Abstract
A set of self-transmissible plasmids with IncQ plasmid-mobilizing capacity was isolated by triparental exogenous isolation from the wheat rhizosphere with an Escherichia coli IncQ plasmid host and a Ralstonia eutropha recipient. Three plasmids of 38 to 45 kb, denoted pIPO1, pIPO2, and pIPO3, were selected for further study. No selectable traits (antibiotic or heavy-metal resistance) were identified in these plasmids. The plasmids were characterized by replicon typing via PCR and hybridization with replicon-specific probes and other hybridizations. pIPO1 and pIPO3 were similar to each other, whereas pIPO2 was different. None of these plasmids belonged to any known incompatibility group. pIPO2 was selected for further work, and a mini-Tn5-tet transposon was inserted to confer selectability. Plasmid pIPO2 had a broad IncQ plasmid mobilization and self-transfer range among the alpha, beta, and gamma subclasses of the Proteobacteria but did not show productive transfer to gram-positive bacteria. Plasmid pIPO2 mobilized IncQ plasmid pIE723 from Pseudomonas fluorescens to diverse indigenous proteobacteria in the rhizosphere of field-grown wheat. Transfer of pIE723 to indigenous bacteria was not observed in the absence of added pIPO2. A specific PCR primer system and a probe were developed for the detection of pIPO2-type plasmids in soil and rhizosphere. Analysis of soil DNA provided evidence for the presence of pIPO2 in inoculated wheat rhizosphere soil in the field study, as well as in the rhizosphere of uninoculated wheat plants growing in soil microcosms. The system failed to identify major reservoirs of pIPO2 in a variety of other soils.
Conjugation is an important gene transfer mechanism for bacteria in the soil and rhizosphere (7, 27, 28), and the genes responsible for mating-aggregate formation and DNA transfer are often carried by self-transmissible plasmids. Such conjugative plasmids are known to be capable of recruiting chromosomal genes as well as mobilizing non-self-transmissible plasmids and hence can provide genetic plasticity to bacterial populations. In cases in which highly promiscuous plasmids are involved, such genetic interactions may occur between a broad range of bacteria (27, 28, 34). Plasmid transfer between introduced bacteria via conjugation in soil has been unequivocally shown in numerous microcosm experiments with different mating combinations including taxonomically diverse ones (15, 21, 27, 28, 31, 44).
However, the abundance and putative role of plasmids present in indigenous bacterial populations in in situ genetic mobilization has so far been rarely studied (11, 19, 36). Hence, the extent to which natural bacterial communities are capable of providing gene-mobilizing capacity under the prevailing conditions in the soil and rhizosphere is still poorly understood. In a pioneering study, Top et al. were able to roughly quantify the prevalence of gene-mobilizing elements in soil by a quantitative exogenous isolation method (36). However, the elements that confer genetic plasticity to bacterial populations, in particular self-transmissible plasmids and conjugative transposons, may occur in fluctuating quantities in soil. Moreover, their prevalence may change as a result of dominating soil ecological factors such as the presence of a rhizosphere or chemical stress (29, 45). To assess such bacterial community responses, a molecular approach assessing the prevalence of plasmid-specific sequences in soil-extracted microbial DNA can be used (29).
Fry and Day suggested a novel approach to directly obtain plasmids with mobilizing capacity from the environment (7). The method, denominated triparental exogenous isolation, involves the coincubation of a selectable plasmid recipient strain with a mixed soil bacterial community in the presence of another strain containing a mobilizable plasmid. Simultaneous selection for the recipient and for a marker(s) of the mobilizable element allows the isolation of indigenous plasmids with plasmid-mobilizing capacity as a result of comobilization (7, 36). As suggested by Top et al. (36), the application of a transfer system consisting of an Escherichia coli plasmid donor strain containing an IncQ plasmid with heavy-metal (Cd, Zn, Co)-resistance genes which are not expressed and a Ralstonia eutropha recipient strain in which they can be expressed to a mixed soil bacterial community allows for the exogenous isolation of plasmid-mobilizing elements with broad-host-range capacity (gene escape system).
The objectives of this study were to gain insight into the natural conjugal gene flow in gram-negative bacterial communities in soil and rhizosphere. For that purpose, plasmids with gene-mobilizing capacity were isolated from soil bacterial populations via the Escherichia coli-Ralstonia eutropha triparental exogenous isolation system. The plasmids obtained, denoted pIPO1, pIPO2, and pIPO3, were characterized by phenotypic and molecular methods. Furthermore, the possible role of one selected plasmid, pIPO2, in in situ mobilization of an IncQ plasmid, as well as its prevalence in soil and the wheat rhizosphere, was assessed by using a plasmid-specific PCR system.
MATERIALS AND METHODS
Strains and plasmids used.
The strains used in this study are listed in Table 1. They were routinely grown at 28°C (Escherichia coli was grown at 37°C) in Luria-Bertani (LB) broth (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl, 1 liter of H2O [pH 7.2]) supplemented with 50 μg of the appropriate antibiotic per ml (Table 1). The soil-isolated unidentified member of the beta proteobacteria F4 was cultured in 0.1× tryptic soy broth (3 g in 1 liter of demineralized water [pH 7.2]), and Rhizobium meliloti L5-30 was grown in TY medium (5 g of tryptone, 3 g of yeast extract, 0.5 g of CaCl2, 1 liter of H2O [pH 6.8]). The strains were maintained as 20% glycerol stocks at −80°C.
TABLE 1.
Strains and plasmids used in this study
| Strain | Resistancesa | Plasmid, reference or source |
|---|---|---|
| E. coli CM990 | App | pMOL187; M. Mergeay, VITO, Mol, Belgium |
| E. coli HB101 | Tcp | pSUP104; IPO-DLOb |
| E. coli CV601 | Rp | H. Tschäpe, BGA, Wernigerode |
| E. coli K12 | Kmp, Smp, Gmp, Sup | pIE723; 35 |
| E. coli Sm10 (pUT/ Tn5::luxAB::tet) | Tcp | A. Glover, University of Aberdeen, Aberdeen, Scotland |
| Enterobacter cloacae BE1 | Rp | IPO-DLO |
| Enterobacter cloacae ECl2 | Rp | IPO-DLO |
| P. fluorescens R33 | Rp, Nx | IPO-DLO |
| P. fluorescens R2f (chr::Tn5) | Km, Sm | IPO-DLO |
| P. fluorescens R2f | Tcp | pSUP104; IPO-DLO |
| P. putida UWC1 | Rp | J. C. Fry, University of Cardiff, Cardiff, Wales |
| P. putida Pp3 | Rp | IPO-DLO |
| R. eutropha AE815 | Rp | M. Mergeay, VITO |
| Acinetobacter calcoaceticus BD413 | Rp | IPO-DLO |
| Agrobacterium sp. strain A3 | Rp | IPO-DLO |
| Bacillus subtilis SEm-2 | Em | IPO-DLO |
| Paenibacillus azotofixans P3L5 | Rp | 25 |
| Rhizobium meliloti L5-30 | Rp | 14 |
| Azospirillum brasilense Sp7R | Rp | 33 |
| Azorhizobium caulinodans ORSR | Rp | 6 |
| Arthrobacter sp. strain ArthR | Rp | J. Jansson, University of Stockholm, Stockholm, Sweden |
| Clavibacter michiganensis CRc | Rp | Department of Botany, MSU,c East Lansing, Mich. |
| Cytophaga sp. strain LTER30 | Rp | 22 |
| Cytophaga heparina | Rp | 22 |
| Unidentified beta proteobacterium F4 | Rp | IPO-DLO |
| Xanthomonas vesicatoria XcR | Rp | 16 |
Rp, rifampicin resistant; Ap, ampicillin resistant; Tc, tetracycline resistant; Nx, nalidixic acid resistant; Km, kanamycin resistant; Sm, streptomycin resistant; Gm, gentamicin resistant; Su, sulfonamide resistant; Em, erythromycin resistant. Superscript p indicates that the resistance is plasmid encoded. Rp resistance, spontaneous chromosomal mutants.
IPO-DLO, strain collection of the Soil Biotechnology Group, Institute for Plant Protection, IPO-DLO.
Kind gift of Dr. D. Fullbright, Department of Botany, Michigan State University (MSU).
Soil, soil microcosms, and sampling procedures.
Flevo silt loam (FSL) soil, freshly collected from a field plot in the vicinity of IPO-DLO (Wageningen, The Netherlands), was used in all experiments. FSL soil has a high clay content and neutral pH, as described previously (21). Polypropylene containers (62 by 65 mm) were used to produce soil microcosms. Portions (60 g) of freshly collected soil were added to the microcosms, and sterile demineralized water was added to bring the moisture content up to about 70% of the water-holding capacity (pF 2; 35% moisture content). The microcosms were either planted with three pregerminated wheat (Triticum aestivum var. Sicco) seeds or left unplanted. They were incubated for 10 days in a climate chamber with a light-dark cycle of 16 and 8 h at 20°C. The moisture status of the microcosm was controlled by weighing, and water was added daily when needed.
Bulk and rhizosphere soil samples were taken by carefully removing the plants from the soil. Plants were shaken to remove excess soil, and the roots were separated from the shoots and blades. Soil not tightly associated with roots was regarded as bulk soil. Portions (5 to 10 g) consisting of either bulk soil or root material with adhering soil were added to Erlenmeier flasks containing 95 ml of either sterile 0.85% NaCl (exogenous isolation) or 0.1% sodium pyrophosphate (direct dilution plating) plus 10 g of gravel (2 to 4 mm in diameter). The flasks were shaken on a Gyrotory shaker at 280 rpm for 10 to 30 min. The soil or rhizosphere suspensions were used in the triparental exogenous isolation procedures or diluted, with aliquots plated for total indigenous bacterial counts (see below).
Triparental exogenous isolation of gene-mobilizing plasmids from bulk and rhizosphere soils.
Soil slurries in 0.85% NaCl prepared as described above were used as sources of donors of gene-mobilizing elements. First, the soil particles were left to settle by leaving the flasks untouched for 10 min. The supernatant (30 ml) was filtered over ordinary filter paper (Selecta faltenfilter; diameter, 185 mm; Schleicher & Schuell, Dassel, Germany), and the filtrate (about 25 ml) was applied to a sterile Millipore membrane filter (pore size, 0.22 μm) with a vacuum filtration unit (Millipore, Bedford, Mass.).
The gene escape system based on the mobilization of the non-self-transmissible plasmid pMOL187 (containing the czc genes, which confer resistance to Cd, Zn, and Co) from E. coli CM990(pMOL187) to the rifampin-resistant plasmidless Ralstonia eutropha AE815 was used (36). It was kindly provided by M. Mergeay (VITO). The czc genes are not expressed in E. coli CM990, and their expression in R. eutropha AE815 allows for selection of transconjugants that received plasmid pMOL187 on Zn- (and/or Cd)-containing agar media. A 1-ml sample of washed overnight cultures of R. eutropha AE815 and of E. coli CM990(pMOL187) was applied to a Millipore membrane filter together with 30 ml of the soil or rhizosphere bacterial suspensions. Inoculated filters were placed on LB agar (1.5%) plates and incubated overnight at 27°C. Following incubation, the filters were vortex shaken in 5 ml of sterile 0.85% NaCl to resuspend the bacterial cells. Serial 10-fold dilutions in 0.85% NaCl were plated onto TMA (azelate) agar (17) containing ZnCl2 (6 mM) and rifampin (50 μg/ml) to enumerate R. eutropha AE815 transconjugants.
Assessment of IncQ plasmid-mobilizing capacity in putative gene-mobilizing strains.
The mobilizing capacity of strains which had obtained the mobilizable plasmid pMOL187 and presumably also contained a gene-mobilizing element was checked by triparental mating involving the IncQ plasmid pSUP104, which carries a selectable tetracycline resistance (20) and a chromosomally marked selectable recipient. The mating mixtures consisted of the putative R. eutropha mobilizer strain, Escherichia coli (pSUP104), and the recipient strain Pseudomonas fluorescens R2f (chr::Tn5). Selection was for the tetracycline resistance marker of pSUP104 and the kanamycin resistance of transposon Tn5. Transconjugant clones were screened, by direct plasmid extraction as well as further mobilization, for the presence of a cotransferred plasmid in addition to pSUP104. For further mobilization, biparental mating between a selected transconjugant and a rifampin-resistant derivative of E. coli CV601 was performed, with selection on LB agar supplemented with 50 μg each of tetracycline and rifampin per ml. Putative transconjugants were scored for the presence of pSUP104 and of the exogenously isolated mobilizing plasmid.
Plasmid extraction.
Plasmids were isolated by alkaline extraction (23). Alternatively, a modified Kado and Liu (12) method (1) was applied. Briefly, 1.5 ml of an overnight cell culture was centrifuged in an Eppendorf centrifuge (10 min at 14,000 rpm); resuspended in 200 μl of a solution containing 50 mM Tris, 3% sodium dodecyl sulfate, and 120 mM NaOH; and incubated at 57°C for 70 min. The DNA was extracted with phenol-chloroform. The aqueous phase was diluted with 200 μl of demineralized H2O, and the DNA was precipitated by adding 50 μl of 3 M sodium acetate (pH 5.2) and 1 ml of absolute ethanol and incubating the mixture at −20°C. The plasmid DNA was taken up in 25 μl of distilled water, and 5 to 8 μl was analysed by agarose gel electrophoresis (0.7% agarose in Tris-borate-EDTA).
To obtain plasmid preparations of sufficient purity to allow digestion by restriction enzymes, DNA was isolated with a mini plasmid preparation kit (Qiagen GmbH, Hilden, Germany). The manufacturer’s protocol was used, except for the use of 20 ml of overnight culture and treatment at 50°C to facilitate the elution of DNA from the column.
Molecular characterization of plasmids.
Homology between plasmids pIPO1, pIPO2, and pIPO3 and other DNA sequences was assessed by hybridization with specific probes. A set of 18 plasmids containing cloned sequences of different replicon types (5) was digested with the appropriate restriction enzymes, and the digests were run on agarose gels. The fragments obtained were characteristic for the incompatibility groups P, N, W, Q, FI (3×), FII, X, B/O, Y, L/M, T, I1, U, HI2, and HI1. A Southern blot of the gel was used as the target for hybridization with two selected plasmids, pIPO2 and pIPO3. In addition, similar filters were prepared with restriction enzyme digests of 17 genes involved in xenobiotic-catabolic processes (xylDLEGFJIHS, catA, benABC, catBCDE, pcaACBDFE, bphC, todC123BADE, alkST, alkBAC, tcbCD, bphABCD, dmpABCD, nahR/nahG, nahAa/Ab, tbuA1, tbmB, and tbmC) (30).
To prepare probes, DNA of the pIPO type plasmids was digested with EcoRI (23) and labeled with digoxigenin by using the megaprime random-labelling system (Boehringer, Mannheim, Germany). Other probes, i.e., whole plasmid RN3 and the IncQ plasmid pMOL187, were prepared similarly.
Hybridizations were carried out as specified by the manufacturer of the membrane filters used (1b), and the blots were washed at high stringency (0.5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] plus 0.1% sodium dodecyl sulfate at 65°C). The CSPD chemiluminescence detection kit (Boehringer) was used, after which X-ray films were exposed to the filters.
PCR and hybridization typing of the isolated plasmids were carried out with the broad-host-range plasmid-specific PCR primer and probe systems and reaction conditions developed by Götz et al. (8). To monitor the efficiency of amplification, the appropriate positive (DNA of prototype plasmids added) and negative (no DNA added) controls were run for each system. All PCR products were run on agarose gels, and the gels were blotted to nylon membranes. The membranes were hybridized with the specific probes for each Inc group (8).
Screening of plasmids for antibiotic and heavy metal resistances.
The presence of plasmid-encoded resistance genes was assessed by streaking plasmid-containing strains onto agar plates (10% strength tryptic soy agar; 0.1× TSA) containing different concentrations of antibiotics or heavy metals (see Table 3). Plasmidless hosts were used as controls. Growth of cells in repeated transfers of the plasmid-loaded strains into single colonies and the absence of such growth in the plasmidless parents were taken as evidence for the presence of the respective resistance genes.
TABLE 3.
Screening for marker genes and PCR BHR Inc group typing of plasmids pIPO1, pIPO2, and pIPO3
| Plasmid | Size (kb) | Resistance
to:
|
Homology to 17 catabolic genesb | PCR Inc group
typingc:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 antibioticsa | 9 heavy metalsa | P
|
N
|
W
|
Q
|
A/C
|
||||||||
| OT | TA1 | KO | OT | KA | OT | OV | OT | OV | REP | |||||
| pIPO1 | 45 | − | − | NDh | − | − | − | +g | − | − | − | +g | +g | − |
| pIPO1T | 46.5 | −d | ND | ND | − | − | − | +f | − | − | − | − | − | − |
| pIPO3 | 45 | − | − | −e | − | − | − | +f | − | − | − | +g | +g | − |
| pIPO3T | 46.5 | −d | ND | ND | − | − | − | +f | − | − | − | − | − | − |
| pIPO2 | 38 | − | − | − | +f | +f | − | − | − | − | − | +g | +g | − |
| pIPO2T | 39.5 | ND | ND | ND | ND | ND | ND | − | − | − | − | − | − | − |
| Controls | ||||||||||||||
| RP4 (IncP) | + | + | + | − | − | − | − | − | − | − | ||||
| RN3 (IncN) | − | − | − | + | + | − | − | − | − | − | ||||
| R388 (IncW) | − | − | − | − | − | + | + | − | − | − | ||||
| RSF1010 (IncQ) | − | − | − | − | − | − | − | + | + | − | ||||
| RA1 (IncA/C) | − | − | − | − | − | − | − | − | − | + | ||||
Antibiotics tested (0, 10, 20, 50, 100, and 200 μg ml−1; ampicillin up to 1,000 μg ml−1): gentamicin, streptomycin, tetracycline, chloramphenicol, kanamycin, trimethoprim, ampicillin, and nalidixic acid. Heavy metals tested: Ag, As, Hg, Cu, Co, Ni, Zn, Cd, Cr. Hosts: Escherichia coli CV601, Pseudomonas fluorescens R2f, Ralstonia eutropha AE815.
Catabolic genes (kindly provided by D. Springael): xylDEGFJIHS (m-toluate meta pathway), catA (catechol-1,2-dioxygenase), benABC (benzoate dioxygenase), catBCDE (catechol ortho-cleavage pathway), pcaACBDFE (protocatechuate pathway), bphC (2,3-dihydroxy-biphenyl dioxygenase), todC123BADE (toluene degradation pathway), alkST (regulatory operon alkane hydroxylase), alkBAC (alkane hydroxylase/alkanol dehydrogenase), tcbCD (chlorocatechol dioxygenase), bphABCD (upper biphenyl pathway), dmpABCD (phenol meta-cleavage pathway), nahR/nahG (regulatory gene naphthalene degradation), nahAa/Ab (naphthalene dioxygenase), tbuA1 (toluene-3-monooxygenase), tbmB (toluene/benzene-2-monooxygenase), and tbmC (toluene/benzene-monooxygenase).
OT, oriT; TA1, trfA1; KO, korA; KA, kikA; OV, oriV; REP, rep.
Except for introduced tetracycline resistance.
A signal was found with the 19.9-kb fragment containing the dmpABCD genes (phenol meta-cleavage pathway).
Nonspecific products.
Product due to the presence of an IncQ plasmid.
ND, not determined.
Host range of mobilization of plasmid pIPO2.
To determine the host range of pIPO2, a Pseudomonas fluorescens R2f (chr::Tn5) donor strain containing both pIPO2 and the IncQ plasmid pIE723 (35) was produced by conjugation. This strain was devoid of any other IncQ determinants due to the selection applied for pIE723. As a negative control for the mating assays, plasmid pIE723 was introduced into the same chromosomal background by electroporation. Plasmid pIE723 has gentamicin, streptomycin, kanamycin, and sulfonamide resistances as selectable markers (35). Plasmid donors were cultured in medium containing 50 μg each of kanamycin and gentamicin per ml.
Rifampin-resistant mutants of strains belonging to a variety of bacterial taxa (Table 1) were isolated by plating dense cultures of these strains onto media containing 50 μg of rifampicin per ml. The plates were incubated for 2 to 4 days at 28°C. Single-mutant colonies were restreaked onto the same medium for final purification. The mutants were used as primary recipients in the mating assays.
Cells from 1-ml overnight cultures were pelleted, washed, and resuspended (to an optical density at 600 nm of 1) in liquid media without antibiotics. The donor and recipient strains were then mixed (1:1), and aliquots (20 μl) were dropped onto LB agar. Alternatively, 100-μl aliquots of washed cultures of both donor and recipient strains were pipetted onto filters which were subsequently placed on LB agar. The plates were incubated overnight at 28°C. After incubation, the mixtures were resuspended in LB broth (drop method) or 0.85% NaCl (filter method), and appropriate dilutions were plated onto transconjugant-selective plates (LB agar with 50 μg of rifampin and gentamicin or streptomycin per ml), onto plates for enumeration of the donor (LB agar with 50 μg of kanamycin per ml) and of the recipient (LB agar with 50 μg of rifampin per ml). Donor and recipient cultures were also placed separately on filters or plates and plated, to assess the occurrence of antibiotic-resistant mutants on transconjugant-selective plates. The plates were incubated for 2 to 4 days at 28 to 30°C before colony enumeration.
The presence of pIPO2 in the transconjugants was verified by PCR amplification of pIPO2-specific sequences with the primers described below. On the other hand, the functional presence of pIPO2 was verified by matings to secondary recipient strains, i.e., the tetracycline (25 μg ml−1)-resistant E. coli Sm10 (Tn5::luxAB::tet) and the nalidixic acid- and rifampin (50 μg ml−1)-resistant P. fluorescens R33 (Table 1). Matings were performed as above, and secondary transconjugants were enumerated on LB agar containing gentamicin (50 μg ml−1) and either tetracycline or nalidixic acid.
Generation and testing of pIPO2-specific primers and a probe.
DNA fragments obtained after restriction of pIPO2 with EcoRI and SphI were cloned into the narrow-host-range positive-selection vector pKIL19 (1a). In an attempt to isolate the minimal replicon, cloned fragments were pooled and transferred en masse into P. putida UWC1, selecting for ampicillin resistance (1,000 μg ml−1). However, clones containing replicators were not identified. Selected random fragments were then subjected to sequencing with the pKIL19 sequencing primers described previously (1a). A 350-bp sequence obtained was compared to the EMBL database by using a FastA homology search. Specific primers for PCR detection of pIPO2 in colonies as well as in soil DNA were designed based on this sequence. These were pIPO2 forward (GAT GAA GCC TGC CAA GTA CG) and pIPO2 reverse [CCG G(G/C)G ATT AGC CTA TCC]. Both primers spanned a 309-bp region. The primers efficiently amplified the 309-bp product with plasmid pIPO2 as a template, using the PCR reaction mixtures described for pure DNA (41), whereas they also worked well with soil DNA, using the soil DNA setup with MgCl2 at 2 mM (41). Forty cycles of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C were used, followed by a final incubation at 72°C (10 min). About 10 to 100 copies of pIPO2 per reaction mixture were thus detectable. The primers were tested for specificity against a suite of 37 plasmid and 9 chromosomal targets and were found to be strictly specific for pIPO2. The amplification product generated with this PCR system targeting plasmid pIPO2 was used as a specific probe in hybridization studies.
Mobilization of plasmid pIE723 by pIPO2 to indigenous bacteria in the wheat rhizosphere under field conditions.
Two experiments in field microplots containing FSL soil were done to assess the capacity of pIPO2 in mobilizing pIE723 from P. fluorescens R2f Rpr to indigenous bacteria. For that purpose, different combinations of host strains with mobilizing (pIPO2) and mobilizable (pIE723) plasmids were established. P. fluorescens R2f Rpr (pIPO2; pIE723) (mobilizing plasmid donor, treatment I) and P. fluorescens R2f Rpr (pIE723) (treatment II) were produced as described above. P. fluorescens R2f Rpr (pIPO2) was produced by selecting kanamycin-sensitive derivatives in subcultures of P. fluorescens R2f Rpr (pIPO2; pIE723) and checking for the loss of pIE723 and maintenance of the more stable plasmid pIPO2.
Rhizosphere soil from young wheat plants (1 to 3 weeks old; three plants per treatment per time point) was treated in the field plot with 10-ml washed overnight suspensions (109 to 1010 CFU ml−1) of different donor and control strains. The strains were added by slowly pipetting the suspensions onto the 5- to 10-mm-diameter zone surrounding the root of each plant. Treatment I consisted of adding P. fluorescens R2f Rpr (pIPO2; pIE723), treatment II consisted of adding P. fluorescens R2f Rpr (pIE723), treatment III consisted of adding P. fluorescens R2f Rpr (pIPO2) followed after 1 h by P. fluorescens R2f Rpr (pIE723), and treatment IV consisted of adding plasmidless P. fluorescens R2f Rpr.
The temperature in the top 5 cm of the soil measured daily at noon varied from 12 to 15°C (mean temperature, 14°C), and the soil moisture in this layer ranged from 26 to 36% (about 50 to 70% of the soil’s moisture-holding capacity). An average rainfall of about 4 mm per day was recorded in the experimental period.
At different times, three plants per treatment were sampled and their respective rhizospheres were obtained and processed for CFU counts of total bacteria (on 0.1× TSA containing 100 μg of cycloheximide per ml), of the respective donors (0.1× TSA supplemented with donor-selective antibiotics), and of indigenous transconjugants (0.1× TSA supplemented with 50 μg of kanamycin per ml plus 50 μg of streptomycin per ml). Phage φR2f was used for donor counterselections as described previously (27, 28).
Transconjugant-selective plates were screened for the presence of pIE723- and pIPO2-containing colonies by colony filter hybridizations with whole plasmid pIE723 as well as the pIPO2-specific PCR product as probes. Putative transconjugants, identified via colony hybridizations, were picked from transconjugant-selective plates and rechecked by colony hybridization with pIE723 as the probe, as well as by IncQ-oriV- and pIPO2-specific colony PCR. Selected colonies that were positive for the former two criteria were further analyzed by Biolog identification (Biolog Inc., Hayward, Calif.), by REP-PCR (43), by tests for the antibiotic resistance of the donor and for resistance to the unselected antibiotic gentamicin, and by direct plasmid extraction.
Detection of pIPO2 homologous sequences in soils.
Total microbial community DNA was obtained from FSL bulk as well as wheat rhizosphere soils from the field microplot study by established procedures (26, 40). In addition, DNA was obtained from a range of different soils, including Ede loamy sand; Finnish organic soil (39); dichloropropene-treated and untreated Odoorn, Westmaas, and Smilde soils (42); the oil-polluted soils L, LP1, and P2, and the acidic soil C3 (unpublished data); and from mercury-treated FSL soil (29). The presence of pIPO2-homologous sequences in the soil and rhizosphere bacterial populations was assessed by screening total soil DNA by pIPO2-specific PCR and hybridization with a specific probe (the PCR product generated on pIPO2). Controls for amplification efficiency consisted of soil DNA with added diluted plasmid pIPO2 DNA.
RESULTS
Triparental exogenous isolation and further transfer of mobilizing plasmids from soil and rhizosphere.
Triparental exogenous isolation of gene-mobilizing plasmids was performed in three experiments with bacterial populations obtained from mature wheat and maize plants grown in the field. Mobilization of pMOL187 was never found with these populations, which suggested that the transfer frequencies were below the detection limit of roughly 10−10 to 10−11 per donor.
To assess the potential of younger seedlings to develop a bacterial rhizosphere population with greater gene-mobilizing capacity, a microcosm setup with wheat plants growing in FSL soil was used. In three experiments, mobilization of the indicator plasmid pMOL187 from E. coli CM990 to R. eutropha AE815 was observed with the bacterial populations associated with the young wheat roots as donors of plasmid-mobilizing capacity. The transfer frequencies were about 0.5 × 10−10 per donor. Mobilization of pMOL187 was never observed with populations obtained from bulk samples of the same soil. Plasmid isolations performed with a group of 20 putative mobilizers unequivocally showed, in addition to pMOL187, the presence of plasmids with sizes between roughly 40 and 60 kb in several R. eutropha transconjugant clones (data not shown). Three of these clones were selected for further study, and the plasmids they contained were designated pIPO1, pIPO2, and pIPO3. By using hybridizations of the 20 colonies with pIPO2 and pIPO3 as probes, these two plasmids were shown to be representative of 70% (14 of 20) of the clones. More specifically, seven clones reacted only with pIPO2, seven other clones (including the one containing pIPO1) reacted only with pIPO3, and six gave no reaction with either probe. Hence, at least two different types of mobilizing plasmids, pIPO2 and pIPO3, were obtained.
Triparental matings with the R. eutropha AE815 clones containing pIPO1, pIPO2, and pIPO3 with pSUP104 as the (selectable) mobilizable plasmid showed that all three plasmids could mobilize pSUP104 from E. coli to P. fluorescens R2f (chr::Tn5), with high frequencies (Table 2). P. fluorescens transconjugants of each mating contained, in addition to pSUP104, a plasmid with a similar size to that in the R. eutropha AE815 primary transconjugants. Selected transconjugants from each mating were able to transfer pSUP104 further to a homologous strain, P. fluorescens R2f Rpr, as well as to E. coli CV601 Rpr (Table 2). The three plasmids obtained in P. fluorescens were selected for use as prototype broad-host-range (BHR) mobilizers in further experiments.
TABLE 2.
Mobilization of plasmid pSUP104 by plasmids pIPO1, pIPO2, and pIPO3
| Strain | Counts (CFU/filter) of:
|
Frequency observeda | Cotransfer of pIPO | |
|---|---|---|---|---|
| Transconjugants | Recipients | |||
| R. eutrophab | ||||
| AE815(pIPO1) | 7.8 × 107 | 1.8 × 109 | 4.3 × 10−2 | + |
| AE815(pIPO2) | 5.9 × 107 | 2.1 × 108 | 2.8 × 10−1 | + |
| AE815(pIPO3) | 3.5 × 108 | 3.5 × 109 | 9.9 × 10−2 | + |
| P. fluorescensc | ||||
| R2f(pIPO1; pSUP104) | 1.7 × 103 | 1.2 × 1010 | 1.4 × 10−7 | + |
| R2f(pIPO2; pSUP104) | 5.7 × 107 | 1.2 × 1010 | 4.7 × 10−3 | + |
| R2f(pIPO3; pSUP104) | 2.7 × 106 | 6.7 × 109 | 4.0 × 10−4 | + |
Two replicate matings were performed, and the average values are reported.
Mobilization by R. eutropha AE815 helper strains containing pIPO1, pIPO2, or pIPO3, between E. coli (pSUP104) and P. fluorescens R2f (chr::Tn5).
Mobilization from P. fluorescens R2f (chr::Tn5) (pIPO; pSUP104) to E. coli CV601 Rpr.
All three plasmids conferred retromobilization (mobilization in the opposite direction) ability, since P. fluorescens R2f cells harboring pIPO1, pIPO2, or pIPO3 were able to capture plasmid pSUP104 with an average frequency of 7 × 10−7 per recipient.
To remove the IncQ plasmid pMOL187, each of the three primary R. eutropha AE815 transconjugants was mated with an E. coli strain containing the narrow-host-range vector pUT/Km with a mini-Tn5::luxAB::tet reporter transposon (10). Selection for transfer to and stable maintenance of the tetracycline resistance in a P. fluorescens recipient strain, as well as a check for the absence of the czc gene cassette, resulted in P. fluorescens clones containing mini-Tn5::luxAB::tet-marked derivatives pIPO1T, pIPO2T, and pIPO3T (Table 3).
Molecular analysis and comparison of plasmids pIPO1, pIPO2, and pIPO3.
Plasmids pIPO1, pIPO2, and pIPO3 were digested with EcoRI, PstI, and SphI, which resulted in patterns of clearly separable bands on 0.8% agarose gels. The patterns obtained with all three enzymes were similar between pIPO1 and pIPO3, whereas the pIPO2 patterns were different (data not shown). The calculated plasmid sizes were 38 kb (pIPO2) and 45 kb (pIPO1 and pIPO3). Hybridization analysis of the digests with pIPO1, pIPO2, pIPO3, and the prototype IncN plasmid RN3 and pSUP104 (controls) as probes revealed that plasmids pIPO1 and pIPO3 were similar, since identical hybridization patterns were found with pIPO1 and pIPO3 as probes whereas the pIPO2 probe did not show indigenous hybridization signals with pIPO1 and pIPO3 target bands (Fig. 1). Hence, plasmid pIPO2 was unrelated at the sequence level (high stringency) to plasmids pIPO1 and pIPO3, based on the absence of any hybridization signals when used as either the probe or the target. As expected, the mini-Tn5::luxAB::tet-marked pIPO derivatives pIPO1T, pIPO2T, and pIPO3T showed hybridization patterns similar to those of the respective parent plasmids, with the exception of the absence of a ca. 10-kb cross-hybridizing IncQ-specific band (also not visible on the gel, which indicated loss of pMOL187) and a change in the banding patterns due to the insertions. Furthermore, there was no homology between any of the pIPO plasmids and the IncN prototype plasmid RN3, or with its oriT PCR product, as found in the hybridization assays.
FIG. 1.
Southern hybridization of digested plasmids with plasmids pIPO2 (A) and pIPO3 (B) as the probes. Lanes: 1, pIPO1; 2, pIPO2; 3, pIPO3; 4, pIPO1T; 5, pIPO2T; 6, pIPO3T; 7, DIG ladder; 8, RN3; 9, pSUP104; 10, mini-Tn5::tet; 11, pMOL187. Plasmid pSUP104 was still present in the target and probe, as shown by the hybridizing band at about 10 kb, which hybridized strongly with pSUP104 (not shown).
Based on these results, we selected plasmids pIPO2 and pIPO3 for further characterization work. Replicon typing of these plasmids by Southern hybridization with 18 incompatibility (Inc) group-specific (origin of replication) probes (5) showed that pIPO2 and pIPO3 had no homology to any of the replicon-specific fragments used as the targets (data not shown). This included fragments specific for the BHR plasmids of the P, N, W, and Q Inc groups (5). In addition, the IncN-, IncP-, and IncW-specific phages ike and PR4 did not infect P. fluorescens R2f or E. coli cells containing pIPO2 or pIPO3/pIPO1, suggesting that these plasmids did not produce pili specific for plasmids of these Inc groups. Finally, a comparison by hybridization of the three pIPO plasmids with two different sets of new mercury resistance plasmids (29, 42) revealed the absence of homology to these plasmids.
Taken together, these results provided preliminary evidence for the suggestion that pIPO2 and pIPO3 may represent two groups of new BHR plasmids.
Screening for markers on plasmids pIPO2 and pIPO3/pIPO1.
Plasmids pIPO1, pIPO2, and pIPO3 did not confer resistance to any of the antibiotics or heavy metals tested when present in any of the three hosts R. eutropha AE815, P. fluorescens R2f, or E. coli (Table 3). The resistances found were in all cases due to the intrinsic resistance of the host (as shown by comparing the resistance patterns of the plasmid-containing hosts with those of the plasmidless hosts) or to the coresident IncQ plasmid (as shown by comparing the resistance patterns with those of strains with only the IncQ plasmids).
Hybridization of pIPO2 and pIPO3 whole-plasmid probes with 17 different catabolic target genes (Table 3; kindly provided by D. Springael, VITO) showed a general absence of any homology between pIPO2 and, with one exception, pIPO3 and any of the specific targets used. Plasmid pIPO3 revealed the presence of homology to a 19.9-kb fragment representing the phenol meta-cleavage pathway.
PCR replicon typing of pIPO1, pIPO2, and pIPO3.
Since the pIPO plasmids were likely to have a broad host range by virtue of their transfer between members of the beta (R. eutropha) and gamma (E. coli, P. fluorescens) Proteobacteria, we investigated whether they belonged to any of the well-known BHR Inc groups. The PCR and hybridization typing we used involved primer systems based on key genes involved in the maintenance and/or transfer of BHR plasmids of the IncP, IncQ, IncN, IncW (8), and IncA/C (1) groups. Table 3 summarizes the results obtained. Amplification with primers characteristic of the origins of transfer (oriT) of IncW and IncQ groups gave negative results with all three pIPO plasmids. On the other hand, the IncP-specific oriT PCR system yielded an aspecific product with plasmid pIPO2; this product also did not hybridize with the IncP-specific probe generated on the prototype IncP plasmid RP4. The other amplification systems, which were based on the origins of vegetative replication oriV (34) on genes involved in the control of vegetative replication (korA, trfA1, and rep) or on an IncN-specific gene (kikA), produced uniformly negative results with the three plasmids. Hence, no evidence was obtained for the classification of any of the three pIPO plasmids in known BHR plasmid Inc groups.
The PCR amplification system characteristic of oriT of the IncN plasmid RN3 (8) produced a product of the size expected for RN3 with pIPO1 and pIPO3 but not with pIPO2. However, amplification of the products with nested primers specific for the RN3 oriT yielded negative results. Moreover, the primary PCR products also did not give a hybridization signal with an RN3-generated PCR product as a probe. These data suggested that the PCR products obtained with pIPO1 and pIPO3 as the targets were not strongly homologous to the RN3 oriT region.
Development of pIPO2-specific PCR primers.
A specific PCR amplification system was first developed to assess the presence of pIPO2-type plasmids in soil. To generate specific primers, banks of pIPO2 restriction fragments were made in the positive-selection vector pKIL19. Clones containing vectors with inserts were selected, and five small (1- to 2-kb) inserts were screened for specificity for pIPO2 by using colony blots. They all hybridized to the (whole-plasmid) pIPO2 probe used, whereas controls without insert did not. The insert of one selected clone was partially sequenced. This sequence showed no homology to any sequence larger than about 20 bp of the database, except for homology (70%) to a 31-bp region of a plasmid pOAD2 (43.6-kb)-borne nylon oligomere degradative gene of a Flavobacterium sp. (18). A PCR amplification system was based on specific stretches in the sequence, taking into account, by database comparisons, the lowest possibility of amplification of foreign DNA. The specificity of this PCR amplification system for pIPO2 and its derivative pIPO2T and the absence of a product with pIPO3, pIPO1, or any other plasmid or chromosomal target was confirmed in tests with DNA of 37 different plasmids as well as 9 chromosomal backgrounds (data not shown). The system worked well with pure plasmid pIPO2 DNA, with whole cells containing the plasmid, and with soil DNA containing the plasmid.
Host and transfer range of pIPO2.
To determine the host range of pIPO2, matings between a P. fluorescens R2f host and rifampin-resistant recipient strains (Table 1) were performed. The IncQ plasmid pIE723 (35) was used as a marker for pIPO2-mediated conjugal transfer, since its markers allowed for selection of transconjugants. For plate matings, resistance to gentamicin was used as the primary marker. To assess the functional presence of pIPO2 in different taxa, putative transconjugants were mated to the tetracycline-resistant E. coli recipient, strain Sm10 (Tn5::luxAB::tet) or to the nalidixic acid-resistant P. fluorescens strain R33. Colony hybridizations were performed with pIPO2 or the pIPO2-specific PCR product as probes to verify the presence of the mobilizer plasmid in the transconjugants. The results presented in Table 4 indicated that pIPO2 was able to mobilize pIE723, as well as self-transfer, to species of a wide variety of gram-negative bacterial genera. This included members of the alpha, beta, and gamma subclasses of the Proteobacteria. Plasmid pIPO2 was functionally present (as evidenced by its ability to mobilize pIE723 further) in R. eutropha AE815, Azospirillum brasilense Sp7, Acinetobacter calcoaceticus BD413, Enterobacter cloacae Ecl2, E. coli CV601, P. fluorescens R2f, P. putida Pp3, R. meliloti L5-30, Xanthomonas vesicatoria Xc, and the putative member of the beta subclass of the Proteobacteria F4. Taxonomic assignment of F4 was made on the basis of partial 16S rDNA sequencing (data not shown). Furthermore, plasmid pIPO2 transferred pIE723 to Agrobacterium sp. strain A3 but did not detectably self-transfer to this strain. No transfer to Cytophaga spp. was detected. Similarly, transfer to species of the gram-positive bacteria was never detected.
TABLE 4.
Host range of mobilization of the IncQ plasmid pIE723 and self-transfer of plasmid pIPO2 from P. fluorescens R2f (chr::Tn5) to different rifampin-resistant recipient strains
| Taxon | Strain | Transfer of:
|
Transfer frequencyb | |
|---|---|---|---|---|
| pIE723 (phys.a) | pIPO (func.a) | |||
| Proteobacteria | ||||
| Alpha subclass | Agrobacterium sp. strain A3 | + | − | 10−2–10−4 |
| A. caulinodans ORS571 | − | − | NA | |
| A. brasilense Sp7 | + | + | 10−1 | |
| R. meliloti L5-30 | + | + | 10−5 | |
| Beta subclass | R. eutropha AE815 | + | + | ND |
| Unclassified isolate F4 | + | + | 10−5–10−6 | |
| Gamma subclass | A. calcoaceticus BD413 | + | + | 10−4 |
| E. cloacae Ecl2 | + | + | 10−2–10−3 | |
| E. coli CV601 | + | + | 10−1–10−3 | |
| P. fluorescens R2f | + | + | 10−3–10−4 | |
| P. putida Pp3 | + | + | 10−1–10−2 | |
| X. vesicatoria Xc | + | + | 10−1–10−2 | |
| Bacteroides | Cytophaga sp. strain LTER30 | − | − | NA |
| C. heparina | − | − | NA | |
| Gram-positive bacteria | Arthrobacter sp. strain ArthR | − | − | NA |
| B. subtilis SEm-2 | − | − | NA | |
| P. azotofixans P3L5 | − | − | NA | |
| C. michiganensis CR | − | NA | NA | |
phys., physical, as evidenced by colony hybridizations, colony PCR, and plasmid extractions; func., functional, as evidenced by a mobilization assay to E. coli Sm10 (Tn5::tet) or P. fluorescens R2f; +, transfer detected; −, no transfer detected; NA, not applicable; ND, not determined.
Average of frequencies measured in at least two separate experiments, indicated as the order of magnitude or range (when frequencies in the independent experiments differed by more than one order of magnitude).
Plasmid pIPO2 can thus be characterized as a BHR plasmid which mobilizes IncQ plasmids to and spreads primarily among members of the alpha, beta, and gamma subclasses of the Proteobacteria.
Mobilization of plasmid pIE723 by pIPO2 to indigenous bacteria in the rhizosphere of wheat grown in field microplots.
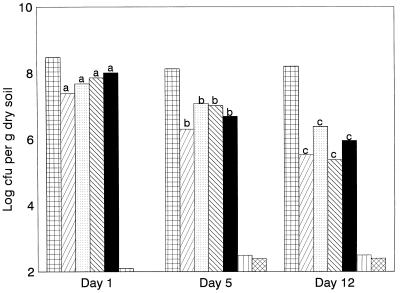
As shown in Fig. 2, all introduced donor strains showed a significant decline in the wheat rhizosphere in FSL soil over 12 days under the prevailing conditions (daily temperatures in the topsoil between 12 and 15°C, moisture content of 26 to 36%) in the field, whereas the levels of indigenous bacteria were roughly stable at around 108 CFU g of dry soil−1. Based on the three data points, there was no difference in behavior among the three different introduced strains, suggesting that plasmid carriage did not drastically affect their fitness. When the mobilizing plasmid pIPO2 was present either in the same strain or in a separately introduced strain, indigenous transconjugants were identified by colony hybridization confirmed by pIE723-specific colony PCR, the presence of the pIE723 resistances, and the absence of donor phenotype (resistance to rifampin, fluorescence on King’s B agar) from day 1 (treatment I) or 5 (treatment III) onward. In contrast, evidence for the occurrence of colonies of indigenous bacteria with the pIE723 phenotype or genotype was not found in the rhizospheres that had received either plasmidless P. fluorescens R2f Rpr or P. fluorescens R2f Rpr (pIE723). In addition, IncQ-oriV specific PCR performed on DNA extracted from the control soil (inoculated with the plasmidless strain) was negative, whereas that with DNA from the pIE723-containing soils revealed positive amplifications (data not shown). On days 1, 5, and 12, the indigenous transconjugants with the pIE723 phenotype were found at levels estimated to be on the order of 102 to 103 CFU g of soil−1 for the jointly introduced plasmids (Fig. 2). For the separately introduced plasmids, this level was also reached on days 5 and 12. The level represented roughly 0.1 to 1% of the colonies developing on the selective media used.
FIG. 2.
Mobilization of plasmid pIE723 by pIPO2 in the wheat rhizosphere in the field. Symbols: , total indigenous bacterial counts on 0.1× TSA in treatment I (all treatments had similar counts throughout); ▨, P. fluorescens R2f Rpr (pIPO2; pIE723), treatment I; ░⃞, P. fluorescens R2f Rpr (pIE723), treatment II; ▧, P. fluorescens R2f Rpr (pIE723), treatment III; ▪, P. fluorescens R2f Rpr (pIPO2), treatment III; ▥, transconjugants, treatment I; ▩, transconjugants, treatment III. For each treatment, a>b>c (P < 0.05). Unlabeled values: no significance assigned because of small numbers (transconjugants) or statistical similarity (indigenous counts).
Twenty-six selected transconjugant colonies, obtained on day 5 from both treatments that had yielded transconjugants, were analyzed. None of these colonies had the donor phenotype, as shown by their sensitivity to rifampin and the absence of fluorescence on King’s B agar. All carried pIE723, as evidenced by their phenotypes (resistances to the antibiotics used for primary selection, kanamycin and streptomycin, as well as to the unselected antibiotic, gentamicin), their positive reaction with the IncQ-specific PCR system, and the physical presence of plasmids with sizes similar to that of pIE723. However, the presence of pIPO2 was evident in only two clones, as shown by pIPO2-specific PCR. Twelve clones obtained from both treatments were identified via the Biolog metabolic fingerprinting system as relatives of members of the gamma subclass of the Proteobacteria Enterobacter sp. (three clones), E. aerogenes, and Klebsiella terrigena (three clones), or the alpha subclass member Agrobacterium radiobacter B (two clones), and Buttiaxella agrestis or remained unidentified (two clones). However, no perfect matches with the Biolog database were obtained.
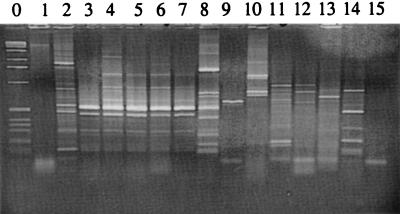
Further analysis by REP-PCR (Fig. 3) revealed a variety of amplification patterns different from that of the donor P. fluorescens R2f, suggesting that a number of transfer events between the added donor and gram-negative indigenous bacteria in the rhizosphere of wheat had taken place. One group of five isolates, consisting of strains 122, 126, 127, 139, and 145 (Fig. 3), showed almost identical repetitive extragenic palindromic (REP)-PCR patterns and might be representative of one recipient group. Two of these strains, 126 and 145, were identified as relatives of Enterobacter spp. by Biolog analysis, whereas three remained unidentified.
FIG. 3.
REP-PCR analysis of selected transconjugants that received plasmid pIE723 in the wheat rhizosphere. Lanes: 0, molecular size ladder; 1, Agrobacterium radiobacter B 11; 2, Klebsiella terrigena 12; 3, unidentified strain 122; 4, Enterobacter agglomerans subgroup A 126; 5 and 6 unidentified strains 127 and 139; 7, E. aerogenes 145; 8, K. terrigena 99; 9, A. radiobacter B 4; 10, Butiaxella agrestis 16; 11, E. aerogenes 39; 12, K. terrigena 62; 13, unidentified strain 94; 14, P. fluorescens R2f Rpr (donor strain); 15, negative control.
Detection of pIPO2-specific sequences in soil.
The PCR system specific for pIPO2 was used to assess the prevalence of this plasmid in different soil samples. Added pIPO2 DNA could be detected in soil DNA extracts down to about 103 to 104 copies g−1, as evidenced by its detection to extinction in a (FSL or Westmaas) soil DNA background (data not shown). Furthermore, FSL rhizosphere soil samples obtained in the field experiment with the introduced pIPO2, as expected, showed the presence of the pIPO2-specific PCR products, whereas pIPO2 was not found in field rhizosphere soil without added pIPO2. In addition, evidence for the natural occurrence of pIPO2 was found in the rhizosphere of microcosm-grown 7-day-old wheat (Fig. 4). A screening of DNA extracts of various soils with widely differing characteristics with respect to soil type and soil treatment, including oil (unpublished data), dichloropropene (42), and mercuric chloride treatments (29), revealed the general absence of pIPO2-specific products, whereas added pIPO2 was readily amplified. Figure 4 shows the products obtained in a selection of the soils. This suggested that irrespective of the stress applied, in all soils the natural levels of pIPO2 plasmids, if present at all, were below 103 to 104 copies g of soil−1.
FIG. 4.
Screening for pIPO2 sequences via PCR analysis of DNA of selected soils and rhizospheres. Lanes (odd numbers, dilute pIPO2 added; even numbers, pIPO2 not added): 0, molecular size ladder; 1 and 2, FSL, bulk; 3 and 4, FSL with mercuric chloride, wheat rhizosphere; 5 and 6, FSL, 7-day wheat rhizosphere; 7 and 8, FSL with mercuric chloride, bulk; 9 and 10, FSL, bulk field; 11 and 12, soil LP1, bulk; 13 and 14, soil P2, bulk; 15 to 18, Odoorn soil, bulk; 19 and 20, soil C3, bulk; 21 and 22, pIPO2 DNA; 23, negative control.
DISCUSSION
In this paper, we provide evidence for the occurrence of novel BHR plasmids in bacterial populations in the rhizosphere of wheat, which are able to mobilize IncQ plasmids to different gram-negative bacterial species. Two plasmids, selected from a group of 20, were shown to be representative of 65% of the clones obtained in one experiment (32.5% for each), suggesting that these identified plasmid types were frequently isolated from the wheat rhizosphere with the gene escape system. The isolation procedure, originally described by Top et al. (36), directly isolates plasmids with BHR mobilizing capacity from soil, irrespective of its original host, by combining the putative plasmid-mobilizing capacity present in soil microbial communities with the discriminatory power of the E. coli (pMOL187)/R. eutropha AE815 plasmid transfer screening system. This approach focuses on the mobilizing capacity of plasmids rather than on selection for known or putative selectable plasmid marker genes such as used in other soil plasmid studies (see, e.g., references 4 and 29) and hence may provide hitherto unknown information on the prevalence and role of gene-mobilizing elements (36).
Generally, a paucity of selectable traits (antibiotic or heavy metal resistance) was found to be present on pIPO2 and pIPO3/pIPO1, indicating that these plasmids were cryptic with respect to such marker genes. Clearly, the exception to this crypticity was their capacity to self-transfer and confer gene-mobilizing capacity to their hosts. We cannot explain the discrepancy between the very similar replicon genotypes of pIPO1 and pIPO3 (evidenced by restriction, hybridization, and replicon typing) and the different transfer frequencies (Table 2). Furthermore, the significance of the homology in pIPO3 to the 19.9-kb fragment containing the dmpABCD genes is still unclear. These points need additional investigation but were not the focus of the present study. The fact that neither of the plasmids could be grouped with any known Inc group by replicon typing with the Couturier et al. (5) probes or with any known BHR plasmid group by PCR typing and probing (8) suggested that they might belong to hitherto undescribed BHR plasmid groups. Top et al. (36) also found that some soil-isolated BHR mobilizing plasmids did not belong to known Inc groups (as evidenced by hybridization with a large number of replicon-specific probes (5). Moreover, Kobayashi and Bailey (13) recently described plasmids in Erwinia and Pseudomonas spp. isolated from the sugarbeet phyllosphere, which could not be assigned to known Inc groups and hence might represent new plasmid groups representative of environmental plasmid pools. Recent work performed in collaboration with M. Couturier, M. Mergeay, and coworkers suggests that several other novel plasmids with BHR characteristics are incompatible with pIPO2. Hence, these plasmids may define at least one new incompatibility group containing BHR plasmids (unpublished data).
Other studies have also addressed the occurrence of mobilizing and other plasmids in soil bacteria. Campbell et al. (4) recently found plasmids in some P. fluorescens soil isolates, but that study did not address the gene-mobilizing capacity of the plasmids. In addition, plasmids of Agrobacterium tumefaciens (e.g., pAK84) and of Rhizobium spp. have been known for a long time, but, again, these have not been assessed for their BHR gene-mobilizing capacity. Boronin suggested that new plasmids able to replicate in Pseudomonas species probably would be BHR plasmids (2). Furthermore, Sayler et al. (24) reviewed the characteristics of a range of catabolic plasmids that are frequently found in soil proteobacteria. Of the many plasmids listed, it is unclear which are BHR plasmids or have gene-mobilizing capacity. Moreover, their in situ gene-mobilizing potential is largely unknown, but these plasmids are clearly important for the enhancement of the breakdown of xenobiotic compounds and have been studied to assess such processes.
The primer system generated for the detection of pIPO2 was found to be specific for this plasmid, as evidenced by PCR amplification of a suite of plasmid and chromosomal DNAs. Hence, we assumed that this system was able to specifically detect pIPO2 via these sequences in soil systems. The experiments performed identified the added pIPO2 in the wheat rhizosphere in the field and also naturally occurring pIPO2 sequences in the rhizosphere of young wheat plants growing in soil microcosms. This and the absence of any evidence for the occurrence of pIPO2 in a range of stressed and unstressed bulk soils thus indicated that young wheat roots were the habitat of selection of this plasmid type and its putative host.
The role of the plasmids obtained initially seemed unclear, since they did not seem to harbor any genes with specific functions. It is possible that the pIPO plasmids possess genes that code for untested functions that may be involved in determining the fitness of their natural hosts. On the other hand, there are indications that so-called core plasmids (BHR plasmids without clearly selectable markers) can be important for mediating genetic plasticity in bacterial populations in natural ecosystems. According to Guiney (9), BHR plasmids have a fundamental biological significance since they provide a means of promiscuous gene exchange. The pIPO plasmids could represent the backbones of particular types of such BHR plasmids. Similarly, Burlage et al. (3) found that several degradative plasmids, i.e., pJP4, pAC25, pSS50, and pBR60, carried different genes for the degradation of chloroaromatic compounds, whereas they have a strongly homologous backbone. Tschäpe also found strong evidence for the presence of cryptic plasmids with presumably similar backbones among enteric bacteria in humans and animals, which acquired streptothricin resistance under conditions of selection (37). These cryptic plasmids might be present in small fractions of natural bacterial populations to serve as tools for capturing beneficial genes and spreading these through these populations in situ. Thus, bacterial populations might be able to quickly adapt to changing environmental conditions. Several other studies provided evidence that pointed in this direction. Verhagen et al. (42) found that after repeated addition of the nematocide 1,3-dichloropropene to several soils, this compound was rapidly degraded. The accelerated breakdown could be ascribed to bacteria containing plasmids harboring the responsible genes. Similarly, Tam et al. (32) encountered accelerated breakdown of 3-ethyl-N,N-dipropyl-thiocarbonate after repeated additions to soil and a concomitant prevalence of bacterial isolates that contained plasmids with degrading capacity. The conclusion of van der Meer (38), that a few common self-transmissible ancestor replicons may have been involved in the acquisition and spread of different catabolic modules, could tie together some of these results. However, the fact that it has so far been impossible to assign many plasmids encountered in environmental samples to known Inc groups (13, 29, 36) via replicon typing with probes based on clinical isolates suggests that they evolved along separate lines instead of from a common ancestor.
The field experiment with introduced plasmid-carrying P. fluorescens strains clearly showed the capacity of pIPO2 to mobilize the IncQ plasmid pIE723 in the wheat rhizosphere to a range of taxonomically diverse members (members of the alpha and gamma subclasses of the Proteobacteria) of the indigenous bacterial community. These transfers were in line with those found in the in vitro experiments, with the indigenous recipients found in some of the same taxons (Enterobacter and Agrobacterium spp.) that had been shown to be able to receive plasmids at high frequencies (Table 4). The low level of cotransfer of plasmid pIPO2 with pIE723 in the field was striking and might be caused by the mobilization of a (small) IncQ plasmid being more efficient in the field than the self-transfer of a larger plasmid (28). The Agrobacterium strain used in the host range study probably also did not receive or maintain pIPO2 at high rate, since it was unable to further mobilize pIE723. The fact that transfer of pIE723 was not detectable when pIPO2 was not introduced into the field rhizosphere soil suggested that naturally occurring pIPO2-like plasmids were either sparsely present or, if present, did not become actively involved in pIE723 mobilization. Indeed, the direct screening of different bulk soil DNAs provided evidence for a general low incidence of pIPO2-like sequences in soils (Fig. 4). It is therefore possible that pIPO2 and like plasmids are present in a minority of bacterial cells in the soil and rhizosphere that support their multiplication and/or maintenance. The presence of a young rhizosphere in combination with optimal conditions, such as encountered with wheat seedlings grown in soil microcosms, possibly favors the natural host of pIPO2, which might be found among enteric bacteria or pseudomonads, taxa that are known to be stimulated by young wheat roots under controlled microcosm conditions.
Since, in spite of a clear involvement in in situ plasmid mobilizations, the ecological role of the pIPO plasmids is still poorly understood, future research might focus on an assessment of the ecological conditions that promote the multiplication and spread of these plasmids. The PCR detection system developed will be helpful in reaching such an objective. Since such conditions very probably also stimulate the growth or activity of the natural plasmid hosts, these might be identified, which may result in a better understanding of plasmid-host relationships as affected by prevailing conditions in their habitat.
ACKNOWLEDGMENTS
We thank Max Mergeay and Dirk Springael for providing some of the strains and catabolic genes used. Andreas Engelberts, Martin Bulsink and Moniek Kemperman are acknowledged for help with some experiments.
This work was supported by the CEC, EU-BIOTECH programme, grant BIO2-CT92-0491.
REFERENCES
- 1.Bailey, M. J. Personal communication.
- 1a.Bernard P, Gabant P, Bahassi E M, Couturier M. Positive-selection vectors using the F plasmid ccdBkiller gene. Gene. 1994;148:71–74. doi: 10.1016/0378-1119(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 1b.Boehringer. The DIG System User’s Guide. Mannheim, Germany: Boehringer; 1995. [Google Scholar]
- 2.Boronin A M. Diversity of Pseudomonas plasmids: to what extent? FEMS Microbiol Lett. 1992;100:461–468. doi: 10.1111/j.1574-6968.1992.tb14077.x. [DOI] [PubMed] [Google Scholar]
- 3.Burlage R S, Bennis L A, Layton A C, Sayler G S, Larimer F. Comparative genetic organization of incompatibility group P degradative plasmids. J Bacteriol. 1990;172:6818–6825. doi: 10.1128/jb.172.12.6818-6825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell J I A, Jacobsen C S, Sörensen J. Species variation and plasmid incidence among fluorescent Pseudomonasstrains isolated from agricultural and industrial soils. FEMS Microbiol Ecol. 1995;18:51–62. [Google Scholar]
- 5.Couturier M, Bex F, Bergquist P L, Maas W K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988;52:375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreyfuss B, Coreia J L, Gillis M. Characterization of Azorhizobium caulinodans gen. nov. sp. nov., a stem-nodulating nitrogen fixing bacterium isolated from Sesbania rostrata. Int J Syst Bacteriol. 1988;38:89–98. [Google Scholar]
- 7.Fry J C, Day M J. Plasmid transfer in the epilithon. In: Fry J C, Day M J, editors. Bacterial genetics in natural environments. London: Chapman & Hall, Ltd.; 1990. pp. 55–80. [Google Scholar]
- 8.Götz A, Pukall R, Smit E, Tietze E, Prager R, Tschäpe H, van Elsas J D, Smalla K. Detection and characterization of broad-host range plasmids in environmental bacteria by PCR. Appl Environ Microbiol. 1996;62:2621–2628. doi: 10.1128/aem.62.7.2621-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guiney D G. Broad host range conjugative and mobilizable plasmids in Gram-negative bacteria. In: Clewell D, editor. Bacterial conjugation. New York, N.Y: Plenum Press; 1993. pp. 75–103. [Google Scholar]
- 10.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill K E, Weightman A J, Fry J C. Isolation and screening of plasmids from the epilithon which mobilize recombinant plasmid pD10. Appl Environ Microbiol. 1992;58:1292–1300. doi: 10.1128/aem.58.4.1292-1300.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi N, Bailey M J. Plasmids isolated from the sugar beet phyllosphere show little or no homology to molecular probes currently available for plasmid typing. Microbiology. 1994;140:289–296. doi: 10.1099/13500872-140-2-289. [DOI] [PubMed] [Google Scholar]
- 14.Kowalski M. Transducing phages of R. meliloti. Acta Microbiol Polon Ser A. 1970;19:109–114. [PubMed] [Google Scholar]
- 15.Lilley A K, Fry J C, Day M J, Bailey M J. In situ transfer of an exogenously isolated plasmid between Pseudomonasspp. in sugar beet rhizosphere. Microbiology. 1994;140:27–33. [Google Scholar]
- 16.Louws F S, Fulbright D W, Stephens C T, de Bruijn F J. Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonaspathovars and strains generated with repetitive sequences and PCR. Appl Environ Microbiol. 1994;60:2286–2295. doi: 10.1128/aem.60.7.2286-2295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mergeay M, Nies D, Schlegel H G, Gerits J, Charles P, van Gijsegem F. Alcaligenes eutrophusis a facultative chemolithotroph with plasmid bound resistance to heavy metals. J Bacteriol. 1985;162:328–334. doi: 10.1128/jb.162.1.328-334.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negoro S, Kakudo S, Urabe I, Okada H. A new nylon oligomer degradation gene (nylC) on plasmid pOAD2 from a Flavobacteriumsp. J Bacteriol. 1992;174:7948–7953. doi: 10.1128/jb.174.24.7948-7953.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell B J, Purdy K J, Thompson I P, Bailey M J. Demonstration of tra+ plasmid activity in bacteria indigenous to the phyllosphere of sugarbeet: gene transfer to a recombinant pseudomonad. FEMS Microbiol Ecol. 1993;12:195–206. [Google Scholar]
- 20.Priefer U B, Simon R, Pühler A. Extension of the host range of Escherichia colivectors by incorporation of RSF1010 replication functions. J Bacteriol. 1985;163:324–330. doi: 10.1128/jb.163.1.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richaume A, Smit E, Fauri G, van Elsas J D. Influence of soil type on the transfer of RP4p from Pseudomonas fluorescensto indigenous bacteria. FEMS Microbiol Ecol. 1992;101:263–292. [Google Scholar]
- 22.Rossbach S, Rasul G, Schneider M, Endley B, de Bruijn F J. Structural and functional conservation of the rhizopine catabolism (moc) locus is limited to selected Rhizobium melilotistrains and unrelated to their geographical origin. Mol Plant-Microbe Interac. 1995;8:549–559. doi: 10.1094/mpmi-8-0549. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Sayler G S, Hooper S W, Layton A C, King J M H. Catabolic plasmids of environmental and ecological significance. Microb Ecol. 1990;19:1–20. doi: 10.1007/BF02015050. [DOI] [PubMed] [Google Scholar]
- 25.Seldin L, van Elsas J D, Penido E G C. Bacillus azotofixans sp. nov., a new nitrogen fixing Bacillusspecies isolated from Brazilian soils and grass roots. Int J Syst Bacteriol. 1984;34:451–456. [Google Scholar]
- 26.Smalla K, Cresswell N, Mendonca-Hagler L C, Wolters A, van Elsas J D. Rapid DNA extraction protocol from soil for polymerase chain reaction-mediated amplification. J Appl Bacteriol. 1993;74:78–85. [Google Scholar]
- 27.Smit E, van Elsas J D, van Veen J A, de Vos W M. Detection of plasmid transfer from Pseudomonas fluorescensto indigenous bacteria in soil by using phage φR2f for donor counterselection. Appl Environ Microbiol. 1991;57:3482–3488. doi: 10.1128/aem.57.12.3482-3488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smit E, Venne D, van Elsas J D. Mobilization of a recombinant IncQ plasmid between bacteria on agar and in soil via co-transfer or retrotransfer. Appl Environ Microbiol. 1993;59:2257–2263. doi: 10.1128/aem.59.7.2257-2263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smit, E., A. Wolters, and J. D. van Elsas. Self-transmissible mercury resistance plasmids with gene-mobilizing capacity in soil bacterial populations: influence of wheat roots and mercury addition. Appl. Environ. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 30.Springael D. Ph.D. thesis. Mol, Belgium: VITO; 1992. [Google Scholar]
- 31.Stotzky G. Gene transfer among bacteria in soil. In: Levy S B, Miller R V, editors. Gene transfer in the environment. New York, N.Y: McGraw-Hill Book Co.; 1989. pp. 165–222. [Google Scholar]
- 32.Tam A C, Behki R M, Khan S K. Isolation and characterization of an S-ethyl-N,N-dipropyl-thiocarbonate degrading Arthrobacter strain and evidence for plasmid-associated S-ethyl-N,N-dipropyl-thiocarbonate degradation. Appl Environ Microbiol. 1987;53:1088–1093. doi: 10.1128/aem.53.5.1088-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarrand J J, Krieg N R, Döbereiner J. A taxonomic study of the Spirillum lipoferum group with the description of a new genus, Azospirillum gen. nov. and two spp., Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilensesp. nov. Can J Microbiol. 1978;24:967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- 34.Thomas C M. Promiscuous plasmids of gram-negative bacteria. London: Academic Press, Ltd.; 1989. [Google Scholar]
- 35.Tietze E, Tschäpe H, Voigt W. Characterization of new resistance plasmids belonging to incompatibility group IncQ. J Basic Microbiol. 1989;29:695–706. doi: 10.1002/jobm.3620291013. [DOI] [PubMed] [Google Scholar]
- 36.Top E, De Smet I, Verstraete W, Dijkmans R, Mergeay M. Exogenous isolation of mobilizing plasmids from polluted soils and sludges. Appl Environ Microbiol. 1994;60:831–839. doi: 10.1128/aem.60.3.831-839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tschäpe H. The spread of plasmids as a function of bacterial adaptability. FEMS Microbiol Ecol. 1994;15:23–32. [Google Scholar]
- 38.Van der Meer J R. Molecular mechanisms of adaptation of soil bacteria to chlorinated benzenes. Ph.D. thesis. Wageningen, The Netherlands: Agricultural University of Wageningen; 1992. [Google Scholar]
- 39.Van Elsas J D, Mäntynen V, Wolters A C. Soil DNA extraction and assessment of the fate of Mycobacterium chlorophenolicumstrain PCP-1 in different soils by 16S ribosomal RNA gene sequence based most-probable-number PCR and immunofluorescence. Biol Fertil Soils. 1997;24:188–195. [Google Scholar]
- 40.Van Elsas J D, Smalla K. Extraction of microbial community DNA from soil. In: Akkermans A D L, van Elsas J D, de Bruijn F J, editors. Molecular microbial ecology manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1.3.3.1–1.3.3.11. [Google Scholar]
- 41.Van Elsas J D, Wolters A C. Polymerase chain reaction (PCR) analysis of soil microbial DNA. In: Akkermans A D L, van Elsas J D, de Bruijn F J, editors. Molecular microbial ecology manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 2.7.2.1–2.7.2.10. [Google Scholar]
- 42.Verhagen C, Smit E, Janssen D B, van Elsas J D. Bacterial dichloropropene degradation in soil: screening of soils and involvement of plasmids carrying a dhlA-like gene. Soil Biol Biochem. 1995;27:1547–1557. [Google Scholar]
- 43.Versalovic J, Schneider M, de Bruijn F J, Lupski J R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol. 1994;5:25–40. [Google Scholar]
- 44.Wellington E M H, Van Elsas J D. Genetic interactions among microorganisms in the natural environment. Oxford, United Kingdom: Pergamon Press; 1992. [Google Scholar]
- 45.Wickham G S, Atlas R M. Plasmid frequency fluctuations in bacterial populations from chemically stressed soil communities. Appl Environ Microbiol. 1988;54:2192–2196. doi: 10.1128/aem.54.9.2192-2196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]