Abstract
Deep brain stimulation (DBS) is an effective therapy for various neurologic and neuropsychiatric disorders, involving chronic implantation of electrodes into target brain regions for electrical stimulation delivery. Despite its safety and efficacy, DBS remains an underutilized therapy. Advances in the field of DBS, including in technology, mechanistic understanding, and applications have the potential to expand access and use of DBS, while also improving clinical outcomes. Developments in DBS technology, such as MRI compatibility and bidirectional DBS systems capable of sensing neural activity while providing therapeutic stimulation, have enabled advances in our understanding of DBS mechanisms and its application. In this review, we summarize recent work exploring DBS modulation of target networks. We also cover current work focusing on improved programming and the development of novel stimulation paradigms that go beyond current standards of DBS, many of which are enabled by sensing-enabled DBS systems and have the potential to expand access to DBS.
Keywords: coordinated reset, deep brain stimulation, evoked potentials, functional magnetic resonance imaging, sweet spot mapping
Introduction
Deep brain stimulation (DBS) is a surgical therapy involving the implantation of electrodes into target brain regions to deliver stimulation. DBS is an effective treatment for various neurologic and neuropsychiatric disorders. The FDA has approved DBS in the treatment of Parkinson's disease (PD), essential tremor (ET), and epilepsy, and granted Humanitarian Device Exemptions for the treatment of dystonia and obsessive compulsive disorder (OCD) (Perlmutter and Mink, 2006). Investigational indications for DBS include depression, Tourette syndrome, Alzheimer's disease, and post-traumatic stress disorder. Although DBS has become standard of care for PD and other neurologic disorders, only a limited number of patients receive this therapy. This may reflect its invasive nature, the high cost of treatment (including surgery and postoperative programming visits), and limited access to specialized care by the patient (Bronstein et al., 2011; Chan et al., 2014; Willis et al., 2014; Kestenbaum et al., 2015).
It is estimated that >244,000 DBS systems have been implanted worldwide, yet the mechanism by which DBS provides its therapeutic benefit remains unclear (Wong et al., 2023). Current research suggests that DBS functions via multiple mechanisms that differ based on indication and symptom (Herrington et al., 2016; Ashkan et al., 2017). The DBS system involves internal and external components, including implanted electrodes, an implantable pulse generator (IPG), extension cables connecting the electrodes and IPG, a clinician programmer for setting and optimizing DBS parameters, and a patient programmer (Fig. 1A) (Sarem-Aslani and Mullett, 2011). Recent innovations in DBS technology include MRI compatibility, rechargeable IPGs, segmented electrodes for directional stimulation (Schüpbach et al., 2017), and bidirectional systems capable of sensing neural activity (local field potentials [LFPs]) while simultaneously delivering therapeutic stimulation (Cummins et al., 2021; Feldmann et al., 2021). These technological advances have enabled further studies of neuromodulation and led to optimization of DBS, including surgical implantation, stimulation parameter selection, and even the timing of stimulation delivery, in search of enhancing clinical outcomes. Furthermore, novelties in DBS technology have contributed to our ever-evolving understanding of DBS mechanisms, which in turn encourages the widespread adoption of DBS therapy across patients, targets, and indications.
Figure 1.

Schematic of internal DBS system components and example results across targeting techniques. A, The DBS system involves internal and external components, including implanted electrodes, an IPG, extension cables connecting the electrodes and IPG. Not shown is the clinician programmer for setting and optimizing DBS parameters, and the patient programmer. Some components of this schematic were created with Biorender.com. B, An example of sweet spot mapping for motor progression and white matter tracts associated with motor progression in patients with Parkinson's disease. Adapted from Hacker et al. (2023). Top left, Coronal. Top right, Axial. Purple outlines the subthalamic nucleus. Red represents red nucleus. White dashed line indicates Bejjani line. C, An example of circuitry characteristics derived from fMRI in a patient with OCD. D, Neuroplastic reductions in OCD-related cortico-striatal hyperconnectivity are also apparent after chronic stimulation, with unique STN-frontostriatal coupling when DBS is off that may reflect disease spread. Caud = Caudate Putamen, VST = Ventral Striatum, ACC = Anterior Cingulate Cortex, STN = Subthalamic nucleus, OFC = orbitofrontal cortex.
Here, we summarize developments in elucidating DBS mechanisms and applying DBS using novel technologies. We begin by providing an overview of recent works that elucidate macro network scale mechanisms of DBS. This is followed by a review of DBS techniques for optimizing the selection of stimulation parameters and the timing of stimulation delivery. We end with a discussion of how advancements in DBS applications may lead to its increased accessibility.
Advances in mechanisms: a network targeting approach
An incomplete understanding of DBS therapeutic mechanisms persists as a fundamental challenge in successfully implementing and optimizing the therapy, especially in investigative indications. Preliminary theories included local suppression of pathologic activity in the target region stemming from the rate model in PD (Boraud et al., 1996; Benazzouz and Hallett, 2000; Dostrovsky et al., 2000), and the decoupling of axon and soma (e.g., an informational lesion) (Grill et al., 2004; McIntyre et al., 2004b; Lowet et al., 2022). Computational modeling (McIntyre et al., 2004a; Farokhniaee and McIntyre, 2019; Bower and McIntyre, 2020) has been used to elucidate single-neuron modulation from stimulation and synaptic plasticity. The extensive and multidisciplinary preclinical and clinical research done to identify the mechanism of DBS action and the resultant divergent conclusions have led to one unified theory: that DBS functions through a multimodal mechanism (for a recent review of DBS mechanisms, see Neumann et al., 2023). A current leading hypothesis postulates that therapeutic benefits of DBS arise from modulating activity throughout target networks (Lozano and Lipsman, 2013; A. Horn et al., 2017; Sobesky et al., 2022; Neumann et al., 2023), which is consistent with the observation that many of the indications treated with DBS are considered network disorders (Prudente et al., 2014; Kaiser et al., 2015; Kim et al., 2017; B. Li et al., 2018; Zhu et al., 2019; Bijanki et al., 2021; Yu et al., 2021). Thus, DBS can be considered a network therapy (Neumann et al., 2023), making the identification of appropriate target networks vital to the optimization of DBS outcomes.
Sweet spot mapping
DBS lead location is a key factor in postsurgical outcomes and underscores the importance of identifying and accurately probing target networks for optimal clinical results (Vitek et al., 2022; Hacker et al., 2023). MRI (Lanotte et al., 2002; Starr et al., 2002; Johnsen et al., 2010) and stereotactic atlas-based targeting (Godinho et al., 2006; Vergani et al., 2007; Zheng et al., 2009) are used for surgical planning to identify and place the electrode in the intended target region. Intraoperatively, microelectrode array recordings can be performed to identify movement-related single units and to map the borders of the target region (D. D. Wang et al., 2018; Koirala et al., 2020), and postoperative imaging is often used to confirm electrode placement (Lanotte et al., 2002; Cintas et al., 2003; Yelnik et al., 2003; Zonenshayn et al., 2004; Maks et al., 2009; Johnsen et al., 2010). Computational models of stimulation informed by electrode location are often used to relate the stimulation area (e.g., volume of tissue activation) to patient outcomes and to define the “sweet spot” within target brain regions (Dembek et al., 2019; Reich et al., 2019; Lumsden et al., 2022; Hacker et al., 2023). Considering DBS' network effects, computational models of fiber activation using techniques, such as fiber filtering, identify possible modulated structural networks implicated in positive DBS outcomes (Neudorfer et al., 2023). A 2022 study compared sweet spots in the globus pallidus for the treatment of cervical versus generalized dystonia (A. Horn et al., 2022) and found that the posterior ventromedial globus pallidus internus (GPi) and modulation of pallidosubthalamic fibers and corticospinal fibers led to optimal treatment of cervical dystonia, while targeting a more anterior and dorsal subregion of the GPi, modulating the pallidothalamic tracts, led to optimal outcomes for generalized dystonia. A recent study by Hacker et al. (2023) describes a potential DBS subthalamic nucleus (STN) sweet spot for early-stage PD that is more ventral and lateral than that for late-stage PD (Fig. 1B) (Yelnik et al., 2003; Caire et al., 2013). Hacker et al. (2023) suggest that targeting the early-stage sweet spot with DBS induces motor improvement and slower motor progression through modulation of fibers projected from the supplementary motor area and primary motor cortex to the STN. Collectively, these works support the hypothesis that DBS functions by modulating networks downstream from the target structure (Neumann et al., 2023).
fMRI
There is a growing consensus that abnormal network oscillations underlie motor dysfunction in movement disorders and certain symptoms of neuropsychiatric conditions treated by DBS (Graybiel and Rauch, 2000; Brown, 2003). This has led to interest in identifying network-based biomarkers of DBS clinical response to verify and guide the selection of therapeutic stimulation parameters. Advances in DBS technology enabling implanted patients to undergo whole-brain 1.5T or 3T MRI while on stimulation (Boutet et al., 2020; Medtronic, 2021) have made fMRI an attractive technique for probing whole-brain DBS effects in the intraoperative and postoperative setting (Fig. 1C) (for a recent systemic review, see Loh et al., 2022).
Timing of imaging and the fMRI paradigm are leading sources of variability within the field. Results are sensitive to either acute stimulation effects, including microlesion phenomena if performed during surgery or in the weeks following, or chronic stimulation effects if performed after DBS parameter optimization (Miao et al., 2022). fMRI paradigms typically involve either (1) continuously cycling stimulation for predetermined intervals during rest or (2) comparisons between rest and task performance during DBS on and off states. Such fMRI paradigms can provide insight into DBS- and task-evoked BOLD response, and stimulation effects on functional connectivity. Despite differences across studies, DBS effects on brain function have largely been reproducible. For example, cycling STN DBS in PD consistently alters BOLD activity of the cerebellum and the cortico-basal ganglia-thalamo-cortical network, although the directionality conflicts across studies (see Loh et al., 2022). Similarly, studies consistently report stimulation-induced increased cortico-thalamic connectivity during rest, but at least one study has shown the opposite (Zhang et al., 2021). Importantly, these effects have been related to and even predictive of PD motor symptom improvement with DBS, which paves the way for future efforts aiming to establish fMRI as a clinical programming tool (Boutet et al., 2021). Other important work includes studies showing DBS effects change overtime, including compared with healthy controls, and how it differs across PD subtypes and patient-specific stimulation parameters (Loh et al., 2022). These studies contribute to our understanding of DBS therapeutic mechanisms.
With GPi now an established target for PD and dystonia, recent efforts have been focused on understanding how GPi-DBS modulates motor networks (Filip et al., 2022; Z. Li et al., 2023). Evidence of GPi-DBS-evoked brain network normalization and cortico-thalamic connectivity changes that correlate with motor improvement overlap with the current understanding of STN-DBS modulatory effects and further highlights similarities across disorders of basal ganglia dysfunction treated with the same target. Such transdiagnostic approaches are emergent for DBS management of neuropsychiatric conditions, where symptom domains overlap and far fewer patients are implanted compared with movement disorders (Allawala et al., 2021). For these conditions and others like dystonia, where the clinical benefits of DBS can take weeks to months to be realized, fMRI stands out as a powerful tool for understanding the time course of DBS effects and establishing a way to choose efficacious stimulation parameters with limited patient feedback during programming. In a recent fMRI study from the Morrison laboratory and collaborators at the University of California–San Francisco, patients with medication refractory OCD receiving DBS to the anterior limb of the internal capsule, underwent a 1 min cycling paradigm during imaging to evaluate differences in functional brain response to therapeutic versus nontherapeutic DBS (Bohara et al., 2023; Slepneva et al., 2023). For each DBS setting, functional connectivity maps (i.e., FCON and FCOFF) seeded in the anterior cingulate cortex (ACC), a gray matter structure connected to the anterior limb of the internal capsule and implicated in OCD, were generated from concatenated stimulation blocks. BOLD activation maps (i.e., BOLDON>OFF) were also computed. Initial findings demonstrated that therapeutic stimulation settings reduced BOLD activity in areas consistent with the default mode network (Fig. 1D) (Slepneva et al., 2023). Similarly, connectivity between the ACC and frontal areas decreased with therapeutic stimulation (Bohara et al., 2023). In novel work performed by the same group, longitudinal analysis of fMRI data from a responder patient in the same cohort showed notable pre-to-post reductions in ACC-striatal connectivity and regional BOLD variability that were persistent with and without active stimulation, implying that DBS has some neuroplastic effects on the brain. More longitudinal fMRI-DBS studies that incorporate adequate time for stimulation effect washout and with thorough symptom assessment at each time point are needed. Additionally, larger cohort studies, including multiparametric and multimodal studies that repeat and/or combine paradigms while incorporating ground truth neural recordings are needed to determine fMRI reliability and further our understanding of DBS circuit engagement.
Here, we have discussed a few of the many approaches used to elucidate mechanisms of DBS, specifically sweet spot mapping and fMRI. These methods may improve DBS clinical outcomes by optimizing lead localization and therapeutic stimulation parameter selection. In indications for which DBS is not commonly used (e.g., OCD and depression), greater efforts should be made to enhance collaboration or establish databases to hasten understanding and widespread implementation of DBS. As discussed, future work may examine how these measurements may be combined with or related to one another in multimodal analyses to decipher causal relationships between applications and therapeutic outcomes across targeted networks.
Advances in DBS applications
Advances in programming: neural activity informed programming
Optimal stimulation parameter selection is as important to DBS outcomes as lead placement. DBS programming aims to determine stimulation parameters that maximize therapeutic benefit while minimizing DBS-induced side effects. Modifiable DBS parameters include stimulation contact(s), contact configurations (e.g., monopolar vs bipolar), amplitude, frequency, and pulse width (see Fig. 3A). DBS programming is a tedious, trial-and-error process that often requires regular reprogramming, which impacts the amount of time patients spend in the clinic (Bronstein et al., 2011). As previously discussed, complexities in programming arise when symptom change is observed on the scale of weeks to months (e.g., psychiatric disorders) compared with immediately [e.g., PD or ET (Crowell et al., 2019)]. The advent of directional leads, which introduced cylindrical contacts segmented into three separate electrodes, has allowed for improved sweet spot targeting and programming by enabling stimulation “steering” (Petry-Schmelzer et al., 2019; Paff et al., 2020; Fricke et al., 2021; Frey et al., 2022). However, directional leads have made an already laborious and time-consuming process even more complicated by increasing the number of possible parameter combinations (Brinke et al., 2018). Thus, there is an urgent need to improve or automate DBS programing to maximize the therapeutic benefit of DBS.
Figure 3.
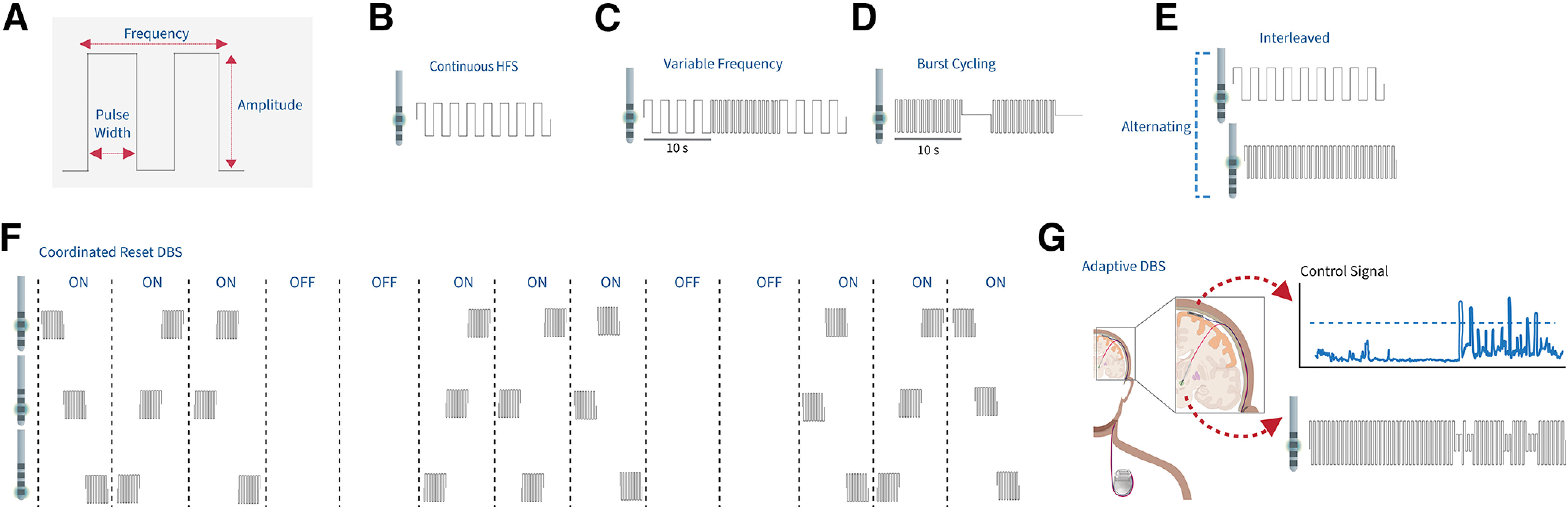
Stimulation paradigms beyond standard high-frequency DBS. A, Schematic of the DBS stimulation pulse, including the amplitude, pulse width, and frequency. B, Continuous high-frequency DBS delivers stimulation at >130 Hz with no change to stimulation amplitude, frequency, pulse width, or active contact. C, Variable frequency stimulation involves stimulating in alternating blocks of high-frequency stimulation and low-frequency stimulation (60-80 Hz). D, Burst cycling DBS delivers bursts of stimulation at the same intraburst frequency as HFS but with an interburst frequency ranging from 4 to 15 Hz. E, Interleaved stimulation alternates between two stimulation programs in which each can have independent amplitude, frequencies, pulse widths, and active contacts. F, Coordinated reset DBS in which the order of the contacts being stimulated is shuffled between each set of stimulation blocks. G, In this example of an adaptive DBS paradigm (components of the schematic were created in Biorender.com), a cortical control signal is being used to control stimulation amplitude on the depth lead within the STN. Once the control signal exceeds a predefined threshold (shown as the blue dotted line), stimulation amplitude decreases to avoid any stimulation-induced symptoms, such as dyskinesia.
Neural sensing for biomarker-driven programming
Next-generation DBS systems capable of sensing LFPs while simultaneously providing therapeutic stimulation have enabled the use of neural biomarker activity as an output for DBS parameter selection. For example, spontaneous oscillatory activity, particularly STN and GPi β (13-30 Hz) activity, is correlated with bradykinesia and rigidity severity in PD (Kühn et al., 2006, 2008). However, contradictory results report this correlation in only 50% of patients treated with STN DBS (Strelow et al., 2022). Nevertheless, recent studies have suggested selecting the contact with the highest beta power for therapeutic stimulation. A 2021 study assessing β spectral power during GPi DBS suggests that reduction in spectral power correlates with PD symptoms (Cagle et al., 2021) and might aid DBS parameter selection. Such findings, together with novel directional leads capable of revealing the spatial distribution of neural biomarkers (Aman et al., 2020), open the door for the development of automated algorithms to guide stimulation parameter selection in PD.
Bidirectional DBS systems have been instrumental in programming optimization beyond movement disorders. It is thought that ventral capsule/ventral striatum (VC/VS) DBS, used to treat OCD, achieves its therapeutic effect by reducing hyperactive fronto-striatal network activity. An ongoing clinical trial aims to identify neural biomarkers of symptom intensity for OCD. In Phase I of the study (NCT03457675), patients received bilateral VC/VS DBS, with leads connected to the investigational Medtronic Summit RC+S device, a second-generation sensing-enabled IPG (Stanslaski et al., 2018). Over 1000 h of bilateral VC/VS LFPs were collected during naturalistic exposures to OCD triggers and used to identify neural activity related to symptom intensity. Low δ-band power emerged as a candidate neural biomarker of OCD symptom intensity during symptom provocation in one individual (Provenza et al., 2021). To expand on initial findings, Phase II of the study (NCT04281134) included chronic electrocorticography (ECoG) electrodes subdurally implanted over orbitofrontal cortex (OFC) to evaluate the utility of orbitofrontal recordings in addition to VC/VS LFPs for biomarker identification. To investigate neural activity related to side effects of stimulation, LFPs were recorded during stimulation amplitude changes in the clinic. Initial electrophysiological analysis by Nicole Provenza from Baylor College of Medicine assessing acute response to bilateral stimulation amplitude increases revealed elevated 30 Hz spectral power specific to lateral OFC associated with increased engagement and approach behaviors that may be a precursor to hypomania (Provenza et al., 2023). These data suggest divergent neural biomarkers of these two competing states (i.e., increased OCD symptoms and hypomania), allowing for simultaneous optimization. Although preliminary, multisite recordings in patients with OCD will enable future investigations of neurophysiological measures of VC/VS-OFC connectivity, bringing us closer to identifying an OCD severity biomarker that may be used for programming optimization.
Evoked potentials (EPs)
Beyond spectral power, stimulation-evoked responses, particularly evoked resonant neural activity (ERNA), have also shown promise as potential biomarkers to guide DBS programming. ERNA is a high-frequency (200-500 Hz) and high-amplitude (∼100-1000 uV) oscillation that occurs in response to each DBS pulse (Fig. 2A,B). ERNA was initially characterized in the STN (Sinclair et al., 2018) and potentially localized to the dorsal region, the optimal target for DBS lead implantation (Sinclair et al., 2019, 2021). A recent study established that ERNA also occurs during GPi DBS and may be localized to a postero-dorsal region spanning the GPi-GPe border (K. A. Johnson et al., 2023). In addition, the contact eliciting the highest amplitude ERNA with STN (Xu et al., 2022) or GPi (K. A. Johnson et al., 2023) DBS may correspond with the therapeutic contact used for chronic DBS. However, the precise localization of ERNA and its potential for tuning based on the direction of stimulation within the GPi or STN remains unknown. Recent work from the de Hemptinne laboratory at the University of Florida by Kara A. Johnson assessed ERNA with intraoperative directional STN or GPi DBS in PD patients. Preliminary results demonstrate direction-specific stimulation ERNA activity, suggesting its promise as a biomarker for effective stimulation parameters while harnessing the full capabilities of directional DBS leads.
Figure 2.
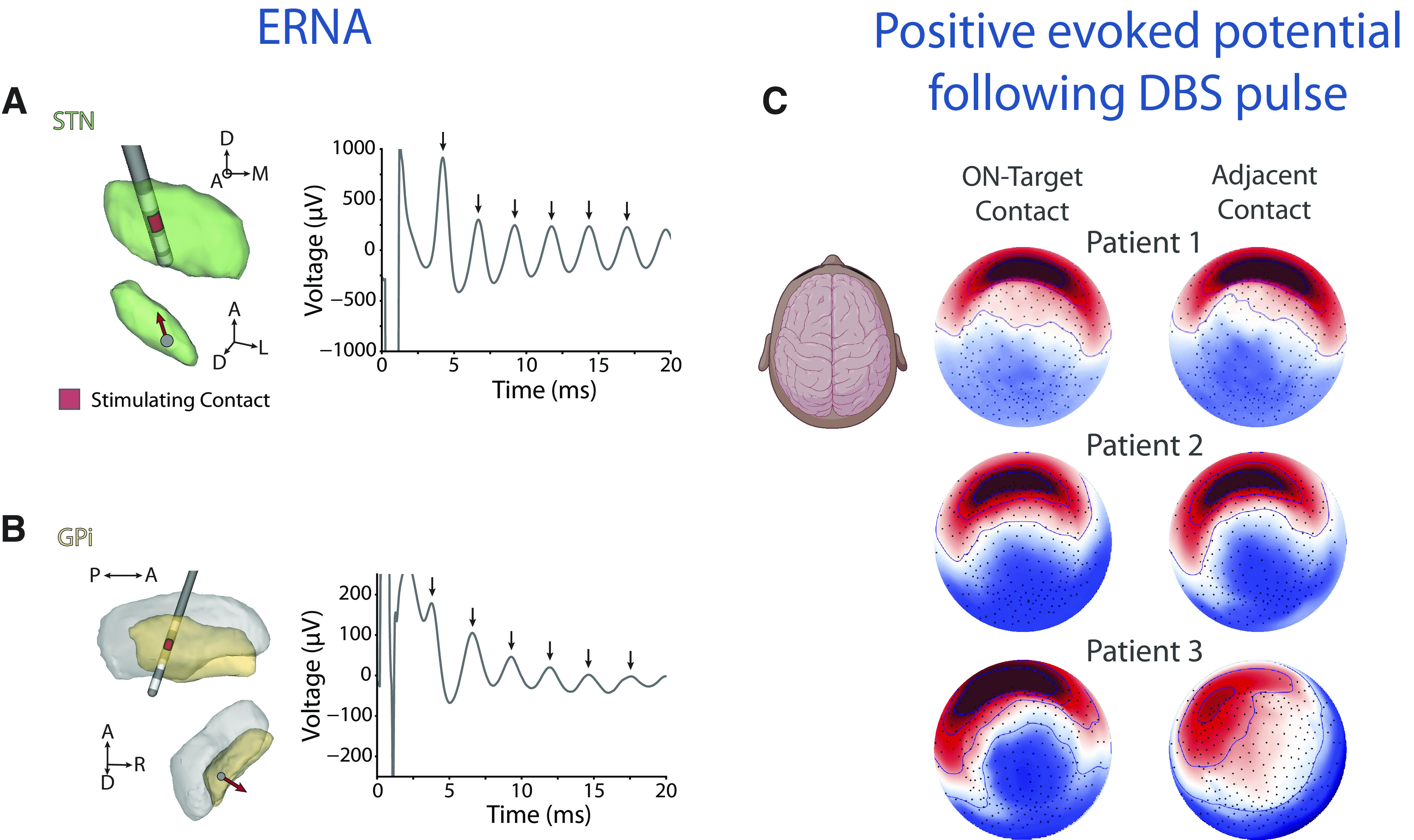
EPs from DBS stimulation both within the target and on the cortex. Examples of intraoperative ERNA with (A) STN DBS and (B) GPi DBS using directional DBS leads. A, B, Left, The electrode locations and stimulation contacts. Red contact represents the contact used for stimulation on the lead. Arrow within the nuclei indicates the directional contact used for stimulation. Each arrow on the time domain plots (A, B, right) indicates an evoked response after a DBS pulse. C, Examples of cortical topography of the evoked response at 40 ms (P40) following a DBS pulse to the on-target contact (top row) and adjacent contact (bottom row) in 3 patients undergoing SCC-DBS for TRD. Saturated color represents higher amplitude (red represents positive; blue represents negative). Included components created with Biorender.com.
Targeting strategies that work for movement disorders may be less applicable to DBS treatment for psychiatric disorders, such as subcallosal cingulate (SCC) DBS for treatment-resistant depression (TRD) (Bergfeld et al., 2018; Crowell et al., 2019). While precise surgical targeting of structures within the SCC region is associated with better treatment outcomes (Riva-Posse et al., 2018, 2020), device parameter adjustment in the clinic is critical to confirm target engagement. A compelling solution to this problem is perturbation mapping, which provides a spatiotemporal pattern of voltage fluctuations conventionally observed in the form of a cortical EP (Waters et al., 2018). Stimulation EPs are derived by averaging an electrophysiological signal across hundreds of pulse-locked, stimulation-recording events and can be obtained using both invasive and noninvasive stimulation and recording strategies. Perturbation maps of the SCC possess the key design characteristics of a viable patient-level biomarker. They are feasibly collected in a clinical setting with EEG (Fig. 2C), and the signal is highly sensitive to programming parameters used to optimize target engagement following device implantation (Waters et al., 2018). EPs are sensitive to changes in dose at the mA-scale, as well as location within the target region at the mm-scale, surpassing other imaging methods that elucidate the effects of parameter adjustments in DBS (Smith et al., 2022). The SCC EP shows remarkable retest reliability and specificity to an individual, while still retaining a population-level structure (Waters et al., 2018).
An important growth area for perturbation mapping research is to decode variance in the EP structure. EPs appear as a complex pattern of electric fields that evolve on the millisecond timescale and can last up to 0.5 s following a pulse of stimulation. These features reflect the causal impact of stimulation on brain-wide dynamics, also called effective connectivity (Entz et al., 2014). The read-out is thus analogous to structural or functional connectivity measures, but it excels in temporal acuity and provides unique information about how and where stimulation perturbs the brain. However, evidence of an association between evoked components and white matter architecture or functional network engagement only partly explains location specificity (Howell et al., 2021). Features of EPs that are sensitive to stimulation location appear to be generated by phase alignment of endogenous oscillatory activity in TRD. This points to a largely untapped oscillatory feature space, which is arguably the most relevant to circuit dynamics (Smith et al., 2022). Further exploration of spectral characteristics of perturbation mapping is warranted to elucidate the mechanism by which stimulation impacts downstream cortical activity. This would enable precision targeting and stimulation programming for TRD and potentially other neurologic disorders to improve clinical efficacy.
Studies exploring a potential relationship between EPs (i.e., ERNA) and oscillatory biomarkers (i.e., beta power) could further clarify the role of such biomarkers in DBS optimization. Collectively, electrophysiological biomarkers could guide automated algorithms to expedite and improve DBS programming. Biomarker localization may elucidate mechanisms of DBS and enable multimodal methods for improved DBS efficacy and efficiency.
Advances in stimulation paradigms: beyond continuous high-frequency DBS
To date, continuous, high-frequency (>130 Hz) stimulation (Fig. 3B) is the gold standard of DBS therapy. However, as clinicians seek the balance between optimal motor outcomes and managing stimulation-induced side effects, alternative paradigms of stimulation have emerged. A recent review (Najera et al., 2023) provides an in-depth report on alternative stimulation paradigms assessed in human subjects.
Variable frequency stimulation
Many emerging stimulation patterns rely on polyrhythmic stimulation. One example is variable frequency stimulation (VFS), which alternates between two frequencies. For example, VFS may alternate between high- and low-frequency stimulation (HFS and LFS, respectively; Fig. 3C). HFS and LFS appear to have differential actions. HFS, and not LFS, improves rigidity and tremor (Benabid et al., 1991; Limousin et al., 1995; Volkmann et al., 2002). Conversely, LFS may improve axial symptoms and speech abnormalities, symptoms that HFS can exacerbate (Moreau et al., 2008; Grover et al., 2019). However, reports assessing the effects of LFS versus HFS on different PD symptoms have varied, except for tremor, which seems to respond best to HFS (Su et al., 2018). Studies have shown that VFS can exploit the hypothesized benefits of both HFS and LFS. A 2018 study compared VFS to HFS in 4 PD patients with freezing of gait (Jia et al., 2018) and reported a 14% improvement in the Unified Parkinson's disease rating scale III (UPDRS-III) motor score using VFS over HFS. Burst cycling, including theta burst stimulation, is another example of a polyrhythmic stimulation pattern, which alternates between periods of ON and OFF stimulation (Fig. 3D) (Bentley et al., 2020; Sáenz-Farret et al., 2021; Wong et al., 2021). The effectiveness of burst cycling may depend on the movement disorder: eliciting variable PD symptom improvement (Montgomery, 2005; M. A. Horn et al., 2020; Dayal et al., 2021; Sáenz-Farret et al., 2021; Wong et al., 2021), no improvement in postural tremor (Kuncel et al., 2012), and promising results for dystonia (Tai et al., 2011; MacLean et al., 2023). Burst cycling is most promising in treating epilepsy, where its application results in decreased seizure occurrence using various DBS targets (Min et al., 2013; Vázquez-Barrón et al., 2021).
Interleaved stimulation
Another promising approach is interleaved stimulation (ILS). Like VFS, ILS takes advantage of alternating between two distinct stimulation settings that maximize symptom alleviation while avoiding adverse stimulation-induced side effects. Unlike VFS, which alternates between frequencies on the same contact, ILS uses programs set at two distinct contacts on an electrode, which allows for different amplitudes, pulse widths, and frequencies (Fig. 3E). ILS has been implemented in DBS for PD, ET, and dystonia and has reduced various motor symptoms and stimulation induced effects. For instance, ILS appears to reduce dyskinesia in PD (Ramirez-Zamora et al., 2015). ILS reduces stimulation-induced side effects (e.g., dysarthria) in both PD (Wojtecki et al., 2011) and ET (Barbe et al., 2014) while maintaining clinical benefit. ILS may also benefit dystonia patients who are otherwise unresponsive to DBS (Kovács et al., 2012). Although promising, further exploration is needed to fully understand the usefulness of ILS across symptoms and indications.
Coordinated reset DBS
Various novel stimulation paradigms have been developed using computational modeling and later tested in human subjects. One example is coordinated reset (CR) DBS, which is based on the theory that HFS stimulation works by disrupting synchronized neural activity. CR DBS is designed to counteract exaggerated synchronization in neuronal populations by using phase-shifted neural activation (Tass, 2003). Instead of delivering continuous, HFS, CR DBS delivers consecutive brief high-frequency pulse trains (i.e., burst stimulation) at a considerably lower stimulation amplitude through different contacts of the DBS lead at different times (Fig. 3F), ultimately dividing the neuronal population into subpopulations, desynchronizing pathologic connectivity and activity. It is suggested that CR is superior to standard continuous DBS (cDBS) because of the significantly reduced energy needed to achieve a therapeutic effect and its reduction in stimulation-induced adverse effects (Tass, 2003). Modeling studies suggest that the network's desynchronization state can sustain after CR DBS is ceased (i.e., carryover effect), suggesting long-lasting motor benefits, which further reduces the need for stimulation and risk of stimulation-induced adverse effects.
When CR DBS was first applied in vivo using nonhuman primate (NHP) models of PD, Tass et al. (2012) demonstrated that STN CR DBS had carryover effects on akinesia improvement, even 35 d after stimulation cessation. A 2016 study validated the acute benefits of CR DBS and a carryover effect of up to 2 weeks on a wider range of parkinsonian symptoms, including akinesia, tremor, rigidity, and bradykinesia (J. Wang et al., 2016). In a CR DBS proof-of-concept clinical study in 6 individuals with PD, Adamchic et al. (2014) applied CR DBS for 3 consecutive days in two daily sessions of up to 2 h. After the third daily dose of CR DBS, motor scores assessed using the UPDRS-III showed a significant improvement compared with the evening score from the first day, signifying cumulative aftereffects of CR DBS. While these studies suggest the superiority of CR DBS compared with standard cDBS, further validation of CR DBS is needed in more patients with PD to clinically translate this novel DBS approach.
CR DBS parameter optimization and underlying mechanisms of therapeutic effect need to be validated to expedite its clinical translation. Modeling studies have suggested that variations in CR DBS parameters (i.e., stimulation frequency, intensity, and number of stimulation sites) can significantly impact its acute and long-lasting stimulation outcomes (Manos et al., 2018). Furthermore, a 2022 study demonstrated that shuffling the order of burst stimulation across DBS contacts, rather than continually repeating the same sequence, improves CR DBS motor outcomes in two NHPs (J. Wang et al., 2022). Studies of CR DBS mechanisms suggest CR DBS desynchronizes oscillatory activity, with one study reporting reduced STN β and theta power following STN CR DBS (Adamchic et al., 2014) and another reporting decreased coherence between left and right primary motor cortex after CR DBS (Chelangat Bore et al., 2022). Ongoing work in Jing Wang's laboratory at the University of Minnesota suggests that STN CR DBS induces acute STN beta phase desynchronization followed by a sustained reduction in the cortical-subcortical (STN-M1 and STN-premotor cortex) coherence that correlates with carryover motor improvement. These results indicate that STN CR DBS therapeutic effect is associated with both local and network neuronal desynchronization, although further validation is needed. Given its desynchronizing effect, CR DBS can also be expanded to other neurologic and neuropsychiatric disorders that involve exaggerated neural synchronization. For example, an ongoing clinical trial (NCT05897775) is assessing the safety and efficacy of thalamic CR DBS in ET.
Adaptive DBS (aDBS)
Bidirectional DBS systems have enabled novel stimulation protocols, such as closed-loop or aDBS, in which stimulation is delivered in response to a control signal (e.g., a neural biomarker) exceeding a defined threshold (Fig. 3G). Trials of aDBS are currently ongoing across industry (NCT04547712) and academia (NCT03582891, NCT04806516, NCT04106466, NCT02649166). The motivation behind aDBS is to enhance therapeutic efficacy by delivering stimulation only when needed based on the dynamics of neural data, potentially avoiding stimulation-induced adverse side effects (e.g., dysarthria) and improving clinical outcomes compared with standard cDBS (Little et al., 2013, 2016b; Piña-Fuentes et al., 2020). aDBS was initially pioneered in an 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated NHP, driving stimulation to the GPi using neuronal spikes recorded from either GPi or motor cortex as control signals (Rosin et al., 2011). aDBS reduced akinesia, pallidal discharge rates, and pathologic oscillations more than standard HFS. Most studies reporting on aDBS outcomes use STN beta power as the control signal because of its relationship to bradykinesia, rigidity severity, and sensitivity to therapy (medication and DBS) states (Kühn et al., 2006, 2008). These studies have been acute, in-clinic trials in PD patients using externalized leads (Little et al., 2013, 2016a; Piña-Fuentes et al., 2020). Nevertheless, they have demonstrated the potential augmented benefits of aDBS over cDBS on motor outcomes.
Researchers have now begun replicating acute aDBS trials in embedded systems that have the potential of at-home testing and enable exploration of different feedback signals across the frequency spectrum or even across implicated pathologic networks using multisite sensing. Using a first-generation bidirectional DBS device (Medtronic PC+S), Swann et al. (2016) demonstrated that reducing stimulation amplitude when narrowband cortical γ was increased, signifying the hyperkinetic state, reduced electrical energy expenditure while maintaining therapeutic efficacy (Swann et al., 2018). Another study using the Medtronic PC+S pioneered a dual threshold control algorithm on STN beta power in 13 patients with PD that reduced bradykinesia and tremor while delivering half the electrical energy as cDBS (Velisar et al., 2019). The first implementation of aDBS in an at-home setting used a second-generation bidirectional device (Medtronic RC+S) (Gilron et al., 2021a). The authors implemented both an STN β- and cortical γ-driven algorithm during awake hours in 2 patients with PD, demonstrating decreased “on-time” dyskinesia in 1 patient during aDBS compared with cDBS using both subjective and objective measures. To better understand the benefits of aDBS, future blinded clinical trials in naturalistic environments are needed (Oehrn et al., 2023).
The potential benefits of aDBS extend to disorders beyond PD. Studies involving ET patients receiving DBS to the ventral intermediate nucleus of the thalamus implanted with the Medtronic PC+S and subdural ECoG electrodes have used beta band desynchronization for movement detection and subsequent stimulation delivery (Opri et al., 2020). aDBS demonstrated equivalent tremor suppression measured by wearable devices and clinical scales, while reducing battery expenditure. Other neural-based aDBS systems have been developed for Tourette syndrome (Cagle et al., 2022), dystonia (V. Johnson et al., 2021), sleep classification (Gilron et al., 2021b), tremor detection (He et al., 2021), freezing of gait in PD (Petrucci et al., 2020; Molina et al., 2021), and epilepsy (Heck et al., 2014). Additionally, wearables for control signal detection have been explored for adaptive paradigms for ET (Cernera et al., 2021) and PD (Malekmohammadi et al., 2016; Louie et al., 2021), providing alternatives to neural biomarkers as control signals. Finally, other types of aDBS control algorithms that use either the phase of a control signal [either a wearable (Cagnan et al., 2017) or neural signal (Holt et al., 2019)] have been pursued to achieve high stimulation temporal specificity or proportional adaptive stimulation (Rosa et al., 2017; Arlotti et al., 2018; Bocci et al., 2021), in which stimulation amplitude increase corresponds to control signal increases (e.g., beta power).
Advances in DBS therapy: toward eliminating barriers to DBS
Collectively, this review highlights ongoing work leading to advancements in elucidating DBS mechanisms and applications, including electrode localization, optimizing DBS parameter selection, and stimulation delivery. These endeavors involve a multidisciplinary approach incorporating expertise from neuroscientists, engineers, and clinicians. Consequently, various modalities will help elucidate DBS mechanisms and application, and urge technological growth. Several of these modalities are highlighted within this review, including sweet spot mapping, EPs, fMRI for circuit engagement, CR DBS, and adaptive stimulation. Although promising, many of the applications reviewed, such as CR or aDBS and various stimulation paradigms, have only been tested in a small number of patients and within in-clinic settings. Future endeavors will need to capitalize on novel bidirectional systems, or firmware upgrades to corroborate in-clinic outcomes in naturalistic settings.
DBS remains an underutilized therapy, despite its proven benefit. The recent advancements highlighted throughout this review may contribute to its widespread adoption. A contributing factor to its underutilization may be clinician apprehension, requiring significant advocacy and knowledge on the patient's part to overcome (Hamberg and Hariz, 2014). In OCD, a major barrier to receiving DBS is lack of coverage for the treatment by private insurance companies (Pinckard-Dover et al., 2021; Visser-Vandewalle et al., 2022). Limited access to specialized care is a major barrier to DBS for those with movement disorders (Auffret et al., 2023). A recent study of >685,000 Medicare beneficiaries living with PD revealed that just 9% of PD beneficiaries see a movement disorders specialist, which is a major barrier to receiving DBS because specialist referrals are often needed to qualify for DBS (Pearson et al., 2023). Once implanted, patients often need regular follow-up visits with a clinician trained in programming and optimization, which is largely empirical and requires extensive clinical experience that is limited to a few expert centers. Limited or no access to such centers is a barrier to receiving DBS shared by potential patients across all indications (Nuttin et al., 2014; Pinckard-Dover et al., 2021; Cabrera et al., 2022; Esper et al., 2022). These challenges highlight the urgent need to streamline or automate the DBS programming process to enable a broad range of advanced clinicians to provide quality care for DBS patients. Further complicating individuals' access to DBS are the racial, gender, and socioeconomic disparities in DBS utilization (Hemming et al., 2011; Auffret et al., 2023). Studies have found that income correlates with DBS outcomes, which may be because of the inability of lower income patients to attend frequent follow-up visits for DBS programming optimization because of difficulty taking time off work, limited access to transportation, or limited support (Willis et al., 2014; Genc et al., 2016). Thus, automated DBS programming may increase accessibility of DBS to vulnerable populations, and when coupled with advanced stimulation paradigms, such as CR and aDBS, patients may experience a decreased need for frequent visits to the clinic and see improved clinical outcomes.
Footnotes
The authors declare no competing financial interests.
References
- Adamchic I, Hauptmann C, Barnikol UB, Pawelczyk N, Popovych O, Barnikol TT, Silchenko A, Volkmann J, Deuschl G, Meissner WG, Maarouf M, Sturm V, Freund HJ, Tass PA (2014) Coordinated reset neuromodulation for Parkinson's disease: proof-of-concept study. Mov Disord 29:1679–1684. 10.1002/mds.25923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allawala A, Bijanki KR, Goodman W, Cohn JF, Viswanathan A, Yoshor D, Borton DA, Pouratian N, Sheth SA (2021) A novel framework for network-targeted neuropsychiatric deep brain stimulation. Neurosurgery 89:E116–E121. 10.1093/neuros/nyab112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman JE, Johnson LA, Sanabria DE, Wang J, Patriat R, Hill M, Marshall E, MacKinnon CD, Cooper SE, Schrock LE, Park MC, Harel N, Vitek JL (2020) Directional deep brain stimulation leads reveal spatially distinct oscillatory activity in the globus pallidus internus of Parkinson's disease patients. Neurobiol Dis 139:104819. 10.1016/j.nbd.2020.104819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotti M, Marceglia S, Foffani G, Volkmann J, Lozano AM, Moro E, Cogiamanian F, Prenassi M, Bocci T, Cortese F, Rampini P, Barbieri S, Priori A (2018) Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology 90:e971–e976. 10.1212/WNL.0000000000005121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkan K, Rogers P, Bergman H, Ughratdar I (2017) Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol 13:548–554. 10.1038/nrneurol.2017.105 [DOI] [PubMed] [Google Scholar]
- Auffret M, Weiss D, Stocchi F, Vérin M, Jost WH (2023) Access to device-aided therapies in advanced Parkinson's disease: navigating clinician biases, patient preference, and prognostic uncertainty. J Neural Transm (Vienna). Advance online publication. Retrieved Jul 12, 2023. 10.1007/s00702-023-02668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MT, Dembek TA, Becker J, Raethjen J, Hartinger M, Meister IG, Runge M, Maarouf M, Fink GR, Timmermann L (2014) Individualized current-shaping reduces DBS-induced dysarthria in patients with essential tremor. Neurology 82:614–619. 10.1212/WNL.0000000000000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J (1991) Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337:403–406. 10.1016/0140-6736(91)91175-t [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Hallett M (2000) Mechanism of action of deep brain stimulation. Neurology 55:S13–S16. [PubMed] [Google Scholar]
- Bentley JN, Irwin ZT, Black SD, Roach ML, Vaden RJ, Gonzalez CL, Khan AU, El-Sayed GA, Knight RT, Guthrie BL, Walker HC (2020) Subcortical intermittent theta-burst stimulation (iTBS) increases theta-power in dorsolateral prefrontal cortex (DLPFC). Front Neurosci 14:41. 10.3389/fnins.2020.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld IO, Mantione M, Figee M, Schuurman PR, Lok A, Denys D (2018) Treatment-resistant depression and suicidality. J Affect Disord 235:362–367. 10.1016/j.jad.2018.04.016 [DOI] [PubMed] [Google Scholar]
- Bijanki KR, Pathak YJ, Najera RA, Storch EA, Goodman WK, Simpson HB, Sheth SA (2021) Defining functional brain networks underlying obsessive–compulsive disorder (OCD) using treatment-induced neuroimaging changes: a systematic review of the literature. J Neurol Neurosurg Psychiatry 92:776–786. 10.1136/jnnp-2020-324478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci T, Prenassi M, Arlotti M, Cogiamanian FM, Borellini L, Moro E, Lozano AM, Volkmann J, Barbieri S, Priori A, Marceglia S (2021) Eight-hours conventional versus adaptive deep brain stimulation of the subthalamic nucleus in Parkinson's disease. NPJ Parkinsons Dis 7:88. 10.1038/s41531-021-00229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohara S, Slepneva N, Norbu T, Motzkin J, Sugrue L, Lee AM, Morrison M (2023) Patterns of fMRI connectivity during cycling stimulation associated with effective DBS for OCD. Brain Stimul 16:347. 10.1016/j.brs.2023.01.664 [DOI] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross C (1996) High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett 215:17–20. 10.1016/s0304-3940(96)12943-8 [DOI] [PubMed] [Google Scholar]
- Boutet A, Chow CT, Narang K, Elias GJ, Neudorfer C, Germann J, Ranjan M, Loh A, Martin AJ, Kucharczyk W, Steele CJ, Hancu I, Rezai AR, Lozano AM (2020) Improving safety of MRI in patients with deep brain stimulation devices. Radiology 296:250–262. 10.1148/radiol.2020192291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutet A, et al. (2021) Predicting optimal deep brain stimulation parameters for Parkinson's disease using functional MRI and machine learning. Nat Commun 12:3043. 10.1038/s41467-021-23311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower KL, McIntyre CC (2020) Deep brain stimulation of terminating axons. Brain Stimul 13:1863–1870. 10.1016/j.brs.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinke T, Odekerken VJ, Dijk JM, Munckhof P, van den Schuurman PR, Bie R (2018) Directional deep brain stimulation: first experiences in centers across the globe. Brain Stimul 11:949–950. 10.1016/j.brs.2018.04.008 [DOI] [PubMed] [Google Scholar]
- Bronstein JM, et al. (2011) Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 68:165. 10.1001/archneurol.2010.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P (2003) Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord 18:357–363. 10.1002/mds.10358 [DOI] [PubMed] [Google Scholar]
- Cabrera LY, Miller MM, Achtyes ED, McCright AM, Bluhm R (2022) Jumping through the hoops: barriers and other ethical concerns regarding the use of psychiatric electroceutical interventions. Psychiatry Res 313:114612. 10.1016/j.psychres.2022.114612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle JN, Okun MS, Cernera S, Eisinger RS, Opri E, Bowers D, Ward H, Foote KD, Gunduz A (2022) Embedded human closed-loop deep brain stimulation for Tourette syndrome: a nonrandomized controlled trial. JAMA Neurol 79:1064–1068. 10.1001/jamaneurol.2022.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle JN, Wong JK, Johnson KA, Foote KD, Okun MS, de Hemptinne C (2021) Suppression and rebound of pallidal beta power: observation using a chronic sensing DBS device. Front Hum Neurosci 15:749567. 10.3389/fnhum.2021.749567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnan H, Pedrosa D, Little S, Pogosyan A, Cheeran B, Aziz T, Green A, Fitzgerald J, Foltynie T, Limousin P, Zrinzo L, Hariz M, Friston KJ, Denison T, Brown P (2017) Stimulating at the right time: phase-specific deep brain stimulation. Brain 140:132–145. 10.1093/brain/aww286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caire F, Ranoux D, Guehl D, Burbaud P, Cuny E (2013) A systematic review of studies on anatomical position of electrode contacts used for chronic subthalamic stimulation in Parkinson's disease. Acta Neurochir (Wien) 155:1647–1654. 10.1007/s00701-013-1782-1 [DOI] [PubMed] [Google Scholar]
- Cernera S, Alcantara JD, Opri E, Cagle JN, Eisinger RS, Boogaart Z, Pramanik L, Kelberman M, Patel B, Foote KD, Okun MS, Gunduz A (2021) Wearable sensor-driven responsive deep brain stimulation for essential tremor. Brain Stimul 14:1434–1443. 10.1016/j.brs.2021.09.002 [DOI] [PubMed] [Google Scholar]
- Chan AK, McGovern RA, Brown LT, Sheehy JP, Zacharia BE, Mikell CB, Bruce SS, Ford B, McKhann GM II (2014) Disparities in access to deep brain stimulation surgery for Parkinson disease: interaction between African American race and Medicaid use. JAMA Neurol 71:291–299. 10.1001/jamaneurol.2013.5798 [DOI] [PubMed] [Google Scholar]
- Chelangat Bore J, Campbell B, Cho H, Pucci F, Gopalakrishnan R, Machado A, Baker K (2022) Long-lasting effects of subthalamic nucleus coordinated reset deep brain stimulation in the non-human primate model of parkinsonism: a case report. Brain Stimul 15:598–600. 10.1016/j.brs.2022.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintas P, Simonetta-Moreau M, Ory F, Brefel-Courbon C, Fabre N, Chaynes P, Sabatier J, Sol JC, Rascol O, Berry I, Lazorthes Y (2003) Deep brain stimulation for Parkinson's disease: correlation between intraoperative subthalamic nucleus neurophysiology and most effective contacts. Stereotact Funct Neurosurg 80:108–113. 10.1159/000075169 [DOI] [PubMed] [Google Scholar]
- Crowell AL, Riva-Posse P, Holtzheimer PE, Garlow SJ, Kelley ME, Gross RE, Denison L, Quinn S, Mayberg HS (2019) Long-term outcomes of subcallosal cingulate deep brain stimulation for treatment-resistant depression. Am J Psychiatry 176:949–956. 10.1176/appi.ajp.2019.18121427 [DOI] [PubMed] [Google Scholar]
- Cummins DD, Kochanski RB, Gilron R, Swann NC, Little S, Hammer LH, Starr PA (2021) Chronic sensing of subthalamic local field potentials: comparison of first and second generation implantable bidirectional systems within a single subject. Front Neurosci 15:725797. 10.3389/fnins.2021.725797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayal V, Rajabian A, Jahanshahi M, Aviles-Olmos I, Cowie D, Peters A, Day B, Hyam J, Akram H, Limousin P, Hariz M, Zrinzo L, Foltynie T (2021) Pedunculopontine nucleus deep brain stimulation for Parkinsonian disorders: a case series. Stereotact Funct Neurosurg 99:287–294. 10.1159/000511978 [DOI] [PubMed] [Google Scholar]
- Dembek TA, Roediger J, Horn A, Reker P, Oehrn C, Dafsari HS, Li N, Kühn AA, Fink GR, Visser-Vandewalle V, Barbe MT, Timmermann L (2019) Probabilistic sweet spots predict motor outcome for deep brain stimulation in Parkinson disease. Ann Neurol 86:527–538. 10.1002/ana.25567 [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM (2000) Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol 84:570–574. 10.1152/jn.2000.84.1.570 [DOI] [PubMed] [Google Scholar]
- Entz L, Tóth E, Keller CJ, Bickel S, Groppe DM, Fabó D, Kozák LR, Eross L, Ulbert I, Mehta AD (2014) Evoked effective connectivity of the human neocortex. Hum Brain Mapp 35:5736–5753. 10.1002/hbm.22581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper CD, Merola A, Himes L, Patel N, Bezchlibnyk YB, Falconer D, Weiss D, Luca C, Cheeran B, Mari Z (2022) Necessity and feasibility of remote tele-programming of deep brain stimulation systems in Parkinson's disease. Parkinsonism Relat Disord 96:38–42. 10.1016/j.parkreldis.2022.01.017 [DOI] [PubMed] [Google Scholar]
- Farokhniaee A, McIntyre CC (2019) Theoretical principles of deep brain stimulation induced synaptic suppression. Brain Stimul 12:1402–1409. 10.1016/j.brs.2019.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann LK, Neumann WJ, Krause P, Lofredi R, Schneider GH, Kühn AA (2021) Subthalamic beta band suppression reflects effective neuromodulation in chronic recordings. Eur J Neurol 28:2372–2377. 10.1111/ene.14801 [DOI] [PubMed] [Google Scholar]
- Filip P, Jech R, Fečíková A, Havránková P, Růžička F, Mueller K, Urgošík D (2022) Restoration of functional network state towards more physiological condition as the correlate of clinical effects of pallidal deep brain stimulation in dystonia. Brain Stimul 15:1269–1278. 10.1016/j.brs.2022.08.025 [DOI] [PubMed] [Google Scholar]
- Frey J, Cagle J, Johnson KA, Wong JK, Hilliard JD, Butson CR, Okun MS, de Hemptinne C (2022) Past, present, and future of deep brain stimulation: hardware, software, imaging, physiology and novel approaches. Front Neurol 13:825178. 10.3389/fneur.2022.825178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke P, Nickl R, Breun M, Volkmann J, Kirsch D, Ernestus RI, Steigerwald F, Matthies C (2021) Directional leads for deep brain stimulation: technical notes and experiences. Stereotact Funct Neurosurg 99:305–312. 10.1159/000512231 [DOI] [PubMed] [Google Scholar]
- Genc G, Abboud H, Oravivattanakul S, Alsallom F, Thompson NR, Cooper S, Gostkowski M, Machado A, Fernandez HH (2016) Socioeconomic status may impact functional outcome of deep brain stimulation surgery in Parkinson's disease. Neuromodulation 19:25–30. 10.1111/ner.12324 [DOI] [PubMed] [Google Scholar]
- Gilron R, et al. (2021a) Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson's disease. Nat Biotechnol 39:1078–1085. 10.1038/s41587-021-00897-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron R, Little S, Wilt R, Perrone R, Anso J, Starr PA (2021b) Sleep-aware adaptive deep brain stimulation control: chronic use at home with dual independent linear discriminate detectors. Front Neurosci 15:732499. 10.3389/fnins.2021.732499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho F, Thobois S, Magnin M, Guenot M, Polo G, Benatru I, Xie J, Salvetti A, Garcia-Larrea L, Broussolle E, Mertens P (2006) Subthalamic nucleus stimulation in Parkinson's disease: anatomical and electrophysiological localization of active contacts. J Neurol 253:1347–1355. 10.1007/s00415-006-0222-z [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL (2000) Toward a neurobiology of obsessive-compulsive disorder. Neuron 28:343–347. 10.1016/s0896-6273(00)00113-6 [DOI] [PubMed] [Google Scholar]
- Grill WM, Snyder AN, Miocinovic S (2004) Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport 15:1137–1140. 10.1097/00001756-200405190-00011 [DOI] [PubMed] [Google Scholar]
- Grover T, Georgiev D, Kalliola R, Mahlknecht P, Zacharia A, Candelario J, Hyam J, Zrinzo L, Hariz M, Foltynie T, Limousin P, Jahanshahi M, Tripoliti E (2019) Effect of low versus high frequency subthalamic deep brain stimulation on speech intelligibility and verbal fluency in Parkinson's disease: a double-blind study. J Parkinsons Dis 9:141–151. 10.3233/JPD-181368 [DOI] [PubMed] [Google Scholar]
- Hacker ML, Rajamani N, Neudorfer C, Hollunder B, Oxenford S, Li N, Sternberg AL, Davis TL, Konrad PE, Horn A, Charles D (2023) Connectivity profile for subthalamic nucleus deep brain stimulation in early stage Parkinson disease. Ann Neurol 94:271–284. 10.1002/ana.26674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg K, Hariz GM (2014) The decision-making process leading to deep brain stimulation in men and women with Parkinson's disease: an interview study. BMC Neurol 14:89. 10.1186/1471-2377-14-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Baig F, Mostofi A, Pogosyan A, Debarros J, Green AL, Aziz TZ, Pereira E, Brown P, Tan H (2021) Closed-loop deep brain stimulation for essential tremor based on thalamic local field potentials. Mov Disord 36:863–873. 10.1002/mds.28513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck CN, et al. (2014) Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 55:432–441. 10.1111/epi.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming JP, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ, Shulman LM (2011) Racial and socioeconomic disparities in parkinsonism. Arch Neurol 68:498–503. 10.1001/archneurol.2010.326 [DOI] [PubMed] [Google Scholar]
- Herrington TM, Cheng JJ, Eskandar EN (2016) Mechanisms of deep brain stimulation. J Neurophysiol 115:19–38. 10.1152/jn.00281.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AB, Kormann E, Gulberti A, Pötter-Nerger M, McNamara CG, Cagnan H, Baaske MK, Little S, Köppen JA, Buhmann C, Westphal M, Gerloff C, Engel AK, Brown P, Hamel W, Moll CK, Sharott A (2019) Phase-dependent suppression of beta oscillations in Parkinson's disease patients. J Neurosci 39:1119–1134. 10.1523/JNEUROSCI.1913-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Reich M, Vorwerk J, Li N, Wenzel G, Fang Q, Schmitz-Hübsch T, Nickl R, Kupsch A, Volkmann J, Kühn AA, Fox MD (2017) Connectivity predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol 82:67–78. 10.1002/ana.24974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, et al. (2022) Optimal deep brain stimulation sites and networks for cervical vs. generalized dystonia. Proc Natl Acad Sci USA 119:e2114985119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn MA, Gulberti A, Gülke E, Buhmann C, Gerloff C, Moll CK, Hamel W, Volkmann J, Pötter-Nerger M (2020) A new stimulation mode for deep brain stimulation in Parkinson's disease: theta burst stimulation. Mov Disord 35:1471–1475. 10.1002/mds.28083 [DOI] [PubMed] [Google Scholar]
- Howell B, Waters AC, Choi KS, Veerakumar A, Obatusin M, Mayberg HS, McIntyre CC (2021) Connectomic predictive modeling guides selective perturbation of tracts in the subcallosal cingulate white matter. In: International IEEE/EMBS Conference on Neural Engineering, pp 271–274. NER: IEEE Computer Society. [Google Scholar]
- Jia F, Wagle Shukla A, Hu W, Almeida L, Holanda V, Zhang J, Meng F, Okun MS, Li L (2018) Deep brain stimulation at variable frequency to improve motor outcomes in Parkinson's disease. Mov Disord Clin Pract 5:538–541. 10.1002/mdc3.12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen EL, Sunde N, Mogensen PH, Ostergaard K (2010) MRI verified STN stimulation site–gait improvement and clinical outcome. Eur J Neurol 17:746–753. 10.1111/j.1468-1331.2010.02962.x [DOI] [PubMed] [Google Scholar]
- Johnson KA, Cagle JN, Lopes JL, Wong JK, Okun MS, Gunduz A, Shukla AW, Hilliard JD, Foote KD, de Hemptinne C (2023) Globus pallidus internus deep brain stimulation evokes resonant neural activity in Parkinson's disease. Brain Commun 5:fcad025. 10.1093/braincomms/fcad025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V, Wilt R, Gilron R, Anso J, Perrone R, Beudel M, Piña-Fuentes D, Saal J, Ostrem JL, Bledsoe I, Starr P, Little S (2021) Embedded adaptive deep brain stimulation for cervical dystonia controlled by motor cortex theta oscillations. Exp Neurol 345:113825. 10.1016/j.expneurol.2021.113825 [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015) Large-scale network dysfunction in major depressive disorder: meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72:603–611. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestenbaum M, Ford B, Louis ED (2015) Estimating the proportion of essential tremor and Parkinson's disease patients undergoing deep brain stimulation surgery: five-year data from Columbia University Medical Center (2009-2014). Mov Disord Clin Pract 2:384–387. 10.1002/mdc3.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Criaud M, Cho SS, Díez-Cirarda M, Mihaescu A, Coakeley S, Ghadery C, Valli M, Jacobs MF, Houle S, Strafella AP (2017) Abnormal intrinsic brain functional network dynamics in Parkinson's disease. Brain 140:2955–2967. 10.1093/brain/awx233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala N, Serrano L, Paschen S, Falk D, Anwar AR, Kuravi P, Deuschl G, Groppa S, Muthuraman M (2020) Mapping of subthalamic nucleus using microelectrode recordings during deep brain stimulation. Sci Rep 10:19241. 10.1038/s41598-020-74196-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács N, Janszky J, Nagy F, Balás I (2012) Changing to interleaving stimulation might improve dystonia in cases not responding to pallidal stimulation. Mov Disord 27:163–165. 10.1002/mds.23962 [DOI] [PubMed] [Google Scholar]
- Kühn AA, Kempf F, Brücke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, Nuttin B, Brown P (2008) High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci 28:6165–6173. 10.1523/JNEUROSCI.0282-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn AA, Kupsch A, Schneider GH, Brown P (2006) Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci 23:1956–1960. 10.1111/j.1460-9568.2006.04717.x [DOI] [PubMed] [Google Scholar]
- Kuncel AM, Birdno MJ, Swan BD, Grill WM (2012) Tremor reduction and modeled neural activity during cycling thalamic deep brain stimulation. Clin Neurophysiol 123:1044–1052. 10.1016/j.clinph.2011.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanotte MM, Rizzone M, Bergamasco B, Faccani G, Melcarne A, Lopiano L (2002) Deep brain stimulation of the subthalamic nucleus: anatomical, neurophysiological, and outcome correlations with the effects of stimulation. J Neurol Neurosurg Psychiatry 72:53–58. 10.1136/jnnp.72.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Friston K, Mody M, Wang H, Lu H, Hu D (2018) A brain network model for depression: from symptom understanding to disease intervention. CNS Neurosci Ther 24:1004–1019. 10.1111/cns.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lai Y, Li J, He N, Li D, Yan F, Zhang Y, Zhang C, Sun B, Wei H (2023) BOLD frequency-dependent alterations in resting-state functional connectivity by pallidal deep brain stimulation in patients with Parkinson's disease. J Neurosurg 1–12. 10.3171/2023.1.JNS221858 [DOI] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL (1995) Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 345:91–95. 10.1016/s0140-6736(95)90062-4 [DOI] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, Foltynie T, Limousin P, Ashkan K, FitzGerald J, Green AL, Aziz TZ, Brown P (2013) Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 74:449–457. 10.1002/ana.23951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Beudel M, Zrinzo L, Foltynie T, Limousin P, Hariz M, Neal S, Cheeran B, Cagnan H, Gratwicke J, Aziz TZ, Pogosyan A, Brown P (2016a) Bilateral adaptive deep brain stimulation is effective in Parkinson's disease. J Neurol Neurosurg Psychiatry 87:717–721. 10.1136/jnnp-2015-310972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Tripoliti E, Beudel M, Pogosyan A, Cagnan H, Herz D, Bestmann S, Aziz T, Cheeran B, Zrinzo L, Hariz M, Hyam J, Limousin P, Foltynie T, Brown P (2016b) Adaptive deep brain stimulation for Parkinson's disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J Neurol Neurosurg Psychiatry 87:1388–1389. 10.1136/jnnp-2016-313518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh A, Gwun D, Chow CT, Boutet A, Tasserie J, Germann J, Santyr B, Elias G, Yamamoto K, Sarica C, Vetkas A, Zemmar A, Madhavan R, Fasano A, Lozano AM (2022) Probing responses to deep brain stimulation with functional magnetic resonance imaging. Brain Stimul 15:683–694. 10.1016/j.brs.2022.03.009 [DOI] [PubMed] [Google Scholar]
- Louie KH, Lu C, Abdallah T, Guzior JC, Twedell E, Netoff TI, Cooper SE (2021) Gait phase triggered deep brain stimulation in Parkinson's disease. Brain Stimul 14:420–422. 10.1016/j.brs.2021.02.009 [DOI] [PubMed] [Google Scholar]
- Lowet E, Kondabolu K, Zhou S, Mount RA, Wang Y, Ravasio CR, Han X (2022) Deep brain stimulation creates informational lesion through membrane depolarization in mouse hippocampus. Nat Commun 13:7709. 10.1038/s41467-022-35314-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N (2013) Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 77:406–424. 10.1016/j.neuron.2013.01.020 [DOI] [PubMed] [Google Scholar]
- Lumsden DE, Tambirajoo K, Hasegawa H, Gimeno H, Kaminska M, Ashkan K, Selway R, Lin JP (2022) Probabilistic mapping of deep brain stimulation in childhood dystonia. Parkinsonism Relat Disord 105:103–110. 10.1016/j.parkreldis.2022.11.006 [DOI] [PubMed] [Google Scholar]
- MacLean J, Olaya J, Liker M, Sanger T (2023) Theta-burst cycling for deep brain stimulation. Brain Stimul 16:235–236. 10.1016/j.brs.2023.01.357 [DOI] [Google Scholar]
- Maks CB, Butson CR, Walter BL, Vitek JL, McIntyre CC (2009) Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg Psychiatry 80:659–666. 10.1136/jnnp.2007.126219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekmohammadi M, Herron J, Velisar A, Blumenfeld Z, Trager MH, Chizeck HJ, Brontë-Stewart H (2016) Kinematic adaptive deep brain stimulation for resting tremor in Parkinson's disease. Mov Disord 31:426–428. 10.1002/mds.26482 [DOI] [PubMed] [Google Scholar]
- Manos T, Zeitler M, Tass PA (2018) How stimulation frequency and intensity impact on the long-lasting effects of coordinated reset stimulation. PLoS Comput Biol 14:e1006113. 10.1371/journal.pcbi.1006113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM, Sherman DL, Thakor NV (2004a) Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol 91:1457–1469. 10.1152/jn.00989.2003 [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL (2004b) Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115:1239–1248. 10.1016/j.clinph.2003.12.024 [DOI] [PubMed] [Google Scholar]
- Medtronic (2021) MRI guidelines for Medtronic deep brain stimulation systems. https://www.medtronic.com/content/dam/emanuals/neuro/CONTRIB_228155.pdf.
- Miao J, Tantawi M, Koa V, Zhang AB, Zhang V, Sharan A, Wu C, Matias CM (2022) Use of functional MRI in deep brain stimulation in Parkinson's diseases: a systematic review. Front Neurol 13:849918. 10.3389/fneur.2022.849918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Guoming L, Jian Z (2013) Treatment of mesial temporal lobe epilepsy with amygdalohippocampal stimulation: a case series and review of the literature. Exp Ther Med 5:1264–1268. 10.3892/etm.2013.968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina R, Hass CJ, Cernera S, Sowalsky K, Schmitt AC, Roper JA, Martinez-Ramirez D, Opri E, Hess CW, Eisinger RS, Foote KD, Gunduz A, Okun MS (2021) Closed-loop deep brain stimulation to treat medication-refractory freezing of gait in Parkinson's disease. Front Hum Neurosci 15:633655. 10.3389/fnhum.2021.633655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery EB (2005) Effect of subthalamic nucleus stimulation patterns on motor performance in Parkinson's disease. Parkinsonism Relat Disord 11:167–171. 10.1016/j.parkreldis.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Moreau C, Defebvre L, Destée A, Bleuse S, Clement F, Blatt JL, Krystkowiak P, Devos D (2008) STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 71:80–84. 10.1212/01.wnl.0000303972.16279.46 [DOI] [PubMed] [Google Scholar]
- Najera RA, Mahavadi AK, Khan AU, Boddeti U, Del Bene VA, Walker HC, Bentley JN (2023) Alternative patterns of deep brain stimulation in neurologic and neuropsychiatric disorders. Front Neuroinform 17:1156818. 10.3389/fninf.2023.1156818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudorfer C, et al. (2023) Lead-DBS v3.0: mapping deep brain stimulation effects to local anatomy and global networks. Neuroimage 268:119862. 10.1016/j.neuroimage.2023.119862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann WJ, Steiner LA, Milosevic L (2023) Neurophysiological mechanisms of deep brain stimulation across spatiotemporal resolutions. Brain awad239. 10.1093/brain/awad239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttin B, et al. (2014) Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J Neurol Neurosurg Psychiatry 85:1003–1008. 10.1136/jnnp-2013-306580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehrn CR, Cernera S, Hammer LH, Shcherbakova M, Yao J, Hahn A, Wang S, Ostrem JL, Little S, Starr PA (2023) Personalized chronic adaptive deep brain stimulation outperforms conventional stimulation in Parkinson's disease. medRxiv 23293450. 10.1101/2023.08.03.23293450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opri E, Cernera S, Molina R, Eisinger RS, Cagle JN, Almeida L, Denison T, Okun MS, Foote KD, Gunduz A (2020) Chronic embedded cortico-thalamic closed-loop deep brain stimulation for the treatment of essential tremor. Sci Transl Med 12:eaay7680. 10.1126/scitranslmed.aay7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paff M, Loh A, Sarica C, Lozano AM, Fasano A (2020) Update on current technologies for deep brain stimulation in Parkinson's disease. J Mov Disord 13:185–198. 10.14802/jmd.20052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C, Hartzman A, Munevar D, Feeney M, Dolhun R, Todaro V, Rosenfeld S, Willis A, Beck JC (2023) Care access and utilization among Medicare beneficiaries living with Parkinson's disease. NPJ Parkinsons Dis 9:108. 10.1038/s41531-023-00523-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW (2006) Deep brain stimulation. Annu Rev Neurosci 29:229–257. 10.1146/annurev.neuro.29.051605.112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucci MN, Neuville RS, Afzal MF, Velisar A, Anidi CM, Anderson RW, Parker JE, O'Day JJ, Wilkins KB, Bronte-Stewart HM (2020) Neural closed-loop deep brain stimulation for freezing of gait. Brain Stimul 13:1320–1322. 10.1016/j.brs.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry-Schmelzer JN, et al., EUROPAR and the IPMDS Non-Motor PD Study Group (2019) Non-motor outcomes depend on location of neurostimulation in Parkinson's disease. Brain 142:3592–3604. 10.1093/brain/awz285 [DOI] [PubMed] [Google Scholar]
- Piña-Fuentes D, van Dijk JM, van Zijl JC, Moes HR, van Laar T, Oterdoom DL, Little S, Brown P, Beudel M (2020) Acute effects of adaptive deep brain stimulation in Parkinson's disease. Brain Stimul 13:1507–1516. 10.1016/j.brs.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinckard-Dover H, Ward H, Foote KD (2021) The decline of deep brain stimulation for obsessive-compulsive disorder following FDA humanitarian device exemption approval. Front Surg 8:642503. 10.3389/fsurg.2021.642503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenza NR, et al. (2021) Long-term ecological assessment of intracranial electrophysiology synchronized to behavioral markers in obsessive-compulsive disorder. Nat Med 27:2154–2164. 10.1038/s41591-021-01550-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenza N, et al. (2023) Identification of candidate neural biomarkers of obsessive-compulsive symptom intensity and response to deep brain stimulation. Biol Psychiatry 93:S64. 10.1016/j.biopsych.2023.02.174 [DOI] [Google Scholar]
- Prudente CN, Hess EJ, Jinnah HA (2014) Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience 260:23–35. 10.1016/j.neuroscience.2013.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Zamora A, Kahn M, Campbell J, DeLaCruz P, Pilitsis JG (2015) Interleaved programming of subthalamic deep brain stimulation to avoid adverse effects and preserve motor benefit in Parkinson's disease. J Neurol 262:578–584. 10.1007/s00415-014-7605-3 [DOI] [PubMed] [Google Scholar]
- Reich MM, et al. (2019) Probabilistic mapping of the antidystonic effect of pallidal neurostimulation: a multicentre imaging study. Brain 142:1386–1398. 10.1093/brain/awz046 [DOI] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, McIntyre CC, Gross RE, Mayberg HS (2018) A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry 23:843–849. 10.1038/mp.2017.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva-Posse P, Crowell AL, Wright K, Waters AC, Choi K, Garlow SJ, Holtzheimer PE, Gross RE, Mayberg HS (2020) Rapid antidepressant effects of deep brain stimulation and their relation to surgical protocol. Biol Psychiatry 88:e37–e39. 10.1016/j.biopsych.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M, Arlotti M, Marceglia S, Cogiamanian F, Ardolino G, Fonzo AD, Lopiano L, Scelzo E, Merola A, Locatelli M, Rampini PM, Priori A (2017) Adaptive deep brain stimulation controls levodopa-induced side effects in Parkinsonian patients. Mov Disord 32:628–629. 10.1002/mds.26953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E, Bergman H (2011) Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72:370–384. 10.1016/j.neuron.2011.08.023 [DOI] [PubMed] [Google Scholar]
- Sáenz-Farret M, Loh A, Boutet A, Germann J, Elias GJ, Kalia SK, Chen R, Lozano AM, Fasano A (2021) Theta burst deep brain stimulation in movement disorders. Mov Disord Clin Pract 8:282–285. 10.1002/mdc3.13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarem-Aslani A, Mullett K (2011) Industrial perspective on deep brain stimulation: history, current state, and future developments. Front Integr Neurosci 5:46. 10.3389/fnint.2011.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach WM, Chabardes S, Matthies C, Pollo C, Steigerwald F, Timmermann L, Visser Vandewalle V, Volkmann J, Schuurman PR (2017) Directional leads for deep brain stimulation: opportunities and challenges. Mov Disord 32:1371–1375. 10.1002/mds.27096 [DOI] [PubMed] [Google Scholar]
- Sinclair NC, McDermott HJ, Bulluss KJ, Fallon JB, Perera T, Xu SS, Brown P, Thevathasan W (2018) Subthalamic nucleus deep brain stimulation evokes resonant neural activity. Ann Neurol 83:1027–1031. 10.1002/ana.25234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair NC, McDermott HJ, Fallon JB, Perera T, Brown P, Bulluss KJ, Thevathasan W (2019) Deep brain stimulation for Parkinson's disease modulates high-frequency evoked and spontaneous neural activity. Neurobiol Dis 130:104522. 10.1016/j.nbd.2019.104522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair NC, McDermott HJ, Lee WL, Xu SS, Acevedo N, Begg A, Perera T, Thevathasan W, Bulluss KJ (2021) Electrically evoked and spontaneous neural activity in the subthalamic nucleus under general anesthesia. J Neurosurg 1–10. 10.3171/2021.8.JNS204225 [DOI] [PubMed] [Google Scholar]
- Slepneva N, Frank A, Norbu T, Motzkin J, Sugrue L, Morrison M, Lee AM (2023) BOLD fMRI response to therapeutic and nontherapeutic deep brain stimulation in obsessive-compulsive disorder. Brain Stimul 16:375. 10.1016/j.brs.2023.01.741 [DOI] [Google Scholar]
- Smith EE, Choi KS, Veerakumar A, Obatusin M, Howell B, Smith AH, Tiruvadi V, Crowell AL, Riva-Posse P, Alagapan S, Rozell CJ, Mayberg HS, Waters AC (2022) Time-frequency signatures evoked by single-pulse deep brain stimulation to the subcallosal cingulate. Front Hum Neurosci 16:939258. 10.3389/fnhum.2022.939258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobesky L, Goede L, Odekerken VJ, Wang Q, Li N, Neudorfer C, Rajamani N, Al-Fatly B, Reich M, Volkmann J, de Bie RM, Kühn AA, Horn A (2022) Subthalamic and pallidal deep brain stimulation: are we modulating the same network? Brain 145:251–262. 10.1093/brain/awab258 [DOI] [PubMed] [Google Scholar]
- Stanslaski S, Herron J, Chouinard T, Bourget D, Isaacson B, Kremen V, Opri E, Drew W, Brinkmann BH, Gunduz A, Adamski T, Worrell GA, Denison T (2018) A chronically implantable neural coprocessor for investigating the treatment of neurological disorders. IEEE Trans Biomed Circuits Syst 12:1230–1245. 10.1109/TBCAS.2018.2880148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA, Christine CW, Theodosopoulos PV, Lindsey N, Byrd D, Mosley A, Marks WJ (2002) Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. J Neurosurg 97:370–387. 10.3171/jns.2002.97.2.0370 [DOI] [PubMed] [Google Scholar]
- Strelow JN, Dembek TA, Baldermann JC, Andrade P, Jergas H, Visser-Vandewalle V, Barbe MT (2022) Local field potential-guided contact selection using chronically implanted sensing devices for deep brain stimulation in Parkinson's disease. Brain Sci 12:1726. 10.3390/brainsci12121726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D, Chen H, Hu W, Liu Y, Wang Z, Wang X, Liu G, Ma H, Zhou J, Feng T (2018) Frequency-dependent effects of subthalamic deep brain stimulation on motor symptoms in Parkinson's disease: a meta-analysis of controlled trials. Sci Rep 8:14456. 10.1038/s41598-018-32161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, de Hemptinne C, Miocinovic S, Qasim S, Wang SS, Ziman N, Ostrem JL, San Luciano M, Galifianakis NB, Starr PA (2016) Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in Parkinson's disease. J Neurosci 36:6445–6458. 10.1523/JNEUROSCI.1128-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, de Hemptinne C, Thompson MC, Miocinovic S, Miller AM, Gilron R, Ostrem JL, Chizeck HJ, Starr PA (2018) Adaptive deep brain stimulation for Parkinson's disease using motor cortex sensing. J Neural Eng 15:046006. 10.1088/1741-2552/aabc9b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CH, Wu RM, Liu HM, Tsai CW, Tseng SH (2011) Meige syndrome relieved by bilateral pallidal stimulation with cycling mode: case report. Neurosurgery 69:E1333–E1337. 10.1227/NEU.0b013e31822a9ad2 [DOI] [PubMed] [Google Scholar]
- Tass PA (2003) A model of desynchronizing deep brain stimulation with a demand-controlled coordinated reset of neural subpopulations. Biol Cybern 89:81–88. 10.1007/s00422-003-0425-7 [DOI] [PubMed] [Google Scholar]
- Tass PA, Qin L, Hauptmann C, Dovero S, Bezard E, Boraud T, Meissner WG (2012) Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol 72:816–820. 10.1002/ana.23663 [DOI] [PubMed] [Google Scholar]
- Vázquez-Barrón D, Cuéllar-Herrera M, Velasco F, Velasco AL (2021) Electrical stimulation of subiculum for the treatment of refractory mesial temporal lobe epilepsy with hippocampal sclerosis: a 2-year follow-up study. Stereotact Funct Neurosurg 99:40–47. 10.1159/000510295 [DOI] [PubMed] [Google Scholar]
- Velisar A, Syrkin-Nikolau J, Blumenfeld Z, Trager MH, Afzal MF, Prabhakar V, Bronte-Stewart H (2019) Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul 12:868–876. 10.1016/j.brs.2019.02.020 [DOI] [PubMed] [Google Scholar]
- Vergani F, Landi A, Antonini A, Parolin M, Cilia R, Grimaldi M, Ferrarese C, Gaini SM, Sganzerla EP (2007) Anatomical identification of active contacts in subthalamic deep brain stimulation. Surg Neurol 67:140–146; discussion 146-147. 10.1016/j.surneu.2006.06.054 [DOI] [PubMed] [Google Scholar]
- Visser-Vandewalle V, et al. (2022) Deep brain stimulation for obsessive-compulsive disorder: a crisis of access. Nat Med 28:1529–1532. 10.1038/s41591-022-01879-z [DOI] [PubMed] [Google Scholar]
- Vitek JL, Patriat R, Ingham L, Reich MM, Volkmann J, Harel N (2022) Lead location as a determinant of motor benefit in subthalamic nucleus deep brain stimulation for Parkinson's disease. Front Neurosci 16:1010253. 10.3389/fnins.2022.1010253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann J, Herzog J, Kopper F, Deuschl G (2002) Introduction to the programming of deep brain stimulators. Mov Disord 17 Suppl 3:S181–S187. 10.1002/mds.10162 [DOI] [PubMed] [Google Scholar]
- Wang DD, de Hemptinne C, Miocinovic S, Ostrem JL, Galifianakis NB, San Luciano M, Starr PA (2018) Pallidal deep-brain stimulation disrupts pallidal beta oscillations and coherence with primary motor cortex in Parkinson's disease. J Neurosci 38:4556–4568. 10.1523/JNEUROSCI.0431-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Nebeck S, Muralidharan A, Johnson MD, Vitek JL, Baker KB (2016) Coordinated reset deep brain stimulation of subthalamic nucleus produces long-lasting, dose-dependent motor improvements in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine non-human primate model of parkinsonism. Brain Stimul 9:609–617. 10.1016/j.brs.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fergus SP, Johnson LA, Nebeck SD, Zhang J, Kulkarni S, Bokil H, Molnar GF, Vitek JL (2022) Shuffling improves the acute and carryover effect of subthalamic coordinated reset deep brain stimulation. Front Neurol 13:716046. 10.3389/fneur.2022.716046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AC, Veerakumar A, Choi KS, Howell B, Tiruvadi V, Bijanki KR, Crowell A, Riva-Posse P, Mayberg HS (2018) Test–retest reliability of a stimulation-locked evoked response to deep brain stimulation in subcallosal cingulate for treatment resistant depression. Hum Brain Mapp 39:4844–4856. 10.1002/hbm.24327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis AW, Schootman M, Kung N, Wang XY, Perlmutter JS, Racette BA (2014) Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology 82:163–171. 10.1212/WNL.0000000000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtecki L, Vesper J, Schnitzler A (2011) Interleaving programming of subthalamic deep brain stimulation to reduce side effects with good motor outcome in a patient with Parkinson's disease. Parkinsonism Relat Disord 17:293–294. 10.1016/j.parkreldis.2010.12.005 [DOI] [PubMed] [Google Scholar]
- Wong JK, Hu W, Barmore R, Lopes J, Moore K, Legacy J, Tahafchi P, Jackson Z, Judy JW, Raike RS, Wang A, Tsuboi T, Okun MS, Almeida L (2021) Safety and tolerability of burst-cycling deep brain stimulation for freezing of gait in Parkinson's disease. Front Hum Neurosci 15:651168. 10.3389/fnhum.2021.651168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, et al. (2023) Proceedings of the 10th Annual Deep Brain Stimulation Think Tank: advances in cutting edge technologies, artificial intelligence, neuromodulation, neuroethics, interventional psychiatry, and women in neuromodulation. Front Hum Neurosci 16:1084782. 10.3389/fnhum.2022.1084782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SS, Lee WL, Perera T, Sinclair NC, Bulluss KJ, McDermott HJ, Thevathasan W (2022) Can brain signals and anatomy refine contact choice for deep brain stimulation in Parkinson's disease? J Neurol Neurosurg Psychiatry jnnp-2021-327708. 10.1136/jnnp-2021-327708 [DOI] [PubMed] [Google Scholar]
- Yelnik J, Damier P, Demeret S, Gervais D, Bardinet E, Bejjani BP, François C, Houeto JL, Arnule I, Dormont D, Galanaud D, Pidoux B, Cornu P, Agid Y (2003) Localization of stimulating electrodes in patients with Parkinson disease by using a three-dimensional atlas-magnetic resonance imaging coregistration method. J Neurosurg 99:89–99. 10.3171/jns.2003.99.1.0089 [DOI] [PubMed] [Google Scholar]
- Yu Y, Escobar Sanabria D, Wang J, Hendrix CM, Zhang J, Nebeck SD, Amundson AM, Busby ZB, Bauer DL, Johnson MD, Johnson LA, Vitek JL (2021) Parkinsonism alters beta burst dynamics across the basal ganglia-motor cortical network. J Neurosci 41:2274–2286. 10.1523/JNEUROSCI.1591-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Lai Y, Li J, He N, Liu Y, Li Y, Li H, Wei H, Yan F, Horn A, Li D, Sun B (2021) Subthalamic and pallidal stimulations in patients with Parkinson's disease: common and dissociable connections. Ann Neurol 90:670–682. 10.1002/ana.26199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Zhang Y, Li J, Zhang X, Zhuang P, Li Y (2009) Subthalamic deep brain stimulation for Parkinson's disease: correlation of active contacts and electrophysiologically mapped subthalamic nucleus. Chin Med J (Engl) 122:2419–2422. [PubMed] [Google Scholar]
- Zhu H, Huang J, Deng L, He N, Cheng L, Shu P, Yan F, Tong S, Sun J, Ling H (2019) Abnormal dynamic functional connectivity associated with subcortical networks in Parkinson's disease: a temporal variability perspective. Front Neurosci 13:80. 10.3389/fnins.2019.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonenshayn M, Sterio D, Kelly PJ, Rezai AR, Beric A (2004) Location of the active contact within the subthalamic nucleus (STN) in the treatment of idiopathic Parkinson's disease. Surg Neurol 62:216–225. 10.1016/j.surneu.2003.09.039 [DOI] [PubMed] [Google Scholar]


