Abstract
Objectives:
To explore the impact of preexisting comorbidities on immunotherapy response, overall and progression-free survival, and immune-related adverse events (irAEs) of patients with advanced head and neck cancer (HNC) treated with immunotherapy.
Patients and Methods:
93 patients treated with immunotherapy were identified and stratified into comorbidity absent or present (CCI < 1 and CCI ≥1, respectively), and clinical outcomes were compared between these two groups.
Results:
Patients with no comorbidities had longer overall survival (aHR = 2.74, 95%CI: [1.18, 6.40], p = 0.02) and progression-free survival (aHR = 2.07, 95%CI [1.03, 4.16], p = 0.04) and a higher tumor response rate (32% in CCI <1 vs. 14% in CC ≥ 1, p = 0.05). Risk for irAEs was higher in the comorbidity absent group (p = 0.05).
Conclusion:
Comorbidity should be considered as a significant prognostic factor in clinical decision-making for patients with advanced HNC undergoing immunotherapy.
Keywords: Advanced Head and Neck Cancer, Comorbidity, Charlson comorbidity index, Comorbidities, Immunotherapy
Introduction:
Comorbid conditions affect adult patients with cancer at increasing frequencies as populations get older. Data from Medicare beneficiaries in the US indicate that at least 68.4% of patients aged 65+ have two or more chronic conditions.1 Risk factors for head and neck cancer (HNC), such as tobacco and alcohol consumption, place those patients at particularly high risk for concurrent conditions such as chronic obstructive pulmonary disease (COPD). The presence of comorbidities has been reported to be a strong prognostic factor in various malignancies, including HNC, with increased mortality rates reported in patients with high comorbidity burden.2–4
Over the last decade, immunotherapies targeting the pathway of programmed cell death receptor/ligand 1 (PD1/PD-L1) have been found to have clear and sustained effects on survival of patients with recurrent and metastatic HNC.5 Inhibiting the interaction of PD-L1 constitutively expressed on tumor cells and PD1 expressed on activated T cells markedly enhances T cell function, resulting in anti-tumor activity. The promising efficacy of PD1/PD-L1 inhibitors, including pembrolizumab and nivolumab, in clinical trials has prompted their approval for the treatment of recurrent or metastatic HNC by the US Food and Drug Administration, while other immunotherapy agents, including CTLA-4 inhibitors, are being considered for approval.
In clinical trials, strict inclusion and exclusion criteria are often employed to minimize risks of autoimmune toxicity with checkpoint blockades, leading to exclusion or underrepresentation of a variety of patient populations with underlying conditions that may alter not only tumor biology, but also response to immune reprogramming and suppression. However, no studies to our knowledge have investigated the influence of comorbidities on immunotherapy outcomes in patients with HNC.
Although this relationship may be complex, comorbidity was shown to be associated with immune dysregulation, most notably, in recent single-cell sequencing studies examining patients with COVID-19.6 Similarly, if a substantial proportion of deaths among HNC patients treated with immunotherapy is associated with comorbidity, this knowledge can help optimize treatment planning for patients with HNC.
The aim of this study is to explore the association of comorbidities with clinical outcomes in a cohort of patients with advanced HNC undergoing immune checkpoint blockade therapy.
Materials and Methods:
Study Design and Patient Population
This single-center retrospective cohort study was approved by the Institutional Review Board of the Johns Hopkins Hospital. Patients with stage IV HNC who underwent treatment with nivolumab, pembrolizumab, durvalumab, or ipilimumab between July 2015 through January 2020, were included in this analysis. Patients were excluded from the study if immunotherapy treatment was not for a primary tumor of the head and neck, the primary tumor site was unknown, patient death prior to post-treatment imaging, or for being under 18 years of age.
Data Collection and Categorization
The electronic medical records were reviewed for eligible patients. Patient demographics and clinical characteristics were retrieved and included age, sex, race, smoking and alcohol use history, tumor grade, Eastern Cooperative Oncology Group (ECOG) score, primary tumor site, HPV status, BMI, and type of immune checkpoint inhibition received.
Comorbidity Assessment
We reviewed patient charts for preexisting comorbid conditions at the time of initial diagnosis of stage IV HNC. Information on preexisting comorbidity was derived from secondary diagnoses coded according to International Classification of Diseases, 10th revision (ICD-10), the patients’ charts and drug plans.
The Charlson Comorbidity Index (CCI) is one of the most extensively validated scoring systems for assessing comorbidities and predicting prognosis and has previously been validated in patients with HNC.7,8 It is a scaled measure that incorporates 19 different medical categories, each weighted according to its potential to impact on mortality. In head-to-head comparisons against other comorbidity indices in the HNC population, no instrument has been shown to clearly perform better than the CCI.9,10 To determine the association between comorbidity status and clinical outcomes, we stratified our cohort into two groups based on CCI score: < 1 and ≥ 1, denoting absence or presence of preexisting comorbidities, respectively, as used by Pylväläinen et al.11
Clinical Endpoints
Overall survival (OS) was calculated as time between start date of immunotherapy treatment until date of death from any cause. Progression-free survival (PFS) was calculated as time between start date of immunotherapy treatment until the occurrence of disease progression according to RECIST criteria (V1.1). Patients with complete response (CR) or partial response (PR) according to the RECIST criteria were considered to have a clinical response to immunotherapy. Patients with disease progression (PD) or stable disease of fewer than 6 months were considered to have not responded to immunotherapy.
We defined immune-related adverse events (irAEs) as any adverse events previously reported to be associated with the mechanism of action of nivolumab, pembrolizumab, durvalumab, or ipilimumab therapy. We only evaluated irAEs that occurred after receipt of immunotherapy and any event temporally linked to this. Severity of irAEs was graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (V5.0).
Statistical Analysis
Statistical analysis was performed using R statistical software (version 4.3.0).
To analyze the association between covariates and overall survival, we performed Cox proportional hazards regression analysis stratified by comorbidity status and adjusted for age, sex, race, HPV status, primary site, BMI, and smoking status.
Univariate analysis and multivariate cox proportional hazards model were used to analyze demographic and clinical characteristics in relation to OS and PFS. Survival curves were generated using the Kaplan–Meier estimator. Patient characteristics across various CCI distributions in the cohort were analyzed using the Wilcoxon rank sum test for continuous variables and Pearson’s chi-squared test or Fisher’s exact test for qualitative variables. All p values ≤ 0.05 were considered statistically significant.
Results:
Cohort Characteristics
There were 93 patients included in the analysis. In terms of baseline preliminary characteristics, no significant differences were observed between these two groups in terms of age, sex, alcohol consumption, and tumor grade (Table 1). However, the cohort with preexisting comorbidities reported higher rates of 0–10 and >10 pack-year smoking history than the cohort with no preexisting comorbidities (26% and 50% vs. 1% and 26%, respectively; p = 0.01). There were no significant differences observed in performance status, primary site, HPV status, BMI group, or immunotherapy type.
Table 1.
Demographic and Clinical Characteristics by Comorbidity Group
| Characteristic | CCI < 1 No. (%) | CCI ≥ 1 No. (%) | P value |
|---|---|---|---|
|
| |||
| Number | 56 | 37 | |
| Age | |||
| Median (IQR) | 64 (55, 69) | 65 (59, 72) | 0.20 |
| < 50 years | 8 (14%) | 2 (5.4%) | 0.56 |
| 50–59 years | 14 (25%) | 8 (22%) | |
| 60–69 years | 21 (38%) | 14 (38%) | |
| 70–79 years | 10 (18%) | 11 (30%) | |
| ≥ 80 years | 3 (5.4%) | 2 (5.4%) | |
| Sex | |||
| Female | 8 (14%) | 5 (14%) | >0.9 |
| Male | 48 (86%) | 32 (86%) | |
| Race | 0.42 | ||
| White | 38 (68%) | 28 (76%) | |
| Other | 18 (32%) | 9 (24%) | |
| Body Mass Index (N = 92) | 0.22 | ||
| Non-Obese | 49 (88%) | 28 (78%) | |
| Obese | 7 (12%) | 8 (22%) | |
| Smoking Status (N = 92) | 0.01 | ||
| Non-Smoker | 28 (50%) | 8 (22%) | |
| Smoker | 28 (50%) | 29 (78%) | |
| Smoking History (N = 84) | 0.01 | ||
| 0 pack-years | 28 (56%) | 8 (24%) | |
| 0 to 10 pack-years | 9 (1%) | 9 (26%) | |
| Over 10 pack-years | 13 (26%) | 17 (50%) | |
| Active Excess Alcohol Use | 0.11 | ||
| No | 37 (66%) | 31 (84%) | |
| Former | 3 (5.4%) | 2 (5.4%) | |
| Yes | 16 (29%) | 4 (11%) | |
| Primary Tumor Site | >0.9 | ||
| Oropharynx | 33 (59%) | 22 (59%) | |
| Non-oropharynx | 23 (41%) | 15 (41%) | |
| HPV Status (N = 61) | |||
| HPV-negative | 14 (39%) | 13 (52%) | 0.31 |
| HPV-positive | 22 (61%) | 12 (48%) | |
| ECOG Status | |||
| 0 | 1 (1.8%) | 1 (2.7%) | >0.9 |
| 1 | 52 (93%) | 34 (92%) | |
| 2 | 3 (5.4%) | 2 (5.4%) | |
| Final Grade (N = 72) | 0.29 | ||
| Well-Differentiated | 5 (11%) | 3 (11%) | |
| Moderately Differentiated | 8 (18%) | 9 (32%) | |
| Poorly Differentiated | 24 (55%) | 15 (54%) | |
| Undifferentiated | 7 (16%) | 1 (3.6%) | |
| Immunotherapy | 0.75 | ||
| Anti-PD-L1 | 2 (3.6%) | 1 (2.7%) | |
| Anti-PD1 | 51 (91%) | 32 (86%) | |
| Anti-PD1 + Anti-CTLA-4 | 3 (5.4%) | 4 (11%) | |
Note: Wilcoxon rank sum test; Pearson’s chi-square test; Fisher’s exact test. Bold values are statistically significant. Abbreviations: HPV, human papilloma virus; SD, standard deviation.
Survival Outcomes
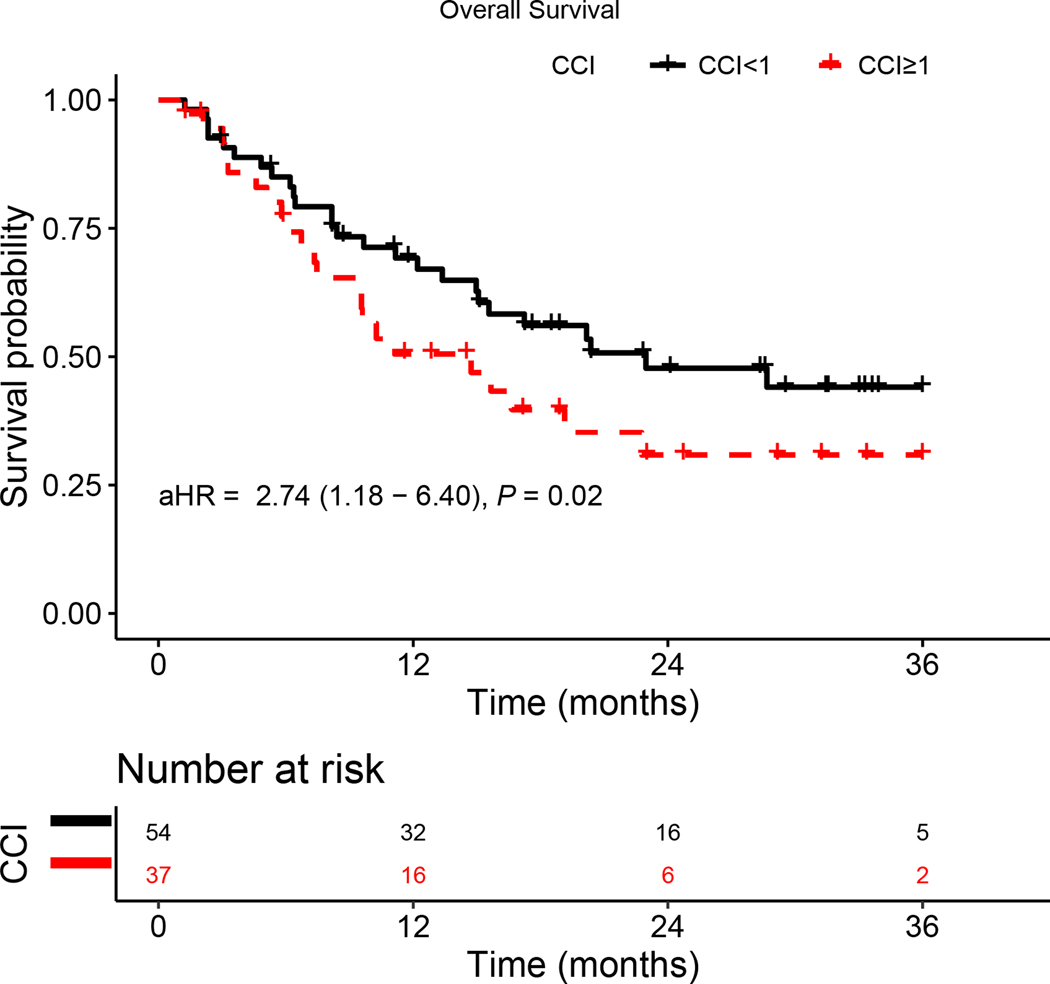
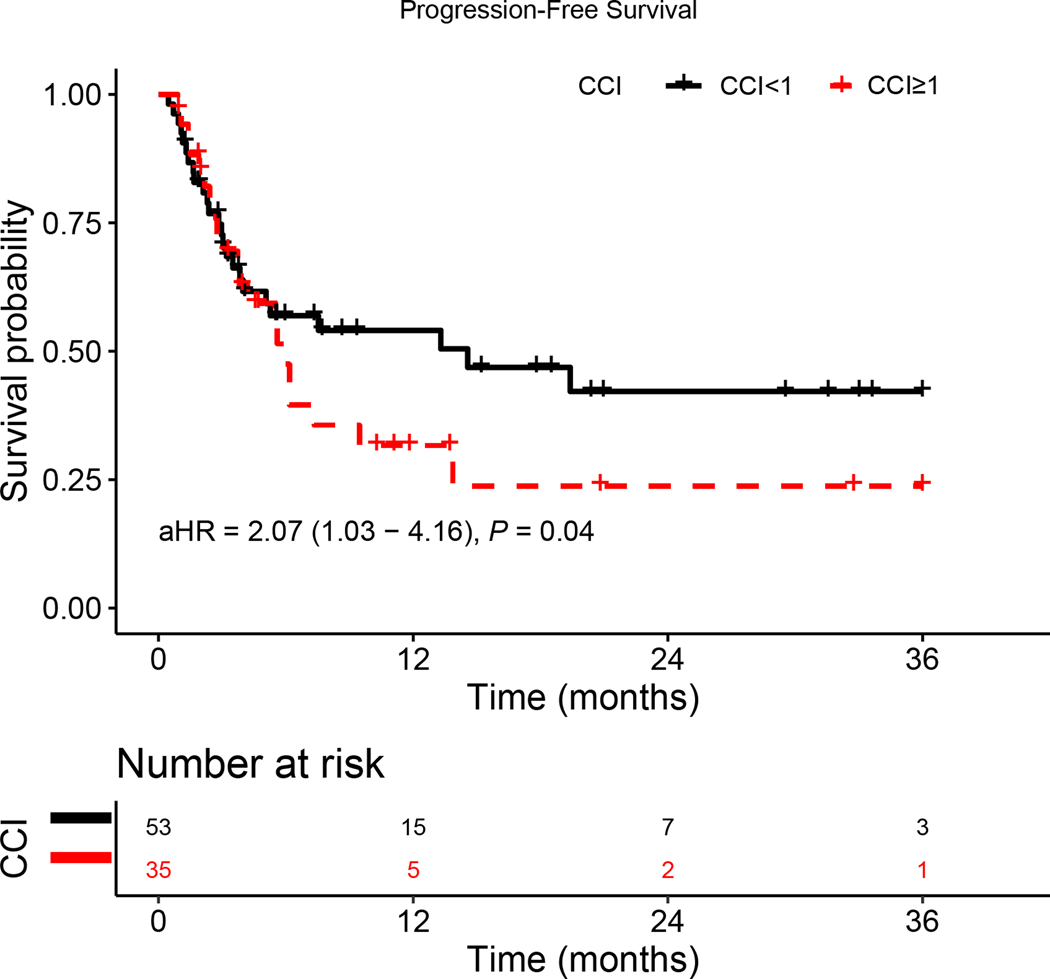
CCI ≥ 1 was associated with increased risk for OS and PFS on multivariate regression analysis (Table 2 and Table 3, respectively). Kaplan-Meier analysis adjusted for age, sex, race, HPV status, primary site, BMI, and smoking status showed presence of comorbidities was significantly associated with both worse OS (aHR = 2.74, 95%CI: [1.18, 6.40]; p = 0.02) and PFS (aHR = 2.07, 95%CI: [1.03, 4.16]; p = 0.04) (Figure 1A and 1B, respectively).
Table 2.
Overall survival analysis by comorbidity group in patients with advanced HNC
| OS HR (95% CI) |
||||
|---|---|---|---|---|
| Variable | Unadjusted | P value | Adjusteda | P value |
| Age | ||||
| < 50 years | [Reference] | [Reference] | ||
| 50–59 years | 0.41 (0.17, 1.03) | 0.06 | 0.28 (0.06, 1.30) | 0.10 |
| 60–69 years | 0.39 (0.17, 0.87) | 0.02 | 0.18 (0.04, 0.76) | 0.02 |
| 70–79 years | 0.33 (0.13, 0.81) | 0.02 | 0.14 (0.03, 0.68) | 0.01 |
| ≥ 80 years | 0.12 (0.01, 0.92) | 0.04 | 0.04 (0.00, 0.50) | 0.01 |
| Sex | ||||
| Male | [Reference] | [Reference] | ||
| Female | 0.81 (0.34, 1.9) | 0.62 | 1.24 (0.31, 5.02) | 0.76 |
| Race | ||||
| White | [Reference] | [Reference] | ||
| Other | 1.02 (0.55, 1.9) | >0.9 | 0.62 (0.24, 1.63) | 0.33 |
| HPV Status | ||||
| HPV-Negative | [Reference] | [Reference] | ||
| HPV-Positive | 1.40 (0.67, 2.92) | 0.38 | 1.03 (0.24, 4.33) | >0.9 |
| Comorbidity | ||||
| CCI < 1 | [Reference] | [Reference] | ||
| CCI ≥ 1 | 1.55 (0.88, 2.74) | 0.13 | 2.74 (1.18, 6.40) | 0.02 |
| Primary Site | ||||
| Non - Oropharynx | [Reference] | [Reference] | ||
| Oropharynx | 1.12 (0.63, 1.97) | 0.70 | 1.47 (0.42, 5.17) | 0.55 |
| BMI Group | ||||
| Non-Obese | [Reference] | [Reference] | ||
| Obese | 0.45 (0.18, 1.13) | 0.09 | 0.31 (0.08, 1.24) | 0.10 |
| Smoking Status | ||||
| Non-Smoker | [Reference] | [Reference] | ||
| Smoker | 1.08 (0.61, 1.93) | 0.79 | 1.57 (0.65, 3.76) | 0.32 |
Note: Bold values are statistically significant.
Adjusted by age, sex, race, HPV status, primary site, BMI, and smoking status.
Table 3.
Progression-free survival analysis by comorbidity group in patients with advanced HNC
| PFS HR (95% CI) |
||||
|---|---|---|---|---|
| Variable | Unadjusted | P value | Adjusteda | P value |
| Age | ||||
| < 50 years | [Reference] | [Reference] | ||
| 50–59 years | 0.54 (0.24, 1.21) | 0.14 | 0.25 (0.06, 1.00) | 0.05 |
| 60–69 years | 0.52 (0.25, 1.09) | 0.08 | 0.21 (0.06, 0.74) | 0.02 |
| 70–79 years | 0.25 (0.11, 0.59) | <0.01 | 0.04 (0.01, 0.21) | <0.01 |
| ≥ 80 years | 0.20 (0.06, 0.66) | 0.01 | 0.04 (0.01, 0.22) | <0.01 |
| Sex | ||||
| Male | [Reference] | [Reference] | ||
| Female | 0.91 (0.46, 1.83) | 0.80 | 0.73 (0.24, 2.23) | 0.58 |
| Race | ||||
| White | [Reference] | [Reference] | ||
| Other | 0.90 (0.55, 1.47) | 0.66 | 0.52 (0.24, 1.13) | 0.10 |
| HPV Status | ||||
| HPV-Negative | [Reference] | [Reference] | ||
| HPV-Positive | 1.03 (0.59, 1.79) | >0.9 | 0.47 (0.17, 1.30) | 0.15 |
| Comorbidity | ||||
| CCI < 1 | [Reference] | [Reference] | ||
| CCI ≥ 1 | 1.3 (0.83, 2.05) | 0.25 | 2.07 (1.03, 4.16) | 0.04 |
| Primary Site | ||||
| Non - Oropharynx | [Reference] | [Reference] | ||
| Oropharynx | 0.94 (0.60, 1.48) | 0.80 | 1.59 (0.64, 3.95) | 0.32 |
| BMI Group | ||||
| Non-Obese | [Reference] | [Reference] | ||
| Obese | 1.13 (0.62, 2.05) | 0.69 | 0.58 (0.17, 1.90) | 0.37 |
| Smoking Status | ||||
| Non-Smoker | [Reference] | [Reference] | ||
| Smoker | 1.08 (0.69, 1.71) | 0.73 | 2.60 (1.27, 5.31) | 0.01 |
Note: Bold values are statistically significant.
Adjusted by age, sex, race, HPV status, primary site, BMI, and smoking status.
Figure 1.
Kaplan–Meier survival curves displaying (a) OS and (b) PFS according to comorbidity group; aHR—adjusted hazard ratio, calculated from a Cox model controlling for age, gender, HPV status, primary site, BMI, and smoking status.
RECIST Tumor Response Rates
Overall response rate, defined as complete response and partial response, was higher in patients with CCI < 1 compared to patients with CCI ≥ 1 (32% vs. 14%, respectively; p = 0.05) (Table 4).
Table 4.
RECIST Tumor Response by Comorbidity Group
| Response | CCI < 1 (N=53) | CCI ≥ 1 (N=36) | P value |
|---|---|---|---|
| Clinical Responders (CR + PR) | 17 (32%) | 5 (14%) | 0.05 |
| Complete Response (CR) | 6 (11%) | 2 (5.6%) | |
| Partial Response (PR) | 11 (21%) | 3 (8.3%) | |
| Stable Disease (SD) | 12 (23%) | 13 (36%) | |
| Progressive Disease (PD) | 24 (45%) | 18 (50%) |
Note: Bold values are statistically significant.
Incidence of Immunotherapy Related Adverse Events (irAEs)
Treatment-related irAEs of any grade were reported in 24 (25.8%) patients based on medical records, with the vast majority of all irAEs reported to be Grade 1–2. The most common adverse event reported was skin rash (29%) followed by colitis (18%) for patients without comorbidity and fatigue (43%) and pneumonitis (43%) in the CCI ≥ 1 category. Risk for irAEs was significantly associated with absence of comorbidities compared to presence of comorbidities (32% vs. 19%, respectively; p = 0.05) (Table 5).
Table 5.
Immune Related Adverse Events by Comorbidity Group
| CCI < 1 (N=17) | CCI ≥ 1 (N=7) | P value | |
|---|---|---|---|
|
| |||
| Immune Related Adverse Event | 0.05 | ||
| Cardiac Tamponade | 1 (5.9%) | 0 (0%) | |
| Colitis | 3 (18%) | 1 (14%) | |
| Fatigue | 0 (0%) | 3 (43%) | |
| Hypothyroidism | 2 (12%) | 0 (0%) | |
| Myocarditis | 1 (5.9%) | 0 (0%) | |
| Nephritis | 1 (5.9%) | 0 (0%) | |
| Pneumonitis | 2 (11%) | 3 (43%) | |
| Rash | 5 (29%) | 0 (0%) | |
| Thrombocytopenia | 2 (12%) | 0 (0%) | |
Note: Bold values are statistically significant.
Discussion:
This retrospective study investigated clinical outcomes of 93 patients who underwent treatment with anti-PD-1 and/or anti-CTLA-4 antibodies for stage IV HNC. Absence of preexisting comorbidities was associated with improved OS and PFS rates as well as response to immunotherapy.
In this study, comorbidity was assessed according to CCI score. CCI, a comprehensive index of multi-morbidities, is an established indicator of a patient’s global status clinically.7,8 Consistent with our findings, CCI has also been shown to be an independent prognosticator for patient outcomes in HNC.12–14 In a Surveillance, Epidemiology, and End Results (SEER) Program analysis of 9386 patients with HNC, Reid et al. found that CCI scores of 1, and 2+ had estimated relative hazards (RHs) of 1.33 (95% CI: 1.21, 1.47) and 1.83 (95% CI: 1.64, 2.05) (p-value for trend < 0.0001).15,16 More recently, in a prospective cohort study of 600 patients with HNC in Canada, Wang et al. demonstrated that after adjustment for age, anatomic subsite, stage, and treatment intent, CCI score was significantly associated with OS on multivariate analysis (p = .01).14 However, this is the largest study to our knowledge that investigates the predictive significance of CCI in patients with stage IV HNC receiving treatment with immunotherapies in particular. In a retrospective analysis of patients with advanced stage recurrent HNC treated with immunotherapy, Konuthula et al. found CCI score to not be predictive of overall survival; however, this analysis was limited by a small sample size (N=44).17 Further research is needed to confirm these findings for other stages of HNC disease.
While the presence of preexisting comorbidities in our study was associated with shorter OS and PFS, these patients were less likely to develop irAEs. Interestingly, in a two-institution cohort study (N = 108) of metastatic head and neck cancer patients, Foster et al. reported that development of irAEs was strongly associated with clinical benefit, including OS, PFS, and immunotherapy response.18 We have similarly observed that the CCI <1 group had greater risk for irAEs and better OS, PFS, and clinical response. Moreover, irAEs were likewise reported to have better treatment outcomes in melanoma and non-small cell lung cancer (NSCLC).19–22 Further evaluation of irAE occurrence as a predictive clinical biomarker for immunotherapy response in HNC is warranted.
Although the mechanism behind the immune alterations resulting from comorbidity burden remains unclear, it is possible that patients with underlying medical conditions may have a weaker immune system and may be more likely to take medications that suppress the immune response, which could potentially decrease the amount of irAEs and lead to worse outcomes. Additionally, smoking, which is a well-established risk factor for many comorbid conditions, was found to be statistically more prevalent in patients with CCI ≥ 1 (p = 0.01) and was associated with worse PFS (p = 0.01). It was previously shown that tumors with genetic smoking signatures have lower immune infiltration as well as worse survival outcomes.23
Surprisingly, increase in age was associated with better OS and PFS. In melanoma, a significant association was observed between age and immunotherapy outcomes, whereas a greater age correlated with a greater survival benefit (p = 0.013).24 Future work is needed to better characterize the impact of age on immunotherapy outcomes in HNC.
A systematic review by Wu et al. of 16 trials with 9795 patients demonstrated that older patients (≥ 65 years) have a more significant overall survival advantage when compared to younger patients.25 Additionally, older patients that were treated with PD-1/L1 inhibitors had a longer OS than younger patients. The review also reported different magnitudes of efficacy based on cancer type (melanoma, non-small-cell lung cancer, renal cell carcinoma, urothelial carcinoma, and gastric tumors), with greater efficacy of immune checkpoint inhibitors in older patients than younger patients. Older patients also had better PFS. The review also suggested that difference in survival were more prominent in PD1/L1 inhibitors compared to CTLA-4 inhibitors. The effect of age difference on survival in melanoma patients was greater than in non-small-cell lung cancer, suggesting variability based on tumor histology. However, the review did not find a significant difference in patients greater than 75 years of age treated with immune checkpoint inhibitors, noting the limitation that those patients accounted for a small part of the cohort and conclusions drawn may not be reliableFuture studies with a larger sample size should confirm these results.
Though the findings of this study are novel and clinically significant, this analysis has limitations that warrant discussion. First, this is a single-institution cohort study, which limits the generalizability of our findings. In addition, causation cannot be established due to the retrospective cohort study design. Data collection in the study was limited by reliance on the accuracy and completion of documentation in the electronic medical records and a small sample size. While the variables in the CCI model are readily available and scores can be easily calculated by physicians, the model was not developed specifically in cancer patients. Although various comorbid ailments are common to all populations, the prevalence, distribution, and relative prognostic impact of each condition to the primary disease process may vary. Future studies should validate our findings using alternative comorbidity indices, such as the Adult Comorbidity Evaluation-27 (ACE-27), American Society of Anesthesiologists’ (ASA) class, and Washington University Head and Neck Comorbidity Index (WUHNCI), in different patient cohorts.10,26 Additionally, when a larger sample size becomes available, future studies should stratify patients by the specific comorbidities reported.
Conclusions:
This investigation is the largest to directly examine the relationship between comorbidity and immunotherapy outcomes in patients with advanced HNC. Our analysis provides new evidence that while comorbidities are associated with worse clinical prognosis of head and neck patients treated with immune checkpoint blockade, absence of comorbidities appears to be associated with greater risk for immune-related adverse events. These advances in the understanding of treatment outcomes are particularly important as attention towards immunotherapy development increases worldwide. Future studies should seek to confirm these findings.
Funding:
This research received no external funding. CM was supported by the NIH training grant 2T32DC000027 during the course of this work.
T.Y.S. reports grants outside of the submitted work from Merck/MSD, Genentech/Roche, BMS, AstraZeneca, Cue Biopharma, Innate, Kura, and Nanobiotix and served in advisory roles for or received honoraria from Innate Pharma, Nanobiotix, Merck/MSD, AstraZeneca, Cue Biopharma, Coherus, Sanofi, Eisei, and Vir Biotechnology.
Footnotes
Ethics approval statement: The current study was conducted according to the Declaration of Helsinki and approved by the approved by the Johns Hopkins Human Research Ethics Committee Institutional Review Board (Protocol Number: IRB00281281).
Conflict of interest disclosure:
The rest of the authors declare no conflicts of interest.
Data availability statement:
The datasets used during the present study are available from the corresponding author upon request.
References:
- 1.Lochner KA. Prevalence of Multiple Chronic Conditions Among Medicare Beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10. doi: 10.5888/pcd10.120137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvo-Espinos C, De Gaona-Lana ER, Gonzalez-Anguren C, Lama-Gay M. Assessment of the impact of comorbidity on the survival of cancer patients treated by palliative care teams. Palliat Support Care. 2015;13(4):1049–1055. doi: 10.1017/S1478951514000832 [DOI] [PubMed] [Google Scholar]
- 3.Bøje CR, Dalton SO, Primdahl H, et al. Evaluation of comorbidity in 9388 head and neck cancer patients: a national cohort study from the DAHANCA database. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2014;110(1):91–97. doi: 10.1016/j.radonc.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 4.Ruud Kjær EK, Jensen JS, Jakobsen KK, et al. The Impact of Comorbidity on Survival in Patients With Head and Neck Squamous Cell Carcinoma: A Nationwide Case-Control Study Spanning 35 Years. Front Oncol. 2021;10. Accessed May 1, 2023. https://www.frontiersin.org/articles/10.3389/fonc.2020.617184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019;99:104460. doi: 10.1016/j.oraloncology.2019.104460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreutmair S, Kauffmann M, Unger S, et al. Preexisting comorbidities shape the immune response associated with severe COVID-19. J Allergy Clin Immunol. 2022;150(2):312–324. doi: 10.1016/j.jaci.2022.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 8.Singh B, Bhaya M, Stern J, et al. Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. The Laryngoscope. 1997;107(11 Pt 1):1469–1475. doi: 10.1097/00005537-199711000-00009 [DOI] [PubMed] [Google Scholar]
- 9.Reid BC, Alberg AJ, Klassen AC, et al. A comparison of three comorbidity indexes in a head and neck cancer population. Oral Oncol. 2002;38(2):187–194. doi: 10.1016/s1368-8375(01)00044-6 [DOI] [PubMed] [Google Scholar]
- 10.Piccirillo JF, Spitznagel EL, Vermani N, Costas I, Schnitzler M. Comparison of comorbidity indices for patients with head and neck cancer. Med Care. 2004;42(5):482–486. doi: 10.1097/01.mlr.0000124254.88292.a1 [DOI] [PubMed] [Google Scholar]
- 11.Pylväläinen J, Talala K, Murtola T, et al. Charlson Comorbidity Index Based On Hospital Episode Statistics Performs Adequately In Predicting Mortality, But Its Discriminative Ability Diminishes Over Time. Clin Epidemiol. 2019;11:923–932. doi: 10.2147/CLEP.S218697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccirillo JF. Importance of comorbidity in head and neck cancer. The Laryngoscope. 2000;110(4):593–602. doi: 10.1097/00005537-200004000-00011 [DOI] [PubMed] [Google Scholar]
- 13.Datema FR, Ferrier MB, van der Schroeff MP, Baatenburg de Jong RJ. Impact of comorbidity on short-term mortality and overall survival of head and neck cancer patients. Head Neck. 2010;32(6):728–736. doi: 10.1002/hed.21245 [DOI] [PubMed] [Google Scholar]
- 14.Wang JR, Habbous S, Espin-Garcia O, et al. Comorbidity and performance status as independent prognostic factors in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38(5):736–742. doi: 10.1002/hed.23947 [DOI] [PubMed] [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03 [DOI] [PubMed] [Google Scholar]
- 16.Reid BC, Alberg AJ, Klassen AC, et al. Comorbidity and survival of elderly head and neck carcinoma patients. Cancer. 2001;92(8):2109–2116. doi: [DOI] [PubMed] [Google Scholar]
- 17.Konuthula N, Do OA, Gobillot T, et al. Oncologic outcomes of salvage surgery and immune checkpoint inhibitor therapy in recurrent head and neck squamous cell carcinoma: A single-institution retrospective study. Head Neck. 2022;44(11):2465–2472. doi: 10.1002/hed.27162 [DOI] [PubMed] [Google Scholar]
- 18.Foster CC, Couey MA, Kochanny SE, et al. Immune-related adverse events are associated with improved response, progression-free survival, and overall survival for patients with head and neck cancer receiving immune checkpoint inhibitors. Cancer. 2021;127(24):4565–4573. doi: 10.1002/cncr.33780 [DOI] [PubMed] [Google Scholar]
- 19.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res. 2016;22(4):886–894. doi: 10.1158/1078-0432.CCR-15-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Socinski MA, Jotte RM, Cappuzzo F, et al. Association of Immune-Related Adverse Events With Efficacy of Atezolizumab in Patients With Non–Small Cell Lung Cancer: Pooled Analyses of the Phase 3 IMpower130, IMpower132, and IMpower150 Randomized Clinical Trials. JAMA Oncol. 2023;9(4):527–535. doi: 10.1001/jamaoncol.2022.7711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haratani K, Hayashi H, Chiba Y, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non–Small-Cell Lung Cancer. JAMA Oncol. 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bastacky ML, Wang H, Fortman D, et al. Immune-Related Adverse Events in PD-1 Treated Melanoma and Impact Upon Anti-Tumor Efficacy: A Real World Analysis. Front Oncol. 2021;11. Accessed April 25, 2023. https://www.frontiersin.org/articles/10.3389/fonc.2021.749064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandal R, Şenbabaoğlu Y, Desrichard A, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1(17). doi: 10.1172/jci.insight.89829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain V, Hwang W, Venigalla S, et al. Association of Age with Efficacy of Immunotherapy in Metastatic Melanoma. The Oncologist. 2020;25(2):e381–e385. doi: 10.1634/theoncologist.2019-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q, Wang Q, Tang X, et al. Correlation between patients’ age and cancer immunotherapy efficacy. Oncoimmunology. 2019;8(4):e1568810. doi: 10.1080/2162402X.2019.1568810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid BC, Alberg AJ, Klassen AC, Koch WM, Samet JM. The American Society of Anesthesiologists’ class as a comorbidity index in a cohort of head and neck cancer surgical patients. Head Neck. 2001;23(11):985–994. doi: 10.1002/hed.1143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon request.