Abstract
To develop the use of electrophoretic lipopolysaccharide profiles for Bradyrhizobium strain identification, we studied the feasibility of using electrophoresis of whole legume nodule homogenates to obtain distinctive lipopolysaccharide profiles. The electrophoretic patterns were the same whether we used nodule extracts, bacteroids, or cultured bacteria as samples, and there was no evidence of changes in the ladder-like pattern during the nodulation process. To assess the reliability of using lipopolysaccharide profiling performed with individual nodules for studying the diversity and microdistribution of the rhizobia nodulating wild shrub legumes, we used a population of Adenocarpus foliolosus seedlings. We obtained 75 different profiles from the 147 nodules studied. There was no dominant profile in the sample, and a plant with different nodules generally produced different profiles. Electrophoresis of legume root nodules proved to be a fast and discriminating technique for determining the diversity of a bradyrhizobial population, although it did not allow the genetic relationships among the nodulating strains to be studied.
The usual way to study natural populations of rhizobia to collect soil samples and then catch the rhizobia by using the appropriate legume host as a trap. After this, the bacteria are isolated from the nodules, and their diversity is evaluated by different methods. This approach is useful for isolating nodulating rhizobial strains present in the soil and for evaluating their competition under controlled conditions. However, all of the information concerning the original short-range distribution of the bacteria in the undisturbed soil is lost. In fact, the distribution and diversity of rhizobia in soil at the microscale level have received little attention (2), and little is known about the distribution of bacteria nodulating perennial legumes in the wild, including the microdistribution of different nodule strains in the soil, the presence of different strains in the same plant, and the frequency of nodule occupation by individual strains.
A strain identification procedure performed directly with individual nodules is of great interest for studies involving large numbers of samples, as is analysis of natural populations. Serology (1) and different types of PCR-based methods, such as enterobacterial repetitive intervening consensus sequence (ERIC)-PCR (15), have been used to identify strains directly from nodule homogenates. Here we describe the use of lipopolysaccharide (LPS) profiling of nodule squashes as a reliable and discriminating technique for identifying the strains in nodules from a natural population. The rationale for using LPS profiles is as follows: (i) it is feasible to use LPS profiling to study the diversity of rhizobia and other gram-negative bacteria (8–10, 18, 19, 25, 27); (ii) LPS molecules are present exclusively in gram-negative bacteria, so contamination by plant components is not possible; and (iii) the number of rhizobia inside the nodules and the sensitivity of the technique allowed us to obtain LPS profiles directly from the nodules without amplification of the bacteria or the molecules themselves. Thus, nodule LPS profiling could be fast, cheap, and not subject to artifacts due to contamination or to a lack of specificity of the amplification procedure.
To determine the reliability of using LPS profiling performed with individual nodules to study the diversity and microdistribution of the rhizobia nodulating wild shrub legumes, we used an undisturbed plot in the pine forest of Tenerife Island in which a perennial legume shrub, Adenocarpus foliolosus, is the dominant plant in the undergrowth.
MATERIALS AND METHODS
Reference strains and culture conditions.
The strains used in this study were Bradyrhizobium japonicum USDA 110 and Bradyrhizobium strains isolated from various Canary Island legume shrubs. Bradyrhizobium (Chamaecytisus) strains BGA-1 and BTA-1 (17) and Bradyrhizobium strains BCO-1, BES-2, BES-3, BES-6, BGA-2, BRT-3, BRT-5, and BTA-2 (25) have been described previously. Cells were maintained at −80°C in yeast extract-mannitol (YM) medium (30) containing 20% glycerol and were grown in YM medium. The samples used for electrophoresis were obtained from cultures grown in YM broth for 5 days at 28°C. The bacteria were harvested by centrifugation, and the sediments were washed with 0.85% NaCl in water. To obtain nodules, plants were axenically grown from germinating seeds (soybean cultivar Williams for B. japonicum and Chamaecytisus proliferus for the Canary Islands strains) in plastic boxes by using Hewitt medium (13) and vermiculite-sand (1:1) as the substrate. The plants were inoculated twice, once at the moment of sowing and then 12 days later. After 3 months (1 month for soybeans) cultured plants were removed, and the nodules were used for electrophoresis.
Preparation of samples, electrophoresis, and silver staining.
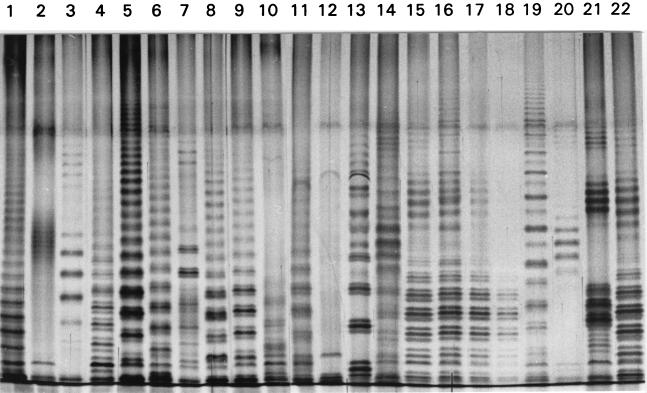
The LPS were purified as described by Westphal and Jann (31). B. japonicum USDA 110 cells from 7-day-old YM medium cultures and purified B. japonicum USDA 110 bacteroids were used as LPS sources. The bacteroids were obtained from soybean nodules by the method of Tao et al. (28). The proteinase K-treated bacterial samples used for electrophoresis were prepared as described by Siverio et al. (27). Electrophoresis was carried out by the method of Laemmli (16) in the presence of 1% sodium dodecyl sulfate (SDS) at 25°C in 0.5-mm-thick 12.5% polyacrylamide slab gels. Silver staining of the LPS was performed by the method of Tsai and Frasch (29). The stained gels were examined with a Pharmacia-LKB Ultroscan XL densitometer, and the software used for gel analysis and comparison was Pharmacia-LKB Analysis GelScan XL software. The comparison of the profiles involved the following three steps: (i) normalization of the profiles to the same length; (ii) grouping of the profiles on the basis of their main characteristics, including ladder-like patterns (see Fig. 3, lanes 1, 3, 4, and 5), groups of stacked bands (see Fig. 3, lane 2), and profiles without any discernible periodicity (see Fig. 3, lane 12); and (iii) direct comparison of profiles of each group by superimposing the normalized densitometric traces. Two profiles were considered identical when 95% of the bands in the profiles coincided.
FIG. 3.
LPS profiles of some A. foliolosus nodules. Lanes 15 to 20 contained samples from six nodules of one seedling. The profiles in lanes 15 to 18 and 22 were ascribed to the same strain; the profiles in all other lanes were different, so they were adscribed to different strains.
Location studied and naturally growing nodule collection.
Nodules were taken from A. foliolosus seedlings growing in a plot (75 by 75 m) in Chipeque (Tenerife, Canary Island; UTM coordinates 28RCS5639; altitude, 1,920 m). This plot is in a pine forest that was planted between 1949 and 1952 and has not been cultivated since. Seedlings were harvested twice; 55 plants were harvested at the end of the growth season in April, and 51 plants were harvested in July during the summer resting period. The shoot length ranged from 3 to 12 cm, and the main root length ranged from 3 to 14 cm. The samples taken in April had swollen pink nodules; the roots were cleaned, and the nodules were processed immediately after harvesting. In contrast, the nodules obtained in July were obtained under drought conditions, and homogenization of these nodules was difficult, so the plants were placed in water for 12 h to allow the nodules to swell before processing.
Nodule LPS electrophoresis.
Nodules were washed, crushed individually in water (15 μl/mm of nodule diameter) by using a 1-ml glass potter (Tissue Grind Micro; Kontes Scientific Glassware, Vineland, N.J.), and suspended (1:1, vol/vol) in 10% dithiothreitol–4% SDS–20% glycerol–0.035% bromphenol blue in 1 M Tris (pH 8.0). The procedure used was the procedure used for the cultured cells. Nodules that had a diameter of about 1 mm produced enough sample for five analyses.
RESULTS AND DISCUSSION
Usefulness of nodule LPS profile analysis for bacterial identification.
Analysis of the LPS profiles of whole-cell homogenates of cultured rhizobia has been used previously as a highly discriminating technique for strain identification (8, 9, 19, 25). However, there are no previously published data on the use of nodule squashes as samples for electrophoresis. Figures 1 to 3 show LPS profiles obtained by performing polyacrylamide gel electrophoresis (PAGE) with nodules, and these profiles prove that it is possible to obtain clear and easily distinguishable LPS profiles directly from nodules.
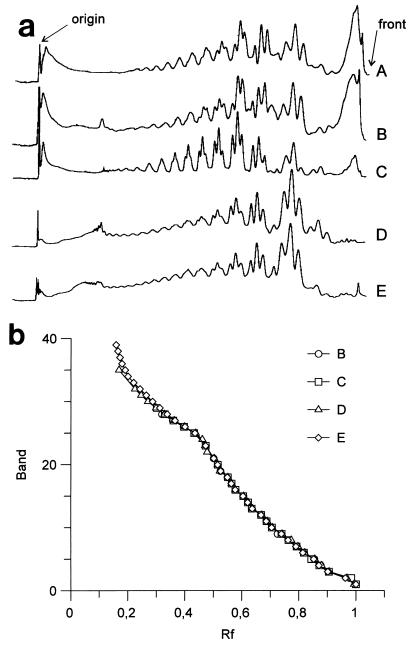
FIG. 1.
Electrophoretic profiles of the LPS from B. japonicum USDA 110. (a) Densitometric traces of the patterns. (b) Relationship between the number of the band and its Rf value. The samples used were a nodule squash lysate (A), a bacteroid lysate (B), a free-living cell lysate (C), purified LPS from free-living bacteria (D), and purified bacteroid LPS (E). The characteristic triplets of many Bradyrhizobium strains are clearly present in the zone between Rf 0.4 and Rf 0.7.
A second question concerns the similarity between the LPS profiles of free-living bacteria and the LPS profiles of nodules. As Fig. 1 shows, the profiles of purified LPS from bacteria and from bacteroids are identical; the same is true for the LPS profiles of whole-cell lysates, bacteroids, or nodule squashes. This suggests that no changes occur during nodulation, although the LPS of bacteria, bacteroids, or nodules produced a band in the front of the lane that is not present in the profiles of purified LPS. Despite this difference, all of the patterns were identical in the zone examined, with bands having Rf values between 0.15 and 0.8 (Fig. 1b). Figure 2 shows the LPS profiles of free-living bacteria and nodules induced in their host plants; samples of the roots, treated in the same way as the nodules, produced a faint streak in the gel without any noticeable band (data not shown). Like the B. japonicum USDA 110 trace, the densitometric traces (data not shown) revealed that there were no differences in the profiles except in the high-mobility zone, where some of the profiles contained a wide streak.
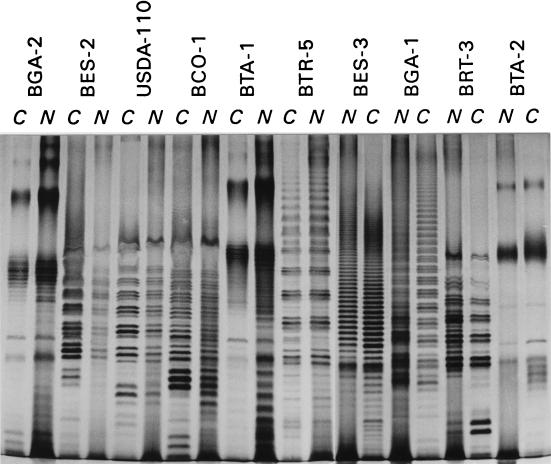
FIG. 2.
Electrophoretic profiles of LPS from free-living and nodule squashes from various Bradyrhizobium (Chamaecytisus) strains isolated from Canary Island legumes and B. japonicum USDA 110. Lanes C, Whole-cell lysate; lanes N, nodule squash lysate.
Our results could disagree with the accepted claim that rhizobial LPS change in the nodules, but this discrepancy is only fictitious. There are previously published data which show that there are differences in the LPS profiles of bacteria and bacteroids; however, in many instances these differences are detectable only when monoclonal antibodies are used, and there is no alteration of the electrophoretic pattern (22, 28). In other cases the differences in the LPS profiles are related to differences in host specificity or to the loss of a symbiotic plasmid (7, 26). Thus, the silver-stained LPS profiles from nodules have the same value as the isolated cell LPS profiles for strain identification purposes.
What is the meaning of an electrophoretic LPS profile?
Both multilocus enzyme electrophoresis and nucleic acid comparison procedures allow simultaneous differentiation between bacterial strains and deduction of genetic relationships between strains. This is not the case for LPS profile comparison, and is the main limitation of the technique. The LPS structure is due to the specificity of the enzymes involved in the synthesis of LPS (11, 32), so different LPS profiles reflect differences in such enzymes. In this sense LPS profile comparison is similar to multilocus enzyme electrophoresis analysis, but the lack of a direct relationship between enzyme specificity and LPS profile precludes any genetic analysis. In addition, the positions of the bands in an LPS profile are not mutually independent, and, as a consequence, the software used to quantify differences between restriction fragment length polymorphism or similar electrophoretic profiles, which assumes that each band is independent of the other bands in the same lane, is not useful for quantitative comparison of LPS profiles. This is due to the mechanism of LPS biosynthesis, which involves the sequential addition of identical subunits to a growing O-antigen chain, and to the fact that LPS are separated by SDS-PAGE on the basis of their sizes (23). This means that the position of band n is related by a monotonous function to the positions of n-1, n-2, and other bands (Fig. 1) (11, 23). However, this does not apply to the LPS profiles without a regular ladder-like pattern, such as the LPS profiles of many Rhizobium strains (25).
Study of a natural population: a real application of the method.
To assess the reliability of the LPS profile technique for studying the nodule occupants in a legume population, we used naturally nodulated A. foliolosus seedlings from a small forest plot. There were no differences in plant size or in the number of nodules per plant between the samples taken in April and the samples taken in July. The seedlings were poorly nodulated; of the 106 seedlings studied, 25 lacked any nodules, 46 had only one nodule, 13 had two nodules, 11 had three nodules, 9 had four nodules, and 2 had six nodules. This could have been due to the fact that these symbiotic couples have an intrinsically low nodulation rate. Three strains isolated from root nodules obtained in Chipeque were tested for nodulating ability under presumably optimal conditions, and the numbers of nodules recovered from 3-month-old plants were as follows: 0 to 9 nodules (mean, 3.6 nodules) for isolate BES-2, 1 to 20 nodules (mean, 9.1 nodules) for isolate BES-3, and 0 to 4 nodules (mean, 1.4 nodules) for isolate BES-6. These figures are in contrast to those obtained for cultured legumes, for which the numbers of nodules both in soil and in synthetic media are much greater. A possible explanation for this is the different life cycles of the legumes studied. Cultured annual legumes are fast growers with short life cycles, whereas in one growing season Adenocarpus or Chamaecytisus seedlings reach only a small size, so a few nodules could be enough to supply their low nitrogen requirements.
We obtained 155 nodules from the population studied; a sample of the LPS profiles obtained from these nodules is shown in Fig. 3. The nodules obtained in the summer under drought conditions generally produced LPS profiles that were less well-resolved than the LPS profiles obtained in the spring, but all but seven nodules could be analyzed. These seven nodules produced only faint streaks with no evidence of LPS; one nodule from a spring sample also failed to produce a clear LPS pattern. These samples were not used in the analysis described below. The remaining 147 nodules produced 75 different LPS profiles. All of the profiles were typical Bradyrhizobium profiles, with a ladder-like pattern and no evidence of the characteristic Rhizobium LPS-I (25). There was not a clearly dominant profile in the population; 43 profiles appeared only once, 16 profiles appeared twice, 7 profiles appeared three times, 2 profiles appeared four times; 1 profile appeared five times, 5 profiles appeared six times, and 1 profile appeared seven times. A plant with multiple nodules was generally nodulated by more than one strain (Table 1), and neighboring nodules on the same plant were generally formed by different strains. When a strain appeared more than once, it was found in different plants.
TABLE 1.
Number of different LPS profiles obtained with A. foliolosus seedlings in which more than one nodule was found
| No. of profiles detected | No. of seedlings with:
|
|||
|---|---|---|---|---|
| 2 nodules | 3 nodules | 4 nodules | 6 nodules | |
| 1 | 3 | 1 | 1 | 0 |
| 2 | 10 | 4 | 3 | 0 |
| 3 | 6 | 5 | 1 | |
| 4 | 0 | 1 | ||
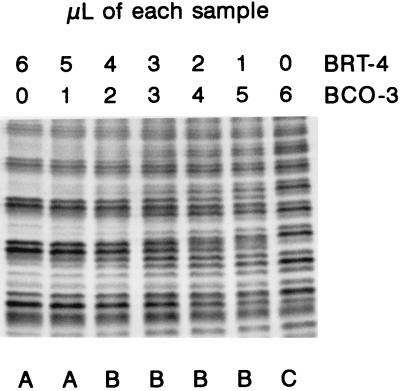
However, a word of caution must be given: the number of LPS profiles can overestimate the actual number of strains. Diversity studies based on whole-nodule lysates performed by using LPS profiling, serology, or ERIC-PCR have the problem that multiple nodule occupation could result in complex patterns that can be wrongly ascribed to new strains. For instance, Labes et al. (15) studied pea nodules by ERIC-PCR and concluded that mixed nodule infections caused by two strains could result in classification of each resulting nodule as a nodule formed by a new strain. In this regard the results of Noel and Brill are noteworthy; in a sample of nine soybean nodules taken at random, seven contained two or more different B. japonicum strains, as defined by protein profiles on PAGE gels (21). Clearly, if this situation is common, any technique that depends on a comparison of band sets is prone to produce an elevated number of false strains. To evaluate the sensitivity of LPS profiling to this problem, we electrophoresed mixtures of two strains in different proportions; the results depended on the differences between the profiles, but in general if the second strain accounted for less than 20% of the population, the profile of the main strain did not change. With 50:50 mixtures, the resulting profiles were clearly different and more complex than the profiles obtained for isolated samples (Fig. 4). If we take this information into account, of the 75 different profiles that we found in our sample, 10 could be produced by the presence in the same nodule of two or more strains; all 10 of these profiles appeared only once in the sample. Thus, we concluded that the number of actually different strains in the 147 nodules studied was between 65 (if we assumed that all complex patterns were due to multiple nodule occupation) to 75 (if we assumed that there was no multiple occupation). LPS profiling of nodules increases our knowledge of the spatial distribution of the nodulating bradyrhizobia in an undisturbed soil. Our sample was composed of a large number of different strains, each one recovered from only one or a few nodules, and in many instances neighboring nodules were formed by different strains. This could be explained by assuming that the population is a mosaic of different strains, each one occupying small areas in the soil; an alternative explanation is that the rhizobial population is distributed more or less evenly in the soil, but each nodule is formed by a random strain from the whole population. Our data did not support either of these alternatives. The diversity that we have found in the population studied was higher than the diversity found in other studies (3, 4, 6, 12, 14, 15, 20). As any individual method detects only a fraction of the total strains in a population (24) and the strains recovered from nodules may be only a fraction of soil population (5), the number of different Adenocarpus-nodulating strains in the Tenerife forest could be enormous.
FIG. 4.
Effect of mixing whole-cell extracts of isolated Bradyrhizobium (Chamaecytisus) strains on LPS profiles. The centers of the electrophoretic gels, in which the differences are clearer, are shown. Note that the isolated strains produced a pattern in which triplets of bands are clearly evident, whereas the mixtures produced more complex patterns containing groups of four to six bands. The profiles in the lanes marked with the same letter were ascribed to the same strain in a blind assay.
ACKNOWLEDGMENTS
This work was supported by a grant from the Canary Islands Government.
We thank E. Meléndez-Hevia for the use of his facilities for the densitometric analysis and for helpful discussions and Carlos Corzo for help with nodule collection.
REFERENCES
- 1.Asanuma S, Thottappilly G, Ayanaba A, Rao V R. Use of the enzyme-linked immunosorbent assay (ELISA) in the detection of Rhizobium both in culture and from root nodules of soybeans and cowpeas. Can J Microbiol. 1985;31:524–528. [Google Scholar]
- 2.Barnet Y M. Ecology of legume root-nodule bacteria. In: Dilworth M J, Glenn A R, editors. Biology and biochemistry of nitrogen fixation. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1991. pp. 199–228. [Google Scholar]
- 3.Bottomley P J, Cheng H, Strain S R. Genetic structure and symbiotic characteristics of a Bradyrhizobium population recovered from a pasture soil. Appl Environ Microbiol. 1994;60:1754–1761. doi: 10.1128/aem.60.6.1754-1761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromfield E S P, Sinha I B, Wolynetz M S. Influence of location, host cultivar, and inoculation on the composition of naturalized populations of Rhizobium meliloti in Medicago sativa nodules. Appl Environ Microbiol. 1986;51:1077–1084. doi: 10.1128/aem.51.5.1077-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bromfield E S P, Barran L R, Wheatcroft R. Relative genetic structure of a population of Rhizobium meliloti isolated directly from soil and from nodules of alfalfa (Medicago sativa) and sweet clover (Melilotus alba) Mol Ecol. 1995;4:183–188. [Google Scholar]
- 6.Brunel B, Rome S, Ziani R, Cleyet-Marel J C. Comparison of nucleotide diversity and symbiotic properties of Rhizobium meliloti populations from annual Medicago species. FEMS Microbiol Ecol. 1996;19:71–82. [Google Scholar]
- 7.Carlson R W, Yadav M. Isolation and partial characterization of the extracellular polysaccharides and lipopolysaccharides from fast-growing Rhizobium japonicum USDA 205 and its Nod− mutant, HC205, which lacks the symbiotic plasmid. Appl Environ Microbiol. 1985;50:1219–1224. doi: 10.1128/aem.50.5.1219-1224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casella S, Rossi N, Toffanin A. Cell surface lipopolysaccharides of different rhizobia. FEMS Microbiol Lett. 1992;93:213–220. [Google Scholar]
- 9.De Maagd R A, van Rossum C, Lugtenberg B J J. Recognition of individual strains of fast-growing rhizobia by using profiles of membrane proteins and lipopolysaccharides. J Bacteriol. 1988;170:3782–3785. doi: 10.1128/jb.170.8.3782-3785.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Weger L A, Jann B, Jann K, Lugtenberg B. Lipopolysaccharides of Pseudomonas spp. that stimulate plant growth: composition and use for strain identification. J Bacteriol. 1987;169:1441–1446. doi: 10.1128/jb.169.4.1441-1446.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman R C, Hunt F. Mechanism of O-antigen distribution in lipopolysaccharide. J Bacteriol. 1990;172:5352–5359. doi: 10.1128/jb.172.9.5352-5359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann A, Amarger N. Genotypic diversity of an indigenous Rhizobium meliloti field population assessed by plasmid profiles, DNA fingerprinting, and insertion sequence typing. Can J Microbiol. 1991;37:600–608. [Google Scholar]
- 13.Hewitt E J. Sand and water culture methods used in the study of plant nutrition. Technical Communication 22. United Kingdom: Farnhan Royal Brahs Commonwealth Agricultural Bureau; 1952. [Google Scholar]
- 14.Kamicker B J, Brill W J. Identification of Bradyrhizobium japonicum nodule isolates from Wisconsin soybean farms. Appl Environ Microbiol. 1986;51:487–492. doi: 10.1128/aem.51.3.487-492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labes G, Ulrich A, Lentzsch P. Influence of bovine slurry deposition on the structure of nodulating Rhizobium leguminosarum bv. viciae soil populations in natural habitat. Appl Environ Microbiol. 1996;62:1717–1722. doi: 10.1128/aem.62.5.1717-1722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.León-Barrios M, Gutiérrez-Navarro A M, Pérez-Galdona R, Corzo J. Characterization of Canary Island isolates of Bradyrhizobium sp. (Chamaecytisus proliferus) Soil Biol Biochem. 1991;23:487–489. [Google Scholar]
- 18.Lindström K, Lipsanen P, Kaijalainen S. Stability of markers used for identification of two Rhizobium galegae inoculant strains after five years in the field. Appl Environ Microbiol. 1990;56:444–450. doi: 10.1128/aem.56.2.444-450.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindström K, Zahran H H. Lipopolysaccharide patterns in SDS-PAGE of rhizobia that nodulate leguminous trees. FEMS Microbiol Lett. 1993;107:327–330. [Google Scholar]
- 20.Madrzak C J, Golinska B, Króliczak J, Pudelko K, Lazewska D, Lampka B, Sadowsky M J. Diversity among field populations of Bradyrhizobium japonicum in Poland. Appl Environ Microbiol. 1995;61:1194–1200. doi: 10.1128/aem.61.4.1194-1200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noel K D, Brill W J. Diversity and dynamics of indigenous Rhizobium japonicum populations. Appl Environ Microbiol. 1980;40:931–938. doi: 10.1128/aem.40.5.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noel K D, Duelli D M, Tao H, Brewin N J. Antigenic change in the lipopolysaccharide of Rhizobium etli CFN42 induced by exudates of Phaseolus vulgaris. Mol Plant Microbe Interact. 1996;9:180–186. [Google Scholar]
- 23.Palva E T, Mäkelä P H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107:137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- 24.Raffetti D, Scotti C, Gnocchi S, Fancelli S, Bazzicalupo M. Genetic diversity of an Italian Rhizobium meliloti population from different Medicago sativa varieties. Appl Environ Microbiol. 1996;62:2279–2285. doi: 10.1128/aem.62.7.2279-2285.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santamaría M, Corzo J, León-Barrios M, Gutiérrez-Navarro A M. Characterisation and differentiation of indigenous rhizobia isolated from Canarian shrub legumes of agricultural and ecological interest. Plant Soil. 1997;190:143–152. [Google Scholar]
- 26.Shindu S S, Brewin N J, Kannenberg E L. Immunochemical analysis of lipopolysaccharides from free-living and endosymbiotic forms of Rhizobium leguminosarum. J Bacteriol. 1990;172:1804–1813. doi: 10.1128/jb.172.4.1804-1813.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siverio F, Cambra M, Gorris M T, Corzo J, López M M. Lipopolysaccharides as determinants of serological variability in Pseudomonas corrugata. Appl Environ Microbiol. 1993;59:1805–1812. doi: 10.1128/aem.59.6.1805-1812.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao H, Brewin N J, Noel K D. Rhizobium leguminosarum CFN42 lipopolysaccharide antigenic changes induced by environmental conditions. J Bacteriol. 1992;174:2222–2229. doi: 10.1128/jb.174.7.2222-2229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 30.Vincent J M. A manual for the practical study of the root nodule bacteria. IBP handbook. Oxford, United Kingdom: Blackwell Scientific Publications, Ltd.; 1970. [Google Scholar]
- 31.Westphal O, Jann K. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 32.Whitfield C. Biosynthesis of lipopolysaccharide O antigens. Trends Microbiol. 1995;3:178–185. doi: 10.1016/s0966-842x(00)88917-9. [DOI] [PubMed] [Google Scholar]