Abstract
Focal cortical dysplasia type II (FCDII) is the most common cause of drug-resistant focal epilepsy in children. Herein, we performed a deep histopathology-based genotype–phenotype analysis to further elucidate the clinico-pathological and genetic presentation of FCDIIa compared to FCDIIb. Seventeen individuals with histopathologically confirmed diagnosis of FCD ILAE Type II and a pathogenic variant detected in brain derived DNA whole-exome sequencing or mTOR gene panel sequencing were included in this study. Clinical data were directly available from each contributing centre. Histopathological analyses were performed from formalin-fixed, paraffin-embedded tissue samples using haematoxylin–eosin and immunohistochemistry for NF-SMI32, NeuN, pS6, p62, and vimentin. Ten individuals carried loss-of-function variants in the GATOR1 complex encoding genes DEPDC5 (n = 7) and NPRL3 (n = 3), or gain-of-function variants in MTOR (n = 7). Whereas individuals with GATOR1 variants only presented with FCDIIa, i.e., lack of balloon cells, individuals with MTOR variants presented with both histopathology subtypes, FCDIIa and FCDIIb. Interestingly, 50% of GATOR1-positive cases showed a unique and predominantly vacuolizing phenotype with p62 immunofluorescent aggregates in autophagosomes. All cases with GATOR1 alterations had neurosurgery in the frontal lobe and the majority was confined to the cortical ribbon not affecting the white matter. This pattern was reflected by subtle or negative MRI findings in seven individuals with GATOR1 variants. Nonetheless, all individuals were seizure-free after surgery except four individuals carrying a DEPDC5 variant. We describe a yet underrecognized genotype–phenotype correlation of GATOR1 variants with FCDIIa in the frontal lobe. These lesions were histopathologically characterized by abnormally vacuolizing cells suggestive of an autophagy-altered phenotype. In contrast, individuals with FCDIIb and brain somatic MTOR variants showed larger lesions on MRI including the white matter, suggesting compromised neural cell migration.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40478-023-01675-x.
Introduction
Histopathological assessment of epilepsy surgery human brain specimens revealed focal cortical dysplasia ILAE type II (FCDII) as the single most common cause of drug-resistant focal epilepsy in children and the third most common in adults [12]. Almost all individuals with FCDII present with epileptic seizures at an early age and continuous rhythmic spiking in electroencephalography [14, 48, 63]. The ILAE classification scheme for FCD further separates subtype IIa with dysmorphic neurons from IIb with dysmorphic neurons and balloon cells [13, 44]. The co-registration of intracerebral electroencephalography with histopathology suggested dysmorphic neurons as the cellular source for interictal and ictal neurophysiological events in FCDII [53]. This correlation was not reported for balloon cells, which is another important signature cell population in FCDIIb [15, 53]. Dysmorphic neurons are microscopically defined by abnormal orientation, enlarged cell bodies, cytoplasmic accumulation of neurofilament proteins and pS6 immunoreactivity indicating constitutive activation of the mTOR signalling pathway [26, 27]. Indeed, most published cases with histopathologically confirmed FCDII can be genetically defined by brain somatic mosaicism of mTOR signalling pathway genes [9, 24]. Pathogenic variants in AKT3, DEPDC5, MTOR, NPRL2, NPRL3, PIK3CA, PTEN, RHEB, TSC1, and TSC2 have been reported previously [2, 17, 33, 35, 40, 47, 51]. The diagnostic yield of such mTOR-related variants in all cortical dysplasias is in the range of 20–80% of individuals, mainly depending on the histopathology subtype (hemimegalencephaly > FCDII), diagnostic genetic testing methods (digital droplet PCR > panel sequencing) and access to histopathologically characterized tissue before extracting DNA [24]. Notably, laser microdissection experiments could allocate these pathogenic variants directly to the affected population of dysmorphic neurons and balloon cells [2, 36].
The most commonly recognized brain somatic variants in FCDII are affecting MTOR [34, 37, 47], especially in FCDIIb [21, 45, 61]. Gain-of-function MTOR variants activate the mTOR complex 1, a central module of the mTOR signalling pathway, regulating cell growth [28, 32, 39], survival [28, 50] and migration [1]. Experimental in-utero electroporation of gain-of-function MTOR variants into the developing mouse cortex cause cytomegalic, pS6 immunoreactive neurons, cortical dyslamination and intractable epilepsy [37]. In contrast, most DEPDC5 variants are loss-of-function germline variants [4, 20, 22]. Together with NPRL2 and NPRL3, DEPDC5 is part of the GATOR1 complex which senses the amino acid content of the cell as a negative regulator of mTORc1 [3]. As confirmed in independent individual series, an additional brain somatic second hit in DEPDC5 is likely necessary to constitutively activate mTORC1 in the affected tissue [2, 4, 36, 40, 54, 56].
Despite advances in our understanding of FCDII, diagnostic methods and disease classification schemes, recognition of FCDII subtypes remains challenging in clinical practice [13, 44, 49, 57]. Magnetic resonance imaging (MRI) techniques became most helpful in identifying these cortical malformations [5], which range in size from hemispheric dysplasias, i.e. hemimegalencephaly, to subtle bottom-of-sulcus FCDII [21, 63]. Postsurgical outcomes often correlate with the visibility of the lesion by MRI, as it compromises and delays the decision-making for surgical treatment and completeness of the surgical resection field when MRI is negative [59, 62]. Herein, we performed a deep histopathology-based genotype–phenotype analysis to study the value of an integrated molecular, histopathology and clinical diagnosis of FCDIIa and IIb subtypes as recently suggested by ILAE’s FCD classification update from 2022 [44].
Material and methods
Individuals included in this study
We included 17 individuals (8 female, 9 male, Table 1) with drug-resistant focal epilepsy, who underwent epilepsy surgery, received a diagnosis of FCDII, and positive genetic testing of DNA obtained from brain tissue (for more details see Table 1 and [40, 47]). Their mean age at seizure onset was 3.47 years [0–17.5; median 1.75 years], and the mean disease duration until surgery was 10.8 years [0.4–32.5; median 7 years]. Clinical data were retrieved from hospital archives. Presurgical MRI findings were reviewed by experienced neurologists and classified into (a) ‘negative’, (b) ‘subtle’, e.g., T1/T2/FLAIR signal intensity changes, blurred grey/white matter junction, with or without alterations in gyral patterning, (c) ‘distinct’ with the additional recognition of a transmantle sign or (d) ‘hemispheric’ lesions with involvement of more than one lobe. The postsurgical outcome was classified according to Engel [23]. The University of Erlangen ethical review board approved the study under agreement number 193_18B.
Table 1.
Individuals included in the study
| ID | Age | Onset | Diagnosis | Gene variant/Ref. | VAF | Family history | FU |
|---|---|---|---|---|---|---|---|
| 1 | 33 | 0.5 | FCD IIa | DEPDC5 (SNV) [47] | Germline | None | IVA (48) |
| 2 | 20 | 4.5 | FCD IIa | DEPDC5 (SNV) [2] | Germline | None | IIA (24) |
| 3 | 42 | 5.5 | FCD IIa | DEPDC5 (CNN-LOH) [37] | Somatic (3.7%) | Father’s sister | IIB (24) |
| 4 | 12 | 2.8 | FCD IIa | DEPDC5 (SNV + LOH) [37] | Two-hit* | None | IIA (48) |
| 5 | 36 | 9 | FCD IIa | DEPDC5 (SNV) [37] | Germline | Brother son | IA (24) |
| 6 | 3 | 0.25 | FCD IIa | DEPDC5 (CNN-LOH) [37] | Somatic (4.3%) | None | IA (24) |
| 7 | 1.5 | 0.4 | FCD IIa | DEPDC5 (SNV)§ | Germline | Sister | IA (24) |
| 8 | 5 | 0.7 | FCD IIa | NPRL3 (SNV) [37] | Germline | None | IA (24) |
| 9 | 0.4 | 0 | FCD IIa | NPRL3 (SNV) [37] | Germline | None | IA (16) |
| 10 | 17 | 3.5 | FCD IIa | NPRL3 (SNV) [47] | Somatic (4.5%) | None | IA (24) |
| 11 | 0.5 | 0.1 | FCD Iib/HME | MTOR (SNV) [37] | Somatic (10.5%) | None | IA (24) |
| 12 | 40 | 17.5 | FCD IIa | MTOR (SNV) [37] | Somatic (5%) | None | IA (24) |
| 13 | 0.8 | 0.3 | FCD IIa/HME | MTOR (SNV) [37] | Somatic (3.4%) | None | IA (24) |
| 14 | 10 | 3 | FCD IIb | MTOR (SNV) [37] | Somatic (5.3%) | None | IA (24) |
| 15 | 19 | 9.5 | FCD IIb | MTOR (SNV) [37] | Somatic (3.5%) | None | IA (24) |
| 16 | 0.9 | 0.2 | FCD IIb | MTOR (SNV) [37] | Somatic (3.1%) | None | IA (24) |
| 17 | 0.6 | 0 | FCD IIb/PMG | MTOR (SNV) [37] | Somatic (4.3%) | None | IA (24) |
Age at surgery in years; Onset: age at seizure onset; sex not shown with 50% males versus 50% females for FCD IIa and FCD IIb, respectively; Lobe: Epileptogenic focus (resulting from EEG, MRI and clinical evaluation)
F frontal, T temporal, P parietal, mult multiple lobes, O occipital; Gene variant results obtained from genetic testing, SNV single nucleotide variant, CNN-LOH copy number neutral loss of heterozygosity, VAF variant allelic frequency, Ref. specific mutation described in cited reference
*Germline variant at VAF = 43.1% and second hit somatic loss of heterozygosity at VAF = 3.7%
$Variant not previously published and specified below; Diagnosis histopathology diagnosis, HME hemimegalencephaly, PMG polymicrogyria, FU Follow-up 2 years after surgery according to Engel`s classification: IA completely seizure free, IIA initially seizure-free, rare seizures now, IIB rare seizures since surgery, IVA no worthwhile improvement (latest available FU in month after surgery)
Histopathological analysis
Native surgical specimens were sectioned into 5 mm thick slabs. Representative slab(s) were fresh frozen in liquid nitrogen and stored at − 80 °C until further use [8]. The remaining tissue slabs were formalin-fixed overnight and paraffin-embedded (FFPE). Four µm thin FFPE sections were cut for each block and stained with haematoxylin–eosin. Selected blocks were further processed for Bielschowski silver staining and/or immunohistochemistry including antibodies directed against: Neurofilament-non phosphorylated (NF-SMI; clone SMI32, mouse, BioLegend, dilution 1:500), Phospho-S6 Ribosomal Protein (Ser235/236) (pS6, rabbit, Cell Signaling, dilution 1:1000), Vimentin (Vim, rabbit, Thermo, dilution 1:1000), Neuronal Nuclei (NeuN, clone A60, mouse, Millipore, dilution 1:500), Ubiquitin-binding p62/ Anti-Sequestosome 1 (p62/SQSTM1, clone P0067, rabbit, Sigma Aldrich, dilution 1:100). Immunofluorescence stainings were also performed with the p62-antibody (dilution 1:1000) and NF-SMI (dilution 1:1000) and further studies using confocal laser scanning microscopy (LSM 780, ZEISS, Germany).
Immunohistochemical stainings were performed on the Ventana BenchMark ULTRA Immunostainer using the OptiView Universal DAB Detection Kit (Ventana Medical Systems, Tucson, AZ, USA). All slides were digitalized by the Hamamatsu Nanozoomer S60. Further digital analysis was performed with QuPath v.0.3.0. We established a quantitative cell analysis for dysmorphic neurons (DN) via a single-pixel classifier. This classifier was trained individually for every sample with six representative dysmorphic neurons each and applied to two 1 mm2 tiles in the region of interest. DN were counted automatically as classifier-created objects, with a minimum size of 400 µm2 imposed. Balloon-cell quantification had to be done manually, as they were not susceptible to the pixel classifier. Here we used Vimentin staining and analysed three 1 mm2 tiles, respectively. In addition, we have reviewed our case series with reference to displaced dysmorphic neurons remote from the focal abnormality (defined as > 5 mm), which was graded into three levels: + = single DN > 5 mm remote from the lesion, ++ = small clusters of DN > 5 mm remote from the lesion; +++ = abundant DN > 5 mm and in continuity to the core lesion. The remote DN were identified by pS6- and SMI32 immunoreactivity.
Genetic analysis
Whole exome sequencing was performed in DNA obtained from 13 individuals as described by Lopez-Rivera and coworker [37] (see Table 1). Variants detected in surgical brain tissue of two other individuals were previously described by Niestroj and coworker [47]. One DEPDC5 germline variants was previously described by Baldassari and coworker [2]. The DEPDC5 variant of individual #7 was also identified from blood cell derived DNA.
Results
Genetic analysis
All 17 individuals showed pathogenic variants related to the mTOR signalling pathway, i.e., DEPDC5 (n = 7) and NPRL3 (n = 3) associated with the GATOR1 complex, and MTOR (n = 7, Table 1). The mean variant allele frequency was 0.4329 for germline variants [± 0.0162] and 0.0467 for somatic variants [± 0.0194]. Among the GATOR1c variants, seven individuals had germline variants, two of which had a family history, and four individuals had somatic variants (one of them revealing both). All MTOR variants were brain mosaicism.
Histopathology
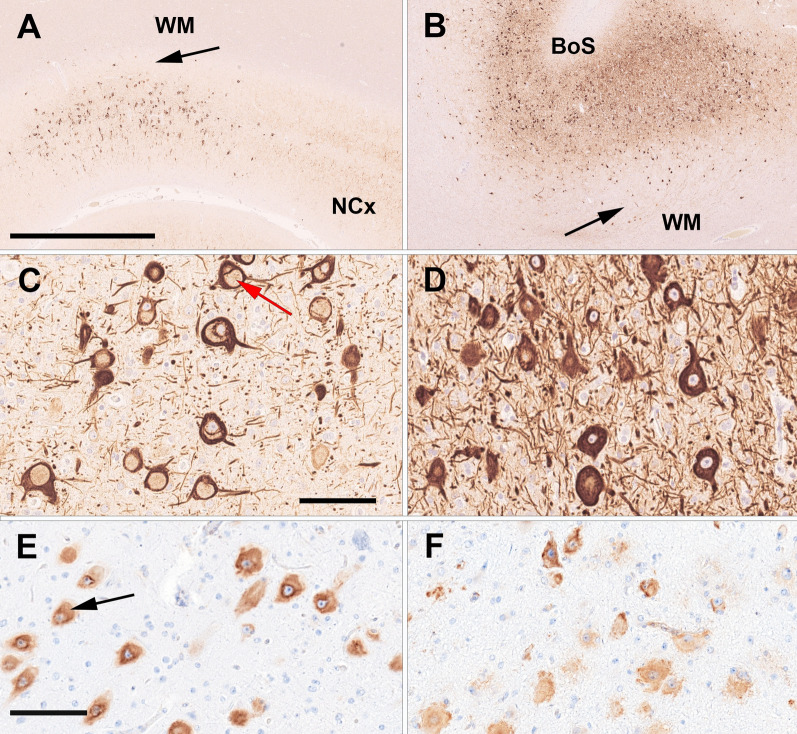
In all cases, a systematic histopathological analysis revealed an association between GATOR1 variants DEPDC5 and NPRL3 with FCD ILAE type IIa (Table 1). In contrast, five specimens with MTOR variants also revealed balloon cells, defining them as FCD ILAE type IIb. Three individuals with MTOR brain mosaicism had additional pathologies (HME n = 2, PMG n = 1). pS6 immunoreactivity was performed in all tissue specimens and confirmed activation of mTOR signalling in affected dysmorphic neurons and/or balloon cells (Fig. 1E/F). All FCDIIa lesions affected only the neocortex without spread into adjacent white matter (Fig. 1A). This observation was in contrast to MTOR-associated FCDIIb, with all lesions affecting the neocortex and the white matter (Fig. 1B).
Fig. 1.
Histopathology findings of FCD ILAE Type IIa and IIb. A 42-year-old male individual with frontal lobe epilepsy since age 5 years (ID3). The MRI was suspicious for a bottom-of-sulcus FCD with transmantel sign. Histopathology confirmed, however, FCDIIa and a pathogenic DEPDC5 mosaicism. The arrow points to the sharp border between the cortical FCDIIa and the normal-appearing white matter (WM). NCx—adjacent normal 6-layered neocortex. Neurofilament SMI32 immunohistochemistry. Scale bar = 2.5mm (applies also to B). Higher magnification in C reveals dysmorphic neurons with a predominant vacuolizing phenotype (red arrow) suggesting accumulation of lipofuscins and an altered autophagy pathway. Scale bar = 100 µm (applies also to D). Same neurons were also labeled with antibodies directed against the phospho-S6-Ser236 epitope (E). The black arrow in (E) points to a neuron with a vacuolizing phenotype. Scale bar in E = 100 µm, applies also to (F); B 19-year-old male individual with frontal lobe epilepsy since age nine years (ID15), histopathological confirmed FCDIIb at a bottom-of-sulcus (BoS; higher magnification in D) and a pathogenic MTOR mosaicism. Dysmorphic neurons and balloon cells were aggregated in the neocortex and white matter (arrow in B) compatible with a migration-deficient phenotype. F both, dysmorphic neurons and balloon cells were labelled with antibodies directed against the pS6 Ser236 epitope
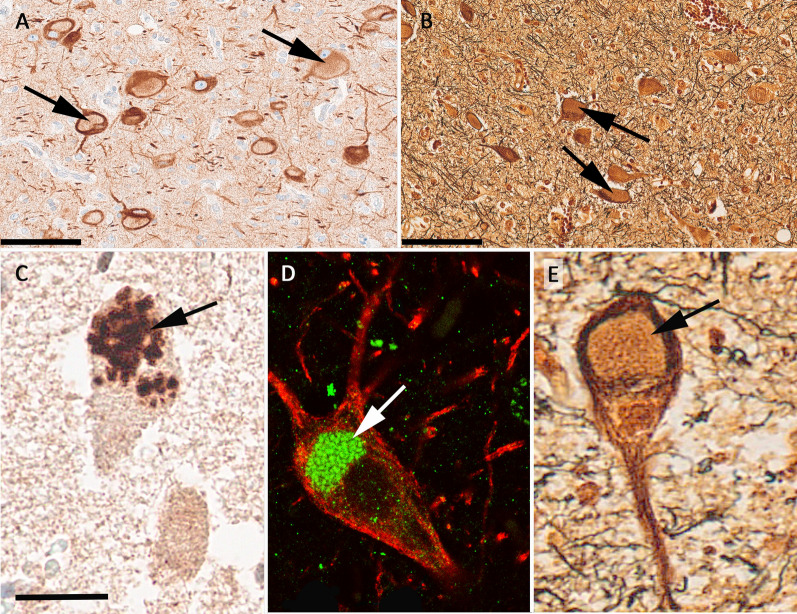
Automatized measurement of the density of dysmorphic neurons did not show any significant difference between the genetic subgroups associated with GATOR1c or MTOR (p = 0.0876). A correlation between VAF and the density of either balloon cells or dysmorphic neurons was also not seen. Five out of 10 cases with GATOR-complex variants had a unique vacuolizing predominant phenotype recognized in neurofilament stainings (NF-SMI32), which could not be detected in any case with MTOR variants (Fig. 2). This was confirmed by a distinct vesicular p62-immunoreactivity pattern (Fig. 2D). Such juxtanuclear p62 accumulation was not recognizable in any sample of FCDIIb carrying MTOR variants. In addition, the cytopathology was mainly localized to the neocortex, i.e., a focal abnormality. This did not exclude the presence of individual pS6- and SMI32-immunoreactive dysmorphic neurons in areas remote from the focal abnormality irrespective from the affected genetic variant, VAF, or germline mutation (Table 2). However, 70% of GATOR positive compared to 28% of MTOR positive FCDII only had single remote DN.
Fig. 2.
Autophagocytic phenotype in DEPDC5 altered FCDIIa. Images were taken from a 42-year-old male individual with drug-resistant focal epilepsy and FCDIIa, DEPDC5 altered (ID3). A juxtanuclear accumulation of autophagosomes can be anticipated from the displacement of neurofilaments (arrow in A as an example, NF-SMI32) and silver impregnation (arrow in B, Bielschowski silver staining, see also higher magnification in E). C p62-immunoreactivity highlighted the aggregation of autophagosomes in FCDIIa. D Further confirmation of the autophagocytic phenotype in FCDIIa by double immunofluorescence and laser scanning microscopy of NF-SMI32 (in red) and p62 (in green). Scale bar in A and B = 100 µm. The scale bar in C = 25 µm, applies also to (D) and (E)
Table 2.
Correlation of neuropathology with neuroimaging findings
| ID | MRI localization | MRI class | MRI alterations in GM and/or WM | Histopath of WM | Balloon cells | vacuolizing phenotype | Remote DN | DN density |
|---|---|---|---|---|---|---|---|---|
| 1 | Left F | Subtle | T2 blurred GM/WM junction with cortical dimple | − | − | + | + | 67 [± 0] |
| 2 | Right F | Negative | None visible | − | − | − | + | NA |
| 3 | Right F | Distinct | FLAIR blurred GM/WM junction, TMS | − | − | + | + | 42.5 [± 3.5] |
| 4 | Right F | Negative | None visible | − | − | + | + | 58 [± 8] |
| 5 | Right F | Subtle | FLAIR blurred GM/WM junction, T1 cortex hyperintensity, no TMS | − | − | + | + | 43.5 [± 11.5] |
| 6 | Left F | Subtle | Cortical thickening, GM/WM T2 hypointense and T1 hyperintense at 3 months, no TMS | − | − | − | ++ | 96.5 [± 9.5] |
| 7 | Left F | Distinct | Cortical thickening, GM/WM T2 hypointense and T1 hyperintense at 10 months, no TMS | − | − | − | ++ | 89 [± 3] |
| 8 | Left TP | Subtle | FLAIR cortical thickening, FLAIR blurred GM/WM junction, no TMS | − | − | − | + | NA |
| 9 | Right FI | Hem | HME—cortical thickening, GM/WM T2 hypo-/T1-hyperintense at 5 months, no TMS | − | − | − | ++ | 42.5 [± 8.5] |
| 10 | Left F | Subtle | T2 + FLAIR cortical thickening T2 + FLAIR blurred GM/WM junction, no TMS | − | − | + | + | 95.5 [± 12.5] |
| 11 | Right FPO | Hem | HME—cortical thickening, GM/WM T2 hypointense at 3 months, no TMS | + | + | − | +++ | 37 [± 2] |
| 12 | Left F | Uncertain | MRI of low quality, pacemaker implantation | + | − | − | ++ | 27 [± 5] |
| 13 | Right H | Hem | HME—cortical thickening, GM/WM T2 hypointense at 3 months, no TMS | − | − | − | ++ | 14.5 [± 4.5] |
| 14 | Left F | Distinct | GM/WM FLAIR hyperintense, T1 hypointense, TMS | + | + | − | +++ | 33 [± 4] |
| 15 | Right F | Distinct | FLAIR blurred GM/WM junction, TMS | + | + | − | + | 73.5 [± 12.5] |
| 16 | Left PO | Distinct | Thickened cortex, GM/WM T2 hyperintense, T1 isointense at 7 months, no TMS | − | + | − | + | 44 [± 1] |
| 17 | Right F | Distinct | Cortical thickening w sulcal dimple, GM/WM T2 hypointense at 5 months, no TMS | + | + | − | +++ | 70 [± 17] |
LID same as in Table 1; MRI findings: All images from MRI datasets were reviewed and classified as “distinct”, e.g., thickened neocortex with distinct signal intensity change in T1, T2 and/or FLAIR with or without transmantle sign (TMS), or “subtle”, e.g. grey/white matter (GM/WM) blurring and/or altered gyration patterns, hem hemispheric, HME hemimegalencephaly, PMG polymicrogyria, WM white matter affection, Balloon cells and vacuolizing phenotype: + positive, − negative, DN density dysmorphic neurons per mm2 as mean ± standard deviation, NA surgical specimen fragmented and not available for analysis; Remote DN dysmorphic neurons remote from the focal abnormality (> 5 mm) graduated in three different levels: + = single DN > 5 mm remote from the lesion, ++ = small clusters of DN > 5 mm remote from the lesion; +++ = abundant DN > 5 mm in continuity to the core lesion; MRI Localization localization of lesion on MRI with side and lobe(s), F frontal, P parietal, O occipital, T temporal, H hemisphere, FLAIR fluid attenuated inversion recovery, T1 T1 weighted image, T2 T2 weighted image
Neuroimaging analysis and postsurgical outcome
Thirteen individuals were seizure-free after surgical treatment (76.5% Engel IA; Table 1). All five individuals not being seizure free shared the same histopathology diagnosis of FCDIIa and carried DEPDC5 variants (Table 1). Two individuals with DEPDC5 germline variants also had a positive family history of epilepsy. There was no significant difference between the histopathologically defined FCDIIa and FCDIIb subtypes concerning age at seizure onset or disease duration. However, we recognized a higher age of GATOR positive patients with vacuolated cells (i.e. 12, 17, 33, 36 and 42 years old at surgery) compared to GATOR positive patients not showing vacuolated neurons (age 0.4, 5, 1.5, 3, 20). Importantly, presurgical MRI findings were subtle in five and negative in two individuals with FCDIIa compared to distinct lesions in four and a hemispheric lesion of individuals with FCDIIb (Fig. 3). All individuals with DEPDC5 variants had their neurosurgical resection confined to the frontal lobe, while three individuals with MTOR variants had multilobar lesions and frontal involvement in six of seven cases. There was no difference in the hemispheric side of FCDIIa or FCDIIb.
Fig. 3.
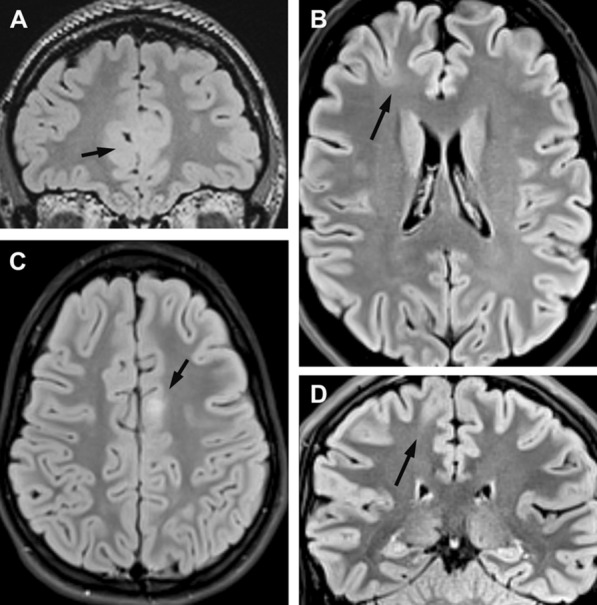
Representative FLAIR neuroimaging findings of FCDIIa, DEPDC5 altered and FCDIIb, MTOR altered. A individual ID4 with histopathologically confirmed FCDIIa and a two-hit DEPDC5 variant. The MRI showed no definite lesion and the right fronto-mesial lobe was surgically removed (arrow); B individual ID5, classified as bottom-of-sulcus FCD; C individual ID14 with a thickened neocortex and distinct signal intensity change in FLAIR; D individual ID15 with a distinct transmantle sign; Individual #14 and #15 both revealed somatic MTOR variants
Discussion
Our study revealed a hitherto under-recognized genotype–phenotype association for FCDIIa with (1) GATOR1 complex variants in the frontal lobe, i.e., DEPDC5 and NPRL3, (2) subtle MRI visibility of the lesion, and (3) the lesion restricted to the neocortex at the microscopy level and autophagosome accumulation in dysmorphic neurons. Importantly, four of ten individuals with FCDIIa were not seizure-free following surgical treatment. This contrasted the genotype–phenotype association of FCDIIb, as all individuals carried a brain somatic MTOR variant, had distinct and clearly visible MRI lesions, and were seizure-free after surgery. At the microscopy level, MTOR-positive FCDIIb revealed a migration-deficient phenotype retaining dysmorphic neurons and balloon cells in the white matter.
Our current literature search for studies reporting the genotype of histopathologically well-characterized FCD ILAE Type II lesions identified ten articles (Table 3). We can readily anticipate from these studies a predominant association of 77% of FCDIIb with mTORC1 alterations, whereas GATOR1 alterations were detected in 67% of all genetically positive FCDIIa. In our case series, however, all GATOR1-positive lesions were histopathologically characterized by FCDIIa, whereas two other FCDIIa lesions were associated with MTOR variants (Table 1). This observation is in line with five of the seven published series reporting genetically confirmed FCDIIa (Table 3), raising this association to 22 of 23 cases (96%). Notwithstanding, it also reiterates the issue of small sample numbers in such case studies and the difficulty of reliably classifying the FCD subtype at the histopathology level [10, 16, 18].
Table 3.
Previously published FCDII lesions with a positive genetic finding and histopathologically proven using the 2011 ILAE classification scheme
| References | # of FCDIIgenetic+ of all individuals | FCDIIaGATOR1+ of all FCD IIa | FCDIIaMTORC1 of all FCD IIa | FCDIIbMTORC1 of all FCD IIb | FCDIIbGATOR1+ of all FCD IIb |
|---|---|---|---|---|---|
| Lim et al. [37] | 12/12 | – | 5/12 | 7/12 | – |
| Nakashima et al. [45] | 6/6 | – | – | 6/6 | – |
| Baulac et al. [4] | 2/4 | 2/2 | – | – | – |
| D’Gama et al. [21] | 5/18 | 2/2 | – | 2/3 | – |
| Ying et al. [63] | 1/10 | 1/1 | – | – | – |
| Niestroj et al. [47] | 4/15 | 2/3 | – | 1/1 | – |
| Baldassari et al. [2] | 34/43 | 5/19 | 10/19 | 9/15 | – |
| Blümcke et al. [10] | 4/22 | 1/3 | 1/3 | 1/1 | – |
| Wang et al. [61] | 10/20 | – | – | 10/10 | – |
| Wang et al. [60] | 15/50 | 15/15 | – | – | – |
| This study | 17/17 | 10/12 | 2/12 | 5/5 | – |
| Total | 110/217 | 38/57 (67%) | 18/46(39%) | 41/53 (77%) | – |
# of FCDIIgenetic+ of all individuals number of histopathologically confirmed FCDII with a genetic lesion/of all reported individuals, FCDIIaGATOR1+ FCD ILAE Type IIa with a variant directly affecting the GATOR1 complex, e.g. DEPDC5 or NPRL3/of all reported FCDIIagenetic+ in that study, FCDIIbMTORC1 FCD ILAE Type IIb with a variant in MTORC1 activating genes of all reported FCDIIbgenetic+ in that study, FCDIIbGATOR1+ FCD ILAE Type IIb with a variant in GATOR1 complex of all reported FCDIIbgenetic+ in that study, FCDIIaMTORC1 FCD ILAE Type IIa with a variant in MTOR complex 1 activating genes of all reported FCDIIagenetic+ in that study. The publication of Lopez-Rivera et al. [40] was excluded herein as it included same cases used in the present study
Interestingly, a previous case study described dysmorphic neurons with significant lipofuscin accumulation as a new disease entity in six individuals, i.e., focal neuronal lipofuscinosis (NFL), distinct from FCD Type IIa or IIb [38], as confirmed by a recent and independent case report [43]. However, these studies did not include any genetic analysis. At the same time the clinico-pathological similarity to our individual series is overwhelming [38]: (1) loss of DEPDC5 immunoreactivity in the population of dysmorphic neurons, (2) activation of the autophagy pathway, (3) lesions confined to the neocortex not involving the white matter, and (4) subtle MRI findings, respectively. Their observation prompted them to conclude a new disease entity separate from FCD ILAE Type II, when compared to six individuals with FCDIIb or seven individuals without any histopathology findings used as control. Although we cannot exclude that p62-immunoreactive aggregates include other material than just lipofuscins or that the Ser236 antibody used herein may have different labelling properties compared to the Ser240 epitope, we suggest our five cases and NFL being the same disease to be classified according to the updated FCD classification scheme of 2022 [44] as: MRI-positive DEPDC5- or NPRL3-altered Focal Cortical Dysplasia ILAE Type IIa.
Loss-of-function variants of the GATOR1 subunits DEPDC5, NPRL2, and NPRL3 in FCDIIa result in constitutive activation of mTORC1 [3, 4, 58] and are likely similar to that mediated by activating MTOR variants. Yet, we cannot explain the predominant autophagocytic phenotype in dysmorphic neurons of FCDIIa and could not directly measure an up or down regulation of autophagy in the FFPE tissue samples [25]. However, evidence for a change in autophagy rate was provided by the study proposing the NLF phenotype [38].
Autophagy and neuronal migration dysregulations can be assigned to the portfolio of the mTOR signalling pathway [30, 37]. In contrast to GATOR1 complex variants, individuals with FCDIIb and brain somatic MTOR variants showed larger lesions, including the white matter in histopathology, which was often reflected also by MRI findings [61]. This suggested a stronger pathogenic focus on neuronal migration in the latter group. Experimental evidence showed that activation of mTORC1 leads to impaired cortical lamination of cytomegalic neurons and cortical hyperplasia in adult animals [19, 21, 31]. Intriguingly, a migration defect was primarily attenuated in our FCDIIaGATOR1+ group. These phenotypic differences associated with MTOR or GATOR1 variants implicate divergent mechanisms during cortical development despite convergent mTORC1 hyperactivation. The timing of the acquired gene alteration and the targeted cell population may explain these differences, as previously shown in experimental animal models [10, 21, 37, 46]. In addition a DEPDC5 two-hit model may be needed to cause FCDIIa [2, 4, 36, 40, 54, 56], as observed in this study's carrier of a germline/somatic DEPDC5 double-hit variant.
The predictability of postsurgical seizure freedom is of great concern for individual management and often guides the decision-making process [29]. MRI visibility of a circumscript lesion and its surgical accessibility are regarded as key factors for good outcome, however. Interestingly, the limitation of FCDIIa, GATOR1-altered lesions, to the neocortex represented a histopathological correlate for subtle or negative MRI findings in our small case series, e.g., lack of a transmantle sign (Fig. 3). This may help to understand another observation in the landscape of epilepsy-related GATOR1 variants, where the most frequent entities are focal epilepsies and often present with MRI-negative/-subtle findings [4]. We also reviewed the published literature on postsurgical outcome in genetically proven FCDII and identified a recent review of 8 children with GATOR positive drug resistant focal epilepsy, of which 4 children did not become seizure free [55]. Their further literature review of GATOR positive cases, where the most encountered pathology was FCDIIa, indicated an overall seizure freedom rate of 60%, a number very similar to ours reported in the current study [55]. Although there was no evidence of any residual dysplasia on postoperative MRI in our cases, that were not seizure free, glial scarring at the resection borders may have obscured such findings.
Genotype–phenotype associations come of age in neuropathology, foremost diagnosing brain tumours, as controversies in microscopic agreement compromised the liability of the histopathology report for decades [7, 11, 52]. Insights into the molecular pathogenesis to better understand the underlying cause, disease prognosis, or targeted treatment options further promoted the integration of molecular neuropathology into clinical practice [41, 42]. A classic example is that of mixed oligo-astrocytoma which became obsolete in the 5th edition of the WHO brain tumour classification scheme [42]. The international consensus FCD classification update 2022 also introduced an integrated classification scheme of histopathological and molecular layers [6, 44]. Such integrated diagnosis will improve our understanding of FCD subtypes and their clinical management and help develop targeted treatment options. Increasing the availability and access to smart drugs targeting mTOR-associated genes will further strengthen the ILAE approach to classify FCD at an integrated molecular pathology level. However, the distinction between FCDIIa and FCDIIb remained often academic as the presence or absence of balloon cells can be a subjective measure when trying to differentiate reactive, gemistocytic astrocytes in areas targeted by intracerebral EEG recordings or from surgical sampling errors in small lesions or functional hemispherotomy. Interrater disagreement due to lack of training facilities or access to specific laboratory resources and protocols may represent another obstacle, e.g., an immunohistochemistry panel for epilepsy [8, 10]. The current individual cohort took all available precaution to rule out such bias and revealed a robust genotype–phenotype association impacting surgical outcomes.
In conclusion, our study revealed phenotypic and genotypic signatures in FCD subtype IIa and IIb despite a close relationship of affected mTOR pathway genes, i.e., DEPDC5, NPRL3, and MTOR, as well as converging histopathology findings, e.g., cortical dyslamination and dysmorphic neurons. Their association with MRI visibility or adverse surgical outcome will gain attention. It will have consequences in the search for precision medicine tools, e.g., presurgical germline testing for GATOR1-associated candidate genes, as more personalized treatment options will become available. This strategy aligns with contemporary disease classification schemes in histopathology integrating the clinical, microscopic and molecular level to help better understand difficult-to-diagnose FCD.
Supplementary Information
Additional file 1. Genetic findings with detailed variant descriptions.
Acknowledgements
The present work was performed in fulfilment of the requirements of the Friedrich-Alexander Universität Erlangen-Nürnberg (FAU) for obtaining the degree ‘Dr. med.’ of Jonas Honke. We kindly thank our colleague Manfred Kudernatsch from the Schoen-Klinik in Vogtareuth for providing tissue samples. Lisa-Marie Niestroj provided the genetic analysis of two individuals included herein. We also thank Julia Salzseiler, Birte Rings, Verena Kollera and Monika Bröckl from Erlangen for their excellent help with the histopathology work up of the FFPE tissue samples.
Author contributions
JH, LH, RC and IB planed the research, microscopically studied all surgical tissue samples and wrote the first draft of the manuscript. KK, CL, JRL, SaB, SB, DL and PN studied the genetic landscape of the tissue specimens included in this research and edited the manuscript. TP, TH, CGB, FW, TC, TK, AG, HH, SBr, KR, AD and SR provided clinical information, review3ed neuroimaging data, shared surgical tissue samples and edited the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. KK, AD, SR and IB are supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Project Number 460333672 – CRC1540 Exploring Brain Mechanics. IB, PN, and DL received further support from the German Research Council (Bl421/4-1).
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional file 1.
Declarations
Ethics approval and consent to participate
The University of Erlangen ethical review board approved the study under agreement number 193_18B.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andrews MG, Subramanian L, Kriegstein AR. mTOR signaling regulates the morphology and migration of outer radial glia in developing human cortex. Elife. 2020;9:e58737. doi: 10.7554/eLife.58737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldassari S, Ribierre T, Marsan E, Adle-Biassette H, Ferrand-Sorbets S, Bulteau C, Dorison N, Fohlen M, Polivka M, Weckhuysen S, et al. Dissecting the genetic basis of focal cortical dysplasia: a large cohort study. Acta Neuropathol. 2019;138:885–900. doi: 10.1007/s00401-019-02061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baulac S, Ishida S, Marsan E, Miquel C, Biraben A, Nguyen DK, Nordli D, Cossette P, Nguyen S, Lambrecq V, et al. Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 variants. Ann Neurol. 2015;77:675–683. doi: 10.1002/ana.24368. [DOI] [PubMed] [Google Scholar]
- 5.Bernasconi A, Cendes F, Theodore WH, Gill RS, Koepp MJ, Hogan RE, Jackson GD, Federico P, Labate A, Vaudano AE, et al. Recommendations for the use of structural magnetic resonance imaging in the care of patients with epilepsy: a consensus report from the International League Against Epilepsy Neuroimaging Task Force. Epilepsia. 2019;60:1054–1068. doi: 10.1111/epi.15612. [DOI] [PubMed] [Google Scholar]
- 6.Blumcke I. It is time to move on: commentary to: genotype–phenotype correlations in focal malformations of cortical development: a pathway to integrated pathological diagnosis in epilepsy surgery. Brain Pathol. 2019;29:467–468. doi: 10.1111/bpa.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blümcke I, Aronica E, Becker A, Capper D, Coras R, Honavar M, Jacques TS, Kobow K, Miyata H, Mühlebner A, et al. Low-grade epilepsy-associated neuroepithelial tumours—the 2016 WHO classification. Nat Rev Neurol. 2016;12:732–740. doi: 10.1038/nrneurol.2016.173. [DOI] [PubMed] [Google Scholar]
- 8.Blumcke I, Aronica E, Miyata H, Sarnat HB, Thom M, Roessler K, Rydenhag B, Jehi L, Krsek P, Wiebe S, et al. International recommendation for a comprehensive neuropathologic workup of epilepsy surgery brain tissue: a consensus Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2016;57:348–358. doi: 10.1111/epi.13319. [DOI] [PubMed] [Google Scholar]
- 9.Blumcke I, Budday S, Poduri A, Lal D, Kobow K, Baulac S. Neocortical development and epilepsy: insights from focal cortical dysplasia and brain tumours. Lancet Neurol. 2021;20:943–955. doi: 10.1016/S1474-4422(21)00265-9. [DOI] [PubMed] [Google Scholar]
- 10.Blumcke I, Coras R, Busch RM, Morita-Sherman M, Lal D, Prayson R, Cendes F, Lopes-Cendes I, Rogerio F, Almeida VS, et al. Toward a better definition of focal cortical dysplasia: an iterative histopathological and genetic agreement trial. Epilepsia. 2021;62:1416–1428. doi: 10.1111/epi.16899. [DOI] [PubMed] [Google Scholar]
- 11.Blumcke I, Coras R, Wefers AK, Capper D, Aronica E, Becker A, Honavar M, Stone TJ, Jacques TS, Miyata H, et al. Challenges in the histopathological classification of ganglioglioma and DNT: microscopic agreement studies and a preliminary genotype-phenotype analysis. Neuropathol Appl Neurobiol. 2019;45:95–107. doi: 10.1111/nan.12522. [DOI] [PubMed] [Google Scholar]
- 12.Blümcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, Pfafflin M, Elger C, Widman G, Schramm J, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med. 2017;377:1648–1656. doi: 10.1056/NEJMoa1703784. [DOI] [PubMed] [Google Scholar]
- 13.Blümcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, Jacques TS, Avanzini G, Barkovich AJ, Battaglia G, et al. The clinico-pathological spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boonyapisit K, Najm I, Klem G, Ying Z, Burrier C, LaPresto E, Nair D, Bingaman W, Prayson R, Luders H. Epileptogenicity of focal malformations due to abnormal cortical development: direct electrocorticographic-histopathologic correlations. Epilepsia. 2003;44:69–76. doi: 10.1046/j.1528-1157.2003.08102.x. [DOI] [PubMed] [Google Scholar]
- 15.Cepeda C, Hurst RS, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Boylan MK, Calvert CR, Jocoy EL, Nguyen OK, Andre VM, et al. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J Neurosci Res. 2003;72:472–486. doi: 10.1002/jnr.10604. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain WA, Cohen ML, Gyure KA, Kleinschmidt-DeMasters BK, Perry A, Powell SZ, Qian J, Staugaitis SM, Prayson RA. Interobserver and intraobserver reproducibility in focal cortical dysplasia (malformations of cortical development) Epilepsia. 2009;50:2593–2598. doi: 10.1111/j.1528-1167.2009.02344.x. [DOI] [PubMed] [Google Scholar]
- 17.Chung C, Yang X, Bae T, Vong KI, Mittal S, Donkels C, Westley Phillips H, Li Z, Marsh APL, Breuss MW, et al. Comprehensive multi-omic profiling of somatic variants in malformations of cortical development. Nat Genet. 2023;55:209–220. doi: 10.1038/s41588-022-01276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coras R, de Boer OJ, Armstrong D, Becker A, Jacques TS, Miyata H, Thom M, Vinters HV, Spreafico R, Oz B, et al. Good interobserver and intraobserver agreement in the evaluation of the new ILAE classification of focal cortical dysplasias. Epilepsia. 2012;53:1341–1348. doi: 10.1111/j.1528-1167.2012.03508.x. [DOI] [PubMed] [Google Scholar]
- 19.Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12:379–392. doi: 10.1038/nrneurol.2016.81. [DOI] [PubMed] [Google Scholar]
- 20.D'Gama AM, Geng Y, Couto JA, Martin B, Boyle EA, LaCoursiere CM, Hossain A, Hatem NE, Barry BJ, Kwiatkowski DJ, et al. Mammalian target of rapamycin pathway variants cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol. 2015;77:720–725. doi: 10.1002/ana.24357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Gama AM, Woodworth MB, Hossain AA, Bizzotto S, Hatem NE, LaCoursiere CM, Najm I, Ying Z, Yang E, Barkovich AJ, et al. Somatic variants activating the mTOR pathway in dorsal telencephalic progenitors cause a continuum of cortical dysplasias. Cell Rep. 2017;21:3754–3766. doi: 10.1016/j.celrep.2017.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dibbens LM, de Vries B, Donatello S, Heron SE, Hodgson BL, Chintawar S, Crompton DE, Hughes JN, Bellows ST, Klein KM, et al. Variants in DEPDC5 cause familial focal epilepsy with variable foci. Nat Genet. 2013;45:546–551. doi: 10.1038/ng.2599. [DOI] [PubMed] [Google Scholar]
- 23.Engel JJ, Van Ness P, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel JJ, editor. Surgical treatment of the epilepsies. New York: Raven; 1993. pp. 609–621. [Google Scholar]
- 24.Gerasimenko A, Baldassari S, Baulac S. mTOR pathway: insights into an established pathway for brain mosaicism in epilepsy. Neurobiol Dis. 2023;182:106144. doi: 10.1016/j.nbd.2023.106144. [DOI] [PubMed] [Google Scholar]
- 25.Hui KK, Tanaka M. Autophagy links MTOR and GABA signaling in the brain. Autophagy. 2019;15:1848–1849. doi: 10.1080/15548627.2019.1637643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iffland PH, 2nd, Carson V, Bordey A, Crino PB. GATORopathies: the role of amino acid regulatory gene variants in epilepsy and cortical malformations. Epilepsia. 2019;60:2163–2173. doi: 10.1111/epi.16370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iffland PH, 2nd, Crino PB. Focal cortical dysplasia: gene variants, cell signaling, and therapeutic implications. Annu Rev Pathol. 2017;12:547–571. doi: 10.1146/annurev-pathol-052016-100138. [DOI] [PubMed] [Google Scholar]
- 28.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 29.Jehi L, Braun K. Does etiology really matter for epilepsy surgery outcome? Brain Pathol. 2021;31:e12965. doi: 10.1111/bpa.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassai H, Sugaya Y, Noda S, Nakao K, Maeda T, Kano M, Aiba A. Selective activation of mTORC1 signaling recapitulates microcephaly, tuberous sclerosis, and neurodegenerative diseases. Cell Rep. 2014;7:1626–1639. doi: 10.1016/j.celrep.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 33.Lai D, Gade M, Yang E, Koh HY, Lu J, Walley NM, Buckley AF, Sands TT, Akman CI, Mikati MA, et al. Somatic variants in diverse genes leads to a spectrum of focal cortical malformations. Brain. 2022;145:2704–2720. doi: 10.1093/brain/awac117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JH, Huynh M, Silhavy JL, Kim S, Dixon-Salazar T, Heiberg A, Scott E, Bafna V, Hill KJ, Collazo A, et al. De novo somatic variants in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet. 2012;44:941–945. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee WS, Leventer RJ, Lockhart PJ. Droplet digital PCR as a first-tier molecular diagnostic tool for focal cortical dysplasia type II. Brain. 2022;145:e119–e121. doi: 10.1093/brain/awac320. [DOI] [PubMed] [Google Scholar]
- 36.Lee WS, Stephenson SEM, Howell KB, Pope K, Gillies G, Wray A, Maixner W, Mandelstam SA, Berkovic SF, Scheffer IE, et al. Second-hit DEPDC5 variant is limited to dysmorphic neurons in cortical dysplasia type IIA. Ann Clin Transl Neurol. 2019;6:1338–1344. doi: 10.1002/acn3.50815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim JS, Kim WI, Kang HC, Kim SH, Park AH, Park EK, Cho YW, Kim S, Kim HM, Kim JA, et al. Brain somatic variants in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med. 2015;21:395–400. doi: 10.1038/nm.3824. [DOI] [PubMed] [Google Scholar]
- 38.Liu JY, Reeves C, Diehl B, Coppola A, Al-Hajri A, Hoskote C, Mughairy SA, Tachrount M, Groves M, Michalak Z, et al. Early lipofuscin accumulation in frontal lobe epilepsy. Ann Neurol. 2016;80:882–895. doi: 10.1002/ana.24803. [DOI] [PubMed] [Google Scholar]
- 39.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Rivera JA, Leu C, Macnee M, Khoury J, Hoffmann L, Coras R, Kobow K, Bhattarai N, Perez-Palma E, Hamer H, et al. The genomic landscape across 474 surgically accessible epileptogenic human brain lesions. Brain. 2022 doi: 10.1093/brain/awac376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 42.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mhatre R, Jagtap SA, Kurwale N, Santhoshkumar R, Deshmukh Y, Mahadevan A. Frontal lobe epilepsy with focal neuronal lipofuscinosis—case report of a rare entity. Epilepsy Behav Rep. 2020;14:100369. doi: 10.1016/j.ebr.2020.100369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Najm I, Lal D, Vanegas MA, Cendes F, Lopes-Cendes I, Palmini A, Paglioli E, Sarnat H, Walsh CA, Wiebe S, et al. The ILAE consensus classification of focal cortical dysplasia (FCD): an update proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2022;63:1899–1919. doi: 10.1111/epi.17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakashima M, Saitsu H, Takei N, Tohyama J, Kato M, Kitaura H, Shiina M, Shirozu H, Masuda H, Watanabe K, et al. Somatic variants in the MTOR gene cause focal cortical dysplasia type IIb. Ann Neurol. 2015;78:375–386. doi: 10.1002/ana.24444. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen LH, Bordey A. Convergent and divergent mechanisms of epileptogenesis in mtoropathies. Front Neuroanat. 2021;15:664695. doi: 10.3389/fnana.2021.664695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niestroj LM, May P, Artomov M, Kobow K, Coras R, Pérez-Palma E, Altmüller J, Thiele H, Nürnberg P, Leu C, et al. Assessment of genetic variant burden in epilepsy-associated brain lesions. Eur J Hum Genet. 2019;27:1738–1744. doi: 10.1038/s41431-019-0484-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, Tampieri D, Gloor P, Quesney F, Andermann E, et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476–487. doi: 10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- 49.Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, Jackson G, Luders HO, Prayson R, Spreafico R, et al. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–8. doi: 10.1212/01.WNL.0000114507.30388.7E. [DOI] [PubMed] [Google Scholar]
- 50.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pirozzi F, Berkseth M, Shear R, Gonzalez L, Timms AE, Sulc J, Pao E, Oyama N, Forzano F, Conti V, et al. Profiling PI3K-AKT-MTOR variants in focal brain malformations reveals new insights for diagnostic care. Brain. 2022;145:925–938. doi: 10.1093/brain/awab376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramaswamy V, Taylor MD. Fall of the optical wall: freedom from the tyranny of the microscope improves glioma risk stratification. Cancer Cell. 2016;29:137–138. doi: 10.1016/j.ccell.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Rampp S, Rossler K, Hamer H, Illek M, Buchfelder M, Doerfler A, Pieper T, Hartlieb T, Kudernatsch M, Koelble K, et al. Dysmorphic neurons as cellular source for phase-amplitude coupling in focal cortical dysplasia type II. Clin Neurophysiol. 2021;132:782–792. doi: 10.1016/j.clinph.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Ribierre T, Deleuze C, Bacq A, Baldassari S, Marsan E, Chipaux M, Muraca G, Roussel D, Navarro V, Leguern E, et al. Second-hit mosaic variant in mTORC1 repressor DEPDC5 causes focal cortical dysplasia-associated epilepsy. J Clin InvestIG. 2018;128:2452–2458. doi: 10.1172/JCI99384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahly A, Whitney R, Costain G, Chau V, Otsubo H, Ochi A, Donner E, Cunningham J, Jones K, Widjaja E, Ibrahim G, Jain P, et al. Epilepsy surgery outcomes in patients with GATOR1 gene complex variants: Report of new cases and review of literature. Seizure Eur J Epilepsy. 2023;107:13–20. doi: 10.1016/j.seizure.2023.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Sim NS, Ko A, Kim WK, Kim SH, Kim JS, Shim KW, Aronica E, Mijnsbergen C, Spliet WGM, Koh HY, et al. Precise detection of low-level somatic variant in resected epilepsy brain tissue. Acta Neuropathol. 2019;138:901–912. doi: 10.1007/s00401-019-02052-6. [DOI] [PubMed] [Google Scholar]
- 57.Tassi L, Colombo N, Garbelli R, Francione S, Lo Russo G, Mai R, Cardinale F, Cossu M, Ferrario A, Galli C, et al. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain. 2002;125:1719–1732. doi: 10.1093/brain/awf175. [DOI] [PubMed] [Google Scholar]
- 58.van Kranenburg M, Hoogeveen-Westerveld M, Nellist M. Preliminary functional assessment and classification of DEPDC5 variants associated with focal epilepsy. Hum Mutat. 2015;36:200–209. doi: 10.1002/humu.22723. [DOI] [PubMed] [Google Scholar]
- 59.Von Oertzen J, Urbach H, Jungbluth S, Kurthen M, Reuber M, Fernandez G, Elger CE. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry. 2002;73:643–647. doi: 10.1136/jnnp.73.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, Liu W, Zhang Y, Liu Q, Cai L, Jiang Y. Seizure features and outcomes in 50 children with GATOR1 variants: a retrospective study, more favorable for epilepsy surgery. Epilepsia Open. 2023;8:969–979. doi: 10.1002/epi4.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Yu T, Blumcke I, Cai Y, Sun K, Gao R, Wang Y, Fu Y, Wang W, Wang Y, et al. The clinico-pathological characterization of focal cortical dysplasia type IIb genetically defined by MTOR mosaicism. Neuropathol Appl Neurobiol. 2023;49(1):e12874. doi: 10.1111/nan.12874. [DOI] [PubMed] [Google Scholar]
- 62.Wang ZI, Jones SE, Jaisani Z, Najm IM, Prayson RA, Burgess RC, Krishnan B, Ristic A, Wong CH, Bingaman W, et al. Voxel-based morphometric magnetic resonance imaging (MRI) postprocessing in MRI-negative epilepsies. Ann Neurol. 2015;77:1060–1075. doi: 10.1002/ana.24407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ying Z, Wang I, Blümcke I, Bulacio J, Alexopoulos A, Jehi L, Bingaman W, Gonzalez-Martinez J, Kobow K, Niestroj LM, et al. A comprehensive clinico-pathological and genetic evaluation of bottom-of-sulcus focal cortical dysplasia in patients with difficult-to-localize focal epilepsy. Epileptic Disord. 2019;21:65–77. doi: 10.1684/epd.2019.1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Genetic findings with detailed variant descriptions.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional file 1.