Abstract
Sepsis is one of the leading causes of death worldwide. While mortality is high regardless of inciting infection or comorbidities, mortality in patients with cancer and sepsis is significantly higher than mortality in patients with sepsis without cancer. Cancer patients are also significantly more likely to develop sepsis than the general population. The mechanisms underlying increased mortality in cancer and sepsis patients are multifactorial. Cancer treatment alters the host immune response and can increase susceptibility to infection. Preclinical data also suggests that cancer, in and of itself, increases mortality from sepsis with dysregulation of the adaptive immune system playing a key role. Further, preclinical data demonstrate that sepsis can alter subsequent tumor growth while tumoral immunity impacts survival from sepsis. Checkpoint inhibition is a well-accepted treatment for many types of cancer, and there is increasing evidence suggesting this may be a useful strategy in sepsis as well. However, preclinical studies of checkpoint inhibition in cancer and sepsis demonstrate results that could not have been predicted by examining either variable in isolation. As sepsis management transitions from a ‘one size fits all’ model to a more individualized approach, understanding the mechanistic impact of cancer on outcomes from sepsis represents an important strategy towards delivering on the promise of precision medicine in the intensive care unit.
Sepsis
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. The inciting infectious agent can be bacterial, viral or fungal. In an attempt to contain and eliminate the infection, the immune system responds by releasing cytokines and inflammatory mediators triggering the activation of the coagulation cascade, altering metabolism and endocrine responses. Damage occurs on all scales in the host from the cellular (increased apoptosis, necroptosis, decreased mitochondrial respiration) to whole body, where damage can be seen in all organs [2].
Sepsis affects nearly 50 million people worldwide, accounting for approximately 20% of deaths in the world prior to the COVID-19 pandemic [3]. Due to these devastating statistics, the World Health Organization has recognized sepsis as a global health priority [4]. Despite intensive research into sepsis and septic shock, 30-day mortality from septic shock has stayed stable worldwide for the past decade [5]. In the United States alone, sepsis affects 1.7 million people and results in approximately 300,000 deaths annually [6]. Further, sepsis is present in 35% of all hospitalizations that end in death [6]. The costs associated with sepsis are enormous – whether measured in human suffering or financially. Many sepsis survivors end up with post-intensive care syndrome where they have physical, emotional and cognitive abnormalities months or years after their hospitalization [7,8]. In addition, costs for Medicare beneficiaries in the United States with sepsis exceed $60 billion, twice as high as previous estimates [9]. However, despite the significant morbidity and mortality attached to sepsis, no targeted treatments are available beyond antibiotic therapy and source control [10]. Improved outcomes from sepsis are possible with earlier and standardized treatment and following evidence based guidelines [10–13].
A large number of randomized controlled trials related to sepsis have been published, yet no pharmacologic intervention has consistently reduced mortality [14]. While the reasons related to this lack of success are assuredly multifactorial, one commonly agreed upon reason is that entry criteria in clinical trials of sepsis are very broad. Sepsis trials include patients with diverse inciting organisms, genetics, age, sex, environment and co-morbidities. This has led to a movement to leave behind a ‘one size fits all’ approach to trials towards an approach where trials have more stringent, biologically defined entry criteria [15]. Indeed, numerous retrospective studies have demonstrated that a variety of approaches can distinguish seemingly similar sepsis patients into phenotypes that have different outcomes that cannot be predicted upon severity of illness and that will respond differently to the same therapeutic agents [16–22].
Cancer
Cancer is the second most common cause of death in the United States [23]. There is an enormous psychological burden associated with cancer, and cancer patients are 71% more likely to experience tax liens, foreclosures, debt collections and bankruptcy with a direct correlation between the declaration of bankruptcy and death [24].
Cancer is characterized by the uncontrollable growth and spread of abnormal cells. Malignant cells have the ability to hide from the immune system and create microenvironments that optimize tumor survival [25]. This allows for the progression of the disease process with inflammatory cells contributing to fibrosis, angiogenesis, and remodeling of the cellular environment. In addition, immune cells that exist within the tumor environment are subjected to anti-inflammatory signals that serve to decrease their intended function, such as the phagocytic ability of macrophages and the activation of dendritic and T cells [26]. When T cells in the tumor microenvironment enter states of relative dysfunction, exhaustion and senesce, tumor proliferation can occur further [27].
Sepsis and cancer
Historically, patients with cancer have been shown to be nearly ten times more likely to develop sepsis than the general population [28], and the leading cause of intensive care unit admissions in patients with cancer is sepsis. Further, although outcomes can vary by a patient’s type of cancer, treatment and access to medical care, it has been estimated that approximately 30% of mortality due to cancer is from sepsis [29].
Cancer, in isolation, and therapy for cancer (e.g. chemotherapy, radiation, surgery, bone marrow transplantation) also increase the risk for sepsis. Both chemotherapy and radiation induce neutropenia which is a key risk factor for infection, related to severity and duration of both neutrophil and monocyte depletion [30]. In addition, there may also be impairment of chemotaxis and phagocytosis even when counts are preserved. Further, multiple chemotherapy regimens induce T or B cell lymphopenia as well as functional changes in both lymphocytes and NK cells. Invasive catheters required for treating tumors (central venous catheters for administration of chemotherapeutic effects) or palliating tumors (long-term catheters for urinary obstruction) can also play a role in development of sepsis. In addition, ethnicity may play a role in defining risk. As an example, even though Native Hawaiians have a lower incidence of colorectal cancer compared with other ethnicities, they have the highest mortality risk from cancer-associated sepsis. A transcriptomic analysis of tumors and adjacent non-tumor tissue was therefore performed on adult patients of Native Hawaiian and Japanese ethnicity who died from cancer-associated sepsis with a median survival of 5 and 117 months respectively [31]. Analyses identified two distinct sepsis gene signatures that were significantly altered in Native Hawaiian patients. Analysis of canonical pathways revealed alterations in mechanisms of viral exit from host cells as well as in epithelial junction remodeling.
With multiple revolutionary advances rapidly changing outcomes and side effects of cancer therapy, it is possible that both risk for sepsis as well as outcomes from sepsis has recently changed in cancer patients. To address this, a group of 1.1 million sepsis hospitalizations were examined using all-payer claims for nearly 50% of the United States population [32]. Over 230,000 sepsis admissions were cancer-related, meaning that over 20% of sepsis hospitalizations are in patients with cancer. Of patients hospitalized with sepsis who had cancer, 63.4% had solid tumors, 18.4% had hematologic malignancies while the remainder were unknown. Mortality in septic patients varies with type of cancer as in-hospital mortality is higher with hematologic tumors than solid tumors. Cancer patients had fewer non-cancer comorbidities. While overall organ dysfunction was similar in septic patients with and without cancer, those with cancer were more likely to have hematologic dysfunction but less likely to have pulmonary or renal dysfunction. Septic patients with cancer were also more likely to have bacteremia, fungemia and gastrointestinal infection.
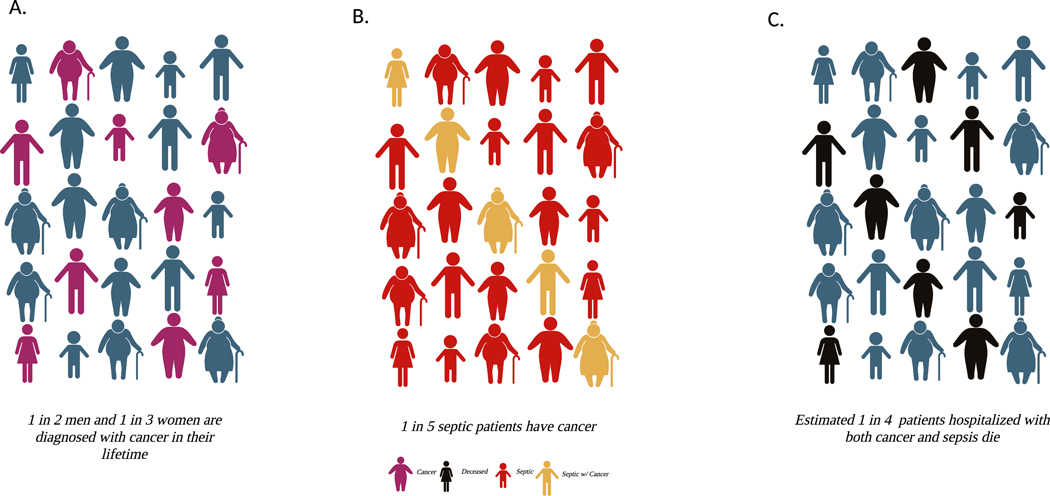
Notably, compared with septic patients without cancer, septic patients with cancer had a markedly higher in-hospital mortality (27.9% versus 19.5%, P<0.001), and mortality was consistently higher in subgroups defined by infection site and burden of acute organ dysfunction [32]. This increase in hospital mortality was age dependent in a manner that might not have been predicted. In general, sepsis is a disease of the extremes of age with incidence and mortality highest in the elderly and neonates. However, the biggest difference in mortality between sepsis patients with and without cancer was in younger adults, with the relative risk highest in patients ages 18–44. The difference in mortality then declines with age until finally there is no difference in patients aged 85 and older. Additionally, 30-day readmission rates were higher in septic patients with cancer for both recurrent sepsis and all causes. An overview of the lifetime risk of developing cancer [33], the percentage of septic patients who have cancer as a pre-existing comorbidity [32], and the mortality of hospitalized patients with cancer and sepsis [32] is shown in Figure 1.
Figure 1. Lifetime Incidence Portrayal: Cancer, Sepsis and Mortality.
(A)Lifetime incidence of developing cancer, (B) percentage of septic patients who have cancer, and (C) mortality of hospitalized patients with cancer and sepsis. Created with BioRender.com.
A complementary study examined over 19 million sepsis hospitalizations from the National Inpatient Sample database from 2008 to 2017 [34]. Of these, 20.4% were associated with cancer, of which approximately 80% were solid cancers. In-hospital mortality was higher in septic patients with cancer than those without cancer (17.88% vs. 12.15%) and hematologic cancers had a slightly higher mortality rate than solid cancers as well as longer lengths of stay and higher hospital charges. Notably, unadjusted mortality rates declined in septic patients with cancer from 23.25% in 2008 to 15% in 2017, a decrease seen in both solid and hematologic cancers. A similar age-dependent stratification was seen in this large database study with odds of death in septic patients with cancer highest in patients aged 18–44 years (odds ratio 3.40, 95% CI: 3.24–3.57). This trend decreased with age until finally septic patients with cancer over 85 years of age actually had a slightly lower risk of mortality than those without cancer.
A meta-analysis of nine studies (seven retrospective, one prospective, one case–control) published between 2015 and 2021 also examined the impact of cancer on sepsis [35]. While acknowledging that the above data represented the largest dataset in the meta-analysis, this meta-analysis gives a broader view since it was international in nature, as opposed to being specifically from the United States. Cancer was found to significantly increase the risk of mortality in patients with sepsis (OR = 2.7, 95%CI: 1.07–6.84). Further, there was a non-significant tendency towards increased early mortality in sepsis patients with cancer (OR = 2.77, 95%CI: 0.88–8.66) and a significant increased risk of late mortality (OR = 2.46, 95%CI: 1.42–4.25). Notably, meta-regression indicated that the presence of pulmonary disease and renal disease as comorbid conditions increased mortality in cancer septic patients. Of note, a complementary meta-analysis of 10 studies including 6605 patients demonstrated pooled ICU, hospital, and 28/30-day mortality rates of 48%, 62%, and 50% respectively, albeit with substantial between-study heterogeneity [36].
A study of 2,062 cancer patients admitted to seven European ICUs further examined the relationship between cancer and sepsis [37]. This had a different tumor type than previous studies outlined, as 82% had hematologic malignancy, including 12% who underwent allogeneic hematopoietic stem cell transplantation, with nearly one third of patients having neutropenia at ICU admission. Overall 30-day mortality was 40% in this patient population. Notably, mechanical ventilation (odds ratio: 3.25; 95%CI: 2.52–4.19) and to a lesser degree vasopressors use (odds ratio: 1.42; 95%CI: 1.10–1.83; P<0.01) were independently associated with 30-day mortality, while type of tumor, stem cell transplant and neutropenia were not predictive of mortality. Delirium has also been shown to be associated with a significant increase in mortality in critically ill patients with cancer [38]. This is complementary to a smaller study of 271 septic shock patients admitted to the ICU of a specialized cancer center which showed that 69.4% of patients had died within 28 days of ICU admission [39]. Risk factors associated with not surviving the ICU included advanced cancer, poor performance status, high lactate level, and concomitant acute respiratory failure.
The complexity of outcomes in cancer and sepsis are potentially amenable to machine learning algorithms to predict outcome. Recently, clinical data of 1584 patients with solid tumors and sepsis were obtained from Medical Information Mart for Intensive Care-IV database and randomly assigned to a training cohort and a validation cohort to predict in-hospital mortality [40]. The least absolute shrinkage and selection operator regression and logistic regression analysis were used to feature selection and model development. A total of nine clinical features were associated with in-hospital mortality, leading to an area under the curve of 0.809 in the training cohort and 0.770 in the validation cohort. As such, artificial intelligence may play a future role in assessing in-hospital mortality of septic patients with solid tumors in the ICU.
Sepsis represents the most severe form of infection, as, by definition, organ dysfunction occurs in sepsis. However, most infections do not result in sepsis, and infection is common in cancer patients prior to the onset of sepsis. Antibiotic use is common in cancer patients, both for treatment and also for prophylaxis in patients with chemotherapy-induced neutropenia [41,42]. Notably, cancer patients have a higher risk of developing infections with antimicrobial-resistant organisms [43] although models of resistant infections in this patient population are heterogeneous [44]. Antibiotic treatment has been associated with worsened survival in subsets of advanced cancer patients receiving chemotherapy [45] and antimicrobial therapy also alters the microbiome, which may impact the efficacy of radiation therapy in cancer patients [46].
While sepsis is cancer patients is therefore of vital importance, it is also important for clinicians to understand that there are numerous cancer-related conditions and drug reactions that mimic sepsis or septic shock that are not caused by infection. Aggressive hematological malignancies can drive organ dysfunctions through spontaneous or treatment-triggered mechanisms via a variety of mechanisms (reviewed in [30]). Additionally, newer cancer therapies such as CAR-T can cause acute systemic inflammation that can mimic sepsis [47].
When sepsis is present in a patient with pre-existing malignancy, general principles of sepsis management (including rapid initiation of appropriately broad antibiotics, resuscitation) should be followed [10]. Additionally, since septic patients with cancer are more prone to have immunosuppression and a worse outcome, special attention should be paid to this patient population [48] and consideration should be given to newer diagnostics that may provide earlier and more accurate specific identification of infections that lead to sepsis [49].
Preclinical modeling of cancer and sepsis
In order to understand potential mechanisms through which pre-existing malignancy increases mortality in sepsis, our lab created mouse models to mimic the human condition [50–52]. Cancer was induced using syngeneic models of both pancreatic cancer and lung cancer, chosen because they are the most common solid tumors associated with sepsis development. Sepsis was induced using either cecal ligation and puncture (CLP), a mouse model of polymicrobial intraabdominal sepsis or Pseudomonas aeruginosa pneumonia. By varying both cancer type and sepsis model, this allowed a gross evaluation of which elements of the host response were generalizable vs. specific to either injury. Regardless of whether cancer type or sepsis model was varied, the combination of pre-existing malignancy followed by sepsis increased mortality in each model, consistent with findings in patients.
A model of pancreatic cancer followed by pneumonia demonstrated increased bacteremia without alterations in local pulmonary infection in cancer septic animals compared with previously healthy ones [50]. In addition, cancer septic animals had increased gut epithelial apoptosis although unexpectedly they had decreased T- and B-lymphocyte apoptosis compared with previously healthy septic mice. Notably, the increased mortality seen in sepsis was not associated with changes in multiple endpoints including serum and pulmonary cytokines, lung histology, complete blood counts, and intestinal proliferation.
In contrast with the diminished T-cell apoptosis in pancreatic cancer/pneumonia, a model of lung cancer followed by CLP demonstrated increased splenic CD4+ T lymphocyte apoptosis, leading to decreases in both their number and frequency [51]. This was not associated with changes in splenic CD8+ T cell numbers. Also distinct from pancreatic cancer/pneumonia, intestinal proliferation was decreased in cancer septic mice compared with previously healthy septic mice although gut epithelial apoptosis was unchanged. Further, cancer septic mice had increased local infection (bacterial burden in the peritoneal cavity), without changes in peritoneal cytokine, neutrophil or dendritic cell responses. Cancer septic mice had biochemical evidence of worsened kidney function with increased creatinine and blood urea nitrogen, but there was no histologic evidence of renal injury.
The differences between pancreatic cancer/pneumonia and lung cancer/CLP cannot distinguish the individual roles of cancer or sepsis in light of the fact that each variable was changed in the above experiments. We therefore created a new cancer/sepsis model giving mice a syngeneic model of pancreatic cancer followed by CLP to allow a comparison to prior studies by holding one variable constant and changing a different one [52]. Cancer septic mice had decreased CD4+ T cell apoptosis as well as increased CD4+ T cells and CD8+ T cells. Notably, splenic CD8+ T cell activation was decreased in cancer septic mice while no differences were noted in gut apoptosis or proliferation, local bacterial burden, or renal, liver injury.
Taken together, these findings allow comparison of the importance of cancer versus the importance of sepsis. If findings were similar in lung cancer/CLP and pancreatic cancer/CLP but different from pancreatic cancer/pneumonia, this would suggest that the type of sepsis plays a greater role in the host response than the type of cancer. Alternatively, if findings were similar in pancreatic cancer/pneumonia and pancreatic cancer/CLP but different from lung cancer/CLP, this would suggest the opposite – that type of cancer plays a greater role in the host response than the type of sepsis. The reality, however, appears to lie in the middle. While there is minimal overlap between pancreatic cancer/pneumonia and lung cancer/CLP beyond increased mortality in the endpoints measured, there is significant overlap of the pancreatic cancer/CLP with both. This suggests that both the chronic dysregulated host state induced by cancer and the acute dysregulated host response induced by sepsis each play a role in mediating mortality in septic hosts with pre-existing malignancy.
Checkpoint blockade
Immune checkpoint blockade has resulted in remarkable results in a variety of solid tumors in sensitive patients [53–55]. Septic patients also have increased levels of multiple checkpoint receptors [56–59], and there is a robust pre-clinical experience showing increased survival from targeting checkpoint blockade in septic mice [60–63]. This has led to phase I trials targeting PD-1 and PD-L1 in septic patients, demonstrating these agents are safe and increase HLA-DR [64–66].
Numerous parallels exist in the host response to both cancer and sepsis [30,67].
Septic patients have down-regulation of multiple immune response pathways suggesting that impaired innate and adaptive immunity may be fundamental to the immunosuppression that characterizes the disorder. Sepsis causes a more pronounced effect on gene transcription in CD4+ T cells than in CD8+ T cells with up-regulation of Arg-1, SOCS-1, SOCS-3, TIGIT, Lag-3, PD-1, and CTLA-4 transcripts [68]. Although cancer has much more profound effects on gene transcripts in CD8 T cells, common immunosuppressive mechanisms are present in both cancer and sepsis. While checkpoint inhibition is beneficial in both cancer (in patients) and sepsis (in preclinical studies) in isolation, it is plausible that the combination of cancer and sepsis could lead to augmented benefit or abrogated benefit compared with sepsis without pre-existing malignancy or no impact. To test this in a preclinical study, immune dysregulation was first examined in a model of lung cancer followed by CLP [69]. A non-biased, unsupervised analysis of phenotypic differences of CD4+ T cell compartments was conducted on splenocytes from cancer septic animals and previously healthy septic animals using Spanning-tree Progression Analysis of Density-normalized Events. Cancer septic animals had more resting memory CD4+ T cells, more activated CD4+ effector T cells, and less naïve CD4+ T cells during sepsis. Further, cancer septic animals had expansion of two distinct subsets of CD4+ T cells including increases in both a PD-1hi population and a 2B4hi BTLAhi LAG-3hi population. By combining phenotypic analysis of exhaustion markers with functional analysis of cytokine production, it was determined that PD-1+ CD4+ T cells had decreased cytokine production following CLP in cancer septic animals while 2B4+ PD-1lo cells secreted increased TNF.
In light of these findings and clinical data of checkpoint blockade in patients with cancer and to a lesser degree sepsis, the efficacy of PD-1 blockade was examined in the setting of sepsis following preexisting malignancy [70]. PD-1 blockade did not alter survival in mice subjected to a model of lung cancer followed by CLP compared with those treated with vehicle. The lack of efficacy of PD-1 blockade in cancer septic animals (as opposed to either to either variable in isolation) was associated with a decrease in PD-1+ responder cells as CD8+ T cells isolated from these mice had decreased CD28 expression as well as decreased frequency of CXCR5+PD-1+ stem cell-like CD8+ T cells. Further, PD-1 blockade was ineffective at inhibiting lymphocyte apoptosis in cancer septic animals.
T-cell signaling is complex with multiple non-redundant co-inhibitory molecules. In light of the lack of efficacy of PD-1 blockade, flow cytometric analysis of T cells isolated from cancer septic animals was performed to determine if other checkpoint inhibitors were up-regulated. This demonstrated up-regulation of 2B4 in cancer septic animals. Kinetic analysis demonstrated increased 2B4 expression on both CD4+ and CD8+ T cells following CLP. Notably, 2B4 blockade led to significantly improved survival after cancer/sepsis compared with vehicle. This was associated with increased T cell costimulatory receptor expression and decreased expression of PD-1 on CD4+ cells, TIGIT on CD8+ cells and CTLA-4 on both cell populations. In addition, intracellular cytokine staining following ex vivo restimulation showed that 2B4 blockade in cancer septic animals led to increased TNF- and IL-2–producing CD4+ T cells and increased frequencies of IFN-γ–, TNF-, and IL-2–producing CD8+ T cells demonstrating that blocking 2B4 improves T cell effector function in cancer septic animals. Blockade of 2B4 also decreased the frequency of Foxp3+ among CD4+ cells. This was associated with decreased CTLA-4 expression in Foxp3+ cells, suggesting Tregs may have lower suppressive activity after 2B4 blockade.
TIGIT is a coinhibitory receptor that is preferentially up-regulated in the setting of cancer [71–73]. Using a similar model of lung cancer followed by CLP, TIGIT was determined to be higher on Foxp3+ Treg and NK cells following cancer alone, a difference that persisted after the onset of sepsis [74]. In cancer sepsis animals, TIGIT+ Treg had a PD-1+ CTLA-4+ ICOS+ Helios+ phenotype, consistent with highly suppressive Treg, while TIGIT expression was associated with decreased function of T effector cells. Notably, anti-TIGIT mAb specifically decreased mortality in cancer and sepsis, as this treatment improved survival in mice with lung cancer followed by CLP but had no impact on survival on previously healthy mice subjected to CLP. Additionally, anti-TIGIT treatment decreased the frequency of PD-1+ cells in CD4+ T cells, CD8+ T cells and Foxp3+ Treg in cancer septic animals without altering these in previously healthy septic mice. Anti-TIGIT also reversed sepsis-induced loss of splenic CD4+ T cells, CD8+ T cells, Foxp3+ Treg, and CD19+ B cells. This was due to decreased apoptosis following anti-TIGIT in cancer septic animals without an alteration in lymphocyte proliferation.
Together, these results suggest a complicated relationship between checkpoint inhibition and outcome in pre-clinical cancer sepsis models related to immunological alterations that differ between previously healthy septic hosts and those with pre-existing cancer. This runs the spectrum from PD-1-blockade losing efficacy when a host has cancer prior to the onset of sepsis to 2B4-blockade retaining similar efficacy regardless of the presence of cancer to anti-TIGIT which has targeted efficacy seen in cancer sepsis not seen in previously healthy sepsis. This range suggests cancer cannot simply be thought of as one of many co-morbidities seen prior to the onset sepsis. As phase 2 and 3 trials are designed to examine checkpoint blockade in sepsis, the presence or absence of cancer should be considered in study design to determine whether this therapy can potentially translate to survival advantage in cancer/septic patients.
Influence of infection on tumor growth
There is emerging evidence that infection alters tumor growth. After initial success deliberately injecting Streptococcus pyogenes into a patient with inoperable cancer in 1891, Dr. William Coley, a bone sarcoma surgeon, injected over 1000 cancer patients with bacteria or bacterial products [75,76]. These products known as “Coley’s toxins” resulted in tumor remission in a significant number of his patients. Unsurprisingly, this strategy disappeared from use with lack of belief amongst contemporaries and more modern cancer therapies. However, the concept of alterations in cancer following infection has been proven to have merit. Intratumoral injections of an attenuated strain of Clostridium novyi led to a microscopically precise, tumor-localized response in a rat glioma model, and intratumoral injection of the same strain led to responses in nearly 40% of dogs with spontaneous solid tumors [77]. Similar results were seen in a recent first-in-human trial in which 24 patients with refractory solid tumors received a single intratumoral injection of non-toxic Clostridium novyi with lysis of tumor masses in over 40% of patients, albeit with toxicity of sepsis or gas gangrene in 3 patients [78].
Using a Medicare linked database, a case-control study of approximately 400,000 adults in the United States (half with cancer, half without), identified associations between sepsis and subsequent cancer formation [79]. Sepsis was associated with increased risk of cancers of the colon, rectum, liver, lung, and cervix as well as acute myeloid leukemia, chronic myeloid leukemia and myelodysplastic syndrome. In contrast, sepsis was associated with decreased risk of cancer of breast, prostate, kidney, and thyroid as well as for melanoma, diffuse large B-cell lymphoma, and follicular lymphoma.
Tumor-specific T cells
The role of sepsis on tumor growth has also been tested mechanistically in preclinical models. Mice with melanoma followed by CLP early during tumor development had CD8+ T cell-dependent attenuation of tumor growth [80]. This was accompanied by an increase in in vivo activation of sepsis-resistant CD8+ tumor-infiltrating T cells. Further, there was increased expression of co-inhibitory receptors PD-1 and LAG-3 due to liberation of sepsis-induced tumor antigens. Further, sepsis-reinvigorated CD8+ tumor-infiltrating T cells were also amenable to checkpoint inhibition leading to further prolongation of cancer survival following sepsis.
In contrast, mice that have CLP followed by lung cancer have higher numbers of Tregs following sepsis which have more suppressive activity in vitro than controls [81]. Notably, these post-septic mice had increased tumor growth compared with sham mice which was speculated to mechanistically be related to the ability of post septic Tregs to impair the antitumor response mediated by CD8+ T cells.
While these studies address how sepsis or bacterial infection impact both tumor-specific T cells and tumor growth, they do not address the converse question of if tumor-specific cells alter the host response and mortality from sepsis. While there is a clear association between altered host response and mortality in cancer patients who are septic, studying the impact of tumor-specific cells requires a mechanistic laboratory-based approach. To address this question, the impact of tumor-specific CD8+ T cells on the immune response was examined in a mouse model of syngeneic lung cancer followed by CLP [82]. In order to determine the contribution of tumor-specific T cells, an antigen-specific tumor model was used wherein OVA-expressing cells were used to generate tumors, and OVA-specific CD8+ OT-I T cells were used to track the tumor-specific CD8+ T cell response. OT-I cells represented a higher percentage of CD8+ T cells in tumors and tumor draining lymph nodes than in the spleen, and intra-tumoral OT-I cells had a higher frequency of co-inhibitory receptor positive cells including PD-1+, 2B4+, and Tim-3+ compared with splenic OT-I cells. To determine the role of tumor-specific T cells in mortality following sepsis, either anti-Thy1.1 antibody or isotype control was given to deplete OT-I cells after adoptive transfer of Thy1.1+ OT-I and lung cancer-OVA tumor induction followed by CLP. Notably, depletion of tumor-specific T cells led to improved survival following sepsis. Similar findings were seen in a model of melanoma followed by CLP, demonstrating the findings are not specific to a single type of cancer and suggesting that tumor-specific T cells play a role in the higher mortality seen in cancer septic animals [82]. Examination of potential reasons for the survival difference showed that frequency of activated CD44+ endogenous CD8+ T cells was lower following anti-Thy1.1 treatment, suggesting that tumor-specific T cells contribute to the activation of endogenous CD8+ T cells. Further, tumor-specific T cells led to increased apoptosis in endogenous CD8+ T cells following sepsis. Additionally, while sepsis causes a decrease of both tumor-specific and endogenous T cells in tumor draining lymph nodes as well as spleen, CLP does not alter the number of tumor-specific CD8+ T cells within the tumor. Tumor-specific T cells also have impaired IFN-γ secretion in the tumor, tumor draining lymph node, and spleen following CLP.
NK cells
To address the impact of sepsis on a pre-established cancer model, mice were inoculated with MCA205 fibrosarcoma cells either subcutaneously (to mimic local disease) or intravenously (to mimic metastatic disease) and then subjected to CLP [83]. Of note, mice with sarcoma did not have increased mortality to CLP in distinction to the lung cancer or pancreatic cancer models followed by CLP or pneumonia described above. However, septic mice had decreased tumor growth in the local cancer model and decreased number and size of pulmonary lesions in the metastatic model. Since toll-like receptors (TLRs) play a major role in the immune response to sepsis following recognition of pathogen-associated molecular patterns, similar experiments were performed in mice deficient for TLR2, TLR4 or their mutual adaptive protein MyD88. Whereas TLR2−/− mice appeared similar to WT mice following cancer and sepsis, the decrease in tumor size seen in WT mice was not seen in either TLR4−/− or MyD88−/− mice. Similarly, CLP-induced inhibition of lung metastasis seen in WT mice was abrogated in TLR4−/− mice. Data from TLR2 and TLR4 agonists in cancer and sepsis confirmed a regulatory role of TLR4 signaling in shaping anti-tumor immune responses. Since MCA205 cells have low MHC class I expression (similar to the classical NK-sensitive YAC-1 cell line), this left open the possibility that these neoplastic cells could also be targeted by NK cells. This was examined by administering endotoxin, which resulted in expansion of the tumor-associated CD11b+CD27+ and CD11b+CD27− cytotoxic NK subsets as well as increased expression of the NKG2D activating receptor.
Intracellular expression of IFNγ was up-regulated on tumor-associated NK cells following CLP. Further, NK cells had intracellular depletion of perforin and granzyme B and increased outer membrane expression of the degranulation marker CD107a following endotoxemia, suggesting that degranulation had already occurred. To address the role of NK cells in mediating sepsis-induced decreases in tumor growth, mice were depleted of NK cells at time of CLP or endotoxemia. While NK depletion did not change mortality following CLP, it restored full tumor growth following CLP in WT mice. In contrast, NK depletion did not impact LPS-induced tumor inhibition, suggesting a sepsis-specific anti-tumoral effect of these cells. To test this, splenic NK cells were obtained following CLP and then co-cultured with MHC-1lo MCA205 target cells. NK cells from septic (CLP) mice had a low-activation pattern, yet when they were in the presence of MCA205 cells, they had increased IFNγ production and cytotoxic functions, consistent with exacerbation of the anti-tumoral properties of NK cells. In contrast, CD8+ T lymphocytes were unchanged following either CLP or endotoxemia.
Beneficial therapeutic preclinical approaches in sepsis that are harmful in cancer and sepsis
In understanding why mortality may be higher in cancer and sepsis, one possibility is that strategies that are effective in previously healthy septic hosts may not be effective in septic hosts with pre-existing malignancy. CXCR4 is a chemokine receptor that plays an important role in T cell co-signaling, formation of the immunological synapse, and directing cells to bone marrow niches following ligation by CXCL12. Following CLP, CXCR4 is selectively up-regulated on naïve CD4+ and CD8+ T cells as well as CD4+ central memory T cells following CLP. Blockade of CXCR4 using the agent Plerixafor led to improved survival following CLP, associated with increased peripheral CD4+ and CD8+ T cells and decreased CD4+ T cell exhaustion [84].
This preclinical finding using a drug that has been in clinical usage for cancer treatment for over a decade led to the question of whether it would be similarly efficacious in cancer and sepsis. Mice with lung cancer followed by CLP had increased CXCR4 expression on CD4+ and CD8+ T cells with expression mainly up-regulated on naïve T cells and central memory T cells compared with cancer, consistent with findings comparing mice in CLP without cancer [85]. However, in marked contrast with sepsis in previously healthy mice, CXCR4 blockade given to cancer septic animals led to decreased survival compared with vehicle. This has significant translatability concerns in that a therapeutic approach that is beneficial in septic hosts without co-morbidities is actually harmful in sepsis with pre-existing malignancy. This led to an exploration of possible mechanisms underlying this profound change in efficacy. Whereas CXCR4 blockade led an increase in the absolute number of CD4+ T cells and CD8+ T cells following CLP (in contrast to reductions caused by sepsis alone), CXCR4 failed to restore these in cancer and sepsis, nor was there an improvement in any T cell subsets. Similarly, while CXCR4 blockade reduced markers of T cell exhaustion following sepsis in previously healthy animals, the same strategy in sepsis failed to reduce T cell expression of PD-1, 2B4, and TIGIT following cancer and sepsis. CXCR4 blockade also fails to promote effective egress of T cells from the bone marrow in cancer septic animals. This was not due to differences in splenic or bone marrow T cell CXCR4 expression between septic animals and cancer septic animals. In contrast, cancer septic animals had less CXCL12 as well fewer CD4+ and CD8+ T cells in the bone marrow, suggesting that the failure of CXCR4 blockade to promote T cell egress from the bone marrow is related to the presence of cancer. Additionally, increased CD69 expression on naïve bone marrow T cells was mechanistically associated with their inability to egress from the bone marrow following CXCR4 blockade in cancer septic animals.
Another strategy that has been shown to improve survival in preclinical sepsis is apoptosis prevention. Sepsis preferentially increases apoptosis in both lymphocytes and the gut epithelium in both septic patients and in animal models of sepsis [86–90]. Multiple studies have demonstrated that prevention of either lymphocyte apoptosis or gut epithelial apoptosis via a variety of different strategies is associated with a marked survival advantage following models of both monomicrobial and polymicrobial sepsis from both pulmonary and intraabdominal sources [91–95].
However, the identical approach leads to markedly different results in cancer sepsis animals. Using a model of pancreatic cancer followed by Pseudomonas aeruginosa pneumonia, lymphocyte prevention was examined using transgenic mice overexpressing human Bcl-2 in both T and B lymphocytes. This led to the expected decreased levels of T lymphocyte apoptosis compared with WT mice [96]. However, even though transgenic mice had decreased lymphocyte apoptosis, they had a paradoxical increase in mortality following cancer and sepsis compared with WT littermates. Notably, neither pneumonia severity nor tumor size was different in transgenic mice although there was an up-regulation of Th1 cytokines in BAL fluid in transgenic mice. In light of these surprising findings, similar studies were performed on Bim−/− mice to determine if the results were generalizable. Septic Bim−/− mice with cancer also had increased mortality compared with WT mice with cancer. To determine if the effect of altering sepsis-induced apoptosis was lymphocyte specific, similar studies were performed in transgenic mice that overexpress Bcl-2 in the intestinal epithelium which demonstrated no difference in survival. Thus, three different transgenic or knockout mice that have been shown to improve survival in previously healthy septic mice had either worsened mortality or had no beneficial impact in cancer septic animals, demonstrating the importance of pre-existing cancer in outcomes from sepsis.
Conclusions and future directions
Thereislittlequestionthatmortalityishigherinpatientswithcancerandsepsisthanintheoverallpopulationofseptic patients without cancer, and the mechanisms underlying are complex. Some of this is unavoidable (for now) as certain cancer treatments such as cytotoxic chemotherapy and bone marrow transplantation predispose cancer patients to infections. This highlights a key distinction between human cancer and sepsis and mouse studies trying to mimic the human condition. The increased mortality in septic patients with cancer is often directly related to a therapy (chemotherapy, immunotherapy) intended to improve outcome from cancer but that has a side effect of making a patient more susceptible to sepsis. In contrast, the majority of mouse studies look at cancer without adjunctive therapy as a risk factor for sepsis. The relevance of this to the human condition is not clear as strong data linking cancer alone (in the absence of anti-tumoral therapy) and poor outcomes from sepsis is lacking in patients.
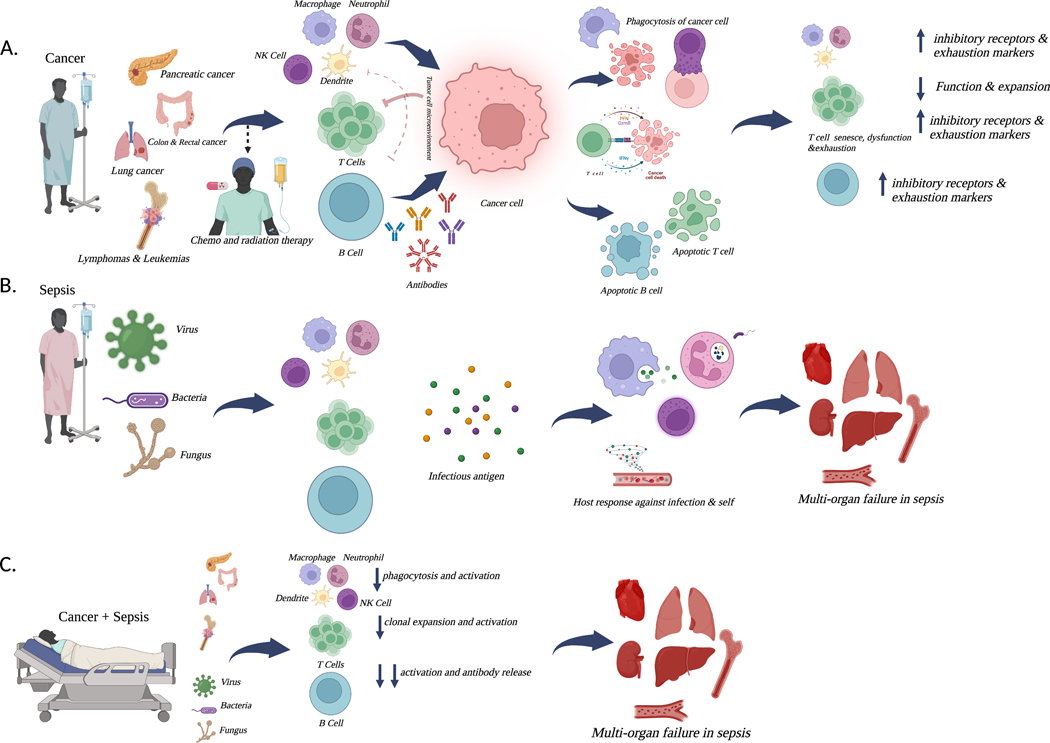
There are numerous opportunities, however, to potentially improve survival in this common patient population that unfortunately is associated with a very poor outcome. While research at preventing, curing or at least improving outcomes in cancer in isolation should theoretically decrease the incidence of cancer and sepsis, there should equally be an understanding that in the short-intermediate time frame, sepsis will continue to be a highly morbid complication in cancer patients. As such, parallel research from both the oncology community and the critical care community targeting translation of pre-clinical findings of cancer and sepsis to the bedside should be a priority. Further, mechanistic studies need to be performed to understand how the unique tumor microenvironment impacts outcomes from sepsis and how sepsis impacts subsequent tumor growth. Since neither cancer nor sepsis is a single unique disease, an approach of lumping all cancers (and all cancer therapy) and all septic patients into a single group will assuredly not result in successful therapy. It is self-evident that an in situ breast cancer does not carry the same risk as stage IV pancreatic cancer and that there are biologic differences between solid and hematologic malignancies. The same holds true in sepsis with different inciting organisms in hosts with different genetics, environments and multi-level omics. Understanding unique physiologic states induced by cancer in sepsis is a complex undertaking that lies within two distinct yet, at times, overlapping fields. Figure 2 represents a schematic of cancer, sepsis and the combination of both. A combination of better understanding of the host response at the bench and then undertaking clinical trials aimed at specific patient populations hold promise in the future for better, more personalized approaches in cancer and sepsis.
Figure 2. Pathophysiologic Insights: Sepsis, Cancer and the Immunologic Response.
Cancer (A) is a common and lethal disease. In the context of mortality from sepsis, risk varies significantly with cancer type, with pancreatic and lung cancer carrying the highest risk in solid tumors and hematologic malignancies also carrying a high risk. While ideally the immune system would robustly respond to a tumor, cancer cells can evade the immune system, allowing for a microenvironment that optimizes their survival and progression of cancer. Immune cells in the tumor microenvironment are also subjected to anti-inflammatory signals that decrease their effectiveness. The result is a maladaptive host response with increased exhaustion and decreased functionality in multiple immune cells. Sepsis (B) can be caused by a diverse array of microbes, including bacteria, fungi and viruses. Invading microbes lead to a host response, intended to contain the initiating infection. When this is unsuccessful, the host responsive can become maladaptive, resulting in cellular damage, ultimately leading to organ dysfunction. Cancer and sepsis (C) leads to higher mortality than sepsis in isolation and is responsible for a significant amount of mortality from cancer. Different tumor types are more susceptible to the development of sepsis, and the infections causing sepsis in cancer patients are more likely to be due anti-microbial resistant organisms. The presence of chronic immune dysfunction in cancer amplifies the acute dysregulated host response in sepsis, leading to increased organ dysfunction and elevated mortality. Created with BioRender.com.
Funding
This work was supported by funding from the National Institutes of Health [grant numbers GM148217, GM095442, and AI149274].
Abbreviations
- CI
confidence interval
- CLP
cecal ligation and puncture
- IL
interleukin
- OR
odds ratio
- TNF
tumor necrosis factor
Footnotes
CRediT Author Contribution
Jeroson C. Williams: Writing—original draft. Mandy L. Ford: Conceptualization, Writing—review & editing. Craig M. Coopersmith: Conceptualization, Writing—original draft.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Data Availability
Data sharing is not applicable.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M. et al. (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810, 2492881 [pii], 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno R, Rhodes A, Piquilloud L, Hernandez G, Takala J, Gershengorn HB et al. (2023) The Sequential Organ Failure Assessment (SOFA) Score: has the time come for an update? Crit. Care 27, 15, 10.1186/s13054-022-04290-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudd KE., Johnson SC., Agesa KM., Shackelford KA., Tsoi D., Kievlan DR. et al. (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395, 200–211, 10.1016/S0140-6736(19)32989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD and Finfer S. (2017) Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N. Engl. J. Med. 377, 414–417, 10.1056/NEJMp1707170 [DOI] [PubMed] [Google Scholar]
- 5.Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J. and Adam D. (2020) Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit. Care 24, 239, 10.1186/s13054-020-02950-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ et al. (2017) Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009–2014. JAMA 318, 1241–1249, 2654187 [pii], 10.1001/jama.2017.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh S, Mikkelsen ME, O’Connor M. and Bowles KH (2022) Why Sepsis Survivors Need an ICD-10 Code for “Sepsis Aftercare”. Chest 162, 979–981, 10.1016/j.chest.2022.06.011 [DOI] [PubMed] [Google Scholar]
- 8.Inoue S, Nakanishi N, Sugiyama J, Moriyama N, Miyazaki Y, Sugimoto T. et al. (2022) Prevalence and Long-Term Prognosis of Post-Intensive Care Syndrome after Sepsis: A Single-Center Prospective Observational Study. J. Clin. Med. 11, 5257–5270, 10.3390/jcm11185257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchman TG, Simpson SQ, Sciarretta KL, Finne KP, Sowers N, Collier M. et al. (2020) Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012–2018. Crit. Care Med. 48, 276–288, 10.1097/CCM.0000000000004224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith CM., French C. et al. (2021) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 49, e1063–e1143, 10.1097/CCM.0000000000005337 [DOI] [PubMed] [Google Scholar]
- 11.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS et al. (2017) Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 376, 2235–2244, 10.1056/NEJMoa1703058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy MM, Gesten FC, Phillips GS, Terry KM, Seymour CW, Prescott HC et al. (2018) Mortality Changes Associated with Mandated Public Reporting for Sepsis. The Results of the New York State Initiative. Am. J. Respir. Crit. Care Med. 198, 1406–1412, 10.1164/rccm.201712-2545OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R. et al. (2015) Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5-year study. Crit. Care Med. 43, 3–12, 10.1097/CCM.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 14.Santacruz CA, Pereira AJ, Celis E. and Vincent JL (2019) Which Multicenter Randomized Controlled Trials in Critical Care Medicine Have Shown Reduced Mortality? A Systematic Review. Crit. Care Med. 47, 1680–1691, 10.1097/CCM.0000000000004000 [DOI] [PubMed] [Google Scholar]
- 15.Maslove DM, Tang B, Shankar-Hari M, Lawler PR, Angus DC, Baillie JK et al. (2022) Redefining critical illness. Nat. Med. 28, 1141–1148, 10.1038/s41591-022-01843-x [DOI] [PubMed] [Google Scholar]
- 16.Wong HR, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT et al. (2015) Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am. J. Respir. Crit. Care Med. 191, 309–315, 10.1164/rccm.201410-1864OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davenport EE, Burnham KL, Radhakrishnan J, Humburg P, Hutton P, Mills TC et al. (2016) Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir. Med. 4, 259–271, S2213–2600(16)00046–1 [pii], 10.1016/S2213-2600(16)00046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scicluna BP, van Vught LA, Zwinderman AH, Wiewel MA, Davenport EE, Burnham KL et al. (2017) Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir. Med. 5, 816–826, 10.1016/S2213-2600(17)30294-1 [DOI] [PubMed] [Google Scholar]
- 19.Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z. et al. (2019) Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 321, 2003–2017, 10.1001/jama.2019.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhavani SV., Carey KA., Gilbert ER., Afshar M., Verhoef PA. and Churpek MM. (2019) Identifying Novel Sepsis Subphenotypes Using Temperature Trajectories. Am. J. Respir. Crit. Care Med. 200, 327–335, 10.1164/rccm.201806-1197OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhavani SV, Semler M, Qian ET, Verhoef PA, Robichaux C, Churpek MM et al. (2022) Development and validation of novel sepsis subphenotypes using trajectories of vital signs. Intensive Care Med. 48, 1582–1592, 10.1007/s00134-022-06890-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalantar KL, Neyton L, Abdelghany M, Mick E, Jauregui A, Caldera S. et al. (2022) Integrated host-microbe plasma metagenomics for sepsis diagnosis in a prospective cohort of critically ill adults. Nature Microbiology 7, 1805–1816, 10.1038/s41564-022-01237-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto K, Imasato M, Yamazaki Y, Tanaka H, Watanabe M, Eguchi H. et al. (2014) Claudin 2 deficiency reduces bile flow and increases susceptibility to cholesterol gallstone disease in mice. Gastroenterology 147, 1134.e10–1145.e10, 10.1053/j.gastro.2014.07.033 [DOI] [PubMed] [Google Scholar]
- 24.Shankaran V, Li L, Fedorenko C, Sanchez H, Du Y, Khor S. et al. (2022) Risk of Adverse Financial Events in Patients With Cancer: Evidence From a Novel Linkage Between Cancer Registry and Credit Records. J. Clin. Oncol. 40, 884–891, 10.1200/JCO.21.01636 [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez H, Hagerling C. and Werb Z. (2018) Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 32, 1267–1284, 10.1101/gad.314617.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim R, Emi M. and Tanabe K. (2005) Cancer cell immune escape and tumor progression by exploitation of anti-inflammatory and pro-inflammatory responses. Cancer Biol. Ther. 4, 924–933, 10.4161/cbt.4.9.2101 [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y., Shao Q. and Peng G. (2020) Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol. Immunol. 17, 27–35, 10.1038/s41423-019-0344-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danai PA, Moss M, Mannino DM and Martin GS (2006) The epidemiology of sepsis in patients with malignancy. Chest 129, 1432–1440, 10.1378/chest.129.6.1432 [DOI] [PubMed] [Google Scholar]
- 29.Rosolem MM, Rabello LS, Lisboa T, Caruso P, Costa RT, Leal JV et al. (2012) Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J. Crit. Care 27, 301–307, S0883–9441(11)00253-X [pii], 10.1016/j.jcrc.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 30.Mirouse A., Vigneron C., Llitjos JF., Chiche JD., Mira JP., Mokart D. et al. (2020) Sepsis and Cancer: An Interplay of Friends and Foes. Am. J. Respir. Crit. Care Med. 202, 1625–1635, 10.1164/rccm.202004-1116TR [DOI] [PubMed] [Google Scholar]
- 31.Glibetic N, Shvetsov YB, Aan FJ, Peplowska K, Hernandez BY and Matter ML (2022) Transcriptome profiling of colorectal tumors from patients with sepsis reveals an ethnic basis for viral infection risk and sepsis progression. Sci. Rep. 12, 20646, 10.1038/s41598-022-24489-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hensley MK., Donnelly JP., Carlton EF. and Prescott HC. (2019) Epidemiology and Outcomes of Cancer-Related Versus Non-Cancer-Related Sepsis Hospitalizations. Crit. Care Med. 47, 1310–1316, 10.1097/CCM.0000000000003896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel RL, Miller KD, Wagle NS and Jemal A. (2023) Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48, 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 34.Sharma A., Nguyen P., Taha M. and Soubani AO. (2021) Sepsis Hospitalizations With Versus Without Cancer: Epidemiology, Outcomes, and Trends in Nationwide Analysis From 2008 to 2017. Am. J. Clin. Oncol. 44, 505–511, 10.1097/COC.0000000000000859 [DOI] [PubMed] [Google Scholar]
- 35.Xiang MJ and Chen GL (2022) Impact of cancer on mortality rates in patients with sepsis: A meta-analysis and meta-regression of current studies. World J. Clin. Cases 10, 7386–7396, 10.12998/wjcc.v10.i21.7386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nazer L, Lopez-Olivo MA, Cuenca JA, Awad W, Brown AR, Abusara A. et al. (2022) All-cause mortality in cancer patients treated for sepsis in intensive care units: a systematic review and meta-analysis. Support. Care Cancer 30, 10099–10109, 10.1007/s00520-022-07392-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemiale V, Pons S, Mirouse A, Tudesq JJ, Hourmant Y, Mokart D. et al. (2020) Sepsis and Septic Shock in Patients With Malignancies: A Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique Study. Crit. Care Med. 48, 822–829, 10.1097/CCM.0000000000004322 [DOI] [PubMed] [Google Scholar]
- 38.Tao J, Seier K, Marasigan-Stone CB, Simondac JS, Pascual AV, Kostelecky NT et al. (2023) Delirium as a Risk Factor for Mortality in Critically Ill Patients With Cancer. JCO Oncol. Pract. 30, Op2200395, 10.1200/OP.22.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuenca JA, Manjappachar NK, Ram´ırez CM, Hernandez M, Martin P, Gutierrez C. et al. (2022) Outcomes and Predictors of 28-Day Mortality in Patients With Solid Tumors and Septic Shock Defined by Third International Consensus Definitions for Sepsis and Septic Shock Criteria. Chest 162, 1063–1073, 10.1016/j.chest.2022.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma C, Sun G, Yang X. and Yang S. (2023) A clinically applicable prediction model for the risk of in-hospital mortality in solid cancer patients admitted to intensive care units with sepsis. J. Cancer Res. Clin. Oncol. 149, 157–166, 10.1007/s00432-023-04661-x [DOI] [PubMed] [Google Scholar]
- 41.Caro J, Madero-Marroquin R, Zubizarreta N, Moshier E, Tremblay D, Coltoff A. et al. (2022) Impact of Fluoroquinolone Prophylaxis on Neutropenic Fever, Infections, and Antimicrobial Resistance in Newly Diagnosed AML Patients. Clin. Lymphoma Myeloma Leuk. 22, 903–911, 10.1016/j.clml.2022.08.001 [DOI] [PubMed] [Google Scholar]
- 42.Moreno-Sanchez F. and Gomez-Gomez B. (2022) Antibiotic Management of Patients with Hematologic Malignancies: From Prophylaxis to Unusual Infections. Curr. Oncol. Rep. 24, 835–842, 10.1007/s11912-022-01226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nanayakkara AK., Boucher HW., Fowler VG Jr, Jezek A, Outterson K. et al. (2021) Antibiotic resistance in the patient with cancer: Escalating challenges and paths forward. CA Cancer J. Clin. 71, 488–504, 10.3322/caac.21697 [DOI] [PubMed] [Google Scholar]
- 44.Danielsen AS, Franconeri L, Page S, Myhre AE, Tornes RA, Kacelnik O. et al. (2023) Clinical outcomes of antimicrobial resistance in cancer patients: a systematic review of multivariable models. BMC Infect. Dis. 23, 247, 10.1186/s12879-023-08182-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chambers LM, Kuznicki M, Yao M, Chichura A, Gruner M, Reizes O. et al. (2020) Impact of antibiotic treatment during platinum chemotherapy on survival and recurrence in women with advanced epithelial ovarian cancer. Gynecol. Oncol. 159, 699–705, 10.1016/j.ygyno.2020.09.010 [DOI] [PubMed] [Google Scholar]
- 46.Poonacha KNT., Villa TG. and Notario V. (2022) The Interplay among Radiation Therapy, Antibiotics and the Microbiota: Impact on Cancer Treatment Outcomes. Antibiotics (Basel) 11, 331, 10.3390/antibiotics11030331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chohan KL, Siegler EL and Kenderian SS (2023) CAR-T Cell Therapy: the Efficacy and Toxicity Balance. Curr. Hematol. Malig. Rep. 18, 9–18, 10.1007/s11899-023-00687-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gudiol C, Albasanz-Puig A, Cuervo G. and Carratala J. (2021) Understanding and Managing Sepsis in Patients With Cancer in the Era of` Antimicrobial Resistance. Front. Med. 8, 636547, 10.3389/fmed.2021.636547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fizza Haider S, Sloss R, Jhanji S, Nicholson E. and Creagh-Brown B. (2023) Management of adult patients with haematological malignancies in critical care. Anaesthesia 77, 263–276, 10.1111/anae.15955 [DOI] [PubMed] [Google Scholar]
- 50.Fox AC, Robertson CM, Belt B, Clark AT, Chang KC, Leathersich AM et al. (2010) Cancer causes increased mortality and is associated with altered apoptosis in murine sepsis. Crit. Care Med. 38, 886–893, 10.1097/CCM.0b013e3181c8fdb1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyons JD, Mittal R, Fay KT, Chen CW, Liang Z, Margoles LM et al. (2016) Murine Lung Cancer Increases CD4+ T Cell Apoptosis and Decreases Gut Proliferative Capacity in Sepsis. PloS ONE 11, e0149069, 10.1371/journal.pone.0149069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyons JD, Chen CW, Liang Z, Zhang W, Chihade DB, Burd EM et al. (2019) Murine Pancreatic Cancer Alters T Cell Activation and Apoptosis and Worsens Survival After Cecal Ligation and Puncture. Shock 51, 731–739, 10.1097/SHK.0000000000001203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santiago-Sánchez GS, Hodge JW and Fabian KP (2022) Tipping the scales: Immunotherapeutic strategies that disrupt immunosuppression and promote immune activation. Front. Immunol. 13, 993624, 10.3389/fimmu.2022.993624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dutta S, Ganguly A, Chatterjee K, Spada S. and Mukherjee S. (2023) Targets of Immune Escape Mechanisms in Cancer: Basis for Development and Evolution of Cancer Immune Checkpoint Inhibitors. Biology (Basel) 12, 218, 10.3390/biology12020218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E. et al. (2022) Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 386, 24–34, 10.1056/NEJMoa2109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F. et al. (2011) Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit. Care 15, R99, 10.1186/cc10112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan L, Chen Y, Han Y. and Tong C. (2022) Role of CD8(+) T cell exhaustion in the progression and prognosis of acute respiratory distress syndrome induced by sepsis: a prospective observational study. BMC Emerg. Med. 22, 182, 10.1186/s12873-022-00733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Q, Xue M, Song Q, Xie J, Yang Y. and Liu S. (2022) Expression of PD-1 on Memory T Lymphocytes Predicts 28-Day Mortality of Patients with Sepsis: A Prospective Observational Study. J. Inflamm. Res. 15, 5043–5052, 10.2147/JIR.S376897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y, Ding R, Chang Y, Li J. and Ma X. (2021) Immune checkpoint molecule TIGIT manipulates T cell dysfunction in septic patients. Int. Immunopharmacol. 101, 108205, 10.1016/j.intimp.2021.108205 [DOI] [PubMed] [Google Scholar]
- 60.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y. et al. (2009) PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. U. S. A. 106, 6303–6308, 06 [pii], 10.1073/pnas.0809422106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang T, Yu-Jing L. and Ma T. (2023) Role of regulation of PD-1 and PD-L1 expression in sepsis. Front Immunol. 14, 1029438, 10.3389/fimmu.2023.1029438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inoue S., Bo L., Bian J., Unsinger J., Chang K. and Hotchkiss RS. (2011) Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock 36, 38–44, 10.1097/SHK.0b013e3182168cce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen CW, Mittal R, Klingensmith NJ, Burd EM, Terhorst C, Martin GS et al. (2017) Cutting Edge: 2B4-Mediated Coinhibition of CD4(+) T Cells Underlies Mortality in Experimental Sepsis. J. Immunol. 199, 1961–1966, jimmunol.1700375 [pii], 10.4049/jimmunol.1700375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED et al. (2019) Immune Checkpoint Inhibition in Sepsis: A Phase 1b Randomized, Placebo-Controlled, Single Ascending Dose Study of Antiprogrammed Cell Death-Ligand 1 Antibody (BMS-936559). Crit. Care Med. 47, 632–642, 10.1097/CCM.0000000000003685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hotchkiss RS, Colston E, Yende S, Crouser ED, Martin GS, Albertson T. et al. (2019) Immune checkpoint inhibition in sepsis: a Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med. 45, 1360–1371, 10.1007/s00134-019-05704-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe E, Nishida O, Kakihana Y, Odani M, Okamura T, Harada T. et al. (2020) Pharmacokinetics, Pharmacodynamics, and Safety of Nivolumab in Patients With Sepsis-Induced Immunosuppression: A Multicenter, Open-Label Phase 1/2 Study. Shock 53, 686–694, 10.1097/SHK.0000000000001443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hotchkiss RS. and Moldawer LL. (2014) Parallels between cancer and infectious disease. N. Engl. J. Med. 371, 380–383, 10.1056/NEJMcibr1404664 [DOI] [PubMed] [Google Scholar]
- 68.Washburn ML, Wang Z, Walton AH, Goedegebuure SP, Figueroa DJ, Van Horn S. et al. (2019) T Cell- and Monocyte-Specific RNA-Sequencing Analysis in Septic and Nonseptic Critically Ill Patients and in Patients with Cancer. J. Immunol. 203, 1897–1908, 10.4049/jimmunol.1900560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie J, Robertson JM, Chen CW, Zhang W, Coopersmith CM and Ford ML (2018) Pre-existing malignancy results in increased prevalence of distinct populations of CD4+ T cells during sepsis. PloS ONE 13, e0191065, 10.1371/journal.pone.0191065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen CW., Xue M., Zhang W., Xie J., Coopersmith CM. and For ML. (2019) 2B4 but not PD-1 blockade improves mortality in septic animals with preexisting malignancy. JCI Insight 4, e127867, 10.1172/jci.insight.127867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin S, Zhang Y, Zhou F, Chen X, Sheng J. and Zhang J. (2022) TIGIT: A promising target to overcome the barrier of immunotherapy in hematological malignancies. Front Oncol. 12, 1091782, 10.3389/fonc.2022.1091782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rousseau A, Parisi C. and Barlesi F. (2023) Anti-TIGIT therapies for solid tumors: a systematic review. ESMO Open 8, 101184, 10.1016/j.esmoop.2023.101184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang D., Gu Y., Yan X., Huo C., Wang G., Zhao Y. et al. (2022) Role of CD155/TIGIT in Digestive Cancers: Promising Cancer Target for Immunotherapy. Front Oncol. 12, 844260, 10.3389/fonc.2022.844260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang W, Anyalebechi JC, Ramonell KM, Chen CW, Xie J, Liang Z. et al. (2021) TIGIT modulates sepsis-induced immune dysregulation in mice with preexisting malignancy. JCI Insight 6, 10.1172/jci.insight.139823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCarthy EF (2006) The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 26, 154–158 [PMC free article] [PubMed] [Google Scholar]
- 76.DeWeerdt S. (2013) Bacteriology: a caring culture. Nature 504, S4–S5, 10.1038/504S4a [DOI] [PubMed] [Google Scholar]
- 77.Roberts NJ, Zhang L, Janku F, Collins A, Bai RY, Staedtke V. et al. (2014) Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 6, 249ra111, 10.1126/scitranslmed.3008982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janku F, Zhang HH, Pezeshki A, Goel S, Murthy R, Wang-Gillam A. et al. (2021) Intratumoral Injection of Clostridium novyi-NT Spores in Patients with Treatment-refractory Advanced Solid Tumors. Clin. Cancer Res. 27, 96–106, 10.1158/1078-0432.CCR-20-2065 [DOI] [PubMed] [Google Scholar]
- 79.Liu Z, Mahale P. and Engels EA (2019) Sepsis and Risk of Cancer Among Elderly Adults in the United States. Clin. Infect. Dis. 68, 717–724, 10.1093/cid/ciy530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Danahy DB, Jensen IJ, Griffith TS and Badovinac VP (2019) Cutting Edge: Polymicrobial Sepsis Has the Capacity to Reinvigorate Tumor-Infiltrating CD8 T Cells and Prolong Host Survival. J. Immunol. 202, 2843–2848, 10.4049/jimmunol.1900076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cavassani KA, Carson W.Ft., Moreira AP, Wen H, Schaller MA, Ishii M. et al. (2010) The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood 115, 4403–4411, 10.1182/blood-2009-09-241083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen CW, Bennion KB, Swift DA, Morrow KN, Zhang W, Oami T. et al. (2021) Tumor-Specific T Cells Exacerbate Mortality and Immune Dysregulation during Sepsis. J. Immunol. 206, 2412–2419, 10.4049/jimmunol.2000865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vigneron C, Mirouse A, Merdji H, Rousseau C, Cousin C, Alby-Laurent F. et al. (2019) Sepsis inhibits tumor growth in mice with cancer through Toll-like receptor 4-associated enhanced Natural Killer cell activity. Oncoimmunology 8, e1641391, 10.1080/2162402X.2019.1641391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramonell KM., Zhang W., Hadley A., Chen CW., Fay KT., Lyons JD. et al. (2017) CXCR4 blockade decreases CD4+ T cell exhaustion and improves survival in a murine model of polymicrobial sepsis. PloS ONE 12, e0188882, 10.1371/journal.pone.0188882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang W, Chihade DB, Xie J, Chen CW, Ramonell KM, Liang Z. et al. (2020) Preexisting malignancy abrogates the beneficial effects of CXCR4 blockade during sepsis. J. Leukoc. Biol. 107, 485–495, 10.1002/JLB.3A1019-502R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM et al. (1999) Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 27, 1230–1251, 10.1097/00003246-199907000-00002 [DOI] [PubMed] [Google Scholar]
- 87.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG and Karl IE (1997) Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit. Care Med. 25, 1298–1307, 10.1097/00003246-199708000-00015 [DOI] [PubMed] [Google Scholar]
- 88.Hotchkiss RS, Dunne WM, Swanson PE, Davis CG, Tinsley KW, Chang KC et al. (2001) Pseudomonas aeruginosa pneumonia induces profound lymphocyte but not bronchial epithelial apoptosis. JAMA 287, 1716–1721 [Google Scholar]
- 89.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM and Karl IE (2005) Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J. Immunol. 174, 5110–5118, 10.4049/jimmunol.174.8.5110 [DOI] [PubMed] [Google Scholar]
- 90.Hotchkiss RS., Swanson PE., Knudson CM., Chang KC., Cobb JP., Osborne DF. et al. (1999) Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J. Immunol. 162, 4148–4156, 10.4049/jimmunol.162.7.4148 [DOI] [PubMed] [Google Scholar]
- 91.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P. et al. (2000) Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat. Immunol. 1, 496–501, 10.1038/82741 [DOI] [PubMed] [Google Scholar]
- 92.Coopersmith CM., Chang KC., Swanson PE., Tinsley KW., Stromberg PE., Buchman TG. et al. (2002) Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit. Care Med. 30, 195–201, 10.1097/00003246-200201000-00028 [DOI] [PubMed] [Google Scholar]
- 93.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Buchman TG et al. (2002) Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA 287, 1716–1721, 10.1001/jama.287.13.1716 [DOI] [PubMed] [Google Scholar]
- 94.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P. et al. (2000) Both poly-caspase and selective caspase 3 inhibitors prevent lymphocyte apoptosis and improve survival in sepsis – studies with pharmacologic agents and caspase 3−/− mice. Nat. Immunol, 1, 496–501,, 10.1038/82741 [DOI] [PubMed] [Google Scholar]
- 95.Schwulst SJ, Muenzer JT, Peck-Palmer OM, Chang KC, Davis CG, McDonough JS et al. (2008) Bim siRNA decreases lymphocyte apoptosis and improves survival in sepsis. Shock 30, 127–134, 10.1097/SHK.0b013e318162cf17 [DOI] [PubMed] [Google Scholar]
- 96.Fox AC., Breed ER., Liang Z., Clark AT., Zee-Cheng BR., Chang KC. et al. (2011) Prevention of lymphocyte apoptosis in septic mice with cancer increases mortality. J. Immunol. 187, 1950–1956, jimmunol.1003391 [pii], 10.4049/jimmunol.1003391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable.