Abstract
Background
Sepsis is a common cause of mortality in critically ill patients, and chronic obstructive pulmonary disease (COPD) is one of the most common comorbidities in septic patients. However, the impact of COPD on patients with sepsis remained unclear. Therefore, the purpose of this study aimed to assess the effect of COPD on the prognosis of septic patients based on Medical Information Mart for Intensive Care (MIMIC-III) database.
Methods
In this retrospective study based on the (MIMIC)-III database version 1.4 (v1.4), we collected clinical data and 28-day all-cause mortality from patients with sepsis in intensive care unit (ICU) and these patients met the diagnostic criteria of Sepsis 3 on ICU admission between 2008 and 2012. International Classification of Diseases (ICD-9) (4660, 490, 4910, 4911, 49120, 49121, 4918, 4919, 4920, 4928, 494, 4940, 4941, 496) was used to identified COPD. We applied Kaplan–Meier analysis to compare difference of 28-day all-cause mortality between septic patients with and without COPD. Cox proportional-hazards model was applied to explore the risk factor associated with 28-day all-cause mortality in patients with sepsis.
Results
Six thousand two hundred fifty seven patients with sepsis were included in this study, including 955 (15.3%) patients with COPD and 5302 patients without COPD (84.7%). Compared with patients without COPD, patients with COPD were older (median: 73.5 [64.4, 82.0] vs 65.8 [52.9, 79.1], P < 0.001), had higher simplified acute physiology score II (SAPSII) (median: 40.0 [33.0, 49.0] vs 38.0 [29.0,47.0], P < 0.001) and greater proportion of mechanical ventilatory support (MV) (55.0% vs 48.9%, P = 0.001). In our study, septic patients with COPD had higher 28-day all-cause mortality (23.6% vs 16.4%, P < 0.001) than patients without COPD. After adjusting for covariates, the results showed that COPD was an independent risk factor for the 28-day all-cause mortality of patients with sepsis (HR 1.30, 95%CI: 1.12–1.50, P = 0.001).
Conclusions
COPD was an independent risk factor of 28-day all-cause mortality in septic patients. Clinically, septic patients with COPD should be given additional care.
Keywords: Sepsis, Chronic obstructive pulmonary disease, MIMIC-III, All-cause mortality
Background
Sepsis is a life-threatening organ dysfunction caused by the dysregulated host response to infection [1]. It is one of the major health problems in the world. Although progress in medical sciences has led to a decrease in sepsis prevalence and mortality, about 20% of deaths worldwide were attributed to sepsis [2].
Chronic obstructive pulmonary disease (COPD) emerged as a critical factor contributing to the therapy and prognosis of patients with sepsis [3–5]. Approximately 6.9%-16.5% of septic patients had COPD, and COPD was one of the most prevalent chronic complications in patients with sepsis [6–13]. There existed a potential interconnection between sepsis and COPD on pathogenesis. COPD patients not only exhibited pulmonary inflammation but a state of persistent systemic inflammation. A meta-analysis of W Gan et al. [14] demonstrated that compared with patients without COPD, patients with COPD had elevated levels of circulating leucocytes, fibrinogen, C-reactive protein (CRP) and tumour necrosis factor-α (TNF-α). In addition to systemic inflammation, patients with stable COPD exhibited a prothrombotic state. An observational study revealed that patients with stable COPD showed elevated levels of crucial coagulation factors (FII, FV, FVIII and FX), while exhibiting reduced levels of coagulation inhibitors (protein S and antithrombin) [15]. Oxidative stress played a significant role in the pathogenesis of COPD. In another study, COPD patients presented higher level of 8-isoprostane (a marker of ongoing oxidative stress) in sputum compared with control subject [16]. Given that dysregulated immune response, coagulation disorders and excessive production of oxidants were inherent features of sepsis [17, 18], systemic inflammatory [14], prothrombotic state [15, 19] and oxidative stress [20] in patients with COPD may contribute to the progressions of sepsis. Likewise, the occurrence of sepsis can also serve as a trigger for acute exacerbations of COPD. Chen et al. [21] found that the onset of sepsis was associated with a poor long-term prognosis characterized by increased risks of severe exacerbations, mortality, pneumonia, and serious pneumonia in patients with COPD. However, the relationship between COPD and progressions of sepsis remained controversial. One retrospective study included 22,354 sepsis patients found that COPD was associated with 60-day mortality [6]. On the contrary, several multi-center studies had reported that COPD was not associated with in-hospital mortality in septic patients [7–9, 22–24]. Furthermore, the relationship between COPD and short-term mortality in septic patients remained unclear.
Therefore, this retrospective study aimed to explore the impact of COPD on 28-day all-cause mortality in patients with sepsis through the Medical Information Mart for Intensive Care (MIMIC)-III database version 1.4 (v1.4) spinning from 2018–2012.
Methods
Database
Data from MIMIC-III v1.4 which is a large, single-center, publicly available critical care database were acquired for conducting this study. This database was composed of adult patients (aged 16 years or above) admitted to critical care units in the Beth Israel Deaconess Medical Center (BIDMC, Boston Massachusetts, USA) between 2001 and 2012. The database consisted of records involving demographics, vital signs, laboratory tests, fluid balance and vital status; documents International Classification of Diseases and Ninth Revision (ICD-9) codes; documents hourly physiologic data from bedside monitors confirmed by intensive care unit (ICU) nurses; and stores written evaluations of radiologic films by specialists covering in the corresponding time period. One author (Yubiao Chen) completed the CITI Data or Specimens Only Research couse, and was approved to gain access to the database and took responsibility for data extraction (certification number 40416369). Since this study was conducted using an anonymous public database that satisfies the protocol of the review board, the requirement of ethical consent is not necessary.
Study population
We focused on septic patients defined by sepsis-3 criteria [1]; Briefly, patients with documented or suspected infection and an acute change in total Sequential Organ Failure Assessment (SOFA) [25] score of ≥ 2 points were defined as sepsis. COPD was identified using International Classification of Diseases (ICD-9) codes (4660, 490, 4910, 4911, 49,120,49,121, 4918, 4919, 4920, 4928, 494, 4940, 4941, 496) [26]. We removed patients admitted to ICU before 2008 as the method was described previously [27], because antibiotic prescriptions have only been recorded since 2003, and groups admitted between 2008 and 2012 were easily identified in the database.
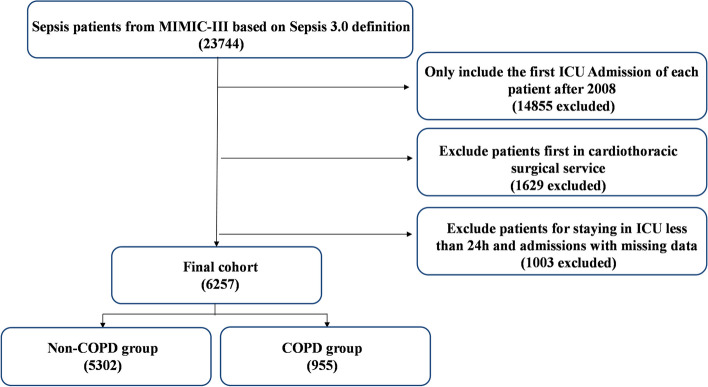
Thirty eight thousand six hundred five MIMIC-III subjects in admissions were included in our study, and 23,744 were identified as sepsis. Then 14,855 secondary (or more) ICU admission were removed, and we included only 8889 first ICU admissions for patients from 2008 – 2012. 1629 patients who were first in cardiothoracic surgical service were excluded since their post-operative physiologic derangements do not translate to the same mortality risk as the other ICU patients. After excluding 1003 patients with less than 24 h of ICU stay and admissions with missing data, we finally included 6257 septic patients in our cohort, of which 955 (15.3%) were known to have COPD (Fig. 1).
Fig. 1.
Study cohort. Illustration of exclusion and inclusion criteria as utilized to select the final cohort of 6257 patients
Data extraction
Structured Query Language (SQL), PostgreSQL tools (version 9.6) and STATA version 17.0 were applied for data extraction and management. Data about age, sex, ethnicity, comorbidities, the first laboratory parameters after ICU admission, two scoring systems containing the SOFA score and simplified acute physiology score II (SAPSII) [28], mechanical ventilation (MV) when ICU admission, the length of hospital and ICU stay and date of dead of patients were extracted directly or calculated. The comorbidities identified by ICD-9 code included atrial fibrillation (AFIB), coronary artery disease (CAD), congestive heart failure (CHF), diabetes, malignancy, chronic renal disease, liver disease, stroke and the laboratory parameters included hemoglobin, platelet counts, white blood cell counts, the percentage of lymphocyte and neutrophil, neutrophil-to-lymphocyte ratio (NLR), PH, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), bicarbonate, partial-thromboplastin time (PTT), prothrombin time (PT), glucose, urea nitrogen, creatinine, lactate, creatine kinase, MB Isoenzyme (CK-MB), creatine kinase, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and albumin. Outcomes in our study contained 7-, 14-, 21- and 28-day all-cause mortality, mortality in the ICU, in-hospital mortality and length of ICU and hospital stay.
Statistical analysis
Baseline characteristics and the laboratory parameters of all patients were stratified by the comorbidity of COPD. After test of normality distribution by Kolmogorov–Smirnov test, continuous data were presented as median and interquartile range (IQR) and significant differences between two group was tested by Wilcoxon rank-sum test. Categorical data were reported as frequencies and percentages and were compared using chi-square test. To determine the association between the comorbidity of COPD with 28-day all-cause mortality, we presented Kaplan–Meier curve and analyzed the result using log-rank tests. Furthermore, the Cox proportional-hazards model was used to analyze the independent effects of COPD on 28-day all-cause mortality. Forward stepwise method was applied for variables selection for Cox proportional-hazards models. Sensitive analyses stratified by sex and MV were performed to explore the association between COPD and 28-day all-cause mortality. Except that Kaplan–Meier approach for survival analysis was performed by R software, the data were analyzed with the statistical package IBM SPSS Statistics software (SPSS) (v16.0; IBM, Armonk, NY). A two-tailed P < 0.05 was considered as statistically significant.
Results
Population characteristics
Baseline characteristics and laboratory parameters of the study population were shown in Tables 1 and 2. Patients with COPD were significantly older (median: 73.5 [64.4, 82.0] vs 65.8 [52.9, 79.1], P < 0.001), had higher SAPSII scores (median: 40.0 [33.0, 49.0] vs 38.0 [29.0, 47.0], P < 0.001), and lager percentage of receiving mechanical ventilation at admission (55.0% vs 48.9%, P = 0.001) than patients without COPD. Septic patients with COPD were more likely to have atrial fibrillation (38.8% vs 26.8%, P < 0.001), coronary artery disease (33.4% vs 23.8% P < 0.001) and congestive heart failure (44.1% vs 25.7%, P < 0.001). There was no significant difference in proportion of diabetes (31.3% vs 29.8%, P = 0.337) and malignancy (22.6% vs 21.3%, P = 0.366) between two groups (Table 1). Compared with patients without COPD, patients with COPD exhibited a higher percentage of neutrophil (median: 84.0 [75.8, 89.6] vs 82.5 [74.0, 88.3], P < 0.001) and a lower percentage of lymphocyte (median: 9.0 [5.0, 14.6] vs 9.8 [5.8, 15.9], P < 0.001), and an elevated neutrophil to lymphocyte ratio (median: 9.0 [5.2, 16.8] vs 8.3 [4.7, 14.6], P = 0.002). Besides, septic patients with COPD had higher platelet counts (median: 208.0 [149.0, 288.3] vs 196.0 [113.0, 273.0], P < 0.001), PTT (median: 31.1 [26.6, 37.9] vs 30.2 [26.3, 36.9], P = 0.036), as well as PCO2 (median: 45.0[38.0, 55.0] vs 40.0 [34.0, 45.0], P < 0.001), and a lower PH (median: 7.37 [7.30, 7.43] vs 7.39 [7.34, 7.44], P < 0.001) and PO2 (median: 92.0 [69.0, 135.0] vs 113.0 [80.0, 171.0], P < 0.001) than septic patients without COPD (Table 2).
Table 1.
Characteristics of patients with sepsis at the admission to ICU according to the presence of COPD
| Independent variable | Total (N = 6257) | Non-COPD (N = 5302) | COPD (N = 955) | P |
|---|---|---|---|---|
| Age, Years | 67.3 (54.3, 79.8) | 65.8 (52.9, 79.1) | 73.5 (64.4, 82.0) | < 0.001 |
| Sex, n (%) | 0.114 | |||
| Male | 3514 (56.2) | 3000 (56.6) | 514 (53.8) | |
| Female | 2743 (43.8) | 2302 (43.4) | 441 (46.2) | |
| Body mass index (kg/m2) | 27.1 (23.6, 31.9) | 27.2 (23.6, 31.6) | 27.0 (23.3, 33.4) | 0.123 |
| Ethnicity, n (%) | < 0.001 | |||
| White | 4549 (72.7) | 3799 (71.7) | 750 (78.5) | |
| Black | 626 (10.0) | 544 (10.3) | 82 (8.6) | |
| Hispanic | 232 (3.7) | 214 (4.0) | 18 (1.9) | |
| Other | 850 (13.6) | 745 (14.1) | 105 (11.0) | |
| Comorbidities, n (%) | ||||
| AFIB | 1793 (28.7) | 1422 (26.8) | 371 (38.8) | < 0.001 |
| CAD | 1582 (25.3) | 1263 (23.8) | 319 (33.4) | < 0.001 |
| CHF | 1782 (28.5) | 1361 (25.7) | 421 (44.1) | < 0.001 |
| Diabetes | 1877 (30.0) | 1578 (29.8) | 299 (31.3) | 0.337 |
| Malignancy | 1346 (21.5) | 1130 (21.3) | 216 (22.6) | 0.366 |
| Renal | 1427 (22.8) | 1165 (22.0) | 262 (27.4) | < 0.001 |
| Liver | 558 (8.9) | 495 (9.3) | 63 (6.6) | 0.006 |
| Stroke | 577 (9.2) | 510 (9.6) | 67 (7.0) | 0.001 |
| Scoring systems, median | ||||
| SOFA | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | 0.240 |
| SAPSII | 38.0 (30.0, 48.0) | 38.0 (29.0,47.0) | 40.0 (33.0, 49.0) | < 0.001 |
| Intervention | ||||
| MV, n (%) | 3119 (49.8) | 2594 (48.9) | 525 (55.0) | 0.001 |
Abbreviations: AFIB Atrial fibrillation, CAD Coronary artery disease, CHF Congestive heart failure, COPD Chronic obstructive pulmonary disease, MV Mechanical ventilation, SOFA Sequential organ failure assessment, SAPSII Simplified acute physiology score II. Bold text indicates P < 0.05
Table 2.
Laboratory parameters of patients with sepsis at the admission to ICU according to the presence of COPD
| Laboratory parameters, median | Total (N = 6257) | Non-COPD (N = 5302) | COPD (N = 955) | P |
|---|---|---|---|---|
| Hemoglobin, g/dL | 10.3 (9.1, 11.7) | 10.3 (9.1, 11.8) | 10.3 (9.1, 11.6) | 0.496 |
| Platelet counts, 103/μL | 197.0 (133.0, 275.0) | 196.0 (113.0, 273.0) | 208.0 (149.0, 288.3) | < 0.001 |
| White blood cell, 103/μL | 10.3 (7.4, 14.0) | 10.3 (7.4, 14.0) | 10.5 (7.4, 14.2) | 0.368 |
| Neutrophil, % | 82.8 (74.0, 88.6) | 82.5 (74.0, 88.3) | 84.0 (75.8, 89.6) | < 0.001 |
| Lymphocyte, % | 9.7 (5.7, 15.7) | 9.8 (5.8, 15.9) | 9.0 (5.0, 14.6) | < 0.001 |
| NLR | 8.4 (4.8, 14.9) | 8.3 (4.7, 14.6) | 9.0 (5.2, 16.8) | 0.002 |
| Eosinophil, % | 0.4 (0.1, 1.4) | 0.5 (0.1, 1.4) | 0.4 (0.1, 1.2) | 0.121 |
| PH | 7.39 (7.33 7.44) | 7.39 (7.34, 7.44) | 7.37 (7.30, 7.43) | < 0.001 |
| PaO2, mm Hg | 109.0 (77.0, 166.0) | 113.0 (80.0, 171.0) | 92.0 (69.0, 135.0) | < 0.001 |
| PaCO2, mm Hg | 40.0 (35.0, 47.0) | 40.0 (34.0, 45.0) | 45.0 (38.0, 55.0) | < 0.001 |
| Bicarbonate, mEq/L | 24.0 (21.0, 27.0) | 24.0 (21.0, 27.0) | 26.0 (23.0, 30.0) | < 0.001 |
| INR | 1.2 (1.1, 1.5) | 1.2 (1.1, 1.5) | 1.2 (1.1, 1.5) | 0.541 |
| PT, s | 14.2 (12.9, 16.5) | 14.3 (12.9, 16.6) | 14.0 (12.8, 16.1) | 0.052 |
| PTT, s | 30.3 (26.4, 37.1) | 30.2 (26.3, 36.9) | 31.1 (26.6, 37.9) | 0.036 |
| Glucose, mg/dL | 123.0 (102.0, 153.0) | 122.0 (102.0, 152.0) | 124.0 (103.0, 157.0) | 0.180 |
| Creatinine, mg/dL | 1.0 (0.7, 1.6)) | 1.0 (0.7, 1.7) | 1.0 (0.7, 1.6) | 0.465 |
| Urea nitrogen, mg/dL | 22.0 (14.0, 37.0) | 21.0 (14.0, 36.0) | 25.0 (16.0, 40.0) | < 0.001 |
| Lactate, mmol/L | 1.6 (1.1, 2.4) | 1.6 (1.2, 2.4) | 1.4 (1.0, 2.0) | < 0.001 |
| CK-MB, ng/mL | 5.0 (3.0, 9.0) | 5.0 (3.0, 9.0) | 5.0 (3.0, 8.0) | 0.611 |
| Creatine kinase, IU/L | 113.0 (52.0, 321.0) | 121.0 (55.0, 346.0) | 90.0 (42.5, 210.0) | < 0.001 |
| ALT, IU/L | 28.0 (17.0, 59.0) | 29.0 (17.5, 61.0) | 24.0 (15.0, 52.5) | < 0.001 |
| AST, IU/L | 37.0 (23.0, 75.0) | 38.0 (23.0, 77.5) | 31.0 (20.8, 64.2) | < 0.001 |
| ALP, IU/L | 86.0 (61.0, 129.8) | 87.0 (62.0, 133.0) | 83.0 (60.5, 115.0) | 0.021 |
| Albumin, g/dL | 3.1 (2.6, 3.6) | 3.1 (2.6, 3.6) | 3.1 (2.7, 3.5) | 0.964 |
Abbreviations: ALP Alkaline phosphatase, AST Aspartate transaminase, ALT Alanine transaminase, CK-MB Creatine kinase, MB Isoenzyme, INR International Normalized Ratio, NLR Neutrophil-to-Lymphocyte Ratio, PT Prothrombin Time, PTT Partial-thromboplastin time. Bold text indicates P < 0.05
Outcomes of septic patients with and without COPD
Compared with septic patients without COPD, septic patients with COPD had higher mortality at any visit periods (especially 28-day all-cause mortality [23.6% vs 16.4%, P < 0.001]), higher mortality in ICU (13.5% vs 8.9%, P < 0.001), in-hospital mortality (17.1% vs 12.3%, P < 0.001) and longer length of ICU stay (3.1 days vs 2.9 days, P = 0.005) (Table 3).
Table 3.
Outcomes of patients with sepsis according to the presence of COPD
| Outcome | Total (N = 6257) | Non-COPD (N = 5302) | COPD (N = 955) | P |
|---|---|---|---|---|
| Mortality, n (%) | ||||
| 7-day | 536 (8.6) | 437 (8.2) | 99 (10.4) | 0.031 |
| 14-day | 797 (12.7) | 647 (12.2) | 150 (15.7) | 0.003 |
| 21-day | 975 (15.6) | 778 (14.7) | 197 (20.6) | < 0.001 |
| 28-day | 1097 (17.5) | 872 (16.4) | 225 (23.6) | < 0.001 |
| ICU | 600 (9.6) | 471 (8.9) | 129 (13.5) | < 0.001 |
| Hospital | 813 (13.0) | 650 (12.3) | 163 (17.1) | < 0.001 |
| Length of stay, median (IQR) | ||||
| Hospital, day | 7.2 (4.6, 12.1) | 7.2 (4.6, 12.1) | 7.1 (4.7, 12.1) | 0.489 |
| ICU, day | 2.9 (1.8, 5.8) | 2.9 (1.8, 5.6) | 3.1 (1.9, 6.4) | 0.005 |
Abbreviations: ICU, Intensive care unit. Bold text indicates P < 0.05
Risk factors associated with 28-day all-cause mortality in septic patients
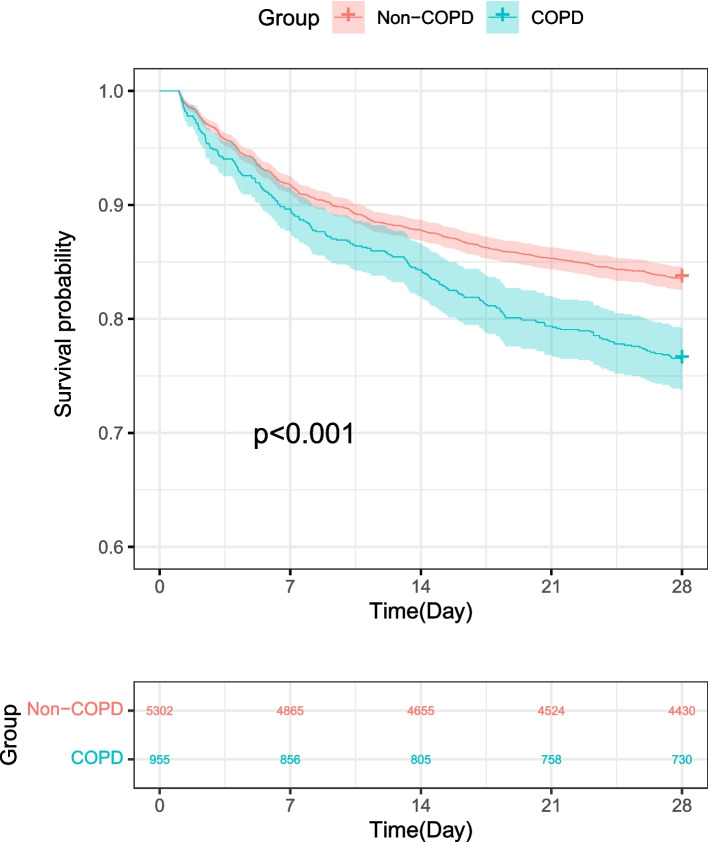
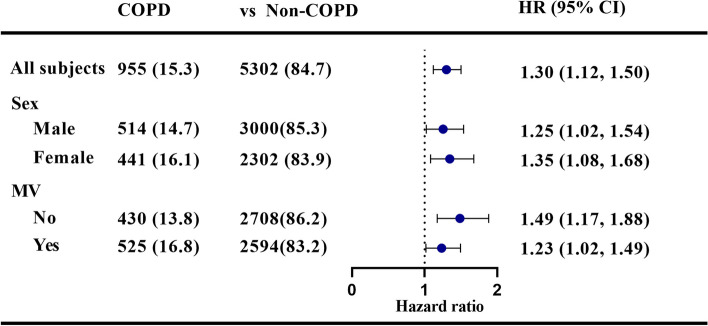
Kaplan–Meier curves in Fig. 2 showed that septic patients with COPD had worse short-term survival rates. Furthermore, we performed univariate analysis to explore risk factors associated with 28-day all-cause mortality in septic patients and found age, ethnicity, comorbidities (COPD, AFIB, CHF, Malignancy, Renal, Liver, Stroke), scoring systems (SOFA, SAPSII) and mechanical ventilation were associated with 28-day all-cause mortality in septic patients, and similar result was found in the multivariate analysis. As a result, the comorbidity of COPD remained a significant risk factor of 28-day all-cause mortality (HR 1.30, 95%CI: 1.12–1.50, P = 0.001) (Table 4). Besides, sensitive analyses stratified by sex and MV were employed to evaluate the association between COPD and 28-day all-cause mortality in septic patients, and the results in each subgroup were in accordance with those in overall patients (Fig. 3).
Fig. 2.
Kaplan–Meier analysis of the probability of survival of patients with sepsis according to the presence of COPD
Table 4.
Cox proportional hazard models exploring the association between COPD and 28-day mortality among septic patients
| Univariate model | Multivariate model | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| COPD | 1.48 (1.28, 1.71) | < 0.001 | 1.30 (1.12,.1.50) | 0.001 |
| Age (Years) | 1.026 (1.02, 1.03) | < 0.001 | 1.01 (1.007, 1.02) | < 0.001 |
| Sex (Male) | 0.98 (0.87, 1.10) | 0.687 | Not selected | - |
| Ethnicity | ||||
| White | Reference | Reference | - | |
| Black | 0.77 (0.62, 0.97) | 0.021 | 0.86 (0.69, 1.08) | 0.197 |
| Hispanic | 0.80 (0.57, 1.13) | 0.210 | 1.13 (0.79, 1.60) | 0.508 |
| Other | 1.30 (1.10, 1.52) | 0.002 | 1.25 (1.06, 1.47) | 0.007 |
| Comorbidities | ||||
| AFIB | 1.72 (1.52, 1.94) | < 0.001 | 1.20 (1.06, 1.37) | 0.006 |
| CAD | 0.99 (0.87, 1.14) | 0.933 | 0.83 (0.72, 0.95) | 0.009 |
| CHF | 1.36 (1.20, 1.54) | < 0.001 | Not selected | |
| Diabetes | 0.99 (0.87, 1.13) | 0.909 | Not selected | |
| Malignancy | 1.87 (1.64, 2.11) | < 0.001 | 1.58 (1.38, 1.80) | < 0.001 |
| Renal | 1.32 (1.15,1.50) | < 0.001 | Not selected | |
| Liver | 1.52 (1.27, 1.82) | < 0.001 | 1.50 (1.24, 1.82) | < 0.001 |
| Stroke | 1.62 (1.36, 1.93) | < 0.001 | 1.80 (1.51, 2.14) | < 0.001 |
| Scoring systems, median | ||||
| SOFA | 1.18 (1.16, 1.20) | < 0.001 | 1.05 (1.02,1.07) | < 0.001 |
| SAPSII | 1.054 (1.05,1.06) | < 0.001 | 1.04 (1.03,1.05) | < 0.001 |
| Intervention | ||||
| MV | 1.39 (1.23,1.56) | < 0.001 | Not selected | |
Multivariate model was adjusted for age, ethnicity, comorbidities (COPD, AFIB, CAD, Malignancy, Liver, Stroke), and scoring systems (SOFA, SAPSII). Abbreviations: AFIB Atrial fibrillation, CAD Coronary artery disease, CHF Congestive heart failure, COPD Chronic obstructive pulmonary disease, MV Mechanical ventilation, SOFA Sequential organ failure assessment, SAPSII Simplified acute physiology score II. Bold text indicates P < 0.05
Fig. 3.
Sensitive analysis of the associations between COPD and 28-day mortality among sepsis patients. Abbreviations: MV Mechanical ventilation
Discussion
This study has revealed two significant findings. Our results showed that septic patients with COPD were older, had more comorbidities, higher SAPSII score, and higher proportion of mechanical ventilation than septic patients without COPD. Secondly, we found that COPD was an independent risk factor for 28-day all-cause mortality in patients with sepsis.
This study showed that 15.3% COPD existed in patients with sepsis, and this result was consisted with the previous studies that the proportion of COPD accounted for 6.9%-16.5% in patients with sepsis [6–13]. A global audit of ICU data reported that there were 454 (15.2%) septic patients with COPD [9]. A demographic study from Sweden showed that in critically ill patients, COPD accounted for 10.5% of 22,354 septic patients [6]. The multi-center, large sample study reported that 12.1% of septic patients were with COPD [13]. These studies indicated that COPD was one of the most common comorbidities of patients with sepsis.
We found that patients with COPD were older and had more comorbidities, higher SAPSII score, as well as higher proportion of mechanical ventilation support than those without COPD in patients with sepsis. Moreover, septic patients with COPD had higher NLR, lower PO2, lower PH, and higher PCO2, due to systemic inflammation [14], chronic hypoxemia [29] and hypercapnia [30]. Taken together, the results revealed that septic patients with COPD had worse status than those without COPD when admitted in ICU.
We further explored the risk factors of 28-day all-cause mortality in patients with sepsis. The result of cox proportional-hazards model showed that COPD was an independent risk factor for 28-day all-cause mortality in patients with sepsis. Our study was consistent with the study of Björn Ahlström et al. [6] that COPD was the risk factor for 60-day mortality in septic patients. However, some studies had contradictory results. Multi-center studies from China reported that COPD was not associated with sepsis-related in-hospital mortality in patients with sepsis [7, 8]. Furthermore, a study from multiple ICUs around the world also showed that COPD was not associated with in-hospital mortality in septic patients [9]. The contradictory results may be due to the differences in the definition of sepsis and the admission time of patients with sepsis in the ICU. In this study, the criteria of sepsis were based on the sepsis-3, which greatly assessed the magnitude of the problem of patients with sepsis [1]. Besides, this study only included patients diagnosed as sepsis within 24 h before and after admission to the ICU to avoid the interference of different sepsis infection patterns caused by acquired infections in the ICU.
We tried to figure out the reason why septic patients with COPD had poorer prognosis. Firstly, patients with COPD were older than those without COPD, and older age may be associated with the poor prognosis of these patients [10, 31, 32]. Secondly, cardiovascular diseases were associated with poor prognosis in patients with COPD [33], and also was associated with poor prognosis in patients with sepsis [34, 35]. In this study, septic patients with COPD had higher proportion of cardiovascular system diseases. Systemic inflammation, hypoxia, and prothrombotic status in COPD patients may increase the risk of cardiovascular events in septic patients [36, 37]. In addition, the results of laboratory tests showed that compared with septic patients without COPD, the platelet counts and NLR were higher in sepsis patients with COPD. NLR was an indicator of systemic inflammation based on complete blood count values, and associated with the onset and development of inflammatory diseases [38, 39]. A recent meta-analysis study also showed that higher NLR was associated with poor prognosis in sepsis [40]. Delayed neutrophil apoptosis, increased release of immature neutrophils and lymphocyte apoptosis can lead to significant increase in circulating neutrophils and lymphopenia in septic patients, and resulted in sepsis progression through enhancing neutrophil-mediated killing and dysregulated innate immune responses [41]. Therefore, except the common risk factors, poorer prognosis of septic patients with COPD may also be related to dysregulated innate immune responses.
This study also existed some limitations. Firstly, COPD was defined by ICD-9 codes [26] rather than based on the spirometry (post-bronchodilator forced expiratory volume in one second/forced vital capacity ratio < 0.70) [42], resulting in the overdiagnosis of COPD [43]. Secondly, since forced expiratory volume in one second and smoking history were important risk factors for patients with COPD [42], due to lack of the two indicators in the MIMIC-III database, the interference caused by these two factors cannot be ruled out.
Conclusions
Our study provided evidence that COPD was an independent risk factor for 28-day all-cause mortality in septic patients. These findings highlighted the importance of providing specialized care for septic patients with COPD. However, it is crucial to recognize that the association between COPD and higher mortality in septic patients is still hypothetical and requires further investigation through appropriately designed studies.
Acknowledgements
We appreciate the excellent work of MIMIC team (Massachusetts Institute of Technology Laboratory for Computational Physiology) to collect bedside data continuously and make the database available for every intensive care researcher.
Abbreviations
- AFIB
Atrial fibrillation
- CAD
Coronary artery disease
- CHF
Congestive heart failure
- COPD
Chronic obstructive pulmonary disease
- NLR
Neutrophil-to-Lymphocyte Ratio
- PO2
Partial pressure of oxygen
- PCO2
Partial pressure of carbon dioxide
- PTT
Partial-thromboplastin time
- PT
Prothrombin time
- CK-MB
Creatine Kinase, MB Isoenzyme
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- ALP
Alkaline phosphatase
Authors’ contributions
YBC, LFL, XCL and BYL conceived the study idea participated in the study conception and design, statistical analysis, data interpretation, drafting and revisions of the manuscript. YBC, YXZ, YZ, YZ, KW, YRP, XNL, ZJW and YTF participated in the data acquisition and revisions of data analysis and interpretation. YBH and YML participated in study conception and design, data interpretation and revisions of the manuscript, also supervised the whole study. All authors provided their final approval for manuscript submission.
Funding
The study was funded by National Key R&D Program of China (2023YFC3041700), the National Natural Science Foundation of China (81870069, 81970071 and 82270085), R&D Program of Guangzhou Laboratory (No. SRPG23-001) and The Science and Technology Program of Guangzhou (202201020444). The funding sources were not involved in study design; collection, analysis, and interpretation of data; or writing of the manuscript.
Availability of data and materials
Available upon request. Corresponding to Drs. Yongbo Huang (yongbo2046@163.com) or Yimin Li (dryiminli@vip.163.com).
Declarations
Ethics approval and consent to participate
The MIMIC-III databases have received ethical approval from the Institutional Review Boards at Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology. As the databases do not contain protected health information, a waiver of informed consent was included in the approval from the Institutional Review Boards at Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology. Therefore, this manuscript does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee. All methods in this study were carried out in accordance with relevant guidelines and regulations (declarations of Helsinki).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yubiao Chen, Lifei Lu, Xicong Li and Baiyun Liu contributed equally to this article and should be regarded as co-first author.
Contributor Information
Yongbo Huang, Email: yongbo2046@163.com.
Yimin Li, Email: dryiminli@vip.163.com.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esper AM, Martin GS. The impact of comorbid [corrected] conditions on critical illness. Crit Care Med. 2011;39:2728–2735. doi: 10.1097/CCM.0b013e318236f27e. [DOI] [PubMed] [Google Scholar]
- 4.Mrus JM, Braun L, Yi MS, Linde-Zwirble WT, Johnston JA. Impact of HIV/AIDS on care and outcomes of severe sepsis. Crit Care. 2005;9:R623–630. doi: 10.1186/cc3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129:1432–1440. doi: 10.1378/chest.129.6.1432. [DOI] [PubMed] [Google Scholar]
- 6.Ahlstrom B, Frithiof R, Larsson IM, Strandberg G, Lipcsey M, Hultstrom M. A comparison of impact of comorbidities and demographics on 60-day mortality in ICU patients with COVID-19, sepsis and acute respiratory distress syndrome. Sci Rep. 2022;12:15703. doi: 10.1038/s41598-022-19539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie J, Wang H, Kang Y, Zhou L, Liu Z, Qin B, Ma X, Cao X, Chen D, Lu W, et al. The Epidemiology of Sepsis in Chinese ICUs: A National Cross-Sectional Survey. Crit Care Med. 2020;48:e209–e218. doi: 10.1097/CCM.0000000000004155. [DOI] [PubMed] [Google Scholar]
- 8.Qu Z, Zhu Y, Wang M, Li W, Zhu B, Jiang L, Xi X. Prognosis and Risk Factors of Sepsis Patients in Chinese ICUs: A Retrospective Analysis of a Cohort Database. Shock. 2021;56:921–926. doi: 10.1097/SHK.0000000000001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakr Y, Jaschinski U, Wittebole X, Szakmany T, Lipman J, Namendys-Silva SA, Martin-Loeches I, Leone M, Lupu MN, Vincent JL, Investigators I. Sepsis in Intensive Care Unit Patients: Worldwide Data From the Intensive Care over Nations Audit. Open Forum Infect Dis. 2018;5:ofy313. doi: 10.1093/ofid/ofy313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Abe T, Ogura H, Shiraishi A, Kushimoto S, Saitoh D, Fujishima S, Mayumi T, Shiino Y, Nakada TA, Tarui T, et al. Characteristics, management, and in-hospital mortality among patients with severe sepsis in intensive care units in Japan: the FORECAST study. Crit Care. 2018;22:322. doi: 10.1186/s13054-018-2186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Qian C, Zhao M, Yu X, Kang Y, Ma X, Ai Y, Xu Y, Liu D, An Y, et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS ONE. 2014;9:e107181. doi: 10.1371/journal.pone.0107181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 14.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyriakopoulos C, Chronis C, Papapetrou E, Tatsioni A, Gartzonika K, Tsaousi C, Gogali A, Katsanos C, Vaggeli A, Tselepi C, et al. Prothrombotic state in patients with stable COPD: an observational study. ERJ Open Res. 2021;7(4):00297–2021. [DOI] [PMC free article] [PubMed]
- 16.Louhelainen N, Rytila P, Haahtela T, Kinnula VL, Djukanovic R. Persistence of oxidant and protease burden in the airways after smoking cessation. BMC Pulm Med. 2009;9:25. doi: 10.1186/1471-2466-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantzarlis K, Tsolaki V, Zakynthinos E. Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies. Oxid Med Cell Longev. 2017;2017:5985209. doi: 10.1155/2017/5985209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husebo GR, Gabazza EC, D'Alessandro Gabazza C, Yasuma T, Toda M, Aanerud M, Nielsen R, Bakke PS, Eagan TML. Coagulation markers as predictors for clinical events in COPD. Respirology. 2021;26:342–351. doi: 10.1111/resp.13971. [DOI] [PubMed] [Google Scholar]
- 20.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144:266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Lai CC, Wang YH, Wang CY, Wang HC, Yu CJ, Chen L, Taiwan clinical trial consortium for respiratory D. The impact of sepsis on the outcomes of COPD patients: A population-based cohort study. J Clin Med. 2018;7(11):393. [DOI] [PMC free article] [PubMed]
- 22.Vucelic V, Klobucar I, Duras-Cuculic B, Gveric Grginic A, Prohaska-Potocnik C, Jajic I, Vucicevic Z, Degoricija V. Sepsis and septic shock - an observational study of the incidence, management, and mortality predictors in a medical intensive care unit. Croat Med J. 2020;61:429–439. doi: 10.3325/cmj.2020.61.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grozdanovski K, Milenkovikj Z, Demiri I, Spasovska K, Cvetanovska M, Saveski V, Grozdanovska B. Epidemiology of Community-Acquired Sepsis in Adult Patients: A Six Year Observational Study. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2018;39:59–66. doi: 10.2478/prilozi-2018-0024. [DOI] [PubMed] [Google Scholar]
- 24.Yao L, Zhang L, Zhou C. Analysis of Prognostic Risk Factors of Sepsis Patients in Intensive Care Unit Based on Data Analysis. J Healthc Eng. 2022;2022:3746640. doi: 10.1155/2022/3746640. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 26.Feng M, McSparron JI, Kien DT, Stone DJ, Roberts DH, Schwartzstein RM, Vieillard-Baron A, Celi LA. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med. 2018;44:884–892. doi: 10.1007/s00134-018-5208-7. [DOI] [PubMed] [Google Scholar]
- 27.Johnson AEW, Aboab J, Raffa JD, Pollard TJ, Deliberato RO, Celi LA, Stone DJ. A Comparative Analysis of Sepsis Identification Methods in an Electronic Database. Crit Care Med. 2018;46:494–499. doi: 10.1097/CCM.0000000000002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 29.Lacasse Y, Casaburi R, Sliwinski P, Chaouat A, Fletcher E, Haidl P, Maltais F. Home oxygen for moderate hypoxaemia in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2022;10:1029–1037. doi: 10.1016/S2213-2600(22)00179-5. [DOI] [PubMed] [Google Scholar]
- 30.Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J. 2007;30:993–1013. doi: 10.1183/09031936.00082507. [DOI] [PubMed] [Google Scholar]
- 31.Brown H, Dodic S, Goh SS, Green C, Wang WC, Kaul S, Tiruvoipati R. Factors associated with hospital mortality in critically ill patients with exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2361–2366. doi: 10.2147/COPD.S168983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:631–639. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- 34.Abou Dagher G, Hajjar K, Khoury C, El Hajj N, Kanso M, Makki M, Mailhac A, Bou Chebl R. Outcomes of patients with systolic heart failure presenting with sepsis to the emergency department of a tertiary hospital: a retrospective chart review study from Lebanon. BMJ Open. 2018;8:e022185. doi: 10.1136/bmjopen-2018-022185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alon D, Stein GY, Korenfeld R, Fuchs S. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PLoS ONE. 2013;8:e72476. doi: 10.1371/journal.pone.0072476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goedemans L, Bax JJ, Delgado V. COPD and acute myocardial infarction. Eur Respir Rev. 2020;29(156):190139. [DOI] [PMC free article] [PubMed]
- 37.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 38.Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, Tattevin P, Thomas R, Fauchet R, Drenou B. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18:487–494. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Jiang J, Liu R, Yu X, Yang R, Xu H, Mao Z, Wang Y. The neutrophil-lymphocyte count ratio as a diagnostic marker for bacteraemia: A systematic review and meta-analysis. Am J Emerg Med. 2019;37:1482–1489. doi: 10.1016/j.ajem.2018.10.057. [DOI] [PubMed] [Google Scholar]
- 40.Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med. 2020;38:641–647. doi: 10.1016/j.ajem.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Jimenez MF, Watson RW, Parodo J, Evans D, Foster D, Steinberg M, Rotstein OD, Marshall JC. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg. 1997;132:1263–1269. doi: 10.1001/archsurg.1997.01430360009002. [DOI] [PubMed] [Google Scholar]
- 42.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Criner GJ, Frith P, Halpin DMG, Han M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. [DOI] [PubMed]
- 43.Manganas H, Lacasse Y, Bourgeois S, Perron J, Dagenais F, Maltais F. Postoperative outcome after coronary artery bypass grafting in chronic obstructive pulmonary disease. Can Respir J. 2007;14:19–24. doi: 10.1155/2007/378963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon request. Corresponding to Drs. Yongbo Huang (yongbo2046@163.com) or Yimin Li (dryiminli@vip.163.com).