Quality improvement, efforts to optimize the safety, efficacy, and value of medical interventions has become an increasingly important focus in health care systems worldwide.1 Although quality measures have been established for many medical specialties, translation to discrete reproducible metrics has presented challenges. Quality metrics are broadly categorized as structure, process, and outcome measures. The most common metrics are those that evaluate individual provider performance related to process metrics. Challenges for metric development include ensuring the linkage between a process measure and a meaningful clinical outcome, reproducibility across providers and health systems, and providing means for quality improvement. An example of a process measure in gastroenterology practice includes conducting colorectal cancer screening at appropriate intervals, whereas outcome measures include colorectal cancer incidence in the screening population. Automated methods for measurement are ideal, given the resources and potential for subjectivity and human error involved in manual assessment of quality metrics. Beyond measurement, development of infrastructure to report process metrics, and an actionable plan for quality improvement to improve outcomes are necessary.
The hepatology community has several proposed quality metrics for patients with cirrhosis, such as assessment of esophageal varices, vaccination for hepatitis A and B, appropriate referral for liver transplantation, appropriate treatment of hepatic encephalopathy, and surveillance for hepatocellular carcinoma (HCC).2 These metrics have been endorsed by the American Association for the Study of Liver Disease (AASLD) Practice Metrics Committee; however, operationalizing the measurement of many of these metrics remains a challenge. One barrier for metric measurement in the ambulatory care setting is that many tests and procedures may be performed at local hospitals and not within the health system. Despite increasing access to electronic health records (EHR), most health systems in the United States are not connected and information cannot be pooled and searched. As a result, few health systems have hepatology practice metrics integrated into quality reporting. At the University of Michigan, specialty care, including hepatology, have been required to develop practice metrics for quality assessment for clinical providers. Herein, we describe the development and implementation of a best practice advisory (BPA) and measurement platform for HCC surveillance in the University of Michigan Epic-based EHR.
Hepatocellular Carcinoma Surveillance
HCC is the 4th most common cause of cancer death worldwide and is a leading cause of death in patients with compensated cirrhosis.3 The benefits of HCC surveillance have been evaluated in case-control and cohort studies, showing improvements in early detection, curative treatment receipt, and overall survival.4 HCC surveillance, using semiannual abdominal ultrasound with or without serum alpha fetoprotein measurement, is recommended in patients with cirrhosis of any etiology and in certain populations with noncirrhotic chronic hepatitis B infection by several international societies, including the AASLD.5 Unfortunately, surveillance rates are low in clinical practice, with a recent meta-analysis estimating that only 24% of at-risk patients underwent surveillance.6 Poor utilization is in part attributable to barriers to screening reported at the patient and provider level.7,8 Furthermore, the frequency of HCC surveillance contributes to inconsistent adherence in many clinical settings. Several interventions have been trialed to improve surveillance rates, including patient-facing interventions (mailed outreach, patient navigators) and provider-facing interventions (best practice advisories, provider dashboards.)6 However, these interventions have been limited by the need for significant resources or lack of applicability outside of a specific health system.
Best Practice Advisory Logic
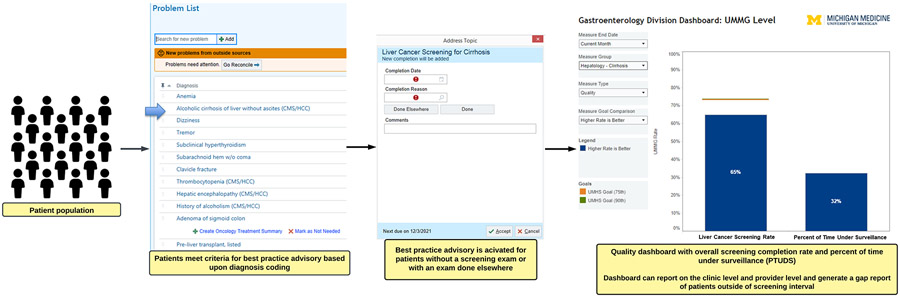
We developed an HCC surveillance BPA nested within the Epic-based EHR at the University of Michigan targeted toward gastroenterology/hepatology subspecialty providers. We engaged the University of Michigan Health Information & Technology services for the development of the measure in the EHR. We provided the rationale for the measure, and worked closely with their team over the course of 3 months to develop the logic and pilot test the BPA, with further refinement over an additional 6-month period before implementation. The BPA was designed to capture all patients recommended to undergoing HCC surveillance, namely patients with cirrhosis of any etiology, and high-risk patients with noncirrhotic chronic hepatitis B per AASLD guidelines.5 We used diagnosis codes within the EHR, in addition to relevant demographic information to identify eligible patients (Supplementary Table 1). We excluded patients with a history of HCC using International Classification of Diseases-10 code C22.0. We identified qualifying surveillance examinations by Current Procedural Terminology codes, which included abdominal ultrasounds, contrast-enhanced multiphasic abdominal computed tomography, or magnetic resonance imaging (Supplementary Table 1). The BPA interval was set at surveillance attainment every 6 months. Additionally, within the BPA there is a prompt to allow acknowledgement of imaging completion at an outside facility, with the date of completion included (Figure 1). The BPA is nested within the “Health Maintenance” tab in Epic.
Figure 1.
Hepatocellular carcinoma screening patient identification, best practice advisory example, and reporting dashboard. UMHS, University of Michigan Health System; UMMG, University of Michigan Medical Group.
Hepatocellular Carcinoma Surveillance Reporting
In parallel with the BPA, we have developed a quality reporting dashboard, which is updated monthly. Here the surveillance attainment rate is reported and can be stratified by clinic location and provider (Figure 1). Provider metrics are reported as percentage of patients who have received HCC surveillance every 6 months with a 2-month grace period (ie, completion of surveillance testing within an 8-month interval satisfies the criteria for surveillance adherence) and the proportion of patients stratified by time under surveillance. The percentage of time under surveillance is the proportion of time over the preceding 12 months the patients have been under a 6-month surveillance window. Additionally, a gap report can be generated to identify which patients are outside their surveillance window allowing providers to send reminders to patients. Because virtually all patients with cirrhosis are managed by hepatologists in our system, the gap reports are available for hepatology practitioners only. The quality metric is semiannually reported as part of the provider’s quality evaluation along with other metrics, such as patient satisfaction scores and colonoscopy adenoma detection rate. These reports are shared with the provider and the service chief, with a remediation plan consisting of a tutorial on BPA usage and discussion of how to address the gap list of patients outside of surveillance for those providers >10% below the group average. The group average is discussed at quarterly hepatology provider meetings, with discussion around gaps and techniques to improve and maintain the surveillance rate.
Limitations of Hepatocellular Carcinoma Surveillance Best Practice Advisory
There are several limitations to the HCC surveillance BPA. First, we are unable to automatically exclude populations that may not benefit from HCC surveillance, such as those patients with Child-Pugh C cirrhosis who are not transplant candidates. However, providers can deactivate the BPA for those patients who would not benefit or refuse HCC surveillance. Although the BPA is satisfied with completion of radiology examinations and we can enter the date of completion of outside examinations into the BPA, we cannot verify if the reports were reviewed and acted on. Provisions, such as natural language processing of reports, could help better ensure reports are reviewed and acted on in a timely manner. The BPA relies on diagnosis coding for cirrhosis and chronic hepatitis B; however, if patients have undiagnosed cirrhosis or do not have appropriate coding entered the BPA is not activated. Undiagnosed cirrhosis is a known barrier to lack of receipt of adequate HCC surveillance.9 The BPA is only provider-facing at this time, but with patients increasingly engaged with the patient portal, a patient-facing version of the BPA could be useful in empowering patients in the surveillance process. Finally, BPAs can create alert fatigue for clinical providers. BPAs often have an attenuated effect because providers often bypass or do not routinely address BPAs, which is an aspect we will monitor with longitudinal assessment of the BPA. We have attempted to simplify the BPA with automated deactivation for 6 months; however, the performance and capture of surveillance examinations outside of our health system remains a challenge.
Operationalization of Other Quality Metrics
There are several proposed metrics for ambulatory hepatology care including ascites management; variceal screening; hepatic encephalopathy treatment; and cirrhosis-related preventative care, such as vaccination against hepatitis A and B. The automation of measurement of many of these metrics remains a challenge. For example, variceal screening recommendations may vary based on a patient’s history, liver stiffness measurement, and platelet count (ie, Baveno VII criteria). Given the limitations of endoscopy availability in some settings, empiric β-blockers for primary prophylaxis in low-risk patients is a viable strategy, whereas other patients may choose to have their endoscopies done locally These varying care pathways for variceal screening make automated tracking challenging. Similarly, vaccination for hepatitis A and B may not be necessary in patients with natural immunity or in those patients who were previously vaccinated. A combination of measurement of immune status, administration of vaccination in clinic, and documentation of receipt of vaccination in local facility may be a viable strategy for implementation of vaccination against viral hepatitis as a quality metric. We have previously successfully implemented a hepatitis C BPA for screening, as recommended by the US Preventative Services Task Force, which identified patients within that birth cohort with no prior diagnosis of hepatitis C or hepatitis C testing, and provided test order and educational material for primary care clinic staff and patients regarding rationale for screening.10
Conclusions
HCC surveillance has been associated with improvement in clinical outcomes in patients with cirrhosis; however, it remains underused. We have designed and implemented a provider-facing BPA within Epic that can be readily implemented at external sites that manage patients with cirrhosis. We have designed the BPA to allow for measurement and feedback for continuous monitoring and quality improvement. This model may be applied to other quality metrics as well; however, the details of operationalization and automation of quality reporting still requires further refinement for many potential measures. The HCC surveillance metric will be used to understand gaps in surveillance receipt at our center and longitudinally assess the success of the BPA in improving HCC surveillance.
Supplementary Material
Funding
Neehar D. Parikh is supported by NIH U01 CA230669 and U01 DK130113. Elliot B. Tapper is supported by U01 DK130113. Anna S. F. Lok is supported by NIH U01 CA230669.
Footnotes
Conflicts of interest
This author discloses the following: Neehar D. Parikh has served as a consultant for Bristol Myers-Squibb, Exact Sciences, Eli Lilly, and Freenome; has served on advisory boards of Genentech, Eisai, Bayer, Exelixis, and Wako/Fujifilm; and has received research funding from Bayer, Target RWE, Exact Sciences, Genentech, and Glycotest. The remaining authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2022.12.009.
References
- 1.Tapper EB, Parikh ND. The future of quality improvement for cirrhosis. Liver Transpl 2021;27:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanwal F, Tapper EB, Ho C, et al. Development of quality measures in cirrhosis by the Practice Metrics Committee of the American Association for the Study of Liver Diseases. Hepatology 2019;69:1787–1797. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 4.Singal AG, Zhang E, Narasimman M, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a systematic review and meta-analysis. J Hepatol 2022;77:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 6.Wolf E, Rich NE, Marrero JA, et al. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta-analysis. Hepatology 2021;73:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singal AG, Tiro JA, Murphy CC, et al. Patient-reported barriers are associated with receipt of hepatocellular carcinoma surveillance in a multicenter cohort of patients with cirrhosis. Clin Gastroenterol Hepatol 2021;19:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons OL, Feng Y, Parikh ND, et al. Primary care provider practice patterns and barriers to hepatocellular carcinoma surveillance. Clin Gastroenterol Hepatol 2019;17:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh ND, Tayob N, Al-Jarrah T, et al. Barriers to surveillance for hepatocellular carcinoma in a multicenter cohort. JAMA Netw Open 2022;5:e2223504–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konerman MA, Thomson M, Gray K, et al. Impact of an electronic health record alert in primary care on increasing hepatitis C screening and curative treatment for baby boomers. Hepatology 2017;66:1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.