Abstract
Physarum polycephalum (P. polycephalum) is a unicellular protist with unique properties, such as learning and remembering in its cultured environment without a brain or central nervous system. The organism has been extensively used in morphology, taxis, and positive feedback dynamics studies. However, the lack of standardization of materials and substrate designs used in P. polycephalum studies has significantly limited conducting such studies, increasing the cost and time. In this study, we introduce a method to control the direction and migration of P. polycephalum by drawing hydrophobic lines and patterns. Our study succeeded in controlling the movement of P. polycephalum by setting a variety of hydrophobic designs such as complete barrier, single-slit barrier, taper barrier, dumbbell barrier, and one-side-opened rectangular barrier, suggesting the effectiveness of the hydrophobic barrier in regulating the propulsion and navigation of the organisms. Moreover, we demonstrated that utilizing such geometric constraints can reduce the experimental time required for toxicity testing based on P. polycephalum by more than 300%. Our techniques open new possibilities for studying the biophysical properties and behaviors of P. polycephalum, while also facilitating toxicity testing.
Introduction
Slime molds, also known as myxomycetes, are a group of protist organisms that have long fascinated scientists and nonexperts. Found in various environments, including forests, grasslands, and urban areas, these mysterious creatures are known for their ability to adapt to their surroundings as they migrate in search of food. One species of slime mold, P. polycephalum, is a unicellular organism that belongs to the phylum Myxomycota, commonly found in damp environments and has a complex life cycle that includes both a vegetative and a reproductive phase.1−4 In particular, P. polycephalum has been shown to exhibit guided behavior, which is the ability to move toward or away from a specific stimulus.5 This behavior is believed to be influenced by the organism’s response to various environmental signals, including its sensitivity to biotic stressors, such as nutritional imbalances, as well as abiotic stressors, like exposure to chemicals or light.6−8
Recently, there has been a growing interest in using P. polycephalum as a model organism for studying various biological and computational problems.9−11 Due to its ability to perform complex tasks, P. polycephalum has been used as a model organism for multiple research areas, including computer science, engineering, and biology.12−14 For example, researchers have used the behavior of P. polycephalum to optimize the layout of transportation networks such as road networks and subway systems.15−17 In specific, P. polycephalum can find the shortest path between food sources, and this behavior can be mimicked to find the most efficient route between different transportation nodes.15,18 Similarly, the ability of P. polycephalum to solve mazes could be replicated in a robot or biosolver by using algorithms that allow it to navigate through complex environments.19,20 Another potential application of P. polycephalum guidance is in medicine. P. polycephalum can sense and respond to changes in the chemical composition of its environment, which could be helpful in the development of new diagnostic tools.21 Additionally, P. polycephalum could be used as a biosensor to detect changes in environmental conditions such as water quality or air pollution by observing its behavior in response to humidity or other environmental factors.22
To understand the intelligence and decision-making capabilities of P.polycephalum, various experimental studies have been conducted to control the movement of P.polycephalum.6,23,24 As one of the methodological examples of P.polycephalum guidance, organism was placed in a dish and exposed to a light source at a specific area.25 The organism was able to navigate toward the light source, demonstrating its ability to sense and respond to light. Similarly, P.polycephalum has been shown to navigate toward or away from areas with high or low humidity, suggesting that it is also sensitive to changes in moisture levels.26 Furthermore, the electric field can guide the movement of P.polycephalum by applying electric fields to its surface and observing the organism’s behavior. This method can be used to study the influence of electric fields on the organism’s behavior, such as electrotaxis or galvanotaxis.24,27 Chemical gradients can also be used to control the direction of P. polycephalum. This is done by placing a chemical attractant or repellent on one side of the surface and observing the movement of the organism as it encounters the chemical gradient.7,28 Overall, these guidance methods for P. polycephalum provide researchers with a powerful tool for studying the underlying mechanisms of guidance and understanding the network algorithms. However, these methods still need improvement, such as not providing precise control over the organism’s movement and navigation and not being easy to use with other methods.
In this study, we introduce a method to create a simple geometric barrier with a hydrophobic pen drawing to control the movement and direction of the slime mold (P. polycephalum). These hydrophobic constraints provide a physical and chemical barrier that the organisms cannot traverse, allowing for precise control while being simple and easy to use. By drawing on each culture medium, five types of hydrophobic barriers were designed: complete barrier, single-slit barrier, taper barrier, dumbbell barrier, and one-side-opened rectangular barrier. To confirm the growth direction and migration length of P. polycephalum according to the type of hydrophobic barrier designs, we prepared a culture medium containing hydrophobic barriers and oatmeal, and cultured P. polycephalum on it. Our results showed that P. polycephalum exhibited various growth patterns depending on the types of hydrophobic barriers and that it grew best and regulated its growth direction in a one-side-opened rectangular hydrophobic barrier. Additionally, we confirmed the responsiveness of the slime mold to poisonous substances (i.e., acidic oatmeal) within hydrophobic constraints. Our study demonstrated that the movement and direction of P.polycephalum could be controlled by setting a mere hydrophobic barrier accompanied by the food-induced organism’s chemotaxis properties. Furthermore, it is validated that our method makes convenient P. polycephalum-based toxicity testing feasible.
Experimental Section
Chemical Materials
P. polycephalum (Plasmodium, Living, Plate) was purchased from Carolina Biological Supply (Burlington, NC, USA). Advanced PAP pen (liquid blocker, 3-bromopropane 65%, ligroin 10%), agar, and distilled water were purchased from Sigma-Aldrich (Burlington, MA, USA). The oatmeal flake was purchased from Quaker Oats Company (Chicago, IL, USA).
Preparation and Culturing of P. polycephalum
To culture P. polycephalum, which is sensitive to light and moisture, a humidifier was installed in an opaque plastic box covered with a blackout cloth. The temperature was maintained at 18–20 °C and humidity at 90–95% throughout the experiment and culture.29,30 Distilled water was used in the humidifier, and the entire plastic box was washed with isopropyl alcohol once a week. Also, all tools used were sterilized with alcohol and flames before use. These controlled environments prevented slime mold contamination. To prevent overgrowth, P. polycephalum was cultured on one Petri dish for up to a week and then subcultured into a new Petri dish (Figure S1).
The subculture method varied depending on the state of P. polycephalum. In the “plasmodial state”, the oatmeal with the grown plasmodium was collected with a spatula or agar gel with the plasmodium was sliced into 1 × 1 cm2 pieces and placed on freshly prepared non-nutrient agar gel (2% w/v).31 Approximately 15 oatmeal flakes were placed on the non-nutrient agar to supply sufficient nutrients (Figure S2). For P. polycephalum in the “sclerotial state”, a drop of distilled water was added to the filter paper where the individual was grown, and it was then cultured in non-nutrient agar to form plasmodium.
Experimental Procedure
An oatmeal flake was placed directly underneath P. polycephalum to provide minimal nutrients, and another oatmeal flake was placed 4 cm away to induce chemotaxis (Figure 1a). A hydrophobic pattern was drawn on the agar medium using a hydrophobic pen, as shown in Figure 1b, confirming the directionality and propulsion ability of P. polycephalum according to the hydrophobic pattern. In addition, printed design paper was placed on the bottom of a Petri dish in which P. polycephalum was cultured, and a hydrophobic pattern was drawn to maintain a pattern of uniform width and shape. The hydrophobic pattern was set to 5 types (complete barrier, single-slit barrier, taper barrier, dumbbell barrier, and one-side-opened rectangular barrier) to investigate the directionality and propulsion ability of the P. polycephalum according to the hydrophobic constraints, and all slime molds were cultured in the same environment.
Figure 1.
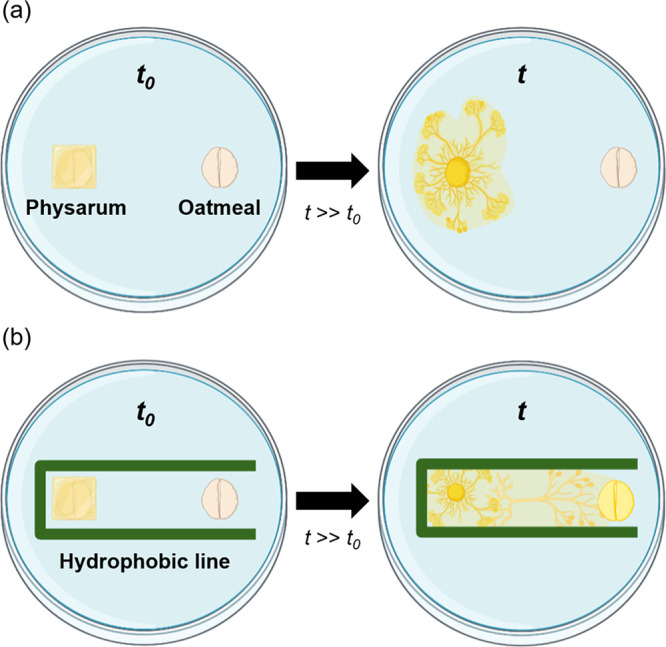
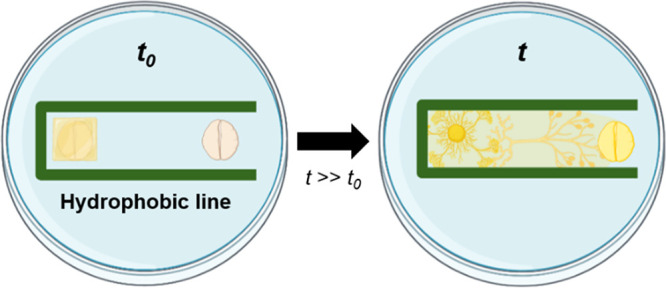
Our hypothesis of directing the movement of P. polycephalum on agarose gel in different environments. (a) Schematic illustration of a culture medium and oatmeal without hydrophobic lines. Despite the chemotactic conditions, P. polycephalum migrates radially rather than toward the oatmeal. (b) Schematic illustration of an agarose gel and oatmeal with a one-side-opened rectangular hydrophobic barrier. The P. polycephalum migrates toward the oatmeal under the chemotactic conditions with the aid of geometric constraints.
To investigate the effect of hydrophobic barrier wall-to-wall distance (dww) on P. polycephalum, it was cultured and observed their behaviors within a one-side-opened rectangular barrier with two different dww (1.5 and 3 cm). In addition, to evaluate and observe the response of P. polycephalum to poisonous substances, oatmeal flakes treated with a strongly acidic solution (pH 2) were used as a food source, inducing chemotaxis.
Imaging of P. polycephalum and Data Analysis Method
To ensure sufficient growth of P. polycephalum, the culture was maintained for up to 60 h, with a minimum of three replicates for each experimental condition. To observe the direction and extent of growth, photographs were taken every 3 h using an iPhone 12 Pro camera under a consistent angle and lighting conditions. The growth distance of P. polycephalum was measured from the side of the oatmeal flake on which it was cultured by using ImageJ software. The growth distance was recorded in centimeters, and the growth rate was calculated as a percentage of the area where P. polycephalum grew based on the 1 × 1 cm2 agar slice (Figure S3).
Results and Discussion
Growth Characteristics and Food Detection Ability of P. polycephalum
P. polycephalum exhibits a characteristic of proliferating in random directions in the absence of specific stimuli, provided that appropriate temperature and sufficient humidity are maintained (Figure 2). When it detects a food source, it changes its growth pattern from random to toward the food direction, which is guided by chemotaxis (Figure S4). Specifically, P. polycephalum finds the shortest paths and detects the presence of food at great distances through 'exploration and exploitation', 'positive chemotaxis', and 'reinforcement learning'.32−34 At this point, the growth rate of P. polycephalum slows down and then accelerates, as the slime mold discovers the shortest path to its food source and modifies the randomly distributed network to grow along this shortest path.35
Figure 2.
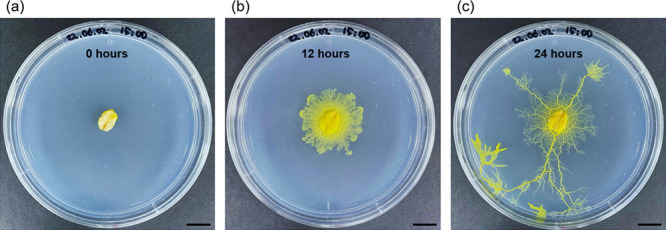
P. polycephalum photographs at (a) seed point (0 h), (b) early growth (12 h), and (c) full growth (24 h). Scale bar, 1 cm.
Manipulation of P. polycephalum Growth Using Hydrophobic Barriers on the Substrate
P. polycephalum can memorize its surroundings, detect stimuli through learning, and change its growth pattern accordingly despite lacking a nervous system. In this study, we created a hydrophobic barrier on an agar medium to control the growth direction of P. polycephalum. We selected five different hydrophobic barriers and observed the growth of P. polycephalum in each design (Figure 3a).
Figure 3.
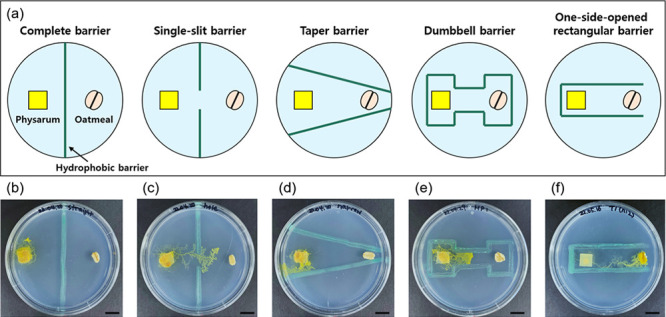
Migration of P. polycephalum using various patterns of hydrophobic barriers. (a) Schematic illustration of 5 different shapes (complete barrier, single-slit barrier, taper barrier, dumbbell barrier, and one-side-opened rectangular barrier). Photographs of P. polycephalum with (b) complete barrier, (c) single-slit barrier, (d) taper barrier, (e) dumbbell barrier, and (f) one-side-opened rectangular barrier after 48 h of incubation. The scale bar represents 1 cm.
Complete Barrier and Single-Slit Barrier
We first designed two types of directional patterns. The first was a complete barrier line that passed through the center of the Petri dish, which blocked the path of P. polycephalum to oatmeal flakes (Figure 3b). The second was a single-slit barrier pattern with a 1 cm gap in the center of the straight line (Figure 3c). In both patterns, P. polycephalum was slower than when only the food source was present without any hydrophobic patterns. In the case of the complete barrier pattern, it showed almost no growth, suggesting completely blocking the chemotactic response induced by the food source from reaching P. polycephalum. In contrast, in the single-slit barrier pattern, P. polycephalum grew toward the food source, indicating a chemotactic response through the open gap. Interestingly, while approaching the food source, P. polycephalum came close to the hydrophobic barrier but did not pass through it and instead (turned back) took a detour. This implied that the hydrophobic barriers serve as both physical and chemical constraints.
Taper-Shaped Barrier
Next, we examined a taper-shaped hydrophobic barrier that gradually narrowed toward oatmeal and observed the growth of P. polycephalum. In detail, we drew two hydrophobic lines on the medium, with a width of 4 cm between the widest portions of the lines and a minimum width of 0.25 cm between the narrowest parts. The lines were arranged in a tapered form where their distance gradually decreased from the left to right. This pattern was designed to guide P. polycephalum toward the oatmeal, but interestingly, it grew in the opposite direction (i.e., the opposite direction from the food source) toward the wider width (Figure 3d). This may be due to the hydrophobic barriers’ chemotactic (repellent) response, which makes it difficult for P. polycephalum to detect the food sources. Specifically, the observed phenomenon can be explained by the fact that P. polycephalum has no preference for the eastern direction, where the hydrophobic patterns’ chemotactic (repellent) response is the highest among the four directions (east, west, south, and north). These results give us insight that an equidistant hydrophobic barrier may be necessary to guide P. polycephalum to the food source efficiently (Figure 3d).
Dumbbell-Shaped Barrier
To investigate the growth of P. polycephalum when it was surrounded by hydrophobic barriers, we designed a dumbbell-shaped pattern that enclosed both P. polycephalum and oatmeal flakes with hydrophobic constraints. Specifically, the area containing P. polycephalum and oatmeal flakes was 2.5 × 1.5 cm2 and the channel width (i.e., dww) between the two chambers (P. polycephalum and oatmeal) was set at 1 cm. As a result, we observed that P. polycephalum grew toward the oatmeal flake, unlike previous patterns, and showed a faster growth rate. In time, however, the growth was hampered by the narrower-than-chamber channel that resembled the results from the tapered pattern (Figure 3e). These results allow us to gain insight into two important conditions to promote the rapid growth and migration of P. polycephalum. First, the dww condition of the hydrophobic barriers should be wider than 1 cm. Second, a pattern of ‘one-side open’ is required not to disrupt the chemotactic attraction of the food source.
One-Side-Opened Rectangular Barrier
Finally, we designed a one-side-opened rectangular pattern where the growth inhibitory effect of P. polycephalum can be minimized by blocking three sides with hydrophobic barriers, except for one direction toward the oat flakes. The channel dww was 1.5 cm, and P. polycephalum was placed at the center of the hydrophobic lines. The P. polycephalum showed clear growth toward the oatmeal flake in this geometric constraint. Specifically, this hydrophobic pattern effectively controlled the growth direction of P. polycephalum toward the food source and exhibited higher growth rates compared to the other patterns (Figure 3f). Furthermore, P. polycephalum continued to grow even after it reached the food source. In summary, we verified that the one-side-opened rectangular hydrophobic barrier was among the best in controlling the growth direction and increasing the growth rate of P. polycephalum.
Effect of dww of the Hydrophobic Barrier on P. polycephalum’s Propulsion
We conducted a detailed investigation to understand how hydrophobic barriers and channel width (i.e., dww) influence the growth of P. polycephalum. Specifically, while maintaining a uniform distance (4 cm) between P. polycephalum and the oatmeal, we experimented under conditions both without a hydrophobic barrier and within a one-side-opened rectangular barrier, adjusting the dww value to 1.5 and 3.0 cm. We quantified the P. polycephalum’s progression toward the food source as the length of its eastward movement (MLEast) and determined the growth rate. In the absence of a hydrophobic barrier, P. polycephalum did not reach the oatmeal flake within 48 h because of the extensive distance between P. polycephalum and oatmeal, instead expanding radially (Figures 4a, S5). It is well-known that when P. polycephalum is not sufficiently close to the oatmeal, its ability to seek the 'shortest path' diminishes. However, under a narrow dww (1.5 cm) condition, P. polycephalum reached the oatmeal flake within 48 h (Figure 4b). The average MLEast was measured as 0.25 cm at 12 h, 1.07 cm at 24 h, 3.51 cm at 36 h, and 3.84 cm at 48 h (Figure 4d). Remarkably, MLEast exhibited a sigmoid curve over time, suggesting that P. polycephalum accelerates toward oatmeal and decelerates as it nears its target. Moreover, the average growth rate was 127% at 12 h, 206% at 24 h, 324% at 36 h, and 405% at 48 h (Figure 4e). This result indicates that P. polycephalum not only moved eastward but also experienced overall growth. In essence, while actively moving toward the identified oatmeal, it simultaneously explored other locations.
Figure 4.
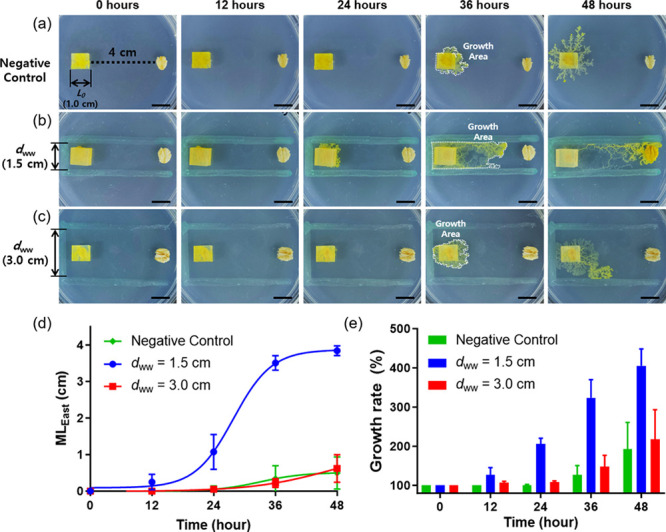
Time-lapse photographs of P. polycephalum without (a) and with a one-side-opened rectangular barrier (b and c). The wall-to-wall distance (dww) of hydrophobic barriers is (b) 1.5 cm and (c) 3.0 cm, respectively. (d) Plot of the MLEast of P. polycephalum over time. (e) Plot of the growth rate of P. polycephalum over time. The growth rate was calculated by growth area/L02 × 100 (%), where L0 is the initial length of P. polycephalum. The scale bar represents 1 cm.
In contrast to the results obtained at dww = 1.5 cm, P. polycephalum cultured under dww = 3.0 cm condition did not reach the oatmeal flakes even after 48 h (Figure 4c). The average MLEast was 0.05 cm at 24 h, 0.22 cm at 36 h, and 0.62 cm at 48 h (Figure 4d). This is nearly at the same level as the negative control, suggesting that the hydrophobic barrier had little effect under these conditions (dww = 3.0 cm). The average growth rates were 107% at 12 h, 109% at 24 h, 148% at 36 h, and 217% at 48 h (Figure 4e). These results suggest that P. polycephalum’s ability to reach the oatmeal is comparable to growth rates observed without a hydrophobic barrier. Taken together, under our experimental conditions, P. polycephalum robustly promotes its own growth and movement at a dww value equivalent to 150% of its initial length (L0). It underscores that the right dww settings are crucial to maximize the effect of the hydrophobic barrier depending on the specific objectives and conditions of the experiment.
P. polycephalum-Based Toxicity Assay
Based on the results of the above experiments, we designed a toxicity test using P. polycephalum to explore to what extent finding optimal dww conditions could be helpful. Since the strongly acidic conditions harm most living organisms, we used poisonous food by immersing an oatmeal flake in a pH 2 solution for 5 s (Figure 5a). Under the narrow dww toxic condition, growth was slower compared to the nontoxic condition, as observed for up to 48 h (Figure 5b). The average MLEast was 0.13 cm at 24 h, 1.02 cm at 36 h, and 1.93 cm at 48 h, with no growth observed until 12 h. The growth rates were 105% at 12 h, 121% at 24 h, 223% at 36 h, and 267% at 48 h (Figure 5c,d). Similar to the nontoxic condition, the growth rate rapidly increased after 24 h but did not reach the food source. The interesting point is that the growth of P. polycephalum occurred extensively in the west, north, and south directions. This seems to be the result of struggling to find a solution path while avoiding the poisonous substance located in the east. In the wide dww under the toxic condition, the MLEast and growth rate were the lowest. After being cultivated, P. polycephalum did not exhibit any growth for the first 24 h but showed signs of growth thereafter. The average MLEast was 0.21 cm at 36 h and 0.44 cm at 48 h. The growth rates were 103% at 12 h, 106% at 24 h, 120% at 36 h, and 167% at 48 h (Figure 5c,d). The trend was similar to the results shown in Figure 4c, although the overall growth was retarded and inhibited. P. polycephalum attempted to migrate to the surrounding areas (north, south, and west) for survival but was obstructed by a hydrophobic barrier. When it moved eastward, it ceased further migration upon recognizing toxic substances. In other words, stimulating P. polycephalum with appropriate dww conditions corresponds to the “stick” strategy in the “carrot and stick” approach, allowing for forced control of the initial movement and activity of P. polycephalum when toxicity testing begins. This movement allows for the confirmation of the initial health status of P. polycephalum, and the degree of toxicity can be clearly identified through subsequent movements of P. polycephalum.
Figure 5.
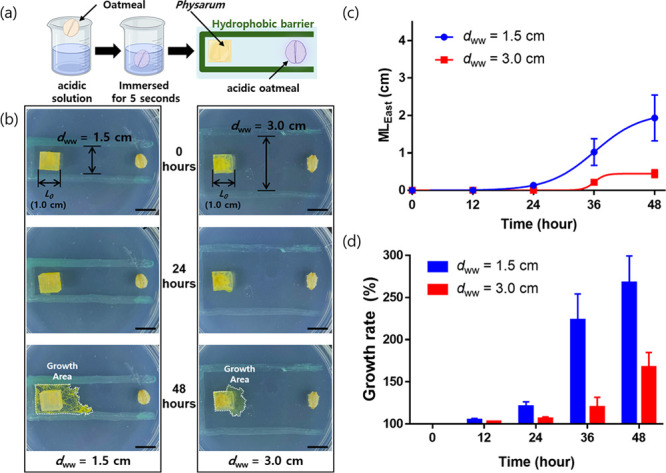
Migration of P. polycephalum with poisonous materials (acidic oatmeal) under chemotactic conditions with one-side-opened rectangular barriers. (a) Schematic illustration of the experimental setup. (b) Time-lapse photographs of P. polycephalum with poisonous materials with one-side-opened rectangular barriers (dww = 1.5 and 3.0 cm). (c) Plot of the MLEast of P. polycephalum over time. (d) Plot of the growth rate of P. polycephalum over time. The scale bar represents 1 cm.
Conclusions
We confirmed the possibility of controlling the growth direction and migration rate of P. polycephalum using various hydrophobic patterns, including complete barrier, single-slit barrier, taper barrier, dumbbell barrier, and one-side-opened rectangular barrier. It has also been experimentally proven that the growth direction and migration rate of P. polycephalum can be controlled by drawing a hydrophobic pattern on the medium. This method will be helpful when we control the growth direction of P. polycephalum effectively without additional materials, manufacturing techniques, and installation processes. The findings of this study can enhance our understanding of the biophysical and behavioral characteristics of P. polycephalum and the dynamic relationship between them. Additionally, it would be beneficial to examine the decoupling effects of the physical and chemical barriers to gain a deeper understanding of the behavior and characteristics of P. polycephalum.
Acknowledgments
Following are results of a study on the “Leaders in Industry-university Cooperation 3.0” (202302170001). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (No. 2020R1A2C2102262 and 2021R1A4A1028969). This study was also sponsored by a Korea University Grant and the BK21 FOUR (Fostering Outstanding Universities for Research).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c05560.
Overgrowth and network formation of P. polycephalum according to distribution of multiple nutritional sources; subculture conditions of P. polycephalum; P. polycephalum growth rate equation; multidirectional growth of P. polycephalum in response to spatially dispersed nutrient sources; and migration length of P. polycephalum over time (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Kamiya N. Physical and Chemical Basis of Cytoplasmic Streaming. Annu. Rev. Plant Physiol. 1981, 32 (1), 205–236. 10.1146/annurev.pp.32.060181.001225. [DOI] [Google Scholar]
- Wright M.; Albertini C.; Planques V.; Salles I.; Ducommun B.; Gely C.; Akhavan-Niaki H.; Mir L.; Moisand A.; Oustrin M.-L. Microtubule cytoskeleton and morphogenesis in the amoebae of the myxomycete Physarum polycephalum. Biol. Cell 1988, 63 (2), 239–248. 10.1016/0248-4900(88)90061-5. [DOI] [PubMed] [Google Scholar]
- Mohberg J.; Rusch H. P. Isolation and DNA content of nuclei of Physarum polycephalum. Exp. Cell Res. 1971, 66 (2), 305–316. 10.1016/0014-4827(71)90682-3. [DOI] [PubMed] [Google Scholar]
- Latty T.; Beekman M. Speed-accuracy trade-offs during foraging decisions in the acellular slime mould Physarum polycephalum. Proc. Biol. Sci. 2011, 278 (1705), 539–545. 10.1098/rspb.2010.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid C. R.; Latty T.; Dussutour A.; Beekman M. Slime mold uses an externalized spatial “memory” to navigate in complex environments. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (43), 17490–17494. 10.1073/pnas.1215037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisseau R. P.; Vogel D.; Dussutour A. Habituation in non-neural organisms: evidence from slime moulds. Proc. R. Soc. B 2016, 283 (1829), 20160446 10.1098/rspb.2016.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino-Ramirez F.; Boussard A.; Arson C.; Dussutour A. Substrate composition directs slime molds behavior. Sci. Rep. 2019, 9 (1), 15444. 10.1038/s41598-019-50872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briard L.; Goujarde C.; Bousquet C.; Dussutour A. Stress signalling in acellular slime moulds and its detection by conspecifics. Philos. Trans. R. Soc., B 1802, 2020 (375), 20190470 10.1098/rstb.2019.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann A.; Adamatzky A. Physarum Spatial Logic. New Math. Nat. Comput. 2011, 07 (03), 483–498. 10.1142/S1793005711002037. [DOI] [Google Scholar]
- Tero A.; Kobayashi R.; Nakagaki T. Physarum solver: A biologically inspired method of road-network navigation. Phys. A 2006, 363 (1), 115–119. 10.1016/j.physa.2006.01.053. [DOI] [Google Scholar]
- Mayne R.; Adamatkzy A. Cellular automata modelling of slime mould actin network signalling. Nat. Comput. 2019, 18 (1), 5–12. 10.1007/s11047-016-9559-0. [DOI] [Google Scholar]
- Berzina T.; Dimonte A.; Adamatzky A.; Erokhin V.; Iannotta S. Biolithography: Slime mould patterning of polyaniline. Appl. Surf. Sci. 2018, 435, 1344–1350. 10.1016/j.apsusc.2017.11.162. [DOI] [Google Scholar]
- Vallverdú J.; Castro O.; Mayne R.; Talanov M.; Levin M.; Baluška F.; Gunji Y.; Dussutour A.; Zenil H.; Adamatzky A. Slime mould: The fundamental mechanisms of biological cognition. Biosystems 2018, 165, 57–70. 10.1016/j.biosystems.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Adamatzky A. Physarum wires: Self-growing self-repairing smart wires made from slime mould. Biomed. Eng. Lett. 2013, 3 (4), 232–241. 10.1007/s13534-013-0108-9. [DOI] [Google Scholar]
- Tero A.; Takagi S.; Saigusa T.; Ito K.; Bebber D. P.; Fricker M. D.; Yumiki K.; Kobayashi R.; Nakagaki T. Rules for Biologically Inspired Adaptive Network Design. Science 2010, 327 (5964), 439–442. 10.1126/science.1177894. [DOI] [PubMed] [Google Scholar]
- Kay R.; Mattacchione A.; Katrycz C.; Hatton B. D. Stepwise slime mould growth as a template for urban design. Sci. Rep. 2022, 12 (1), 1322. 10.1038/s41598-022-05439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunita I.; Yoshihara K.; Tero A.; Ito K.; Lee C. F.; Fricker M. D.; Nakagaki T.. Adaptive Path-Finding and Transport Network Formation by the Amoeba-Like Organism Physarum. Natural Computing and Beyond, Tokyo, 2013//, Suzuki Y., Nakagaki T., Eds.; Springer: Japan, 2013; 14–29. [Google Scholar]
- Evangelidis V.; Jones J.; Dourvas N.; Tsompanas M.-A.; Sirakoulis G. C.; Adamatzky A. Physarum machines imitating a Roman road network: the 3D approach. Sci. Rep. 2017, 7 (1), 7010. 10.1038/s41598-017-06961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagaki T.; Yamada H.; Tóth Á. Maze-solving by an amoeboid organism. Nature 2000, 407 (6803), 470–470. 10.1038/35035159. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Guo J.; Lao Z.; Zhang S.; Yan X. Swarm Robot Exploration Strategy for Path Formation Tasks Inspired by Physarum polycephalum. Complexity 2021, 2021, 6698421 10.1155/2021/6698421. [DOI] [Google Scholar]
- Ahmed M. E; Sevindik M.; Baba H.; Bal C.; Colak O. F. Antioxidant, oxidant and antimicrobial capacities of Physarum album. J. Bacteriol. Mycol. Open Access 2018, 6 (6), 317–320. 10.1155/2021/6698421. [DOI] [Google Scholar]
- Beekman M.; Latty T. Brainless but Multi-Headed: Decision Making by the Acellular Slime Mould Physarum polycephalum. J. Mol. Biol. 2015, 427 (23), 3734–3743. 10.1016/j.jmb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Murugan N. J.; Kaltman D. H.; Jin P. H.; Chien M.; Martinez R.; Nguyen C. Q.; Kane A.; Novak R.; Ingber D. E.; Levin M. Mechanosensation Mediates Long-Range Spatial Decision-Making in an Aneural Organism. Adv. Mater. 2021, 33 (34), 2008161 10.1002/adma.202008161. [DOI] [PubMed] [Google Scholar]
- Kato S.Galvanotaxis of the Plasmodium of Physarum Polycephalum. In Integral Biomathics: Tracing the Road to Reality, Simeonov P. L., Smith L. S., Ehresmann A. C., Eds.; Springer: Berlin Heidelberg, 2012; 57–62. [Google Scholar]
- Zhu L.; Kim S. J.; Hara M.; Aono M. Remarkable problem-solving ability of unicellular amoeboid organism and its mechanism. R. Soc. Open Sci. 2018, 5 (12), 180396 10.1098/rsos.180396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J. W.; Baldwin H. H.. Chapter 2 Methods of Culture for Plasmodial Myxomycetes. In Methods in Cell Biology, Prescott D. M. Ed.; Academic Press, 1964, 1, 9–41.
- Tsuda S.; Jones J.; Adamatzky A.; Mills J. Routing Physarum with electrical flow/current. Int. J. Nanotechnol. Mol. Comput. 2011, 3 (2), 56–70. 10.4018/jnmc.2011040104. [DOI] [Google Scholar]
- Nakagaki T.; Kobayashi R.; Nishiura Y.; Ueda T. Obtaining multiple separate food sources: behavioural intelligence in the Physarum plasmodium. Proc. R. Soc. Lond. B. Biol. Sci. 2004, 271 (1554), 2305–2310. 10.1098/rspb.2004.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywda A.; Petelenz E.; Michalczyk D.; Płonka P. Sclerotia of the acellular (true) slime mould Fuligo septica as a model to study melanization and anabiosis. Cell. Mol. Biol. Lett. 2008, 13 (1), 130–143. 10.2478/s11658-007-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczyk-Wetula D.; Jakubowska M.; Felska M.; Skarżyński D.; Mąkol J.; Płonka P. M. Tyrophagus putrescentiae (Sarcoptiformes: Acaridae) in the in vitro cultures of slime molds (Mycetozoa): accident, contamination, or interaction?. Exp. Appl. Acarol. 2021, 84 (2), 445–458. 10.1007/s10493-021-00608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry M. M.; Murugan N. J.; Levin M.. Studying Protista WBR and Repair Using Physarum polycephalum. Methods Mol. Biol., Blanchoud S., Galliot B., Eds.; Springer US, 2022; 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar M.; Alim K. Encoding memory in tube diameter hierarchy of living flow network. Proc. Natl. Acad. Sci. U. S. A. 2021, 118 (10), e2007815118 10.1073/pnas.2007815118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino-Ramirez F.; Arson C.; Dussutour A. Substrate and cell fusion influence on slime mold network dynamics. Sci. Rep. 2021, 11 (1), 1498. 10.1038/s41598-020-80320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid C. R.; Latty T. Collective behaviour and swarm intelligence in slime moulds. FEMS Microbiol. Rev. 2016, 40 (6), 798–806. 10.1093/femsre/fuw033. [DOI] [PubMed] [Google Scholar]
- Nakagaki T.; Iima M.; Ueda T.; Nishiura Y.; Saigusa T.; Tero A.; Kobayashi R.; Showalter K. Minimum-Risk Path Finding by an Adaptive Amoebal Network. Phys. Rev. Lett. 2007, 99 (6), 068104 10.1103/PhysRevLett.99.068104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


