Abstract
A new fungus, Ophiocordyceps alboperitheciata, parasitic on the larva of Noctuidae (Lepidoptera) was identified from a survey of entomopathogenic fungi in Kunming Wild Duck Forest Park, Yunnan Province, China. It can be primarily distinguished from relatives by its longer fertile parts, sterile tips, superficial perithecia, narrower asci, and smaller septa of ascospores. As revealed from phylogenetic analyses inferred from nrSSU, nrLSU, tef-1α, rpb1, and rpb2 sequence data, O. alboperitheciata belongs to the Hirsutella citriformis clade in the genus Ophiocordyceps of Ophiocordycipitaceae, and forms a separated clade from other related species. The uniqueness of the taxon is significantly evidenced by both molecular phylogeny and morphology. Furthermore, the interspecific relationships in the H. citriformis clade are discussed.
Keywords: New species, Hirsutella, Ophiocordyceps, phylogenetic analyses
1. Introduction
The entomopathogenic Ophiocordyceps Petch is the largest genus with the maximum number of species in the family Ophiocordycipitaceae (Hypocreales) [1–3]. The genus was originally established by Petch based on four species, i.e., the type O. blattae Petch occurring on a cockroach, O. unilateralis (Tul. & C. Tul.) Petch on ants, O. peltata (Wakef.) Petch on a coleopteran larva, as well as O. rhizoidea (Höhn.) Petch on a coleopteran larva [4]. Ophiocordyceps was separated from Cordyceps sensu lato by the existing phylogenetic classification [5], accommodating over 270 names of accepted species to date [3,6,7]. Main hosts of Ophiocordyceps pertain to Lepidoptera, Coleoptera, Hymenoptera, Hemiptera, Diptera, Orthoptera, and Odonata, most of which are larvae of Lepidoptera or Coleoptera inhabiting wood or soil [5,8]. Species of Ophiocordyceps are distributed worldwide, primarily located in temperate, subtropical to tropical areas [9]. It is noteworthy that the species diversity of Ophiocordyceps appears to be the maximal in East and Southeast Asia [5].
Numerous associated-asexual morphs of Ophiocordyceps were reported, (e.g., Hirsutella Pat., Hymenostilbe Petch, Paraisaria Samson & B.L. Brady, and Syngliocladium Petch) [2,3,5,10,11]. On the whole, asexual types of Ophiocordyceps are discovered from Hymenostilbe and Hirsutella, and the latter is recognized as the most common isolation source. The genus Hirsutella was erected by Patouillard based on the type species H. entomophila Pat., as suggested to attack an adult beetle collected from Ecuador [12]. First, the genus Hirsutella was defined as a clavariaceous hymenomycete with simple sterigmate basidia. Subsequently, this genus was critically investigated and then identified as anamorphic insect pathogens [13]. The generic name Hirsutella was affected by the ending of dual nomenclature for various morphs of pleomorphic fungi in 2011 [14]. Next, Simmons et al. initially adopted the Ophiocordyceps name for a novel species, O. myrmicarum D.R. Simmons & Groden, described only from the asexual Hirsutella morph [15]. The suppression of the generic name could positively impact the study on those groups in phylogeny and facilitate taxonomic revisions of the family Ophiocordycipitaceae. Thus far, over 100 asexual morphs of the genus Ophiocordyceps were identified (Index Fungorum: http://www.indexfungorum.org), as associated with more than 30 sexual species [16]. The Hirsutella consists of six groups, i.e., H. citriformis Speare, H. thompsonii F.E. Fisher, H. nodulosa Petch, H. guyana Minter & B. L. Brady, H. sinensis Liu, Guo, Yu & Zang, and Hirsutella ant pathogen clades.
A series of surveys were conducted to reveal the species diversity of entomopathogenic fungi in Kunming, Yunnan Province, China [16–20]. To be specific, the richness of cordycipitoid fungi was found to be relatively higher in Kunming Wild Duck Lake Forest Park. On the whole, 41 species were found here (with 20 species proposed as new species), belonging to eight genera of three families (i.e., Clavicipitaceae, Cordycipitaceae and Ophiocordycipitaceae), which are Flavocillium, Cordyceps, Beauveria, Samsoniella, Simplicillium, Ophiocordyceps, Polycephalomyces, and Metarhizium. Among these species, a fungus attacking caterpillar was determined as a novel taxon of Ophiocordyceps by conducting the analyses of both morphology and molecular phylogeny. This study attempts to introduce the new species and investigate its biological and phylogenetic status.
2. Materials and methods
2.1. Specimen collection and strain isolation
In the present study, a specimen of the novel species was collected from the Kunming Wild Duck Lake Forest Park, Yunnan Province, China, in August 2018. The isolate was obtained with the methods presented by Wang et al. [18]. The specimen was deposited in Yunnan Herbal Herbarium (YHH), Yunnan University. The cultures were deposited at Yunnan Fungal Culture Collection (YFCC), Yunnan University. To describe the new species, the macro- and micromorphological characteristics were observed by complying with Wang et al. [20].
2.2. Morphological observations
The sample was photographed with a digital camera and Olympus SZ61 (Tokyo, Japan) stereomicroscope. Subsequently, the macromorphological characteristics were recorded (e.g., texture, shape, color, length, diameter of the stroma and color, shape, length, diameter of the fertile head, and host type). Furthermore, Olympus CX40 (Tokyo, Japan) and BX53 (Tokyo, Japan) microscopes were employed to observe the micromorphological characteristics of perithecia, asci, asci-caps and ascospores Next, the morphology of cultures was characterized by using the method presented by Wang et al. [16].
2.3. DNA extraction, PCR, and sequencing
The total genomic DNAs were extracted by employing the CTAB method of Liu et al. [21]. Five nuclear gene regions were amplified and sequenced, i.e., the small subunit of ribosomal DNA (nrSSU), the large subunit of ribosomal DNA (nrLSU), translation extension factor 1-gene (tef-1α), the largest subunit of RNA polymerase II (rpb1), as well as the second largest subunit of RNA polymerase II (rpb2) [5,22,23]. Polymerase chain reaction (PCR) was performed by adopting the method presented by Wang et al. [20]. Moreover, amplifications were conducted in 25 µL, and PCR conditions were referenced from Sung et al. [5]. Furthermore, PCR products were sequenced by the Beijing Genomics Institute (Shenzhen, China).
2.4. Phylogenetic analyses
Five-gene sequences (i.e., nrSSU, nrLSU, tef-1α, rpb1, and rpb2) of taxa pertaining to Hirsutella, Ophiocordyceps, and Polycephalomyces were downloaded from GenBank, and combined with those generated in here. Table 1 lists the specimen accession information and GenBank numbers of the five loci. Sequences were aligned by employing the programs Clustal X2.0 and MEGA5 [24]. Phylogenetic analyses were conducted with Bayesian inference (BI) and maximum-likelihood (ML) methods with the use of the programs MrBayes v.3.1.2 and RaxML7.0.3 [25,26], respectively. In addition, the BI analysis was conducted on MrBayes v.3.1.2 for five million generations with the GTR + G + I model, as determined by jModelTest version 2.1.4 [27]. Specific to the ML analysis based on RaxML7.0.3, GTR + I acted as the optimal model, and 500 fast bootstrap replications were conducted on the five-locus dataset. Trees were sampled every 100 generations. The first 25% trees were discarded as burn-in and the remaining trees were used to create a consensus tree using the sumt demand.
Table 1.
Specimen information and GenBank accession number for sequences used in this study.
| Species | Isolate no./ specimen no. | Host | GenBank accession no. |
||||
|---|---|---|---|---|---|---|---|
| nrSSU | nrLSU | tef1-α | rpb1 | rpb2 | |||
| Ophiocordyceps alboperitheciata | YHH 16755 | Lepidoptera | MT222278 | MT222279 | MT222280 | MT222281 | |
| Hirsutella citriformis | CHE-CNRCB 335 | KY587216 | KY587203 | KY587213 | |||
| Hirsutella citriformis | CHE-CNRCB 339 | KY587217 | KY587204 | KY587214 | |||
| Hirsutella citriformis | ARSEF 490 | Hemiptera | KM652103 | KM651987 | |||
| Hirsutella citriformis | ARSEF 591 | Hemiptera | KM652104 | KM651988 | |||
| Hirsutella citriformis | ARSEF 1035 | Hemiptera | KM652064 | KM652105 | KM651989 | KM652030 | |
| Hirsutella citriformis | ARSEF 1446 | Hemiptera | KM652065 | KM652106 | KM651990 | KM652031 | |
| Hirsutella citriformis | ARSEF 2598 | Hemiptera | KM652107 | KM651991 | |||
| Hirsutella citriformis | CHE-CNRCB 375 | KY587218 | KY587205 | KY587215 | |||
| Hirsutella cryptosclerotium | ARSEF 4517 | Hemiptera | KM652066 | KM652109 | KM651992 | KM652032 | |
| Hirsutella eleutheratorum | ARSEF 13375 | MH057734 | MH057732 | MH057733 | |||
| Hirsutella fusiformis | ARSEF 5474 | Coleoptera | KM652067 | KM652110 | KM651993 | KM652033 | |
| Hirsutella gigantea | ARSEF 30 | Hymenoptera | JX566977 | JX566980 | KM652034 | ||
| Hirsutella guyana | ARSEF 878 | Hemiptera | KM652068 | KM652111 | KM651994 | KM652035 | |
| Hirsutella haptospora | ARSEF 2226 | Ixodida | KM651995 | KM652036 | |||
| Hirsutella illustris | ARSEF 5539 | Hemiptera | KM652069 | KM652112 | KM651996 | KM652037 | |
| Hirsutella kirchneri | ARSEF 5551 | Ixodida | KM652070 | KM652113 | KM651997 | ||
| Hirsutella lecaniicola | ARSEF 8888 | Hemiptera | KM652071 | KM652114 | KM651998 | KM652038 | |
| Hirsutella liboensis | ARSEF 9603 | Lepidoptera | KM652072 | KM652115 | |||
| Hirsutella necatrix | ARSEF 5549 | Ixodida | KM652073 | KM652116 | KM651999 | KM652039 | |
| Hirsutella nodulosa | ARSEF 5473 | Lepidoptera | KM652074 | KM652117 | KM652000 | KM652040 | |
| Hirsutella radiate | ARSEF 1369 | Diptera | KM652076 | KM652119 | KM652002 | KM652042 | |
| Hirsutella rhossiliensis | ARSEF 3747 | Tylenchida | KM652080 | KM652123 | KM652006 | KM652045 | |
| Hirsutella satumaensis | ARSEF 996 | Lepidoptera | KM652082 | KM652125 | KM652008 | KM652047 | |
| Hirsutella sp. | ARSEF 8378 | Hemiptera | KM652084 | KM652127 | KM652010 | KM652049 | |
| Hirsutella stilbelliformis var. myrmicarum | IMI 396397 | Hymenoptera | GQ866966 | GQ866964 | |||
| Hirsutella strigosa | ARSEF 2197 | Hemiptera | KM652085 | KM652129 | KM652012 | KM652050 | |
| Hirsutella subulata | ARSEF 2227 | Lepidoptera | KM652086 | KM652130 | KM652013 | KM652051 | |
| Hirsutella thompsonii | ARSEF 2800 | Ixodida | KM652095 | KM652142 | KM652023 | KM652058 | |
| Hirsutella thompsonii | ARSEF 3323 | Ixodida | KM652096 | KM652143 | KM652024 | KM652059 | |
| Hirsutella thompsonii | ARSEF 1947 | Ixodida | KM652146 | KM652026 | |||
| Hirsutella versicolor | ARSEF 1037 | Hemiptera | KM652102 | KM652150 | KM652029 | KM652063 | |
| Hirsutella shennongjiaensis | GZUIFR-Snj121022 | KY945357 | KY945364 | ||||
| Ophiocordyceps aciculari | OSC 128580 | Coleoptera | DQ522543 | DQ518757 | DQ522326 | DQ522371 | DQ522423 |
| Ophiocordyceps agriotidis | ARSEF 5692 | Arthropoda | DQ522540 | DQ518754 | DQ522322 | DQ522368 | DQ522418 |
| Ophiocordyceps aphodii | ARSEF 5498 | Coleoptera | DQ522541 | DQ518755 | DQ522323 | DQ522419 | |
| Ophiocordyceps appendiculata | NBRC 106959 | JN941729 | JN941412 | AB968578 | JN992463 | AB968540 | |
| Ophiocordyceps brunneipunctata | OSC 128576 | Coleoptera | DQ522542 | DQ518756 | DQ522324 | DQ522369 | DQ522420 |
| Ophiocordyceps coenomyia | NBRC 106964 | AB968385 | AB968413 | AB968571 | AB968533 | ||
| Ophiocordyceps elongata | OSC 110989 | Lepidoptera | EF468808 | EF468748 | EF468856 | ||
| Ophiocordyceps entomorrhiza | KEW 53484 | Coleoptera | EF468954 | EF468809 | EF468749 | EF468857 | EF468911 |
| Ophiocordyceps formicarum | TNS F18565 | KJ878921 | KJ878888 | KJ878968 | KJ879002 | KJ878946 | |
| Ophiocordyceps formosana | TNM F13893 | KJ878908 | KJ878956 | KJ878988 | KJ878943 | ||
| Ophiocordyceps forquignonii | OSC 151908 | KJ878922 | KJ878889 | KJ879003 | KJ878947 | ||
| Ophiocordyceps gracilis | EFCC 8572 | Lepidoptera | EF468956 | EF468811 | EF468751 | EF468859 | EF468912 |
| Ophiocordyceps gracilis | OSC 151906 | Lepidoptera | KJ878923 | KJ878890 | KJ878969 | ||
| Ophiocordyceps heteropoda | EFCC 10125 | Hemiptera | EF468957 | EF468812 | EF468752 | EF468860 | EF468914 |
| Ophiocordyceps humbertii | MF116A | MK874747 | MK875537 | MK863828 | |||
| Ophiocordyceps humbertii | MF116B | MK874748 | MK875536 | MK863829 | |||
| Ophiocordyceps irangiensis | OSC 128577 | DQ522546 | DQ518760 | DQ522329 | DQ522374 | DQ522427 | |
| Ophiocordyceps irangiensis | OSC 128578 | DQ522556 | DQ518770 | DQ522345 | DQ522391 | DQ522445 | |
| Ophiocordyceps kniphofioides | MF90 | Hymenoptera | MK874746 | MK875538 | MK863827 | ||
| Ophiocordyceps konnoana | EFCC 7295 | Coleoptera | EF468958 | EF468862 | EF468915 | ||
| Ophiocordyceps konnoana | EFCC 7315 | Coleoptera | EF468959 | EF468753 | EF468861 | EF468916 | |
| Ophiocordyceps lanpingensis | YHOS 0705 | Lepidoptera | KC417458 | KC417460 | KC417462 | KC417464 | KC456333 |
| Ophiocordyceps lloydii | OSC 151913 | KJ878924 | KJ878891 | KJ878970 | KJ879004 | KJ878948 | |
| Ophiocordyceps longissima | EFCC 6814 | Hemiptera | EF468817 | EF468757 | EF468865 | ||
| Ophiocordyceps myrmicarum | CG1357 | Hymenoptera | MG922559 | MG922561 | MG922554 | MG922556 | |
| Ophiocordyceps nigrella | EFCC 9247 | EF468963 | EF468818 | EF468758 | EF468866 | EF468920 | |
| Ophiocordyceps nutans | OSC 110994 | DQ522549 | DQ518763 | DQ522333 | DQ522378 | ||
| Ophiocordyceps pseudocommunis | NHJ 12581 | Isoptera | EF468973 | EF468831 | EF468775 | EF468930 | |
| Ophiocordyceps ravenelii | OSC 151914 | KJ878932 | KJ878978 | KJ879012 | KJ878950 | ||
| Ophiocordyceps rhizoidea | NHJ 12522 | EF468970 | EF468825 | EF468764 | EF468873 | EF468923 | |
| Ophiocordyceps rubiginosiperitheciata | NBRC 106966 | JN941704 | JN941437 | AB968582 | JN992438 | AB968544 | |
| Ophiocordyceps sinensis | EFCC 7287 | Lepidoptera | EF468971 | EF468827 | EF468767 | EF468874 | EF468924 |
| Ophiocordyceps sinensis | ARSEF 6282 | Lepidoptera | KM652083 | KM652126 | KM652009 | KM652048 | |
| Ophiocordyceps sobolifera | KEW 78842 | Hemiptera | EF468972 | EF468828 | EF468875 | EF468925 | |
| Ophiocordyceps sp. | OSC 151904 | KJ878935 | KJ878899 | KJ878980 | KJ879014 | ||
| Ophiocordyceps sp. | OSC 151909 | KJ878937 | KJ878900 | KJ878981 | KJ879016 | KJ878952 | |
| Ophiocordyceps sphecocephala | OSC 110998 | DQ522551 | DQ518765 | DQ522336 | DQ522381 | DQ522432 | |
| Ophiocordyceps unilateralis | OSC 128574 | Hymenoptera | DQ522554 | DQ518768 | DQ522339 | DQ522385 | DQ522436 |
| Ophiocordyceps unituberculata | YHH HU1301 | KY923213 | KY923215 | KY923217 | KY923219 | ||
| Ophiocordyceps unituberculata | YFCC HU1301 | KY923214 | KY923216 | KY923218 | KY923220 | ||
| Ophiocordyceps variabilis | ARSEF 5365 | Diptera | DQ522555 | DQ518769 | DQ522340 | DQ522386 | DQ522437 |
| Ophiocordyceps variabilis | OSC 111003 | Diptera | EF468985 | EF468839 | EF468779 | EF468885 | EF468933 |
| Ophiocordyceps yakusimensis | HMAS 199604 | KJ878938 | KJ878902 | KJ879018 | KJ878953 | ||
| Ophiocordyceps pulvinata | TNS-F-30044 | GU904208 | GU904209 | GU904210 | |||
| Ophiocordyceps crinalis | GDGM 17327 | KF226253 | KF226254 | KF226256 | KF226255 | ||
| Cordyceps militaris | OSC 93623 | AY184977 | AY184966 | DQ522332 | DQ522377 | ||
| Cordyceps tenuipes | TBRC 7265 | MF140707 | MF140827 | MF140776 | MF140800 | ||
3. Results
3.1. Phylogenetic analyses
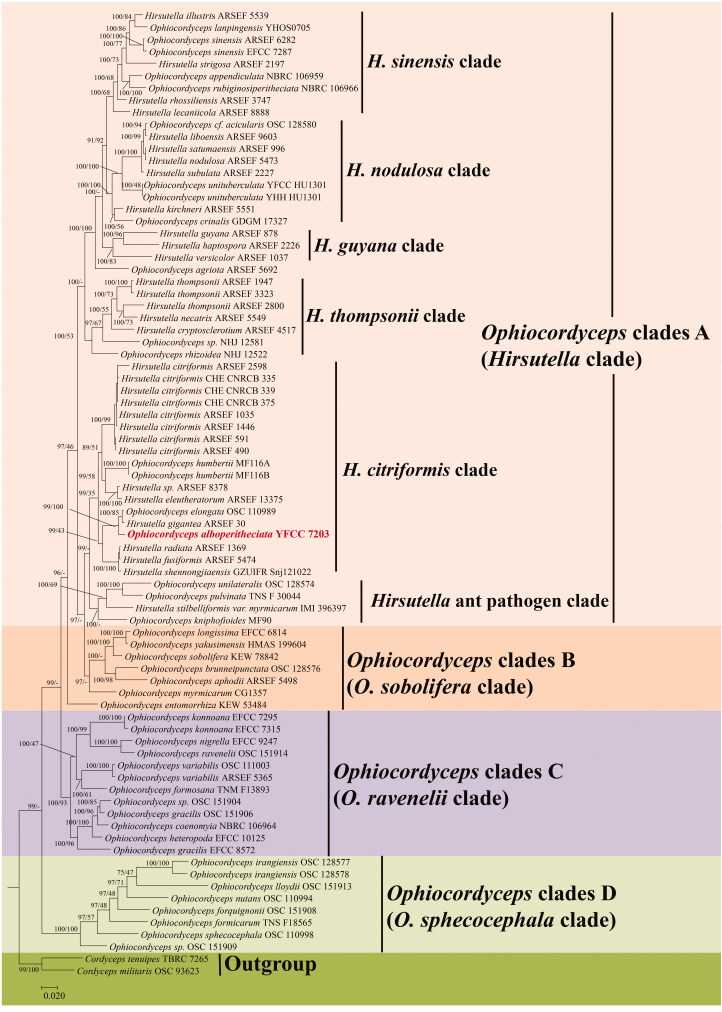
In ML and BI phylogenetic analyses, five-gene sequences of eighty taxa from Hirsutella, Ophiocordyceps, and the outgroup taxa Cordyceps tenuipes (Peck) Kepler, B. Shrestha & Spatafora and C. militaris (L.) Fr. were retrieved from GenBank, which were combined with those generated in the present study. The combined dataset consisted of 4082 bp (i.e., 794 bp for tef-1α, 859 bp for nrLSU, 999 bp for nrSSU, 543 bp for rpb1, as well as 887 bp for rpb2). Phylogenetic trees analyzed by ML and BI exhibited the nearly identical overall topologies (Figure 1). The mentioned results shared similar phylogenetic structures with existing analyses [14,15,18,19]. The phylogenetic trees recognized four statistically well-supported clades in Ophiocordyceps, designated here as Ophiocordyceps clades A (the Hirsutella clade), B (the O. sobolifera clade), C (the O. ravenelii clade), and D (the O. sphecocephala clade) (Figure 1). The Ophiocordyceps clade A (Hirsutella clade) consisted of six major subclades, i.e., H. nodulosa, H. citriformis, H. thompsonii, H. guyana, H. sinensis, as well as Hirsutella ant pathogen clades. As revealed from phylogenetic analyses, the new species O. alboperitheciata clustered into the H. citriformis subclade and isolated a distinct clade from other related species with 100% statistical support.
Figure 1.
Phylogenetic placement of Ophiocordyceps alboperitheciata infered from BI and ML analyses based on five-gene (nrSSU, nrLSU, tef-1α, rpb1, and rpb2) sequence dataset. Values at the nodes before and after the backslash are BI posterior probabilities and ML bootstrap proportions, respectively. Support values of ML bootstrap proportions greater than 40% are indicated at the nodes.
3.2. Taxonomy
Ophiocordyceps alboperitheciata H. Yu, Q. Fan & Y.B. Wang, sp. nov. (Figure 2).
Figure 2.
Morphological characteristics of Ophiocordyceps alboperitheciata. (a, b) Stromata on a larva of Noctuidae; (c) Fertile part; (d) Sterile tip; (e–g) Perithecia; (h–j, l) Asci; (k) Ascospores; (m) Clony on PDA. Scale bars: a, b = 1 cm; c = 600 μm; d = 1 mm; e–f = 100 μm; g–j = 50 μm; k, l = 20 μm; m = 1 cm.
MycoBank: MB 834082
Etymology: alboperitheciata, indicating to the color of perithecia from the type specimen, "albo" means white.
Typus: China. Yunnan Province: Kunming City, the Wild Duck Forest Park, at 25°13′N, 102°87′E, alt. 2100 m, on a larva of Noctuidae (Lepidoptera) buried in fallen leaves, 12 August 2018, Hong Yu (holotype, YHH 16755; ex-holotype living culture, YFCC 7203).
Sexual morph: Stromata arising in pairs from the larva of Noctuidae buried in fallen leaves, cylindrical, flexible, light brown to dark brown, unbranched, gradually tapering toward the apex, 69–71 × 0.6–1.2 mm, with a sterile tip, remaining unchanged in 3% KOH. Stipes cylindrical, smooth, dark brown, 0.6–1.2 mm wide. Fertile parts clavate, pale brown, covered by a spinous surface, reaching up to 4.1–4.5 × 0.8–1.4 mm. Perithecia superficial, subtranslucent, scattered or crowded, nearly ovoid, white to pale brown, exhibiting an unequal distribution on the middle of the stromata, covering densely the lower part and aggregating loosely at the upper of stromata, arranged in a disordered manner, 408–549 × 233–321 µm. Asci hyaline, cylindrical, eight-spores, 144–246 × 3.5–4.7 μm, with a hemispheric apical cap of 3.2–4.2 × 2.3–2.5 µm. Ascospores hyaline, cylindrical, multiseptate, 0.5–0.6 µm diameter, with septa of 1.1–1.3 µm long. Part-spores were not examined.
Asexual morph: Colonies on PDA growing very slowly, exhibiting 3.0–3.6 cm diameter in 21 days at 25 °C, fan split, dark brown at the centrum, and white at the edge. Reverse dark brown. Hyphae hyaline, branched, septate, smooth-walled, 1.37–2.05 μm wide. Conidiogenous cells and conidia were not detected.
Host: Larva of Noctuidae (Lepidoptera).
Habitat: Buried in fallen leaves.
Type locality: The Wild Duck Lake Forest Park, Kunming City, Yunnan Province, China.
4. Discussion
The particularity of O. alboperitheciata is revealed by morphological and ecological comparisons with eight other closely related species that possess Hirsutella morphs (Table 2). Ophiocordyceps alboperitheciata is noticeably inconsistent with eight other related species of H. citriformis clade in five aspects: (1) its fertile parts are long, rod-shaped, 4.1–4.5 × 0.8–1.4 mm; (2) its perithecia are superficial and scattered or crowded, which is nearly white; (3) its asci are slender; (4) its septa of ascospores are smaller; (5) it is associated with the larva of Noctuidae buried in fallen leaves. In the H. citriformis clade, sexual morphs of species have been rarely reported, except for O. elongata and O. humbertii Petch [13,28,29]. It is noteworthy, O. alboperitheciata and O. elongata are closely clustered together, whereas the latter exhibits greater sizes of stromata (110 mm long), asci (220 × 8 µm), ascospore septa (4–12 µm long), and immersed perithecia. Ophiocordyceps alboperitheciata synthesizes relatively shorter stromata (54–65 mm long) with sterile tips and fertile parts, stromata in pairs, superficial perithecia, shorter asci (144–246 × 3.5–4.7 μm), as well as ascospore septa (1.1–1.3 µm). As revealed from the mentioned distinct features above indicated that O. alboperitheciata was considerably different from other related species. The hosts comprised five orders of insects in the H. citriformis clade, in which O. alboperitheciata, O. elongate, and H. gigantean clustered together and linked to Lepidoptera, other six species displayed the respective association with Hymenoptera, Hemiptera, Diptera, Orthoptera, Dermaptera, and Anoplura.
Table 2.
A morphological comparison of Ophiocordyceps alboperitheciata and its related species.
| Species | Host | Habitat | Synnemata/stromata | Perithecia | Asci | Ascospores | Conidiogenous cells | Conidia | References |
|---|---|---|---|---|---|---|---|---|---|
| O. alboperitheciata | Larva of Noctuidae (Lepidoptera) | Buried in fallen leaves | Stromata in pairs, rigid, the stalk is smooth, unbranched, long 54–65 mm, light brown to dark brown, with a clavate fertile part, white to light brown, 4.1–4.5 × 0.8–1.4 mm, and a sterile tip. | Perithecia superficial, scattered or crowded, size 0.41–0.55 × 0.23–0.32 mm, nearly ovoid, white nearly light brown. | Asci hyaline, cylindrical, 8-spores, 144–246 × 3.5–4.7 μm, with a hemispheric apical cap, 3.2–4.2 × 2.3–2.5 µm. | Ascospores hyaline, cylindric, multiseptate, 0.5–0.6 µm diameter, with septa 1.1–1.3 µm apart. part-spores were not seen. | Undetermined | Undetermined | This study |
| O. elongata | Pupae and larvae of Apalela americana (Lepidoptera). | Unknown | The stalk is flexuose, longitudinally sulcate and twisted, 110 mm long, pale brown. | The perithecia are immersed, scattered or crowded, ovato-conoid, size 0.5 × 0.3 mm, apex subacute, wall vellow by transmitted light. | The asci are 220 µm long, 8 µm diameter. | Ascospores cylindric, 2 µm diameter, with septa 4–12 µm apart. Part-spores were not seen. | Unknown | Unknown | [28] |
| O. humbertii | Hymenoptera | Unknown | Several, 7 mm long, dark brown, with an oval swelling, 1 × 0.4 mm. | Perithecia, scattered, dark amber, subtranslucent, flask-shaped with a truncate apex, 275 × 120 µm. | The asci are 130 µm long, 10 µm diameter, capitate, fusoid or narrow-clavate. | Ascospores are 75 µm long, 25 µm diameter, narrow-fusoid, septate at intervals of 6–16 µm, not dividing into part-spores. | Unknown | Unknown | [29] |
| H. gigantea | Pupae and larvae of Apatela Americana (Lepidoptera) | On wood | Branched, longitudinally sulcate, glabrous, ashy and minutely setose above, size 40 × 0.6 mm, brown below. | None | None | None | Phialider up to 40 µm high, with a flask-shaped base, 16–20 × 8–9 µm, and a long, stout sterigma, 1 µm diameter. | The spore cluster is lemon-shaped, 10 × 6 µm, becoming globose, 10 µm diameter, and the separate conidia are broadly cymbiform with obtuse tips, 9–10 × 3–4 µm | [28] |
| H. citriformis | Adult of Fulgoridae (Hemiptera) | Unknown | Synnemata usually long, flexible, simple or branched, branches often short and stumpy, and easily detached, brown in color | None | None | None | Sporophores simple, sessile or subsessile, with rather short, delicate sterigmata 20–30 µm | Spores fusoid, hyaline, 5.5–8.5 × 1.5–18 µm | [13] |
| H. radiata | Fly (Diptera) | Unknown | Rrigid, branched, size 18–19 mm, dark brown or rufous brown, cinercous toward the tips, with a matt surface | None | None | None | The phialides have a conical base, 5–8 × 3–4 µm, merging into a stout sterigmata, 9–14 µm long, or a cylindrical base, 6–18 × 2 µm, with a sterigmata 6 µm long | The spore cluster is oval, 9–11 × 6–7 µm, and the individual conidia are cymbiform, 6–9 × 2–2.5 µm, or oval, 7–8 × 3–4 µm | [28] |
| H. fusiformis | Cricket adult (Orthoptera) | Unknown | Synnemata erect, straight, unbranched, uniform in height, measuring 4–5 mm, nearly black in color | None | None | None | Sporophores simple, sessile, the inflated basal portion tapering gradually to rather short 25–35 µm sterigmata | Spores fusoid cylindrical, hyaline, size 9–10 × 2 µm | [28] |
| H. shennongjiaensis | Earwigs (Dermaptera) | Unknown | Synnemata cylindrical, size 60.0 × 1.0–2.0 mm, brown | None | None | None | Conidiogenous cells solitary, phialides cylindrical or awl-like, 14.4–26.1 or 6.3–14.4 µm | Conidia hyaline, aseptate, smooth, sausage-shaped, single or double from the apex of the neck, 6.3–10.8 × 3.6–6.3 µm | [30] |
| H. eleutheratorum | Colaoptera (Anoplura) | Unknown | Synnemata simple or branching, 3–5 mm, cinereous to violaceous gray to dull brown, often paler at the apex | None | None | None | Conidiogenous cells ellipsoid, base 8–10 × 5–6 µm, tapering rather abruptly into a long neck, 30–35 µm long | Conidia cymbiform to narrow ellipsoid, 4–7 × 1–2 µm, forming citriform clusters 8 × 6 µm | [31] |
The family Ophiocordycipitaceae was proposed according to the type genus Ophiocordyceps with the sexual morph characterized by the production of whole septate ascospores, which usually did not disarticulate into part-spores at maturity, and asci had an apical hemispheric cap. The Hirsutella, as the old asexual generic name associated with the Ophiocordyceps, is synonymized under Ophiocordyceps, most species occurring from adult insects are formerly employed in the Ophiocordyceps clade A [1,3,5]. Phylogenetic studies of Hirsutella species from the USA were conducted by three loci providing evidence for taxonomic revisions under novel rules [14,15]. The available molecular data have facilitated the use of the mentioned fungi and associated data to conduct in-depth phylogenetic classification studies on Hirsutella and Ophiocordyceps. The phylogenetic tree of Hirsutella and Ophiocordyceps of this study complies with the existing studies of Ophiocordycipitaceae [5,10,14,15]. The genus Ophiocordyceps with Hirsutella morph comprises six distinct groups, i.e., H. citriformis, H. thompsoni, H. nodulosa, H. guyana, H. sinensis, and Hirsutella ant pathogen clades. The insect pathogen O. alboperitheciata pertains to the H. citriformis clade, which is obviously separated from other allied species.
The present phylogenetic tree covers nine species cluster in the H. citriformis clade. Our result is consistent with existing findings, i.e., H. radiata, H. fusiformis, and O. shennongjiaensis, and H. gigantean and O. elongate group cluster closely, respectively [14,15,32]. Three species, i.e., O. alboperitheciata, O. elongate, and H. gigantea, are closely clustered together, whereas they are noticeably inconsistent with each other in morphological and ecological characteristics. According to both molecular phylogeny and morphology, a consistent relationship between O. alboperitheciata and other relatives in the H. citriformis clade is evidenced. Thus, the novel species O. alboperitheciata is proposed in genus Ophiocordyceps.
Funding Statement
This work was funded by the National Natural Science Foundation of China [No. 31760011 and 31870017], and the Department of Science and Technology of Yunnan Province [No. 2018FY001(-006)].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Wijayawardene NN, Hyde KD, Rajeshkumar KC, et al. Notes for genera: Ascomycota. Fungal Divers. 2017;86(1):1–594. [Google Scholar]
- 2.Araújo JPM, Evans HC, Kepler RM, et al. Zombie-ant fungi across continents: 15 new species and new combinations within Ophiocordyceps. I. Myrmecophilous hirsutelloid species. Stud Mycol. 2018;90:119–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luangsa-Ard JJ, Tasanathai K, Thanakitpipattana D, et al. Novel and interesting Ophiocordyceps spp. (Ophiocordycipitaceae, Hypocreales) with superficial perithecia from Thailand. Stud Mycol. 2018;89:125–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petch T. Notes on entomogenous fungi. Trans Br Mycol Soc. 1931;16(1):55–75. [Google Scholar]
- 5.Sung GH, Hywel-Jones NL, Sung JM, et al. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol. 2007;57:5–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spatafora JW, Quandt CA, Kepler RM, et al. New 1F1N species combinations in Ophiocordycipitaceae (Hypocreales). IMA Fungus. 2015;6(2):357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khonsanit A, Luangsa-Ard JJ, Thanakitpipattana D, et al. Cryptic species within Ophiocordyceps myrmecophila complex on formicine ants from Thailand. Mycol Prog. 2019;18(1–2):147–161. [Google Scholar]
- 8.Mains E. North American entomogenous species of Cordyceps. Mycologia. 1958;50(2):169–222. [Google Scholar]
- 9.Ban S, Sakane T, Nakagiri A.. Three new species of Ophiocordyceps and overview of anamorph types in the genus and the family Ophiocordyceptaceae. Mycol Prog. 2015;14(1):1017. [Google Scholar]
- 10.Quandt CA, Kepler RM, Gams W, et al. Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus. 2014;5(1):121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanjuán T, Franco-Molano AE, Kepler RM, et al. Five new species of entomopathogenic fungi from the Amazon and evolution of neotropical Ophiocordyceps. Fungal Biol. 2015;119(10):901–916. [DOI] [PubMed] [Google Scholar]
- 12.Mains EB. Entomogenous species of Hirsutella, Tilachlidium and Synnematium. Mycologia. 1951;43(6):691–718. [Google Scholar]
- 13.Speare AT. On certain entomogenous fungi. Mycologia. 1920;12(2):62–76. [Google Scholar]
- 14.Simmons DR, Kepler RM, Rehner SA, et al. Phylogeny of Hirsutella species (Ophiocordycipitaceae) from the USA: remedying the paucity of Hirsutella sequence data. IMA Fungus. 2015;6(2):345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons DR, Lund J, Levitsky T, et al. Ophiocordyceps myrmicarum, a new species infecting invasive Myrmica rubra in Maine. J Invertebr Pathol. 2015;125:23–30. [DOI] [PubMed] [Google Scholar]
- 16.Wang YB, Nguyen TT, Dai YD, et al. Molecular phylogeny and morphology of Ophiocordyceps unituberculata sp. nov. (Ophiocordycipitaceae), a pathogen of caterpillars (Noctuidae, Lepidoptera) from Yunnan, China. Mycol Prog. 2018;17(6):1–9. [Google Scholar]
- 17.Fan Q, Wang YB, Tang DX, et al. Species diversity of Cordyceps sensu lato in the wild duck lake forest park of Kunming (in Chinese). Acta Edulis Fungi. 2020;27(2):101–108. [Google Scholar]
- 18.Wang YB, Yu H, Dai YD, et al. Polycephalomyces yunnanensis (Hypocreales), a new species of Polycephalomyces parasitizing Ophiocordyceps nutans and stink bugs (hemipteran adults). Phytotaxa. 2015;208(1):34. [Google Scholar]
- 19.Wang YB, Yu H, Dai YD, et al. Polycephalomyces agaricus, a new hyperparasite of Ophiocordyceps sp. infecting melolonthid larvae in southwestern China. Mycol Prog. 2015;14(9):70–78. [Google Scholar]
- 20.Wang YB, Wang Y, Fan Q, et al. Multigene phylogeny of the family Cordycipitaceae (Hypocreales): new taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Divers. 2020;103(1):1–46. [Google Scholar]
- 21.Liu ZY, Liang ZQ, Whalley AJS, et al. Cordyceps brittlebankisoides, a new pathogen of grubs and its anamorph, Metarhizium anisopliae var. majus. J Invertebr Pathol. 2001;78(3):0–182. [DOI] [PubMed] [Google Scholar]
- 22.Rehner SA, Samuels GJ.. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequence. Mycol Res. 1994;98(6):625–634. [Google Scholar]
- 23.Bischoff JF, Rehner SA, Humber RA.. Metarhizium frigidum sp. nov.: a cryptic species of M. anisopliae and a member of the M. flavoviride complex. Mycologia. 2006;98(5):737–745. [DOI] [PubMed] [Google Scholar]
- 24.Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronquist F, Huelsenbeck JP.. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. [DOI] [PubMed] [Google Scholar]
- 26.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. [DOI] [PubMed] [Google Scholar]
- 27.Darriba D, Taboada GL, Doallo R, et al. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9(8):772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petch T. Notes on entomogenous fungi. Trans Br Mycol Soc. 1937;21(1–2):34–67. [Google Scholar]
- 29.Petch T. Notes on entomogenous fungi. Trans Br Mycol Soc. 1935;19(3):161–194. [Google Scholar]
- 30.Zou X, Zhou JX, Liang ZQ, et al. Hirsutella shennongjiaensis, a new entomopathogenic species infecting Earwig (Dermaptera). Mycosystema. 2016;35(9):1070–1079. [Google Scholar]
- 31.Petch T. Notes on entomogenous fungi. Trans Br Mycol Soc. 1932;16(4):209–245. [Google Scholar]
- 32.Hodge KT. Revisionary studies in Hirsutella (Anamorphic Hyphomycetes: Clavicipitaceae) [Ph.D. dissertation]. America: Cornell University; 1998. [Google Scholar]