Abstract
Serotonergic psychedelics, such as psilocybin and LSD, have garnered significant attention in recent years for their potential therapeutic effects and unique mechanisms of action. These compounds exert their primary effects through activating serotonin 5-HT2A receptors, found predominantly in cortical regions. By interacting with these receptors, serotonergic psychedelics induce alterations in perception, cognition, and emotions, leading to the characteristic psychedelic experience. One of the most crucial aspects of serotonergic psychedelics is their ability to promote neuroplasticity, the formation of new neural connections, and rewire neuronal networks. This neuroplasticity is believed to underlie their therapeutic potential for various mental health conditions, including depression, anxiety, and substance use disorders. In this mini-review, we will discuss how the 5-HT2A receptor activation is just one facet of the complex mechanisms of action of serotonergic psychedelics. They also interact with other serotonin receptor subtypes, such as 5-HT1A and 5-HT2C receptors, and with neurotrophin receptors (e.g., tropomyosin receptor kinase B). These interactions contribute to the complexity of their effects on perception, mood, and cognition. Moreover, as psychedelic research advances, there is an increasing interest in developing nonhallucinogenic derivatives of these drugs to create safer and more targeted medications for psychiatric disorders by removing the hallucinogenic properties while retaining the potential therapeutic benefits. These nonhallucinogenic derivatives would offer patients therapeutic advantages without the intense psychedelic experience, potentially reducing the risks of adverse reactions. Finally, we discuss the potential of psychedelics as substrates for post-translational modification of proteins as part of their mechanism of action.
Introduction
Serotonergic psychedelics are gaining rapid support because of their fast-acting therapeutic effects for a plethora of neuropsychiatric conditions, including depression, anxiety, post-traumatic stress disorder, substance use disorders (SUDs), anorexia, and chronic pain. Serotonergic psychedelics (e.g., LSD, psilocybin [and its active metabolite, psilocin], N,N-dimethyltryptamine [DMT], 2,5-dimethoxy-4-iodoamphetamine [DOI]) are generally defined by their agonism of the serotonin (5-HT) receptor 2A (5-HT2A). However, to say that psychedelics exert all their potential therapeutic effects through 5-HT2A activation is unlikely. As a group of compounds, they target many 5-HT receptors and interact with multiple other neurotransmitter systems.
While the in vivo occupancy of the 5-HT2A receptor correlated with induced psychedelic experiences (Madsen et al., 2019), and 5-HT2A is necessary for the perceptual effects of the serotonergic psychedelics (Halberstadt and Geyer, 2011), the breadth of receptor agonism suggests it may not be sufficient for therapeutic effects (Fig. 1). For example, all tested serotonergic psychedelics showed potent binding to the 5-HT2B and 5-HT2C receptors (Nelson et al., 1999), and recent findings suggest that the neuroplastic effects of psychedelics may be dependent on tropomyosin receptor kinase B (TrkB) receptor binding (Moliner et al., 2023) (Fig. 1). LSD, a high-affinity 5-HT2A agonist, binds to most 5-HT receptor subtypes (Nichols, 2004), multiple dopamine and adrenergic receptors (Ray, 2010; Lewis et al., 2023), and TrkB (Ly et al., 2018; Moliner et al., 2023); thus, these sites may serve a function in the overall positive effects of LSD on psychiatric disorders. While DMT, an indolamine-based psychedelic, appears to exert its effects through 5-HT1A agonism in concert with 5-HT2A (Halberstadt and Geyer, 2011), the phenethylamines (e.g., mescaline) show no affinity for 5-HT1A (Nichols, 2004). Thus, a more rigorous description of the molecular pathways is needed to disentangle the therapeutic action from the various hallucinogenic effects (Ray, 2010; Lewis et al., 2023).
Figure 1.
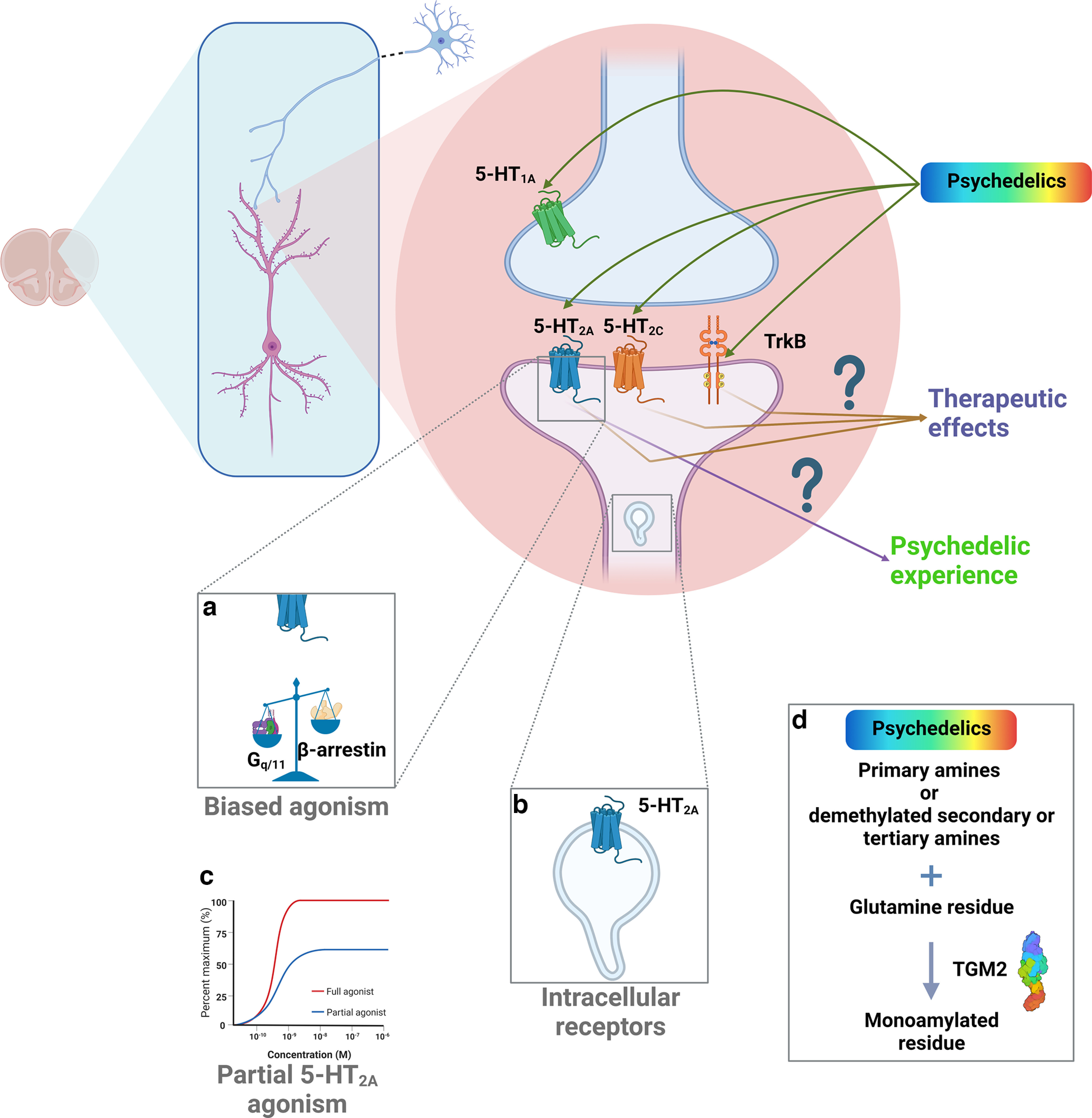
The multiple targets of serotonergic psychedelic drugs. Among the major anatomic targets for serotonergic psychedelics are cortical areas, such as the PFC, where serotonergic receptors are located in pyramidal neurons. Although activation of 5-HT2A receptors is an integral element of the hallucinogenic experience and may contribute to some of the proposed therapeutic effects of these class of drugs, other receptors of the same family (5-HT2C and 5-HT1A) or even the TrkB receptor of the neurotrophin, BDNFs are involved in the therapeutic effects of psychedelic drugs. This suggests a potential to dissociate psychedelics from therapeutic effects. a, Several psychedelic drugs may also induce their effects (psychedelic and or therapeutic) via a biased activation of signaling pathways (G-protein-mediated vs β-arrestin). b, Alternatively, serotonergic psychedelics may target intracellular 5-HT2A receptors to induce therapeutic effects because of their lipophilicity, which allows them to traverse the cell membrane. c, Some derivatives, such as 2-Br-LSD, also target 5-HT2A receptors as partial agonists, inducing synaptic plasticity but lacking hallucinogenic effects. d, Finally, it is also conceivable that several psychedelics and entactogens can be a substrate for TGM2 to monoamylate glutamine restudies in proteins, such as Rac1 or histones, to alter cytoskeleton function or gene expression, respectively, in the induction of lasting therapeutic effects.
Serotonergic psychedelics are being investigated for the treatment of depressive disorders and other mood disorders (Davis et al., 2021; Daws et al., 2022; D'Souza et al., 2022; Goodwin et al., 2022, 2023; Zeifman et al., 2023). In preclinical studies, chronic stress (a risk factor for depression and other mood disorders) results in structural and functional atrophy, primarily in the prefrontal cortex (PFC) and hippocampus (Duman et al., 2016), and such effects are thought to be the basis of deficits of many mood disorders (Russo and Nestler, 2013). Current pharmaceutical treatments, such as monoamine reuptake inhibitors, promote positive neuroplastic changes but only following chronic treatment, and they suffer from high resistance in patient populations (Zhdanava et al., 2021). These drawbacks have motivated preclinical and clinical research on more effective and faster-acting alternatives for mood disorders. Several studies have demonstrated that psychedelics can increase structural and functional neuroplasticity (Cameron et al., 2018; Ly et al., 2018; Shao et al., 2021; Vargas et al., 2023) in the PFC, hippocampus, and other brain regions involved in emotion (Vargas et al., 2021). Importantly, these compounds appear to be rapid-acting and effective in at least some treatment-resistant patients with major depressive disorder (Carhart-Harris et al., 2017; Palhano-Fontes et al., 2019; Davis et al., 2021; Daws et al., 2022; D'Souza et al., 2022; Goodwin et al., 2022, 2023; Zeifman et al., 2023).
Serotonergic psychedelics also show promise in reducing alcohol intake in heavy drinkers (Bogenschutz et al., 2022) and increasing smoking cessation (Johnson et al., 2017). Recently, data from the National Survey on Drug Use and Health found that psilocybin was associated with a 30% decrease in the odds of developing an opioid use disorder (OUD) (Jones et al., 2022), suggesting that serotonergic psychedelics may be efficacious in the treatment of the underlying addiction in many different SUDs. While the evidence is promising, little is known about the modulatory mechanisms of psychedelic serotonergic agonists on the mesocorticolimbic system, which is thought to drive the development of addiction-related behaviors.
Despite the promise of psychedelic-assisted treatments for several psychiatric illnesses, their widespread use, which requires close clinical supervision, represents an economic strain on health care systems. As it currently stands, psychedelic-assisted therapy costs several thousand dollars per session (Marseille et al., 2022; Chrysanthos, 2023), making this effective therapy inaccessible for a large portion of the patient population, especially those from lower socioeconomic areas. Furthermore, regulatory and legal barriers still exist, which makes treatment implementation problematic (Johnson et al., 2008). There are also concerns that the hallucinogenic action of serotonergic psychedelics could produce hallucinogen-persisting perception disorder (Ford et al., 2022) and irreversible psychotic episodes in susceptible populations, which has already led to routinely excluding patients with a family history of bipolar disorder or schizophrenia from participating in psychedelic clinical trials (Johnson et al., 2008). This underscores the importance of determining psychedelics' mechanism of action. Recent findings suggest that it may be possible to decouple the hallucinogenic and therapeutic effects of psychedelics. Thus, there is a growing interest in developing so-called “second-generation” psychedelic analogs with attenuated or absent hallucinogenic effects, which may have similar neuroplastic and behavioral effects to the classic psychedelics.
In this review and the Mini-Symposium at the Society for Neuroscience meetings in 2023, we will review some of the latest findings of serotonergic psychedelic drug actions and introduce the concept of nonhallucinogenic psychedelic analogs with therapeutic potential.
5-HT2A receptor: an integral part of the psychedelic experience
5-HT2A is the most abundant excitatory G-protein-coupled receptor (GPCR) of the 5-HT receptor family (Saha and González-Maeso, 2023). Like 5-HT2B and 5-HT2C, 5-HT2A signals through Gq/11, activating a cytoplasmic protein phospholipase C (PLC), which cleaves the membrane phospholipid, phosphatidylinositol 4,5-bisphosphate, followed by generation of diacylglycerol and inositol triphosphate). Inositol triphosphate releases Ca2+ from intracellular stores (Saha and González-Maeso, 2023).
The PFC layer 5 pyramidal cells express high levels of the 5-HT2A receptor, making drugs that target this receptor, serotonergic psychedelics, ideal for the modulation of pyramidal cell excitability (Weber and Andrade, 2010). Treatment with serotonergic psychedelic compounds increases both the structural (dendritogenesis, spinogenesis, synaptogenesis) and functional plasticity of these cells (Ly et al., 2018; Cameron et al., 2021, 2023; Shao et al., 2021; Vargas et al., 2023).
The 5-HT2A receptor is an integral part of the psychedelic experience (Vollenweider et al., 1998; Kraehenmann et al., 2017), as cotreatment with the antagonist ketanserin blocks the perceptual effects of psychedelic compounds (psilocybin and LSD) in humans (Vollenweider et al., 1998; Kraehenmann et al., 2017). In mice, the head-twitch response, characterized by a rapid back-and-forth head movement, has predictive validity of the hallucinogenic potency of serotonergic psychedelics in humans (Halberstadt et al., 2020). The head-twitch response induced by several serotonergic psychedelics [DOI, 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane, 1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane DOB (DOB), psilocin, mescaline, LSD]; it is prevented by pretreatment with ketanserin, and abolished in 5-HT2A-KO animals (González-Maeso et al., 2003, 2007).
While 5-HT2A receptor activation gives rise to the hallucinogenic part of the drug action, it remains unclear whether this is necessary for all the therapeutic effects of psychedelic-assisted therapy (Fig. 1). The emotional journey characteristic of the psychedelic experience correlates with the lasting therapeutic effects of these compounds (Yaden and Griffiths, 2021). There is also counterevidence: a case study reported an antidepressant effect of psilocybin in the absence of hallucinations while the patient was being cotreated with a 5-HT2A antagonist (Rosenblat et al., 2023). Furthermore, recent testing of “nonhallucinogenic” analogs of serotonergic psychedelic compounds, such as tabernanthalog, AAZ-A-154 (R)-70, lisuride, and 2-bromo-LSD (2-Br-LSD), further suggest that hallucinations may not be necessary for some therapeutic effects (Cameron et al., 2021; Kaplan et al., 2022; Lewis et al., 2023; Dong et al., 2021). However, if any of these nonhallucinogenic compounds has therapeutic effects on patient populations remains to be determined. Clinical trials with novel putative nonhallucinogenic analogs are underway (Delix Therapeutics, 2023). Furthermore, 2-Br-LSD has shown efficacy against cluster headaches (Karst et al., 2010), similar to LSD and psilocybin (Sewell et al., 2006).
There have been contrasting reports regarding the engagement of the 5-HT2A receptor for some cellular and behavioral effects of serotonergic psychedelics. Highly selective 5-HT2A agonists, such as 25CN-NBOH (Jensen et al., 2020), appear to increase cognitive flexibility in mice (Odland et al., 2021). Indeed, neuronal growth elicited by psychedelics (DOI, DMT, psilocybin, and LSD) and spine dynamics in vivo reveals that spine density is inhibited by pretreatment with the 5-HT2A antagonist, ketanserin (Ly et al., 2018; Cameron et al., 2021; Shao et al., 2021), but other aspects, such as spine size, are unaffected (Shao et al., 2021). Furthermore, the effects of psychedelics (DOI, psilocybin, LSD) in behavioral assays for antidepressant efficacy (e.g., forced swim test and sucrose preference test) or social behavior are blocked by 5-HT2A antagonists (De Gregorio et al., 2021; Cameron et al., 2023) in some studies, but not in others (Hesselgrave et al., 2021; Moliner et al., 2023).
Experiments using antagonists to study the 5-HT2A receptor require careful dose titration and determination of adequate treatment timing. In addition, ketanserin has off-target affinity at other targets (Henry et al., 1994; Casey et al., 2022), leading to conflicting results (Hesselgrave et al., 2021). Thus, further confirmation with other selective antagonists or using 5-HT2A-KO mice provides a more precise approach to probing the mechanism of action of serotonergic psychedelic compounds.
For example, the effects of 5-methoxy-DMT (5-MeO-DMT) in the tail-suspension test were abolished in the 5-HT2A-KO mice (Cameron et al., 2023). Furthermore, psilocybin restored the reward-seeking effects after chronic stress in the sucrose preference test in WT mice but not in 5-HT2A-KO mice. In this same vein, other groups have also reported a blunted effect on fear extinction learning in 5-HT2A-KO mice (de la Fuente Revenga et al., 2021). Finally, the neuroplastic growth and functional effects of psychedelic treatment are abolished in 5-HT2A-KO mice (Cameron et al., 2023; Vargas et al., 2023). This supports the necessity of the 5-HT2A receptor in mediating some of the therapeutic and neuroplastic effects of serotonergic psychedelics. However, the constitutive 5-HT2A-KO mice are less anxious than their WT littermates (Weisstaub et al., 2006), which may muddle the interpretation of some of the data. Thus, conditional KO mice would further validate the abovementioned findings while providing temporal and spatial resolution.
One alternative hypothesis posits that therapeutic and hallucinogenic effects diverge via functional selectivity at the 5-HT2A, driving different downstream intracellular signaling cascades (Fig. 1a) (Kwan et al., 2022). It is also possible that signaling through dimers, such as a 5-HT2A-mGluR2 complex, underlie the divergent signals, although this has yet to show physiological validity in more complex in vitro and in vivo systems. Further work to elucidate the downstream signaling effects may be helpful to optimize further and disentangle the psychedelic effects from the hallucinogenic effects.
In addition to their action on membrane surface receptors, there is the intriguing possibility that serotonergic psychedelics may target an intracellular pool of 5-HT2A receptors (Fig. 1b) (Vargas et al., 2023). Indeed, serotonergic psychedelics are highly lipophilic compounds that can easily pass through cell membranes, and some of them (DMT and psilocin) target intracellular 5-HT2A receptors to induce neuronal growth (Vargas et al., 2023). It remains unknown whether the total volume of receptors targeted is sufficient to elicit growth or whether the internal receptors have different properties or signaling pathways leading to these effects.
Serotonergic psychedelics as treatment for OUD: role of 5-HT2A and 5-HT1A
Serotonergic psychedelics are currently being investigated as novel SUD treatments (Johnson et al., 2017; Bogenschutz et al., 2022), with most studies focusing on reducing alcohol and nicotine use. The mystical experience and subjective effects through activation of 5-HT2A are thought to be attributed to the reduction in humans abusing substances. However, the activation of these receptors has been shown to modulate other neurotransmitter systems (e.g., dopaminergic pathways) that may be responsible for producing behavioral changes.
Indeed, excitatory pyramidal neurons from layers II/III and V/VI in both the mPFC (Goldman-Rakic, 1996; Jakab and Goldman-Rakic, 1998) and somatosensory cortex, where 5-HT2A receptors are expressed most abundantly, synapse onto medium spiny neurons in the NAc and other regions in the striatum to promote goal-directed actions (Nestler, 2001; Russo et al., 2010; Scofield et al., 2016). Studies using electrophysiology discovered that the prominent form of plasticity at corticostriatal synapses is NMDA glutamate receptor-dependent LTP, and further studies found positive reinforcement learning specifically engages the mPFC to striatum pathway (Reynolds et al., 2001; Ungless et al., 2001; Lüscher and Malenka, 2011). These pathways and the corresponding behaviors are implicated in SUDs.
The use of serotonergic agonists, such as 5-HT receptor-selective phenethylamines and psilocybin, is understudied regarding the adaptations produced in drug dependence. There is contradictory evidence on how 5-HT2A receptor stimulation in cortical neurons affects DA firing in the NAc and behaviors associated with drug reward and craving. The activation of 5-HT2A receptors on pyramidal neurons projecting to the NAc is thought to influence adaptive value applied to conditioned behavior and may play a significant role in relapse-related behaviors, such as avoiding uncomfortable physical or mental states associated with drug abstinence, reward-seeking, or being triggered by paired contexts (McFarland et al., 2003; Zhang et al., 2021). To this end, studies have assessed the role of 5-HT2A receptor activation in intracranial self-stimulation (Jaster et al., 2022), behavioral sensitization (Pang et al., 2016; Sierra et al., 2022), shifting to drug-dependent states (Vargas-Perez et al., 2017), and withdrawal (Pang et al., 2016).
The 5-HT2A receptor is involved in the acute effects of some, but not all, psychedelics in rodent models of intracranial self-stimulation. All structurally different psychedelics produced a depression in intracranial self-stimulation responses (Sakloth et al., 2019; Jaster et al., 2022), and the selective 5-HT2A receptor antagonist, volinanserin (MDL-100907), was only able to attenuate the effect of the phenethylamine psychedelic, DOI, but not the ergoline, LSD, or the tryptamine, psilocybin (Jaster et al., 2022). This suggests that less 5-HT2A-selective psychedelics exert a mechanism outside the 5-HT2A receptor that contributes to psychedelic action in some models.
Most animal models of substance use tend to assess the acute effects of psychedelics on behavior rather than the sustained behavioral changes, although this is starting to change. One group assessed the effects of LSD on ethanol drinking behaviors up to 46 d following initial administration and found that LSD decreased overall ethanol consumption, and a higher dose produced a decrease in ethanol preference, with no changes in overall fluid intake (Alper et al., 2018). Another study evaluating psilocybin on ethanol consumption in male and female mice found a sex-dependent effect, in which male mice had decreased consumption and preference of alcohol following a single dose of psilocybin, and female mice displayed no changes in ethanol consumption or preference (Alper et al., 2023).
While the mechanisms behind psychedelics' potential therapeutic effects on addiction-related behaviors are still under investigation, feedback inhibition could be one such mechanism to explain how these compounds are modulating behavior, both independently or dependently on 5-HT2A. It is known that drugs, such as selective serotonin reuptake inhibitors (SSRIs), increase the extracellular concentration of serotonin, leading to the activation of 5-HT1A receptors and immediate inhibition of serotonin neurons (Babb et al., 2018). Psychedelics also rapidly increase extracellular serotonin by activating 5-HT2A receptors, most likely producing a similar feedback inhibition. This feedback inhibition is perhaps influencing the incentive salience of drugs of abuse as coded by projections between the mPFC/somatosensory cortex and NAc. Considering that psilocin has an affinity for both the 5-HT1A and 5-HT2A receptors (Fig. 1) (Halberstadt and Geyer, 2011), there might be some equilibrium between the two signaling pathways that are necessary for the potential therapeutic action of psychedelics in this context.
Another potential mechanism of action of serotonergic psychedelics on SUD could be the interaction of downstream signaling pathways or receptor crosstalk, which is currently under investigation. One example is the interaction between serotonin and opioid signaling, where opioid use indirectly alters NMDA signaling by acting on neighboring μ-opioid receptors (MORs), causing crosstalk between the downstream signaling pathways (Rodríguez-Muñoz et al., 2012; Chartoff and Connery, 2014). Evidence also shows that MOR and 5-HT2A receptors colocalize in several rodent brain regions, including the PFC and dorsal/ventral striatum. Further, in neurons expressing MOR and 5-HT2A receptor, upregulation of MOR levels was seen and blocked by selective inhibition of Gq proteins; and when treated with serotonin and morphine, there was desensitization, internalization, and downregulation of MOR (Lopez-Gimenez et al., 2008). A novel mechanism for serotonergic modulation of substance use, specifically opioids, is the activation of pyramidal neurons expressing 5-HT2A receptors in the PFC, which then send projections into the NAc, influencing the dopamine tone that is altered following prolonged drug use. Potential therapeutics using this mechanism would target the pathways involved in drug-liking, craving, and potentially withdrawal, instead of directly targeting the opioid receptors.
The question remains whether these psychedelics can produce therapeutic effects in other models of drug-related behaviors, and whether the combination of behavioral, pharmacological, and molecular techniques can provide insight into the specific receptor and cell targets modulating the underlying circuitry related to these behavioral phenotypes. To that end, the use of conditioned-place preference, reinstatement, and withdrawal assays in combination with FLP/Cre-mediated expression of 5-HT2A receptor in the cortico-accumbal pathway are being explored with structurally different psychedelics to identify critical targets and cell populations involved in their potential to unpair opioid-context interactions and reduce uncomfortable states. By identifying specific neuronal populations and their projections involved in these behaviors associated with opioid use and abuse, it could be possible to produce a novel treatment mechanism for OUD.
The nonhallucinogenic analog 2-Br-LSD also targets 5-HT2A receptors
Several nonhallucinogenic derivatives from serotonergic psychedelics have been investigated in preclinical models, and some are already in clinical testing. Lisuride, for example, is an ergoline on the market as a Parkinson's treatment. Although its mechanism of action for Parkinson's is thought to involve dopamine receptor activation (Horowski and Löschmann, 2019), lisuride binds to TrkB (Moliner et al., 2023), is a 5-HT2A partial agonist (López-Giménez and González-Maeso, 2018), and has recently shown antidepressant potential (Qu et al., 2023).
A newly synthesized analog of ibogaine, tabernanthalog, has been found to promote neuroplasticity in the PFC and reverse depression-like behavior following chronic stress in mice (Cameron et al., 2021). Furthermore, the same group developed the compound AAZ (Dong et al., 2021), which is a nonhallucinogenic analog developed for depression. Indeed, there are already putative nonhallucinogenic analogs in Phase I clinical trials (Delix Therapeutics, 2023).
Most recently, 2-Br-LSD has also been investigated. 2-Br-LSD is a partial 5-HT2A agonist that enhances PFC neuroplasticity and promotes the reversal of depression-like behavior in preclinical models but lacks hallucinogenic effects (Lewis et al., 2023). Synthesized alongside LSD by Albert Hoffman in the early 1960s, it was found to lack LSD's mind-altering side effects and was initially thought to be a 5-HT2A antagonist. Recently, it was shown to be a potential treatment for cluster headaches, a trait shared with other classical psychedelics (Karst et al., 2010). Thus, based on its close structural homology with LSD and apparent neuroactive potential, work began investigating its possible efficacy as a novel therapeutic for mood disorders.
2-Br-LSD shows an interesting receptor-binding profile; while it is a mild to potent agonist of many 5-HT receptors, including 5-HT1B/1D/1F/2A/6, it also shows potent dopamine D2 and D4 receptor agonism (Lewis et al., 2023). However, 2-Br-LSD's receptor profile shows greater receptor selectivity activity than LSD (which showed agonism for all 5-HT receptors assayed). Most importantly, 2-Br-LSD did not show 5-HT2B agonism, a receptor linked to fibrotic cardiac valvulopathy (Rothman and Baumann, 2009), and thus represents a safer alternative to other psychedelic compounds in treating mood disorders. While LSD is a potent agonist of 5-HT2A, 2-Br-LSD only partially activates this receptor; thus, it can partially antagonize it in the presence of serotonin (Fig. 1c). This may explain why it lacks hallucinogenic effects, although its therapeutic effects depend on 5-HT2A activity; a threshold of efficacy may exist below which pathways related to the side effects are not activated. Interestingly, chronic 2-Br-LSD treatment does not induce tolerance, an effect possibly mediated by weak recruitment of β-arrestin2, a pathway thought to underlie desensitization found with the classic psychedelics (Smith et al., 1999; Gresch et al., 2005).
Compared with the classic psychedelics, 2-Br-LSD shows similar 5-HT2A activation-dependent antidepressant and neuroplastic effects, as demonstrated with the selective 5-HT2A antagonist, volinanserin, in vitro and in vivo. In stress-naive mice, 2-Br-LSD increases the probability of active coping behavior in the forced swim test without increasing overall locomotor behavior (Lewis et al., 2023). Further, in chronically stressed mice, 2-Br-LSD reverses the effects of stress on center exploration in the open field assay and deficits in self-grooming in the splash test. Acute 2-Br-LSD treatment also induces cortical spinogenesis, both in vitro and in vivo, and increases dendritic arbor complexity of cortical neurons in culture, effects like that seen with ketamine treatment.
The fact that 2-Br-LSD is not hallucinogenic but shows similar neuroplastic and behavioral effects in mouse models may suggest that the mind-altering characteristic of psychedelics is not a necessary factor in their therapeutic potential. However, human trials will be necessary to demonstrate clinical efficacy. Further, 2-Br-LSD's receptor profile shows that not only is 5-HT2A agonism a necessary component of its mechanism of action, but also that it represents a possibly safer alternative to treatment with the classic psychedelics.
The 5-HT2C receptor as a therapeutic target for SUD
Along with the other 5-HT2 family members, 5-HT2C is a GPCR that canonically couples to Gq/11 subtypes to signal, primarily leading to PLC activation, inositol triphosphate accumulation, intracellular calcium release, and protein kinase C activation (Kim et al., 2020). Previously, GPCRs are understood to couple to one specific canonical G protein subtype (Gq/11, Gi/o, G12/13, Gs/olf) as well as signal through G protein-independent pathways, notably via β-arrestins (Lefkowitz and Whalen, 2004). However, accumulating evidence has revealed that many GPCRs exhibit varying degrees of promiscuous coupling, interacting with multiple G protein subtypes (Inoue et al., 2019; Sandhu et al., 2022). In particular, 5-HT2C has been demonstrated to activate Gi/o and G12/13 subtypes, which lead to distinct downstream signaling pathways (Alberts et al., 1999; Cussac et al., 2002; McGrew et al., 2002). The activation of these additional G proteins complicates our understanding of which 5-HT2C signaling pathways are ultimately responsible for therapeutic versus side effects, mainly as they apply to psychedelics and their emerging therapeutic properties.
The 5-HT2C receptor has emerged as a promising target for therapeutic effects (Palacios et al., 2017). The high sequence homology of 5-HT2A and 5-HT2C has posed a challenge in developing selective agonists for either receptor subtype (Cheng et al., 2016a,b; Palacios et al., 2017), and most clinically studied psychedelics, such as LSD, are nonselective agonists with the 5-HT2 family and across other 5-HT receptors (Nichols, 2004; Lewis et al., 2023).
However, there has been progress in developing several selective 5-HT2C agonists, which have been investigated as potential therapeutics for the treatment of multiple psychiatric disorders, particularly impulse-related disorders, such as SUDs (Higgins et al., 2013; Campbell et al., 2021). A growing body of evidence supports the influence of 5-HT2C on impulse regulation, partly through the modulation of dopaminergic neurotransmission in the VTA (Bubar and Cunningham, 2007; Howell and Cunningham, 2015). 5-HT2C agonists have been shown to decrease substance use behaviors, including the self-administration of ethanol, cocaine, and nicotine in rodents (Grottick et al., 2000; Rocha et al., 2002; Tomkins et al., 2002; Howell and Cunningham, 2015). Additionally, there is abundant evidence that 5-HT2C mediates satiety and food intake. For example, 5-HT2C KO mice display hyperphagia and increased body mass, whereas 5-HT2C agonists suppress food intake (Tecott et al., 1995; Nonogaki et al., 1998; Vickers et al., 1999; Clifton et al., 2000; Grottick et al., 2000; Higgins et al., 2013). For this reason, lorcaserin (Belviq) was an FDA-approved 5-HT2C selective agonist implemented as a treatment for weight loss (Thomsen et al., 2008). Not surprisingly, lorcaserin and other 5-HT2C selective agonists can reduce alcohol drinking in rodent models in addition to anorectic effects (Rezvani et al., 2014; Tabbara et al., 2021; Fletcher et al., 2022). These studies have solidified the potential of 5-HT2C as a pertinent therapeutic drug target for both obesity and SUDs; but unfortunately, lorcaserin was recently withdrawn because of a higher frequency of cancer diagnoses in the lorcaserin-treated group relative to the placebo group (Mazza et al., 2023). Interestingly, psilocybin, which has equal 5-HT2A and 5-HT2C affinities, has shown promise in SUD treatment, while no difference in food intake was found in rodents (Fadahunsi et al., 2022). Thus, further studies into 5-HT2C signaling and how psychedelics signal at this receptor subtype are warranted.
In recent years, the concept of ligand bias for either G-protein-dependent or G-protein-independent signaling pathways has been identified as a means to achieve biased agonists endowed with greater therapeutic value over traditional balanced agonists (Kenakin, 2011; Tan et al., 2018). Therefore, the design of biased agonists and, ultimately, pathway-selective 5-HT2C agonists will likely uncover key signaling pathways necessary for treatments for SUDs, which could avoid many side effects associated with previous 5-HT2C selective agonists. Overall, investigating psychedelics' ability to engage noncanonical downstream signaling for other serotonin receptors, such as 5-HT2C, is crucial for the potential development of more precise and effective therapeutics for mental health disorders.
BDNF and TrkB: key to psychedelic-induced neuroplasticity and therapeutic effects
BDNF is a member of the neurotrophin family of growth factors that regulate neurite outgrowth, synaptogenesis, and acts as a critical regulator of activity-dependent neuronal plasticity through its receptor TrkB (Park and Poo, 2013), BDNF binding leads to TrkB dimerization, autophosphorylation, and signaling through mitogen-activated protein kinase, phosphoinositol-3-kinase, and the PLC γ pathways (Park and Poo, 2013).
BDNF and TrkB are widely recognized as key components that mediate the therapeutic effects of pharmacologically diverse antidepressants (Castrén and Antila, 2017; Vollenweider and Preller, 2020). Recent studies have shown that commonly used antidepressants, such as slow-acting fluoxetine or fast-acting ketamine can directly bind to the transmembrane domain of TrkB, thereby allosterically promoting BDNF effects and producing plasticity- and antidepressant-related effects of antidepressants in mice (Casarotto et al., 2021; Kot et al., 2023).
A recent breakthrough discovery found that psychedelics with clinical potential, such as LSD and psilocin, bind to TrkB with 1000-fold higher affinities than other antidepressants (Moliner et al., 2023). It was shown that binding to TrkB mediates the plasticity and antidepressant-like effects of psychedelics. The binding of psychedelics to the transmembrane domain of TrkB dimers in a different site than BDNF was confirmed using several orthogonal methods: binding assays with radiolabeled LSD, mutagenesis studies, NMR spectroscopy, TrkB dimerization assays, microscale thermophoresis, and extensive molecular dynamics simulations in physiological membrane conditions. Interestingly, the binding pockets and TrkB conformational changes induced by serotonergic psychedelics are different from those seen for conventional antidepressants, which may help explain the faster and longer-lasting antidepressant actions of psychedelics.
Serotonergic psychedelics are not direct TrkB agonists, as extracellular BDNF is required for their effects on TrkB dimerization, spinogenesis, and dendritogenesis. Activity-dependent BDNF release in stimulated synapses selectively stabilizes active synapses at the expense of inactive ones (Park and Poo, 2013), which is critical for Hebbian-type plasticity. Direct agonists are expected to activate TrkB regardless of neuronal activity and BDNF presence in all synapses, worsening signal-to-noise ratios within neuronal networks. In contrast, psychedelics selectively promote, maintain, and strengthen activity-dependent plasticity in active synapses through a positive allosteric modulation of endogenous BDNF signaling.
A point mutation in the transmembrane domain of TrkB that impairs the binding of serotonergic psychedelics prevents induction of neuroplasticity and antidepressant-like behavioral responses to LSD and psilocybin, but does not influence the head-twitch response associated with 5-HT2A activity and hallucinations in humans (Halberstadt et al., 2020; Moliner et al., 2023). Moliner et al. (2023) also report that the relatively selective 5-HT2A antagonist volinanserin does not prevent psychedelics from promoting TrkB dimerization and neurotrophic signaling, spinogenesis, dendritogenesis, or antidepressant-like behavioral effects. These findings suggest that the TrkB-dependent effects of psychedelics on plasticity can be detached from their hallucinogenic-like action via 5-HT2A receptors.
However, several outstanding questions remain about the involvement of TrkB in the effects of serotonergic psychedelics. For example, the binding of tritiated LSD (Meibach et al., 1980) and the localization of TrkB (Yan et al., 1997) appear to be in different brain regions, indicating the need for additional studies to disentangle the contribution of TrkB and serotonin receptors to the psychedelic experience and the different therapeutic effects of these compounds.
In summary, TrkB is gathering increased attention as a common binding site for antidepressants and psychedelics that plays a key role in mediating their plasticity and therapeutic-like actions, independently of hallucinogenic effects.
Psychedelics as protein modifiers in neuronal function and plasticity
Biogenic amine neuromodulators, such as serotonin and dopamine, in addition to binding receptors, are chemically reactive and have been reported to bind to glutamine residues of diverse proteins covalently; this modification is referred to as monoaminylation (Muma and Mi, 2015; Farrelly et al., 2019; Lepack et al., 2020). The covalent addition of amines, such as serotonin and dopamine, to proteins is called transamidation (Lukasak et al., 2022) and is catalyzed by the enzyme transglutaminase 2 (TGM2). Transamidation of serotonin (serotonylation) or dopamine (dopaminylation) of key proteins underlies cytoskeletal rearrangement (Dai et al., 2008; Jones et al., 2009), transcriptional regulation (Farrelly et al., 2019; Lepack et al., 2020), and mitogenesis (Muma and Mi, 2015), processes linked to synapse formation and maturation.
Psychedelic drugs structurally resemble the neuromodulators serotonin and dopamine, produce profound states of altered consciousness, and show promise for treating mental health disorders (Kwan et al., 2022). Psychedelics are so potent, long-lasting, and varied in their effects on cognition and neuroplasticity that additional mechanisms beyond 5-HT2A agonism seem likely. Psychedelics are more lipophilic than their endogenous counterparts, and their cellular entry is not restricted by the presence of specific transporters (Vargas et al., 2023). Therefore, in the context of monoaminylation, psychedelic drugs may serve as exogenous substrates for transamidation by TGM2 in place of endogenous amines. Prior evidence has demonstrated a role for TGM2-mediated transamidation in psychedelic-indced synaptogenesis, as the psychedelic phenethylamine DOI required TGM2 activity to promote the formation of new dendritic spines (Mi et al., 2017). DOI was shown to induce serotonylation of Rac Family Small GTPase 1 (Rac1), which functions in the assembly of the actin cytoskeleton, a process critical for neurite outgrowth (Dai et al., 2008). Serotonylation of Rac1 leads to its constitutive activation and results in a spine growth phenotype. Intriguingly, this activity also required 5-HT2A agonism. As 5-HT2A activity induces Ca2+ fluxes, and TGM2 is a Ca2+-dependent enzyme, this sets up the hypothesis that 5-HT2A and TGM2 work in conjunction to manifest the cellular effects of psychedelics.
Several psychedelic and entactogen compounds (e.g., mescaline, 4-bromo-2,5-dimethoxyphenethylamine) are primary amines and can, theoretically, undergo transamidation by TGM2 (Fig. 1d). Most others are secondary or tertiary amines and would require demethylation (Al-Kachak and Maze, 2023) to become TGM2 substrates (Fig. 1d). The demethylation of psychedelics has been shown to occur in vivo (e.g., tryptamine was identified as a direct and fairly rapid metabolite of DMT in brains) (Kargbo, 2022). Like serotonin, tryptamine is a suitable substrate for TGM2-mediated transamidation of proteins (Lukasak et al., 2022). Several drugs, administered as pro-drugs, require demethylation by CYP3A4 or other enzymes for activation (Ortiz de Montellano, 2013). It is plausible that some psychedelic drugs require similar demethylation for many effects. Interestingly, the commonly prescribed antidepressant medication sertraline functions as a CYP3A4 inhibitor in addition to an SSRI (Masubuchi and Kawaguchi, 2013; Ghosh et al., 2015). Sertraline has been shown to blunt the subjective effects of psychedelic drugs, while other SSRIs, such as escitalopram, which are not CYP3A4 inhibitors, have less impact on these effects (Bonson et al., 1996). This raises the possibility that the subjective and/or therapeutic effects of some psychedelic drugs could be mediated by metabolites, which appears to be the case for ketamine as well (Farmer et al., 2020).
Could the therapeutic effects of psychedelics be mediated, at least somewhat, through TGM2 activity? A landmark study showed that serotonylation of histone H3 promotes permissive expression of genes related to cellular differentiation and maintenance and was required for neurite outgrowth (Farrelly et al., 2019). Serotonylation of histone H3 in neurons of the dorsal raphe nucleus decreases with stress, and patients with major depressive disorder who were not taking antidepressants at the time of death display decreased histone serotonylation (Al-Kachak et al., 2023). In the same study, major depressive disorder patients that had been prescribed an antidepressant before the time of death had increased histone serotonylation, indicating there may be an interaction between SSRIs and histone serotonylation (Al-Kachak et al., 2023). Histone H3 serotonylation occurs at glutamine residue 5 (H3Q5ser) and leads to altered gene expression through the protein WD Repeat Domain 5, a chromatin modulator that reads H3Q5ser and activates transcription (Zhao et al., 2021). In humans, H3Q5ser appears upstream of, and frequently in combination with, histone H3 trimethylated lysine 4 (H3K4me3). H3K4me3 reader enzymes promiscuously bind H3K4me3Q5ser; Q5ser strongly inhibits H3K4 methylation erasers; these conditions favor gene expression in the presence of the H3K4me3Q5ser modification (Zheng et al., 2022). TGM2 is further capable of “writing” and “erasing” monoaminylation based on the concentrations of the amine substrates, lending to the idea that psychedelics could alter these histone marks (Zheng et al., 2022). Further investigation of histone monoaminylation will be required to determine whether this newly identified class of histone marks is part of the molecular underpinnings of depression, anxiety, etc., and if psychedelics impact this process, either by themselves being incorporated by TGM2, or by shifting the equilibrium of incorporated substrates.
Psychedelic drugs are also being trialed and showing great promise in SUDs (Noller et al., 2018; Meinhardt et al., 2021). In vivo, dopaminylation of histone H3 decreases with initial cocaine use and increases during withdrawal, regulating genes linked to cocaine seeking (Lepack et al., 2020). Withdrawal from heroin also increases H3 dopaminylation, resulting in transcriptional and behavioral changes (Fulton et al., 2022). Thus, it is possible that the success of psychedelics in this regimen also stems from the covalent modification of histones and other cellular targets.
Discussion
Psychedelics are gaining rapid momentum for treating neuropsychiatric disorders. Therefore, determining the molecular targets and signaling pathways of classical psychedelics is essential for understanding their effects on brain physiology and behavior and their potential therapeutic targets. While it is well established that the principal molecular target of classical psychedelics to elicit hallucinations and subjective effects is the 5-HT2A receptor widely expressed in cortical pyramidal neurons (González-Maeso et al., 2003; González-Maeso et al., 2007), there is an ongoing debate on whether 5-HT2A agonism is necessary for the plasticity-inducing and therapeutic actions of psychedelics (Fig. 1). A growing body of evidence reinforces the idea that psychedelics display complex polypharmacological profiles that go beyond their 5-HT2A-mediated hallucinogenic activity. Among their many targets, TrkB, 5-HT2C, and 5-HT1A are gathering increased attention as binding sites that play a key role in mediating psychedelics' plasticity and therapeutic actions, independently of hallucinogenic effects. It is possible, and likely, that psychedelics mediate their effects through several of these mechanisms. The spectrum of receptors activated by each compound may be key for optimizing use across different disorders. Furthermore, delineating the downstream effects of these targets may help optimize and refine the therapeutic effects for patients needing help. While technically challenging, there is also an increasing need to dissect circuit-level or brain-wide mechanisms of action of psychedelics, as they may provide further insight into how these compounds elicit their many complex and diverse actions.
Footnotes
This work was supported by National Institutes of Health/National Institute of General Medical Sciences R35GM133421 to J.D.M. MITACS accelerate grants IT27497 and IT24847 to A.A.-V.
The authors declare no competing financial interests.
References
- Alberts GL, Pregenzer JF, Im WB, Zaworski PG, Gill GS (1999) Agonist-induced GTPgamma35S binding mediated by human 5-HT(2C) receptors expressed in human embryonic kidney 293 cells. Eur J Pharmacol 383:311–319. 10.1016/s0014-2999(99)00653-6 [DOI] [PubMed] [Google Scholar]
- Al-Kachak A, Fulton SL, Farrelly LA, Lepack AE, Bastle RM, Kong L, Cathomas F, Newman EL, Menard C, Ramakrishnan A, Chan JC, Safovich P, Lyu Y, Covington HE, Shen L, Gleason K, Tamminga CA, Russo SJ, Maze I (2023) Histone H3 serotonylation dynamics in dorsal raphe nucleus contribute to stress-induced gene expression and behavior. bioRxiv. 10.1101/2023.05.04.539464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kachak A, Maze I (2023) Post-translational modifications of histone proteins by monoamine neurotransmitters. Curr Opin Chem Biol 74:102302. 10.1016/j.cbpa.2023.102302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper K, Dong B, Shah R, Sershen H, Vinod KY (2018) LSD Administered as a Single Dose Reduces Alcohol Consumption in C57BL/6J Mice. Front Pharmacol 9:994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper K, Cange J, Sah R, Schreiber-Gregory D, Sershen H, Vinod KY (2023) Psilocybin sex-dependently reduces alcohol consumption in C57BL/6J mice. Front Pharmacol 13:1074633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb JA, Linnros SE, Commons KG (2018) Evidence for intact 5-HT1A receptor-mediated feedback inhibition following sustained antidepressant treatment in a rat model of depression. Neuropharmacol 141:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Ross S, Bhatt S, Baron T, Forcehimes AA, Laska E, Mennenga SE, O'Donnell K, Owens LT, Podrebarac S, Rotrosen J, Tonigan JS, Worth L (2022) Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry 79:953–962. 10.1001/jamapsychiatry.2022.2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Buckholtz JW, Murphy DL (1996) Chronic administration of serotonergic antidepressants attenuates the subjective effects of LSD in humans. Neuropsychopharmacology 14:425–436. 10.1016/0893-133X(95)00145-4 [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA (2007) Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience 146:286–297. 10.1016/j.neuroscience.2006.12.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LP, Benson CJ, Dunlap LE, Olson DE (2018) Effects of N,N-dimethyltryptamine on rat behaviors relevant to anxiety and depression. ACS Chem Neurosci 9:1582–1590. 10.1021/acschemneuro.8b00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LP, et al. (2021) A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 589:474–479. 10.1038/s41586-020-3008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LP, Patel SD, Vargas MV, Barragan EV, Saeger HN, Warren HT, Chow WL, Gray JA, Olson DE (2023) 5-HT2ARs mediate therapeutic behavioral effects of psychedelic tryptamines. ACS Chem Neurosci 14:351–358. 10.1021/acschemneuro.2c00718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Bonomo Y, Pastor A, Collins L, Norman A, Galettis P, Johnstone J, Lawrence AJ (2021) The 5-HT2C receptor as a therapeutic target for alcohol and methamphetamine use disorders: a pilot study in treatment-seeking individuals. Pharmacol Res Perspect 9:e00767. 10.1002/prp2.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Bolstridge M, Demetriou L, Pannekoek JN, Wall MB, Tanner M, Kaelen M, McGonigle J, Murphy K, Leech R, Curran HV, Nutt DJ (2017) Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep 7:13187. 10.1038/s41598-017-13282-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto PC, et al. (2021) Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 184:1299–1313.e19. 10.1016/j.cell.2021.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey AB, Cui M, Booth RG, Canal CE (2022) 'Selective' serotonin 5-HT2A receptor antagonists. Biochem Pharmacol 200:115028. 10.1016/j.bcp.2022.115028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E, Antila H (2017) Neuronal plasticity and neurotrophic factors in drug responses. Mol Psychiatry 22:1085–1095. 10.1038/mp.2017.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Connery HS (2014) It's MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol 5:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Giguere PM, Schmerberg CM, Pogorelov VM, Rodriguiz RM, Huang XP, Zhu H, McCorvy JD, Wetsel WC, Roth BL, Kozikowski AP (2016a) Further advances in optimizing (2-phenylcyclopropyl)methylamines as novel serotonin 2C agonists: effects on hyperlocomotion, prepulse inhibition, and cognition models. J Med Chem 59:578–591. 10.1021/acs.jmedchem.5b01153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, McCorvy JD, Giguere PM, Zhu H, Kenakin T, Roth BL, Kozikowski AP (2016b) Design and discovery of functionally selective serotonin 2C (5-HT2C) receptor agonists. J Med Chem 59:9866–9880. 10.1021/acs.jmedchem.6b01194 [DOI] [PubMed] [Google Scholar]
- Chrysanthos N (2023) 'It's going to be for people with money': psychedelic treatments tipped to cost at least $25,000. Sydney Morning Herald. https://www.smh.com.au/politics/federal/it-s-going-to-be-for-people-with-money-psychedelic-treatments-tipped-to-cost-at-least-25-000-at-first-20230313-p5crpd.html. [Accessed June 24, 2023].
- Clifton PG, Lee MD, Dourish CT (2000) Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist and D-fenfluramine on feeding patterns in the rat. Psychopharmacology (Berl) 152:256–267. 10.1007/s002130000504 [DOI] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Duqueyroix D, Pasteau V, Millan MJ (2002) Differential activation of Gq/11 and Gi(3) proteins at 5-hydroxytryptamine(2C) receptors revealed by antibody capture assays: influence of receptor reserve and relationship to agonist-directed trafficking. Mol Pharmacol 62:578–589. 10.1124/mol.62.3.578 [DOI] [PubMed] [Google Scholar]
- Dai Y, Dudek NL, Patel TB, Muma NA (2008) Transglutaminase-catalyzed transamidation: a novel mechanism for Rac1 activation by 5-HT2A receptor stimulation. J Pharmacol Exp Ther 326:153–162. 10.1124/jpet.107.135046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, Finan PH, Griffiths RR (2021) Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry 78:481–489. 10.1001/jamapsychiatry.2020.3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, Roseman L, Nutt D, Carhart-Harris R (2022) Increased global integration in the brain after psilocybin therapy for depression. Nat Med 28:844–851. 10.1038/s41591-022-01744-z [DOI] [PubMed] [Google Scholar]
- De Gregorio D, Aguilar-Valles A, Preller KH, Heifets BD, Hibicke M, Mitchell J, Gobbi G (2021) Hallucinogens in mental health: pre-clinical and clinical studies on LSD, psilocybin, MDMA, and ketamine. J Neurosci 41:891–900. 10.1523/JNEUROSCI.1659-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Zhu B, Guevara CA, Naler LB, Saunders JM, Zhou Z, Toneatti R, Sierra S, Wolstenholme JT, Beardsley PM, Huntley GW, Lu C, González-Maeso J (2021) Prolonged epigenetic and synaptic plasticity alterations following single exposure to a psychedelic in mice. bioRxiv 432725. 10.1101/2021.02.24.432725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delix Therapeutics (2023) Delix Therapeutics announces completion of 1st cohort dosing, dose escalation approval in Phase I trial for novel compound DLX-001. https://www.delixtherapeutics.com/news/delix-therapeutics-announces-completion-of-1st-cohort-dosing-dose-escalation-approval-in-phase-i-trial-for-novel-compound-dlx-001/. [Accessed June 2023].
- Dong C, Ly C, Dunlap LE, Vargas MV, Sun J, Hwang IW, Azinfar A, Oh WC, Wetsel WC, Olson DE, Tian L (2021) Psychedelic-inspired drug discovery using an engineered biosensor. Cell 184:2779–2792.e18. 10.1016/j.cell.2021.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Syed SA, Flynn LT, Safi-Aghdam H, Cozzi NV, Ranganathan M (2022) Exploratory study of the dose-related safety, tolerability, and efficacy of dimethyltryptamine (DMT) in healthy volunteers and major depressive disorder. Neuropsychopharmacology 47:1854–1862. 10.1038/s41386-022-01344-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadahunsi N, Lund J, Breum AW, Mathiesen CV, Larsen IB, Knudsen GM, Klein AB, Clemmensen C (2022) Acute and long-term effects of psilocybin on energy balance and feeding behavior in mice. Transl Psychiatry 12:330. 10.1038/s41398-022-02103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer CA, Gilbert JR, Moaddel R, George J, Adeojo L, Lovett J, Nugent AC, Kadriu B, Yuan P, Gould TD, Park LT, Zarate CA Jr (2020) Ketamine metabolites, clinical response, and gamma power in a randomized, placebo-controlled, crossover trial for treatment-resistant major depression. Neuropsychopharmacology 45:1398–1404. 10.1038/s41386-020-0663-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly LA, et al. (2019) Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature 567:535–539. 10.1038/s41586-019-1024-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Li Z, Ji X, Higgins GA, Funk D, Lê AD (2022) Effects of pimavanserin and lorcaserin on alcohol self-administration and reinstatement in male and female rats. Neuropharmacology 215:109150. 10.1016/j.neuropharm.2022.109150 [DOI] [PubMed] [Google Scholar]
- Ford H, Fraser CL, Solly E, Clough M, Fielding J, White O, Van Der Walt A (2022) Hallucinogenic persisting perception disorder: a case series and review of the literature. Front Neurol 13:878609. 10.3389/fneur.2022.878609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton SL, Mitra S, Lepack AE, Martin JA, Stewart AF, Converse J, Hochstetler M, Dietz DM, Maze I (2022) Histone H3 dopaminylation in ventral tegmental area underlies heroin-induced transcriptional and behavioral plasticity in male rats. Neuropsychopharmacology 47:1776–1783. 10.1038/s41386-022-01279-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh C, Hossain M, Spriggs A, Ghosh A, Grant GA, Marchi N, Perucca E, Janigro D (2015) Sertraline-induced potentiation of the CYP3A4-dependent neurotoxicity of carbamazepine: an in vitro study. Epilepsia 56:439–449. 10.1111/epi.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1996) Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA 93:13473–13480. 10.1073/pnas.93.24.13473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC (2003) Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci 23:8836–8843. 10.1523/JNEUROSCI.23-26-08836.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53:439–452. 10.1016/j.neuron.2007.01.008 [DOI] [PubMed] [Google Scholar]
- Goodwin GM, et al. (2022) Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med 387:1637–1648. 10.1056/NEJMoa2206443 [DOI] [PubMed] [Google Scholar]
- Goodwin GM, et al. (2023) Single-dose psilocybin for a treatment-resistant episode of major depression: impact on patient-reported depression severity, anxiety, function, and quality of life. J Affect Disord 327:120–127. 10.1016/j.jad.2023.01.108 [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Smith RL, Barrett RJ, Sanders-Bush E (2005) Behavioral tolerance to lysergic acid diethylamide is associated with reduced serotonin-2A receptor signaling in rat cortex. Neuropsychopharmacology 30:1693–1702. 10.1038/sj.npp.1300711 [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA (2000) Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther 295:1183–1191. [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2011) Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61:364–381. 10.1016/j.neuropharm.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD (2020) Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 167:107933. 10.1016/j.neuropharm.2019.107933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JP, Botton D, Sagne C, Isambert MF, Desnos C, Blanchard V, Raisman-Vozari R, Krejci E, Massoulie J, Gasnier B (1994) Biochemistry and molecular biology of the vesicular monoamine transporter from chromaffin granules. J Exp Biol 196:251–262. 10.1242/jeb.196.1.251 [DOI] [PubMed] [Google Scholar]
- Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, Thompson SM (2021) Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci USA 118:2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Sellers EM, Fletcher PJ (2013) From obesity to substance abuse: therapeutic opportunities for 5-HT2C receptor agonists. Trends Pharmacol Sci 34:560–570. 10.1016/j.tips.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Horowski R, Löschmann PA (2019) Classical dopamine agonists. J Neural Transm (Vienna) 126:449–454. 10.1007/s00702-019-01989-y [DOI] [PubMed] [Google Scholar]
- Howell LL, Cunningham KA (2015) Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev 67:176–197. 10.1124/pr.114.009514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Raimondi F, Kadji FM, Singh G, Kishi T, Uwamizu A, Ono Y, Shinjo Y, Ishida S, Arang N, Kawakami K, Gutkind JS, Aoki J, Russell RB (2019) Illuminating G-protein-coupling selectivity of GPCRs. Cell 177:1933–1947.e25. 10.1016/j.cell.2019.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS (1998) 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA 95:735–740. 10.1073/pnas.95.2.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaster AM, Elder H, Marsh SA, de la Fuente Revenga M, Negus SS, González-Maeso J (2022) Effects of the 5-HT2A receptor antagonist volinanserin on head-twitch response and intracranial self-stimulation depression induced by different structural classes of psychedelics in rodents. Psychopharmacology (Berl) 239:1665–1677. 10.1007/s00213-022-06092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Halberstadt AL, Märcher-Rørsted E, Odland AU, Chatha M, Speth N, Liebscher G, Hansen M, Bräuner-Osborne H, Palner M, Andreasen JT, Kristensen JL (2020) The selective 5-HT2A receptor agonist 25CN-NBOH: structure-activity relationship, in vivo pharmacology, and in vitro and ex vivo binding characteristics of 3H]25CN-NBOH. Biochem Pharmacol 177:113979. 10.1016/j.bcp.2020.113979 [DOI] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R (2008) Human hallucinogen research: guidelines for safety. J Psychopharmacol 22:603–620. 10.1177/0269881108093587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Garcia-Romeu A, Griffiths RR (2017) Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse 43:55–60. 10.3109/00952990.2016.1170135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Ricard JA, Lipson J, Nock MK (2022) Associations between classic psychedelics and opioid use disorder in a nationally-representative U.S. adult sample. Sci Rep 12:4099. 10.1038/s41598-022-08085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P (2009) Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci USA 106:19575–19580. 10.1073/pnas.0905884106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan AL, et al. (2022) Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity. Nature 610:582–591. 10.1038/s41586-022-05258-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargbo RB (2022) Application of deuterated N,N-dimethyltryptamine in the potential treatment of psychiatric and neurological disorders. ACS Med Chem Lett 13:1402–1404. 10.1021/acsmedchemlett.2c00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst M, Halpern JH, Bernateck M, Passie T (2010) The non-hallucinogen 2-bromo-lysergic acid diethylamide as preventative treatment for cluster headache: an open, non-randomized case series. Cephalalgia 30:1140–1144. 10.1177/0333102410363490 [DOI] [PubMed] [Google Scholar]
- Kenakin T (2011) Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336:296–302. 10.1124/jpet.110.173948 [DOI] [PubMed] [Google Scholar]
- Kim K, Che T, Panova O, DiBerto JF, Lyu J, Krumm BE, Wacker D, Robertson MJ, Seven AB, Nichols DE, Shoichet BK, Skiniotis G, Roth BL (2020) Structure of a hallucinogen-activated Gq-coupled 5-HT2A serotonin receptor. Cell 182:1574–1588.e19. 10.1016/j.cell.2020.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot EF, Goncharuk SA, Franco ML, Arseniev AS, Benito-Martínez A, Costa M, Cattaneo A, Vilar M, Mineev KS (2023) Structural basis for the transmembrane signaling and antidepressant-induced activation of the receptor tyrosine kinase TrkB. bioRxiv 543881. 10.1101/2023.06.06.543881. [DOI] [Google Scholar]
- Kraehenmann R, Pokorny D, Vollenweider L, Preller KH, Pokorny T, Seifritz E, Vollenweider FX (2017) Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berl) 234:2031–2046. 10.1007/s00213-017-4610-0 [DOI] [PubMed] [Google Scholar]
- Kwan AC, Olson DE, Preller KH, Roth BL (2022) The neural basis of psychedelic action. Nat Neurosci 25:1407–1419. 10.1038/s41593-022-01177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Whalen EJ (2004) beta-arrestins: traffic cops of cell signaling. Curr Opin Cell Biol 16:162–168. 10.1016/j.ceb.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Lepack AE, et al. (2020) Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science 368:197–201. 10.1126/science.aaw8806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis V, Bonniwell EM, Lanham JK, Ghaffari A, Sheshbaradaran H, Cao AB, Calkins MM, Bautista-Carro MA, Arsenault E, Telfer A, Taghavi-Abkuh FF, Malcolm NJ, El Sayegh F, Abizaid A, Schmid Y, Morton K, Halberstadt AL, Aguilar-Valles A, McCorvy JD (2023) A non-hallucinogenic LSD analog with therapeutic potential for mood disorders. Cell Rep 42:112203. 10.1016/j.celrep.2023.112203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Giménez JF, González-Maeso J (2018) Hallucinogens and serotonin 5-HT2A receptor-mediated signaling pathways. Curr Top Behav Neurosci 36:45–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimemez JF, Vilaro MT, Milligan G (2008) Morphine desensitization, internalization, and down-regulation of the mu opioid receptor is facilitated by serotonin 5-hydroxytryptamine2A receptor coactivation. Mol Pharmacol 74:1278–1297. [DOI] [PubMed] [Google Scholar]
- Lukasak BJ, Mitchener MM, Kong L, Dul BE, Lazarus CD, Ramakrishnan A, Ni J, Shen L, Maze I, Muir TW (2022) TGM2-mediated histone transglutamination is dictated by steric accessibility. Proc Natl Acad Sci USA 119:e2208672119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC (2011) Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69:650–663. 10.1016/j.neuron.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister AK, Ori-McKenney KM, Gray JA, Olson DE (2018) Psychedelics promote structural and functional neural plasticity. Cell Rep 23:3170–3182. 10.1016/j.celrep.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen MK, Fisher PM, Burmester D, Dyssegaard A, Stenbæk DS, Kristiansen S, Johansen SS, Lehel S, Linnet K, Svarer C, Erritzoe D, Ozenne B, Knudsen GM (2019) Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 44:1328–1334. 10.1038/s41386-019-0324-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseille E, Mitchell JM, Kahn JG (2022) Updated cost-effectiveness of MDMA-assisted therapy for the treatment of post-traumatic stress disorder in the United States: findings from a Phase 3 trial. PLoS One 17:e0263252. 10.1371/journal.pone.0263252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi Y, Kawaguchi Y (2013) Time-dependent inhibition of CYP3A4 by sertraline, a selective serotonin reuptake inhibitor. Biopharm Drug Dispos 34:423–430. 10.1002/bdd.1857 [DOI] [PubMed] [Google Scholar]
- Mazza M, Kotzalidis GD, Marano G, De Berardis D, Martinotti G, Romagnoli E, Biondi-Zoccai G, Abbate A, Sani G (2023) Lorcaserin: worthy of further insights? Results from recent research. CNS Neurol Disord Drug Targets. Advance online publication. Retrieved Mar 30, 2023. 10.1002/bdd.1857. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–3537. 10.1523/JNEUROSCI.23-08-03531.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew L, Chang MS, Sanders-Bush E (2002) Phospholipase D activation by endogenous 5-hydroxytryptamine 2C receptors is mediated by Galpha13 and pertussis toxin-insensitive Gbetagamma subunits. Mol Pharmacol 62:1339–1343. 10.1124/mol.62.6.1339 [DOI] [PubMed] [Google Scholar]
- Meibach RC, Maayani S, Green JP (1980) Characterization and radioautography of 3H]LSD binding by rat brain slices in vitro: the effect of 5-hydroxytryptamine. Eur J Pharmacol 67:371–382. 10.1016/0014-2999(80)90178-8 [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, et al. (2021) Psilocybin targets a common molecular mechanism for cognitive impairment and increased craving in alcoholism. Sci Adv 7:eabh2399. 10.1126/sciadv.abh2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Z, Si T, Kapadia K, Li Q, Muma NA (2017) Receptor-stimulated transamidation induces activation of Rac1 and Cdc42 and the regulation of dendritic spines. Neuropharmacol 117:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliner R, et al. (2023) Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat Neurosci 26:1032–1041. 10.1038/s41593-023-01316-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muma NA, Mi Z (2015) Serotonylation and transamidation of other monoamines. ACS Chem Neurosci 6:961–969. 10.1021/cn500329r [DOI] [PubMed] [Google Scholar]
- Nelson DL, Lucaites VL, Wainscott DB, Glennon RA (1999) Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT(2B) and 5-HT2C receptors. Naunyn Schmiedebergs Arch Pharmacol 359:1–6. 10.1007/pl00005315 [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2001) Molecular neurobiology of addiction. Am J Addict 10:201–217. 10.1080/105504901750532094 [DOI] [PubMed] [Google Scholar]
- Nichols DE (2004) Hallucinogens. Pharmacol Ther 101:131–181. 10.1016/j.pharmthera.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Noller GE, Frampton CM, Yazar-Klosinski B (2018) Ibogaine treatment outcomes for opioid dependence from a twelve-month follow-up observational study. Am J Drug Alcohol Abuse 44:37–46. 10.1080/00952990.2017.1310218 [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Strack AM, Dallman MF, Tecott LH (1998) Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med 4:1152–1156. 10.1038/2647 [DOI] [PubMed] [Google Scholar]
- Odland AU, Jessen L, Kristensen JL, Fitzpatrick CM, Andreasen JT (2021) The 5-hydroxytryptamine 2A receptor agonists DOI and 25CN-NBOH decrease marble burying and reverse 8-OH-DPAT-induced deficit in spontaneous alternation. Neuropharmacology 183:107838. 10.1016/j.neuropharm.2019.107838 [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano PR (2013) Cytochrome P450-activated prodrugs. Future Med Chem 5:213–228. 10.4155/fmc.12.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JM, Pazos A, Hoyer D (2017) A short history of the 5-HT2C receptor: from the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology (Berl) 234:1395–1418. 10.1007/s00213-017-4545-5 [DOI] [PubMed] [Google Scholar]
- Palhano-Fontes F, et al. (2019) Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med 49:655–663. 10.1017/S0033291718001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang G, Wu X, Tao X, Mao R, Liu X, Zhang YM, Li G, Stackman RW Jr, Dong L, Zhang G (2016) Blockade of serotonin 5-HT2A receptors suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-treated mice. Front Pharmacol 7:514. 10.3389/fphar.2016.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14:7–23. 10.1038/nrn3379 [DOI] [PubMed] [Google Scholar]
- Qu Y, Chang L, Ma L, Wan X, Hashimoto K (2023) Rapid antidepressant-like effect of non-hallucinogenic psychedelic analog lisuride, but not hallucinogenic psychedelic DOI, in lipopolysaccharide-treated mice. Pharmacol Biochem Behav 222:173500. 10.1016/j.pbb.2022.173500 [DOI] [PubMed] [Google Scholar]
- Ray TS (2010) Psychedelics and the human receptorome. PLoS One 5:e9019. 10.1371/journal.pone.0009019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR (2001) A cellular mechanism of reward-related learning. Nature 413:67–70. 10.1038/35092560 [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley MC, Levin ED (2014) Lorcaserin, a selective 5-HT(2C) receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacol Biochem Behav 125:8–14. 10.1016/j.pbb.2014.07.017 [DOI] [PubMed] [Google Scholar]
- Rocha BA, Goulding EH, O'Dell LE, Mead AN, Coufal NG, Parsons LH, Tecott LH (2002) Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci 22:10039–10045. 10.1523/JNEUROSCI.22-22-10039.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M, Sánchez-Blázquez P, Vicente-Sánchez A, Berrocoso E, Garzón J (2012) The Mu-Opioid Receptor and the NMDA Receptor Associate in PAG Neurons: Implications in Pain Control. Neuropsychopharmacol 37:338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat JD, Leon-Carlyle M, Ali S, Husain MI, MR S (2023) Antidepressant effects of psilocybin in the absence of psychedelic effects. Am J Psychiatry 59:412–413. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH (2009) Serotonergic drugs and valvular heart disease. Expert Opin Drug Saf 8:317–329. 10.1517/14740330902931524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ (2010) The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 33:267–276. 10.1016/j.tins.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, González-Maeso J (2023) The crosstalk between 5-HT2AR and mGluR2 in schizophrenia. Neuropharmacology 230:109489. 10.1016/j.neuropharm.2023.109489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakloth F, Leggett E, Moerke MJ, Townsend EA, Banks ML, Negus SS (2019) Effects of acute and repeated treatment with serotonin 5-HT2A receptor agonist hallucinogens on intracranial self-stimulation in rats. Exp Clin Psychopharmacol 27:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu M, Cho A, Ma N, Mukhaleva E, Namkung Y, Lee S, Ghosh S, Lee JH, Gloriam DE, Laporte SA, Babu MM, Vaidehi N (2022) Dynamic spatiotemporal determinants modulate GPCR: G protein coupling selectivity and promiscuity. Nat Commun 13:7428. 10.1038/s41467-022-34055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW (2016) The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev 68:816–871. 10.1124/pr.116.012484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell RA, Halpern JH, Pope HG Jr (2006) Response of cluster headache to psilocybin and LSD. Neurology 66:1920–1922. 10.1212/01.wnl.0000219761.05466.43 [DOI] [PubMed] [Google Scholar]
- Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, Kwan AC (2021) Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109:2535–2544.e4. 10.1016/j.neuron.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra S, Muchhala KH, Jessup DK, Contreras KM, Shah UH, Stevens DL, Jimenez J, Cuno Lavilla XK, de la Fuente Revenga M, Lippold KM, Shen S, Poklis JL, Qiao LY, Dewey WL, Akbarali HI, Damaj MI, González-Maeso J (2022) Sex-specific role for serotonin 5-HT2A receptor in modulation of opioid-induced antinociception and reward in mice. Neuropharmacology 209:108988. 10.1016/j.neuropharm.2022.108988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E (1999) Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology (Berl) 144:248–254. 10.1007/s002130051000 [DOI] [PubMed] [Google Scholar]
- Tabbara RI, Li Z, Fletcher PJ, Lê AD (2021) The serotonin 2C receptor agonist lorcaserin, alone and in combination with the opioid receptor antagonist naltrexone, attenuates binge-like ethanol drinking. Addict Biol 26:e13040. 10.1111/adb.13040 [DOI] [PubMed] [Google Scholar]
- Tan L, Yan W, McCorvy JD, Cheng J (2018) Biased ligands of G protein-coupled receptors (GPCRs): structure-functional selectivity relationships (SFSRs) and therapeutic potential. J Med Chem 61:9841–9878. 10.1021/acs.jmedchem.8b00435 [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D (1995) Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374:542–546. 10.1038/374542a0 [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D (2008) Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325:577–587. 10.1124/jpet.107.133348 [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Joharchi N, Tampakeras M, Martin JR, Wichmann J, Higgins GA (2002) An investigation of the role of 5-HT(2C) receptors in modifying ethanol self-administration behaviour. Pharmacol Biochem Behav 71:735–744. 10.1016/s0091-3057(01)00710-9 [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411:583–587. 10.1038/35079077 [DOI] [PubMed] [Google Scholar]
- Vargas MV, Meyer R, Avanes AA, Rus M, Olson DE (2021) Psychedelics and other psychoplastogens for treating mental illness. Front Psychiatry 12:727117. 10.3389/fpsyt.2021.727117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, Cameron LP, Patel SD, Hennessey JJ, Saeger HN, McCorvy JD, Gray JA, Tian L, Olson DE (2023) Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 379:700–706. 10.1126/science.adf0435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Grieder TE, Ting-A-Kee R, Maal-Bared G, Chwalek M, van der Kooy D (2017) A single administration of the hallucinogen, 4-acetoxy-dimethyltryptamine, prevents the shift to a drug-dependent state and the expression of withdrawal aversions in rodents. Eur J Neurosci 45:1410–1417. 10.1111/ejn.13572 [DOI] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH (1999) Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl) 143:309–314. 10.1007/s002130050952 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Preller KH (2020) Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci 21:611–624. 10.1038/s41583-020-0367-2 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9:3897–3902. 10.1097/00001756-199812010-00024 [DOI] [PubMed] [Google Scholar]
- Weber E, Andrade R (2010) Htr2a gene and 5-HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci 4:36. 10.3389/fnins.2010.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA (2006) Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313:536–540. 10.1126/science.1123432 [DOI] [PubMed] [Google Scholar]
- Yaden DB, Griffiths RR (2021) The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci 4:568–572. 10.1021/acsptsci.0c00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC (1997) Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol 378:135–157. [DOI] [PubMed] [Google Scholar]
- Zeifman RJ, Wagner AC, Monson CM, Carhart-Harris RL (2023) How does psilocybin therapy work? An exploration of experiential avoidance as a putative mechanism of change. J Affect Disord 334:100–112. 10.1016/j.jad.2023.04.105 [DOI] [PubMed] [Google Scholar]
- Zhang LY, Zhou YQ, Yu ZP, Zhang XQ, Shi J, Shen HW (2021) Restoring glutamate homeostasis in the nucleus accumbens via endocannabinoid-mimetic drug prevents relapse to cocaine seeking behavior in rats. Neuropsychopharmacology 46:970–981. 10.1038/s41386-021-00955-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Chen W, Pan Y, Zhang Y, Sun H, Wang H, Yang F, Liu Y, Shen N, Zhang X, Mo X, Zang J (2021) Structural insights into the recognition of histone H3Q5 serotonylation by WDR5. Sci Adv 7:eabf4291. 10.1126/sciadv.abf4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanava M, Pilon D, Ghelerter I, Chow W, Joshi K, Lefebvre P, Sheehan JJ (2021) The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J Clin Psychiary 82:20m13699. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Bastle RM, Zhao S, Kong L, Vostal L, Ramakrishnan A, Shen L, Fulton SL, Wang H, Zhang B, Upad A, Thompson RE, Molina H, Stransky S, Sidoli S, Muir TW, Li H, David Y, Maze I (2022) Histone monoaminylation dynamics are regulated by a single enzyme and promote neural rhythmicity. bioRxiv 519310. 10.1101/2022.12.06.519310. [DOI] [Google Scholar]


