Abstract
We sought to determine whether microorganisms from the polychlorinated biphenyl (PCB)-contaminated sediment in Woods Pond (Lenox, Mass.) could dehalogenate brominated biphenyls. The PCB dechlorination specificities for the microorganisms in this sediment have been well characterized. This allowed us to compare the dehalogenation specificities for brominated biphenyls and chlorinated biphenyls within a single sediment. Anaerobic sediment microcosms were incubated separately at 25°C with 16 different mono- to tetrabrominated biphenyls (350 μM) and disodium malate (10 mM). Samples were extracted and analyzed by gas chromatography with an electron capture detector and a mass spectrometer detector at various times for up to 54 weeks. All of the tested brominated biphenyls were dehalogenated. For most congeners, including 2,6-dibromobiphenyl (26-BB) and 24-25-BB, the dehalogenation began within 1 to 2 weeks. However, for 246-BB and 2-2-BB, debromination was first observed at 7 and 14 weeks, respectively. Most intermediate products did not persist, but when 2-2-BB was produced as a dehalogenation product, it persisted for at least 15 weeks before it was dehalogenated to 2-BB and then to biphenyl. The dehalogenation specificities for brominated and chlorinated biphenyls were similar: meta and para substituents were generally removed first, and ortho substituents were more recalcitrant. However, the brominated biphenyls were better dehalogenation substrates than the chlorinated biphenyls. All of the tested bromobiphenyls, including those with ortho and unflanked meta and para substituents, were ultimately dehalogenated to biphenyl, whereas their chlorinated counterparts either were not dehalogenation substrates or were only partially dehalogenated. Our data suggest that PCB-dechlorinating microorganisms may be able to dehalogenate brominated biphenyls and may exhibit a relaxed specificity for these substrates.
There have been many studies of the microbial dehalogenation of polychlorinated biphenyls (PCBs) (1–3, 5–7, 9–11, 17–19, 22–24, 26–29), but only two previous studies of dehalogenation of polybrominated biphenyls (PBBs) (15, 16). Microorganisms from a PBB-contaminated site (Pine River, St. Louis, Mich.) (16) and two PCB-contaminated sediments (Silver Lake, Pittsfield, Mass., and Hudson River, river mile 193, Fort Edward, N.Y.) (9, 10) partially dehalogenated the commercial PBB mixture Firemaster BP6 (15). A single hexabromobiphenyl, 2,4,5,2′,4′,5′-hexabromobiphenyl (245-245-BB), which constitutes more than 50% of Firemaster (21), was the primary dehalogenation substrate. Microorganisms from the Pine River site removed 32% of the meta and para bromines from Firemaster in 32 weeks (15). The dehalogenation products were predominantly 24-25-BB and 25-2-BB, with lesser amounts of 25-25-BB and 24-24-BB and a trace of 2-2-BB. In contrast, microorganisms collected from the Pine River upstream of the PBB contamination did not dehalogenate Firemaster. Hudson River and Silver Lake microorganisms removed 12 and 3%, respectively, of the meta and para bromines from Firemaster in 32 weeks, but the main product was 2-2-BB (15). No ortho debromination was observed in any of these experiments. However, small amounts of biphenyl and 2-BB were detected when Firemaster was incubated with a pyruvate enrichment culture of PCB-dechlorinating microorganisms from the Hudson River (15). The latter data suggested the possibility of ortho debromination, since all PBB congeners in Firemaster have at least one ortho bromine.
The PCBs in Hudson River and Silver Lake sediments have undergone extensive microbial dechlorination in situ (9, 10). In addition, many laboratory experiments have confirmed that PCB dechlorinators are present in these sediments and can be eluted from them and transferred to other sediments (1, 17–19). Neither sediment shows any evidence of PBB contamination. Hence, the observation that microorganisms from two PCB-contaminated sites can remove the meta and para bromines from 245-245-BB suggests that PCB dechlorinators might be able to dehalogenate brominated biphenyls. We sought to test this possibility by assessing the ability of the anaerobic microorganisms from the PCB-contaminated sediments of Woods Pond (Lenox, Mass.) to dehalogenate a variety of mono- through tetrabrominated biphenyls.
The PCB dechlorination specificity for the microorganisms in Woods Pond has been well characterized (3, 5, 6, 23, 24, 26–29). This information provided us with the opportunity to directly compare the dehalogenation specificities for PCBs and brominated biphenyls within a single sediment. The microorganisms in Woods Pond sediment can dechlorinate PCBs by removal of flanked meta or para chlorines from 3,4- (34-), 234-, 235-, 236-, 245-, 345-, 2345-, 2346-, 2356-, and 23456-chlorophenyl rings and unflanked para chlorines from 24- and 246-chlorophenyl rings (3, 6, 24, 28, 29). However, the unflanked meta chlorines on 3- and 25-chlorophenyl rings are not dechlorinated by the microorganisms in this sediment and neither is the unflanked para chlorine on 4-chlorophenyl rings (6). Only three PCB congeners, 246-CB, 24-CB, and 2356-CB, are known to be substrates for ortho dechlorination by the microorganisms in Woods Pond (23, 26, 28). Unfortunately, 2-CB, 2-2-CB, 26-CB, and other ortho-chlorinated congeners do not appear to be substrates (23, 26–29). We postulated that brominated biphenyls might be favorable substrates for PCB dechlorinators because they are PCB analogs. Furthermore, their chemistry indicates that brominated biphenyls should be more easily dehalogenated than PCBs. The aryl-bromine bond is weaker than the aryl-chlorine bond (dissociation energies for the C6H5-Br and C6H5-Cl bonds are 80 and 95 kcal/mol, respectively (see Table 5, p. F-243, in reference 25). In addition, chemical dehalogenations carried out by various reaction mechanisms have consistently shown that aryl bromines are more easily removed than aryl chlorines (8, 13, 20).
We investigated the dehalogenation of all commercially available mono-, di-, and tribrominated biphenyls and that of several tetrabrominated biphenyls. These were 2-BB, 3-BB, 4-BB, 24-BB, 25-BB, 26-BB, 2-2-BB, 4-4-BB, 245-BB, 246-BB, 345-BB, 25-2-BB, 25-3-BB, 25-4-BB, 24-25-BB, and 25-25-BB. We expected to observe selective dehalogenation from ortho, meta, and para positions as has been observed for PCB dechlorination by microorganisms from Woods Pond sediment (see above). Furthermore, because bromines should be easier to dehalogenate, we reasoned that the unflanked bromines on 3-, 4-, 25-, and possibly 2- and 26-bromophenyl rings might also be dehalogenated by these microorganisms even though the chlorines on their PCB counterparts are not.
The results confirmed our expectations. All of the brominated biphenyls were completely dehalogenated to biphenyl in live samples, but no dehalogenation occurred in autoclaved controls. Debromination occurred first from the meta and para positions and then from the ortho positions. Most of the congeners were dehalogenated after a lag time of 1 to 2 weeks, but 2-2-BB required an acclimation period of 14 weeks before dehalogenation commenced. Most dehalogenation intermediates did not accumulate, but when 2-2-BB was produced as an intermediate it persisted for at least 15 weeks.
MATERIALS AND METHODS
Chemicals.
Brominated biphenyls (97 to 99% purity) were purchased from AccuStandard (New Haven, Conn.) or Ultra Scientific (North Kingstown, R.I.). l-Malic acid (cell culture reagent quality, catalog no. M-7387) was purchased from Sigma Chemical Corporation (St. Louis, Mo.) and adjusted to pH 7 with sodium hydroxide.
Slurry preparation and incubation.
Multiple core samples (45 cm) of sediment were collected from the west side of Woods Pond (5), a shallow impoundment on the Housatonic River. The core samples were pooled in glass jars, topped with site water, and stored at 4°C until used. On a dry-weight basis, each gram of sediment contained 45.1 μg of partially dechlorinated Aroclor 1260 and 7,100 μg of weathered hydrocarbon oil. Slurries were prepared under an atmosphere of 95 to 97% nitrogen-3 to 5% hydrogen in an anaerobic chamber by mixing wet sediment (2 volumes) with glass-distilled water (3 volumes). The slurries were dispensed into serum bottles, and a bromobiphenyl congener was added from a concentrated stock solution (70 mM in acetone) to give a final concentration of 350 μmol per liter of slurry. (This corresponds to 560, 750, 940, and 1,130 μg of brominated biphenyl per g [dry weight] of sediment for mono- through tetrabrominated biphenyls, respectively.) Except where indicated, disodium malate was also added to give a final concentration of 10 mM. No other nutrients were added. The bottles were crimp sealed with Teflon-lined butyl rubber septa. Sterile controls were prepared by pasteurization (at 75°C for 20 min), followed by incubation (at 23 to 25°C for 24 h) and autoclaving (at 121°C for 3 h). Duplicate samples and controls were incubated in the dark at 23 to 25°C. Although no bicarbonate or CO2 was added, all incubations became methanogenic within 1 to 2 weeks.
The data for dehalogenation of 246-BB were obtained from an experiment set up under different conditions. For this experiment, microorganisms were eluted from a fresh slurry of Woods Pond sediment by two consecutive gravity filtrations of the sediment slurry through several layers of glass wool and then used to inoculate a pasteurized slurry prepared from decomposed freshwater marsh peat collected from a beaver meadow in the Adirondack Mountains (N.Y.). The marsh peat slurry was prepared in the anaerobic chamber, dispensed into serum bottles as described above, and then pasteurized by heating twice to 80°C for 10 min with a 24-h interval at 24°C between heatings. Following pasteurization, 10 ml of the supernatant was removed from each 30-ml sample of marsh slurry and replaced with the microbial inoculum from Woods Pond. 26-BB or 246-BB, disodium malate, and Aroclor 1260 were then added to the resulting inoculated sediment to give final concentrations per liter of 350 μmol, 10 mmol, and 10 mg, respectively. The slurries were incubated at 22 to 25°C and became methanogenic within a week or two.
Extraction and bromobiphenyl analysis.
Aliquots (1 ml each) of the slurries were sampled periodically and extracted by vigorous shaking (24 h) with anhydrous ether (5 volumes) and elemental mercury (1/4 volume, to remove sulfur) in vials with Teflon-lined foam-backed screw caps. Samples were analyzed by gas chromatography (GC) on a 5880A GC (Hewlett-Packard Co., Palo Alto, Calif.) equipped with a Ni63 electron capture detector operated at 300°C and a DB-1 (polydimethylsiloxane) capillary column (30 m long by 0.25 mm [inner diameter; 0.25-μm phase thckness], J & W Scientific, Inc., Folsom, Calif.). We used a two-stage GC temperature program as follows: 2 min at 40°C, increase at 20°C/min to 160°C, hold 3 min, increase at 2°C/min to 260°C, hold 20 min. The carrier and makeup gas was nitrogen.
Bromobiphenyls formed as dehalogenation products were initially identified by matching GC retention times with those of authentic standards. Reference standards were not available for 2-3-BB, 2-4-BB, 34-BB, 35-BB, and 24-2-BB. These intermediates were identified from the possible debromination products based on comparisons of their elution positions, relative to those of the other brominated biphenyl congeners, with the relative elution positions of PCBs with the same substitution patterns (12). All intermediates were subsequently analyzed by selected ion monitoring (electron impact ionization at 70 eV) with a Hewlett-Packard 5890/5971A GC-mass spectrometer (MS) equipped with a DB-1 capillary column as described above and were confirmed to be brominated biphenyls. We used a multistage GC temperature program as follows: 2 min at 50°C, increase at 20°C/min to 150°C, increase at 4°C/min to 210°C, increase at 20°C/min to 270°C, and hold for 10 min.
Several isomers of each homolog class were scanned by GC-MS to determine the fragmentation pattern and to identify the most abundant ions. Scan windows were set to include the earliest and latest eluting isomers of each homolog class. The masses of the most abundant ions of the molecular ion cluster and its characteristic fragments were monitored for each homolog class. These were, in order of relative abundance, m/z 153 and 154 for biphenyl; m/z 232, 234, and 152 for monobromobiphenyls; m/z 312, 314, and 152 for dibromobiphenyls; m/z 390, 392, 230, and 232 for tribromobiphenyls; and m/z 310, 389, 391, and 470 for 24-25-BB and 25-25-BB. The ratios of the areas of these ions were checked for each sample to verify that they matched those of the standards. 26-BB and its products, 2-BB and biphenyl, were quantified by GC-MS with a linear three-point calibration curve.
RESULTS
Stoichiometric dehalogenation of 26-BB to biphenyl.
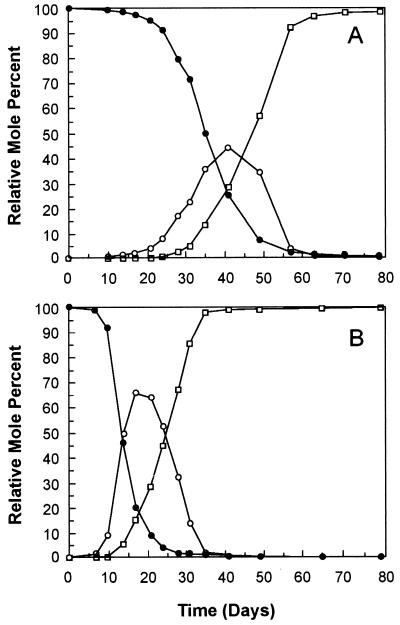
We examined the dehalogenation of 26-BB in our first experiments because we were particularly interested in determining whether the microorganisms in Woods Pond sediment could dehalogenate ortho-brominated biphenyls. As shown in Fig. 1A, dehalogenation of 26-BB was first detected at 2 weeks and the congener was completely dehalogenated to biphenyl within 3 months. 2-BB accumulated as an intermediate while the 26-BB was being dehalogenated, but some of it was further dehalogenated to biphenyl even though significant amounts of 26-BB remained. This result suggests that the rate of dehalogenation of 26-BB is higher than that for 2-BB. No dehalogenation occurred in autoclaved controls.
FIG. 1.
Dehalogenation of 26-BB to biphenyl. 26-BB (350 μmol per liter of slurry) was incubated in anaerobic microcosms of Woods Pond sediment with or without malate (10 mM) as described in the text. The time course of dehalogenation was monitored by GC-MS as described in Materials and Methods. (A) No malate. (B) Malate was added at the beginning of the incubation. •, 26-BB; ○, 2-BB, □, biphenyl.
Effect of malate on dehalogenation of 26-BB.
We had previously determined that disodium malate (10 mM) accelerated the dechlorination of 25-34-CB to 25-3-CB (4). Malate also accelerated the dehalogenation of 26-BB (Fig. 1B), but the degree of acceleration differed depending on the time of year at which the sediment was collected and the length of time it was stored before use. Subsequent experiments showed that the malate was depleted within the first few days of incubation, prior to the onset of dehalogenation. Furthermore, replenishing the malate during the incubation had no effect on the dehalogenation. Hence, it is unlikely that malate was an electron donor for the dehalogenation reaction. Malate also accelerated the dehalogenation at concentrations of 2.5 and 5 mM, but these concentrations were slightly less effective than 10 mM (data not shown). Malate had no significant effect at concentrations of 0.1 or 0.5 mM.
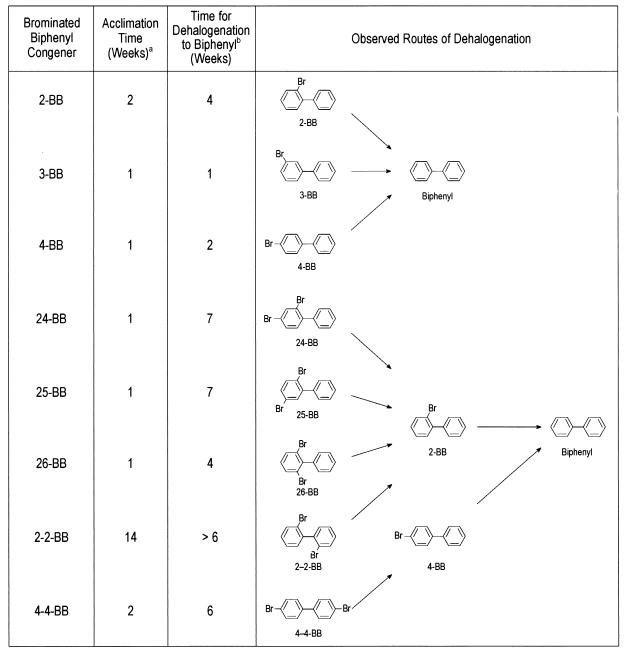
Dehalogenation of mono- and dibrominated biphenyls.
All but one of the mono- and dibromobiphenyls were dehalogenated after only 1 to 2 weeks of acclimation. Following acclimation, more than 90% of each of these congeners was dehalogenated to biphenyl in 1 to 7 weeks (Fig. 2). The meta and para bromines were removed first, and then the ortho bromines were removed (Fig. 2). 4-BB was detected as a transient intermediate of 4-4-BB but was further dehalogenated to biphenyl without accumulating. The 2-BB produced from the dehalogenation of 24- and 25-BB accumulated briefly prior to dehalogenation as seen previously for 26-BB (Fig. 1B). Unlike the other congeners, 2-2-BB required a long acclimation period (14 weeks) before dehalogenation commenced. However, although 2-BB was detected as an intermediate of 2-2-BB, it did not accumulate as in the other slurries but was rapidly dehalogenated to biphenyl.
FIG. 2.
Dehalogenation of mono- and dibrominated biphenyls. a, length of incubation before dehalogenation was observed, as evidenced by the appearance of the first traces of dehalogenation product(s). As little as 1 to 2% dehalogenation of the substrate could be detected. b, length of time after acclimation for dehalogenation of ∼90% of the bromobiphenyl to biphenyl.
Dehalogenation of tribrominated biphenyls.
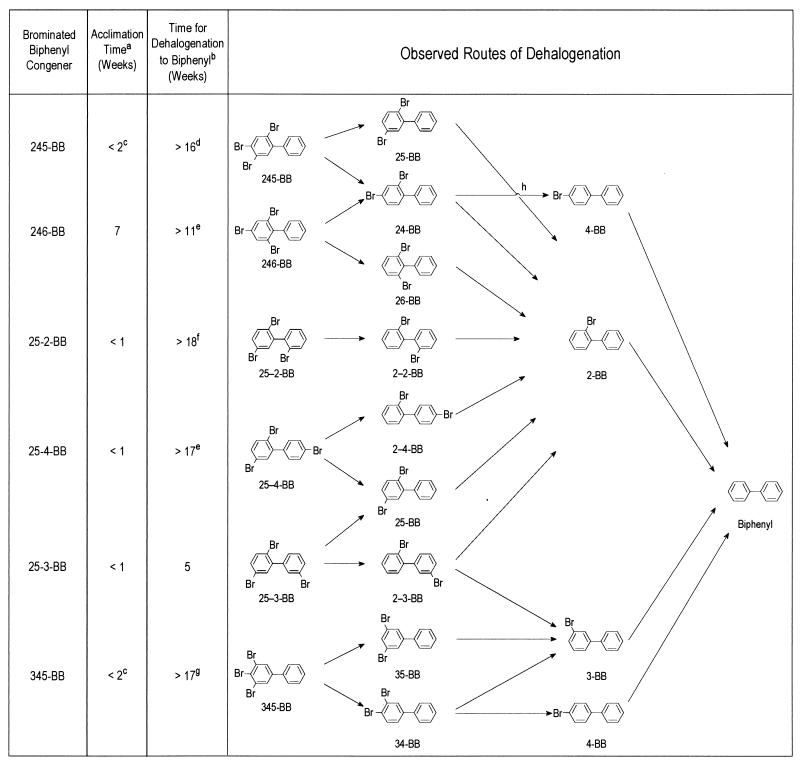
All tribromobiphenyls except 246-BB were also dehalogenated after only 1 to 2 weeks of acclimation, but complete dehalogenation to biphenyl took much longer than for mono- and dibromobiphenyls (Fig. 3). For most tribromobiphenyls, small to moderate amounts of the parent congener persisted for long times, even though most of the congener had already been dehalogenated to biphenyl. Most of the tribromobiphenyls were dehalogenated by two different routes, but it was generally not possible to determine whether one route was favored, because most dibrominated intermediates did not accumulate to substantial levels before further dehalogenation. However, meta and para bromines were generally removed before ortho bromines. When formed, 26-BB and 2-BB briefly accumulated to substantial levels.
FIG. 3.
Dehalogenation of tribrominated biphenyls. a, length of incubation before dehalogenation was observed, as evidenced by the appearance of the first traces of dehalogenation products. b, length of time after acclimation for dehalogenation of ∼90% of the bromobiphenyl to biphenyl. c, the first time point in these incubations was at 14 days; by that time, significant dehalogenation to di- and monobrominated products had already occurred. d, a moderate amount of 245-BB was still present at this time. e, small amounts of 246-BB and 25-4-BB, respectively, persisted in these samples. f, this congener was rapidly dehalogenated to 2-2-BB, which persisted at this time; biphenyl was first observed at 136 days. g, a large amount of 345-BB and a moderate amount of 3-BB persisted. h, this dehalogenation reaction was observed only when 246-BB was the initial substrate.
There were two instances in which ortho debromination occurred prior to complete meta and para dehalogenation (Fig. 3). (i) The 246-BB was dehalogenated to both 24-BB and 26-BB. Subsequently, 2-BB and 4-BB were detected prior to dehalogenation to biphenyl. (ii) The 2-3-BB that was formed as an intermediate from 25-3-BB was dehalogenated to both 2-BB and 3-BB before dehalogenation to biphenyl.
The 345-BB was dehalogenated to 34-BB and 35-BB and then to 3-BB, 4-BB, and biphenyl. The 35-BB and 3-BB both accumulated to high levels for a short time before dehalogenation to biphenyl.
The 25-2-BB was exclusively dehalogenated by the pathway 25-2-BB → 2-2-BB → 2-BB → biphenyl. The initial dehalogenation product, 2-2-BB, was first detected at 6 days and persisted without further dehalogenation for at least 18 weeks. No 2-BB or biphenyl was detected until 136 days.
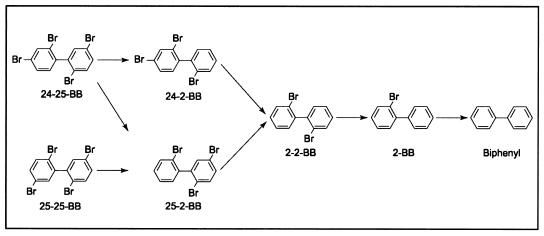
Dehalogenation of 24-25-BB and 25-25-BB.
Figure 4 shows the pathways for dehalogenation of 24-25-BB and 25-25-BB. The meta and para dehalogenation of these tetrabromobiphenyls to 2-2-BB commenced within 2 weeks, but the 2-2-BB accumulated and persisted for at least 15 weeks. When the slurries were sampled again at 54 weeks, some of the tetrabromobiphenyl and 2-2-BB was still present, but most had been dehalogenated to biphenyl. Figure 5 shows the product distribution at various times for one of duplicate incubations of 24-25-BB.
FIG. 4.
Dehalogenation pathway for 24-25-BB and 25-25-BB. Both congeners were dehalogenated to 2-2-BB, which began to accumulate at 14 days and persisted for at least 16 weeks. Dehalogenation of 2-2-BB began sometime after 17 weeks and prior to 54 weeks. In both cases, small amounts of 2-2-BB and tetrabromobiphenyl still persisted at 54 weeks.
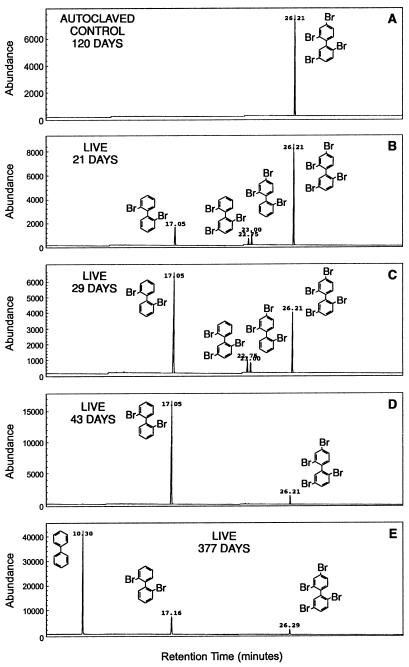
FIG. 5.
Total ion chromatogram of 24-25-BB and its dehalogenation products at various times. The dehalogenation of 24-25-BB was monitored by GC-MS as described in Materials and Methods. The areas plotted for each compound represent the sum of selected ions for that compound and therefore are not directly comparable to concentration. However, the plots do indicate the progress of the dehalogenation. (A) Autoclaved control at 120 days. (B through E) Live samples showing the dehalogenation products present at 21, 29, 43, and 377 days. The retention times have shifted slightly for the sample at 377 days because this sample was analyzed many months later than the other samples.
DISCUSSION
Dehalogenation specificity: brominated versus chlorinated biphenyls.
Despite no history of prior exposure to brominated biphenyls, the microorganisms in Woods Pond sediment dehalogenated all of the tested brominated congeners to biphenyl. As expected, brominated biphenyls were better substrates for microbial dehalogenation than chlorinated biphenyls. Perhaps as a result, the specificity for brominated biphenyl dehalogenation was less stringent than that observed for PCBs. Table 1 compares the dehalogenation of various brominated biphenyls and their chlorinated counterparts in microcosms of Woods Pond sediment. Although all of these brominated biphenyls were dehalogenated, most of their chlorinated analogs were not dehalogenated, despite prolonged incubation. Furthermore, the brominated biphenyls were totally dehalogenated to biphenyl, but the chlorinated biphenyls that were substrates were only partially dehalogenated.
TABLE 1.
Dehalogenation of chlorinated and brominated biphenyls in microcosms of Woods Pond sedimenta
| Chlorinated biphenyls
|
Brominated biphenyls
|
|||||
|---|---|---|---|---|---|---|
| Congener | Product(s) | Acclimation timeb (weeks) | Reference | Congener | Product(s)g | Acclimation time (weeks) |
| 2-CBc | None | (27) | 24, 28 | 2-BB | BP | 2 |
| 4-CBc | None | (27) | 24, 26, 28 | 4-BB | BP | 1 |
| 24-CBd | 2-CB, 4-CB | 8 | 26, 28 | 24-BB | 2-BB, 4-BB, BP | 1 |
| 25-CBd | None | (17) | 24, 26 | 25-BB | 2-BB, BP | 1 |
| 26-CBd | None | (43) | 6, 23, 26, 28 | 26-BB | 2-BB, BP | 1 |
| 2-2-CB | None | (8) | 24 | 2-2-BB | 2-BB, BP | 14 |
| 4-4-CB | None | (8) | 24 | 4-4-BB | 4-BB, BP | 2 |
| 245-CB | 25-CB, (24-CB)e | 3 | 24, 26 | 245-BB | 24-BB, 25-BB, 2-BB, BP | <2 |
| 246-CBf | 24-CB, 2-CB, 4-CB | 8 | 26, 28 | 246-BB | 24-BB, 26-BB, 2-BB, 4-BB, BP | 7 |
| 246-CBf | 26-CB | 8 | 6, 28 | 246-BB | 24-BB, 26-BB, 2-BB, 4-BB, BP | 7 |
| 345-CB | 35-CB | 3 | 26 | 345-BB | 34-BB, 35-BB, 3-BB, 4-BB, BP | <2 |
| 25-3-CBd | None | (8) | 3 | 25-3-BB | 25-BB, 2-3-BB, 2-BB, 3-BB, BP | <1 |
Dehalogenation of four PCB congeners, 2-CB, 4-CB, 2-2-CB, and 4-4-CB, was tested in the same batch of sediment used to test dehalogenation of the brominated biphenyls. Results for dehalogenation of the remaining chlorinated biphenyls are from previous experiments with different batches of sediment. Except for small differences in acclimation time, the results of dehalogenation of chlorinated biphenyls were the same in studies by several different investigators.
The time given is the shortest time observed before any dehalogenation products were observed. For cases in which dechlorination did not occur, the time in parentheses is the time at which the experiment was terminated.
When incubated separately, this congener persisted until the experiments were terminated at 8 weeks (24). When produced as a dehalogenation intermediate of 24-CB, this congener persisted for >27 weeks.
This congener was produced as the dehalogenation product of another congener. The acclimation time was counted only after the congener had accumulated to high levels.
24-CB was a minor product (<1% of the total).
The time given is for incubations in which 246-CB was a product of 2346-CB (28). When the congener was incubated separately, the observed acclimation time was 24 weeks (26).
BP, biphenyl.
For brominated biphenyls, there appeared to be no particular preference for which meta or para bromine was removed first, regardless of whether the bromine was flanked or unflanked. Generally, all possible initial meta and para dehalogenation products were observed (Fig. 2 through 5). Usually all meta and para bromines were removed prior to ortho dehalogenation. The unflanked meta bromines appeared to be slightly more difficult to remove, as evidenced by the brief accumulation of 3-BB and 35-BB when these congeners were produced as intermediates.
In contrast, PCB dehalogenation primarily targets the flanked meta and para chlorines in Woods Pond sediment (Table 1 and references 3, 24, and 26). Furthermore, the substitution pattern of the PCB substrate determines whether meta or para chlorines will be removed. For example, only one product was seen for the dechlorination of 345-CB, 235-CB, 25-34-CB, and 24-34-CB (3, 26). Both possible initial meta and para dechlorination products were observed for 245-CB and 234-CB, but the ratio of the two products was >99:1 (24). The substitution pattern of the added PCB substrate also determines the specificity of the dechlorination of Aroclor 1260 that will be primed in the sediment (24). Unflanked para chlorines on 24- and 246-chlorophenyl groups can also be dehalogenated, but usually only after long acclimation times (6, 26, 28, 29). Unflanked meta dechlorination of PCBs has never been observed in Woods Pond sediment.
For brominated biphenyls, all ortho bromines were ultimately removed, but they were apparently more difficult to remove than the meta and para bromines. This was evidenced by the longer acclimation times for 2-BB, and especially 2-2-BB, and by the accumulation and persistence of these congeners when they were produced as dehalogenation intermediates. For chlorinated biphenyls, the ortho chlorines are not dehalogenation substrates, except for those on 2356-CB, 246-CB, and 24-CB (23, 26, 28).
Despite the differences discussed above, there are similarities in the relative reactivity preferences for brominated and chlorinated biphenyls in Woods Pond sediment. For brominated biphenyls, the observed order of reactivity was as follows: flanked meta ≈ flanked para ≈ unflanked para > unflanked meta > ortho bromines. For PCBs, the order of reactivity is as follows: flanked meta ≈ flanked para > unflanked para ≥ ortho > unflanked meta chlorines.
Morris and colleagues reported that microorganisms collected upstream of the PBB contamination in the Pine River could not dehalogenate Firemaster, but microorganisms eluted from the PBB-contaminated section of the Pine River and from two sites with a history of exposure to PCBs, but not to PBBs, were able to dehalogenate Firemaster (15). These observations suggest that prior acclimation to halogenated biphenyls is necessary for PBB dehalogenation and that PCB dechlorinators might be able to dehalogenate brominated biphenyls. Our data for microorganisms from Woods Pond, which also has no history of PBB contamination, are consistent with this interpretation. Furthermore, the observed similarities in the relative reactivity preferences for chlorinated and brominated biphenyls suggest that the PCB dechlorinators in Woods Pond may exhibit cross-reactivity for brominated biphenyls. However, given that PCBs composed of certain chlorophenyl groups (e.g., those substituted at positions 2-, 3-, 4-, 25-, and 26-) appear to totally resist dehalogenation in this sediment, we were surprised that all of the brominated biphenyls were completely dehalogenated to biphenyl.
Clearly, the difference between PCB and brominated biphenyl dehalogenation is not simply a matter of reduction potential. The reduction potentials of 4-4-CB and 25-25-CB are higher than that of 4-BB (20); yet these PCBs are not dehalogenation substrates in Woods Pond (Table 1 and references 3 and 24). If the same microorganisms do indeed dehalogenate both brominated and chlorinated biphenyls, it appears that the dehalogenases show a relaxed specificity for bromophenyl groups. There is precedent for such relaxed specificity; Desulfomonile tiedje can remove chlorine only from the meta positions of halogenated benzoates, but it can remove iodine and bromine from ortho, meta, and para positions (reviewed in reference 14). A full understanding of our own results will require isolation of the responsible dehalogenating microorganisms and their dehalogenases.
Dehalogenation of 246-BB.
Unexpectedly, the acclimation time for dehalogenation of 246-BB was 7 weeks versus 1 to 2 weeks for all other tribromobiphenyls. Furthermore, although bromines were almost always more readily removed from meta and para positions than from ortho positions, this was not true for 246-BB. As previously observed for 246-CB, the unflanked halogen in the para position proved as difficult to remove as those in the ortho positions. Since the unflanked para bromines on 4-BB and 24-BB and the ortho bromines on 2-BB and 26-BB were removed after very short acclimation times, we do not understand the relative recalcitrance of 246-BB. The incubation conditions for 246-BB were different from those for the other congeners. However, it is unlikely that this explains the difference, because the acclimation time for the dehalogenation of 26-BB was the same under both incubation conditions.
Ability of individual brominated biphenyls to trigger dehalogenation.
Morris and coworkers reported that the microorganisms from three different sediments dehalogenated 245-245-BB only when it was incubated as a component of Firemaster and not when it was incubated alone (15). This was unexpected, because the total concentrations of 245-245-BB in the Firemaster experiments and in the 245-245-BB experiments were comparable. Morris and coworkers proposed that one or more of the other components of Firemaster might be required to elicit a response (e.g., enzyme induction) that triggers dehalogenation. This proposal is not consistent with our results with Woods Pond sediment. Each of the 16 brominated biphenyls that we studied triggered dehalogenation, regardless of the substitution pattern. However, we did not study 245-245-BB.
The relative recalcitrance of 2-2-BB and its implications for the dehalogenation of Firemaster BP6.
Very long acclimation times were required for ortho dehalogenation when ortho bromines were juxtaposed on both rings, as in 2-2-BB, 25-2-BB, 24-25-BB, and 25-25-BB. Prior acclimation for meta and/or para dehalogenation did not facilitate ortho dehalogenation of 2-2-BB, suggesting that the microorganisms that dehalogenate 2-2-BB are distinct from those that dehalogenate the other congeners tested.
Microorganisms from the Pine River, the Hudson River, and Silver Lake meta- and para-dehalogenated the 245-245-BB in Firemaster to di-ortho-substituted products including 2-2-BB, but no ortho dehalogenation was observed in the 32-week incubations (15). This is consistent with the relative recalcitrance of 2-2-BB that we observed in Woods Pond sediment. It is important to note that Morris and coworkers terminated their experiments when fewer than one-third of the meta and para bromines had been removed from Firemaster (15). Given that we observed ortho dehalogenation of congeners with ortho bromines juxtaposed on both rings only after nearly all of the meta and para bromines had been removed, and then only after a long acclimation to 2-2-BB (see Results and Fig. 5), it is possible that ortho dehalogenation to biphenyl would have occurred in the Firemaster experiments if the incubations had been extended further. On the other hand, it is also possible that the microorganisms from the sites studied in the Firemaster experiments are incapable of ortho-dehalogenating brominated biphenyls. It is well established that microorganisms from Woods Pond can ortho-dehalogenate certain PCB congeners, but no conclusive evidence for ortho dechlorination of PCBs has been reported for microorganisms from any of the locations studied by Morris and coworkers (11, 16, 18, and 22 and references 9 and 10 as reevaluated in reference 2). Longer experiments with microorganisms from the Pine River, the Hudson River, and Silver Lake incubated with 245-245-BB, 24-24-BB, 24-25-BB, 25-25-BB, 25-2-BB, and 2-2-BB would determine whether these sediments harbor microorganisms capable of totally dehalogenating the key components of Firemaster BP6.
ACKNOWLEDGMENTS
We thank Ralph J. May for assistance in developing GC-MS methods of analysis, Rosanna Stokes for malate analysis, Lynn Smullen for help in preparing the figures, and Kim DeWeerd for helpful comments on the manuscript.
REFERENCES
- 1.Alder A C, Häggblom M M, Oppenheimer S R, Young L Y. Reductive dechlorination of polychlorinated biphenyls in anaerobic sediments. Environ Sci Technol. 1993;27:530–538. [Google Scholar]
- 2.Bedard D L, Quensen J F., III . Microbial reductive dechlorination of polychlorinated biphenyls. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss Division, John Wiley & Sons, Inc.; 1995. pp. 127–216. [Google Scholar]
- 3.Bedard D L, Bunnell S C, Smullen L A. Stimulation of microbial para-dechlorination of polychlorinated biphenyls that have persisted in Housatonic River sediment for decades. Environ Sci Technol. 1996;30:687–694. [Google Scholar]
- 4.Bedard, D. L., S. C. Bunnell, and H. M. Van Dort. Unpublished data.
- 5.Bedard D L, May R J. Characterization of the polychlorinated biphenyls in the sediments of Woods Pond: evidence for microbial dechlorination of Aroclor 1260 in situ. Environ Sci Technol. 1996;30:237–245. [Google Scholar]
- 6.Bedard D L, Van Dort H M, May R J, Smullen L A. Enrichment of microorganisms that sequentially meta-, para-dechlorinate the residue of Aroclor 1260 in Housatonic River sediment. Environ Sci Technol. 1997;31:3308–3313. [Google Scholar]
- 7.Berkaw M, Sowers K R, May H D. Anaerobic ortho dechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Appl Environ Microbiol. 1996;62:2534–2539. doi: 10.1128/aem.62.7.2534-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown H C, Krishnamurthy S. Selective reductions. XIV. The fast reaction of aryl bromides and iodides with lithium aluminum hydride in tetrahydrofuran. A simple, convenient procedure for the hydrogenolysis of aryl bromides and iodides. J Org Chem. 1969;34:3918–3923. [Google Scholar]
- 9.Brown J F, Jr, Bedard D L, Brennan M J, Carnahan J C, Feng H, Wagner R E. Polychlorinated biphenyl dechlorination in aquatic sediments. Science. 1987;236:709–712. doi: 10.1126/science.236.4802.709. [DOI] [PubMed] [Google Scholar]
- 10.Brown J F, Jr, Wagner R E, Feng H, Bedard D L, Brennan M J, Carnahan J C, May R J. Environmental dechlorination of PCBs. Environ Toxicol Chem. 1987;6:579–593. [Google Scholar]
- 11.Fish K M, Principe J M. Biotransformations of Aroclor 1242 in Hudson River test tube microcosms. Appl Environ Microbiol. 1994;60:4289–4296. doi: 10.1128/aem.60.12.4289-4296.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frame G M, Wagner R E, Carnahan J C, Brown J F, Jr, May R J, Smullen L A, Bedard D L. Comprehensive, quantitative, congener-specific analyses of eight Aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere. 1996;33:603–623. [Google Scholar]
- 13.Kuivila H G, Menapace L W. Reduction of alkyl halides by organotin hydrides. J Org Chem. 1963;28:2165–2167. [Google Scholar]
- 14.Mohn W M, Tiedje J M. Microbial reductive dehalogenation. Microbiol Rev. 1992;56:482–507. doi: 10.1128/mr.56.3.482-507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris P J, Quensen III J F, Tiedje J M, Boyd S A. Reductive debromination of the commercial polybrominated biphenyl mixture Firemaster BP6 by anaerobic microorganisms from sediments. Appl Environ Microbiol. 1992;58:3249–3256. doi: 10.1128/aem.58.10.3249-3256.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris P J, Quensen III J F, Tiedje J M, Boyd S A. An assessment of the reductive debromination of polybrominated biphenyls in the Pine River Reservoir. Environ Sci Technol. 1993;27:1580–1586. [Google Scholar]
- 17.Quensen J F, III, Tiedje J M, Boyd S A. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science. 1988;242:752–754. doi: 10.1126/science.242.4879.752. [DOI] [PubMed] [Google Scholar]
- 18.Quensen J F, III, Boyd S A, Tiedje J M. Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Appl Environ Microbiol. 1990;56:2360–2369. doi: 10.1128/aem.56.8.2360-2369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee G-Y, Bush B, Bethoney C M, DeNucci A, Oh H-M, Sokol R. Reductive dechlorination of Aroclor 1242 in anaerobic sediments: pattern, rate, and concentration dependence. Environ Toxicol Chem. 1993;12:1025–1032. [Google Scholar]
- 20.Rusling J F, Arena J V. Direct reduction of halogenated biphenyls at mercury electrodes. J Electroanal Chem. 1985;186:225–235. [Google Scholar]
- 21.Sundstrom G, Hutzinger O, Safe S. Identification of 2,2′,4,4′,5,5′- hexabromobiphenyl as the major component of flame retardant Firemaster BP-6. Chemosphere. 1976;5:11–14. [Google Scholar]
- 22.Tiedje J M, Quensen III J F, Chee-Sanford J, Schimel J P, Boyd S. Microbial reductive dechlorination of PCBs. Bioremediation. 1993;4:231–240. doi: 10.1007/BF00695971. [DOI] [PubMed] [Google Scholar]
- 23.Van Dort H M, Bedard D L. Reductive ortho and meta dechlorination of a polychlorinated biphenyl congener by anaerobic microorganisms. Appl Environ Microbiol. 1991;57:1576–1578. doi: 10.1128/aem.57.5.1576-1578.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Dort H M, Smullen L A, May R J, Bedard D L. Priming meta-dechlorination of polychlorinated biphenyls that have persisted in Housatonic River sediments for decades. Environ Sci Technol. 1997;31:3300–3307. [Google Scholar]
- 25.Weast R C, Astle M J, editors. CRC handbook of chemistry and physics. 61st ed. Boca Raton, Fla: CRC Press, Inc.; 1980. p. F-243. [Google Scholar]
- 26.Williams W A. Microbial reductive dechlorination of trichlorobiphenyls in anaerobic slurries. Environ Sci Technol. 1994;28:630–635. doi: 10.1021/es00053a015. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Bedard D L, Wiegel J. Influence of incubation temperature on the microbial reductive dechlorination of 2,3,4,6-tetrachlorobiphenyl in two freshwater sediments. Appl Environ Microbiol. 1996;62:4174–4179. doi: 10.1128/aem.62.11.4174-4179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q, Bedard D L, Wiegel J. Effect of incubation temperature on the route of microbial reductive dechlorination of 2,3,4,6-tetrachlorobiphenyl in polychlorinated biphenyl (PCB)-contaminated and PCB-free freshwater sediments. Appl Environ Microbiol. 1997;63:2836–2843. doi: 10.1128/aem.63.7.2836-2843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Q, Bedard D L, Wiegel J. Temperature determines the pattern of anaerobic microbial reductive dechlorination of Aroclor 1260 primed by 2,3,4,6-tetrachlorobiphenyl in Woods Pond sediment. Appl Environ Microbiol. 1997;63:4818–4825. doi: 10.1128/aem.63.12.4818-4825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]