinamide, or exercise regimes could be combined with pharmacological treatments that increase SIRT3 expression levels. The synergistic effect of concomitant increase in enzyme and cofactor levels is shown in the present study to normalize major metabolic disturbances associated with pulmonary hypertension and reprogram the diseased cells toward a homeostatic phenotype. This illustration was created using BioRender.com.
Footnotes
Supported by grant JTC-2019-062 from the European Research Area Cardiovascular Disease Joint Transnational Project.
Originally Published in Press as DOI: 10.1165/rcmb.2023-0199ED on August 16, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Xu W, Janocha AJ, Erzurum SC. Metabolism in pulmonary hypertension. Annu Rev Physiol . 2021;83:551–576. doi: 10.1146/annurev-physiol-031620-123956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao L, Ashek A, Wang L, Fang W, Dabral S, Dubois O, et al. Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation . 2013;128:1214–1224. doi: 10.1161/CIRCULATIONAHA.113.004136. [DOI] [PubMed] [Google Scholar]
- 3. Saygin D, Highland KB, Farha S, Park M, Sharp J, Roach EC, et al. Metabolic and functional evaluation of the heart and lungs in pulmonary hypertension by gated 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Pulm Circ . 2017;7:428–438. doi: 10.1177/2045893217701917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernandez-Saavedra D, Sanders L, Freeman S, Reisz JA, Lee MH, Mickael C, et al. Stable isotope metabolomics of pulmonary artery smooth muscle and endothelial cells in pulmonary hypertension and with TGF-beta treatment. Sci Rep . 2020;10:413–415. doi: 10.1038/s41598-019-57200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:325–334. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest . 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu W, Comhair SAA, Chen R, Hu B, Hou Y, Zhou Y, et al. Integrative proteomics and phosphoproteomics in pulmonary arterial hypertension. Sci Rep . 2019;9:18623–18626. doi: 10.1038/s41598-019-55053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D’Alessandro A, Tauber J, et al. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a MicroRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation . 2017;136:2468–2485. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caruso P, Dunmore BJ, Schlosser K, Schoors S, Dos Santos C, Perez-Iratxeta C, et al. Identification of MicroRNA-124 as a major regulator of enhanced endothelial cell glycolysis in pulmonary arterial hypertension via PTBP1 (polypyrimidine tract binding protein) and pyruvate kinase M2. Circulation . 2017;136:2451–2467. doi: 10.1161/CIRCULATIONAHA.117.028034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Plecitá-Hlavatá L, Tauber J, Li M, Zhang H, Flockton AR, Pullamsetti SS, et al. Constitutive reprogramming of fibroblast mitochondrial metabolism in pulmonary hypertension. Am J Respir Cell Mol Biol . 2016;55:47–57. doi: 10.1165/rcmb.2015-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chelladurai P, Kuenne C, Bourgeois A, Günther S, Valasarajan C, Cherian AV, et al. Epigenetic reactivation of transcriptional programs orchestrating fetal lung development in human pulmonary hypertension. Sci Transl Med . 2022;14:eabe5407. doi: 10.1126/scitranslmed.abe5407. [DOI] [PubMed] [Google Scholar]
- 12. Li M, Plecitá-Hlavatá L, Dobrinskikh E, McKeon BA, Gandjeva A, Riddle S, et al. SIRT3 is a critical regulator of mitochondrial function of fibroblasts in pulmonary hypertension. Am J Respir Cell Mol Biol . 2023;69:570–583. doi: 10.1165/rcmb.2022-0360OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, et al. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci USA . 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diao Z, Ji Q, Wu Z, Zhang W, Cai Y, Wang Z, et al. SIRT3 consolidates heterochromatin and counteracts senescence. Nucleic Acids Res . 2021;49:4203–4219. doi: 10.1093/nar/gkab161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paulin R, Dromparis P, Sutendra G, Gurtu V, Zervopoulos S, Bowers L, et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab . 2014;20:827–839. doi: 10.1016/j.cmet.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 16. Waypa GB, Osborne SW, Marks JD, Berkelhamer SK, Kondapalli J, Schumacker PT. Sirtuin 3 deficiency does not augment hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol . 2013;49:885–891. doi: 10.1165/rcmb.2013-0191OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med . 2021;13:eabd7064. doi: 10.1126/scitranslmed.abd7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walzik D, Jonas W, Joisten N, Belen S, Wüst RCI, Guillemin G, et al. Tissue-specific effects of exercise as NAD+ -boosting strategy: Current knowledge and future perspectives. Acta Physiol (Oxf) . 2023;237:e13921. doi: 10.1111/apha.13921. [DOI] [PubMed] [Google Scholar]
- 19. Grünig E, MacKenzie A, Peacock AJ, Eichstaedt CA, Benjamin N, Nechwatal R, et al. Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J . 2021;42:2284–2295. doi: 10.1093/eurheartj/ehaa696. [DOI] [PubMed] [Google Scholar]