Abstract
Background
Prior studies have demonstrated the negative impact of language barriers on access, quality, and safety of healthcare, which can lead to health disparities in linguistic minorities. As the population ages, those with multiple chronic diseases will require increasing levels of home care and long-term services. This study described the levels of multimorbidity among recipients of home care in Ontario, Canada by linguistic group.
Methods
Population-based retrospective cohort of 510,685 adults receiving home care between April 1, 2010, to March 31, 2018, in Ontario, Canada. We estimated and compared prevalence and characteristics of multimorbidity (2 or more chronic diseases) across linguistic groups (Francophones, Anglophones, Allophones). The most common combinations and clustering of chronic diseases were examined. Logistic regression models were used to explore the main predictors of ‘severe’ multimorbidity (defined as the presence of five or more chronic diseases).
Results
The proportion of home care recipients with multimorbidity and severe multimorbidity was 92% and 44%, respectively. The prevalence of multimorbidity was slightly higher among Allophones (93.6%) than among Anglophones (91.8%) and Francophones (92.4%). However, Francophones had higher rates of cardiovascular and respiratory disease (64.9%) when compared to Anglophones (60.2%) and Allophones (61.5%), while Anglophones had higher rates of cancer (34.2%) when compared to Francophones (25.2%) and Allophones (24.3%). Relative to Anglophones, Allophones were more likely to have severe multimorbidity (adjusted OR = 1.04, [95% CI: 1.02–1.06]).
Conclusions
The prevalence of multimorbidity among Ontarians receiving home care services is high; especially for whose primary language is a language other than English or French (i.e., Allophones). Understanding differences in the prevalence and characteristics of multimorbidity across linguistic groups will help tailor healthcare services to the unique needs of patients living in minority linguistic situations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-023-04267-5.
Keywords: Chronic diseases, Multimorbidity, Home care, Linguistic group, Language barriers, Elders
Introduction
As the population continues to age, the Canadian health care system will be faced with the challenge of providing care to patients with increasing levels of multi morbidity or medical complexity [1]. In 2016, 16.9% of Canadians were over the age of 65; [2] this number is predicted to rise to 25% by 2036 [3]. Older people experience higher rates of health care utilization [4, 5] and are at increased risk of poorer health outcomes [6–10]; this it thought to be a result of the higher prevalence of chronic conditions, multimorbidity, which predisposes these patients to disability and frailty [11–13]. A considerable proportion of Canadians over the age of 65 receive formal support services, including but not limited to home care services. In the fiscal year 2016/17, approximately 760,000 Ontarians (5.8% of the population) received government-funded home care services [14].
Ontario has a publicly funded health care system with a commitment to equitable care for its population, including providing healthcare services in both of Canada’s official languages, English and French [15]. Approximately 5% of Ontarians identify French as their mother tongue; however, the proportion of Francophones can be as high as 30% in regions of the province, such as Eastern Ontario and Northern Ontario [16]. Moreover, approximately 2.5% of Ontarians in 2016 are unable to communicate in one of Canada’s official languages [17]. Despite government efforts to improve the delivery of healthcare services to residents of Ontario living in minority linguistic communities, health disparities related to access, health status (e.g. prevalence of chronic conditions), quality, and safety of care persist across linguistic groups in Ontario [18, 19].
Language is an important socio-cultural factor related to health and wellbeing as well as on access and use of health services [20, 21]. Language barriers impact the amount and quality of information exchanged between a patient and the healthcare providers and their ability to establish rapport, [22] which can impact the quality and safety of care received, and thus affect a patient’s overall health status. While many studies have described the relationship between various socio-demographic characteristics (e.g., sex, age, education, income, immigrant status, and ethnicity) and multimorbidity, [23–25] none have described the prevalence of multimorbidity by linguistic group. In addition, research in Canada have revealed inequalities in the health conditions of official linguistic minorities [19, 26].
The objective of this study was to describe the extent and patterns of multimorbidity among recipients of home care services in Ontario, Canada, stratified by linguistic group. More specifically, we sought to: 1) determine the prevalence of multimorbidity, 2) describe the characteristics and clusters of multimorbidity, and 3) identify the main predictors of severe multimorbidity.
Methods
Study design and population
We conducted a population-based retrospective cohort study in Ontario, Canada, using linked administrative databases. Ontario is Canada’s most populous province, and in 2015–2016, 6.7% of households of the province reported that at least one person received formal home care services in the previous 12 months, which is higher than the national average (6.4%) [27]. The province also account for nearly 80% of the clients admitted in home care based on the assessments conducted in 2020–2021 [28]. Reporting of this study follows guidelines for observational studies using routinely collected health care data (see Appendix 1 in Supplemental material).
We included all people in Ontario who received an assessment using the Resident Assessment Instrument for Home Care (RAI-HC) between April 1, 2010, to March 31, 2018. The RAI-HC is a standardized tool to assess individuals' acute and chronic health care needs and is performed for all people who are referred for publicly-funded home healthcare services in Ontario [29]. For patients with more than one RAI assessment, we used the first assessment in the accrual period as the index event (index RAI assessment) to identify individual characteristics. We excluded individuals who were: older than 105 years of age, not eligible for the Ontario Health Insurance Plan (OHIP), and/or did not have a completed language or age variable on their RAI-HC assessment (see Appendix 2 in Supplementary material).
Data sources
We used administrative databases housed and maintained at ICES (formerly Institute for Clinical Evaluative Sciences). The data were linked using unique encoded identifiers, including: 1) The Resident Assessment Instrument – Home Care (RAI-HC) database, which captures data for Ontario residents receiving publicly funded home care services for at least 60 consecutive days or waiting for admissions to long term care facilities [30]. The RAI-HC database capture data from completed RAI-HC assessments and included baseline sociodemographic (highest-level of education) and health characteristics (Activities of Daily Living Scale [ADL Hierarchy Scale], [31] Cognitive Performance Scale [CPS], [32] Changes in health, End-stage disease, and Signs and Symptoms [CHESS] score, [33] Resource Utilization Grouping III [RUG-III], [34] Charlson index score, a comorbidity index [35] and the number of prescribed medications); 2) the Immigration, Refugees and Citizenship Canada (IRCC) Permanent Resident's Database, which identifies people who immigrated to Canada and became permanent residents after 1985; 3) the Registered Persons Database (RPDB) was used to ascertain demographic characteristics, including age, sex, and resident postal code; 4) the 2016 Statistics Canada Census to generate the study participant’s neighbourhood income quintiles and urban/rural status using postal code data; [36] 5) the Canadian Institute of Health Information (CIHI) Discharge Abstract Database (DAD) and the Ontario Health Insurance Plan (OHIP) database for hospital admissions and physician billings, respectively, to determine chronic disease status using standardized approaches and validated algorithms [37, 38].
In 2007, Ontario’s public healthcare services' planning and distribution were divided into 14 geographically defined local health integration networks (LHINs) [39]. Given the uneven distribution of Francophones in Ontario, we grouped the LHINS into three regions: Northern (comprised of North East and North West LHINs), Eastern (Champlain LHIN), and South-Western (the remaining 11 LHINs).
Exposure
We identified person’s language using the language variable recorded in RAI-HC from the index RAI assessment. During RAI-HC assessments, language is ascertained by the interviewer and, if necessary, by asking the home care recipient report their primary language [40]. We classified Anglophones and Francophones as individuals that spoke English and French, respectively. All remaining individuals were considered as Allophones. We excluded individuals who communicate with non-spoken languages (e.g., artificial languages, sign languages).
Outcomes
Our main outcomes were a) the prevalence of individual chronic disease(s) (see Appendix 2 in Supplementary material) and b) the prevalence of multimorbidity (defined as two or more chronic diseases). The prevalence of chronic diseases was ascertained using algorithms validated and applied in previous studies [38, 41]. We categorized individuals based on their number of chronic diseases (0, 1, 2, 3, 4 and 5 +), and two levels of multimorbidity, 2 + and 3 + [42, 43]. Home care recipients with higher numbers of conditions have been shown to have greater functional decline and poorer health-related quality of life; [41, 44–46] we used the category of five or more chronic diseases (5 + comorbidities) to assess the risk of higher levels of comorbidity by language group in our study.
We used two approaches to examine the patterns of multimorbidity. First, we derived the five most common co-occurring clusters of conditions within each level of comorbidity (i.e., 2, 3, 4, 5) and measured their prevalence (number of individuals with the most common combination in each level of multimorbidity divided by the number of individuals in this level). Second, we followed a non-data driven approach to group the prevalent chronic conditions that uses a clinical criteria, such as the risk adjustment model of the Medicare and Medicaid Services – Hierarchical Conditions Categories (CMS/HCC) [47]. This model applies a risk assessment criteria to create diagnostic categories that are clinically meaningful to clinicians, due to its interpretability and utility for disease management and quality monitoring [48–50]. Similar approaches have been used to define disease groups to study health outcomes and quality of life, and to provide patient-centred care to individuals with multimorbidity [50–52]. Therefore, seven predefined clinical clusters were created (Table 1). First, we included multimorbid individuals with cancer, dementia, or stroke-related condition into one individual cluster. Then, we grouped the remaining multimorbid individuals into the cluster of their first diagnosis.
Table 1.
Clinical clusters of chronic diseases
| Functional Cluster | Chronic disease |
|---|---|
| C1. Cancer | All Cancers |
| C2. Cardio-Respiratory | AMI, Arrhythmia, Asthma, CHF, Coronary Heart Disease, Hypertension, COPD |
| C3. Mental disorders | Mood, anxiety, depression and other nonpsychotic disorders, Other mental health conditions |
| C4. Metabolic-GI-Renal | Diabetes, IBD, Renal disease |
| C5. Muscle-skeletal | Osteoarthritis, Rheumatoid arthritis, Other Arthritis (Synovitis, Fibrositis, Connective tissue disorders, Ankylosing spondylitis, Gout Traumatic arthritis, pyogenic arthritis, Joint derangement, Dupuytren's contracture, Other MSK disorders), Osteoporosis |
| C6. Dementia | Dementia |
| C7. Stroke | Stroke (excluding transient ischemic attack) |
AMI acute myocardial infarction, CHF congestive health failure, COPD chronic obstructive pulmonary disease, IBD inflammatory bowel disease, RA rheumatoid arthritis, MSK Muscle-skeletal
Analysis
We used frequency measures to compare the cohort’s baseline sociodemographic and health characteristics by linguistic group (Francophones, Anglophones, Allophones). We used descriptive analyses to compare the prevalence and characteristics of the comorbidities across linguistic groups.
We fitted multivariable logistic regression models to explore the main predictors of ‘severe’ multimorbidity, adjusted for patient age group (ref. < 50), sex (ref. male), immigration status (ref. long-term residents), neighbourhood income quintile (ref. Q5), rurality (ref. urban), region of the province (ref. southwestern) and the functional health variables (ADL, CPS, CHESS [ref. the lowest scores]).
Ethics approval
ICES is a prescribed entity under Sect. 45 of Ontario's Personal Health Information Protection Act. Section 45 authorizes ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of, allocation of resources to or planning for all or part of the health system. Projects that use data collected by ICES under Sect. 45 of PHIPA, and use no other data, are exempt from REB review. The use of the data in this project is authorized under Sect. 45 and approved by ICES’ Privacy and Legal Office.
Results
A total of 510,685 adults receiving home care services in Ontario met the eligibility criteria and were included in this study. Table 2 presents the cohort’s sociodemographic characteristics. The majority of the cohort were Anglophones (80.2%), followed by Allophones (17.5%) and Francophones (2.3%), 65 years or older (78.9%), and women (58.8%).
Table 2.
Sociodemographic characteristics by linguistic group
| Characteristics | Anglophone | Francophone | Allophone | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| (N = 409,578) | (N = 11,907) | (N = 89,200) | (N = 510,685) | ||||||
| # | % | # | % | # | % | # | % | ||
| Sex | Female | 238,950 | 58.3 | 7,250 | 60.9 | 54,006 | 60.5 | 300,206 | 58.8 |
| Male | 170,628 | 41.7 | 4,657 | 39.1 | 35,194 | 39.5 | 210,479 | 41.2 | |
| Age group | < 50 | 29,004 | 7.1 | 484 | 4.1 | 2,508 | 2.8 | 31,996 | 6.3 |
| 50–64 | 67,277 | 16.4 | 1,567 | 13.2 | 6,798 | 7.6 | 75,642 | 14.8 | |
| 65 + | 313,297 | 76.5 | 9,856 | 82.8 | 79,894 | 89.6 | 403,047 | 78.9 | |
| Immigration | Long-term Resident | 406,177 | 99.2 | 11,828 | 99.3 | 80,171 | 89.9 | 498,176 | 97.6 |
| status | Immigrant | 3,401 | 0.8 | 79 | 0.7 | 9,029 | 10.1 | 12,509 | 2.4 |
| Highest level of | Less than High school | 83,484 | 20.4 | 4,927 | 41.4 | 32,047 | 35.9 | 120,458 | 23.6 |
| education | High school completed | 68,001 | 16.6 | 1,447 | 12.2 | 7,077 | 7.9 | 76,525 | 15.0 |
| Some post-secondary | 57,775 | 14.1 | 1,295 | 10.9 | 6,542 | 7.3 | 65,612 | 12.8 | |
| Univ./Post-sec completed | 43,457 | 10.6 | 895 | 7.5 | 5,616 | 6.3 | 49,968 | 9.8 | |
| Neighborhood | Q1 (Lowest) | 101,561 | 24.8 | 3,350 | 28.1 | 24,610 | 27.6 | 129,521 | 25.4 |
| income | Q2 | 88,490 | 21.6 | 2,867 | 24.1 | 21,384 | 24.0 | 112,741 | 22.1 |
| quintile | Q3 | 77,615 | 18.9 | 2,355 | 19.8 | 17,541 | 19.7 | 97,511 | 19.1 |
| Q4 | 71,583 | 17.5 | 1,877 | 15.8 | 14,659 | 16.4 | 88,119 | 17.3 | |
| Q5 (Highest) | 69,246 | 16.9 | 1,421 | 11.9 | 10,791 | 12.1 | 81,458 | 16.0 | |
| Area of | Urban | 348,928 | 85.2 | 8,411 | 70.6 | 87,527 | 98.1 | 444,866 | 87.1 |
| residence | Rural | 60,329 | 14.7 | 3,477 | 29.2 | 1,614 | 1.8 | 65,420 | 12.8 |
| Region of | Eastern | 28,448 | 6.9 | 5,589 | 46.9 | 3,485 | 3.9 | 37,522 | 7.3 |
| residencea | Northern | 31,637 | 7.7 | 4,380 | 36.8 | 2,430 | 2.7 | 38,447 | 7.5 |
| Southwest | 349,492 | 85.3 | 1,938 | 16.3 | 83,285 | 93.4 | 434,715 | 85.1 | |
aRegions: Northern (Northeast and Northwest LHINs), Eastern (Champlain LHIN), Southwest (remaining 11 LHINs). Ontario is organized in 14 health regions or LHINs (Local Health Integration Network)
A larger proportion of Francophones lived in rural areas (29.2%) compared to Anglophones (14.7%) and Allophones (1.8%). Francophones made a larger proportion of the population in the Northern (36.8%) and Eastern (46.9%) regions of the province. Overall, Anglophones were more highly educated (10.6% had completed a university degree), compared to Francophones (7.5%) and Allophones (6.3%). Anglophones were also more likely to live in the highest income quintile neighbourhood, with nearly 35% of Anglophones living in neighbourhoods with a household income within the 4th or 5th quintiles of income, compared to less than 30% of Allophones and Francophones. Allophones had worse physical and cognitive performance compared to Anglophones and Francophones (see the health-related characteristics of the cohort in Table S1 of Supplementary material).
Chronic diseases and multimorbidity
Overall, 92% of the cohort had two or more chronic diseases, and 44% had 5 or more diseases (Table 3). Compared to Anglophones and Francophones, Allophones had the highest proportion of chronic conditions across all categories of comorbidities (2 + , 3 + , 4 + , 5 +).
Table 3.
Prevalence of chronic diseases by linguistic group—(N = 510,685)
| Health conditions |
Anglophone (N = 409,578) |
Francophone (N = 11,907) |
Allophone (N = 89,200) |
Total (N = 510,685) |
||||
|---|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | # | % | |
| Level of multimorbidity | ||||||||
| 2 + diseases | 375,849 | 91.8 | 11,003 | 92.4 | 83,527 | 93.6 | 470,379 | 92.1 |
| 3 + diseases | 325,253 | 79.4 | 9,636 | 80.9 | 74,011 | 83.0 | 408,900 | 80.1 |
| 4 + diseases | 254,230 | 62.1 | 7,650 | 64.2 | 58,565 | 65.7 | 320,445 | 62.7 |
| 5 + diseases | 178,007 | 43.5 | 5,426 | 45.6 | 41,248 | 46.2 | 224,681 | 44.0 |
| Charlson index score (mean-SD) | 1.62 | 2.04 | 1.64 | 1.96 | 1.56 | 1.91 | 1.63 | 1.87 |
| Prevalence of chronic diseases | ||||||||
| AMI | 6,822 | 1.7 | 226 | 1.9 | 1,370 | 1.5 | 8,418 | 1.6 |
| Arrhythmia | 87,225 | 21.3 | 2,350 | 19.7 | 20,564 | 23.1 | 110,139 | 21.6 |
| Asthma | 74,941 | 18.3 | 2,352 | 19.8 | 16,694 | 18.7 | 93,987 | 18.4 |
| Cancer | 134,630 | 32.9 | 2,854 | 24.0 | 21,011 | 23.6 | 158,495 | 31.0 |
| Congestive Heart Failure (CHF) | 92,830 | 22.7 | 3,052 | 25.6 | 22,301 | 25.0 | 118,183 | 23.1 |
| COPD | 82,749 | 20.2 | 3,258 | 27.4 | 12,227 | 13.7 | 98,234 | 19.2 |
| Coronary Heart Disease (CHD) | 142,916 | 34.9 | 4,617 | 38.8 | 32,825 | 36.8 | 180,358 | 35.3 |
| Dementia | 64,013 | 15.6 | 2,185 | 18.4 | 17,035 | 19.1 | 83,233 | 16.3 |
| Diabetes | 142,894 | 34.9 | 4,624 | 38.8 | 40,408 | 45.3 | 187,926 | 36.8 |
| Hypertension | 306,181 | 74.8 | 9,201 | 77.3 | 74,222 | 83.2 | 389,604 | 76.3 |
| Inflammatory Bowel Disease (IBD) | 5,553 | 1.4 | 113 | 1.0 | 461 | 0.5 | 6,127 | 1.2 |
| Other mental health conditions | 39,790 | 9.7 | 1,060 | 8.9 | 6,843 | 7.7 | 47,693 | 9.3 |
| Non-psych. mood & anxiety dis | 89,229 | 21.8 | 2,634 | 22.1 | 17,030 | 19.1 | 108,893 | 21.3 |
| Osteoarthritis | 290,286 | 70.9 | 8,502 | 71.4 | 65,248 | 73.2 | 364,036 | 71.3 |
| Osteoporosis | 55,145 | 13.5 | 1,433 | 12.0 | 15,794 | 17.7 | 72,372 | 14.2 |
| Renal disease | 67,734 | 16.5 | 1,950 | 16.4 | 15,571 | 17.5 | 85,255 | 16.7 |
| Rheumatoid arthritis | 16,386 | 4.0 | 497 | 4.2 | 2,830 | 3.2 | 19,713 | 3.9 |
| Stroke | 55,090 | 13.5 | 1,502 | 12.6 | 13,215 | 14.8 | 69,807 | 13.7 |
AMI acute myocardial infarction, CHF congestive health failure, COPD chronic obstructive pulmonary disease, IBD inflammatory bowel disease, RA rheumatoid arthritis
The most prevalent chronic diseases were hypertension (76.3%), osteoarthritis [OA] (71.3%), diabetes (36.8%) and coronary heart diseases [CHD] (35.3%). Allophones had the highest prevalence of hypertension (83.2%), OA (73.2%), diabetes (45.3%), dementia (19.1%), osteoporosis (17.7%) and stroke (14.8%). Francophones had the highest prevalence of major cardiovascular diseases such as CHD (38.8%), congestive heart failure [CHF] (25.6%), acute myocardial infarction [AMI] (1.9%), as well as COPD (27.4%). The prevalence of cancer was significantly higher among Anglophones (32.9%) than among Francophones (24.0%) and Allophones (23.6%).
Patterns of multimorbidity clusters
There were no significant differences in the patterns of the top-five common disease combinations per multimorbidity level across linguistic groups (see Table S2 in Supplementary material). The order of the disease combinations was similar across the three linguistic groups, except for the category of 5 or more diseases for allophones and francophones, in which the combination including cancer was the second most common, whereas it was the first one for anglophones.
Top-five combinations of chronic diseases
Hypertension and OA are the most frequent diseases, with at least one appearing in all the top-five common combinations (see Table S2 in Supplementary material). Excluding these two chronic diseases, major cardiovascular diseases (e.g., CHD, CHF), dementia, diabetes, and cancer were the most frequent diseases in the top-five combinations. According to the type of disease present in a combination, Anglophones were overrepresented in combinations categories with cancer, whereas allophones overrepresented in combinations with hypertension, OA, diabetes, and CHD. Francophones were overrepresented in combinations with dementia.
Clinical clusters of diseases
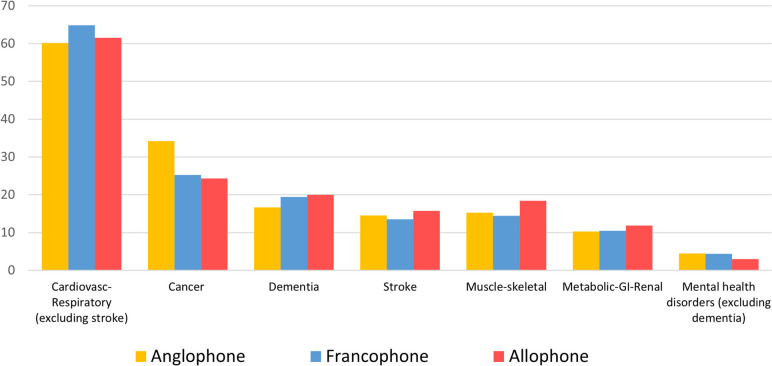
Cardiovascular and respiratory diseases (60.6%) and Cancer (32.2%) clusters were the most important functional clusters of chronic diseases (Fig. 1). These two clusters were the most prevalent across all linguistic groups.
Fig. 1.
The proportion of clinical clusters by linguistic group
The proportion of Francophones was significantly higher in the clusters of cardiovascular-respiratory diseases (64.9%) compared to Anglophones (60.2%) and Allophones (61.5%) (p < 0.001). Consistent with the prevalence of individual diseases, the cancer cluster was significantly more prominent among Anglophones (34.2%) than Francophones (25.2%) and Allophones (24.3%) (p < 0.001). Similarly, a significantly higher proportion of Allophones fell in the dementia cluster than the other linguistic groups. Allophones were notably overrepresented in the stroke and muscle-skeletal diseases clusters (Fig. 1).
Predictors of severe multimorbidity
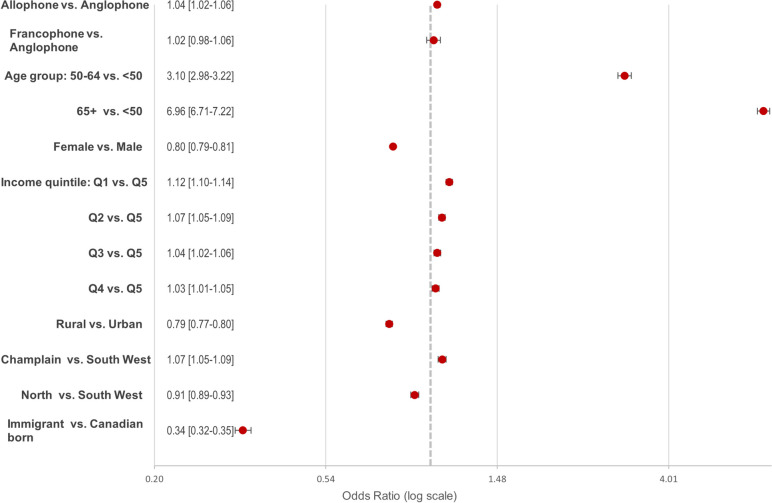
The multivariable regression analysis showed that, relative to Anglophones, Allophones had significantly greater odds of having five or more chronic diseases (adjusted OR = 1.04, [95% CI: 1.02–1.06]) (Fig. 2). Compared to those under 50 years of age, patients in age groups 50–64 years and 65 years and over were three (aOR = 3.10, [95% CI: 2.98–3.22]) and seven (aOR = 6.96, [95% CI: 6.71–7.22]) times more likely to have severe multimorbidity, respectively. Living in Eastern Ontario was also associated with higher odds of severe multimorbidity (aOR = 1.07, [95% CI: 1.05–1.09]). Conversely, being female (aOR = 0.80, [95% CI: 0.79–0.81]), immigrant (aOR = 0.34, [95% CI: 0.32–0.35]) and residing in rural areas (aOR = 0.79, [95% CI: 0.77–0.80]) were significantly associated with lower odds of having severe multimorbidity.
Fig. 2.
Multivariable analysis of the association between sociodemographic and health characteristics and severe multimorbidity (5 or more chronic diseases) (adjusted OR*, 95% CI). * Multivariable logistic regression model, adjusted by age, sex, neighborhood income level, immigrant status, rurality, area, and health characteristics
There was also a clear gradient between neighbourhood income level and severe multimorbidity, from wealthier quintile 4 (aOR = 1.03, [95% CI: 1.01–1.05]) to the poorest quintile (aOR = 1.12, [95% CI: 1.10–1.14]), relative to the wealthiest Q5.
Discussion
This study described multimorbidity in Ontario residents receiving home care, stratified by linguistic group. As expected, a high proportion (92%) of individuals receiving home care have multimorbidity (defined as two or more chronic diseases). The high prevalence is consistent with other studies which found higher levels of multimorbidity among older people and receiving long-term care [53, 54]. Across linguistic groups, Allophones had the highest proportion of multimorbidity, followed by Francophones, then Anglophones.
When considering multimorbidity by combinations of chronic diseases, we found that Hypertension or OA appeared in all top-5 combinations, a finding consistent with previous population-based studies in Ontario [38]. Excluding these two chronic diseases, we found that major cardiovascular diseases (i.e. CHD and CHF), dementia, diabetes, and cancer were the most frequent diseases appearing in common combinations. This pattern was consistent across the linguistic groups. Also, the combinations of chronic diseases showed no differences by linguistic characteristics.
We then examined clusters of chronic diseases, which may be more clinically relevant for healthcare providers. Overall, the most prevalent clinical clusters were cardiovascular/respiratory disease (excluding stroke) and dementia, a finding that is consistent with prior studies of populations of older recipients of home care services [37]. Francophones were overrepresented in the cardiovascular/respiratory disease, which is likely due to the higher rates of cardiovascular risk factors (e.g., smoking, dyslipidemia, family history of CVD), which has been reported in previous studies [55, 56]. Furthermore, a report on the health of seniors in Ontario showed that Franco-Ontarians had higher rates of obesity, especially those living in minority linguistic communities [57]. The overrepresentation of Anglophones in the cancer cluster could be related to a higher cancer survival levels observed in the southwest region of the province, which is predominantly anglophone [58]. This may be also related to health seeking behaviours, as anglophones with cancer seek out home care more often and earlier in life, whereas language and cultural barriers can influence cancer care seeking behaviours among non-anglophone patients [59, 60].
Previous studies have reported an association between high levels of multimorbidity with disability, frailty and poor health outcomes [61–63]. We hypothesized that linguistic minorities (e.g., Francophones and Allophones in Ontario) would have higher levels of multimorbidity due to the effect of language barriers to health services. We found that Allophones were significantly more likely to have 5 + multimorbidity. This may be due to the fact that Allophones may not seek health services due to their limited capacity to communicate in Canada’s official languages or may not seek out publicly funded home care services due to cultural preferences, differences in informal health care services (e.g., large households, family structures), leading to more health complications and worse health outcomes. Moreover, a large proportion of Allophones are recent immigrants, who face additional barriers accessing and using health services [64, 65]. As this linguistic group has poor ability to communicate or are not proficient in one of Canada’s official languages, it makes them more susceptible to barriers in accessing health care services and receiving appropriate care, leading to poorer health outcomes. However, the independent protective effect of immigrants might be related to the healthy immigrant effect, which is widely documented, [66] as well as their lower levels of health care seeking behaviours that have been identified among immigrants [67]. Unfortunately, we were not able to account for other migration-related factors (e.g. length of stay in Canada) to assess that assumption in this study.
Strengths and limitations
This study has several strengths, notably its use of a large population-based cohort and validated datasets. However, this study also has limitations. We obtained an individual's primary language from the RAI-HC assessments. During these assessments, interviewers are instructed to determine the home care recipient's primary language by listening and observing and, if necessary, by asking the home care recipient to specify his or her primary language. For Ontarians who speak multiple languages, it is unclear how interviewers assign primary language. Also, the interviewers do not assess language proficiency, which is particularly important for Ontarians that speak multiple languages. However, our group's preliminary analyses have shown that the language variable of the RAI-HC database have a high agreement (kappa = 0.76) with self-reported language at home from the Canadian Community Health Survey. (Batista et al., unpublished data, 2019). Finally, the approach used to create the clusters could be a limitation, as it can affect the generalizability of the findings and comparisons with other data-driven approaches for clustering chronic diseases.
Conclusions and implications
The prevalence of multimorbidity among Ontarians receiving home care services is high. There exist important clinical differences in the prevalence and characteristics of disease burden across linguistic groups. Understanding the interaction between language and multimorbidity will allow policy makers to implement patient-oriented healthcare strategies that address the needs of linguistic minorities, who face barriers to accessing appropriate healthcare services. This study found that home care recipients whose primary language was other than English or French were more likely to have severe multimorbidity. Hence, healthcare systems should identify individuals living in minority linguistic situations and implement strategies (e.g. care coordinators which can facilitate access to home care services, interpretation services, social work) to provide care to patients with important communication barriers and also more complex care needs. However, additional studies are needed to understand the effect of other linguistic factors (e.g., language of service, patient-provider language discordance, use of interpreters) on the healthcare and the health outcomes of people living with multimorbidity in Ontario.
Supplementary Information
Additional file 1: Appendix 1. The RECORD statement – checklist of items, extended from the STROBE statement. Appendix 2. Cohort creation flowchart. Appendix 3. List of diagnosis codes for defining the 18 selected chronic conditions and Clinical clusters. Appendix 4. Supplementary tables. Table S1. Clinical characteristics of the cohort by linguistic group - (N=510,685). Table S2. Top-five combinations of diseases across multimorbidity levels (2-5 diseases), by linguistic group (N=470,379). Table S3. Risk of severe multimorbidity (5 or more chronic diseases) and linguistic characteristics (adjusted OR*, 95% CI).
Acknowledgements
Parts or whole of this material are based on data and/or information compiled and provided by Immigration, Refugees and Citizenship Canada (IRCC) current to May 31, 2017. However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of IRCC.
Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of CIHI.
Abbreviations
- ADL
Activities of Daily Living
- AMI
Acute myocardial infarction
- AMI
Acute myocardial infarction
- CHD
Coronary heart diseases
- CHESS
Changes in health, End-stage disease, and Signs and Symptoms
- CHF
Congestive health failure
- CHF
Congestive heart failure
- CIHI
Canadian Institute of Health Information
- COPD
Chronic obstructive pulmonary disease,
- CPS
Cognitive Performance Scale
- DAD
Discharge Abstract Database
- IBD
Inflammatory bowel disease
- ICES
Formerly, Institute for Clinical Evaluative Sciences
- IRCC
Immigration, Refugees and Citizenship Canada
- LHINs
Local health integration networks
- MSK
Muscle-skeletal
- OA
Osteoarthritis
- OHIP
Ontario Health Insurance Plan
- RA
Rheumatoid arthritis
- RAI-HC
Resident Assessment Instrument for Home Care
- RPDB
Registered Persons Database
- RUG-III
Resource Utilization Grouping III
Authors’ contributions
RB, DP, DM and PT conceived the original idea. EmS, ER, RR performed a background literature review. RB, MR, EwS, CK, LiB, LuB, DP, DM, and PT designed the study and interpreted the results. ES and MP performed the statistical analyses. RB, MR, MP, CK, LiB, DM and PT reviewed and verified the results of the analyses. RB, RR, EmS, ER, and MR drafted the manuscript. All authors reviewed and provided feedback and approved the version to be published.
Funding
This study was primarily supported by the Institut du Savoir Montfort and the Programme de subventions Savoir Montfort, concours 2018–2019 funded by Fondation Monfort. This study was also supported by ICES, which is funded by an annual grant from the Ontario MOHLTC. ICES has been approved by Ontario's Information and Privacy Commissioner since 2005. The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. ICES collects information most notably for purposes of Sect. 45 of Ontario's Personal Health Information Protection Act (PHIPA). Tanuseputro is supported by a PSI Graham Farquharson Knowledge Translation Fellowship.
Availability of data and materials
The data set from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Declarations
Ethics approval and consent to participate
This project was conducted under Sect. 45 and approved by the ICES Privacy and Compliance Office. ICES is a prescribed entity under Sect. 45 of Ontario's Personal Health Information Protection Act. Section 45 authorizes ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of, allocation of resources to or planning for all or part of the health system.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Canadian Medical Association . The State of Seniors Health Care in Canada. Ottawa: Canadian Medical Association; 2016. p. 20. [Google Scholar]
- 2.Statistics Canada. Census profile, 2016 Census. Statistics Canada. 2017. Available at: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=PR&Code1=01&Geo2=&Code2=&SearchText=Canada&SearchType=Begins&SearchPR=01&B1=All&TABID=1&type=0. (Accessed 16 Aug 2021).
- 3.Statistics Canada. Population Projections for Canada (2018 to 2068), Provinces and Territories (2018 to 2043). Statistics Canada. 2019. Available at: https://www150.statcan.gc.ca/n1/pub/91-520-x/91-520-x2019001-eng.htm. (Accessed July, 2021).
- 4.Spillman BC. Changes in Elderly Disability Rates and the Implications for Health Care Utilization and Cost. Milbank Q. 2004;82:157–194. doi: 10.1111/j.0887-378X.2004.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luppa M, Luck T, Weyerer S, König H-H, Brähler E, Riedel-Heller SG. Prediction of institutionalization in the elderly. A systematic review Age and Ageing. 2010;39:31–38. doi: 10.1093/ageing/afp202. [DOI] [PubMed] [Google Scholar]
- 6.CIHI. Health Care in Canada, 2011- A Focus on Seniors and Aging. Ottawa: Canadian Institute for Health Information; 2011. p. 162.
- 7.Feely A, Lix LM, Reimer K. Estimating multimorbidity prevalence with the Canadian Chronic Disease Surveillance System. Health Promot Chronic Dis Prev Can. 2017;37:215–222. doi: 10.24095/hpcdp.37.7.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts KC, Rao DP, Bennett TL, Loukine L, Jayaraman GC. Prevalence and patterns of chronic disease multimorbidity and associated determinants in Canada. Health Promot Chronic Dis Prev Can. 2015;35:87–94. doi: 10.24095/hpcdp.35.6.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakib MN, Shooshtari S, St John P and Menec V. The prevalence of multimorbidity and associations with lifestyle factors among middle-aged Canadians: an analysis of Canadian Longitudinal Study on Aging data. BMC Public Health. 2019;19:243. [DOI] [PMC free article] [PubMed]
- 10.St Sauver JL, Boyd CM, Grossardt BR, Bobo WV, Finney Rutten LJ, Roger VL, et al. Risk of developing multimorbidity across all ages in an historical cohort study: differences by sex and ethnicity. BMJ Open. 2015;5(2):e006413. [DOI] [PMC free article] [PubMed]
- 11.Gulley SP, Rasch EK, Bethell CD, et al. At the intersection of chronic disease, disability and health services research: A scoping literature review. Disabil Health J. 2018;11:192–203. doi: 10.1016/j.dhjo.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep. 2013;24:10–17. [PubMed] [Google Scholar]
- 13.Villacampa-Fernández P, Navarro-Pardo E, Tarín JJ, Cano A. Frailty and multimorbidity: Two related yet different concepts. Maturitas. 2017;95:31–35. doi: 10.1016/j.maturitas.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Auditor General of Ontario. Annual Report 2017: Follow-Up Report on Audit Recommendations. Toronto: Office of the Auditor General of Ontario; 2017.
- 15.Ministry of Health and Long-Term Care. Guide to Requirements and Obligations Relating to French Language Health Services. Toronto: Ontario Ministry of Health and Long-Term Care; 2017. p. 27.
- 16.Corbeil J-P, Lafrenière S. Portrait of Official-Language Minorities in Canada: Francophones in Ontario. Ottawa: Statistics Canada; 2010. p. 109. [Google Scholar]
- 17.Satistics Canada. Knowledge of Official Languages. Data tables, 2016 Census Catalogue no 98–400-X2016384. 2019–06–17 ed. Ottawa: Satistics Canada; 2016.
- 18.Bouchard L, Gaboury I, Chomienne M-H, Gilbert A, Dubois L. La santé en situation linguistique minoritaire. Healthcare Policy. 2009;4:36–42. [PMC free article] [PubMed] [Google Scholar]
- 19.Picard L and Allaire G. Deuxième Rapport sur la santé des francophones de l'Ontario. Sudbury: Programme de recherche, d’éducation et de développement en santé publique; Institut franco-ontarien, Université Laurentienne; 2005. p. 159.
- 20.DuBard CA, Gizlice Z. Language spoken and differences in health status, access to care, and receipt of preventive services among US Hispanics. Am J Public Health. 2008;98:2021–2028. doi: 10.2105/AJPH.2007.119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponce NA, Hays RD, Cunningham WE. Linguistic Disparities in Health Care Access and Health Status Among Older Adults. J Gen Intern Med. 2006;21:786–791. doi: 10.1111/j.1525-1497.2006.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan SH, Greenfield S, Ware JE. Assessing the Effects of Physician-Patient Interactions on the Outcomes of Chronic Disease. Med Care. 1989;27:S110–S127. doi: 10.1097/00005650-198903001-00010. [DOI] [PubMed] [Google Scholar]
- 23.Agborsangaya CB, Lau D, Lahtinen M, Cooke T, Johnson JA. Multimorbidity prevalence and patterns across socioeconomic determinants: a cross-sectional survey. BMC Public Health. 2012;12:201. doi: 10.1186/1471-2458-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashman JJ, Beresovsky V. Multiple Chronic Conditions Among US Adults Who Visited Physician Offices: Data From the National Ambulatory Medical Care Survey, 2009. Prev Chronic Dis. 2013;10:E64. doi: 10.5888/pcd10.120308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afshar S, Roderick PJ, Kowal P, Dimitrov BD, Hill AG. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health. 2015;15:776. doi: 10.1186/s12889-015-2008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leis A, Bouchard L, Editorial. The Health of Official Language Minority Populations. Can J Public Health. 2013;104:2.
- 27.Gilmour H. Formal home care use in Canada. Health Rep. 2018;29:3–9. [PubMed] [Google Scholar]
- 28.Canadian Institute for Health Information. Profile of Clients in Home Care, 2020–2021. Quick Stats. Ottawa: Canadian Institute for Health Information; 2021.
- 29.InterRAI. interRAI - The international Resident Assessment Instrument (RAI). interRAI Organization; 2018. Available at: http://www.interrai.org/. Accessed 16 Apr 2021.
- 30.Health Quality Ontario. Measuring Home Care Performance in Ontario. Health Quality Ontario. 2017. Available at: https://www.hqontario.ca/system-performance/measuring-system-performance/measuring-home-care. (Accessed 14 Apr 2021).
- 31.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:M546–M553. doi: 10.1093/gerona/54.11.M546. [DOI] [PubMed] [Google Scholar]
- 32.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49:M174–M182. doi: 10.1093/geronj/49.4.M174. [DOI] [PubMed] [Google Scholar]
- 33.Hirdes JP, Frijters DH, Teare GF. The MDS-CHESS Scale: A New Measure to Predict Mortality in Institutionalized Older People. J Am Geriatr Soc. 2003;51:96–100. doi: 10.1034/j.1601-5215.2002.51017.x. [DOI] [PubMed] [Google Scholar]
- 34.Poss JW, Hirdes JP, Fries BE, McKillop I, Chase M. Validation of Resource Utilization Groups Version III for Home Care (RUG-III/HC): Evidence From a Canadian Home Care Jurisdiction. Med Care. 2008;46:380–387. doi: 10.1097/MLR.0b013e31815c3b6c. [DOI] [PubMed] [Google Scholar]
- 35.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 36.Stephenson A, Hux J, Tullis E, Austin PC, Corey M, Ray J. Socioeconomic status and risk of hospitalization among individuals with cystic fibrosis in Ontario Canada. Pediatr Pulmonol. 2011;46:376–384. doi: 10.1002/ppul.21368. [DOI] [PubMed] [Google Scholar]
- 37.Mondor L, Maxwell CJ, Hogan DB, et al. Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: A retrospective analysis of a population-based cohort. PLoS Med. 2017;14:e1002249. doi: 10.1371/journal.pmed.1002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koné Pefoyo AJ, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. doi: 10.1186/s12889-015-1733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barker P. Local Health Integration Networks: The Arrival of Regional Health Authorities in Ontario. London: Brescia University College; 2007. p. 21.
- 40.Morris JN, Hawes C, Mor V, et al. Resident Assessment Instrument (RAI) RAI-MDS 2.0 User's Manual, Canadian Version. 2.0 ed. Washington DC: interRAI; 2010.
- 41.Mondor L, Maxwell CJ, Bronskill SE, Gruneir A, Wodchis WP. The relative impact of chronic conditions and multimorbidity on health-related quality of life in Ontario long-stay home care clients. Qual Life Res. 2016;25:2619–2632. doi: 10.1007/s11136-016-1281-y. [DOI] [PubMed] [Google Scholar]
- 42.Ording AG, Sørensen HT. Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol. 2013;5:199–203. doi: 10.2147/CLEP.S45305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stirland LE, González-Saavedra L, Mullin DS, Ritchie CW, Muniz-Terrera G, Russ TC. Measuring multimorbidity beyond counting diseases: systematic review of community and population studies and guide to index choice. BMJ. 2020;368:m160. doi: 10.1136/bmj.m160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arokiasamy P, Uttamacharya U, Jain K, et al. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: what does the study on global ageing and adult health (SAGE) reveal? BMC Med. 2015;13:178. doi: 10.1186/s12916-015-0402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan A, Wallace E, O'Hara P, Smith SM. Multimorbidity and functional decline in community-dwelling adults: a systematic review. Health Qual Life Outcomes. 2015;13:168. doi: 10.1186/s12955-015-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St John PD, Tyas SL, Menec V, Tate R, Griffith L. Multimorbidity predicts functional decline in community-dwelling older adults: Prospective cohort study. Can Family Physician. 2019;65:e56–e63. [PMC free article] [PubMed] [Google Scholar]
- 47.Pope G, Ellis R, Ash A, et al. Diagnostic Cost Group Hierarchical Condition Category Models for Medicare Risk Adjustment. Final report. Waltham: Health Economics Research, Inc; 2000. p. 293.
- 48.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 49.U. S. Department of Health Human Services. Multiple Chronic Conditions—A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions. Washington, DC: Department of Health Human Services; 2010. p. 18.
- 50.Kronick RG, Bella M, Gilmer TP, Somers SA. The Faces of Medicaid II: Recognizing the Care Needs of People with Multiple Chronic Conditions. Hamilton: Center for Health Care Strategies; 2007.
- 51.González-Chica DA, Hill CL, Gill TK, Hay P, Haag D, Stocks N. Individual diseases or clustering of health conditions? Association between multiple chronic diseases and health-related quality of life in adults. Health Qual Life Outcomes. 2017;15:244. doi: 10.1186/s12955-017-0806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schäfer I, von Leitner E-C, Schön G, et al. Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PloS one. 2010;5:e15941-e. [DOI] [PMC free article] [PubMed]
- 53.Abad-Diez JM, Calderon-Larranaga A, Poncel-Falco A, et al. Age and gender differences in the prevalence and patterns of multimorbidity in the older population. BMC Geriatr. 2014;14:75. doi: 10.1186/1471-2318-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akner G. Analysis of multimorbidity in individual elderly nursing home residents. Development of a multimorbidity matrix. Arch Gerontol Geriatrics. 2009;49:413–9. [DOI] [PubMed]
- 55.Ayoub C, Bernick J, Arasaratnam P, et al. Coronary Artery Disease in French Canadians—Investigation of a Suggested Vulnerable Population. Can J Cardiol. 2016;32:1240–1245. doi: 10.1016/j.cjca.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 56.McNicoll S, Latour Y, Rondeau C, Bouthillier D, Davignon J, Genest J., Jr Cardiovascular risk factors and lipoprotein profile in French Canadians with premature CAD: impact of the National Cholesterol Education Program II. Can J Cardiol. 1995;11:109–116. [PubMed] [Google Scholar]
- 57.Bouchard L, Makvandi E, Sedigh G, van Kemenade S. The Health of the Francophone Population Aged 65 and over in Ontario A region-by-region portrait based on the Canadian Community Health Survey (CCHS). Ottawa: Reseau de recherche appliquée sur la santé des francophones de l’Ontario (RRASFO); 2014. p. 48.
- 58.Ontario Health (Cancer Care Ontario). Ontario Cancer Profiles: Cancer Survival. Cancer Care Ontario. 2021. Available at: https://cancercareontario.ca/ontariocancerprofiles. (Accessed 6 Aug 2021).
- 59.Al Shamsi H, Almutairi AG, Al Mashrafi S, Al KT. Implications of Language Barriers for Healthcare: A Systematic Review. Oman Med J. 2020;35:e122. doi: 10.5001/omj.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uzar-Ozcetin YS, Tee S, Kargin M. Achieving culturally competent cancer care: A qualitative study drawing on the perspectives of cancer survivors and oncology nurses. Eur J Oncol Nurs. 2020;44:101701. doi: 10.1016/j.ejon.2019.101701. [DOI] [PubMed] [Google Scholar]
- 61.Kingston A, Robinson L, Booth H, Knapp M, Jagger C, project M. Projections of multi-morbidity in the older population in England to 2035: estimates from the Population Ageing and Care Simulation (PACSim) model. Age Ageing. 2018;47:374–80. [DOI] [PMC free article] [PubMed]
- 62.Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: A systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- 64.Djiadeu P, Yusuf A, Ongolo-Zogo C, et al. Barriers in accessing HIV care for Francophone African, Caribbean and Black people living with HIV in Canada: a scoping review. BMJ Open. 2020;10:e036885. doi: 10.1136/bmjopen-2020-036885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lebrun LA, Dubay LC. Access to primary and preventive care among foreign-born adults in Canada and the United States. Health Serv Res. 2010;45:1693–1719. doi: 10.1111/j.1475-6773.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu C, Ng E. Healthy immigrant effect by immigrant category in Canada. Health Rep. 2019;30:3–11. doi: 10.25318/82-003-x201900400001-eng. [DOI] [PubMed] [Google Scholar]
- 67.Sarría-Santamera A, Hijas-Gómez AI, Carmona R, Gimeno-Feliú LA. A systematic review of the use of health services by immigrants and native populations. Public Health Rev. 2016;37:28. doi: 10.1186/s40985-016-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. The RECORD statement – checklist of items, extended from the STROBE statement. Appendix 2. Cohort creation flowchart. Appendix 3. List of diagnosis codes for defining the 18 selected chronic conditions and Clinical clusters. Appendix 4. Supplementary tables. Table S1. Clinical characteristics of the cohort by linguistic group - (N=510,685). Table S2. Top-five combinations of diseases across multimorbidity levels (2-5 diseases), by linguistic group (N=470,379). Table S3. Risk of severe multimorbidity (5 or more chronic diseases) and linguistic characteristics (adjusted OR*, 95% CI).
Data Availability Statement
The data set from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.