Abstract
Detection of pathogenic Naegleria fowleri in environmental water samples, which is necessary for the prevention of primary amoebic meningoencephalitis, generally requires concentrating the samples. Two concentration techniques, filtration and centrifugation, were used to study the recovery of N. fowleri, in vegetative or cystic form, that had been mixed with the two other thermotolerant Naegleria species, N. lovaniensis and N. australiensis. Counting of amoebae was performed by the most probable number method on 10 water replicates of 100 ml and 10 ml each. With both concentration methods, recovery was better for cysts than for trophozoites (53% ± 21% versus 5% ± 5% by filtration and 57% ± 25% versus 22% ± 5% by centrifugation). The recovery of Naegleria trophozoites by filtration was very low, and centrifugation was significantly better than filtration in recovery of Naegleria trophozoites (22% ± 5% versus 5% ± 5%; P < 0.001). For cysts, however, filtration appeared as efficient as centrifugation, with equivalent values for recovery (53% ± 21% versus 57% ± 25%; P > 0.7). Although the recovery of cysts of N. fowleri obtained by filtration (51% ± 24%) appeared higher than that by centrifugation (36% ± 23%), the difference was not significant (P > 0.1). Both concentration methods have highly variable recovery rates, making accurate quantification of low concentrations (<100/liter) of N. fowleri in the environment difficult.
Naegleria is a ubiquitous free-living amoeba found in diverse freshwater environmental sites. Within this genus, the pathogenic species Naegleria fowleri is the causative agent of primary amoebic meningoencephalitis, a rare but rapidly fatal central nervous system disease occurring after exposure to contaminated water.
Isolation of pathogenic free-living amoebae and evaluation of their abundance in water samples are difficult and require concentrating the samples because the amoeba content in the natural environment is generally too low to allow a direct evaluation method. The two procedures commonly used for Naegleria concentration are filtration and centrifugation. Numerous workers (3, 5–7, 12) use membrane filtration, while others (4, 8, 9, 14) prefer centrifugation or use one of the two methods, depending on the sample volumes (13). Until now, the accuracy, precision, and analytical sensitivity of the two concentration methods have not been studied in a comparative fashion for free-living amoebae. However, the validity of count results depends on the accuracy of the recovery procedure used for the isolation of amoebae. In the present study, we have attempted to quantify the yield of filtration and we have compared the recovery efficiencies of filtration and centrifugation by using both techniques on the same seeded river water samples.
MATERIALS AND METHODS
The experiments have been conducted with different mixtures of both vegetative and cystic forms of the pathogenic N. fowleri associated with another thermotolerant Naegleria species, either N. lovaniensis or N. australiensis.
River water.
All the experiments were carried out with river water. Amoebae in the river water had been removed by two filtrations (with a 3-μm-pore-size cellulose acetate filter). The concentration of suspended matter in the water samples was adjusted to 20 mg/liter by addition of autoclaved suspended solids. The river water specimens thus obtained (4 to 5 liters) were then seeded with an amoebic suspension in vegetative or cystic form. This decontamination procedure was very effective, since in the course of our study, we never observed or isolated any amoeba other than the Naegleria species used for each experiment.
Amoebae.
Amoeba strains (N. fowleri Na 1104c, N. lovaniensis Ar9Ml, and N. australiensis 4.5.c.3) were grown for 2 to 5 days at 37°C on nonnutrient agar plates (NNA) spread with Escherichia coli. Naegleria trophozoites or cysts were harvested in 2 to 3 ml of amoeba saline solution (10) by gentle scraping of either an advancing front of amoebae or an encysted area. The amoebic concentration of this initial suspension was determined by four counts on a Thoma hemacytometer. After an appropriate dilution, this suspension was added to the sample of river water to obtain a final mixed concentration ranging from 50 to 100 amoebae/liter. The concentration of viable amoebae was controlled for each species from a dilution of the initial suspension by directly spreading on 10 petri plates a volume of 0.2 to 0.5 ml in order to theoretically have 5 to 10 lytic areas per plate. The cumulative number of lytic areas observed on the 10 plates was recorded to calculate the number of viable amoebae actually present in the sample before processing.
Filtration.
To determine the recovery efficiency of filtration, the river water specimens seeded with 50 to 100 amoebae/liter were submitted to continuous magnetic stirring and replicate samples (10 100-ml and 10 10-ml samples) were pressure filtered (with about 3 to 5 kPa) through 1.2-μm-pore-size cellulose acetate filters (Millipore). Filters cut in half were inverted on NNA plates overlaid with E. coli. Plates incubated at 42°C were microscopically examined daily for amoeba outgrowth over a period of 8 to 9 days.
Experiments were performed with the following mixtures of vegetative or cystic forms in different ratios to detect possible interspecific competition: (i) N. fowleri (Na 1104)-N. lovaniensis (Ar9Ml) (ratio from 1:1 to 4:1) and (ii) N. fowleri (Na 1104)-N. australiensis (4.5.c.3) (ratio of 1:1).
Every plaque emerging along the two membrane halves was picked for isolation and subcultured in order to identify the amoebic clone by isoenzyme typing as previously described (11).
Comparison of filtration and centrifugation.
The comparative study of filtration and centrifugation was carried out by processing 10 replicates of 10 ml and 100 ml each from the same seeded river water specimen by the two methods. The river water specimen was seeded with vegetative or cystic forms (100 amoebae/liter) of the following species mixtures: (i) N. fowleri (Na 1104)-N. lovaniensis (Ar9Ml) (ratio of 1:1 or 3:1 for trophozoites and 3:1 for cysts) and (ii) N. fowleri (Na 1104)-N. australiensis (4.5.c.3) (ratio of 1:1 for both trophozoites and cysts). Filtration was performed as described above for the 10- and 100-ml volumes. Centrifugation was carried out at 1,000 × g for 15 min for the 10-ml volumes and at 3,000 × g for 10 min for the 100-ml volumes in a FIRLABO SV11 centrifuge with an SV11E11 swinging rotor. After centrifugation, all but 750 μl of the supernatant was discarded by vacuum aspiration. The tube contents, after vortex stirring, were spread on an NNA plate overlaid with E. coli. Each tube was rinsed carefully with sterile water (750 μl), and the rinsing material was also spread on the same plate.
All the plates for both procedures were incubated at 42°C. Careful daily macroscopical and microscopical examinations of plates allowed us to observe amoebic growth by noting the development of lytic areas over the bacterial coating, and each amoebic plaque was subcultured for species identification by isoenzyme typing. The number of positive plates for total Naegleria and for each Naegleria species was recorded to compare the two concentration methods.
Expression of results and statistical analysis.
Both for filtration and comparison of filtration-centrifugation, the concentration of total Naegleria and of each Naegleria species was expressed as the most probable number (MPN) per liter after recording the number of positive plates for the 10 10-ml replicates and the 10 100-ml replicates (2). The recovery efficiencies were calculated by dividing the number (MPN per liter) of amoebae recovered by each method by the number of viable amoebae, as determined by control plating before processing.
For comparison of filtration and centrifugation, a χ2 test was applied to the paired MPN/liter results obtained with the two methods concurrently applied to the same samples.
RESULTS
Filtration recovery.
The filtration method was better for the recovery of cysts than for vegetative forms, as the yield of filtration, always higher for cysts, demonstrated. For the competing mixed species N. fowleri plus N. lovaniensis and N. fowleri plus N. australiensis, the filtration mean recovery, all species taken as a whole, was only 10% ± 10.3% for vegetative forms but was 54.5% ± 15.6% for the cysts (including the results of comparative experiments on filtration-centrifugation) (Tables 1 and 2). The yield for each species considered separately showed the same tendency, particularly for N. fowleri (10.2% for trophozoites versus 55.3% for cysts). Recovery efficiencies were highly variable for trophozoites and cysts, as evidenced by the standard deviations.
TABLE 1.
Recovery of trophozoites of competing Naegleria species by filtration
| Naegleria spp. | Ratio of the 2 Naegleria speciesa | % Recovery
|
|||
|---|---|---|---|---|---|
| N. fowleri | N. lovaniensis | N. australiensis | Total Naegleria | ||
| N. fowleri + N. lovaniensis | 40:60 (1:1)b | 0 | 12 | 7 | |
| 48:52 (1:1)b | 2.5 | 18.7 | 12.5 | ||
| 69:31 (3:1)b | 0 | 9.5 | 3 | ||
| 73:27 (4:1) | 3 | 7 | 4 | ||
| 77:23 (4:1) | 10 | 10 | 11 | ||
| 78:22 (4:1) | 38 | 5 | 29 | ||
| 80:20 (4:1) | 39 | 0 | 31 | ||
| Mean ± SD | 13.2 ± 17.5 | ||||
| N. fowleri + N. australiensis | 48:52 (1:1) | 3 | 5 | 4 | |
| 52:48 (1:1)b | 2 | 0 | 1 | ||
| 54:46 (1:1) | 7 | 16 | 12 | ||
| 57:43 (1:1)b | 4.2 | 0 | 2.4 | ||
| 57:43 (1:1) | 15 | 7 | 14 | ||
| Mean ± SD | 6.1 ± 5.2 | ||||
| Mean recovery ± SD | 10.2 ± 13.8 | 8.9 ± 5.8 | 5.6 ± 6.4 | 10.0 ± 10.3 | |
The observed ratio and theoretical ratio (shown in parentheses) are given for each mixture of competing Naegleria species.
Filtration performed during comparative experiments with centrifugation.
TABLE 2.
Recovery of cysts of competing Naegleria species by filtration
| Naegleria spp. | Ratio of the 2 Naegleria speciesa | % Recovery
|
|||
|---|---|---|---|---|---|
| N. fowleri | N. lovaniensis | N. australiensis | Total Naegleria | ||
| N. fowleri + N. lovaniensis | 62:38 (1:1) | 52 | 13 | 42 | |
| 64:36 (3:1)b | 26 | 76 | 73 | ||
| 78:22 (3:1)b | 64 | 5 | 49.5 | ||
| 84:16 (4:1) | 67 | 25 | 65 | ||
| 89:11 (4:1) | 84 | 0 | 75 | ||
| Mean ± SD | 58.6 ± 21.7 | ||||
| N. fowleri + N. australiensis | 22:78 (1:1)b | 84 | 6 | 29.5 | |
| 50:50 (1:1) | 50 | 24 | 53 | ||
| 51:49 (1:1)b | 51 | 6 | 37 | ||
| 54:46 (1:1) | 32 | 48 | 53 | ||
| 56:44 (1:1) | 65 | 13 | 48 | ||
| 66:34 (1:1)b | 33 | 31 | 75 | ||
| Mean ± SD | 52.5 ± 19.8 | ||||
| Mean recovery ± SD | 55.3 ± 19.8 | 23.8 ± 30.7 | 21.3 ± 16.4 | 54.5 ± 15.6 | |
The observed ratio and theoretical ratio (shown in parentheses) are given for each mixture of competing Naegleria species.
Filtration performed during comparative experiments with centrifugation.
Filtration generally allowed recovery of the association of the two species in 7 of the 12 tests for trophozoites (Table 1) and in 10 of the 11 tests for the cysts (Table 2) (Fig. 1 and 2).
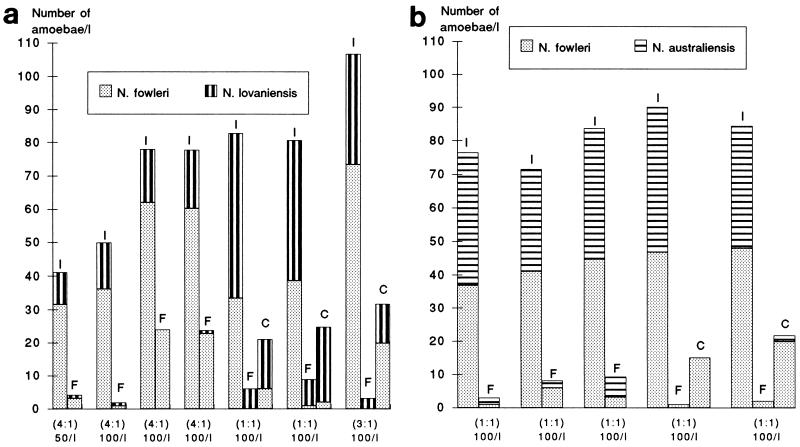
FIG. 1.
Recovery of competing Naegleria trophozoites from seeded river water by filtration and centrifugation for mixtures of N. fowleri-N. lovaniensis (a) and N. fowleri-N. australiensis (b). Values plotted for each experiment are the number (number of viable amoebae per liter according to control plating) of the two species in the inoculum (I) and the MPN of each species obtained by filtration (F) and centrifugation (C). The theoretical ratio of the two species (in parentheses) and the theoretical total amoebic concentration are shown at the bottom of the figure for each experiment.
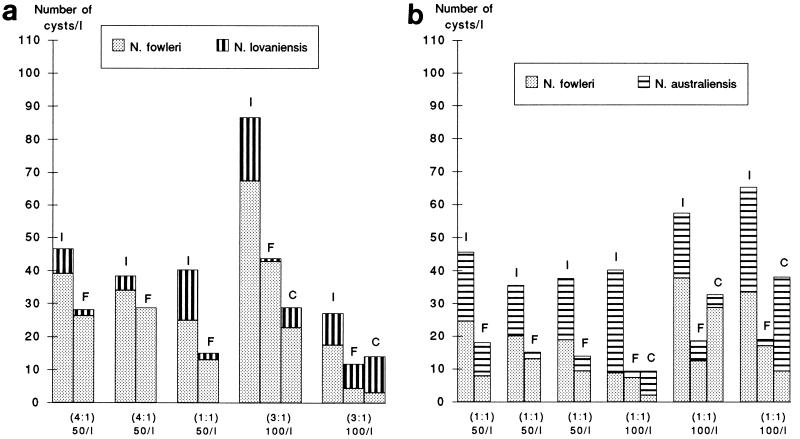
FIG. 2.
Recovery of competing Naegleria cysts from seeded river water by filtration and centrifugation for mixtures of N. fowleri-N. lovaniensis (a) and N. fowleri-N. australiensis (b). Values plotted for each experiment are given as described in the legend to Fig. 1.
The study of N. fowleri-N. lovaniensis mixtures of trophozoites where theoretical species ratios varied significantly (from 1:1 to 4:1) showed that the recovery of N. fowleri tended to be higher when it made up a larger proportion of the mixture (Table 1). However, when the ratio of the two species was close to 1:1, the presence of competing N. lovaniensis species decreased the recovery of N. fowleri trophozoites, which were overgrown especially when the N. lovaniensis concentration reached a threshold value of 30 to 40 amoebae/liter in the mixture (Fig. 1a), as was the case in some experiments with a theoretical ratio of 1:1 or 3:1.
For N. fowleri-N. australiensis mixtures, trophozoites of N. fowleri have been recovered with their usual low yield, but in these experiments the observed ratio, theoretically 1:1, fluctuated less (Table 1 and Fig. 1b).
In the mixtures of cysts (Table 2 and Fig. 2), independently of the species ratio (1:1 to 4:1), filtration appeared more favorable to recovery of N. fowleri cysts (55.3%) than to that of cysts of the two other species, N. lovaniensis and N. australiensis (23.8 and 21.3%, respectively).
Comparative recoveries of filtration and centrifugation.
Generally, for the Naegleria trophozoites, without regard to the species, the centrifugation recovery rate was significantly higher than the filtration recovery rate (22% ± 5% versus 5% ± 5%; χ2 on paired MPN values = 29.65; P ≤ 0.001) (Table 3 and Fig. 1). However, filtration was as efficient as centrifugation for isolation of cysts: the mean recoveries were approximately equivalent with nonsignificant differences (53% versus 57%; χ2 = 2.54; P > 0.7), and the mean recovery by centrifugation was superior to that by filtration in only one of five experiments with cysts.
TABLE 3.
Comparison of recovery efficiencies of filtration and centrifugation for competing Naegleria species in vegetative and cystic forms
| Form and Naegleria spp. | Ratio of the 2 Naegleria species | % Recovery
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Filtration
|
Centrifugation
|
||||||||
| N. fowleri | N. lovaniensis | N. australiensis | Total Naegleria | N. fowleri | N. lovaniensis | N. australiensis | Total Naegleria | ||
| Cysts | |||||||||
| N. fowleri + N. lovaniensis | 78:22 | 64 | 5 | 50 | 34 | 32 | 40 | ||
| 64:36 | 26 | 76 | 73 | 18 | 113 | 63 | |||
| N. fowleri + N. australiensis | 22:78 | 84 | 6 | 30 | 23 | 23 | 23 | ||
| 66:34 | 33 | 31 | 75 | 76 | 21 | 75 | |||
| 51:49 | 51 | 6 | 37 | 28 | 91 | 83 | |||
| Mean ± SD | 51 ± 24 | 41 ± 50 | 15 ± 14 | 53 ± 21 | 36 ± 23 | 72 ± 57 | 45 ± 40 | 57 ± 25 | |
| Trophozoites | |||||||||
| N. fowleri + N. lovaniensis | 40:60 | 0 | 12 | 7 | 18 | 30 | 18 | ||
| 48:52 | 2 | 19 | 13 | 5 | 54 | 28 | |||
| 69:31 | 0 | 10 | 3 | 27 | 36 | 21 | |||
| N. fowleri + N. australiensis | 52:48 | 2 | 0 | 1 | 32 | 0 | 17 | ||
| 57:43 | 4 | 0 | 2 | 41 | 5 | 27 | |||
| Mean ± SD | 2 ± 2 | 14 ± 5 | 0 ± 0 | 5 ± 5 | 25 ± 14 | 40 ± 13 | 3 ± 4 | 22 ± 5 | |
The results of the recovery of each Naegleria species by the two methods were as follows. (i) Typically for N. fowleri in the vegetative form, centrifugation was always significantly superior to filtration, with mean recoveries of 25% ± 14% and 2% ± 2%, respectively (χ2 = 16.64; P < 0.01). For N. fowleri cysts, although the results appeared better for filtration than for centrifugation (51% ± 24% versus 36% ± 23%), the differences were not statistically significant (χ2 = 7.91; P > 0.1). (ii) For N. lovaniensis, in the vegetative and cystic forms, centrifugation always produced better results than filtration (72% ± 57% versus 41% ± 50% for cysts and 40% ± 13% versus 14% ± 5% for trophozoites), but these differences between the two methods were significant only for the trophozoites (χ2 = 10.06; P < 0.02) and not for the cysts (χ2 = 3.37; P > 0.1). (iii) For N. australiensis in cystic form, centrifugation was superior to filtration in two of the three tests (χ2 = 14.05; P < 0.01). No conclusion can be drawn from the experiments with N. australiensis trophozoites because they were not recovered. In addition, the results of the comparative study carried out on N. fowleri-N. lovaniensis or N. fowleri-N. australiensis mixtures showed that, whatever the concentration method, the recovery rates obtained with the cysts were better than with vegetative forms (Table 3). This is in agreement with the results obtained previously for filtration.
DISCUSSION
Among the numerous environmental surveys, most aimed at detecting the presence of pathogenic N. fowleri in correlation with the elevation of water temperature. Only a few investigators have tentatively considered exact numeration of amoebae in the water (1, 4, 9, 13). Indeed, the quantitative determination of Naegleria in water by filtration necessarily requires an indirect counting method because of the presence of the filter membrane on the agar surface, which hides the development of lytic plaques subsequently coalescing under the two filter halves. Therefore, the response expected for the presence of amoebae in a given volume of water treated by filtration (volume ≥ 10 ml) can be only of an all-or-none type: i.e., the presence or absence of at least one amoeba in the volume considered. The precision and accuracy of the best method of determining MPN will be reached only by increasing the serial repetition of different sample volumes. Therefore, the determination of amoebic concentration by the MPN method is tedious and time-consuming, which may explain the scarcity of such studies. Probably for the same reasons, no systematic comparative study between filtration and centrifugation for amoeba recovery has ever been attempted. From a limited number of environmental samples treated simultaneously by the two methods, De Jonckheere (4) demonstrated the isolation of a larger number of strains of Naegleria by centrifugation than by filtration but did not estimate the amoebic concentration.
All the results presented here demonstrate a greater efficiency in recovery of cysts than of vegetative forms regardless of the concentration method used. The filtration isolation procedure largely used by most investigators showed a particularly low recovery yield for vegetative forms of Naegleria, since most of the time it was below 10%, whereas it reached 54.5% for the cysts. These results show that the evaluation of the number of amoebae by filtration leads to an underestimation of the real situation that should be taken into consideration for the interpretation of environmental surveys. Although the filtrates have not been checked for the presence of amoebae, it is very unlikely that this low efficiency was due to a lack of retention of amoebae on the filter membrane. Indeed, because of their size and despite their great plasticity, the Naegleria trophozoites (10 to 35 μm), as well as the cysts (7 to 17 μm), cannot pass through a 1.2-μm-pore-size filter membrane. This fact is clearly confirmed by the total absence of exogenous amoebae during all our experiments (even those normally smaller than Naegleria, like some Hartmannellidae), resulting from decontamination of the river water by 3-μm-pore-size filtration. By comparison, centrifugation allowed higher recovery rates than filtration for vegetative forms but did not appear to improve cyst recovery (Table 3).
These results can be explained as follows. The cytoplasmic membrane of the vegetative form, which is directly exposed to the external conditions, is probably very sensitive to the various adverse conditions met during successive operations of isolation. For example, during filtration, trophozoites may be suddenly lysed by a too rapid and pronounced vacuum. Immediately after filtration, the trophozoites are then covered by the inverted filter membrane and can remain trapped by the layer of suspended matter, which can be very compact when there is a high load of this suspended matter in the water. On the other hand, centrifugation is probably better tolerated by vegetative forms. Indeed, after the pellet resulting from centrifugation is plated, vegetative forms dispersed on the agar surface are in better oxygenation conditions to resume outgrowth and multiplication than when they are flattened by the filter membrane. For cysts, the approximately equivalent mean recovery efficiencies with the two methods can be explained by the fact that they are protected by their cyst wall and resist adverse conditions better than trophozoites whatever the concentration procedure used. Furthermore, centrifugation could allow a direct plaque count of lytic areas on individual plates instead of an estimate of the MPN as we have attempted in our comparative scheme. Consequently, according to our results, since the Naegleria amoebae can assume three different stages during their life cycle (vegetative, cystic, and even flagellate forms), the concentration method should ideally be chosen with the knowledge of the precise form of the pathogen in the environmental sample. If the water contains a high proportion of cysts, the two concentration methods seem equivalent, and conversely, if trophozoites predominate, centrifugation will obviously be better than filtration. Unfortunately, we have no means to foresee the precise Naegleria status at the time of sampling in natural waters.
The second major conclusion that may be drawn from our data is that regardless of the concentration method, there is important imprecision and inaccuracy in the evaluation of Naegleria concentrations in water samples. This imprecision probably results in part from the low Naegleria concentration, which was arbitrarily fixed at 50 to 100 Naegleria per liter in our experiments. However, amoebic contaminations higher than this threshold value do not require concentration any longer: their MPN can be estimated by direct spreading of small volumes (0.1 and 1 ml) on the agar surface. Furthermore, the specific isolation of N. fowleri by filtration, especially for vegetative forms, becomes more uncertain in the presence of direct competitors, such as any other thermotolerant free-living amoebae, and particularly other thermotolerant Naegleria species (N. lovaniensis and N. australiensis). The importance of this competitive phenomenon is evidenced by the fact that according to our own experience of environmental survey (data not published), the small volumes (0.01 liter) used for MPN determination are often more positive for N. fowleri strains than larger volumes (1 liter and 0.1 liter). Similar observations have already been made by De Jonckheere (4) and Tyndall et al. (13). Therefore, for all these reasons, in practice, it will be better to include in the MPN procedure, whenever possible, the use of small volumes (≤10 ml) while increasing the number of replicates. Therefore, this process has two main advantages: first, a limitation of the phenomena of interspecific competition, and second, the elimination of the interfering effects of concentration methods (for 1- and 0.1-ml volumes).
ACKNOWLEDGMENTS
This work was supported by a grant from Electricité de France (Direction des Etudes et Recherches).
We acknowledge the skillful technical assistance of M. C. Testard and thank J. F. De Jonckheere for helpful comments on the manuscript.
REFERENCES
- 1.Cerva L. Studies of limax amoeba in a swimming-pool. Hydrobiologia. 1971;38:141–161. [Google Scholar]
- 2.Champsaur H. Méthodes générales d’examen bactériologique des eaux. In: Rodier J, editor. L’analyse de l’eau. Paris, France: Dunod; 1996. pp. 755–771. [Google Scholar]
- 3.Delattre J M, Oger C. Naegleria fowleri and heated aquatic environments: a possible mechanism. Ann Soc Belg Méd Trop. 1981;61:441–452. [PubMed] [Google Scholar]
- 4.De Jonckheere J F. Quantitative study of Naegleria fowleri in surface water. Protistologica. 1978;14:475–481. [Google Scholar]
- 5.Esterman A, Dorsch M, Cameron S, Roder D, Robinson B, Christy P. The association of Naegleria fowleri with the chemical, microbiological and physical characteristics of South Australian water supplies. Water Res. 1984;18:549–553. [Google Scholar]
- 6.Griffin J L. The pathogenic amoeboflagellate Naegleria fowleri: environmental isolations, competitors, ecologic interactions, and the flagellate empty habitat hypothesis. J Protozool. 1983;30:403–409. doi: 10.1111/j.1550-7408.1983.tb02939.x. [DOI] [PubMed] [Google Scholar]
- 7.Huizinga H W, McLaughlin G L. Thermal ecology of Naegleria fowleri from a power plant cooling reservoir. Appl Environ Microbiol. 1990;56:2200–2205. doi: 10.1128/aem.56.7.2200-2205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John D T, De Jonckheere J. Isolation of Naegleria australiensis from an Oklahoma lake. J Protozool. 1985;32:571–574. doi: 10.1111/j.1550-7408.1985.tb03077.x. [DOI] [PubMed] [Google Scholar]
- 9.Kyle D E, Noblet G P. Vertical distribution of potentially pathogenic free-living amoebae in freshwater lakes. J Protozool. 1985;32:99–105. doi: 10.1111/j.1550-7408.1985.tb03022.x. [DOI] [PubMed] [Google Scholar]
- 10.Page F C. An illustrated key to freshwater and soil amoebae. Ambleside, Cumbria, England: Freshwater Biological Association; 1976. [Google Scholar]
- 11.Pernin P, Grelaud G. Application of isoenzymatic typing to the identification of nonaxenic strains of Naegleria (Protozoa, Rhizopoda) Parasitol Res. 1989;75:595–598. doi: 10.1007/BF00930954. [DOI] [PubMed] [Google Scholar]
- 12.Sykora J L, Keleti G, Martinez A J. Occurrence and pathogenicity of Naegleria fowleri in artificially heated waters. Appl Environ Microbiol. 1983;45:974–979. doi: 10.1128/aem.45.3.974-979.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyndall R L, Ironside K S, Melter P L, Tan E L, Hazen T C, Fliermans C B. Effect of thermal additions on the density and distribution of thermophilic amoebae and pathogenic Naegleria fowleri in a newly created cooling lake. Appl Environ Microbiol. 1989;55:722–732. doi: 10.1128/aem.55.3.722-732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellings F M, Amuso P T, Chang S L, Lewis A L. Isolation and identification of pathogenic Naegleria from Florida lakes. Appl Environ Microbiol. 1977;34:661–667. doi: 10.1128/aem.34.6.661-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]