Abstract
Background
The triglyceride glucose-body mass (TyG-BMI) index is acknowledged as both a reliable indicator of the risk of cardiovascular disease and an accurate surrogate biomarker for evaluating insulin resistance (IR). The importance of the TyG-BMI index among people with heart failure (HF), however, requires more investigation. The objective of this study was to inquire about the relationship between HF patients’ TyG-BMI index and their risk of 360-day mortality.
Methods
The Medical Information Mart for Intensive Care (MIMIC-IV) database provided the study’s patient data, which were divided into quartiles according to their TyG-BMI index. The endpoint was mortality from all causes within 360 days. Kaplan-Meier analysis was used to compare this primary endpoint amongst the four groups indicated above. The association between the TyG-BMI index and the endpoint was investigated using restricted cubic splines and Cox proportional hazards analysis.
Results
The study enrolled a total of 423 patients with HF (59.2% male), of whom 70 patients (16.9%) died within 360 days. Patients with higher TyG-BMI indexes had significantly lower mortality risks, according to the Kaplan-Meier analysis (log-rank P = 0.003). Furthermore, the restricted cubic spline analysis illustrated a decrease in the risk of all-cause mortality with an increasing TyG-BMI index. Additionally, multivariable Cox proportional hazards analyses showed that the risk of 360-day death from all causes was considerably higher in the lowest quartile of TyG-BMI. In comparison to the lowest TyG-BMI group, the fully adjusted Cox model yielded a hazard ratio (HR) of 0.24 (95% CI: 0.10, 0.59; p = 0.002) for 360-day mortality.
Conclusions
In patients diagnosed with HF, a lower TyG-BMI index is strongly related to a higher risk of 360-day mortality. This index can be employed to categorize the risk levels of patients with HF and predict their one-year all-cause mortality .
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-02047-4.
Keywords: Heart Failure, Triglyceride glucose-body mass (TyG-BMI), Insulin resistance, Prognosis, MIMIC-IV
Introduction
Heart failure (HF) presents a considerable medical challenge, impacting approximately 64 million individuals globally. Despite stable incidence rates globally, its mortality and incidence rates remain elevated, which imposes a substantial societal burden [1]. An important factor in the occurrence and development of HF is insulin resistance (IR). Likewise, HF can cause IR, contributing to a deleterious cycle [2, 3]. The significant advantages seen in HF patients who used Sodium-glucose co-transporter 2 (SGLT2) inhibitors imply that Diabetocardiology may be a focal point in the treatment of HF patients, deserving heightened attention [4].
Currently, there are no validated methods available for accurately determining insulin resistance (IR). The hyperinsulinemic-euglycemic clamp method, which is regarded as the most accurate method for determining insulin resistance, has drawbacks including complexity, high expense, and invasiveness [5]. Therefore, the homeostasis model assessment-estimated insulin resistance (HOMA-IR) index is extensively utilized currently. However, its applicability is restricted to individuals undergoing insulin treatment or those with non-functional beta cells [6]. The triglyceride-glucose (TyG) index, which has superiority in assessing IR in both patients with and without diabetes and applicability to all subjects regardless of their status as recipients of insulin treatment, was created to get over this limitation [7, 8]. More importantly, recent research has shown that the TyG index’s effectiveness in assessing IR can be significantly increased by combining it with obesity indicators including the waist-to-height ratio (WtHR), body mass index (BMI), and waist circumference (WC) [9, 10]. Other investigations discovered that the TyG-BMI index outperformed other indicators in assessing IR in the relevant association [11, 12]. Furthermore, Studies have demonstrated that the TyG-BMI index has a substantial correlation with a number of illnesses, including nonalcoholic fatty liver disease, hyperuricemia, diabetes mellitus (DM), and coronary heart disease (CHD) [13–16]. Nevertheless, the current literature lacks studies investigating the correlation between the TyG-BMI index and HF patients’ prognosis.
The study aims to elucidate the connection between the TyG-BMI index and the one-year all-cause mortality of HF patients. The findings of this study may help create fresh approaches for improving patient prognosis in this population while providing insightful information about the predictive value of the TyG-BMI index for HF patients’ clinical results.
Methods
Study participants
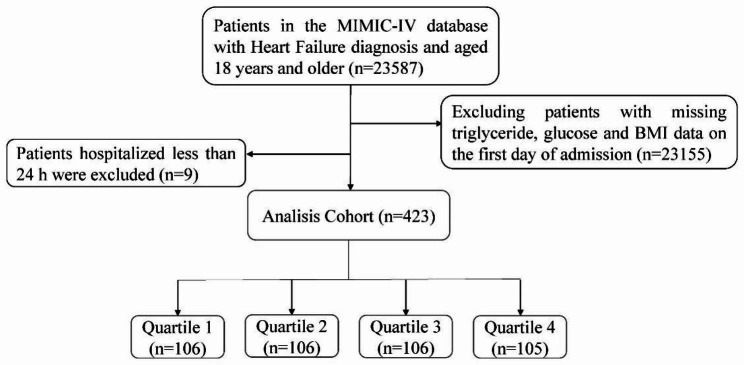
The Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (BIDMC) collaborated in developing the Medical Information Mart for Intensive Care (MIMIC-IV) electronic database (version 2.2), which was used in the present study. The database comprises pertinent information concerning patients who underwent inpatient treatment at BIDMC between 2008 and 2019. The Institutional Review Board (IRB) of BIDMC has waived informed consent and permitted the sharing of research resources since all data have undergone de-identification [17]. Prior to data extraction, the author Jiahao Dou fulfilled all the necessary requirements to access the database. The study included adult first-time hospitalized patients diagnosed with heart failure, based on the Ninth and Tenth Revisions of the International Classification of Diseases. The ICD codes for HF patients are listed in Additional file 2. Patients lacking data on admission day blood glucose, triglycerides, and BMI were excluded, as well as patients with a hospital stay shorter than 24 h. The flowchart for patient screening is presented in Fig. 1.
Fig. 1.
Flowchart of patient selection
Data extraction
The pgAdmin PostgreSQL tool (version 6.1) was used to extract information from the MIMIC-IV database, specifically from the following five categories: (1) Demographics, encompassing age, gender, race, marital status, and BMI; (2) Laboratory examination, including serum sodium, serum creatinine, aspartate aminotransferase (AST), red blood cell distribution width (RDW), white blood cell (WBC), red blood cell (RBC), hemoglobin, hematocrit, glucose, glycated hemoglobin A1c (HbA1c), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and N-terminal pro-B-type natriuretic peptide (NT-proBNP), C-reactive protein (CRP) ; (3) Comorbidities, such as CHD, atrial fibrillation (AF), DM, chronic obstructive pulmonary disease (COPD), and chronic kidney disease (CKD); (4) Medication treatments, consisting of diuretics, beta-blockers, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (ACEI/ARB); and (5) Duration of hospitalization, follow-up survival status, and follow-up survival time (with the database offering 1-year follow-up information for all discharged patients). The TyG-BMI index was computed according to the TG, BMI and fasting blood glucose (FBG) concentrations according to the equation: ln [TG (mg/dL) × FBG (mg/dL)/2] × BMI [18]. All laboratory variables were exclusively obtained from the initial 24 h after patient admission, and in cases of multiple results, the average value was utilized. Variables with missing values exceeding 20% were excluded to mitigate potential bias. Variables with less than 20% missing values were imputed using the random forest imputation method implemented in the missForest package of R software [19].
Outcomes
The main objective of this study was 360-day all-cause mortality. Secondary outcomes focused on 28-day all-cause mortality.
Statistical analysis
Statistics were conducted using t-tests or analysis of variance (ANOVA), and constant variables were reported as the mean ± standard deviation, or median (interquartile range, IQR). For categorical variables, numbers (proportions) were employed for presentation, and their analysis was carried out by means of chi-square tests, corrected chi-square tests, or Fisher’s exact test. Kaplan-Meier survival analysis was employed to evaluate the incidence rate of major outcome events across distinct stratified groups based on the TyG-BMI index. Log-rank tests were utilized to assess any observed disparities. Univariable Cox regression analysis was performed to examine the association between the TyG-BMI index and the mortality rate at 360 days. Variables with clinical significance and a significance level of P < 0.05 were incorporated into the multivariable Cox proportional hazards model. The model 1 solely included the TyG-BMI index without any further adjustments. In Model 2, adjustments were made for age, gender, and race. Model 3 incorporated further adjustments for laboratory tests and comorbidities such as LDL-C, HDL-C, COPD, CKD. The reference category for all models was the lowest quartile of the TyG-BMI index. We assessed the predictive ability, sensitivity, and specificity of the TyG-BMI index for predicting the primary outcome by employing a receiver-operating characteristic (ROC) curve analysis. We used a restricted cubic spline analysis to capture the dose-effect relationship connection with the TyG-BMI index and the risk of the primary result. Furthermore, we performed stratified analyses to evaluate the consistency of the TyG-BMI index’s prognostic values with the primary outcome. The analyses took into account gender, age (≤ 68 years and > 68 years), as well as the presence of comorbidities such as DM, CHD, and AF. The data analyses were conducted using R software (version 4.2.2) and SPSS statistical software (Version 26.0). For all analyses, a two-sided P-value below 0.05 was considered statistically significant.
Results
Baseline characteristics
Based on the quartiles of the TyG-BMI index, Table 1 (in Additional file 1) lists the baseline characteristics for HF patients. The participants had a mean age of 68 years, with 59.2% of them being male. Patients in the higher TyG-BMI index group were generally younger and had elevated levels of RBC, Platelet, Hemoglobin, HbA1c, TC, and HCT, as well as lower levels of HDL and NT-proBNP. Furthermore, they exhibited a higher prevalence of DM but a lower prevalence of AF compared to the lower group. Additionally, the higher TyG-BMI group had an increased use of diuretics (all p < 0.05). With increasing TyG-BMI index, 360 days mortality (25.5% vs. 19.8% vs. 12.3% vs. 8.5%, P = 0.004) decreased gradually. However, no significant differences were observed in 28-day mortality (p = 0.090).
Primary outcomes
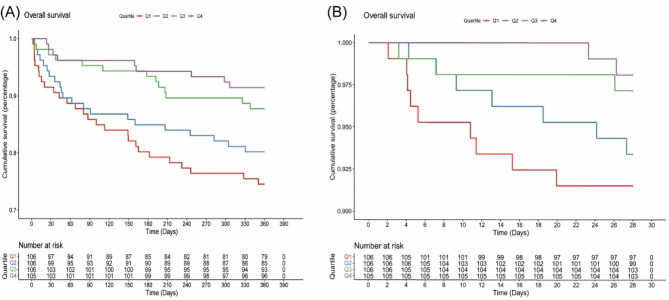
The Kaplan-Meier survival analysis curves in Fig. 2 show the prevalence of all-cause mortality in several groups that have been divided based on the TyG-BMI index quartiles.
Fig. 2.
Kaplan–Meier survival analysis curves for all-cause mortality
TyG-BMI index: Q1 (44.30–209.09), Q2 (209.09–254.27), Q3 (254.27–314.09), Q4 (314.09–575.70). Kaplan–Meier curves showing the cumulative probability of all-cause mortality according to groups at 28-day (A), and 360-day (B)
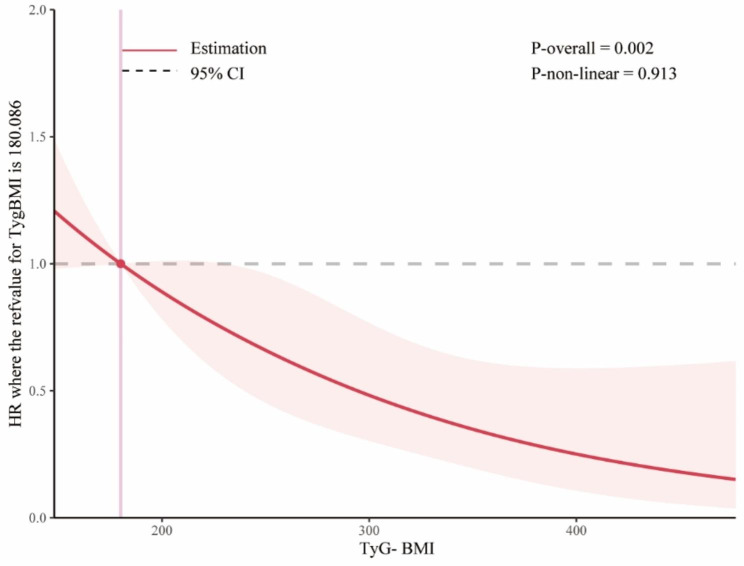
Patients with a higher TyG-BMI index demonstrated a significantly higher 360-day survival rate compared to those with a lower index (log-rank P = 0.003). In contrast, there was no significant difference observed in the 28-day timeframe (log-rank P = 0.085). The restricted cubic spline (RCS) model revealed an L-shaped connection between the index and the all-cause death rate over a 360-day period using the TyG-BMI index as a constant variable (Fig. 3).
Fig. 3.
Restricted cubic spline regression analysis of TyG-BMI index with in all-cause mortality
Abbreviation :TyG-BMI index: triglyceride glucose-body mass index
The inflection point of the RCS curve was determined to be at TyG-BMI = 289. Based on this inflection point, the data were divided into two groups. Segmented regression analysis was conducted on each group individually, presenting the outcomes as follows:
HR = 0.73 (95% CI 0.57–0.92, P = 0.009) for TyG-BMI < 289, and HR = 0.52 (95% CI 0.25–1.07, P = 0.075) for TyG-BMI ≥ 289. The ROC curve revealed a moderate ability of TyG-BMI to predict 360 days mortality (AUC = 0.647 (0.581, 0.715), the ideal cut-off point was 244.35, the specificity was 59.2%, and the sensitivity was 67.1% (Fig. 4).
Fig. 4.
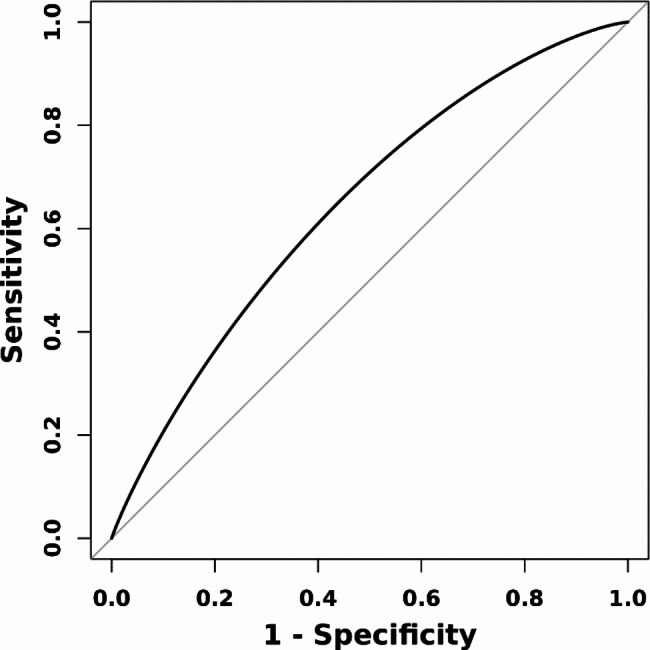
ROC curve assesses the predictive capability of the TyG-BMI index for all-cause mortality
Abbreviation: ROC, receiver-operating characteristic; TyG-BMI, triglyceride glucose-body mass index
When categorizing the TyG-BMI quartiles, patients in the higher TyG-BMI quartile were significantly associated with a higher 360-day survival rate in the three established Cox proportional hazards models that were constructed and exhibited an increasing trend with higher TyG-BMI indices(Table 2 in Additional file 1).
Subgroup analysis
The subgroup analysis demonstrated consistent associations among the TyG-BMI index quartiles and risks of primary results in various subgroups, such as sex, marital status, AF, CHD, COPD, hyperlipidemia, and the usage of statins, diuretics, antiplatelet medications, and ACEI/ARB (Table 3 in Additional file 1). The relationship between TyG and risk of 360-day mortality was consistent among male patients, married patients, those diagnosed with AF, hyperlipidemia, without CHD, COPD, and individuals using statins, diuretics, and antiplatelet medications, but not ACEI/ARB patients. It is worth noting that similar associations exist in both patients with and without DM or CKD. Additionally, no significant interactions between the factors in the subgroup examination and the TyG-BMI index were found (all p-values for interaction > 0.05).
Discussion
The TyG-BMI index and mortality from all causes in patients with HF were investigated for the first time in this study, which found a strong inverse association between the two variables. Despite adjusting for a wide variety of confounding factors, higher TyG-BMI index levels are significantly correlated with lower mortality rates, and this conclusion remains consistent. This correlation may be attributed to the “obesity paradox”.
IR refers to the reduced or impaired responsiveness of insulin-dependent organs and tissues to both endogenous and exogenous insulin, which has a close relationship between the occurrence and development of HF [3]. On one hand, IR can cause HF. In a 9-year prospective study conducted in Sweden, involving nearly 12,000 participants without HF, it was discovered that insulin IR precedes HF and acts as an independent risk factor, regardless of other risk factors such as diabetes [20]. Held et al. demonstrated that for patients with diabetes, each rise of one millimole per liter in fasting blood glucose is related to a 1.10. times greater risk of hospitalization due to congestive heart failure [21]. The specific mechanisms include: reduced capacity for myocardial energy metabolism and disorder in substrate metabolism, abnormal calcium signaling pathway, and impaired NO signaling pathway between myocardial cells and endothelial cells [22–26]. On the other hand, HF can also cause IR. According to the findings of the Leibniz Institute for Prevention Research and Epidemiology (BIPS), individuals with HF have a 13% increased likelihood of having diabetes compared to the general population [27]. Research has shown that patients with HF not only have systemic IR but also often manifest myocardial IR [28, 29]. The mechanisms include overactive stimulation of the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system (SNS), as well as imbalanced secretion of cytokines [30, 31].
Undoubtedly, IR contributes significantly as a risk variable to the onset and progression of HF. The value and accuracy of the TyG-BMI index in assessing IR also have been validated in numerous studies. The relationship between TyG index and heart failure has been confirmed by multiple studies. Multiple studies have demonstrated a significant association between a high TyG index and an increased risk of heart failure, mortality, and readmission in patients with heart failure. Moreover, relevant studies have consistently reported the excellent diagnostic and predictive capabilities of the TyG index [32–34]. Therefore, the “obesity paradox” may be the explanation for the negative correlation between the TyG-BMI index and all-cause mortality in HF patients.
Although a greater BMI is linked to a higher risk of developing heart failure, the connection between BMI and outlook in those who have been diagnosed with HF is unexpectedly complex [35]. Scientific literature on HF has extensively shown the association between this prognosis and conventional risk factors [36]. Higher BMI categories were strongly linked with a decreased risk of death, according to a massive random control trial comprising 7599 patients with HF [37]. Furthermore, a substantial finding was also found in research examining the relationship between BMI and death in the hospital in a sample of 108,927 patients with decompensated heart failure.For each 5-unit increment in BMI, the results showed a 10% reduction in the mortality (P < 0.001) [38]. With a total of 22,807 participants, a meta-analysis was performed aimed at looking at the connection between BMI and outcomes for those with HF. Findings demonstrated that individuals with a low BMI were at the highest risk for HF hospitalizations and all-cause mortality, while those classified as overweight had the lowest risk [39].
The “obesity paradox” in HF can be attributed to the following predominant mechanisms. Firstly, patients with HF are generally in a catabolic state, and obesity or overweight may suggest a greater presence of physiological reserves and can potentially lead to a more favorable prognosis [36]. Secondly, B-natriuretic peptide levels are frequently decreased in obese people, demonstrating that this specific subset of people has better hemodynamic properties [40]. These patients are capable of maintaining elevated blood pressures, potentially preserving renal function and allowing individuals to endure more beneficial medications for prognosis such as neprilysin inhibitors, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors (ACEI), and beta-blockers [41]. Thirdly, those patients may derive advantages from the preventative properties that anti-inflammatory adipokines exert. In HF, elevated amounts of tumor necrosis factor-alpha (TNF-α) may induce heart damage due to its pro-apoptotic and detrimental impact on the myocardium. Nevertheless, adipose tissue generates soluble TNF-α receptors that demonstrate cardioprotective properties by counteracting the biological actions of TNF-α [42, 43]. Additionally, obesity is linked to reduced levels of adiponectin, an adipokine that enhances energy expenditure and promotes weight loss. However, these effects are detrimental for HF patients in a catabolic state [44]. Finally, the presence of adipose tissue is frequently linked to elevated muscular strength and enhanced cardiopulmonary function in HF patients, which is advantageous for them [45]. The research findings have significant implications for clinical practice and patient self-management. Firstly, TyG-BMI index can serve as an effective predictive indicator in the management of HF patients. Patients who have lower TyG-BMI index experience an increased probability of death. Secondly, paying close attention to the BMI and nutritional status of HF patients is highly significant, and providing early nutritional support is vital for those with low body weight or poor nutritional status. Furthermore, gastrointestinal edema in HF patients may result in reduced appetite and weight loss, which might manifest before obvious symptoms like limb edema and breathing difficulties. The survival rate of patients with HF may be increased by the prompt identification and management of these symptoms.
But there were certain restrictions on our study as well. Firstly, this retrospective investigation depends on observational research, which limits the ability to establish a definitive causal relationship. Although we utilized multivariable adjustments and subgroup analysis, potential confounders may still affect the clinical prognosis. For instance, cardiac ultrasound data was not publicly accessible in the most recent version of the database, while variables like NT-proBNP and APACHE scores were not considered due to a significant amount of missing data. Furthermore, given the database limitations, it is not possible to ascertain whether all glucose and lipid measurements were obtained from fasting individuals. Thirdly, this study exclusively focused on evaluating the baseline TyG-BMI index. Further investigation is required to determine the prognostic significance of dynamic changes in the TyG-BMI index during hospitalization and follow-up.Fourthly, we were unable to compare the TyG-BMI index with other widely recognized IR measuring methods due to the absence of relevant information in the database. Finally, this study had a sample size of moderate magnitude, a relatively short follow-up duration, and was solely conducted at a single center, potentially introducing selection bias. Future research should diligently address the aforementioned issues to the fullest extent possible, with the aim of providing robust evidence to bolster our findings.
Conclusion
In spite of additional risk variables, we found that the TyG-BMI index indicated a better one-year all-cause mortality outcome for those with HF. The tyG-BMI index may serve as an effective tool for classifying and treating the risks associated with HF patients. Moreover, for patients diagnosed with heart failure, BMI should receive attention as it significantly affects prognosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We greatly acknowledge the diligent efforts of the personnel responsible for the design and maintenance of the MIMIV database.
Abbreviations
- ACEI/ARB
Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker
- AF
Atrial fibrillation
- BIDMC
Beth Israel Deaconess Medical Center
- BIPS
Leibniz Institute for Prevention Research and Epidemiology
- BMI
Body mass index
- CHD
Coronary heart disease
- CKD
Chronic kidney disease
- COPD
Chronic obstructive pulmonary disease
- CRP
C-reactive protein
- DM
Diabetes mellitus
- HbA1c
Glycated hemoglobin A1c
- HF
Heart failure
- HOMA-IR
Homeostasis model assessment-estimated insulin resistance
- IR
Insulin resistance
- IRB
Institutional Review Board
- LDL-C
Low-density lipoprotein cholesterol
- MIMIC-IV
Medical Information Mart for Intensive Care
- MIT
Massachusetts Institute of Technology
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- RAAS
Renin-angiotensin-aldosterone system
- RBC
Red blood cell
- RCS
Restricted cubic spline
- ROC
Receiver-operating characteristic
- SGLT2i
Sodium-glucose co-transporter 2 inhibitors
- SNS
Sympathetic nervous system
- TC
Total cholesterol
- TG
Triglyceride
- TNF-α
Tumor necrosis factor-alpha
- TyG-BMI index
Triglyceride glucose-body mass index
- TyG
Triglyceride-glucose
- WBC
White blood cell
- WC
Waist circumference
- WtHR
Waist-to-height ratio
Authors’ contributions
JHD, CG, RYW , QQL, HZ, SFS, XLS, and JW were responsible for the study concept, and JHD, YWW, ZHP, CG, RYW, and JW for the study design. Data extraction was undertaken by JHD. YWW, ZHP, QQL, HZ, and RYW were responsible for data analysis. Drafting of the manuscript: JHD, SFS, XLS, and CG. Critical revision of the manuscript for important intellectual content: JW. All authors read and approved the final manuscript.
Funding
This work was supported by grants from Shaanxi Provincial Science and Technology Department (NO.2020ZDLSF02-09).
Data Availability
The corresponding author can be contacted to receive the datasets generated and utilized in this work upon reasonable request and with MIMIC’s permission.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shahim B, Kapelios CJ, Savarese G, Lund LH. Global Public Health Burden of Heart Failure: an updated review. Card Fail Rev. 2023;9:e11. doi: 10.15420/cfr.2023.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannel WB, Hjortland M, Castelli WP. Role of Diabetes in Congestive Heart Failure: the Framingham study. AM J CARDIOL. 1974;34(1):29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 3.Erqou S, Adler AI, Challa AA, Fonarow GC, Echouffo-Tcheugui JB. Insulin resistance and incident Heart Failure: a meta-analysis. EUR J HEART FAIL. 2022;24(6):1139–41. doi: 10.1002/ejhf.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunwald E. Diabetocardiology: a new subspecialty? Eur Heart J 2023. [DOI] [PubMed]
- 5.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in Cardiovascular Diseases: landscape and limitations. CARDIOVASC DIABETOL. 2022;21(1):68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minh HV, Tien HA, Sinh CT, Thang DC, Chen CH, Tay JC, Siddique S, Wang TD, Sogunuru GP, Chia YC, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with Hypertension. J CLIN HYPERTENS. 2021;23(3):529–37. doi: 10.1111/jch.14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. METAB SYNDR RELAT D. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 8.Placzkowska S, Pawlik-Sobecka L, Kokot I, Piwowar A. Indirect insulin resistance detection: current clinical trends and laboratory limitations. BIOMED PAP. 2019;163(3):187–99. doi: 10.5507/bp.2019.021. [DOI] [PubMed] [Google Scholar]
- 9.Bala C, Gheorghe-Fronea O, Pop D, Pop C, Caloian B, Comsa H, Bozan C, Matei C, Dorobantu M. The Association between six surrogate insulin resistance indexes and Hypertension: a Population-based study. METAB SYNDR RELAT D. 2019;17(6):328–33. doi: 10.1089/met.2018.0122. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez-Velez R, Perez-Sousa MA, Gonzalez-Ruiz K, Cano-Gutierrez CA, Schmidt-RioValle J, Correa-Rodriguez M, Izquierdo M, Romero-Garcia JA, Campos-Rodriguez AY, Triana-Reina HR et al. Obesity- and Lipid-Related Parameters in the Identification of Older Adults with a High Risk of Prediabetes According to the American Diabetes Association: An Analysis of the 2015 Health, Well-Being, and Aging Study. Nutrients 2019, 11(11). [DOI] [PMC free article] [PubMed]
- 11.Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride glucose-body Mass Index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11(3):e149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PLoS ONE. 2019;14(3):e212963. doi: 10.1371/journal.pone.0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Wang R, Fu X, Song H. Non-insulin-based insulin resistance indexes in predicting severity for coronary artery Disease. DIABETOL METAB SYNDR. 2022;14(1):191. doi: 10.1186/s13098-022-00967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng W, Kong F, Chen S. Comparison of the predictive value of four insulin resistance surrogates for the prevalence of Hypertension: a population-based study. DIABETOL METAB SYNDR. 2022;14(1):137. doi: 10.1186/s13098-022-00907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Shi Z, Chen X, Wang J, Ding J, Geng S, Sheng X, Shi S. Relationship between six insulin resistance surrogates and nonalcoholic fatty Liver Disease among older adults: a cross-sectional study. DIABET METAB SYND OB. 2023;16:1685–96. doi: 10.2147/DMSO.S409983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Y, Hu H, Li Q, Deng Z, Liu D. Triglyceride glucose-body mass index and the risk of progression to Diabetes from prediabetes: a 5-year cohort study in Chinese adults. FRONT PUBLIC HEALTH. 2023;11:1028461. doi: 10.3389/fpubh.2023.1028461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson A, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, Pollard TJ, Hao S, Moody B, Gow B, et al. MIMIC-IV, a freely accessible electronic health record dataset. SCI DATA. 2023;10(1):1. doi: 10.1038/s41597-022-01899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y, Fang Z, Zhang X, Wen Y, Lu J, He S, Xu B. Association between triglyceride glucose-body mass index and cardiovascular outcomes in patients undergoing percutaneous coronary intervention: a retrospective study. CARDIOVASC DIABETOL. 2023;22(1):75. doi: 10.1186/s12933-023-01794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stekhoven DJ, Buhlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–8. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 20.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of Congestive Heart Failure. JAMA-J AM MED ASSOC. 2005;294(3):334–41. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 21.Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, Sleight P, Teo K. Glucose levels predict hospitalization for Congestive Heart Failure in patients at high cardiovascular risk. Circulation. 2007;115(11):1371–5. doi: 10.1161/CIRCULATIONAHA.106.661405. [DOI] [PubMed] [Google Scholar]
- 22.How OJ, Aasum E, Severson DL, Chan WY, Essop MF, Larsen TS. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes. 2006;55(2):466–73. doi: 10.2337/diabetes.55.02.06.db05-1164. [DOI] [PubMed] [Google Scholar]
- 23.Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5(11):715–24. doi: 10.1038/ncpcardio1347. [DOI] [PubMed] [Google Scholar]
- 24.Nagoshi T, Yoshimura M, Rosano GM, Lopaschuk GD, Mochizuki S. Optimization of cardiac metabolism in Heart Failure. CURR PHARM DESIGN. 2011;17(35):3846–53. doi: 10.2174/138161211798357773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuunanen H, Knuuti J. Metabolic remodelling in human Heart Failure. CARDIOVASC RES. 2011;90(2):251–7. doi: 10.1093/cvr/cvr052. [DOI] [PubMed] [Google Scholar]
- 26.Falcao-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. HEART FAIL REV. 2012;17(3):325–44. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 27.Bell DS. Functional class in patients with Heart Failure is associated with the development of Diabetes. AM J MED. 2003;115(5):412. doi: 10.1016/S0002-9343(03)00392-9. [DOI] [PubMed] [Google Scholar]
- 28.Mamas MA, Deaton C, Rutter MK, Yuille M, Williams SG, Ray SG, New J, Gibson JM, Neyses L. Impaired glucose tolerance and insulin resistance in Heart Failure: underrecognized and undertreated? J CARD FAIL. 2010;16(9):761–8. doi: 10.1016/j.cardfail.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 29.Heck PM, Dutka DP. Insulin resistance and Heart Failure. Curr Heart Fail Rep. 2009;6(2):89–94. doi: 10.1007/s11897-009-0014-8. [DOI] [PubMed] [Google Scholar]
- 30.Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and Hypertension of the metabolic syndrome. AM J PHYSIOL-HEART C. 2012;302(6):H1219–30. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lastra G, Dhuper S, Johnson MS, Sowers JR. Salt, aldosterone, and insulin resistance: impact on the cardiovascular system. NAT REV CARDIOL. 2010;7(10):577–84. doi: 10.1038/nrcardio.2010.123. [DOI] [PubMed] [Google Scholar]
- 32.Zheng H, Chen G, Wu K, Wu W, Huang Z, Wang X, Chen Z, Cai Z, Cai Z, Lan Y, et al. Relationship between cumulative exposure to triglyceride-glucose index and Heart Failure: a prospective cohort study. CARDIOVASC DIABETOL. 2023;22(1):239. doi: 10.1186/s12933-023-01967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalaji A, Behnoush AH, Khanmohammadi S, Ghanbari MK, Sharifkashani S, Sahebkar A, Vinciguerra C, Cannavo A. Triglyceride-glucose index and Heart Failure: a systematic review and meta-analysis. CARDIOVASC DIABETOL. 2023;22(1):244. doi: 10.1186/s12933-023-01973-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Q, Yang J, Tang H, Guo Z, Dong W, Wang Y, Meng X, Zhang K, Wang W, Shao C, et al. High triglyceride-glucose (TyG) index is associated with poor prognosis of Heart Failure with preserved ejection fraction. CARDIOVASC DIABETOL. 2023;22(1):263. doi: 10.1186/s12933-023-02001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavie CJ, Kokkinos P, Lin GM. Obesity paradox is still alive in heart failure. Heart 2023. [DOI] [PubMed]
- 36.Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity Paradox in Heart Failure. PROG CARDIOVASC DIS. 2018;61(2):151–6. doi: 10.1016/j.pcad.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Lavie CJ, Sharma A, Alpert MA, De Schutter A, Lopez-Jimenez F, Milani RV, Ventura HO. Update on obesity and obesity Paradox in Heart Failure. PROG CARDIOVASC DIS. 2016;58(4):393–400. doi: 10.1016/j.pcad.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute Heart Failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. AM HEART J. 2007;153(1):74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, Arbab-Zadeh A, Mukherjee D, Lazar JM. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic Heart Failure. AM J CARDIOL. 2015;115(10):1428–34. doi: 10.1016/j.amjcard.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED. Obesity and suppressed B-type natriuretic peptide levels in Heart Failure. J AM COLL CARDIOL. 2004;43(9):1590–5. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 41.Lavie CJ, Milani RV, Ventura HO. Obesity and Cardiovascular Disease: risk factor, paradox, and impact of weight loss. J AM COLL CARDIOL. 2009;53(21):1925–32. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 42.Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li YY, McTiernan C. The role of Tumor necrosis factor in the pathophysiology of Heart Failure. J AM COLL CARDIOL. 2000;35(3):537–44. doi: 10.1016/S0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 43.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble Tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277(6):E971–5. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 44.Packer M. Leptin-Aldosterone-Neprilysin Axis: identification of its distinctive role in the pathogenesis of the three phenotypes of Heart Failure in people with obesity. Circulation. 2018;137(15):1614–31. doi: 10.1161/CIRCULATIONAHA.117.032474. [DOI] [PubMed] [Google Scholar]
- 45.Artero EG, Lee DC, Lavie CJ, Espana-Romero V, Sui X, Church TS, Blair SN. Effects of muscular strength on cardiovascular risk factors and prognosis. J CARDIOPULM REHABIL. 2012;32(6):351–8. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The corresponding author can be contacted to receive the datasets generated and utilized in this work upon reasonable request and with MIMIC’s permission.