Abstract
The immune system responds to cancer in two main ways. First, there are prewired responses involving myeloid cells, innate lymphocytes and innate-like adaptive lymphocytes that either reside in premalignant tissues or traffic directly to tumours, and second, there are antigen priming-dependent [Au:OK?] responses, in which adaptive lymphocytes are primed in secondary lymphoid organs before homing to tumours. Transforming growth factor-β (TGFβ) — one of the most potent and pleiotropic regulatory cytokines — controls almost every stage of the tumour-elicited immune response, from leukocyte development in primary lymphoid organs, to their priming in secondary lymphoid organs and their effector functions in the tumour itself. The complexity of TGFβ-regulated immune cell circuitries, as well as the contextual roles of TGFβ signalling in cancer cells and tumour stromal cells, necessitate the use of rigorous experimental systems that closely recapitulate human cancer to uncover the underlying immunobiology. The diverse functions of TGFβ in healthy tissues further complicate the search for effective and safe cancer therapeutics targeting the TGFβ pathway. Here, we discuss the contextual complexity of TGFβ signalling in tumour-elicited immune responses and explain how understanding this may guide the development of mechanism-based cancer immunotherapy. [Au:OK?]
[H1] Introduction
Members of the haematopoietic cell lineage make up the most diverse and dynamic cell populations in the cancer environment. Frequent infiltration of leukocytes in a growing tumour was observed as early as 1863, which prompted Rudolf Virchow to propose sites of chronic inflammation as origins of neoplastic malignancy. At the turn of the 20th century, Paul Ehrlich postulated an anti-cancer function for the immune system, proposing it eliminates aberrant cells generated during the course of fetal and post-fetal development. With an increasing understanding of leukocyte differentiation and regulatory mechanisms, the anti-cancer and pro-cancer functions of the immune system have begun to be elucidated. Based on how leukocytes are activated and recruited to the tumour, cancer-associated immune responses are either prewired or are priming-dependent (Box 1). The prewired immune response involves myeloid cells as well as innate lymphocytes and innate-like adaptive lymphocytes that are derived from progenitors generated in primary lymphoid organs. By contrast, the priming-dependent immune response mobilizes adaptive lymphocytes that are activated by antigens in secondary lymphoid organs before homing to cancerous tissues.
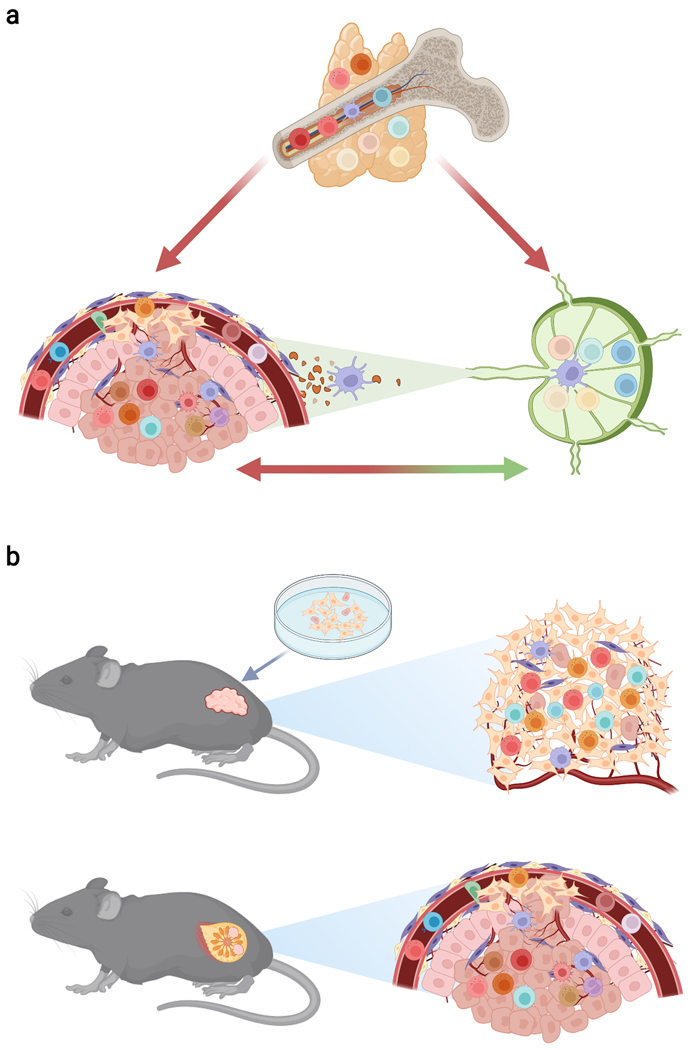
Box 1: A holistic immuno-oncology perspective and implications for preclinical cancer model choice.
The immune-recognition characteristics of leukocytes are used to define innate immunity and adaptive immunity, two arms of the immune system that mediate host defence against challenges such as cancer. Immune responses to cancer can also be defined by the behavioural features of leukocytes, which include pre-wired immunity and priming-dependent immunity (see Box Figure, part (a)). Pre-wired immunity is mediated by innate myeloid cells as well as innate lymphocytes and innate-like adaptive lymphocytes, which are pre-programmed to exert their effector functions and can traffic directly from the bone marrow or thymus to peripheral organs and tumours. Priming-dependent immunity is mediated by adaptive lymphocytes that require an antigenic activation [Au:OK?] step in secondary lymphoid organs, such as the lymph node, before they can mediate effector functions in the tumour. Crosstalk between the tumour and its draining lymph node involves the trafficking of immune cells, such as the migration of dendritic cells carrying antigen from the tumour to the draining lymph node and the trafficking of primed lymphocytes from the lymph node to the tumour. [Au: Edit OK?]
Many mouse models are employed to investigate cancer immunobiology (see Box Figure, part (b)). These include transplantable tumour models (top panel), where cancer cell lines are propagated in vitro and inoculated into target tissues of recipient mice. In this context, epithelial cancer cell lines may have become more mesenchymal-like during propagation76, and the growing tumour may not be integrated into the organ of interest, which alters the composition and function of tumour-infiltrating leukocytes. Autochthonous tumour models (bottom panel) involve tumour initiation by cells of an endogenous organ in an intact animal via processes such as oncogene expression or carcinogen exposure. Compared with transplantable models, autochthonous models more accurately recapitulate the endogenous tumour microenvironment, including cues of tissue homeostatic disruption202, and thus provide a better model of human disease.
In this Review, we focus on transforming growth factor β (TGFβ)-mediated control of immune cell responses in cancer, a topic of substantial recent discussion1–3. We cover the pleiotropic effects of TGFβ on prewired and priming-dependent immune cell responses and consider how these are regulated by cross-communications between cancer cells and the endogenous cancer environment — such responses are best recapitulated in autochthonous tumour models in mice, though we also discuss relevant data from transplantable models (Box 1). Finally, we consider how our growing understanding of these pathways can inform therapeutic targeting of the multi-functional TGFβ pathway for cancer immunotherapy. [Au: Edits OK?]
[H1] TGFβ and cellular regulation
The TGFβ signalling pathway is an intercellular communication pathway with pleiotropic effects on almost all metazoan cell lineages4. There are three mammalian TGFβ family members (TGFβ1, TGFβ2 and TGFβ3) with TGFβ1 playing a major role in immune regulation5 and TGFβ2 and TGFβ3 functioning in other cellular contexts, including fibrosis6. TGFβ is synthesized and assembled as a latent complex made of two copies of the N-terminal latency-associated peptide (LAP) and the C-terminal active cytokine, which can be covalently linked to latent TGFβ binding proteins (LTBPs) or leucine-rich repeat-containing proteins including LRRC32 (also known as GARP) and LRRC33. The LTBP-bound LAP–TGFβ complex is targeted to the extracellular matrix (ECM) by binding to fibrillin or fibronectin, while the plasma membrane-localized GARP or LRRC33 anchors LAP–TGFβ to the plasma membrane (Figure 1).
Figure 1. Molecular modalities of TGFβ activation and signaling.
TGFβ is produced in a latent complex involving two copies of latency-associated peptide (LAP) and the active cytokine. This complex can be covalently linked to latent TGFβ-binding proteins (LTBPs) which can be found in the extracellular matrix (ECM), or to leucine-rich repeat-containing proteins such as LRRC32 (also known as GARP), which can be found on the cell surface. The integrin αvβ6, which interacts with the cytoskeleton, and integrin αvβ8 can liberate and expose the receptor-binding site of active TGFβ. TGFβ binds to the heterotetrameric TGFβ type II and type I serine/threonine kinase receptors (TGFBR2 and TGFBR1), triggering TGFBR2 phosphorylation of TGFBR1, which then phosphorylates SMAD2 and SMAD3. These two phosphorylated proteins can modulate gene expression in two ways: (1) through forming a heterotrimeric complex with SMAD4 and translocating to the nucleus, controlling target gene expression, and (2) through binding the SMAD4–SKI–SKIL complex, which normally represses transcription where it binds, and leads to its degradation, thus alleviating transcriptional repression. Phosphorylated SMAD3 also has transcription-independent cell signalling functions including liberation of protein kinase A (PKA) from an inactive PKA complex. Only the mediators of TGFβ signalling discussed in the text are displayed.
The arm and ‘straightjacket’ domains of LAP form a ring around TGFβ, masking its receptor interaction sites7, with TGFβ activation primarily mediated by integrin molecules. The LAP domains of TGFβ1 and TGFβ3 contain an integrin-binding RGD (Arg-Gly-Asp) motif, and substitution of RGD with RGE (Arg-Gly-Glu) abolishes integrin binding. Mice harbouring RGE mutant alleles of Tgfb1 manifest an autoimmune phenotype indistinguishable from that of TGFβ1-deficient mice8. In addition, mice devoid of the αv family integrins αvβ6 and αvβ8 phenocopy TGFβ1- and TGFβ3-deficient mice9. αvβ6 liberates ECM-associated TGFβ through physical force generated from its association with the cytoskeleton10. αvβ8 does not interact with the cytoskeleton, but its interaction with the GARP-associated LAP–TGFβ complex effectively exposes the receptor-binding site of TGFβ11 (Figure 1).
Active TGFβ mediates its biological functions by binding to heterotetrameric TGFβ type II and type I serine/threonine kinase receptors (TGFBR2 and TGFBR1), which triggers TGFBR2-mediated phosphorylation of TGFBR1 that in turn phosphorylates the SMAD2 and SMAD3 proteins (Figure 1). Phosphorylated SMAD2 and SMAD3 form heterotrimeric complexes with SMAD4, and translocate to the nucleus to control target gene expression4. SMAD4 can also constitutively bind to target gene loci in complex with SKI and SKIL to suppress gene transcription, and binding of phosphorylated SMAD2 and SMAD3 to the SMAD4–SKI–SKIL complex induces SKI–SKIL degradation12,13. Clinically relevant SKI mutations abolish SKI interaction with phosphorylated SMAD2 and SMAD3, but not SMAD4, thereby preventing SKI degradation and markedly blunting TGFβ-regulated gene expression14. Thus, the TGFβ receptor-activated SMAD2–SMAD3 complex can engage two opposing modes of transcriptional regulation in terms of SMAD4 function: a cooperative mode that involves the SMAD2–SMAD3–SMAD4 complex directly controlling gene transcription, and an antagonistic mode in which SMAD2–SMAD3 relieves transcriptional repression of TGFβ target genes by the SMAD4–SKI–SKIL complex by promoting SKI–SKIL degradation [Au: Edit OK?] (Figure 1). In addition, SMAD complexes exhibit non-transcriptional functions including activation of protein kinase A (PKA)15 (Figure 1). The TGFβ signalling pathway also involves negative regulators. SMAD7 works to suppress TGFβ signalling by binding to TGFBR1 and blocking SMAD2–SMAD3 from being activated, blocking SMAD2–SMAD3 from complexing with SMAD4, and triggering ubiquitin-mediated degradation of TGFBR116. TGFβ signalling induces expression of SMAD7 and SKIL, and these negative feedback loops ensure TGFβ responses are tightly regulated.
[H1] TGFβ in adaptive lymphocytes in cancer
The adaptive immune system consists of T cells and B cells that express antigen-specific T cell receptors (TCRs) and B cell receptors (BCRs) on their cell surfaces, with BCRs also secreted as antibodies. TGFβ critically regulates the development, activation and differentiation of these lymphocytes with pleiotropic effects in cancer.
[H2] TGFβ in adaptive T cell responses in cancer.
In the thymus during T cell development, αβ lineage CD4+ and CD8+ T cells are positively selected by low affinity self-peptide antigens presented by MHC class II and MHC class I molecules, respectively, while a fraction of highly self-reactive T cells differentiate into CD4+ regulatory T (Treg) cells and CD8+ memory phenotype (MP) T cells [Au: Reference this statement?]. Following priming in secondary lymphoid organs by antigen-presenting cells (APCs) bearing their cognate antigens, naive CD4+ and CD8+ T cells as well as Treg cells and MP CD8+ T cells can exert effector and regulatory functions in cancer.
[H2] T helper cells.
TGFβ signalling promotes IL-7 receptor α (IL-7Rα) expression in low-affinity thymic CD4+ T cells to support their maintenance in peripheral tissues17, while dampening agonistic antigen-driven negative selection of CD4+ T cells18. In addition, TGFβ inhibits activation, proliferation and effector differentiation of peripheral autoreactive T cells19, and in mice conditional ablation of TGFBR2 or SMAD2 and SMAD3 at the CD4+CD8+ stage of T cell development causes lethal autoimmunity[Au:OK?]20–23. The TGFβ-induced SMAD3–SMAD4 complex activates PKA to trigger C-terminal SRC kinase (CSK)-mediated suppression of proximal TCR signalling to prevent inadvertent T cell priming24,25. TGFβ also downregulates the transcription factors T-bet and GATA3 to suppress CD4+ T cell differentiation into T helper 1 (Th1) and Th2 cells, respectively26–28. In contrast, TGFβ synergizes with IL-6 to induce the differentiation of IL-17A-producing Th17 cells29,30, driven in part by STAT3 attenuation of the SMAD3–SMAD4-mediated suppression of TCR signalling25, and SMAD2–SMAD3 reversal of the SMAD4–SKI–SKIL-mediated transcriptional repression of RORγt31. Thus, TGFβ functions as a pivotal regulator of CD4+ T cell activation and proliferation as well as guiding fate specification of the three major Th cell subsets (Figure 2).
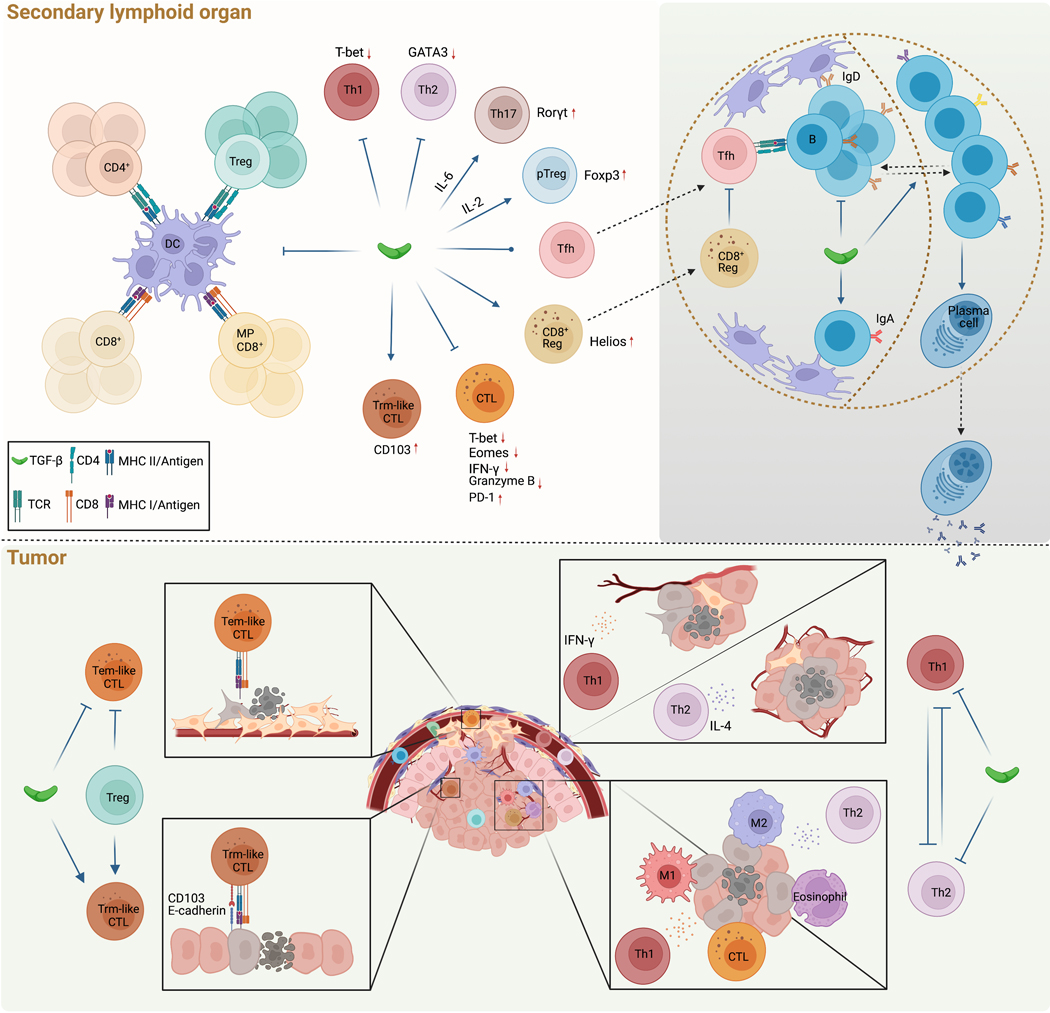
Figure 2. TGFβ control of priming-dependent lymphocyte responses in cancer.
a, In secondary lymphoid organs, TGFβ inhibits naive CD4+ T cell, regulatory T (Treg) cell, naïve CD8+ T cell and memory-phenotype (MP) CD8+ T cell priming by dendritic cells (DCs). b, TGFβ also inhibits Th1 and Th2 cell, but promotes Th17 cell and peripheral Treg (pTreg) cell, differentiation by regulating expression of the transcription factors T-bet, GATA3, RORγt, and FOXP3, while its effect on T follicular helper (Tfh) cell differentiation is contextual. Among CD8+ T cells, TGFβ promotes differentiation of regulatory CD8+ T cells, in part by promoting expression of the transcription factor Helios. These cells reside in the B cell follicle where they can inhibit Tfh cell responses. In addition, TGFβ promotes tissue resident memory (Trm)-like cytotoxic T lymphocytes (CTLs) through expression of the integrin CD103, while also repressing CTL differentiation by suppressing expression of T-bet and Eomes, IFNγ and granzyme B, and promoting expression of the inhibitory receptor PD-1.
c, TGFβ inhibits B cell proliferation, and promotes both IgA class-switching and migration from the light zone (LZ) and the dark zone (DZ) in the germinal centre.
d-e, In the tumour, TGFβ, in part regulated by Treg cells, inhibits circulating T effector memory (Tem)-like CTL responses against mesenchymal phenotype cancer cells (d), while promoting Trm-like CTL responses, including cytotoxicity against epithelial cancer cells through CD103 interaction with E-cadherin (e).
f-g, TGFβ also inhibits both Th1 and Th2 effector states. Should this inhibition be removed, IFNγ-producing Th1 cells can impact angiogenesis (f), ‘M1-like’ macrophage polarization and CTL function (g), while IL-4-producing Th2 cells can influence tissue-level vascularization and tumour tissue healing (f), ‘M2-like’ macrophage polarization and eosinophil responses (g), which collectively suppress tumor development by targeting cancer cells (g) and the cancer environment (f).
TGFβ-mediated control of peripheral Th cell responses in cancer has recently been investigated in a transgenic model of breast cancer, where TGFBR2 was depleted in mature CD4+ T cells32. Blockade of TGFβ signalling results in enhanced CD4+ T cell activation and differentiation into Th1 and Th2 cells in tumour-draining lymph nodes32. Of note, TGFBR2-deficient CD4+ T cells predominantly localize in the tumour stroma, and they promote tumour tissue healing and blood vasculature reconfiguration to trigger hypoxia and starvation-induced cancer cell death32. Ablation of IL-4 reverses vasculature remodelling and tumour suppression in this system32, supporting the idea that a tissue-level cancer defence mechanism is mediated by Th2 cells in this model. Amplified Th1 cell responses following TGFβ blockade has also been shown to suppress cancer progression. Metastasis of prostate cancer to bone is associated with enhanced Th17 cell, but not Th1 cell, differentiation in patients treated with the immune checkpoint inhibitor anti-CTLA433. In a mouse model, blocking TGFβ along with anti-CTLA-4 and anti-PD-1 treatment diminishes Th17 cell, but enhances Th1 cell, differentiation in association with clonal expansion of CD8+ T cells and this leads to regression of prostate cancer bone metastasis33. These and other studies have started to reveal that TGFβ is a major negative regulator of Th1 and Th2 cell-driven anti-tumour responses in different cancer settings34 (Figure 2).
In contrast, Th17 cells mostly exhibit pro-tumour activities34. In patients with colon cancer, a high proportion of IL-22-expressing Th17 cells in the tumour tissue is associated with increased TGFβ1 expression35. TGFβ1 induces IL-22 expression in Th17 cells under conditions of strong co-stimulation35. In an azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced mouse model of colon cancer, ablation of TGFBR2 in Th17 cells suppresses IL-17A and IL-22 expression and leads to diminished tumour growth35. Similarly, overexpression of SMAD7 in T cells blocks TGFβ signalling, causing enhanced Th1 cell, but diminished Th17 cell, differentiation and tumour inhibition in AOM/DSS and transplantable colon cancer models36,37. Taken together, TGFβ generally inhibits both CD4+ T cell priming and differentiation of anti-tumour Th1 and Th2 cells while fostering pro-tumour Th17 cell responses, although type 1 and type 2 cytokines can also have pro-tumour functions38–41.
T follicular helper (Tfh) cells also exhibit potent anti-tumour functions in models expressing B cell neoantigens42. Tfh cells are preferentially differentiated from IL-2-producing CD4+ T cells that have undergone strong TCR stimulation during priming43. In this context, TGFβ may limit Tfh cell generation by inhibiting TCR signalling. Alternatively, TGFβ-mediated inhibition of IL-2R expression can insulate CD4+ T cells from IL-2 signalling, and thus promote Tfh cell generation44. Thus, TGFβ can exhibit opposing roles in control of Tfh cell differentiation (Figure 2), and future studies will be needed to unravel how it affects Tfh cell responses in the context of cancer.
[H2] Regulatory T cells.
Thymic Treg (tTreg) cell differentiation is promoted by TGFβ signalling45. TGFβ-mediated support of tTreg cell generation does not involve induction of the lineage-defining transcription factor FOXP346,47, but rather acts indirectly via attenuation of agonistic antigen-triggered T cell clonal deletion18. Peripheral Treg (pTreg) cells can also differentiate from naïve CD4+ T cells in the presence of TGFβ48, in part via SMAD3-mediated induction of Foxp3 transcription49, but to what degree the induction of pTreg cells by TGFβ contributes to tumour-associated immune regulation is incompletely understood. Studies in mouse models as well as in human melanoma, gastrointestinal and ovarian cancers have revealed that Treg cells and other CD4+ T cells exhibit a largely non-overlapping TCR repertoire in different cancer settings50–52. This suggests that pTreg cell conversion does not occur in the tumour, although substantial TCR overlap was observed between intratumoral Treg cells and other intratumoral CD4+ T cells in patients with breast cancer53. TGFβ plays a more prominent role in controlling Th cell differentiation than in driving pTreg cell differentiation, and this is likely because a higher level of TGFβ is needed to induce FOXP3 expression than to regulate Th cell lineage-specifying transcription factors54. Beyond influencing pTreg cell differentiation, TGFβ can suppress Treg cell expansion in an autocrine manner, as demonstrated by inducible depletion of TGFBR2 in CD4+ T cells or Treg cell-specific ablation of TGFβ1, which both result in enhanced Treg cell proliferation55,56. Depletion of TGFBR2 or TGFβ1 in Treg cells does not impact tumour growth in transgenic models of breast and prostate cancers32,57. Thus, although TGFβ induction of Foxp3 expression is a widely used assay for in vitro studies (Figure 2), pTreg cell differentiation may not majorly contribute to tumour-infiltrating Treg cells. Furthermore, Treg cell maintenance and function in the tumour does not depend on TGFβ signaling in a transgenic model of breast cancer32. In a transplantable squamous cell carcinoma model, neutralization of TGFβ did not affect Treg cell frequency in tumours, but it diminished the anti-PD-1-induced expansion of Treg cell populations and synergized with anti-PD-1 to suppress tumour development58. Whether TGFβ promotes pTreg cell differentiation under the condition of anti-PD-1 treatment remains to be determined.
[H2] Cytotoxic T lymphocytes.
TGFβ promotes IL-7Rα expression and thymic CD8+ T cell lineage commitment17, whereas it suppresses antigen-driven proliferation of peripheral CD8+ T cells59, partly via DGKζ- and PTPN22-dependent mechanisms60,61. In addition, FOXP1 [Au:OK?] interacts with TGFβ-activated SMAD2 and SMAD3 to repress the MYC and JUN transcription factors that drive proliferation of CD8+ T cells62. TGFβ also inhibits expression of T-bet and Eomes, which support CD8+ T cell differentiation into cytotoxic T lymphocytes (CTLs)63. Furthermore, SMAD2 and SMAD3 partner with ATF1 to inhibit expression of CTL effector molecules, including IFNγ and granzyme B64. Chronic antigen stimulation of CTLs results in a dysfunctional state of T cell ‘exhaustion’ characterized by high expression of inhibitory receptors including PD-1. TGFβ enhances PD-1 expression in a SMAD3-dependent manner in association with SMAD3 recruitment to the Pdcd1 promoter region65. Thus, TGFβ engages a number of gene expression programmes to attenuate CTL differentiation and function (Figure 2).
TGFβ has been shown to inhibit cancer immunosurveillance by CTLs in transplantable tumour models. Blocking TGFβ signalling in CD8+ T cells inhibits the growth of transplanted thymoma cells and this is associated with expansion of antigen-specific CTLs66. Mice with CD8+ T cell-specific deletion of TGFBR1 or treated with a TGFBR1 kinase inhibitor display increased rejection of an implanted colon cancer cell line, and this is associated with high expression of CXC-chemokine receptor 3 (CXCR3), which supports CTL trafficking to the tumour67. High numbers of Treg cells are frequently observed in transplanted tumours, and their depletion results in CTL-mediated tumour inhibition68. Of note, Treg cells express high levels of integrin αvβ8, which activates TGFβ, and T cell- or Treg cell-specific deletion of Itgb8 (which encodes integrin β8) or treatment with an integrin β8-blocking antibody suppresses tumour growth in a CD8+ T cell-dependent manner69,70. Treg cells also express high levels of the TGFβ-tethering molecule GARP, and Treg cell-specific deletion of GARP or treatment with a GARP–TGFβ1 blocking antibody enhances the anti-tumour activity of anti-PD-1 in association with increased CTL effector function71. These findings suggest that Treg cells may suppress CTL-mediated cancer immunity by presenting and activating TGFβ1 in the tumour microenvironment, although αvβ8 and TGFβ1 expressed by cancer cells may also promote tumor immune evasion70,72,73.
However, the impact of CD8+ T cell-targeted TGFβ signalling blockade on cancer progression is not robust in genetic models of solid tumours. In a transgenic model of prostate cancer, transfer of tumour-antigen-reactive CD8+ T cells that express a dominant-negative mutant of TGFBR2 (TGFDNR) delays, but does not prevent, tumour progression74, although in another study with CD8+ T cells recognizing a different oncoprotein antigen, the anti-tumour effect of the transferred cells can be substantially enhanced if other lymphocytes are depleted[Au:OK? The meaning of the second part of this sentence isn’t clear to me]75. In a transgenic model of breast cancer, deletion of TGFBR2 specifically in CD8+ T cells results in enhanced CD8+ T cell activation in tumour-draining lymph nodes with tumour-infiltrating CD8+ T cells displaying high levels of granzyme B and low levels of PD-1 expression. However, tumour development is not inhibited32. The discrepancy between transplantable and autochthonous cancer models could be because the commonly used epithelial tumour-derived cancer cell lines exhibit a more mesenchymal phenotype76, and are more likely to be suppressed by CTLs with a blood-circulating effector–memory-like state, which manifests predominantly under conditions of TGFβ inhibition (Figure 2).
Of note, recent studies have revealed a critical function for TGFβ in promoting the establishment of an epithelial tissue residency programme in CTLs. In infection models, TGFβ signalling is required at multiple stages of resident memory CTL development: from naive T cell priming in secondary lymphoid organs by αvβ8-expressing dendritic cells (DCs)77 to T cell retention in non-lymphoid tissues. The latter [Au: Edit OK?] can be mediated by keratinocyte integrin activation of CD8+ T cell-produced TGFβ1 in the epidermis78, and by T-bet-expressing Treg cells that are recruited to the vicinity of CD8+ T cells to activate TGFβ1 via αvβ879. An important target of TGFβ in promoting epithelial tissue residency is the αE integrin (also known as CD103), which is likely induced in CD8+ T cells through SMAD2–SMAD3-mediated reversal of transcriptional repression by SMAD4–SKI–SKIL80,81. Indeed, SMAD4 suppresses TGFβ target genes including CD103, both transcriptionally and epigenetically, in CD8+ T cells prior to TGFβ exposure, and SMAD4 deletion leads to microbiota-mediated accumulation and epithelial cell-induced activation of CD8+ T cells, resulting in severe intestinal inflammation82. Paired with β7 integrin, CD103 binds to E-cadherin on epithelial cells, and facilitates CTL-mediated cancer immunosurveillance by enhancing target cell cytotoxicity83. Importantly, tumour-infiltrating CTLs that resemble CD103+ resident memory T cells have been associated with improved patient prognosis in a number of epithelial cancer types84. In a lung cancer model, CD103 is required for CTL recruitment within epithelial tumour islets, and TGFβ signalling enhances CD103-dependent T cell adhesion and integrin-linked kinase (ILK)-mediated signalling to promote cancer cell killing85. These findings reveal that TGFβ promotes resident memory-like CTL-mediated cancer immunosurveillance within the epithelial cancer microenvironment (Figure 2), and findings from transplantable tumour models should be interpreted with caution. [H2] Regulatory CD8+ T cells. Unprimed mice harbour populations of highly self-reactive MP CD8+ T cells that express Eomes and the IL-2/IL-15 receptor β chain CD12286. A subset of these cells express inhibitory receptors of the LY49 family and CXC-chemokine receptor 5 (CXCR5), and are preferentially localized near or within B cell follicles to inhibit Tfh cell-mediated humoral immune responses87. Similarly to tTreg cells, thymic selection of CD122+LY49+ regulatory CD8+ T cells is promoted by TGFβ signalling at a young age88, which might be mediated by TGFβ suppression of clonal deletion of autoreactive T cell progenitors. In addition, ablation of TGFBR2 in mature T cells results in diminished expression of the transcription factor Helios in regulatory CD8+ T cells, which synergizes with Eomes deletion to deplete regulatory CD8+ T cells and trigger autoimmunity89. Notably, follicular lymphoma patients have a population of tumour-associated cytotoxic CXCR5+CD8+ T cells that inhibit Tfh cell-mediated B cell differentiation90. These cells can be induced and expanded by TGFβ and IL-23, and exhibit a gene expression profile similar to that of regulatory CD8+ T cells90. Furthermore, this gene signature is positively associated with survival of follicular lymphoma patients90, supporting a lymphoma surveillance function of this unique TGFβ-induced regulatory CD8+ T cell subset (Figure 2).
[H2] TGFβ in innate-like T cell responses in cancer.
A fraction of αβ T cells that react strongly to self-peptides presented by MHC class I or MHC class II molecules are agonistically selected and differentiate into intraepithelial T lymphocytes (IELs)91. In addition, lipids presented by the MHC class I-related molecule CD1d drive differentiation of invariant natural killer T (iNKT) cells and subsets of γδ T cells92. γδ T cell populations can also be selected by non-polymorphic proteins such as the butyrophilin family93. These unconventional lineages of αβ T cells and γδ T cells can directly traffic to and reside in the tumour without experiencing priming in secondary lymphoid organs.
[H2] Cytotoxic innate-like T cells.
In transgenic models of epithelial cancers, a population of tissue-resident αβTCR+ lineage killer innate-like T cells (ILTCKs) that express the innate lymphocyte activation receptor NK1.1 and the integrin CD103 expand in tumours94. αβTCR+ ILTCKs exhibit potent lytic granule-mediated cytolytic activities against cancer cells, and are phenotypically distinct from CD8+PD-1+ T cells that do not kill cancer cells94. Following early encounter with cognate antigens, αβTCR+ ILTCKs arise from FcεRIγ-expressing IEL-like thymic progenitors, and seed healthy and tumour tissues independently of priming in secondary lymphoid organs95. Expansion and effector differentiation of αβTCR+ ILTCKs in the tumour are driven by the cancer cell-expression of IL-1595 and TGFβ signalling (B.G.N, unpublished observations). Thymic selection of IELs has also been shown to require TGF-β signaling as a likely consequence of attenuated clonal deletion18,96. In addition, FcεRIγ-expressing TCRαβ ILTCKs are induced in human colon cancer95. These findings suggest that TGF-β promotes thymic development and peripheral differentiation of TCRαβ ILTCKs to support their prewired anti-tumor function (Figure 3).
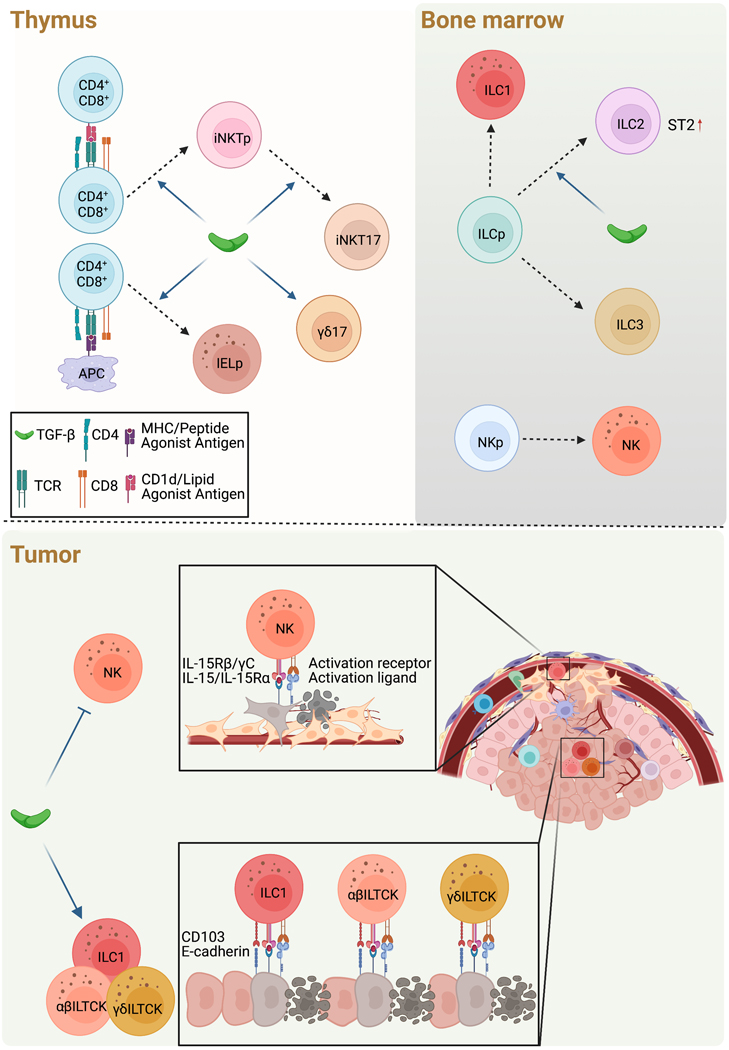
Figure 3. TGFβ control of prewired innate lymphocyte and innate-like T cell responses in cancer.
a, In the thymus, TGFβ is required for development of CD1d/lipid agonist antigen-reactive invariant Natural Killer T (iNKT) precursor (iNKTp) and MHC/peptide agonist antigen-reactive intraepithelial lymphocyte (IEL) precursor (IELp), likely through attenuated clonal deletion. In addition, TGFβ is required for the differentiation of IL-17-producing iNKT cells (iNKT17) and IL-17-producing ɣδ lineage T cells.
b, In the bone marrow, the innate lymphoid cell progenitor (ILCp) gives rise to ILC1, ILC2, and ILC3, while the natural killer (NK) progenitor (NKp) gives rise to NK cells. TGFβ promotes differentiation of ILC2 in part via ST2 expression.
c-d, In the tumor, TGFβ inhibits NK cell activation and effector function against mesenchymal phenotype cancer cells (c), while promoting cytotoxic ILC1s as well as killer innate-like T cells (ILTCKs) of both αβ and γδ T cell lineages that co-localize with epithelial cancer cells in part through CD103 interaction with E-cadherin (d). The cancer surveillance functions of NK cells, ILC1s and ILTCKs are additionally regulated by IL-15 and activation receptor signaling pathways.
In addition to TCRαβ ILTCKs, a population of tumor-resident NK1.1+CD103+ TCRγδ ILTCKs expand in epithelial cancers94, and they are also dependent on cancer cell-expressed IL-15 as well as TGF-β signaling for terminal differentiation (B. G. N., E.R.K, unpublished observations). In mice, development of γδ T cells commences in the fetal thymus and occurs in overlapping waves, with each wave expressing specific pairs of TCRγ and TCRδ chains97; yet the TCR usage and thymic origin of γδTCR+ ILTCKs remain to be determined. A subset of γδ T cells that expresses high levels of cytotoxic molecules and innate lymphocyte-associated markers expands in human breast tumours, and its gene expression signature predicts better patient survival98,99. Notably, these cells can express the tissue residency marker CD103, and are functionally skewed towards cytolysis and IFNγ production99. In vitro studies have revealed that TGFβ enhances cell contact-dependent cytotoxicity of γδ T cells through upregulation of CD103100, supporting a positive role of TGFβ in γδTCR+ ILTCK-mediated cancer immunosurveillance (Figure 3).
[H2] Helper innate-like T cells.
iNKT cells are cytokine-producing T cells whose development is supported by TGFβ signalling in part through enhanced survival of iNKT precursors20,21,101 (Figure 3). Three subsets of mature iNKT cells — iNKT1, iNKT2 and iNKT17 cells — are characterized by their expression of IFNγ, IL-4 and IL-17, respectively, and their differentiation initiates in the thymus. Thymic development of iNKT17 cells is dependent on TGFβ signalling102 (Figure 3), and SMAD4 is required for IL-17 production by these cells [Au:OK?] in peripheral tissues102. There is evidence for both pro- and anti-tumour functions of iNKT cells. Mice deficient in CD1d show protection against tumour development [Au:OK?] in multiple cancer models103,104, but administering α-galactosylceramide, which induces iNKT activation and cytokine production, also shows a protective effect in models of cancer [Au:OK?] 105,106. Whether and how TGFβ regulates iNKT cell responses in cancer remains to be determined.
Thymic development of IL-17-producing γδ T cells is dependent on TGFβ1107 (Figure 3), and these cells promote cancer progression in part through recruitment of neutrophils108. In contrast, the development of skin-resident dendritic epidermal T cells (DETCs), another population of γδ T cells, is not affected in TGFβ1-deficient mice109. DETCs produce high levels of IL-13, which act on epithelial cells to suppress cutaneous carcinogenesis110. DETCs also promote antibody class-switching to IgE by directing an IL-4-dependent Th cell response, which protects against skin-tumour formation by inhibiting epithelium damage111. Whether TGFβ regulates DETC-mediated type 2 cancer immunity is open to future investigation.
[H2] TGFβ in B cell responses in cancer.
B cells mediate humoral immune responses by secreting antibodies, and this involves the activation of conventional B2 cells in secondary lymphoid organs. Blockade of TGFβ signalling in B cells causes enhanced activation and proliferation, whereas class switching to IgA is attenuated112. Peyer’s patches are major sites of IgA production and IgA responses are dependent on B cell expression of GARP and follicular dendritic cell (FDC) expression of αvβ8113,114. However, blockade of TGFβ signalling does not affect IgA production in lymph nodes, and delayed inhibition of TGFβ signalling in germinal center (GC) B cells does not impair IgA class switching in Peyer’s patches115. Iterative cycling of GC B cells between the light zone, where they are stimulated by Tfh cells and FDCs, and the dark zone, where they undergo somatic hypermutation, is crucial for antibody affinity maturation. GC B cells show high levels of TGFβ-dependent SMAD2 phosphorylation, and TGFβ signalling in GC B cells promotes their transition from the light zone to the dark zone of the GC as well as antibody affinity maturation115. Thus, TGFβ has pleiotropic functions in control of B cell responses (Figure 2).
In mouse models of breast cancer with high mutational burdens, B cells are crucial for efficacious immune checkpoint blockade (ICB) therapies that promote production of cancer cell-reactive IgG116. Increased numbers of B cells and B cell-associated tertiary lymphoid structures have also been associated with better ICB outcomes in a number of cancer types117–119. Whether and how TGFβ regulates the anti-cancer B cell response will be an interesting topic for future study. In contrast, depletion of B cells enhances CTL-dependent inhibition of prostate tumours in mice treated with oxaliplatin, a platinum-based chemotherapy[Au:OK?]120. Oxaliplatin induces tumour infiltration by a population of IgA+PDL1+IL-10+ plasma cells, and blockade of TGFβ signalling in B cells attenuates their differentiation into plasma cells [Au:OK?], potentiating the oxaliplatin-induced inhibition of tumour growth120. Considering that TGFβ signalling is selectively required for IgA class switching at an early stage of B cell activation in Peyer’s patches115, TGFβ may be playing a similar role in the prostate tumour-associated IgA+ plasma cell response, but where this effect occurs and whether it occurs in other tumour contexts remain unknown.
[H1] TGFβ in innate immunity in cancer
The innate immune system includes lymphoid and myeloid cell lineages that form the first line of defense against immune challenges and regulate adaptive immune responses. Similar to its influence over T and B cells, TGFβ exhibits pleiotropic functions in control of innate immune cell functions in cancer.
[H2] TGFβ in innate lymphocyte responses in cancer.
Innate lymphocytes manifest effector functions that mirror those of T cells and innate-like T cells. Natural killer (NK) cells recirculate and can induce target cell cytotoxicity, while innate lymphoid cells (ILCs) reside in peripheral tissues, produce an array of inflammatory cytokines, and can also trigger lytic granule-mediated cytotoxicity.
[H2] Cytotoxic innate lymphocytes.
Depletion of TGFBR2 from NKp46-expressing innate lymphocytes, which include NK cells, [Au:OK?] inhibits cancer cell metastasis121, while expression of an active form of TGFBR1 in these cells enhances cancer cell metastasis121 and accelerates growth of methylcholanthrene-induced fibrosarcoma122. In addition, deletion of SMAD4 causes enhanced cancer cell metastasis, which is associated with a phenotypical change in NK cells similar to what occurs in cells expressing an active form of TGFBR1123. This suggests that TGFβ suppresses NK cell-mediated surveillance of cancer metastasis via release of SMAD4 transcriptional repressive activity, such as that mediated through SMAD4–SKI–SKIL. However, it remains possible this effect is due to signalling by other TGFβ superfamily members that also use SMAD4. TGFβ inhibits IL-15-induced activation of the metabolic regulator mTORC1 in NK cells, which might account for their reduced proliferation, diminished expression of activation receptors, and impaired cytotoxic activities121. In addition, TGFβ impedes IL-2-induced glycolysis and oxidative phosphorylation in human NK cells, which may limit their anti-tumour activity [Au:OK?] 124. These findings collectively indicate that TGFβ can suppress NK cell-mediated cancer surveillance (Figure 3).
While NK cells recirculate, tumour-resident cytotoxic innate lymphocytes are induced in transgenic models of epithelial cancers, and these cells also suppress tumour development94,125. These cells do not develop from mature NK cells, but differentiate from ILC progenitors125. Maintenance of CD103+ group 1 ILCs (ILC1s) is dependent on cancer cell-expressed IL-15 as well as TGFβ signalling, and ablation of TGFBR2 in ILC1s results in accelerated tumour growth125. Cytotoxic ILC1s are also induced in human epithelial cancers and these cells are associated with better survival rates in patients with chromophobe renal cell carcinoma126. In co-cultures with head and neck cancer cells, the differentiation of CD94+NKp80+CD16− ILC progenitors into cytotoxic ILC1s is dependent on TGFβ signalling127. Thus, similar to what is seen with resident memory-like CTLs and ILTCKs, tissue-resident [Au:OK?] cytotoxic ILC1s require TGFβ signalling for surveillance of epithelial cancers (Figure 3).
[H2] Helper innate lymphocytes.
ILC2s produce inflammatory cytokines in response to ‘alarmins’ such as IL-33. Blockade of TGFβ signalling reduces ILC2 progenitor numbers and attenuates ILC2 development in association with low expression of the IL-33 receptor ST2128 (Figure 3). Administration of IL-33 expands ILC2s and suppresses tumour development in a mouse pancreatic cancer model via recruitment of DCs that activate CD8+ T cells in tumours129. In a mouse melanoma model, ILC2-derived GM-CSF promotes the expansion and effector function of eosinophils to suppress tumour development130. In line with these observations, high levels of ILC2 infiltration track with good clinical prognosis in patients with pancreatic cancer or melanoma129,130. In contrast to these positive effects of TGFβ in promoting anti-tumour ILC2 responses, in the AOM–DSS-induced colorectal cancer model, TGFβ signalling promotes conversion of ILC3s into a population of IL-10-producing regulatory-type ILCs, and co-transfer of these regulatory ILCs with cancer cells results in accelerated tumour growth131.
[H2] TGFβ in mononuclear phagocyte responses in cancer.
DCs, monocytes, and macrophages constitute the mononuclear phagocytes of the immune system. DCs present antigens to T and B cells in secondary lymphoid organs, and are critical regulators of priming-dependent adaptive immunity. Monocytes circulate in blood and differentiate to tissue macrophages which, together with macrophages seeded early during development, maintain tissue homeostasis and control immune responses.
[H2] Dendritic cells.
As sentinels of antigenic challenge, DCs differentiate from the common DC progenitor distinct from monocytes, and include conventional DC subset 1 (cDC1) and cDC2132. Conditional ablation of Tgfbr2 with CD11c-Cre transgenic mice shows no overt phenotypic changes in DCs in secondary lymphoid organs and yet results in lethal autoimmunity133. This discrepancy may be due to poor DC specificity of CD11c-Cre, which targets other leukocyte populations including activated T cells134. Notably, blockade of TGF-β signaling selectively impairs differentiation of intestinal CD103+CD11b+ cDC2s, but not CD103+CD11b− cDC1s, in association with defective pTreg and Th17 cell differentiation134. How this distinct TGF-β DC regulation pathway affects T cell responses in intestinal malignancy has not been explored.
[H2] Monocytes and macrophages.
Tissue macrophages can differentiate from embryonic progenitors, including epidermal Langerhans cells and alveolar macrophages, which both require autocrine TGFβ1 for their maintenance135,136. In a model of cutaneous squamous cell carcinoma, mice with constitutive depletion of Langerhans cells are protected from DMBA-induced tumour development as a consequence of defective conversion of DMBA to DMBA-trans-3,4-diol137. In contrast, inducible depletion of Langerhans cells causes accelerated tumour growth due to failed recruitment of NK1.1+ innate lymphocytes into the epidermis138. While TGFβ plays key roles in development of these cells, whether and how it further influences their function in cancer remains to be explored. [Au: Can you explain what this is telling us about TGFb in the context of cancer here?]
In addition to tissue-resident macrophages, monocytes are frequently recruited to tumours and differentiate into tumour-associated macrophages139. TGFβ can induce chemotaxis of monocytes140 and can influence the monocyte-derived macrophage phenotype, such as in the mammary gland141 and in the intestine142. In the context of cancer, blockade of TGFβ signalling in myeloid cells, including macrophages, results in diminished tumour growth and reduced cancer cell metastasis143–146. Likewise, in a DMBA-induced breast cancer model, inducible expression of a dominant-negative mutant of TGFBR2 in macrophages leads to reduced tumour incidence147. Administration of a STING agonist induces type 1 interferon production and suppresses growth of mammary tumours orthotopically transplanted into the fat pad, but not the growth of mammary tumours that spontaneously arise in transgenic mice[Au:OK?]148. The differential response is due to excessive macrophage TGFβ signalling in the genetic model, which inhibits STING-induced IRF3 phosphorylation and the ensuing type 1 interferon production148. Conversely, activated IRF3 suppresses TGFβ-induced SMAD3 phosphorylation149. These findings reveal cross-inhibitory functions of TGFβ and the IRF3 pathway, which may account for the immunosuppressive function of TGFβ in macrophages in the setting of cancer [Au:OK?].
[H2] TGFβ in granulocyte responses in cancer.
Neutrophils are the most abundant granulocytes in the circulation and are frequently recruited to tumours. They predominantly have pro-tumour functions, driving cancer cell genome instability, cancer cell proliferation and tumour angiogenesis150. In a transgenic model of colon cancer, neutrophil-specific deletion of Tgfbr1 inhibits metastasis in association with reduced numbers of tumour-infiltrating neutrophils151 — this is in line with the chemotactic activities of TGFβ on neutrophils152,153. Thus, TGFβ may induce neutrophil recruitment to promote tumour development.
[H1] Targeting TGFβ for cancer immunotherapy
The pleiotropic roles of TGFβ in the context of cancer make it a prominent, but complex, target for therapy. Although TGFβ can promote cancer cell invasion and dissemination, its tumour suppressor functions can be observed [Au:OK?] in advanced cancers154. Furthermore, loss-of-function mutations affecting TGFβ receptors in cancer cells can lead to a spillover effect, with TGFβ instead acting on non-cancer cell populations in the tumour microenvironment155,156. In addition, while mesenchymal stromal cell TGFβ signalling inhibits T cell infiltration into tumours157–160, blockade of TGFβ signaling in fibroblasts induces forestomach carcinoma as a consequence of excessive inflammation-induced DNA damage161,162. Systemic blockade of TGFβ signalling can also trigger cardiac toxicity163, limiting safety profiles of some drug programmes. Cancer immunotherapies that block inhibitory pathways such as PD-1 can show great efficacy but are often associated with immune-related adverse events (IRAEs)164. Given the role of TGFβ in inhibiting peripheral autoreactive lymphocytes19, such immune-related toxicities remain potential side effects of TGFβ-blocking drugs. Despite these constraints, several TGFβ-targeting programmes are being developed for cancer immunotherapy.
[H2] Systemic blockade of the TGFβ pathway.
Interest in the pro-tumour functions of TGFβ has led to the development of antagonists that intervene with almost all steps of TGFβ signalling (Figure 4). In preclinical studies, these drug programmes show immune system-dependent therapeutic efficacy particularly in settings of combination therapies.
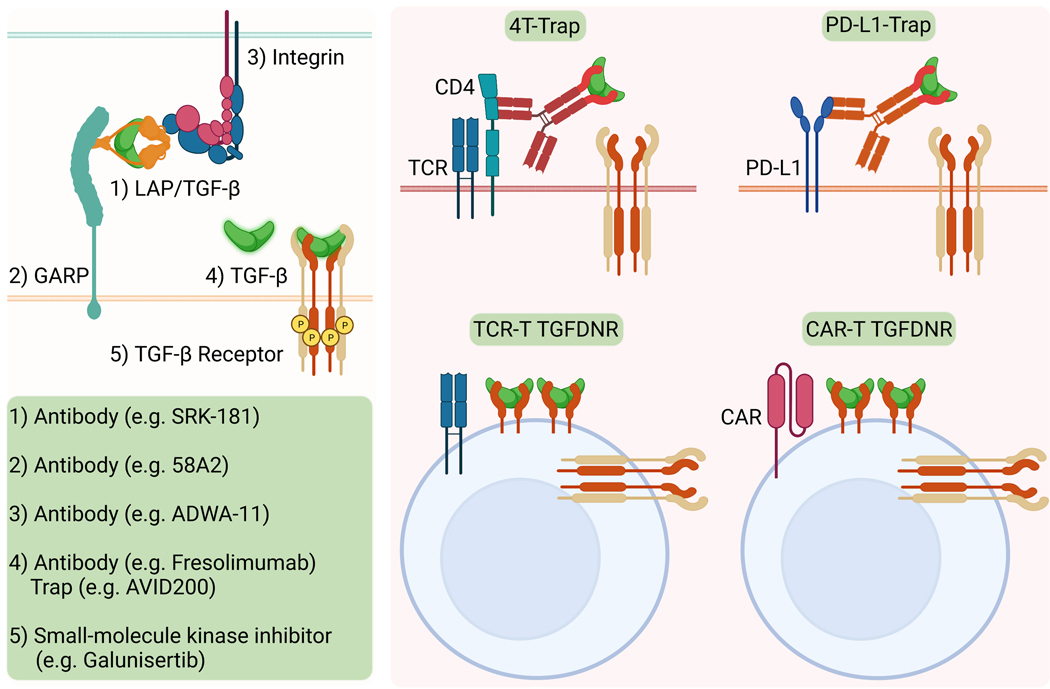
Figure 4. Strategies to target the TGFβ pathway for cancer therapy.
Pharmacological interventions of the TGFβ pathway are grouped into two categories: first, systemic blockade that acts on 1) the latency-associated peptide (LAP) and TGFβ complex, 2) the LAP/TGFβ tethering molecule GARP, 3) the LAP/ TGFβ activating integrin, 4) the active form of TGFβ, or 5) the TGFβ receptor with antibodies and the ectodomain of TGFBR2 (TGFβ Trap)-based biologics as well as small-molecule kinase inhibitors; and second, targeted blockade with bispecific molecules to deliver TGFβ Trap to targeted cell populations such as CD4+ T cells with 4T-Trap or PD-L1-expressing cancer cells with PD-L1-Trap, or overexpressing a dominant-negative mutant of TGFBR2 (TGFDNR) in tumor antigen-specific T cell receptor (TCR) T cells or cancer cell-reactive chimeric antigen receptor (CAR) T cells for cell therapy.
[H2] Blocking antibodies.
SRK-181 is a blocking antibody that selectively binds to latent TGFβ1 and inhibits its activation165. In transplantable tumour models, SRK-181 synergized with anti-PD-1 to suppress tumour development in association with the expansion of CD8+ T cell and Treg cell populations in the tumours165. Of note, unlike 12.7, which is a high-affinity antibody that neutralizes all TGFβ family members, SRK-181 exhibits low cardiovascular toxicities165. Robust CD8+ T cell-dependent anti-tumour immunity has also been reported following treatment with TW7–28G11 and TW7–16B4, which are mouse [Au:OK?] antibodies that bind to the TGFβ1–LAP complex or LAP, respectively, and inhibit TGFβ1 activation166,167. Of note, unlike SRK-181, these antibodies deplete LAP+ T cells, including Treg cells, which likely contributes to their single-agent effects.
Docking of the TGFβ1–LAP complex on the T cell surface is mediated by GARP. 58A2 is an antibody that binds to the GARP–TGFβ1–LAP complex and inhibits TGFβ1 activation71. A 58A2 variant that does not bind Fc receptors synergizes with anti-PD-1 to suppress growth of CT26 tumours in association with enhanced effector functions of tumour-infiltrating CD8+ T cells71. Treg cell-specific deletion of GARP nullifies the anti-tumour effects of 58A2[Au:OK?]71, suggesting that the GARP–TGFβ1–LAP complex expressed on Treg cells is the functional target, although endothelial cells may also be a target in an MC38 tumour model168.
As discussed above, latent TGFβ1 and TGFβ3 complexes are activated by the integrins αvβ6 and αvβ8. ADWA-11 is an αvβ8 blocking antibody, and a non-Fc-binding form of ADWA-11 can synergize with anti-PD-1, anti-CTLA4, an agonist antibody against 4–1BB and radiation therapy to suppress the growth of transplanted tumours[Au: Edit OK?]69. Itgb8 mRNA is enriched in tumour-associated Treg cells, and T cell-specific deletion of Itgb8 recapitulates the tumour suppression phenotype of ADWA-1169. In contrast, experiments using an αvβ8 blocking antibody, C6D4, showed that cancer cell-expressed αvβ8 activates TGFβ1–LAP produced by T cells72,73. Activation of TGFβ can also be mediated by cancer cell-expressed αvβ6 in pancreatic cancers, and its blockade either enhances or suppresses cancer progression in tumour-bearing mice treated with the chemotherapy gemcitabine169,170. It is currently unknown to what extent the opposing outcomes are due to pleiotropic functions of TGFβ on cancer cells and/or stromal cells.
Blocking antibodies against active forms of TGFβ are among the first antagonists tested in preclinical and clinical studies. Administration of a pan-TGFβ blocking [Au:OK?] antibody 1D11 revives T cell-dependent cancer immunity in combination with radiation, anti-CTLA4 or anti-PD-133,171,172. A humanized version of 1D11, fresolimumab, has entered a phase 2 trial for metastatic breast cancers, and prolongs patient median survival when combined with radiation therapy173. Several other pan-TGFβ neutralizing antibodies as well as an antibody that blocks TGFβ1 and TGFβ2, but not TGFβ3, have also shown efficacy in preclinical studies58,174,175. However, systemic blockade of TGFβ can suppress anti-tumour immune responses as well. In a model of head and neck cancer, vaccination-triggered cancer immunity is attenuated by a pan-TGFβ antibody, as it inhibits the generation of tissue-resident CD8+ T cells176.
[H2] TGFβ traps.
AVID200 is a fusion protein of the TGFBR2 ectodomain to human Fc, and selectively neutralizes TGFβ1 and TGFβ3177. A phase 1 dose escalation study showed that AVID200 is well tolerated as a monotherapy, with some cancer patients experiencing stable disease after treatment. The ectodomain of TGFBR2 has also been fused with anti-PD-L1 as a bifunctional anti-PD-L1–TGFβ-Trap molecule, M7824, that better suppresses tumour development than anti-PD-L1 alone in transplantable tumour models178. M7824 (Bintrafusp alfa) shows a good safety profile and clinical efficacy in phase 1 trials179, but does not produce better outcomes as a monotherapy in lung and biliary tract cancers than anti-PD-1 or anti-PDL1. Additional anti-PD-L1 or anti-CTLA4 and TGFBR2 extracellular domain fusion proteins have been generated, and shown better efficacies in preclinical models than anti-PD-L1 or anti-CTLA4 monotherapy180. Anti-CTLA4–TGFβ-Trap effectively depletes Treg cells180, but its safety profile has not been examined. [Au: Edit OK?]
[H2] Small-molecule inhibitors.
Galunisertib (LY2157299) is an orally available TGFBR1 kinase inhibitor, and has shown anti-tumour activity as a single-agent or in combination with anti-PD-L1 in preclinical models159,181,182. An intermittent dosing of galunisertib appeared safe, and prolonged survival in a subset of patients with hepatocellular carcinoma in combination with the tyrosine kinase inhibitor sorafenib in phase 1b/2 clinical trials183–185. In addition, galunisertib increased median survival time of patients with unresectable pancreatic cancer in combination with gemcitabine186, but had limited clinical activity in combination with the anti-PD-L1 antibody durvalumab187. Several other TGFBR1 kinase inhibitors, including vactosertib (TEW-7197), LY3200882 and PF06952229, are also being tested in clinical trials.
[H2] Targeted blockade of the TGFβ pathway.
The pleiotropic functions of TGFβ engender complex outcomes when using a systemic TGFβ blockade approach. Non-selective drug delivery also limits the maximum-tolerated dose of TGFβ antagonists that can be achieved in therapeutically relevant targets. To overcome these limitations, targeted blockade of TGFβ signalling in selected leukocyte populations have emerged as a novel immunotherapy strategy (Figure 4).
[H2] Antibody-based targeting.
Following the finding that TGFβ primarily targets CD4+ T cells to induce tumour immune tolerance32, a bispecific receptor decoy 4T-Trap was developed with fusion of the extracellular domain of TGFBR2 to ibalizumab, a non-immunosuppressive CD4-specific antibody188. 4T-Trap effectively suppresses TGFβ signalling in CD4+ T cells, and triggers vasculature remodelling and starvation-associated cancer cell death, which is further enhanced by VEGF neutralization188. The bi-functional molecule anti-CTLA4–TGFβ-Trap (M7824) and the PD-L1–TGFβ bispecific antibody YM101 can also be classified as targeted TGFβ blockers178,180,189. Notably, anti-CTLA4–TGFβ–Trap exhibits better anti-tumour activity than the combination of anti-CTLA4 and a systemic TGFβ antagonist180. M7824 and YM101 are delivered to PD-L1-expressing cells, including APCs and cancer cells, but this targeted delivery does not appear to exert better cancer immunity compared to combinations of anti-PD-L1 and a nontargeted TGFβ antagonist178,189, and may induce undesirable outcomes by blocking TGFβ signaling in cancer cells.
[H2] Cell engineering-based targeting.
Expression of TGFDNR in tumour-reactive CD4+ or CD8+ T cells effectively suppresses cancer progression in a transplantable melanoma model190. In a transgenic model of prostate cancer, TGFDNR also enhances CD8+ T cell-mediated cancer immunity with varying efficacies74,75. This therapeutic strategy has shown efficacy in a small group of Epstein-Barr virus (EBV)+ Hodgkin lymphoma patients transfused with autologous EBV antigen-specific CD8+ T cells191. In addition to antigen-specific T cells, chimeric antigen receptor (CAR)-based T cell therapy can be enhanced through expression of TGFDNR, deletion of the Tgfbr2 gene, or expression of a secreted form of an anti-PD-1–TGFBR2 extracellular domain fusion protein in preclinical models192–194. A recent phase 1 trial of TGFDNR-expressing CAR T cells targeting the prostate-specific membrane antigen (PSMA) appears feasible and generally safe with one patient experienced a marked clonal CAR cell expansion195. Of note, high and persistent expression of TGFDNR in CD8+ T cells can trigger lymphoproliferative disorders in mouse models196,197, cautioning potential safety liabilities of this approach.
TGFβ signalling can be repurposed to enhance cancer T cell immunity through the expression of a TGFBR2–41BB chimeric molecule to ectopically induce signalling of 41BB, a co-stimulatory T cell receptor198. Targeting TGFβ with an engineered anti-TGFβ single-chain variable fragment-based CAR has also been shown to effectively activate T cells in response to TGFβ199. In addition to T cells, NK cells exhibit enhanced anti-cancer immunity in preclinical models when engineered to express TGFDNR, a SMAD3 shRNA or a chimeric molecule combining TGFBR2 with DAP12 (a transmembrane signalling adapter protein in NK cells)200,201.
[H1] Concluding remarks
As an evolutionarily ancient regulatory cytokine, TGFβ has pleiotropic functions within and beyond the immune system. Its pro- or anti-tumour immune activities depend on its source, dose, context and its leukocyte targets, as well as on the cancer type and disease stage. TGFβ regulates priming-dependent immune responses during lymphocyte activation and differentiation in secondary lymphoid organs and in the tumour itself. Similarly, prewired innate immune cell and innate-like adaptive lymphocyte responses can be influenced by TGFβ, both during their development and in the tumour.
Although TGFβ-mediated inhibition of Th1 and Th2 cell differentiation and induction of Th17 cell differentiation generally fosters tumour progression, TGFβ has been shown to promote both pro-tumour and anti-tumour functions of cytotoxic lymphocyte lineages. TGFβ-mediated suppression of effector memory-like CTL and NK cell responses promotes immune evasion of mesenchymal-type cancer cells, but it bolsters immunosurveillance of epithelial cancers by resident memory-like CTLs as well as by tumour-resident ILC1s and ILTCKs.
A deeper understanding of how TGFβ influences tissue-associated immune responses in homeostasis will further inform the role TGFβ plays when cancer disrupts this balance. Such disruption can lead to pathological TGFβ signaling, impacting both immune and non-immune cells and leading to unexpected outcomes. A spillover effect is observed when cancer cells lose responsiveness to TGFβ through somatic mutations in TGFβ receptors, thus leading to an increase in TGFβ signaling in non-cancer cells and modulation of the tumour microenvironment.
Given the multifaceted physiological roles of TGFβ, both in the immune system and beyond, therapeutic approaches to target this cytokines for cancer therapy need to be stringently vetted for safety, as treatments targeting other immune inhibitory pathways can trigger IRAEs. Potential therapies also need to be rigorously tested for efficacy in tumour models, particularly in autochthonous tumor models that more closely recapitulate human malignancies. The pipeline of TGFβ antagonists has expanded to cover almost all steps of the molecular signalling pathway, and cell-specific targeting of TGFβ function has also been leveraged for targeted immunotherapy. It is hoped that our growing appreciation of the intricacies of TGFβ signalling in various cancers will reinvigorate attempts to target this pathway for cancer treatment in the future.
Acknowledgements
The authors apologize to those whose work they could not cite owing to space constraints. They thank former and current Li laboratory members for discussion. This work was supported by the US National Institutes of Health Memorial Sloan Kettering Cancer Center Core Grant (P30 CA08748). The figures were created with BioRender.com.
Glossary
- Innate immunity
immunity mediated by leukocytes that express germline-encoded receptors that recognize common, broad patterns associated with pathogens and cell stress
- Adaptive immunity
immunity mediated by leukocytes that express an antigen-specific receptor (T cell receptor or B cell receptor) that drives both their development in primary lymphoid organs and their function in the periphery
- Prewired immunity
immune responses involving innate myeloid cells as well as innate lymphocytes and innate-like adaptive lymphocytes that reside in premalignant tissues or traffic to tumours directly after development in primary lymphoid organs
- Priming-dependent immunity
immune responses involving adaptive lymphocytes that are primed in secondary lymphoid organs before homing to tumours
- Autochthonous tumor model
tumour model where tumour initiation is by cells of an endogenous organ in an intact animal via processes such as oncogene expression or carcinogen exposure
Footnotes
Competing Interests
Memorial Sloan Kettering Cancer Center owns a patent on “Methods and Compositions For Targeting TGF-β Signaling in CD4+ Helper T Cells for Cancer Immunotherapy” with M.L. listed as an inventor.
References
- 1.Batlle E. & Massague J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 50, 924–940, doi: 10.1016/j.immuni.2019.03.024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derynck R, Turley SJ & Akhurst RJ TGFbeta biology in cancer progression and immunotherapy. Nat Rev Clin Oncol 18, 9–34, doi: 10.1038/s41571-020-0403-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Bulk J, de Miranda N. & Ten Dijke P. Therapeutic targeting of TGF-beta in cancer: hacking a master switch of immune suppression. Clin Sci (Lond) 135, 35–52, doi: 10.1042/CS20201236 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David CJ & Massague J. Contextual determinants of TGFbeta action in development, immunity and cancer. Nat Rev Mol Cell Biol 19, 419–435, doi: 10.1038/s41580-018-0007-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li MO, Wan YY, Sanjabi S, Robertson AK & Flavell RA Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24, 99–146, doi: 10.1146/annurev.immunol.24.021605.090737 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Sun T. et al. TGFbeta2 and TGFbeta3 isoforms drive fibrotic disease pathogenesis. Sci Transl Med 13, doi: 10.1126/scitranslmed.abe0407 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Shi M. et al. Latent TGF-beta structure and activation. Nature 474, 343–349, doi: 10.1038/nature10152 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z. et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol 176, 787–793, doi: 10.1083/jcb.200611044 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aluwihare P. et al. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 122, 227–232, doi: 10.1242/jcs.035246 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong X. et al. Force interacts with macromolecular structure in activation of TGF-beta. Nature 542, 55–59, doi: 10.1038/nature21035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell MG et al. Cryo-EM Reveals Integrin-Mediated TGF-beta Activation without Release from Latent TGF-beta. Cell 180, 490–501 e416, doi: 10.1016/j.cell.2019.12.030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagano Y. et al. Arkadia induces degradation of SnoN and c-Ski to enhance transforming growth factor-beta signaling. J Biol Chem 282, 20492–20501, doi: 10.1074/jbc.M701294200 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Levy L. et al. Arkadia activates Smad3/Smad4-dependent transcription by triggering signal-induced SnoN degradation. Mol Cell Biol 27, 6068–6083, doi: 10.1128/MCB.00664-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gori I. et al. Mutations in SKI in Shprintzen-Goldberg syndrome lead to attenuated TGF-beta responses through SKI stabilization. Elife 10, doi: 10.7554/eLife.63545 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L. et al. A transforming growth factor beta-induced Smad3/Smad4 complex directly activates protein kinase A. Mol Cell Biol 24, 2169–2180, doi: 10.1128/MCB.24.5.2169-2180.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derynck R. & Budi EH Specificity, versatility, and control of TGF-beta family signaling. Sci Signal 12, doi: 10.1126/scisignal.aav5183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouyang W. et al. TGF-beta cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor alpha expression. Immunity 39, 335–346, doi: 10.1016/j.immuni.2013.07.016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang W, Beckett O, Ma Q. & Li MO Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32, 642–653, doi: 10.1016/j.immuni.2010.04.012 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh SA et al. Foxp3-independent mechanism by which TGF-beta controls peripheral T cell tolerance. Proc Natl Acad Sci U S A 114, E7536–E7544, doi: 10.1073/pnas.1706356114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li MO, Sanjabi S. & Flavell RA Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and - independent mechanisms. Immunity 25, 455–471, doi: 10.1016/j.immuni.2006.07.011 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Marie JC, Liggitt D. & Rudensky AY Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25, 441–454, doi: 10.1016/j.immuni.2006.07.012 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Takimoto T. et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J Immunol 185, 842–855, doi: 10.4049/jimmunol.0904100 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Gu AD, Wang Y, Lin L, Zhang SS & Wan YY Requirements of transcription factor Smad-dependent and -independent TGF-beta signaling to control discrete T-cell functions. Proc Natl Acad Sci U S A 109, 905–910, doi: 10.1073/pnas.1108352109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cattley RT, Lee M, Boggess WC & Hawse WF Transforming growth factor beta (TGF-beta) receptor signaling regulates kinase networks and phosphatidylinositol metabolism during T-cell activation. J Biol Chem 295, 8236–8251, doi: 10.1074/jbc.RA120.012572 (2020). # These studies demonstrate a biochemical mechanism by which TGFβ regulates T cell receptor signaling in the absence or presence of the proinflammatory cytokine IL-6 in CD4+ T cells.
- 25. Prado DS et al. Synergistic and additive interactions between receptor signaling networks drive the regulatory T cell versus T helper 17 cell fate choice. J Biol Chem 297, 101330, doi: 10.1016/j.jbc.2021.101330 (2021). # These studies demonstrate a biochemical mechanism by which TGFβ regulates T cell receptor signaling in the absence or presence of the proinflammatory cytokine IL-6 in CD4+ T cells.
- 26.Gorelik L, Constant S. & Flavell RA Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med 195, 1499–1505, doi: 10.1084/jem.20012076 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorelik L, Fields PE & Flavell RA Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol 165, 4773–4777, doi: 10.4049/jimmunol.165.9.4773 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Heath VL, Murphy EE, Crain C, Tomlinson MG & O’Garra A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol 30, 2639–2649, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 29.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM & Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189, doi: 10.1016/j.immuni.2006.01.001 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Bettelli E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238, doi: 10.1038/nature04753 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Zhang S. et al. Reversing SKI-SMAD4-mediated suppression is essential for TH17 cell differentiation. Nature 551, 105–109, doi: 10.1038/nature24283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu M. et al. TGF-beta suppresses type 2 immunity to cancer. Nature 587, 115–120, doi: 10.1038/s41586-020-2836-1 (2020). #This study reveals helper T cells as a crucial target of TGFβ-induced tumour immune tolerance in a transgenic model of breast cancer, blockade of which results in a Th2 cellmediated tissue-level cancer defense response associated with tumour vasculature remodeling.
- 33. Jiao S. et al. Differences in Tumor Microenvironment Dictate T Helper Lineage Polarization and Response to Immune Checkpoint Therapy. Cell 179, 1177–1190 e1113, doi: 10.1016/j.cell.2019.10.029 (2019). # This study shows that blocking TGFβ synergizes with anti-CTLA4 and anti-PD-1 treatment to suppress bone metastasis in a murine model of castration-resistant prostate cancer, in association with decreased Th17 cell, but enhanced Th1 cell, differentiation.
- 34.Gao S, Hsu TW & Li MO Immunity beyond cancer cells: perspective from tumor tissue. Trends Cancer 7, 1010–1019, doi: 10.1016/j.trecan.2021.06.007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perez LG et al. TGF-beta signaling in Th17 cells promotes IL-22 production and colitis-associated colon cancer. Nat Commun 11, 2608, doi: 10.1038/s41467-020-16363-w (2020). # This study demonstrates that TGFβ signaling sustains Th17 cell differentiation and IL-22 production, which promotes tumour development in a murine model of colitis-associated colon cancer model.
- 36.Rizzo A. et al. Smad7 expression in T cells prevents colitis-associated cancer. Cancer Res 71, 7423–7432, doi: 10.1158/0008-5472.CAN-11-1895 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Rizzo A. et al. Smad7 induces plasticity in tumor-infiltrating Th17 cells and enables TNF-alpha-mediated killing of colorectal cancer cells. Carcinogenesis 35, 1536–1546, doi: 10.1093/carcin/bgu027 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Malmberg KJ et al. IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J Clin Invest 110, 1515–1523, doi: 10.1172/JCI15564 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Syu LJ et al. Transgenic expression of interferon-gamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol 181, 2114–2125, doi: 10.1016/j.ajpath.2012.08.017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeNardo DG et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16, 91–102, doi: 10.1016/j.ccr.2009.06.018 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maier B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262, doi: 10.1038/s41586-020-2134-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui C. et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell 184, 6101–6118 e6113, doi: 10.1016/j.cell.2021.11.007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiToro D. et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 361, doi: 10.1126/science.aao2933 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall HD et al. The transforming growth factor beta signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa. Elife 4, e04851, doi: 10.7554/eLife.04851 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y. et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 9, 632–640, doi: 10.1038/ni.1607 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Zheng Y. et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463, 808–812, doi: 10.1038/nature08750 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlenner SM, Weigmann B, Ruan Q, Chen Y. & von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med 209, 1529–1535, doi: 10.1084/jem.20112646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W. et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198, 1875–1886, doi: 10.1084/jem.20030152 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tone Y. et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 9, 194–202, doi: 10.1038/ni1549 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Hindley JP et al. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res 71, 736–746, doi: 10.1158/0008-5472.CAN-10-1797 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malchow S. et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339, 1219–1224, doi: 10.1126/science.1233913 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmadzadeh M. et al. Tumor-infiltrating human CD4(+) regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity. Sci Immunol 4, doi: 10.1126/sciimmunol.aao4310 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xydia M. et al. Common clonal origin of conventional T cells and induced regulatory T cells in breast cancer patients. Nat Commun 12, 1119, doi: 10.1038/s41467-021-21297-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou L. et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453, 236–240, doi: 10.1038/nature06878 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutcher I. et al. Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity 34, 396–408, doi: 10.1016/j.immuni.2011.03.005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sledzinska A. et al. TGF-beta signalling is required for CD4(+) T cell homeostasis but dispensable for regulatory T cell function. PLoS Biol 11, e1001674, doi: 10.1371/journal.pbio.1001674 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donkor MK, Sarkar A. & Li MO Tgf-beta1 produced by activated CD4(+) T Cells Antagonizes T Cell Surveillance of Tumor Development. Oncoimmunology 1, 162–171, doi: 10.4161/onci.1.2.18481 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dodagatta-Marri E. et al. alpha-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by alpha-TGFbeta antibody to promote durable rejection and immunity in squamous cell carcinomas. J Immunother Cancer 7, 62, doi: 10.1186/s40425-018-0493-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson LD & Jameson SC TGF-beta sensitivity restrains CD8+ T cell homeostatic proliferation by enforcing sensitivity to IL-7 and IL-15. PLoS One 7, e42268, doi: 10.1371/journal.pone.0042268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arumugam V. et al. TCR signaling intensity controls CD8+ T cell responsiveness to TGF-beta. J Leukoc Biol 98, 703–712, doi: 10.1189/jlb.2HIMA1214-578R (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brownlie RJ et al. Resistance to TGFbeta suppression and improved anti-tumor responses in CD8(+) T cells lacking PTPN22. Nat Commun 8, 1343, doi: 10.1038/s41467-017-01427-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stephen TL et al. Transforming growth factor beta-mediated suppression of antitumor T cells requires FoxP1 transcription factor expression. Immunity 41, 427–439, doi: 10.1016/j.immuni.2014.08.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackay LK et al. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 43, 1101–1111, doi: 10.1016/j.immuni.2015.11.008 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Thomas DA & Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380, doi: 10.1016/j.ccr.2005.10.012 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Park BV et al. TGFbeta1-Mediated SMAD3 Enhances PD-1 Expression on Antigen-Specific T Cells in Cancer. Cancer Discov 6, 1366–1381, doi: 10.1158/2159-8290.CD-15-1347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorelik L. & Flavell RA Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 7, 1118–1122, doi: 10.1038/nm1001-1118 (2001). [DOI] [PubMed] [Google Scholar]
- 67. Gunderson AJ et al. TGFbeta suppresses CD8(+) T cell expression of CXCR3 and tumor trafficking. Nat Commun 11, 1749, doi: 10.1038/s41467-020-15404-8 (2020). # This study reveals that blockade of TGFβ signaling in CD8+ T cells synergizes with chemoradiation to suppress tumour growth in a transplantable tumour model, which is associated with increased CXCR3 expression and CD8+ T cell tumour trafficking.
- 68.Luo CT, Liao W, Dadi S, Toure A. & Li MO Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature 529, 532–536, doi: 10.1038/nature16486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dodagatta-Marri E. et al. Integrin alphavbeta8 on T cells suppresses anti-tumor immunity in multiple models and is a promising target for tumor immunotherapy. Cell Rep 36, 109309, doi: 10.1016/j.celrep.2021.109309 (2021). #These studies demonstrate that the Treg cell-expressed αvβ8 integrin promotes TGFβ1 activation and suppresses cancer immunosurveillance in transplantable tumour models, and αvβ8 is potential immuno-oncology target.
- 70. Laine A. et al. Regulatory T cells promote cancer immune-escape through integrin alphavbeta8-mediated TGF-beta activation. Nat Commun 12, 6228, doi: 10.1038/s41467-021-26352-2 (2021). #These studies demonstrate that the Treg cell-expressed αvβ8 integrin promotes TGFβ1 activation and suppresses cancer immunosurveillance in transplantable tumour models, and αvβ8 is potential immuno-oncology target.
- 71. de Streel G. et al. Selective inhibition of TGF-beta1 produced by GARP-expressing Tregs overcomes resistance to PD-1/PD-L1 blockade in cancer. Nat Commun 11, 4545, doi: 10.1038/s41467-020-17811-3 (2020). # This study shows that Treg cell-expressed GARP promotes tumour immune escape, which can be targeted in synergy with anti-PD-1 to inhibit tumour growth in a transplantable tumour model.
- 72.Takasaka N. et al. Integrin alphavbeta8-expressing tumor cells evade host immunity by regulating TGF-beta activation in immune cells. JCI Insight 3, doi: 10.1172/jci.insight.122591 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seed RI et al. A tumor-specific mechanism of Treg enrichment mediated by the integrin alphavbeta8. Sci Immunol 6, doi: 10.1126/sciimmunol.abf0558 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chou CK et al. Cell-intrinsic abrogation of TGF-beta signaling delays but does not prevent dysfunction of self/tumor-specific CD8 T cells in a murine model of autochthonous prostate cancer. J Immunol 189, 3936–3946, doi: 10.4049/jimmunol.1201415 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bendle GM, Linnemann C, Bies L, Song JY & Schumacher TN Blockade of TGF-beta signaling greatly enhances the efficacy of TCR gene therapy of cancer. J Immunol 191, 3232–3239, doi: 10.4049/jimmunol.1301270 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Zhong W. et al. Comparison of the molecular and cellular phenotypes of common mouse syngeneic models with human tumors. BMC Genomics 21, 2, doi: 10.1186/s12864-019-6344-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mani V. et al. Migratory DCs activate TGF-beta to precondition naive CD8(+) T cells for tissue-resident memory fate. Science 366, doi: 10.1126/science.aav5728 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirai T. et al. Competition for Active TGFbeta Cytokine Allows for Selective Retention of Antigen-Specific Tissue- Resident Memory T Cells in the Epidermal Niche. Immunity 54, 84–98 e85, doi: 10.1016/j.immuni.2020.10.022 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ferreira C. et al. Type 1 Treg cells promote the generation of CD8(+) tissue-resident memory T cells. Nat Immunol 21, 766–776, doi: 10.1038/s41590-020-0674-9 (2020). # This study reveals that T-bet-expressing type 1 Treg cells are recruited in close proximity with CD8+ T cells, and can promote differentiation of tissue-resident memory T cells via αvβ8-mediated activation of TGFβ1.
- 80.Hu Y, Lee YT, Kaech SM, Garvy B. & Cauley LS Smad4 promotes differentiation of effector and circulating memory CD8 T cells but is dispensable for tissue-resident memory CD8 T cells. J Immunol 194, 2407–2414, doi: 10.4049/jimmunol.1402369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu B. et al. The SKI proto-oncogene restrains the resident CD103(+)CD8(+) T cell response in viral clearance. Cell Mol Immunol 18, 2410–2421, doi: 10.1038/s41423-020-0495-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Igalouzene R. et al. SMAD4 TGF-beta-independent function preconditions naive CD8+ T cells to prevent severe chronic intestinal inflammation. J Clin Invest 132, doi: 10.1172/JCI151020 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le Floc’h A. et al. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med 204, 559–570, doi: 10.1084/jem.20061524 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoffmann JC & Schon MP Integrin alphaE(CD103)beta7 in Epithelial Cancer. Cancers (Basel) 13, doi: 10.3390/cancers13246211 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Boutet M. et al. TGFbeta Signaling Intersects with CD103 Integrin Signaling to Promote T-Lymphocyte Accumulation and Antitumor Activity in the Lung Tumor Microenvironment. Cancer Res 76, 1757–1769, doi: 10.1158/0008-5472.CAN-15-1545 (2016). # This study shows that TGFβ promotes CD103-dependent CD8+ T cell adhesion, signaling, and anti-tumour functions in a human lung tumour slice culture system, supporting a positive role for TGFβ in epithelial cancer immunity.
- 86.Miller CH et al. Eomes identifies thymic precursors of self-specific memory-phenotype CD8(+) T cells. Nat Immunol 21, 567–577, doi: 10.1038/s41590-020-0653-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim HJ, Verbinnen B, Tang X, Lu L. & Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 467, 328–332, doi: 10.1038/nature09370 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCarron MJ & Marie JC TGF-beta prevents T follicular helper cell accumulation and B cell autoreactivity. J Clin Invest 124, 4375–4386, doi: 10.1172/JCI76179 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mishra S. et al. TGF-beta and Eomes control the homeostasis of CD8+ regulatory T cells. J Exp Med 218, doi: 10.1084/jem.20200030 (2021). # This study demonstrates that TGFβ cooperates with Eomes to promote homeostasis of regulatory CD8+ T cells, deficiency of which results in lethal autoimmunity.
- 90.Chu F. et al. CXCR5(+)CD8(+) T cells are a distinct functional subset with an antitumor activity. Leukemia 33, 2640–2653, doi: 10.1038/s41375-019-0464-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McDonald BD, Jabri B. & Bendelac A. Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat Rev Immunol 18, 514–525, doi: 10.1038/s41577-018-0013-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cohen NR, Garg S. & Brenner MB Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol 102, 1–94, doi: 10.1016/S0065-2776(09)01201-2 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Nielsen MM, Witherden DA & Havran WL gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol 17, 733–745, doi: 10.1038/nri.2017.101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dadi S. et al. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell 164, 365–377, doi: 10.1016/j.cell.2016.01.002 (2016). # These studies demonstrate that induction of tissue-resident phenotype killer innate lymphocytes and innate-like T cells defines a discrete prewired cancer surveillance response, and TCRαβ+ killer innate-like T cells are a distinct lineage of self-reactive T cells.
- 95. Chou C. et al. Programme of self-reactive innate-like T cell-mediated cancer immunity. Nature, doi: 10.1038/s41586-022-04632-1 (2022). # These studies demonstrate that induction of tissue-resident phenotype killer innate lymphocytes and innate-like T cells defines a discrete prewired cancer surveillance response, and TCRαβ+ killer innate-like T cells are a distinct lineage of self-reactive T cells.
- 96.Konkel JE et al. Control of the development of CD8alphaalpha+ intestinal intraepithelial lymphocytes by TGF-beta. Nat Immunol 12, 312–319, doi: 10.1038/ni.1997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vantourout P. & Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol 13, 88–100, doi: 10.1038/nri3384 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Y. et al. An innate-like Vdelta1(+) gammadelta T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci Transl Med 11, doi: 10.1126/scitranslmed.aax9364 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boufea K. et al. Single-cell RNA sequencing of human breast tumour-infiltrating immune cells reveals a gammadelta T-cell subtype associated with good clinical outcome. Life Sci Alliance 4, doi: 10.26508/lsa.202000680 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peters C. et al. TGF-beta enhances the cytotoxic activity of Vdelta2 T cells. Oncoimmunology 8, e1522471, doi: 10.1080/2162402X.2018.1522471 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doisne JM et al. iNKT cell development is orchestrated by different branches of TGF-beta signaling. J Exp Med 206, 1365–1378, doi: 10.1084/jem.20090127 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Havenar-Daughton C, Li S, Benlagha K. & Marie JC Development and function of murine RORgammat+ iNKT cells are under TGF-beta signaling control. Blood 119, 3486–3494, doi: 10.1182/blood-2012-01-401604 (2012). [DOI] [PubMed] [Google Scholar]
- 103.Moodycliffe AM, Nghiem D, Clydesdale G. & Ullrich SE Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol 1, 521–525, doi: 10.1038/82782 (2000). [DOI] [PubMed] [Google Scholar]
- 104.Terabe M. et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med 202, 1627–1633, doi: 10.1084/jem.20051381 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kobayashi E, Motoki K, Uchida T, Fukushima H. & Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res 7, 529–534 (1995). [PubMed] [Google Scholar]
- 106.Toura I. et al. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with alpha-galactosylceramide. J Immunol 163, 2387–2391 (1999). [PubMed] [Google Scholar]
- 107.Do JS et al. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J Immunol 184, 1675–1679, doi: 10.4049/jimmunol.0903539 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coffelt SB et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348, doi: 10.1038/nature14282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Borkowski TA, Letterio JJ, Farr AG & Udey MC A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med 184, 2417–2422, doi: 10.1084/jem.184.6.2417 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dalessandri T, Crawford G, Hayes M, Castro Seoane R. & Strid J. IL-13 from intraepithelial lymphocytes regulates tissue homeostasis and protects against carcinogenesis in the skin. Nat Commun 7, 12080, doi: 10.1038/ncomms12080 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crawford G. et al. Epithelial damage and tissue gammadelta T cells promote a unique tumor-protective IgE response. Nat Immunol 19, 859–870, doi: 10.1038/s41590-018-0161-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cazac BB & Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 13, 443–451, doi: 10.1016/s1074-7613(00)00044-3 (2000). [DOI] [PubMed] [Google Scholar]
- 113.Reboldi A. et al. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 352, aaf4822, doi: 10.1126/science.aaf4822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wallace CH et al. B lymphocytes confer immune tolerance via cell surface GARP-TGF-beta complex. JCI Insight 3, doi: 10.1172/jci.insight.99863 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Albright AR et al. TGFbeta signaling in germinal center B cells promotes the transition from light zone to dark zone. J Exp Med 216, 2531–2545, doi: 10.1084/jem.20181868 (2019). # This study uncovers discrete types of TGFβ signaling that influence B cell biology, with signaling in the Peyer’s patches inducing IgA induction in activated B cells, and signaling in the germinal center required for transition from light zone to dark zone.
- 116.Hollern DP et al. B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer. Cell 179, 1191–1206 e1121, doi: 10.1016/j.cell.2019.10.028 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Helmink BA et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555, doi: 10.1038/s41586-019-1922-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Petitprez F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560, doi: 10.1038/s41586-019-1906-8 (2020). [DOI] [PubMed] [Google Scholar]
- 119.Cabrita R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565, doi: 10.1038/s41586-019-1914-8 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Shalapour S. et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98, doi: 10.1038/nature14395 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Viel S. et al. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci Signal 9, ra19, doi: 10.1126/scisignal.aad1884 (2016). # This study reveals that TGFβ suppresses activation, cytolytic activity, and cancer metastasis surveillance function of NK cells in association with TGFβ control of mTORC1 signaling.
- 122.Gao Y. et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol 18, 1004–1015, doi: 10.1038/ni.3800 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Cortez VS et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat Immunol 18, 995–1003, doi: 10.1038/ni.3809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zaiatz-Bittencourt V, Finlay DK & Gardiner CM Canonical TGF-beta Signaling Pathway Represses Human NK Cell Metabolism. J Immunol 200, 3934–3941, doi: 10.4049/jimmunol.1701461 (2018). [DOI] [PubMed] [Google Scholar]
- 125. Nixon BG et al. Cytotoxic granzyme C-expressing ILC1s contribute to antitumor immunity and neonatal autoimmunity. Sci Immunol 7, eabi8642, doi: 10.1126/sciimmunol.abi8642 (2022). # This study demonstrates that ILC1s can exhibit the lytic granule-mediated cytotoxicity, and TGFβ signaling is required to maintain the anti-tumour function of CD103+ ILC1s in a transgenic model of breast cancer.
- 126.Kansler ER et al. Cytotoxic innate lymphoid cells sense cancer cell-expressed interleukin-15 to suppress human and murine malignancies. Nat Immunol 23, 904–915, doi: 10.1038/s41590-022-01213-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moreno-Nieves UY et al. Landscape of innate lymphoid cells in human head and neck cancer reveals divergent NK cell states in the tumor microenvironment. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2101169118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang L. et al. TGF-beta induces ST2 and programs ILC2 development. Nat Commun 11, 35, doi: 10.1038/s41467-019-13734-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moral JA et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 579, 130–135, doi: 10.1038/s41586-020-2015-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jacquelot N. et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat Immunol 22, 851–864, doi: 10.1038/s41590-021-00943-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang S. et al. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res 30, 610–622, doi: 10.1038/s41422-020-0312-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murphy TL et al. Transcriptional Control of Dendritic Cell Development. Annu Rev Immunol 34, 93–119, doi: 10.1146/annurev-immunol-032713-120204 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramalingam R. et al. Dendritic cell-specific disruption of TGF-beta receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J Immunol 189, 3878–3893, doi: 10.4049/jimmunol.1201029 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bain CC et al. TGFbetaR signalling controls CD103(+)CD11b(+) dendritic cell development in the intestine. Nat Commun 8, 620, doi: 10.1038/s41467-017-00658-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaplan DH et al. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med 204, 2545–2552, doi: 10.1084/jem.20071401 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu X. et al. The Cytokine TGF-beta Promotes the Development and Homeostasis of Alveolar Macrophages. Immunity 47, 903–912 e904, doi: 10.1016/j.immuni.2017.10.007 (2017). [DOI] [PubMed] [Google Scholar]
- 137.Modi BG et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science 335, 104–108, doi: 10.1126/science.1211600 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ortner D. et al. Langerhans cells and NK cells cooperate in the inhibition of chemical skin carcinogenesis. Oncoimmunology 6, e1260215, doi: 10.1080/2162402X.2016.1260215 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Franklin RA et al. The cellular and molecular origin of tumor-associated macrophages. Science 344, 921–925, doi: 10.1126/science.1252510 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wahl SM et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A 84, 5788–5792, doi: 10.1073/pnas.84.16.5788 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun X, Robertson SA & Ingman WV Regulation of epithelial cell turnover and macrophage phenotype by epithelial cell-derived transforming growth factor beta1 in the mammary gland. Cytokine 61, 377–388, doi: 10.1016/j.cyto.2012.12.002 (2013). [DOI] [PubMed] [Google Scholar]
- 142.Rani R, Smulian AG, Greaves DR, Hogan SP & Herbert DR TGF-beta limits IL-33 production and promotes the resolution of colitis through regulation of macrophage function. Eur J Immunol 41, 2000–2009, doi: 10.1002/eji.201041135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ryzhov SV et al. Role of TGF-beta signaling in generation of CD39+CD73+ myeloid cells in tumors. J Immunol 193, 3155–3164, doi: 10.4049/jimmunol.1400578 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Novitskiy SV et al. Deletion of TGF-beta signaling in myeloid cells enhances their anti-tumorigenic properties. J Leukoc Biol 92, 641–651, doi: 10.1189/jlb.1211639 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pang Y. et al. TGF-beta signaling in myeloid cells is required for tumor metastasis. Cancer Discov 3, 936–951, doi: 10.1158/2159-8290.CD-12-0527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meng X. et al. Myeloid-specific TGF-beta signaling in bone promotes basic-FGF and breast cancer bone metastasis. Oncogene 35, 2370–2378, doi: 10.1038/onc.2015.297 (2016). [DOI] [PubMed] [Google Scholar]
- 147.Sun X. et al. Attenuated TGFB signalling in macrophages decreases susceptibility to DMBA-induced mammary cancer in mice. Breast Cancer Res 23, 39, doi: 10.1186/s13058-021-01417-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Guerin MV et al. TGFbeta blocks IFNalpha/beta release and tumor rejection in spontaneous mammary tumors. Nat Commun 10, 4131, doi: 10.1038/s41467-019-11998-w (2019). # This study reveals differences in macrophage responsiveness to STING activation in transplantable versus spontaneous tumour models, showing spontaneous tumours to be higher for TGFβ, which limits the interferon-induced tumour regression.
- 149.Xu P. et al. Innate antiviral host defense attenuates TGF-beta function through IRF3-mediated suppression of Smad signaling. Mol Cell 56, 723–737, doi: 10.1016/j.molcel.2014.11.027 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hedrick CC & Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol, doi: 10.1038/s41577-021-00571-6 (2021). [DOI] [PubMed] [Google Scholar]
- 151.Jackstadt R. et al. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell 36, 319–336 e317, doi: 10.1016/j.ccell.2019.08.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brandes ME, Mai UE, Ohura K. & Wahl SM Type I transforming growth factor-beta receptors on neutrophils mediate chemotaxis to transforming growth factor-beta. J Immunol 147, 1600–1606 (1991). [PubMed] [Google Scholar]
- 153.Fava RA et al. Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J Exp Med 173, 1121–1132, doi: 10.1084/jem.173.5.1121 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang Y. et al. The Outcome of TGFbeta Antagonism in Metastatic Breast Cancer Models In Vivo Reflects a Complex Balance between Tumor-Suppressive and Proprogression Activities of TGFbeta. Clin Cancer Res 26, 643–656, doi: 10.1158/1078-0432.CCR-19-2370 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Novitskiy SV et al. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov 1, 430–441, doi: 10.1158/2159-8290.CD-11-0100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dhainaut M. et al. Spatial CRISPR genomics identifies regulators of the tumor microenvironment. Cell 185, 1223–1239 e1220, doi: 10.1016/j.cell.2022.02.015 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chakravarthy A, Khan L, Bensler NP, Bose P. & De Carvalho DD TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun 9, 4692, doi: 10.1038/s41467-018-06654-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mariathasan S. et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548, doi: 10.1038/nature25501 (2018). # These studies demonstrate that increased TGFβ levels in tumour tissue is associated with T cell exclusion in metastatic cancer patients, and blockade of TGFβ signaling synergizes with anti-PD-L1 to promote anti-tumour T cell immunity in transplantable primary and metastatic tumour models.
- 159. Tauriello DVF et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543, doi: 10.1038/nature25492 (2018). # These studies demonstrate that increased TGFβ levels in tumour tissue is associated with T cell exclusion in metastatic cancer patients, and blockade of TGFβ signaling synergizes with anti-PD-L1 to promote anti-tumour T cell immunity in transplantable primary and metastatic tumour models.
- 160.Grauel AL et al. TGFbeta-blockade uncovers stromal plasticity in tumors by revealing the existence of a subset of interferon-licensed fibroblasts. Nat Commun 11, 6315, doi: 10.1038/s41467-020-19920-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bhowmick NA et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851, doi: 10.1126/science.1090922 (2004). [DOI] [PubMed] [Google Scholar]
- 162.Achyut BR et al. Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-beta signaling. PLoS Genet 9, e1003251, doi: 10.1371/journal.pgen.1003251 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Anderton MJ et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol 39, 916–924, doi: 10.1177/0192623311416259 (2011). [DOI] [PubMed] [Google Scholar]
- 164.Conroy M. & Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun 13, 392, doi: 10.1038/s41467-022-27960-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martin CJ et al. Selective inhibition of TGFbeta1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci Transl Med 12, doi: 10.1126/scitranslmed.aay8456 (2020). [DOI] [PubMed] [Google Scholar]
- 166.Oida T. & Weiner HL TGF-beta induces surface LAP expression on murine CD4 T cells independent of Foxp3 induction. PLoS One 5, e15523, doi: 10.1371/journal.pone.0015523 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gabriely G. et al. Targeting latency-associated peptide promotes antitumor immunity. Sci Immunol 2, doi: 10.1126/sciimmunol.aaj1738 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bertrand C. et al. Combined Blockade of GARP:TGF-beta1 and PD-1 Increases Infiltration of T Cells and Density of Pericyte-Covered GARP(+) Blood Vessels in Mouse MC38 Tumors. Front Immunol 12, 704050, doi: 10.3389/fimmu.2021.704050 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hezel AF et al. TGF-beta and alphavbeta6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res 72, 4840–4845, doi: 10.1158/0008-5472.CAN-12-0634 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Reader CS et al. The integrin alphavbeta6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J Pathol 249, 332–342, doi: 10.1002/path.5320 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vanpouille-Box C. et al. TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res 75, 2232–2242, doi: 10.1158/0008-5472.CAN-14-3511 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rodriguez-Ruiz ME et al. TGFbeta Blockade Enhances Radiotherapy Abscopal Efficacy Effects in Combination with Anti-PD1 and Anti-CD137 Immunostimulatory Monoclonal Antibodies. Mol Cancer Ther 18, 621–631, doi: 10.1158/1535-7163.MCT-18-0558 (2019). [DOI] [PubMed] [Google Scholar]
- 173.Formenti SC et al. Focal Irradiation and Systemic TGFbeta Blockade in Metastatic Breast Cancer. Clin Cancer Res 24, 2493–2504, doi: 10.1158/1078-0432.CCR-17-3322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Greco R. et al. Pan-TGFbeta inhibition by SAR439459 relieves immunosuppression and improves antitumor efficacy of PD-1 blockade. Oncoimmunology 9, 1811605, doi: 10.1080/2162402X.2020.1811605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Terabe M. et al. Blockade of only TGF-beta 1 and 2 is sufficient to enhance the efficacy of vaccine and PD-1 checkpoint blockade immunotherapy. Oncoimmunology 6, e1308616, doi: 10.1080/2162402X.2017.1308616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nizard M. et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat Commun 8, 15221, doi: 10.1038/ncomms15221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lin HY et al. The soluble exoplasmic domain of the type II transforming growth factor (TGF)-beta receptor. A heterogeneously glycosylated protein with high affinity and selectivity for TGF-beta ligands. J Biol Chem 270, 2747–2754, doi: 10.1074/jbc.270.6.2747 (1995). [DOI] [PubMed] [Google Scholar]
- 178. Lan Y. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-beta. Sci Transl Med 10, doi: 10.1126/scitranslmed.aan5488 (2018). # This study reveals that the bifunctional molecule M7824 with fusion of the extracellular domain of TGFBR2 (TGFβ Trap) to a PD-L1 antibody suppresses primary tumour growth and metastasis more effectively than anti-PD-L1 or TGFβ Trap alone in transplantable tumour models.
- 179.Strauss J. et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFbeta, in Advanced Solid Tumors. Clin Cancer Res 24, 1287–1295, doi: 10.1158/1078-0432.CCR-17-2653 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ravi R. et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFbeta enhance the efficacy of cancer immunotherapy. Nat Commun 9, 741, doi: 10.1038/s41467-017-02696-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yingling JM et al. Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-beta receptor type I inhibitor. Oncotarget 9, 6659–6677, doi: 10.18632/oncotarget.23795 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Holmgaard RB et al. Targeting the TGFbeta pathway with galunisertib, a TGFbetaRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer 6, 47, doi: 10.1186/s40425-018-0356-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kelley RK et al. A Phase 2 Study of Galunisertib (TGF-beta1 Receptor Type I Inhibitor) and Sorafenib in Patients With Advanced Hepatocellular Carcinoma. Clin Transl Gastroenterol 10, e00056, doi: 10.14309/ctg.0000000000000056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ikeda M. et al. A phase 1b study of transforming growth factor-beta receptor I inhibitor galunisertib in combination with sorafenib in Japanese patients with unresectable hepatocellular carcinoma. Invest New Drugs 37, 118–126, doi: 10.1007/s10637-018-0636-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Harding JJ et al. Phase 1b study of galunisertib and ramucirumab in patients with advanced hepatocellular carcinoma. Cancer Med 10, 3059–3067, doi: 10.1002/cam4.3880 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Melisi D. et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer 119, 1208–1214, doi: 10.1038/s41416-018-0246-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Melisi D. et al. Safety and activity of the TGFbeta receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Immunother Cancer 9, doi: 10.1136/jitc-2020-002068 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Li S. et al. Cancer immunotherapy via targeted TGF-beta signalling blockade in TH cells. Nature 587, 121–125, doi: 10.1038/s41586-020-2850-3 (2020). # This study shows that a CD4+ T cell-directed TGFβ inhibitor 4T-Trap with fusion of the extracellular domain of TGFBR2 (TGFβ Trap) to a CD4 antibody, but not a nontargeted TGFβ Trap, suppresses tumour growth in a transgenic model of breast cancer.
- 189.Yi M. et al. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-beta and PD-L1. J Hematol Oncol 14, 27, doi: 10.1186/s13045-021-01045-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang L. et al. Inhibition of TGF-beta signaling in genetically engineered tumor antigen-reactive T cells significantly enhances tumor treatment efficacy. Gene Ther 20, 575–580, doi: 10.1038/gt.2012.75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bollard CM et al. Tumor-Specific T-Cells Engineered to Overcome Tumor Immune Evasion Induce Clinical Responses in Patients With Relapsed Hodgkin Lymphoma. J Clin Oncol 36, 1128–1139, doi: 10.1200/JCO.2017.74.3179 (2018). # This study shows that adoptive transfer of Epstein Barr virus antigen-specific T cells overexpressing a dominant-negative mutant of TGFBR2 is safe, and can induce complete responses even in Hodgkin lymphoma patients with resistant disease.
- 192.Kloss CC et al. Dominant-Negative TGF-beta Receptor Enhances PSMA-Targeted Human CAR T Cell Proliferation And Augments Prostate Cancer Eradication. Mol Ther 26, 1855–1866, doi: 10.1016/j.ymthe.2018.05.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tang N. et al. TGF-beta inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight 5, doi: 10.1172/jci.insight.133977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen X. et al. Secretion of bispecific protein of anti-PD-1 fused with TGF-beta trap enhances antitumor efficacy of CAR-T cell therapy. Mol Ther Oncolytics 21, 144–157, doi: 10.1016/j.omto.2021.03.014 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Narayan V. et al. PSMA-targeting TGFbeta-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med, doi: 10.1038/s41591-022-01726-1 (2022). # This study shows that adoptive transfer of a PSMA-targeting chimeric antigen receptor T cells overexpressing a dominant-negative mutant of TGFBR2 is generally safe in metastatic castration-resistant prostate cancer patients, and evidence of tumour regression was observed in one of the 13 patients.
- 196.Lucas PJ et al. Transforming growth factor-beta pathway serves as a primary tumor suppressor in CD8+ T cell tumorigenesis. Cancer Res 64, 6524–6529, doi: 10.1158/00085472.CAN-04-0896 (2004). [DOI] [PubMed] [Google Scholar]
- 197.Ishigame H, Mosaheb MM, Sanjabi S. & Flavell RA Truncated form of TGF-betaRII, but not its absence, induces memory CD8+ T cell expansion and lymphoproliferative disorder in mice. J Immunol 190, 6340–6350, doi: 10.4049/jimmunol.1300397 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Roth TL et al. Pooled Knockin Targeting for Genome Engineering of Cellular Immunotherapies. Cell 181, 728–744 e721, doi: 10.1016/j.cell.2020.03.039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chang ZL et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat Chem Biol 14, 317–324, doi: 10.1038/nchembio.2565 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Burga RA et al. Engineering the TGFbeta Receptor to Enhance the Therapeutic Potential of Natural Killer Cells as an Immunotherapy for Neuroblastoma. Clin Cancer Res 25, 4400–4412, doi: 10.1158/1078-0432.CCR-18-3183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang QM et al. Enhanced Cancer Immunotherapy with Smad3-Silenced NK-92 Cells. Cancer Immunol Res 6, 965–977, doi: 10.1158/2326-6066.CIR-17-0491 (2018). [DOI] [PubMed] [Google Scholar]
- 202.Guerin MV, Finisguerra V, Van den Eynde BJ, Bercovici N. & Trautmann A. Preclinical murine tumor models: a structural and functional perspective. Elife 9, doi: 10.7554/eLife.50740 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]