Abstract
Cordyceps is a genus of ascomycete fungi and is well known as one of the important medical fungi in Chinese, Korea, and other Asian countries, because of its various beneficial effects on human health. The pharmacological activities of Cordyceps extract are mainly focused on anti-cancer, anti-metastatic, and immune modulating effects. In the present study, we investigated whether the antiplatelet effect of ethanol extract of cultured Cordyceps militaris (CMEE) with FeCl3-induced arterial thrombosis model. We observed that CMEE exhibited a significant inhibitory effect against ADP and collagen-induced platelet aggregation. However, there were no significant differences in prothrombin time (PT) and activated partial thromboplastin time (aPTT). These results suggest that antithrombotic activity of CMEE is related to antiplatelet effect rather than anticoagulation effect, and CMEE may be a positive effect on improving blood circulation against vessel injury and occlusion.
Keywords: Cordyceps militaris, cordycepin, antiplatelet, antithrombotic, Ginkgo biloba extract
1. Introduction
Many published papers have been demonstrated that platelet plays important roles in thrombosis and hemostasis, and the hyperreactivity of platelet is related to arterial thrombosis, which is the common cause of cardiovascular diseases, such as atherosclerosis and myocardial infarction [1–4]. Antithrombotic drugs are classified with anticoagulant drugs (aspirin, clopidogrel, and glycoprotein IIb/IIIa receptor antagonist) and antiplatelet drugs (heparin and warfarin) [5]. Because these antithrombotic drugs have an inherent risk of bleeding, the development of novel antithrombotic drugs without bleeding remains an unmet medical need [6]. Recently, the antithrombotic activity of bioactive components in natural products with no or minimal side effect have been reported [7,8], therefore, interest in medical plants and mushrooms is increasing.
Cordyceps militaris (C. militaris) is the most famous medicinal mushroom and shows various pharmacological properties, such as anti-viral, anti-tumor, anti-oxidant, immune-modulating, and tissue and organ protective effects including renal, neural, hepatic, and cardiovascular system [9–12]. For this reason, C. militaris has been widely used in China, Korea, and other Asian countries for centuries. Although many artificial cultured and extraction methods of C. militaris have been development [13], the detailed mechanisms of its biological functions have not yet been fully elucidated.
Therefore, in this study we used a ferric chloride (FeCl3)-induced carotid arterial thrombosis model to evaluate the antithrombotic activity of C. militaris ethanol extracts (CMEE) and to elucidate its molecular mechanism by which antiplatelet or anticoagulation function.
2. Materials and methods
2.1. Reagents
The Ginkgo biloba leaf extract (Ginkgo), a comparative material, was purchased from GNC (Pittsburgh, PA, USA). Collagen and adenosine diphosphate (ADP) were purchased from Sigma (St. Louis, MO, USA).
2.2. Cordyceps militaris ethanol extract (CMEE) preparation
Artificial cultured C. militaris (Figure 1) was provided from Mushtech Co., Ltd. (Hoengseong-gun, Republic of Korea). Cultivated whole fruiting bodies of C. militaris, containing 2.33 mg/g of cordycepin, were dried at 50 °C and crushed in a blender and then the crude powder was extracted with ethanol at 85 °C for 6 h. The ethanol extract was vacuum filtered using a filter paper (Whatman No. 2) and then was evaporated at 65 °C by an evaporator (N-1000, Eyela, Tokyo, Japan) under reduced pressure. The concentrated extract was frozen at −80 °C and then lyophilized using a freeze-dryer.
Figure 1.
Natural (A) and cultivated (B) C. militaris.
2.3. Fecl3-induced arterial thrombosis model
Male Sprague-Dawley (SD) rats (5 weeks) were purchased from DBL Co., Ltd. (Eumseong, Republic of Korea), and FeCl3-induced arterial thrombosis was performed according to the previously described method [14]. All animals were housed in colony cages under standard laboratory conditions (temperature 22 ± 2 °C, relative humidity 50 ± 5%, and 12 hr light/dark cycle) and had free access to food and water. All experiment protocols were approved by the Institutional Animal Care and Use Committee of the EBO (Cheonwon-gun, Chungcheongbuk-do, Republic of Korea, Certificate No: EBOA-2015-12).
2.4. Preparation of blood samples
Male SD rats (180–200 g) were anesthetized with isoflurane (Piramal, PA, USA). Blood samples were collected 60 min after last sample treatment from the abdominal aorta into a syringe containing 3.8% sodium citrate. The ratio of blood to 3.8% sodium citrate was adjusted to 1:9 v/v. After centrifugation at 950 rpm for 10 min at room temperature, supernatants (platelet-rich plasma, PRP) were used for the aggregation study. The platelet count in PRP was finally adjusted to approximately 3.8 × 108/ml. Platelet-poor plasma (PPP) was obtained by centrifuging the PRP at 3000 rpm for 10 min for the coagulation assay.
2.5. In vivo antithrombotic activity assay
Male SD rats (180–200 g) were orally administered with Ginkgo (100 mg/kg), or CMEE at doses of 30, 100, and 300 mg/kg daily for 3 weeks. At 45 min after the last treatment, the rats were anesthetized using isoflurane (Piramal, PA, USA), and a segment of the right carotid artery was exposed. At 60 min after the last treatment, thrombosis was induced by placing 2 mm2 of Whatman No. 1 filter paper saturated with 70% FeCl3 on the carotid artery near the probe for 10 min, and the blood flow rate was measured using a laser Doppler flowmeter (FLO-C1, Omegawave, Tokyo, Japan). Then the removal of Whatman filter paper, the blood flow rate was measured for 30 min. The occlusion time was set as the time for the blood flow rate to slow to 10% of the initial blood flow rate.
2.6. Ex vivo platelet aggregation assay
Platelet aggregation was measured by using a Chrono-Log aggregometer (Chrono-Log, Havertown, USA). PRP was incubated at 37 °C for 10 min in the aggregometer with stirring at 1,200 rpm. ADP (final concentration, 10 μM) and collagen (final concentration, 5 μg/mL) were used as stimulators. The extent of platelet aggregation was estimated by measuring the maximum height above the baseline reached by the aggregation curve within 15 min after stimulation.
2.7. Ex vivo coagulation assay
To evaluate the anticoagulation effect of CMEE, prothrombin time (PT) and activated thromboplastin time (aPTT) were measured by using an Automated Coagulation Laboratory (ACL) 100 Instrument (Instrumentation Laboratory Company, Milano, Italy). PPP was incubated at 37 °C for 7 min, and then 50 μL of the incubated PPP was mixed with 100 μL of the PT reagent (RecombiPlasTin 2 G; Instrumentation Laboratory, Bedford, MA, USA). Additionally, 50 μL of the incubated PPP was mixed with 53 μL of the aPTT reagent (SynthAsil; Instrumentation Laboratory) and the coagulation was started by addition of 50 μL of CaCl2 with final concentration of 15 mM.
2.8. Statistical analysis
All data are expressed as the mean ± standard error (SE). Statistical analysis was performed using SPSS program (SPSS INC, ver. 21.0). The statistical comparisons were performed using one-way analysis of variance (ANOVA) followed by Dunnett’s test. A significant difference was defined as p < 0.05.
3. Results
3.1. Cordyceps militaris ethanol extract showed antithrombotic activity in a rat arterial model of thrombosis
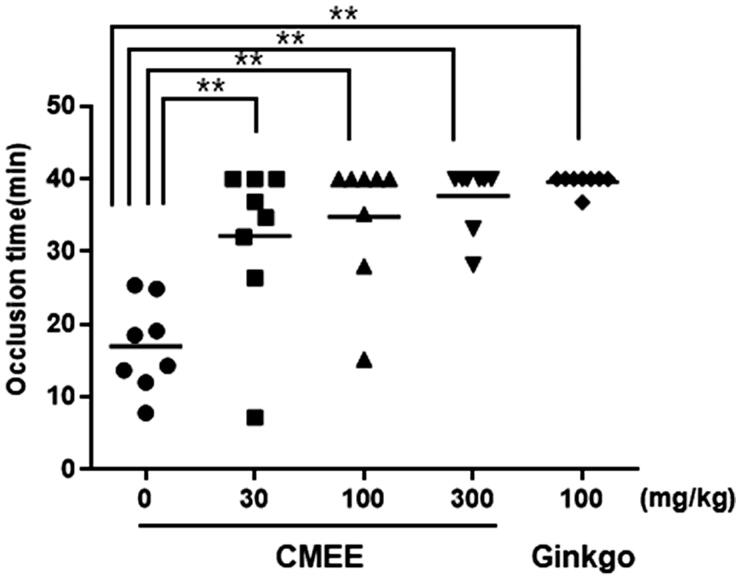
We investigated whether CMEE has antithrombotic effects in FeCl3-induced thrombus formation model using a Doppler flow probe system. There was no difference in body weight between groups during the study. As shown Figure 2, the occlusion time of control group was 17.0 ± 6.2 min, but the occlusion time of CMEE-treated groups were significantly delayed approximately 2 times compare with control group in a dose dependent manner, 32.2 ± 11.1 min at 30 mg/kg, 34.8 ± 9.0 min at 100 mg/kg, and 37.7 ± 4.5 min at 300 mg/kg, respectively. The positive control Ginkgo-treated group was 39.6 ± 1.1 min, similar to that of CMEE 300 mg/kg treated group.
Figure 2.
In vivo antithrombotic effect of C. militaris ethanol extract (CMEE). Data are expressed as the mean ± SE (n = 8). **p < 0.01 compared with the control.
3.2. Cordyceps militaris ethanol extract inhibited platelet aggregation by ADP and collagen
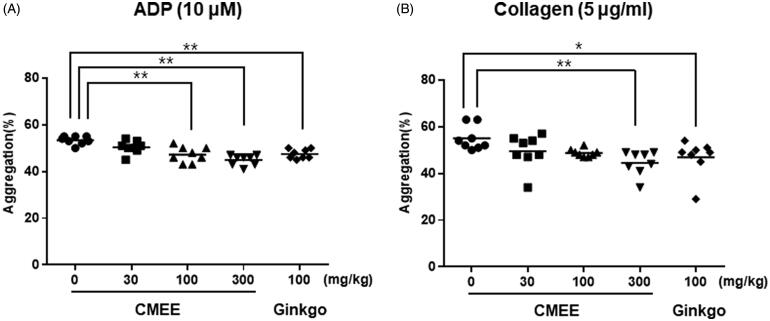
ADP and collagen play an important role in platelet aggregation as agonist [15]. CMEE in a dose-dependent manner inhibited ADP- and collagen-induced platelet aggregation (Figure 3). The values of percentage inhibition in ADP-induced platelet aggregation were 53.4 ± 1.8% at control group, 50.4 ± 2.7% at 30 mg/kg of CMEE-treated group, 45.6 ± 3.6% at 100 mg/kg of CMEE-treated group, 44.9 ± 2.2% at 300 mg/kg of CMEE-treated group, and 47.5 ± 2.0%, at Ginkgo-treated group (Figure 3(A)). A statistically significant inhibition in collagen-induced platelet aggregation, compared to control (55.0 ± 5.2%), was observed to 44.5 ± 5.2% and 46.9 ± 7.7% at 300 mg/kg of CMEE-treated group and Ginkgo-treated group, respectively (Figure 3(B)).
Figure 3.
Ex vivo antiplatelet effect of C. militaris ethanol extract (CMEE). (A) ADP-induced platelet aggregation and (B) Collagen-induced platelet aggregation. Data are expressed as the mean ± SE (n = 8). *p < 0.05 and **p < 0.01 compared with the control.
3.3. Cordyceps militaris ethanol extract had no effect on blood coagulation
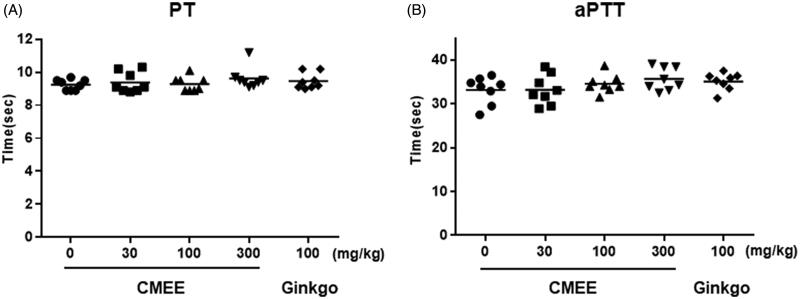
To determine whether CMEE shows anticoagulant activity, coagulation time was evaluated in rat plasma. By contrast of the platelet aggregation results, CMEE and Ginkgo-treated groups did not significantly affect the prolongation of both the PT (Figure 4(A)) and the aPTT (Figure 4(B)).
Figure 4.
Ex vivo anticoagulation effect of C. militaris ethanol extract (CMEE). (A) Prothrombin time (PT) and (B) Activated partial thromboplastin time (aPTT). Data are expressed as the mean ± SE (n = 8).
4. Discussion
Thrombosis is clotting of blood in the arterial or venous circulatory system and is a major causing of myocardial infarction and strokes, therefore the development of antithrombotic therapy is related to reducing the risk factors of cardiovascular diseases [16]. To overcoming bleeding, a critical side effect of antithrombotic drugs, there are suggest new antiplatelet and anticoagulant targets and their pathophysiological mechanisms. In addition, studies with medical herbs or plants have been investigated for development of antiplatelet and anticoagulant agents [17,18].
In the present study, we determined antithrombotic effect of ethanol extract of C. militaris in FeCl3-induced rat carotid artery thrombosis models. CMEE showed a significant prolonged occlusion times in a dose-dependent manner without any abnormal symptoms and body weight change (Figure 2). Although the mechanism of thrombosis is very complex, there are two main processes that platelet aggregation and coagulation cascade [19]. In order to confirm the mechanism of antithrombotic activity of CMEE, we performed ex vivo antiplatelet aggregation assay and anticoagulation assay. As shown Figures 3 and 4, CMEE significantly inhibited ADP- and collagen-induced platelet aggregation, but no influence on coagulation times. Ginkgo biloba leaf, a comparative control, has a high content of flavonoids and has antioxidant, vasodilation, and improving blood flow [20]. The effect of improving blood flow of Ginkgo biloba leaf is also antiplatelet and antithrombotic activities without the prolongation of coagulation time [21]. These data are correlation with our results and supported that C. militaris has similar activity and mechanism in the enhancing blood circulation compared with Ginkgo biloba leaf.
In conclusion, our present study demonstrated that C. militaris has a potential of antithrombotic effect, which is results from its antiplatelet activity rather than anticoagulation activity. Therefore, C. miliatris has a positive influence on the improving blood flow and the recovery of vessel injury. The follow-up of the studies, which are identify more detailed mechanism of antithrombotic and antiplatelet effects of C. militrais, and combinative therapy of other antiplatelet or anticoagulation drugs, might suggest the development of a new dietary supplement or an antithrombotic agent reducing the side-effects.
Funding Statement
This research was supported by Bio-industry Technology Development Program [316025-05] of IPET (Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries), and the National Research Foundation (NRF) grant funded by the Korea government (MSIT) [No. 2019R1A2C2005157].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23(12):2131–2137. [DOI] [PubMed] [Google Scholar]
- 2.Koupenova M, Kehrel BE, Corkrey HA, et al. . Thrombosis and platelets: an update. Eur Heart J. 2017;38(11):785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Silverstein RL. Platelet metabolism meets thrombosis. Blood. 2018;132(11):1089–1091. [DOI] [PubMed] [Google Scholar]
- 5.Watson RD, Chin BS, Lip GY. Antithrombotic therapy in acute coronary syndromes. BMJ. 2002;325(7376):1348–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFadyen JD, Peter K. Novel antithrombotic drugs on the horizon: the ultimate promise to prevent clotting while avoiding bleeding. Circ Res. 2017;121(10):1133–1135. [DOI] [PubMed] [Google Scholar]
- 7.Vilahur G, Badimon L. Antiplatelet properties of natural products. Vascul Pharmacol. 2013;59(3–4):67–75. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Yang FQ, Zhang Q, et al. . Natural products for antithrombosis. Evid Based Complement Alternat Med. 2015;2015:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuli HS, Sandhu SS, Sharma AK. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech. 2014;4(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Gong Z, Su Y, et al. . Cordyceps fungi: natural products, pharmacological functions and developmental products. J Pharm Pharmacol. 2009;61(3):279–291. [DOI] [PubMed] [Google Scholar]
- 11.Qin P, Li X, Yang H, et al. . Therapeutic potential and biological applications of cordycepin and metabolic mechanisms in cordycepin-producing fungi. Molecules. 2019;24(12):2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das SK, Masuda M, Sakurai A, et al. . Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia. 2010;81(8):961–968. [DOI] [PubMed] [Google Scholar]
- 13.Cui JD. Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Crit Rev Biotechnol. 2015;35(4):475–484. [DOI] [PubMed] [Google Scholar]
- 14.Li W, McIntyre TM, Silverstein RL. Ferric chloride-induced murine carotid arterial injury: a model of redox pathology. Redox Biol. 2013;1(1):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon HW, Shin JH, Lim DH, et al. . Antiplatelet and antithrombotic effects of cordycepin-enriched WIB-801CE from Cordyceps militaris ex vivo, in vivo, and in vitro. BMC Complement Altern Med. 2016;16(1):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451(7181):914–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohd Nor NH, Othman F, Mohd Tohit ER, et al. . Medicinal herbals with antiplatelet properties benefit in coronary atherothrombotic diseases. Thrombosis. 2016;2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metharom P, Berndt MC, Baker RI, et al. . Current state and novel approaches of antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2015;35(6):1327–1338. [DOI] [PubMed] [Google Scholar]
- 19.Tomaiuolo M, Brass LF, Stalker TJ. Regulation of platelet activation and coagulation and its role in vascular injury and arterial thrombosis. Interv Cardiol Clin. 2017;6(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshikawa T, Naito Y, Kondo M. Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid Redox Signal. 1999;1(4):469–480. [DOI] [PubMed] [Google Scholar]
- 21.Ryu KH, Han HY, Lee SY, et al. . Ginkgo biloba extract enhances antiplatelet and antithrombotic effects of cilostazol without prolongation of bleeding time. Thromb Res. 2009;124(3):328–334. [DOI] [PubMed] [Google Scholar]