Abstract
Angiotensin II (Ang II)-induced vascular endothelial cell injury and dysfunction are important pathophysiological factors in the occurrence and development of hypertension. In this study, the amelioration effects of two peptides KA-8 (KLHDEEVA) and PG-7 (PSRILYG) from Harpadon nehereus bone on Ang II-induced damage and dysfunction in human umbilical vein endothelial cells (HUVECs) were investigated. The results showed that they could significantly decrease the reactive oxygen species (ROS) level and increase the activity of antioxidant enzymes in Ang II-induced HUVEC. Two peptides, especially PG-7, significantly upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2). In addition, PG-7 significantly reduced the level of expression of endothelin-1(ET-1) and increased the phosphorylation level of phosphoinositide 3-kinase (PI3K), serine/threonine kinase (AKT), and nitric oxide synthase (eNOS). These results indicated that the two peptides, especially PG-7, can ameliorate angiotensin II-induced HUVEC injury and dysfunction through activation of the AKT/eNOS and Nrf2 pathway. Furthermore, PG-7 showed a stronger affinity with angiotensin-converting enzyme (ACE) and ACE inhibitory than KA-8. In conclusion, peptide PG-7 reveals potential in the prevention and treatment of hypertension.
1. Introduction
Vascular endothelial cells secrete a variety of bioactive compounds that maintain blood vessel tone and thereby regulate blood pressure. Vascular endothelial cell injury or apoptosis leads to endothelial cell dysfunction, which is predictive of cardiovascular diseases, such as coronary heart disease and hypertension. Oxidative stress is one of the main factors in endothelial cell injury and dysfunction, which could result in cardiovascular diseases.1,2 Angiotensin II (Ang II) plays a key role in normal vascular physiology and endothelial damage. High levels of Ang II could cause oxidative stress reactions and increase blood pressure, thus inducing endothelial cell apoptosis and hypertension.3 In addition, Ang II also causes vascular dysfunction by inducing other vascular dysfunction-related factors, such as vasoconstrictor endothelin-1 (ET-1) and nitric oxide (NO).4 Therefore, attenuation of Ang II-induced oxidative stress and the secretion of vascular dysfunction-related factors are important strategies to improve cardiovascular diseases.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a master regulator of the cellular antioxidant system.3 Reactive oxygen species (ROS) or Nrf2 agonists stimulate Nrf2 transport to the nucleus, thereby activating the transcription of numerous antioxidant enzyme genes such as NAD(P)H: quinone oxidoreductase 1 (NQO1) and heme-oxygenase (HO-1). Therefore, the Nrf2 pathway is considered to be a key target to mitigate oxidative stress-induced endothelial cell damage. In addition, the classic apoptotic pathway serine/threonine kinase (AKT) is also relevant to endothelial cell damage. Furthermore, previous studies have suggested that AKT is upstream of the Nrf2 pathway. Some natural compounds have been reported to attenuate oxidative damage by the AKT/Nrf2 pathway.5 Bioactive peptides, as important natural compounds, have been widely used in functional foods and pharmaceuticals because of their significant antioxidant, hypotensive, hypolipidemic, antibacterial, and antitumor activities.6 The oligopeptide LSGYGP from tilapia revealed protective effects on oxidative stress and endothelial injury in Ang II-stimulated human umbilical vein endothelial cells (HUVECs).4 However, little is known about peptides from marine fish bone resources for attenuation of endothelial injury induced by Ang II.
Harpadon nehereus is an important economic fish in Asia because of its large yield, low price, and high protein content. Due to the lack of scales, bones are the main byproduct in the processing of Harpadon nehereus. Therefore, the preparation of bioactive compounds from bones is an important way to make full use of Harpadon nehereus resources. We had successfully extracted and characterized the collagen from Harpadon nehereus bones in our previous study.7 Furthermore, the peptides KLHDEEVA (KA-8) and PSRILYG (PG-7) were isolated from the hydrolysate of Harpadon nehereus bone collagen and revealed the protective effects against hyperglycemia-induced HepG2 damage (Scheme 1).8 In order to expand their application prospects and fields, it is necessary to evaluate their other biological activities such as antihypertensive and anti-inflammatory activities. Therefore, this study focuses on investigating whether they are able to protect against HUVEC injury induced by Ang II and the underlying mechanisms, including oxidative stress and dysfunction mediated by Nrf2 and AKT/nitric oxide synthase (eNOS) pathways.
Scheme 1. Molecular Structures of KA-8 and PG-7.

2. Materials and Methods
2.1. Materials
HUVEC was purchased from Shanghai Academy of Biological Sciences, Chinese Academy of Sciences (Shanghai, China). Ang II is obtained from APExBIO Technology LLC (Houston, USA). DMEM medium was purchased from Gibco Co., Ltd. (CA, USA). The ROS, malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) assay kits were purchased from Beyotime (Shanghai, China). All antibodies, including Nrf2, HO-1, NQO1, PI3K, AKT, eNOS, and ET-1, were from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other reagents were analytical grade.
2.2. Cell Culture
HUVEC was maintained in DMEM supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), and fetal bovine serum (10%, v/v) at 37 °C and 5% CO2. A total of 2 × 104 cells/well were inoculated in 96-well plates for culture overnight. The upper layer of medium was carefully aspirated, and 200 μL of medium including 100 μM KA-8 or PG-7 (100 μM) was added to each well. After 24 h, the cell proliferation rates were measured according to the CCK-8 assay instructions. The experiments included a control group, a model group, and peptide groups. The protective effects of peptides on the HUVEC injury were evaluated by treatment with 5 μM Ang II for 24 h.
2.3. Observation of Cell Morphology
The cells were digested into single-cell suspension and diluted with the new medium. 2 mL of cell suspension was added to the 6-well plate, resulting in a final cell concentration of 4 × 105/well. The 100 μM oligopeptides were added and cultured for 4 h. The medium was discarded, and then, 5 μM Ang II was added. After culturing for 24 h, the cells were observed and photographed under an inverted microscope.
2.4. Detection of Intracellular ROS Levels
After culturing, according to Section 5, 2 mL of 10 μM DCFH-DA solution was added to per well of the 6-well plate and returned to the incubator for incubation. After incubation for 20 min at 37 °C and 5% CO2, the 6-well plates were wrapped with tinfoil to prevent fluorescence quenching. The supernatant was removed, and the serum-free cell medium was used to wash off the DCFH-DA solution that did not enter the cells. The fluorescence intensity of the HUVEC was observed under a fluorescence microscope.
2.5. Determination of Antioxidant Enzymes, MDA, and NO Levels
Each well of the 96-well plate was inoculated with 2 × 104 cells and cultured overnight. The medium was removed, and the new medium containing 100 μM KA-8 or PG-7 was added. After 4 h of cultivation, 5 μM Ang II was added for incubation 24 h. After incubation, HUVECs were washed twice with PBS. A precooled cell lysate of 50 μL was added to each well and placed on ice for cleavage. The cells were then transferred to a precooled 1.5 mL centrifuge tube for cleavage for 30 min. The supernatant was obtained by centrifugation at 4 °C, 12,000 r/min for 10 min. The levels of CAT, SOD, GSH-Px, NO, and MDA in the supernatant were determined according to the kit instructions.
2.6. Western Blot Assay
HUVEC was cultured with six-well plates (5 × 106 cells/well). The cells were pretreated with KA-8 and PG-7 (100 μM) for 4 h and then treated with Ang II (5 μM) for 24 h. After treatment, the cells were cleaned with precooled PBS and cleaved with ice lysis buffer for 30 min. After centrifugation at 12,000 × g for 10 min, the supernatant was collected and the protein concentration was determined with the BCA protein detection kit. After incubation at 95 °C for 10 min, the protein (20–40 μg) was carried out with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to the PVDF membrane (Polyvinylidene-Fluoride, Merck). The nonspecific binding site was sealed in 5% bovine serum albumin solution (BSA) at room temperature for 1 h and then incubated with the primary antibody at 4 °C overnight. After washing with TBST (3 times, 10 min/time), the film was incubated with secondary resistance at room temperature for 1 h. After washing with TBST (3 times, 10 min/times), antibody signals were detected by the western blot development system. The dilution ratio of internal reference protein, vascular endothelial injury-related protein, and oxidative stress-related pathway protein was prepared according to the instructions.
2.7. Molecular Docking and Activity Inhibition of ACE with Peptides
The ACE (PDB ID: 5amb) protein was obtained from the PDB database. The molecular docking between ACE and peptides was obtained by using Auto Dock Vina. Pymol 2.7 was used to draw the 3D docking diagram and label the hydrogen bond and the bond length.
10 μL of ACE solution (0.1 U/mL), 50 μL of FAPGG solution (1 mM), and 40 μL of peptide solution (25, 50, and 100 μM) were added to the 96-well plate (the sample group). The peptide solution was replaced with 40 μL of HEPES buffer (80 mM, pH 8.3) in the blank group. The initial absorbance (A1 and B1) and the absorbance (A2 and B2) after reaction 30 min at 37 °C of the blank group and the sample group were measured at 340 nm, respectively. The calculation formula for ACE inhibition activity is as follows:
2.8. Data Analysis
All experiments were performed three times, and the results were expressed as mean ± SD. GraphPad Prism 8.0 software was used for one-way ANOVA and comparison between groups.
3. Results
3.1. Cytoprotective Effects of KA-8 and PG-7 on Ang II-Stimulated HUVEC
The effects of KA-8 and PG-7 on the cell viability of Ang II-stimulated HUVEC were investigated. As shown in Figure 1a, compared to the Con group, the cell viability in the Ang II group decreased significantly. However, the cell viability of Ang II-stimulated HUVEC was significantly increased after KA-8 and PG-7 treatment. In order to more intuitively evaluate the protective effect of KA-8 and PG-7 on Ang II-induced HUVEC damage, the morphology of HUVEC in each group was observed under a microscope. As shown in Figure 1b, HUVEC in the Con group were spindle-shaped and adhered to the wall after inoculation for 4 h. When stimulated by Ang II, the morphology and number of cells changed. In the Ang II group, the cells became polygonal or round with the blurred cell boundaries. In addition, the number of cells in the Ang II group was significantly reduced compared with the Con group. The cell reduction caused by Ang II treatment was significantly reversed by PG-7 pretreatment. Furthermore, the cell morphology in the PG-7 group was similar to that in the Con group. However, KA-8 did not improve the number and morphology of cells as significantly as PG-8.
Figure 1.
Effects of KA-8 and PG-7 on HUVEC viability (a) and morphology (b, 200×). **** means p < 0.0001 compared with the Ang II group.
3.2. KA-8 and PG-7 against ROS Production
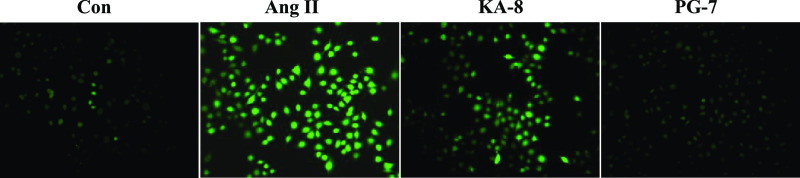
Studies have shown that Ang II stimulation can lead to the increase of the ROS content in HUVECs.9 This experiment explored whether KA-8 and PG-7 could reverse the Ang II-induced increase in the level of ROS in HUVECs. As shown in Figure 2, the Ang II group showed a stronger fluorescence state than the Con group, which was consistent with previous findings.10 After treatment with KA-8 and PG-7, the intracellular fluorescence intensity decreased significantly and the PG-7 group had the best effect. The results showed that two peptides, especially PG-7, could inhibit the production of ROS induced by Ang II in HUVEC, thereby reducing the oxidative damage caused by ROS.
Figure 2.
Effects of KA-8 and PG-7 on ROS in HUVECs (200×).
3.3. Effects of KA-8 and PG-7 on Antioxidant Enzymes Activities and the MDA Content in HUVEC
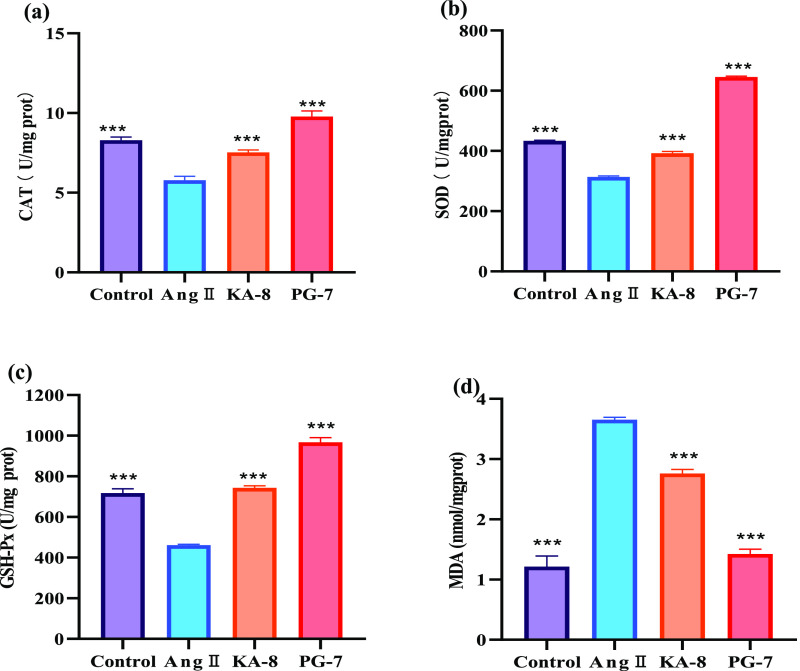
The increase of ROS levels in HUVEC induced by Ang II was closely related to the decrease of the activities of antioxidant enzymes such as SOD, CAT, and GSH-Px.11 As shown in Figure 3, compared to the Con group, the activities of CAT, SOD, and GSH-Px in the Ang II group were significantly reduced, while the content of MDA was significantly increased by 200%. KA-8 and PG-7 treatments significantly reversed the decline of CAT, SOD, and GSH-Px caused by Ang II, especially in the PG-7 group; the activities of antioxidant enzymes significantly rebounded to the levels in the Con group. At the same time, the level of intracellular MDA after PG-7 treatment also decreased to that of the Con group. These results indicated that two peptides, especially PG-7, could significantly increase the antioxidant enzyme activities in Ang II-induced HUVEC, thereby alleviating oxidative damage.
Figure 3.
Effects of KA-8 and PG-7 on the levels of CAT (a), SOD (b), GSH-Px (c), and MDA (d) in HUVECs. *** means p < 0.001 compared with the Ang II group.
3.4. Effects of KA-8 and PG-7 on the Nrf2 Pathway
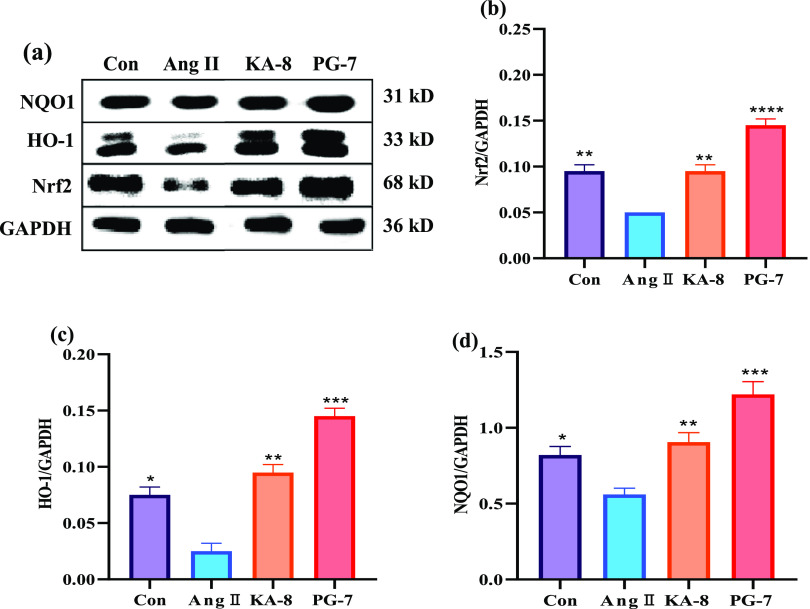
Nrf2 is a key factor regulating cellular oxidative stress response and controls the expression of downstream antioxidant enzyme genes by binding to the antioxidant response element (ARE). Therefore, activation of the Nrf2 pathway can protect cells from oxidative stress-induced damage, thereby inhibiting vascular remodeling and endothelial dysfunction. The above experiments have confirmed that KA-8 and PG-7 can improve the CAT, SOD, and GSH-Px activities in Ang II-induced HUVEC, which may be done by activating the Nrf2 pathway. Therefore, the effects of KA-8 and PG-7 on the expression of proteins associated with the Nrf2 pathway were investigated. It was shown in Figure 4 that Ang II treatment significantly inhibited the expression of Nrf2 protein in HUVEC, but KA-8 and PG-7 reversed the inhibitory effect of Ang II on the expression of Nrf2 protein. In particular, the Nrf2 content in the PG-7 group was even about 50% higher than that in the Con group. The variation trends of antioxidant enzymes, including CAT, SOD, GSH-Px, HO-1, and NQO1 in HUVEC, were basically consistent with that of Nrf2, indicating that KA-8 and PG-7 increased antioxidant enzyme levels by activating the Nrf2 pathway.
Figure 4.
Effects of KA-8 and PG-7 on the expression of Nrf2 pathway proteins (a) in Ang II-stimulated HUVEC. The levels of Nrf2 (b), HO-1 (c), and NQO1 (d) were evaluated by the gray scale analysis of bands. *, **, ***, and **** represent p < 0.05, <0.01, <0.001, and <0.0001 compared with the Ang II group, respectively.
3.5. Effects of KA-8 and PG-7 on NO, eNOS, and ET-1 Expression in HUVEC
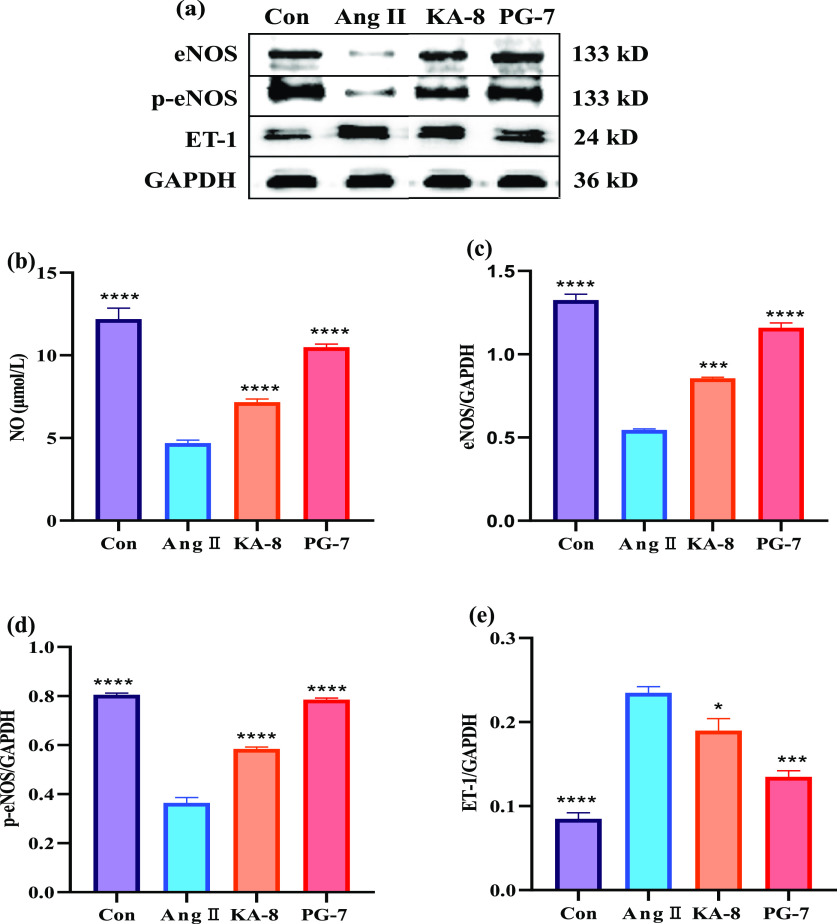
The contents of NO and the expression of eNOS and ET-1 were measured in HUVEC after treatment with two peptides. As shown in Figure 5, the Ang II stimulation had an obvious inhibition on NO levels, eNOS expression, and eNOS phosphorylation (p-eNOS), as compared to the Con group. Remarkably, KA-8 and PG-7 treatment increased the Ang II-stimulated decrease in NO, eNOS, and p-eNOS levels. In particular, the NO, eNOS, and p-eNOS levels in the PG-7 group were more than 100% higher than those in the Ang II group. Furthermore, ET-1 expression mainly increased after exposure to Ang II, but treatment with two peptides, especially PG-7, markedly blocked the Ang II-induced changes. The results revealed that two peptides, especially PG-7, may promote the expression of eNOS and inhibit the production of ET-1, thereby protecting HUVEC from Ang II-induced damage.
Figure 5.
Effects of KA-8 and PG-7 on protein expression (a) and levels of NO (b), eNOS (c), p-eNOS (d), and ET-1 (e) in Ang II-stimulated HUVEC. *, ***, and **** represent p < 0.05, <0.001, and <0.0001 compared with the Ang II group, respectively.
3.6. Effects of KA-8 and PG-7 on the PI3K/AKT Pathway
The effects of KA-8 and PG-7 interventions on the intracellular PI3K/AKT pathway were investigated, and the results are shown in Figure 6. Compared with the Con group, the phosphorylation of PI3K (p-PI3K) and AKT (p-AKT) in the Ang II group was significantly decreased (p < 0.05). However, these results were both reversed by PG-7 intervention (p < 0.0001). The phosphorylation of PI3K in the KA-8 group was significantly increased (p < 0.01), but the phosphorylation of AKT was not significantly different.
Figure 6.
Effects of KA-8 and PG-7 on the protein expression (a) of the PI3K/AKT pathway and levels of p-PI3K (b) and p-AKT (c) in Ang II-stimulated HUVEC. *, **, and **** represent p < 0.05, <0.01, and <0.0001 compared with the Ang II group, respectively.
3.7. Molecular Docking between Two Peptides and ACE
In order to evaluate the potential ACE inhibitory activities of the KA-8 and PG-7, molecular docking was used to study the molecular interactions and binding affinity between two peptides and ACE. The 3D structures of the KA-8 and PG-7 with the ACE-N domain are exhibited in Figure 7a. Fifteen hydrogen bonds were observed in the structure formed by the KA-8 and ACE-N domain (Lys432, Asp393, Glu431, Ser260, His491, Met256, Thr144, Asp140, Arg350, Gln355, Glu362, and Asp354). For PG-7, ten hydrogen bonds were formed between the peptide and eight amino acid residues of the ACE-N domain (Ser357, Gln355, Arg350, Glu431, Thr358, Cys348, His331, and His491). The docking results showed that some amino acid residues formed more than one hydrogen bond with the peptides. In addition to hydrogen bonds, peptides bind to the active sites of ACE through hydrophobic and van der Waals forces. The docking results showed that although KA-8 formed more hydrogen bonds with ACE, PG-7 had lower binding energy with ACE (−10.0 kcal/mol) than KA-8 (−9.2 kcal/mol). This result may be due to the fact that PG-7 contains a higher proportion of hydrophobic amino acids and thus has a stronger hydrophobic force.
Figure 7.
Molecular docking with the N-domain of ACE (a) and ACE inhibitory (b) of KA-8 and PG-7. *** and **** represent p < 0.001 and <0.0001, respectively.
In order to confirm the reliability of the molecular docking results, the inhibitory activities of the two peptides against ACE were investigated in vitro. As shown in Figure 7b, the ACE inhibitory of KA-8 remained at a low level under three concentrations. However, the ACE inhibitory effect of PG-7 increased sharply with the increase of PG-7 concentration and reached 29% at 100 μM concentration. These results indicated that PG-7 has ACE inhibition activity stronger than that of KA-8, which was consistent with molecular docking.
4. Discussion
Endothelial cell dysfunction/injury is considered a predictor of cardiovascular disease (CVD), such as heart disease, arteriosclerosis, stroke, kidney disease, and hypertension.12 Some studies have provided evidence that oxidative stress plays important role in the endothelial dysfunction/injury associated with CVD.13 During the occurrence and development of CVD, Ang II gradually accumulates in the vascular endothelium, thus stimulating endothelial cells to undergo oxidative stress.14 Therefore, Ang II is considered a powerful inducer of reactive oxygen species (ROS) generation, which causes endothelial dysfunction. It was reported that Ang II increased vascular ROS levels by inhibiting antioxidant enzyme activity such as SOD, CAT, and GSH-Px.1 In this study, the results demonstrated that Ang II significantly induced HUVEC reduction and deformation, which was attributed to a high level of ROS generation by decreasing the activities of antioxidant enzymes.
It has been reported that antioxidant peptides could reduce oxidative stress by eliminating the ROS and enhance the antioxidant system of the cell.15 In this study, we found that two peptides, especially PG-7, could significantly eliminate the ROS and increase the activities of SOD, CAT, and GSH-Px in Ang II-induced HUVEC (Figures 2 and 3). Our results demonstrated that peptides KA-8 and PG-7 protected the HUVEC from Ang II-induced injury through attenuating oxidative stress, which was also reflected in the improvement of the cell number and morphology after treatment with peptides (Figure 1). Furthermore, some papers suggested that peptides with a high proportion of hydrophobic amino acids showed high ROS inhibition activity and strong ability to enhance antioxidant enzyme activity.16 In this study, PG-7 exhibited the stronger ROS scavenging and antioxidant enzyme activities than KA-8, which may be attributed to its high proportion of hydrophobic amino acids such as Leu, Pro, and Phe.
Nrf2/ARE is one of the important signaling pathways of the cellular oxidative stress response. Nrf2 regulates the expression of antioxidant enzyme proteins such as HO-1 and NQO1, which are beneficial for protecting endothelial cells from oxidative stress-induced injury.17 Therefore, our research focused on the activation of the Nrf2 pathway to explore the molecular mechanism of antioxidant effects of KA-8 and PG-7. As expected, two peptides increased the expression of Nrf2 and its target products HO-1 and NQO1, thereby reducing the oxidative stress injury induced by Ang II in HUVEC. Compared with KA-8, PG-7 had a better effect on the activation of Nrf2 expression, which was consistent with higher levels of SOD, CAT, HO-1, and NQO1 in the PG-7 group. In addition, it was reported that HO-1 could reduce intracellular ROS levels by increasing the glutathione and bilirubin levels.18 Our results revealed that the HO-1 level in the PG-7 group was higher than that in the KA-8 group, which was consistent with the lower ROS levels in the KA-8 group in Figure 2.
In addition to oxidative stress, Ang II has been reported to induce endothelial cell dysfunction by inhibiting nitric oxide synthase (eNOS) activity and stimulating ET-1 secretion.14,19 eNOS is a key rate-limiting enzyme in the synthesis of NO, which promotes vasodilation and blood pressure reduction. ET-1 is an important vasoconstriction factor and is one of the risk factors for endothelial cell dysfunction and hypertension. Therefore, increasing the level of expression of eNOS and decreasing the content of ET-1 can effectively improve endothelial cell dysfunction and hypertension. In this study, it was observed that two peptides could significantly reverse the abnormal decline of eNOS and the rise of ET-1 in HUVEC caused by Ang II treatment. In addition, PG-7 revealed to be more effective than KA-8 in improving Ang II-induced abnormalities in eNOS and ET-1 levels. These results are in concordance with the previous studies, where peptides IVTNWDDMEK, IPIPATKT, and LSGYGP confirmed the improvement effects in eNOS and ET-1 levels.4,20,21 In summary, our research suggested that two peptides, especially PG-7, may improve endothelial cell dysfunction and decrease blood pressure by regulating the eNOS expression and ET-1 secretion.
The PI3K/AKT pathway plays a key role in many cellular processes, including glucose metabolism, apoptosis, cell proliferation, cell transcription, and cell migration.22 The activated PI3K/AKT pathway promotes the production of HO-1 by up-regulating the expression of Nrf2, thereby alleviating oxidative stress damage.23 Flavonoids derived from deep-sea Arthrinium sp. regulate ox-LDL-induced oxidative damage by activating the AKT/Nrf2/HO-1 pathway in vascular endothelial cells.24 In addition, the PI3K/AKT pathway can also regulate the expression of eNOS and the secretion of ET-1, thereby improving vascular endothelial cell dysfunction.25 In our study, peptides PG-7 and KA-8 protected HUVEC from Ang II-induced injury and dysfunction by regulating the levels of Nrf2, eNOS, and ET-1. Therefore, we further explored whether they achieved this result by activating the PI3K/AKT pathway. As observed in Figure 6, PG-7 restored the levels of phosphorylated PI3K (p-PI3K) and AKT (p-AKT) in Ang II-induced HUVEC. The results suggested that PG-7 may prevent HUVEC against Ang II-induced damage and dysfunction by activating AKT/Nrf2 and AKT/eNOS pathways.
In the prevention and treatment of hypertension, on the one hand, it is necessary to repair the injury/dysfunction of vascular endothelial cells induced by Ang II, and on the other hand, it is also important to inhibit the production of Ang II. Synthetic angiotensin I-converting enzyme (ACE) catalyzes the production of Ang II, which causes endothelial cell damage/dysfunction and high blood pressure. Therefore, inhibiting ACE activity is a well-established approach used in the prevention and treatment of hypertension. Molecular docking is one of the important methods for screening ACE inhibitors, and many ACE inhibitory peptides had been obtained based on it.20,21,26 Our study demonstrated that both peptides, especially PG-7, could ameliorate Ang II-induced endothelial cell damage/dysfunction by inhibiting oxidative stress and modulating eNOS and ET-1 abnormalities. Therefore, it is necessary to further investigate whether they can inhibit Ang II production. Our results showed that PG-7 and KA-8 formed 10 and 15 hydrogen bonds with the N-domain of ACE, respectively. However, PG-7 revealed a lower binding energy (−10.0 kcal/mol) with ACE than KA-8 (−9.2 kcal/mol), which may be attributed to the fact that PG-7 has a stronger hydrophobic force due to its more hydrophobic amino acids. The results showed that the binding force of ACE and peptides was not only related to the number and length of hydrogen bonds but also to other forces such as hydrophobic force and van der Waals force. This result was consistent with the previous research.21 Furthermore, the results of ACE inhibition of two peptides in vitro also showed that PG-7 had ACE inhibition activity stronger than KA-8. In summary, our studies found that the two peptides, especially PG-7, not only ameliorated Ang II-induced HUVEC damage/dysfunction by inhibiting oxidative stress and regulating eNOS and ET-1 levels but also inhibited the ACE activity. This study laid a solid theoretical foundation for the application of peptide PG-7 in the prevention and treatment of hypertension.
5. Conclusions
This study focused on the amelioration effects of two peptides KA-8 (KLHDEEVA) and PG-7 (PSRILYG) from bone collagen hydrolysate on Ang II-induced cell damage/dysfunction of HUVEC. The results showed that they inhibited oxidative stress by decreasing the ROS level and increasing the activity of antioxidant enzymes (CAT, SOD, and GSH-Px), which were mediated by Nrf2 upregulation. In addition, two peptides, especially PG-7, significantly reduced the expression of ET-1 and increased the phosphorylation level of AKT and eNOS. This study demonstrated that the two peptides, especially PG-7, ameliorated Ang II-induced HUVEC damage/dysfunction via the AKT/Nrf2 and AKT/eNOS pathway. In addition, PG-7 revealed a stronger affinity with the ACE protein and ACE inhibitor than KA-8. In conclusion, peptide PG-7 has a certain potential application in the prevention and treatment of hypertension.
Acknowledgments
This work was supported by the Zhoushan Medical and Health Science and Technology Project (2022YA02) and Special fund for clinical research of Zhejiang Medical Association (2022ZYC-Z38).
Author Contributions
M.S. and W.Z.: Investigation, Writing-original draft. K.S.: Supervision, Funding acquisition. H.J.: Conceptualization, Supervision, Writing-review and editing. M.S. and W.Z. contributed equally to this work and shared the first authorship.
The authors declare no competing financial interest.
References
- Li M.; Liu X.; He Y. P.; Zheng Q. Y.; Wang M.; Wu Y.; Zhang Y. P.; Wang C. Y. Celastrol attenuates angiotensin II mediated human umbilical vein endothelial cells damage through activation of Nrf2/ERK1/2 /Nox2 signal pathway. Eur. J. Pharmacol. 2017, 797, 124–133. 10.1016/j.ejphar.2017.01.027. [DOI] [PubMed] [Google Scholar]
- Liu X. Y.; Chen K. K.; Zhu L. J.; Liu H.; Ma T.; Xu Q. Y.; Xie T. Y. Soyasaponin Ab protects against oxidative stress in HepG2 cells via Nrf2/HO-1/NQO1 signaling pathways. J. Funct. Foods 2018, 45, 110–117. 10.1016/j.jff.2018.03.037. [DOI] [Google Scholar]
- Yang Y.; Tian T.; Wang Y.; Li Z.; Xing K.; Tian G. SIRT6 protects vascular endothelial cells from angiotensin II-induced apoptosis and oxidative stress by promoting the activation of Nrf2/ARE signaling. Eur. J. Pharmacol. 2019, 859, 172516 10.1016/j.ejphar.2019.172516. [DOI] [PubMed] [Google Scholar]; ARTN 172516
- Chen J.; Gong F.; Chen M.; Li C.; Hong P.; Sun S.; Zhou C.; Qian Z. In Vitro Vascular-Protective Effects of a Tilapia By-Product oligopeptide on Angiotensin II-Induced Hypertensive Endothelial Injury in HUVEC by Nrf2/NF-kappa B Pathways. Mar. Drugs 2019, 17 (7), 431. 10.3390/md17070431. [DOI] [PMC free article] [PubMed] [Google Scholar]; ARTN 431
- He S.; Zhao W.; Chen X.; Li J.; Zhang L.; Jin H. Ameliorative Effects of Peptide Phe-Leu-Ala-Pro on Acute Liver and Kidney Injury Caused by CCl4 via Attenuation of Oxidative Stress and Inflammation. ACS Omega 2022, 7, 44796–44803. 10.1021/acsomega.2c04851. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jin H. X.; Li Y.; Shen K.; Li J.; Yu F. M.; Yang Z. S. Regulation of H2O2-induced cells injury through Nrf2 signaling pathway: An introduction of a novel cysteic acid-modified peptide. Bioorg. Chem. 2021, 110, 104811 10.1016/j.Bioorg.2021.104811. [DOI] [PubMed] [Google Scholar]; ARTN 104811; Zhang X. G.; Liu A. X.; Zhang Y. X.; Zhou M. Y.; Li X. Y.; Fu M. H.; Pan Y. P.; Xu J.; Zhang J. Q. A diarylheptanoid compound from Alpinia officinarum Hance ameliorates high glucose-induced insulin resistance by regulating PI3K/AKT-Nrf2-GSK3beta signaling pathways in HepG2 cells. J. Ethnopharmacol. 2022, 295, 115397 10.1016/j.jep.2022.115397. [DOI] [PubMed] [Google Scholar]
- Sheng Y.; Qiu Y. T.; Wang Y. M.; Chi C. F.; Wang B. Novel Antioxidant Collagen Peptides of Siberian Sturgeon (Acipenser baerii) Cartilages: The Preparation, Characterization, and Cytoprotection of H2O2-Damaged Human Umbilical Vein Endothelial Cells (HUVECs). Mar. Drugs 2022, 20 (5), 325. 10.3390/md20050325. [DOI] [PMC free article] [PubMed] [Google Scholar]; ARTN 325; Sheng Y.; Wang W. Y.; Wu M. F.; Wang Y. M.; Zhu W. Y.; Chi C. F.; Wang B. Eighteen Novel Bioactive Peptides from Monkfish (Lophius litulon) Swim Bladders: Production, Identification, Antioxidant Activity, and Stability. Mar. Drugs 2023, 21 (3), 169. 10.3390/md21030169. [DOI] [PMC free article] [PubMed] [Google Scholar]; ARTN 169
- Shen K.; Li J.; Zhao W.; Shao M.; Jin H. Physicochemical Properties of Collagen from the Bone of Harpadon nehereus and Its Protective Effects against Angiotensin II-Induced Injury in Human Umbilical Vein Endothelial Cells. ACS Omega 2022, 7 (27), 23412–23420. 10.1021/acsomega.2c01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S.; Xu Z.; Li J.; Guo Y.; Lin Q.; Jin H. Peptides from Harpadon nehereus protect against hyperglycemia-induced HepG2 via oxidative stress and glycolipid metabolism regulation. J. Funct. Foods 2023, 108, 105723 10.1016/j.jff.2023.105723. [DOI] [Google Scholar]
- Cai S.; Pan N.; Xu M.; Su Y.; Qiao K.; Chen B.; Zheng B.; Xiao M.; Liu Z. ACE Inhibitory Peptide from Skin Collagen hydrolysate of Takifugu bimaculatus as Potential for Protecting HUVECs Injury. Mar. Drugs 2021, 19 (12), 655. 10.3390/md19120655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassan M.; Kwon Y.; Munkhsaikhan U.; Sahyoun A. M.; Ishrat T.; Galan M.; Gonzalez A. A.; Abidi A. H.; Kassan A.; Ait-Aissa K. Protective Role of Short-Chain Fatty Acids against Ang- II-Induced Mitochondrial Dysfunction in Brain Endothelial Cells: A Potential Role of Heme Oxygenase 2. Antioxidants 2023, 12 (1), 160. 10.3390/antiox12010160. [DOI] [PMC free article] [PubMed] [Google Scholar]; ShamsEldeen A. M.; Fawzy A.; Ashour H.; Abdel-Rahman M.; Nasr H. E.; Mohammed L. A.; Abdel Latif N. S.; Mahrous A. M.; Abdelfattah S. Hibiscus attenuates renovascular hypertension-induced aortic remodeling dose dependently: the oxidative stress role and Ang II/cyclophilin A/ERK1/2 signaling. Front. Physiol. 2023, 14, 1116705 10.3389/fphys.2023.1116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.; Wang W.; Jin X. Hirudin Protects Ang II-Induced Myocardial Fibroblasts Fibrosis by Inhibiting the Extracellular Signal-Regulated Kinase1/2 (ERK1/2) Pathway. Med. Sci. Monit. 2018, 24, 6264–6272. 10.12659/MSM.909044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.; Channon K. M.; Antoniades C. Therapeutic strategies targeting endothelial function in humans: clinical implications. Curr. Vasc. Pharmacol. 2012, 10 (1), 77–93. 10.2174/157016112798829751. [DOI] [PubMed] [Google Scholar]
- Gu L.; Bai W.; Li S.; Zhang Y.; Han Y.; Gu Y.; Meng G.; Xie L.; Wang J.; Xiao Y.; et al. Celastrol prevents atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS One 2013, 8 (6), e65477 10.1371/journal.pone.0065477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; Zheng L.; Liu S.; Peng Z.; Zhang S. Total flavonoids from Plumula Nelumbinis suppress angiotensin II-induced fractalkine production by inhibiting the ROS/NF-kappaB pathway in human umbilical vein endothelial cells. Exp. Ther. Med. 2014, 7 (5), 1187–1192. 10.3892/etm.2014.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Lu S.; Li Y.; Wang H.; Shi Y.; Zhang L.; Tu Z. Protective effect of antioxidant peptides from grass carp scale gelatin on the H2O2-mediated oxidative injured HepG2 cells. Food Chem. 2022, 373 (Pt B), 131539 10.1016/j.foodchem.2021.131539. [DOI] [PubMed] [Google Scholar]; Tao L.; Gu F.; Liu Y.; Yang M.; Wu X. Z.; Sheng J.; Tian Y. Preparation of antioxidant peptides from Moringa oleifera leaves and their protection against oxidative damage in HepG2 cells. Front. Nutr. 2022, 9, 1062671 10.3389/fnut.2022.1062671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Wu T.; Fang L.; Liu C.; Liu X.; Li H.; Shi J.; Li M.; Min W. Peptides from walnut (Juglans mandshurica Maxim.) protect hepatic HepG2 cells from high glucose-induced insulin resistance and oxidative stress. Food Funct. 2020, 11 (9), 8112–8121. 10.1039/D0FO01753A. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Liu J.; Duan H.; Li R.; Peng W.; Wu C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. 10.1016/j.jare.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Zheng L.; Su G.; Zeng X.; Sun B.; Zhao M. Evaluation and Exploration of Potentially Bioactive Peptides in Casein Hydrolysates against Liver Oxidative Damage in STZ/HFD-Induced Diabetic Rats. J. Agric. Food Chem. 2020, 68 (8), 2393–2405. 10.1021/acs.jafc.9b07687. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Shi R.; Xu C.; Li L. BRD4 expression in patients with essential hypertension and its effect on blood pressure in spontaneously hypertensive rats. J. Am. Soc. Hypertens. 2018, 12 (12), E107–E117. 10.1016/j.jash.2018.11.004. [DOI] [PubMed] [Google Scholar]; Li B.; Fang K.; Lin P.; Zhang Y.; Huang Y.; Jie H. Effect of sacubitril valsartan on cardiac function and endothelial function in patients with chronic heart failure with reduced ejection fraction. Clin. Hemorheol. Microcirc. 2021, 77 (4), 425–433. 10.3233/Ch-201032. [DOI] [PubMed] [Google Scholar]
- Chen M.; Pan D.; Zhou T.; Gao X.; Dang Y. Novel Umami Peptide IPIPATKT with Dual Dipeptidyl Peptidase-IV and Angiotensin I-Converting Enzyme Inhibitory Activities. J. Agr Food Chem. 2021, 69 (19), 5463–5470. 10.1021/acs.jafc.0c07138. [DOI] [PubMed] [Google Scholar]
- Wang C.; Song C.; Liu X.; Qiao B.; Song S.; Fu Y. ACE inhibitory activities of two peptides derived from Volutharpa ampullacea perryi hydrolysate and their protective effects on H2O2 induced HUVECs injury. Food Res. Int. 2022, 157, 111402 10.1016/j.foodres.2022.111402. [DOI] [PubMed] [Google Scholar]; ARTN 111402
- Shariati M.; Meric-Bernstam F. Targeting AKT for cancer therapy. Expert Opin. Invest. Drugs 2019, 28 (11), 977–988. 10.1080/13543784.2019.1676726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Tong L.; Zhang J.; Zhang Y.; Zhang F. Galangin Alleviates Liver Ischemia-Reperfusion Injury in a Rat Model by Mediating the PI3K/AKT Pathway. Cell. Physiol. Biochem. 2018, 51 (3), 1354–1363. 10.1159/000495553. [DOI] [PubMed] [Google Scholar]
- Hou J. R.; Wang Y. H.; Zhong Y. N.; Che T. T.; Hu Y.; Bao J.; Meng N. Protective Effect of Flavonoids from a Deep-Sea-Derived Arthrinium sp. against ox-LDL-Induced Oxidative Injury through Activating the AKT/Nrf2/HO-1 Pathway in Vascular Endothelial Cells. Mar. Drugs 2021, 19 (12), 712. 10.3390/md19120712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Yao F.; Song J.; Fu B.; Sun G.; Song X.; Fu C.; Jiang R.; Sun L. Protective effects of phenolic acid extract from ginseng on vascular endothelial cell injury induced by palmitate via activation of PI3K/Akt/eNOS pathway. J. Food Sci. 2020, 85 (3), 576–581. 10.1111/1750-3841.15071. [DOI] [PubMed] [Google Scholar]; Zhang Y.; Zhong D. L.; Zheng Y. L.; Li Y. X.; Huang Y. J.; Jiang Y. J.; Jin R. J.; Li J. Influence of electroacupuncture on ghrelin and the phosphoinositide 3-kinase/protein kinase B/endothelial nitric oxide synthase signaling pathway in spontaneously hypertensive rats. J. Integr. Med. 2022, 20 (5), 432–441. 10.1016/j.joim.2022.06.007. [DOI] [PubMed] [Google Scholar]; Zheng Q.; Wu Q.; Yang H.; Chen Q. H.; Li X. H.; Guo J. Y. A kappa-OR Agonist Protects the Endothelial Function Impaired by Hyperuricemia Through Regulating the Akt/eNOS Signal Pathway. Probiotics Antimicrob. Proteins 2022, 14 (4), 751–759. 10.1007/s12602-022-09945-1. [DOI] [PubMed] [Google Scholar]
- Chen J.; Tan L.; Li C.; Zhou C.; Hong P.; Sun S.; Qian Z. J. Mechanism Analysis of a Novel Angiotensin-I-Converting Enzyme Inhibitory Peptide from Isochrysis zhanjiangensis Microalgae for Suppressing Vascular Injury in Human Umbilical Vein Endothelial Cells. J. Agric. Food Chem. 2020, 68 (15), 4411–4423. 10.1021/acs.jafc.0c00925. [DOI] [PubMed] [Google Scholar]