Abstract
Bifidobacterium spp. are non-spore-forming Gram-positive anaerobes that are indigenous to the human gastrointestinal tract and vagina. They are believed to be non-pathogenic organisms for humans and thus are widely used as probiotics. An 83-year-old woman taking cephalexin for 4 days was diagnosed with obstructive pyelonephritis. Y-branched Gram-positive rods were found in both anaerobic and aerobic blood culture bottles, and in an anaerobic urine culture. Bifidobacterium breve was finally identified. Ceftriaxone and metronidazole were administered to the patient, and she was discharged after intermittent catheterization for dysuria. Urinary tract infection caused by Bifidobacterium spp. is believed to be rare, but it can develop in patients with underlying urological conditions. Recognition of the characteristic morphology and conducting anaerobic urine culture may help in identifying more cases of Bifidobacterium urinary tract infections.
Keywords: Bifidobacterium breve, urinary tract infection, Bifidobacterium, pyelonephritis
Data Summary
No new data were generated in this case report.
Introduction
Bifidobacterium spp. are non-spore-forming Gram-positive anaerobes that are indigenous to the human gastrointestinal tract and vagina. There are over 50 Bifidobacterium species; however, only 11 have been isolated from the human gut and oral cavity [1].
Bifidobacterium spp. are functionally very important for intestinal health, and are generally considered to be non-pathogenic [2]. They are widely used as a probiotic and are expected to prevent infections and allergies. However, various infections caused by Bifidobacterium spp., (e.g. prosthetic joint infection, pulmonary infection, necrotizing fasciitis and bacteraemia) have been reported [3–6]. Dental caries caused by Bifidobacterium dentium is the most common clinical disease involving Bifidobacterium spp. [7]. Other species, Bifidobacterium adolescentis, Bifidobacterium breve and Bifidobacterium longum, are occasionally isolated from various infections, primarily in immunocompromised individuals [1, 8–11]. However, urinary tract infections (UTIs) caused by these species have been rarely reported.
Case presentation
An 83-year-old woman with diabetes mellitus and dementia visited a local clinic complaining of fever. Although the details were unknown, she was prescribed cephalexin.
However, she gradually developed appetite loss and difficulty in ambulation. Four days later, she had persistent fever and appetite loss and could not move. Thus, her family requested an ambulance, and she was transported to our hospital.
The patient’s vital signs were as follows: blood pressure, 156/110 mmHg; heart rate, 140 min−1 (sinus tachycardia); respiratory rate, 22 min−1 (slight tachypnea); body temperature, 36.4 °C (normal); and Glasgow coma scale score, E4V4M6 (best eye and motor response, but confused verbal response). Neither abdominal tenderness nor costovertebral tenderness was noted in the patient. Other physical examinations were unremarkable. The laboratory findings were as follows: white blood cell count, 6700 µl-1 (normal range, neutrophils, 78.6%); creatinine, 6.5 mg dl−1 (high); and C-reactive protein, 19.0 mg dl−1 (high). The urinalysis results were as follows: pH, 5.0 (low); glucose, 3+; protein 1+; red blood cell count, 11.5/high power field (HPF, high); white blood count, 500/HPF (high); and nitrate, negative (normal). Abdominal CT revealed diffuse bladder wall thickening, bilateral dilation of the renal ureters, hydronephrotic kidneys and perinephric fat stranding; however, there were neither ureteral nor kidney stones.
The patient was diagnosed with obstructive pyelonephritis and admitted to the hospital after foley catheter insertion. Therefore, ceftriaxone 1 g qd was administered intravenously to the patient after obtaining samples for a urinary culture and two sets of blood cultures. The following day, a urologist was consulted. Based on imaging findings and bacteriuria, the urologist determined that she had chronic cystitis. The cause of the obstructive pyelonephritis was unclear; however, invasive procedures were not indicated, considering the patient’s general condition.
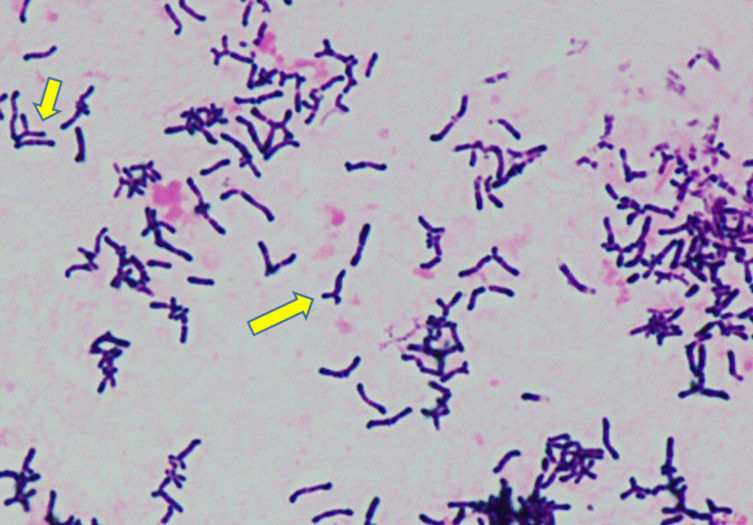
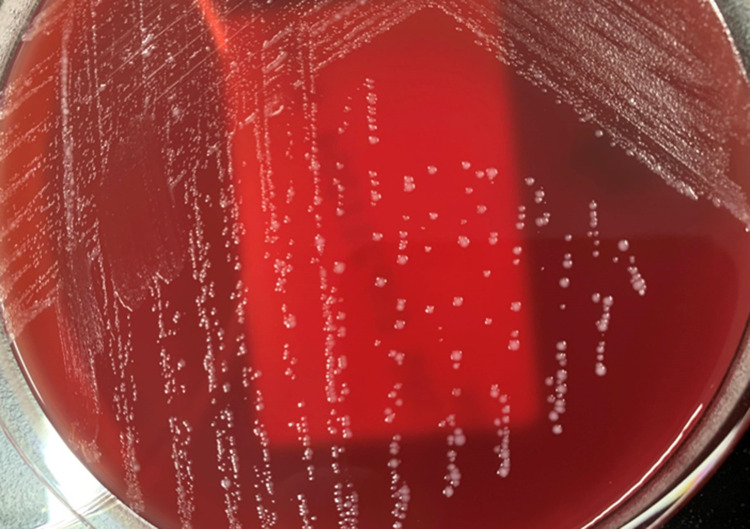
Two sets of anaerobic blood culture bottles (BD BACTEC 22F anaerobic medium; Becton Dickinson and Company, Sparks, NV, USA) and one set of aerobic culture bottles (BD BACTEC 23F aerobic medium) gave positive results after 33, 47 and 132 h, respectively. Gram staining from the blood culture bottles revealed Gram-positive rods (GPRs) with Y-branched forms (Fig. 1). The GPRs were incubated at 35 °C in an anaerobic atmosphere on an ABHK agar plate (Nissui Pharmaceuticals, Tokyo, Japan) and in an aerobic atmosphere supplemented with 5 % CO2 using BBL trypticase soy agar (TSA) with 5 % sheep blood and chocolate II agar LDIP (Becton Dickinson and Company, Sparks, NV, USA). Grey and smooth colonies were observed on the ABHK agar plates after 48 h (Fig. 2). Colonies smaller than those on ABHK agar were found on BBL TSA agar after a 48 h incubation period. All bacteria developed on ABHK agar plates and TSA with 5 % sheep blood and chocolate II agar were identified by two matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) methods. They were identified as B. breve using MBT Compass v. 4.1 and MBT Compass Library v. 9.0.0.0.(8468 MSPs) (Bruker Daltonics, Bremen, Germany; score value 2.11) with a microflex LT/ST system, and as Bifidobacterium spp. using VITEK MS software 4.3.0 and VITEK MS Knowledge Base version 3.0 (bioMérieux, Marcy-l’´Etoile, France). The 16s RNA sequences of the amplified products obtained from the organism matched 100 %.
Fig. 1.
Gram-positive rods at 1,000 x magnification from a blood culture bottle sample (Arrow: Y-branched form).
Fig. 2.
Grey and smooth colonies on the ABHK agar plates after 48 h incubation. na, not applicable; F, female; M, male; nd, no data; MALDI-TOF-MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; UTIs, urinary tract infections; BBG-01, Bifidobacterium breve strain Yakult; CTRX, ceftriaxione; ABPC/SBT, ampicillin–sulbactam; CLDM, clindamycin; PCG, penicillin G; CPFX, ciplofloxacin; CTX, cefotaxime; CMZ, cefmetazole; CEZ, cefazolin; VCM, vancomycin; MEPM, meropenem; LZD, linezolid; PIPC/TAZ, piperacillin–tazobactam; MNZ, metronidazole. *Positive bottles are not described in the reference.
Susceptibility testing was performed using a dry plate EIKEN (Eiken Chemical Co., Ltd, Tokyo, Japan) assay using the microbroth dilution method. Minimum inhibitory concentrations (MICs) were as follows: penicillin G, 0.25 µg ml−1; ceftriaxone, 2 µg ml−1; cefmetazole, >16 µg ml−1; clindamycin, ≦0.25 µg ml−1; vancomycin, ≦1 µg ml−1; and metronidazole, ≦8 µg ml−1.
Gram staining of the urine samples showed only numerous Y-branched GPRs. Antimicrobial-susceptible Klebsiella pneumoniae and Escherichia coli (MIC: cefazolin ≦1 µg ml−1, ceftriaxone ≦0.5 µg ml−1, levofloxacin ≦0.12 µg ml−1), and Enterococcus faecalis (no antimicrobial susceptibility results) grew on BD BBL Prepared Plated: CHROMagar Orientation Media under aerobic conditions, whereas B. breve and Murdociella sp. (no antimicrobial susceptibility results) grew on the ABHK agar plates under anaerobic conditions.
Following catheter drainage and administration of ceftriaxone, the patient soon became afebrile. The clinical course of this case proceeded well. After the organism was identified as B. breve , oral metronidazole 500 mg TID was added to the treatment. Ceftriaxone and metronidazole were administered for 11 and 4 days, respectively. An interview with the patient revealed no history of probiotic or dairy product use. On day 29, the patient was discharged after intermittent catheterization for dysuria.
Two days after discharge from the hospital, the patient was readmitted because of a recurrence of obstructive pyelonephritis, and only extended-spectrum β-lactamase-producing K. pneumoniae (MIC: ceftriaxone >1 µg ml−1, meropenem≦0.25 µg ml−1, levofloxacin 1 µg ml−1) were isolated from blood and urine cultures. During the hospital stay, she developed cerebral embolism and died approximately 7 weeks after the B. breve bacteraemia episode.
Discussion
The clinical significance and incidence of infections caused by Bifidobacterium spp. are unclear. In Norway, 0–2 bacteraemia cases due to Bifidobacterium spp. were reported annually between 2007 and 2012 [6]. Brook et.al. reported that 57 Bifidobacterium spp. were identified in 2033 samples from paediatric patients. Among these, Bifidobacterium spp. could not be identified in 342 blood cultures [8]. Mahlen et al. reported three adult cases of B. breve bacteraemia during 2000–2007 in two US hospitals [10]. Boume et al. reported that 10 Bifidobacterium spp. were detected in 91 493 blood cultures from 1972 to 1977 [12]. Probiotics play a major role in Bifidobacterium bloodstream infections in children [13, 14]. However, it is unclear whether invasive Bifidobacterium infections in adults are associated with probiotics [15].
In total, 14 cases of bloodstream infections with B. breve have been reported in the literature (Table 1) [3, 6, 10, 13, 16, 17]. Although Bifidobacterium spp. are classified as anaerobes, they can grow in aerobic atmospheres supplemented with 5 % CO2. Andriantsoanirina et al. reported that many Bifidobacterium spp. are resistant to oxygen; in fact, 77.8 % of the evaluated B. breve strains were resistant to oxygen [18]. Here, the time to positivity from the blood culture was longer for the aerobic bottles than that for the anaerobic bottles. Moreover, B. breve bacteraemia might be underestimated in children taking probiotics because usually only samples for aerobic cultures are collected from children.
Table 1.
Reviews of bacteremia caused by Bifidobacterium breve
|
No |
Reference |
Age/ Gender |
Blood culture system |
Positive bottle |
Polymicrobial bacteremia |
Time to positivity (hours) |
Identification |
Probiotics |
Underlying disease |
Antibiotics |
Duration of antibiotics (days) |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 |
3 |
42 /M |
nd |
ND/ anaerobic bottle |
– |
nd |
16S rRNA gene, MALDI-TOF-MS |
nd |
Necrotizing fasciitisObesity, dyslipidaemia, type 2diabetes mellitus |
CTRX, ABPC/ SBT+CLDM |
32 |
Recovered |
|
2 |
6 |
49 /F |
nd |
nd |
– |
nd |
MALDI-TOF-MS |
nd |
Recurrent wound infection |
PCG, CPFX, CLDM |
nd |
Recovered |
|
3 |
6 |
84 /F |
nd |
nd |
Bacteroides spp., Candida glabrata |
nd |
nd |
Pyelonephritis, hydronephrosis caused by a kidney stone |
CTX |
nd |
Recovered |
|
|
4 |
10 |
ND/ND |
BacT/ Alert 3DTM |
BacT/ Alert FN or SA* |
nd |
nd |
16S rRNA gene |
nd |
History of decubitis ulcers, frequent admissions for recurrent UTIs |
nd |
nd |
nd |
|
5 |
10 |
ND/ND |
– |
nd |
nd |
Peritonitis |
nd |
nd |
nd |
|||
|
6 |
10 |
ND/ND |
Bacteroides vulgatus Fusobacterium spp. |
nd |
nd |
Stage B prostate cancer, ileal resection; thought to be transient bacteremia |
nd |
nd |
nd |
|||
|
7 |
13 |
0 /M |
BACTEC 9050 TM |
94F paediatric bottle |
– |
170 |
polymorphic DNA analysis |
BBG-01 |
Cloacal exstrophy, postoperative adhesive ileus |
CMZ, CEZ, VCM |
15 |
Recovered |
|
8 |
13 |
0 /F |
– |
138 |
Esophageal atresia, postoperative gastro-esophageal reflux, aspiration pneumonia |
CEZ, VCM |
14 |
Recovered |
||||
|
9 |
13 |
0 /M |
– |
128 |
Necrotizing enterocolitis, gastrointestinal perforation |
ABPC/ SBT, MEPM, VCM, LZD |
14 |
Recovered |
||||
|
10 |
13 |
0 /M |
– |
114 |
Necrotizing enterocolitis, gastrointestinal perforation, congenital heart diseases |
PIPC/ TAZ, VCM |
20 |
Recovered |
||||
|
11 |
13 |
0 /M |
– |
125 |
Food protein-induced enterocolitis syndrome |
ABPC/ SBT, VCM |
14 |
Recovered |
||||
|
12 |
13 |
0 /F |
– |
229 |
Ileal volvulus, food protein-induced enterocolitis syndrome |
CMZ |
5 |
Recovered |
||||
|
13 |
16 |
0/ND |
nd |
nd |
– |
4 days |
polymorphic DNA analysis |
BBG-01 |
Omphalocele |
ABPC/ SBT, MEPM |
12 |
Recovered |
|
14 |
17 |
2 /M |
nd |
ND/anaerobic bottle |
– |
nd |
MALDI-TOF-MS |
– |
Leukaemia, febrile neutropenia |
PIPC/ TAZ, PCG |
14 |
Recovered |
|
This case |
na |
83 /F |
BACTEC FXrt 3DTM |
22F anaerobic medium |
– |
33, 47 |
16S rRNA gene, MALDI-TOF-MS |
– |
Obstractive pyelonephritis, diabetes mellitus |
CTRX+MNZ |
11 |
Recovered |
|
23F aerobic medium |
132 |
*Positive bottles are not described in the reference.
ABPC, ampicillin; ABPC/SBT, ampicillin sulbactam; AIHA, autoimmune hemolytic anaemia; ; BCX, blood culture; CP, chloramphenicol; CTFX, ceftriaxione; CTX, cefotaxime; DM, diabetes mellitus; ESBL, extended-spectrum β-lactamase ; F, female; LT, Left; M, male; MALDI-TOF-MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; MCFG, micafungin; MDS, myelodysplastic syndromes; MEPM, meropenem; MNZ, metronidazole; NA, not applicable; ND, no data; SSPE, subacute sclerosing panencephalitis; UTI, urinary tract infection.
To our knowledge, UTIs caused by Bifidobacterium spp. are quite rare; we only found nine cases (Table 2) [5, 10, 19–23]. Most of the patients had underlying diseases and urological problems. We suspected the following aetiology for the present case: chronic cystitis led to obstructive pyelonephritis caused by polymicrobial pathogens; preceding cephalexin selected B. breve , which is naturally resistant to cephalosporins; and, B. breve bacteraemia developed due to increased pressure in the renal pelvis. Here, Gram staining of urine specimen showed numerous typical Y-branched GPRs; however, no growth was observed in the routine urine culture. Anaerobic urine culture should be considered in patients with an immunocompromised state, urological problems and positive Gram staining but negative routine culture [24]. If Bifidobacterium spp. are suspected based on Gram staining and poor growth of routine urine culture, anaerobic culture is the key to the early identification of this organism.
Table 2.
Reviews of UTIs caused by Bifidobacterium spp
|
No. |
Reference |
Age/gender |
Underlying diseases |
Urological problems |
History of UTI |
Bcx |
Probiotics |
Urine culture |
Identification |
Antibiotics |
Duration (days) |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 |
5 |
51/F |
DM, hypertension, cervical carcinoma |
Obstructive nephropathy |
nd |
nd |
nd |
Bifidobacterium spp. E. coli (ESBL+) |
MALDI-TOF-MS, VITEK® 2 |
MEPM |
nd |
Recovered |
|
2 |
10 |
nd/nd |
Dementia |
nd |
nd |
nd |
nd |
16S rRNA gene |
nd |
nd |
nd |
|
|
3 |
10 |
nd/nd |
nd |
nd |
nd |
nd |
nd |
16S rRNA gene |
nd |
nd |
nd |
|
|
4 |
19 |
66/F |
MDS, liver cirrhosis, DM |
Lt hydronephrosis, Ureter stone |
+ |
nd |
nd |
Bifidobacterium spp. C. glabrata |
RapID ANA II System |
MEPM |
7 |
Recovered |
|
5 |
20 |
80/F |
Breast cancer, hypothyroidism, AIHA |
nd |
+ |
nd |
nd |
16S rRNA gene |
ABPC/SBT |
5 |
Relapsed |
|
|
6 |
21 |
40s/M |
SSPE, DM |
Bilateral hydronephrosis |
– |
– |
– |
16S rRNA gene |
ABPC/SBT |
nd |
Recovered |
|
|
7 |
22 |
7/F |
nd |
Recurrent UTIs |
+ |
nd |
nd |
B. adolescentis, P. asaccharolyticus |
Conventional methods |
ABPC |
10–14 |
Recovered |
|
8 |
23 |
41/M |
Abdominal injury |
Urethral stricture and repeated urethral dilation |
+ |
+ |
– |
nd |
CP |
10 |
Recovered |
|
|
This case |
na |
83/F |
DM, hypertension |
Bilateral hydronephrosis |
+ |
+ |
– |
B. breve, K. pneumoniae, E. coli, E. faecalis |
16S rRNA gene, MALDI-TOF-MS |
CTRX, MNZ |
11 |
Recovered |
ABPC, ampicillin; ABPC/SBT, ampicillin sulbactam; AIHA, autoimmune hemolytic anemia; Bcx, blood culture; CP, chloramphenicol; CTRX, ceftriaxione; CTX, cefotaxime; DM, diabetes mellitus; ESBL, extended-spectrum β-lactamase; F, female; Lt, left; M, male; MALDI-TOF-MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; MCFG, micafungin; MDS, myelodysplastic syndromes; MEPM, meropenem; MNZ, metronidazole; NA, not applicable; ND, no data; SSPE, subacute sclerosing panencephalitis; UTI, urinary tract infection.
Sequencing of the 16s RNA gene has been used as the gold standard for accurate species identification [1, 25]. However, genetic analysis is difficult to perform in actual clinical practice. The identification of Bifidobacterium spp. based on phenotypic characteristics is challenging [26]. Identification by biochemical testing is known to be affected by insufficient growth and poor reproducibility [1]. According to the manufacturer’s instructions, the three commercial kits that can identify Bifidobacterium spp. in each database based on biochemical characterization are the BD BBLTM CRYSTALTM ANR ID System (Becton Dickinson and Company, Sparks, NV, USA), which can identify B. adolescentis , B. dentium and Bifidobacterium spp.; the API 20A (bioMérieux, Marcy-l’Etoile, France), which can identify B. adolescentis , B. dentium, B. breve, B. bifidum and Bifidobacterium spp.; and the API RAPID ID 32A (bioMérieux, Marcy-l’Etoile, France), which can identify B. adolescentis , B. breve , B. longum , B. dentium, B. bifidum and Bifidobacterium spp. [27, 28]. If microbiology technicians think that anaerobic bacteria are absent due to their growth in aerobic bottles, an incorrect identification kit may be used, which can result in misidentification of the bacteria. Although identifying the bacteria to species level by biochemical characterization is sometimes difficult, identification to the genus level is possible if the correct identification kit is selected. Characteristic Gram staining will help in identifying the Bifidobacterium spp. without MALDI-TOF-MS or sequencing of the 16s RNA gene.
MALDI-TOF-MS has been reported to be useful in identifying anaerobic bacteria, including Bifidobacterium spp. [29, 30]. The performance of MALDI-TOF-MS has been believed to surpass that of these commercial kits, with a higher rate of correct identification and fewer misidentifications. As such, the implementation of MALDI-TOF-MS has become a cornerstone in the identification of anaerobic bacteria, including our hospital [1, 31]. Weber et al. reported an adult case of B. longum bacteraemia identified using MALDI-TOF-MS (Bruker Daltonics) [15]. Esaiassen et al. reported that MALDI-TOF-MS (Microflex LT instrument, Bruker Daltonics) can be used for species-level identification of B. longum , B. breve and B. animalis [6]. In our case, Vitek MS did not provide a species-level identification. B. breve is included in Bifidobacterium spp. in VITEK MS Knowledge Base version 3.0–3.2, thus identification of subspecies in this case was impossible using VITEK MS. The accurate identification and compilation of clinical pictures by expanding the library of MALDI-TOF-MS or identification kits will help in identifying differences in the clinical pictures of different Bifidobacterium spp. infections. A greater compilation of Bifidobacterium infection reports will lead to clarifying the pathogenicity, clinical picture and optimal management of infections, especially UTIs.
Conclusion
In this report, we describe a rare case of B. breve bacteraemia and obstructive pyelonephritis. The combination of Bifidobacterium bacteraemia and UTI is believed to be rare; however, there may be undiagnosed cases due to the poor growth in routine urine culture and the difficulty of identification. Recognition of Y-branched GPR and conducting anaerobic urine culture may lead to find more cases of Bifidobacterium UTIs. Clinicians and microbiology technicians need to keep in mind the usefulness and limitation of commercial kits and MALDI-TOF-MS in identifying Bifidobacterium spp.
Funding information
The authors received no specific grant from any funding agency.
Acknowledgements
The authors thank Keisuke Oka for valuable assistance in identifying B. breve.
Author contributions
M.N.: conceptualization, data curation, writing – original draft. H.M.: conceptualization, data curation, writing – review and editing. S.T.: data curation, writing – review and editing. Y.O.: data curation, writing – review and editing. Y.S.: data curation, writing – review and editing. T.O.: data curation, writing – review and editing. A.O.: data curation, writing – review and editing. Y.M.: supervision, writing – review and editing.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Consent to publish
Written consent to publish has been obtained from the patient’s daughter.
Footnotes
Abbreviations: GPRs, gram positive rods; HPF, high power field; MALDI-TOF-MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; MIC, minimum inhibitory concentration; UTIs, urinary tract infections.
References
- 1.Karen CC, Michael AP, Marie LL, Alexander JM, Robin P, et al. Manual of Clinical Microbiology, 12th edition. Wiley; 2019. [Google Scholar]
- 2.Turroni F, Ribbera A, Foroni E, van Sinderen D, Ventura M. Human gut microbiota and bifidobacteria: from composition to functionality. Antonie van Leeuwenhoek. 2008;94:35–50. doi: 10.1007/s10482-008-9232-4. [DOI] [PubMed] [Google Scholar]
- 3.Takeda Y, Ota K, Kondo A, Nishii T, Onishi N, et al. A case of necrotizing fasciitis caused by Bifidobacterium breve . IDCases. 2023;31:e01667. doi: 10.1016/j.idcr.2022.e01667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takiguchi Y, Nagayosi M, Matsuura Y, Akiba Y, Naito A. Peribronchial connective tissue infection caused by Bifidobacterium longum and Veillonella species mimicking lung cancer. Intern Med. 2021;60:453–456. doi: 10.2169/internalmedicine.5120-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butta H, Sardana R, Vaishya R, Singh KN, Mendiratta L. Bifidobacterium: an emerging clinically significant metronidazole-resistant anaerobe of mixed pyogenic infections. Cureus. 2017;9:e1134. doi: 10.7759/cureus.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esaiassen E, Hjerde E, Cavanagh JP, Simonsen GS, Klingenberg C. Norwegian study group on invasive Bifidobacterial I. Bifidobacterium bacteremia: clinical characteristics and a Genomic approach to assess Pathogenicity. J Clin Microbiol. 2017;55:2234–2248. doi: 10.1128/JCM.00150-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chávez de Paz LE, Molander A, Dahlén G. Gram-positive rods prevailing in teeth with apical periodontitis undergoing root canal treatment. Int Endod J. 2004;37:579–587. doi: 10.1111/j.1365-2591.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 8.Brook I. Isolation of non-sporing anaerobic rods from infections in children. J Med Microbiol. 1996;45:21–26. doi: 10.1099/00222615-45-1-21. [DOI] [PubMed] [Google Scholar]
- 9.Brook I, Frazier EH. Significant recovery of nonsporulating anaerobic rods from clinical specimens. Clin Infect Dis. 1993;16:476–480. doi: 10.1093/clind/16.4.476. [DOI] [PubMed] [Google Scholar]
- 10.Mahlen SD, Clarridge JE. Site and clinical significance of Alloscardovia omnicolens and Bifidobacterium species isolated in the clinical laboratory. J Clin Microbiol. 2009;47:3289–3293. doi: 10.1128/JCM.00555-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brook I. Pericarditis caused by anaerobic bacteria. Int J Antimicrob Agents. 2009;33:297–300. doi: 10.1016/j.ijantimicag.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Bourne KA, Beebe JL, Lue YA, Ellner PD. Bacteremia due to Bifidobacterium, Eubacterium or Lactobacillus; twenty-one cases and review of the literature. Yale J Biol Med. 1978;51:505–512. [PMC free article] [PubMed] [Google Scholar]
- 13.Sakurai Y, Watanabe T, Miura Y, Uchida T, Suda N, et al. Clinical and bacteriologic characteristics of six cases of Bifidobacterium breve bacteremia due to pprobiotic administration in the neonatal intensive care unit. Pediatr Infect Dis J. 2022;41:62–65. doi: 10.1097/INF.0000000000003232. [DOI] [PubMed] [Google Scholar]
- 14.Kitajima H, Hirano S. Safety of Bifidobacterium breve (BBG-01) in preterm infants. Pediatr Int. 2017;59:328–333. doi: 10.1111/ped.13123. [DOI] [PubMed] [Google Scholar]
- 15.Weber E, Reynaud Q, Suy F, Gagneux-Brunon A, Carricajo A, et al. Bifidobacterium species bacteremia: risk factors in adults and infants. Clin Infect Dis. 2015;61:482–484. doi: 10.1093/cid/civ347. [DOI] [PubMed] [Google Scholar]
- 16.Ohishi A, Takahashi S, Ito Y, Ohishi Y, Tsukamoto K, et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J Pediatr. 2010;156:679–681. doi: 10.1016/j.jpeds.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 17.Avcin SL, Pokorn M, Kitanovski L, Premru MM, Jazbec J. Bifidobacterium breve sepsis in child with high-risk acute lymphoblastic leukemia. Emerg Infect Dis. 2015;21:1674–1675. doi: 10.3201/eid2109.150097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andriantsoanirina V, Allano S, Butel MJ, Aires J. Tolerance of Bifidobacterium human isolates to bile, acid and oxygen. Anaerobe. 2013;21:39–42. doi: 10.1016/j.anaerobe.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Pathak P, Trilligan C, Rapose A. Bifidobacterium--friend or foe? A case of urinary tract infection with Bifidobacterium species. BMJ Case Rep. 2014;2014:bcr2014205122. doi: 10.1136/bcr-2014-205122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barberis CM, Cittadini RM, Almuzara MN, Feinsilberg A, Famiglietti AM, et al. Recurrent urinary infection with Bifidobacterium scardovii . J Clin Microbiol. 2012;50:1086–1088. doi: 10.1128/JCM.06027-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujioka T NY, Imai K, Matsubara T, Honda K. A case of infection by Bifidobacterium breve detected in urine and Ascites samples. Igakukensa. 68:370–375. n.d. [Google Scholar]
- 22.Brook I. Urinary tract infection caused by anaerobic bacteria in children. Urology. 1980;16:596–598. doi: 10.1016/0090-4295(80)90566-x. [DOI] [PubMed] [Google Scholar]
- 23.Guillard F, Appelbaum PC, Sparrow FB. Pyelonephritis and septicemia due to gram-positive rods similar to Corynebacterium group E (aerotolerant Bifidobacterium adolescentis) Ann Intern Med. 1980;92:635–636. doi: 10.7326/0003-4819-92-5-635. [DOI] [PubMed] [Google Scholar]
- 24.Legaria MC, Barberis C, Famiglietti A, De Gregorio S, Stecher D, et al. Urinary tract infections caused by anaerobic bacteria. Utility of anaerobic urine culture. Anaerobe. 2022;78:102636. doi: 10.1016/j.anaerobe.2022.102636. [DOI] [PubMed] [Google Scholar]
- 25.Justesen US, Holm A, Knudsen E, Andersen LB, Jensen TG, et al. Species identification of clinical isolates of anaerobic bacteria: a comparison of two matrix-assisted laser desorption ionization-time of flight mass spectrometry systems. J Clin Microbiol. 2011;49:4314–4318. doi: 10.1128/JCM.05788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Requena T, Burton J, Matsuki T, Munro K, Simon MA, et al. Identification, detection, and enumeration of human bifidobacterium species by PCR targeting the transaldolase gene. Appl Environ Microbiol. 2002;68:2420–2427. doi: 10.1128/AEM.68.5.2420-2427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.bioMérieux API & ID 32 IDENTIFICATION DATABASES. [ March 29; 2023 ]. https://www.biomerieux-diagnostics.com/sites/clinic/files/9308960-002-gb-b-apiweb-booklet.pdf n.d. accessed.
- 28.Becton Dickinson and Company BBL CRYSTALTM IDENTIFICATION SYSTEMS Anaerobe ID Kit. [ March 29; 2023 ]. https://legacy.bd.com/ds/technicalCenter/clsi/clsi-Crysana.pdf n.d. accessed.
- 29.Alcalá L, Marín M, Ruiz A, Quiroga L, Zamora-Cintas M, et al. Identifying anaerobic bacteria using MALDI-TOF mass spectrometry: a four-year experience. Front Cell Infect Microbiol. 2021;11:521014. doi: 10.3389/fcimb.2021.521014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barreau M, Pagnier I, La Scola B. Improving the identification of anaerobes in the clinical microbiology laboratory through MALDI-TOF mass spectrometry. Anaerobe. 2013;22:123–125. doi: 10.1016/j.anaerobe.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Bächli P, Baars S, Simmler A, Zbinden R, Schulthess B. Impact of MALDI-TOF MS identification on anaerobic species and genus diversity in routine diagnostics. Anaerobe. 2022;75:102554. doi: 10.1016/j.anaerobe.2022.102554. [DOI] [PubMed] [Google Scholar]