Supplemental Digital Content is Available in the Text.
Key Words: bariatric surgery, Roux-en-Y gastric bypass, sleeve gastrectomy, antidepressant, escitalopram
Abstract
Background:
Changes in the gastrointestinal physiology after bariatric surgery may affect the pharmacokinetics of medications. Data on the impact of different surgical techniques on the pharmacokinetics of commonly prescribed antidepressants such as escitalopram are limited.
Methods:
This case-only prospective study investigated escitalopram-treated patients who underwent bariatric surgery at hospitals in Central Norway. Escitalopram concentrations were assessed using serial blood samples obtained during a dose interval of 24 hours preoperatively and at 1, 6, and 12 months, postoperatively. The primary outcomes were changes in the area under the time–concentration curve (AUC0-24) with secondary outcomes, including full pharmacokinetic profiling. We performed repeated-measures analysis of variance for the AUC0-24 and secondary outcomes.
Results:
Escitalopram-treated obese patients who underwent sleeve gastrectomy (n = 5) and Roux-en-Y gastric bypass (n = 4) were included. Compared with preoperative baseline, dose-adjusted AUC0-24 values were within ±20% at all time points, postoperatively in the sleeve gastrectomy and oux-en-Y gastric bypass groups, with the largest changes occurring 1 month postoperatively (+14.5 and +17.2%, respectively). No statistically significant changes in any pharmacokinetic variables over time were reported; however, there was a trend toward increased maximum concentrations after surgery (P = 0.069).
Conclusions:
Our findings suggest that bariatric surgery has no systematic effect on the pharmacokinetics of escitalopram. However, because of the substantial interindividual variation, therapeutic drug monitoring can be considered to guide postoperative dose adjustments.
INTRODUCTION
Epidemiologic data worldwide suggest that obesity is an emerging public health issue with alarming proportions.1 Based on current trends, it is estimated that by 2025, approximately one in 4 adults may suffer from obesity.1 Moreover, patients with severe mental illnesses, including major depression, are at an elevated risk of obesity; factors explaining this risk may include a sedentary lifestyle and an unhealthy diet.2 Affective symptoms2 and metabolic effects of several psychotropic medications may partially account for the increased prevalence of obesity in patients with severe mental illness.3
Despite the high prevalence of obesity in patients with severe mental illness, there are limited data on the effects of obesity on the pharmacokinetics of antidepressants,4 which are a core component of the mainstay treatment of several mental disorders. The effects of obesity on drug disposition vary substantially among antidepressants.5,6 Apart from the knowledge gap regarding the effects of obesity on the pharmacokinetics of numerous antidepressants, evidence regarding the effects of bariatric surgery on the pharmacokinetics of antidepressants is limited.7,8 Nevertheless, as bariatric surgery plays a considerable role in the treatment of morbid obesity,9 patients with psychiatric comorbidities and/or those undergoing antidepressant treatment are frequently referred for bariatric surgery.10,11 The 2 most commonly performed weight loss operations are the sleeve gastrectomy (SG) and the Roux-en-Y gastric bypass (RYGB).12 Over the past decade, there has been a relative increase in the number of SG, which now rank first in the United States, followed by RYGB.12 Both procedures are restrictive, whereas RYGB is also malabsorptive.13 Postoperative changes in gastric mixing, gastric pH, gastric emptying, gastrointestinal transit time, and presystemic metabolism by gut mucosal enzymes may underlie alterations in the bioavailability of drugs. These alterations may also be associated with changes in drug solubility and absorption in the gastrointestinal tract. Moreover, cytochrome P450 (CYP) enzymes 3A4 and 3A5, located in the wall of the proximal intestinal tract, may be less involved in the presystemic metabolism of antidepressants after RYGB14 because of the bypass of the duodenum during RYGB with ingested food and medications directly moving into the jejunum. Long-term pharmacokinetic changes are likely to occur as patients lose weight. For example, a reduction in adipose tissue mass may lead to the redistribution of lipophilic agents, such as psychotropic medications.15 In addition, because there seems to be an inverse relationship between body mass index (BMI) and CYP3A-activity, the oral bioavailability of CYP3A-substrates change during weight loss.16,17 Owing to the multitude of physiologic changes that occur after bariatric surgery, it may be difficult to predict their impact on the pharmacokinetics of antidepressants. This effect may be associated with perioperative changes in treatment response or safety.18
Escitalopram, the active S enantiomer of racemic citalopram, is one of the most commonly prescribed selective serotonin reuptake inhibitors.19 Escitalopram shows very high serotonin transporter selectivity20 and is indicated for the treatment of major depressive disorder in adults and adolescents, and for generalized anxiety disorder in adults.21 Escitalopram metabolism primarily involves CYP2C19-mediated N-demethylation,22 whereas CYP3A4 and CYP2D6 seem to be involved to a lesser extent.23,24 Although CYP2C19, CYP2D6, and CYP3A4 contribute to hepatic metabolism, only CYP3A4/5 is highly expressed in the intestinal mucosa.25
To the best of our knowledge, data on changes in escitalopram pharmacokinetics after bariatric surgery are limited, although escitalopram is a first-line antidepressant. Two cases of escitalopram-treated patients from a selective serotonin reuptake inhibitor-treated cohort undergoing RYGB have been reported.26 Here, the authors reported minimal changes in the area under the time–serum concentration curve (AUC) at one and 6 months postoperatively in one of the patients, whereas in the second patient, there was a >50% decrease in AUC, 1 month after RYGB, gradually increasing up to preoperative levels 6 months postoperatively. Two studies investigated the impact of bariatric surgery on the trough concentrations of escitalopram,27,28 although such measures may be more prone to chance variability than AUCs. In a cohort of 4 escitalopram-treated obese patients undergoing RYGB,27 decreased escitalopram concentrations were observed at 2 and 6 weeks postoperatively in all patients compared with preoperative levels; the decline ranged 4%–71%. In a more recent naturalistic study,28 dose-adjusted concentrations were relatively stable (decreased by approximately 20% on average) within 1 year postoperatively in a pooled group of 17 citalopram- or escitalopram-treated patients undergoing RYGB or SG.
The aim of our study was to assess the effects of RYGB and SG on escitalopram pharmacokinetics assessed at 1, 6, and 12 months postoperatively.
METHODS
Study Design and Population
This case-only prospective study investigated patients treated with escitalopram who underwent bariatric surgery for obesity between November 2016 and March 2022. Data were collected from the Centre for Obesity Research at St. Olav University Hospital, Trondheim, Norway. All patients underwent a psychiatric assessment as part of the multidisciplinary preoperative screening at the Obesity Clinic, were informed regarding the potential influence of the scheduled bariatric surgery on the pharmacokinetics of escitalopram, and were invited to participate in a pharmacokinetic study. The patient did not follow any dietary regimen. Patients who had previously undergone gastrointestinal tract resection were excluded. This pharmacokinetic platform study, “Changes in oral health and pharmacokinetics of drugs after bariatric surgery, BAR-MEDS” has been approved by the Regional Committee for Medical and Health Research Ethics in Mid Norway (ref. 2016/1145). The study was registered at www.clinicaltrials.gov (NCT03460379) and performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Serial blood samples were obtained preoperatively and at 1, 6, and 12 months, postoperatively. Blood samples were obtained at 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 12, and 24 hours after escitalopram administration. After centrifugation and pipetting, serum was stored at −80°C. Comedications with potential inhibitory or inducing effects on CYP isoenzyme activity were also recorded.
Drug Quantification
Serum escitalopram concentrations were analyzed using a validated, enantiomer-selective, ultra-high-performance supercritical fluid chromatography-tandem mass spectrometry (UHPSFC-MS/MS) method developed in our laboratory as described previously.29 Briefly, sample preparation before the UHPSFC-MS/MS analysis consisted of protein precipitation in acetonitrile and filtration through a phospholipid removal plate. This method used a UPC2 Trefoil CEL2 column and a mobile phase consisting of CO2 and methanol/acetonitrile (70:30, v/v) with 10 mmol/L ammonium acetate. MS/MS detection was performed with positive electrospray ionization and multiple reaction monitoring (m/z 325.1 > 262.0 and m/z 325.1 > 109.0). The calibration range was 5–500 nmol/L for R- and S-citalopram. The between-assay relative standard deviations were in the range of 3.4%–4.5%.
Pharmacokinetic Analyses
Before calculations were performed, all concentrations were adjusted to an intake of 10 mg/d; that is, when the dose was 15 mg/d, all measured concentrations were divided by 1.5, whereas when the dose was 20 mg/d, all measured concentrations were divided by 2. After this procedure, trough concentrations (C0, measured immediately before intake of the daily dose of escitalopram), maximum serum concentrations (Cmax), and the time to achieve Cmax (tmax) were obtained directly. Other pharmacokinetic variables were calculated using the pharmacokinetic program package (version 5.0; ThermoFisher Scientific, Waltham, MA).
The AUC from 0 to 24 hours (AUC0-24) was calculated using a mixed log linear model. The apparent clearance (Cl/F) was calculated as dose/AUC. By applying a noncompartmental model, the parameter estimate describing the decrease in log concentration (λz) was calculated using the best-fit log-linear regression line of the samples representing the elimination phase. The elimination half-life (t1/2) was calculated as ln2/λz. Apparent volume of distribution (Vd/F) was calculated as (Cl/F)/λz.
The patients were genotyped for CYP2C19, which is mainly involved in escitalopram metabolism,20 by allele-specific polymerase chain reaction. The inactivating variants *2, *3, and *4, as well as the *17 variant, which causes increased metabolism, were included in the test procedure.
Outcomes
Our primary outcomes were short- and long-term changes in systemic escitalopram exposure, as reflected by the AUC0-24. Other pharmacokinetic variables were considered secondary outcomes. Furthermore, we estimated the relative changes in percent from baseline for AUC0-24, Cmax, body weight, BMI, body fat, muscle mass, and visceral fat area. Body composition was measured using a multifrequency impedance analyzer (InBody 720, Seoul, Korea). Correlations between relative changes in AUC0-24 or Vd/F and relative changes in body weight, body fat, and BMI at the 12-month follow-up were investigated using Spearman rank correlation test. We performed repeated-measures analysis of variance for AUC0-24, CL/F, C0, Cmax, tmax, t1/2 and Vd/F using the Statistical Package for the Social Sciences software (IBM SPSS Statistics for Windows version 28.0, 2016; IBM Corp., Armonk, NY).
RESULTS
Nine escitalopram-treated obese patients were included; 5 underwent SG and 4 RYGB. The detailed demographic and clinical characteristics of the patients are summarized in Table 1. All the patients were Caucasian. Three patients, all from the SG group, had the genotype CYP2C19*1/*1; 3 patients, all from the RYGB group, had CYP2C19*1/*2; 2 patients (one each from the SG and RYGB groups) had CYP2C19*1/*17; and one patient from the SG group had CYP2C19*2/*17. AUC0-24 at baseline was 1442 versus 989 nmol/L × h in patients with and without the inactivating CYP2C19*2 allele, respectively, although we did not perform statistical comparisons because of the small number of patients.
TABLE 1.
Baseline Patient Characteristics
| Sleeve Gastrectomy (n = 5) | RYGB (n = 4) | |
| Age (yr), mean ± SD | 39.4 ± 8.2 | 38.5 ± 12.5 |
| Sex (female–male), n | 3–2 | 4–0 |
| Body weight (kg), mean ± SD | 122.0 ± 10.4 | 106.1 ± 11.5 |
| Body mass index (kg/m2), mean ± SD | 39.0 ± 2.8 | 39.3 ± 4.7 |
| CYP2C19 genotype (n) | ||
| *1/*1–*1/*17 | 3–1 | 0–1 |
| *1/*2–*2/*17 | 0–1 | 3–0 |
| Escitalopam dose (n)* | ||
| 10 mg/d–15 mg/d–20 mg/d | 2–0–3 | 2–1–1 |
| Co-medication with esomeprazole (n) | 1 | 1 |
| Serum creatinine (µmol/L), mean ± SD | 61.4 ± 8.0 | 64.0 ± 9.7 |
| Serum albumine (g/L), mean ± SD | 43.2 ± 1.7 | 42.5 ± 2.7 |
| Serum orosomucoid (g/L), mean ± SD | 0.82 ± 0.24 | 0.86 ± 0.13 |
| Serum ALAT (U/L), mean ± SD | 26.2 ± 13.7 | 22.8 ± 8.8 |
| Serum ASAT (U/L), mean ± SD | 24.6 ± 5.2 | 18.3 ± 4.6 |
| Serum gamma-GT (U/L), mean ± SD | 32.2 ± 15.9 | 33.5 ± 18.8 |
| Serum ALP (U/L), mean ± SD | 61.8 ± 13.9 | 65.8 ± 20.1 |
| Serum bilirubin, total (µmol/L), mean ± SD | 9.4 ± 4.1 | 7.8 ± 3.3 |
| Serum INR, mean ± SD | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Serum CRP (<5 –≥5 mg/L), n | 4–1 | 1–3 |
In the pharmacokinetic calculations, doses of 15 and 20 mg/d were adjusted to 10 mg/d.
ALAT, alanine aminotransferase; ALP, alkaline phosphatase; ASAT, aspartate aminotransferase; gamma-GT, gamma-glutamyltransferase; INR, international normalized ratio.
The key pharmacokinetic data are presented in Table 2. Data for 2 of the 4 RYGB patients were not available at 6 months, whereas for the other 2 concentrations, the data indicated that they were not in a steady state. Therefore, the 6-month results of the RYGB group were excluded. Compared with baseline, AUC0-24 values were within ±20% at 1 month postoperatively in the SG and the RYGB groups (+14.5% and +17.2%, respectively); at 6 months AUC0-24 values slightly declined in the SG group (−3.2% compared with baseline; no data available from the RYGB group) and remained relatively stable at 12 months AUC0-24 values in both groups (+10.9% in the SG and +3.9% in the RYGB groups, respectively, compared to baseline) (Fig. 1A). One month postoperatively, Cmax increased by 14.3% in the SG and by 23.8% in the RYGB group compared with baseline. The increase in Cmax was approximately the same 12 months postoperatively, with a 20.2% and a 14.5% increase from baseline values in the SG and the RYGB groups, respectively (Fig. 1B).
TABLE 2.
Pharmacokinetic and Body Composition Variables Preoperatively (Baseline) and at 1, 6, and 12 Months Postoperatively
| Preoperatively (Baseline) | 1 Month Postoperatively | 6 Months Postoperatively< | 12 Months Postoperatively | |||||
| Sleeve Gastrectomy | RYGB | Sleeve Gastrectomy | RYGB | Sleeve Gastrectomy | RYGB | Sleeve Gastrectomy | RYGB | |
| C0, nmol/L | 35.0 (30.0, 37.5) | 41.7 (26.2, 56.5) | 40.5 (30.0, 42.0) | 54.0 (36.0, 68.1) | 32.0 (31.0, 40.0) | NA* | 37.5 (35.0, 45.0) | 38.0 (31.8, 46.0) |
| Cmax, nmol/L | 57.0 (54.5, 63.0) | 68.5 (53.0, 86.7) | 68.0 (59.0, 70.0) | 88.0 (64.5, 110.1) | 62.0 (61.0, 64.5) | NA* | 68.0 (62.0, 68.5) | 74.2 (68.2, 88.0) |
| Cmax, % change | — | — | 14.3 (−6.3, 19.3) | 23.8 (16.3, 27.7) | 2.4 (−1.6, 13.1) | NA* | 20.2 (−1.6, 27.0) | 14.5 (−1.3, 32.9) |
| Cmax/C0 ratio | 1.6 (1.5, 1.6) | 1.9 (1.7, 2.0) | 1.7 (1.5, 1.7) | 1.7 (1.7, 1.8) | 1.7 (1.6, 1.9) | NA* | 1.8 (1.7, 1.8) | 2.0 (1.9, 2.2) |
| tmax, h | 3.0 (3.0,6.0) | 3.2 (2.5, 4.0) | 3.0 (2.5, 3.0) | 2.7 (2.4, 3.0) | 3.0 (3.0, 5.6) | NA* | 4.0 (3.0, 6.0) | 1.5 (1.5, 1.7) |
| AUC0-24, nmol/L x h | 1024 (1001, 1153) | 1301 (918, 1731) | 1210 (1146, 1291) | 1653 (1136, 2078) | 1096 (1069, 1104) | NA* | 1230 (1200, 1278) | 1178 (1092, 1412) |
| AUC0-24, % change† | — | — | 14.5 (−4.8, 17.0) | 17.2 (6.5, 26.1) | −3.2 (−7.3, 9.5) | NA* | 10.9 (−2.5, 40.9) | 3.9 (−6.0, 13.0) |
| t1/2, h | 31.2 (28.8, 36.4) | 28.7 (27.1, 35.7) | 36.7 (29.5, 40.2) | 28.7 (26.2, 36.2) | 30.1 (28.7, 31.7) | NA* | 33.1 (31.6, 34.1) | 26.4 (25.9, 27.4) |
| CL/F, mL/min | 502 (446, 513) | 431 (298, 560) | 424 (398, 448) | 332 (247, 465) | 469 (466, 481) | NA* | 418 (402, 428) | 437 (381, 474) |
| Vd/F, L | 1280 (1190, 1367) | 1330 (1140, 1382) | 1298 (1281, 1413) | 1055 (896, 1177) | 1285 (1187, 1294) | NA* | 1179 (1169, 1289) | 1043 (874, 1149) |
| Body weight (kg) | 119.0 (115.9, 127.6) | 109.4 (103.8, 111.8) | 105.6 (100.9, 112.3) | 97.1 (88.8, 102.8) | 86.0 (82.4, 103.6) | NA* | 84.4 (79.1, 100.9) | 78.7 (67.5, 86.3) |
| Body weight, % change† | — | — | −12.6 (−15.2, −12.0) | −11.0 (−14.0, −8.0) | −25.8 (−30.8, −21.5) | NA* | −29.1 (−31.7, −21.1) | −30.0 (−35.1, −24.6) |
| BMI, kg/m2 | 37.3 (36.6, 41.7) | 39.5 (35.3, 43.6) | 33.0 (32.8, 35.3) | 35.2 (30.4, 39.8) | 27.1 (26.5, 28.8) | NA* | 26.9 (25.7, 29.5) | 28.3 (26.5, 29.5) |
| BMI, % change | — | — | −12.0 (−15.3, −11.8) | −11.1 (−13.3, −9.0) | −26.0 (−30.0, −21.6) | NA* | −29.3 (−31.1, −20.8) | −30.4 (−35.0, −25.1) |
| Body fat, kg | 54.2 (43.9, 58.4) | 53.2 (46.4, 59.4) | 43.9 (36.2, 45,9) | 45.5 (37.6, 53.0) | 22.6 (20.2, 26.0) | NA* | 21.2 (21.1, 27.0) | 29.8 (23.9, 32.8) |
| Body fat, % change† | — | — | −19.0 (−21.4, −18.9) | −14.8 (−19.0, −10.7) | −54.0 (−55.5, −40.7) | NA* | −53.8 (−60.9, −36.8) | −49.0 (−56.9, −41.7) |
| Muscle mass, kg | 36.8 (33.8, 41.2) | 29.8 (27.1, 32.2) | 34.4 (30.4–39.4) | 27.6 (25.1, 29.3) | 34.7 (31.1, 36.8) | NA* | 33.7 (32.0, 35.4) | 26.2 (23.3, 29.0) |
| Muscle mass, % change† | — | — | −7.2 (−10.1, −6.5) | −8.1 (−9.7, −7.4) | −8.0 (−10.7, −7.2) | NA* | −8.4 (−14.1, −6.2) | −13.0 (−15.2, −9.6) |
| Visceral fat area, cm2 | 224.3 (201.0, 226.6) | 247.7 (232.7, 265.3) | 169.9 (169.8, 186.4) | 208.2 (196.4, 223.6) | 93.1 (86.3, 158.9) | NA* | 99.4 (80.9, 150.5) | 119.6 (93.9, 146.5) |
| Visceral fat area, % change† | — | — | −16.9 (−25.1, −16.6) | −15.9 (−17.7, −13.8) | −51.8 (−61.5, −32.4) | NA* | −55.7 (−59.7, −35.1) | −55.1 (−59.7, −47.7) |
All data are presented as medians, with interquartile ranges in parentheses. Data for 2 of the 4 patients with RYGB were not available at 6 months, whereas for the other 2 concentrations, the data indicated that they were not at a steady state.
Changes relative to baseline.
Vd/L, apparent volume of distribution; NA, not applicable.
FIGURE 1.
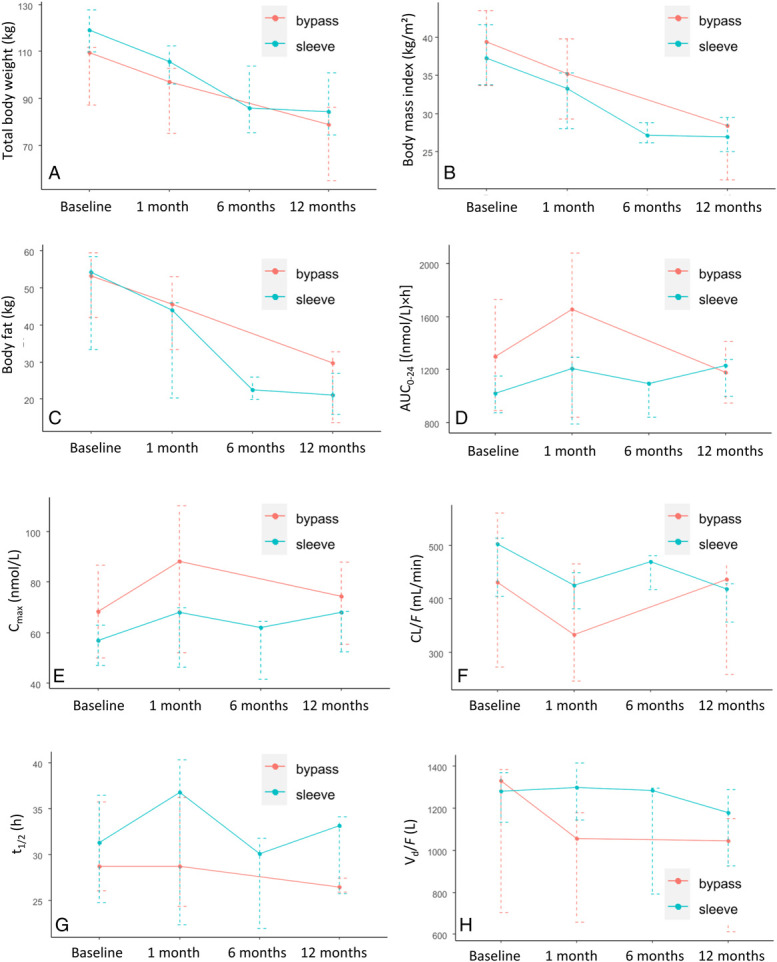
Total body weight (A), body mass index (B), body fat (C), AUC0-24 (D), Cmax (E), CL/F (F), t1/2 (G), and Vd/F (H) preoperatively (baseline) and 1, 6, and 12 months after bariatric surgery in 5 patients undergoing sleeve gastrectomy (“sleeve”) and 4 patients undergoing Roux-en-Y gastric bypass (“bypass”). Data are presented as medians with interquartile ranges.
The calculated % changes in body fat and weight, BMI, muscle mass, and visceral fat are shown in Table 2.
The repeated-measures analysis of variance model did not report any significant changes in AUC0-24, CL/F, C0, tmax, t1/2 and Vd/F (P = 0.39, P = 0.47, P = 0.42, P = 0.23, P = 0.40, and P = 0.12, respectively), but showed a trend for Cmax (P = 0.069). Changes in body weight and fat, BMI, CL/F, t1/2, and Vd/F over time are shown in Figures 1C–H.
One SG-treated patient used esomeprazole concomitantly throughout the study period, another RYGB-treated patient used esomeprazole at baseline and 1 month, and a third RYGB-treated patient used esomeprazole at 1 month. We present pharmacokinetic data for individual patients, including those comedicated with esomeprazole and those with the inactivating CYP2C19*2 variant in Supplemental Digital Content (see Supplementary Fig. 1a–f, http://links.lww.com/TDM/A677); however, we were unable to perform statistical analyses for comedication effects separately because of low power, because of the small size of the group.
We did not detect any correlation between relative changes for AUC0-24 or Vd/F and relative changes for body weight, fat, or BMI at 12-month follow-up (P-values ranging 0.16–0.64 for Spearman rank correlation).
DISCUSSION
The main finding in our prospective cohort of escitalopram-treated patients undergoing SG or RYGB was that these bariatric operations did not exert any substantial or predictable effect on escitalopram concentrations in the short- or long-term, at the group or individual level. This lack of pronounced changes may be explained by the activation of factors compensating for, for example, the expected decrease in absorption after RYGB treatment,30 and for weight loss. Although we did not detect any statistically significant changes, we observed a trend in Cmax changes. Interestingly, these changes may have followed somewhat different patterns between patients undergoing SG and RYGB; in those undergoing SG, Cmax was relatively stable at 1 and 6 months (+14.3 and +2.4%, respectively), followed by an increase 12 months postoperatively (+20.2%), whereas in the RYGB group, Cmax increased by 23.8% compared with baseline at 1 month and subsequently decreased to 14.5% higher than the baseline 12 months after RYGB. This may imply different adaptation processes in gastrointestinal physiology or anatomical changes after SG and RYGB,30 although it should be emphasized that none of the differences were statistically significant, and that the changes were small compared with the relatively wide therapeutic index of escitalopram.
SG may reduce gastric volume without inducing malabsorption, because no part of the small intestine is bypassed, and pyloric function remains preserved.31 Therefore, we did not expect any significant impact of SG on escitalopram pharmacokinetics, with the exception of increased Cmax and decreased Tmax. Previous evidence is limited, and we are aware of the findings of a study by Wallerstedt et al.28 The authors investigated a mixed cohort of patients who underwent RYGB or SG. However, it was not specified which patients were treated with which type of bariatric surgery; therefore, it was not possible to draw any conclusions on the effects of SG on escitalopram pharmacokinetics. Interpretation of their findings was further hindered by the pooling of citalopram- and escitalopram-treated patients. The authors reported no significant changes in citalopram/escitalopram dose-adjusted trough concentrations at postoperative follow-up.28 However, data on the effects of RYGB on the pharmacokinetics of escitalopram are limited. However, it is challenging to compare our findings with those of previous studies. Specifically, the use of different pharmacokinetic assessments and follow-up time windows did not provide a comprehensive overview of the effects of RYGB on escitalopram pharmacokinetics. Among previous publications, the study with the design closest to ours reported AUC changes in 2 escitalopram-treated patients out of an antidepressant-treated cohort undergoing RYGB at one, 6, and 12 months postoperatively.26 In one patient, AUC changes were negligible at follow-up assessments; in the second patient, AUC values were approximately halved in the first month, slowly increasing to almost reach preoperative levels at 6 months, and finally 30% increased at 12 months.26 A more recent study of 4 severely obese patients treated with RYGB suggested decreased trough concentrations of escitalopram, ranging 4%–71% of baseline concentrations at 2 and 6 weeks postoperatively.27 However, the AUC may be a more reliable measure of systemic exposure than single-concentration measurements. The interpretation of these findings is further complicated by the contrasting evidence on the effects of weight loss after RYGB on CYP2C19 activity; some studies have suggested increased activity,16 whereas others have indicated no major changes.32
In light of our findings and previous evidence, we hypothesized that pharmacokinetic changes after bariatric surgery may also present strong interindividual variations.28 Understanding the individual adaptation of gastrointestinal physiology postoperatively and its effects on drug bioavailability may require a better understanding of the potential confounders. For example, the role of comedication with inhibitory effects has been previously suggested,27 but remains unclear. In our cohort, we were unable to address potentially distinct patterns of pharmacokinetic alterations postoperatively in patients comedicated with esomeprazole, a CYP2C19 inhibitor shown to increase escitalopram concentrations by approximately 80%,33 because of an underpowered sample. CYP2C19 genotype may also be a factor that needs to be considered. Of the 4 patients with the highest AUC values at baseline in the outcohort, 3 had the inactivating CYP2C19*2 variant, of whom one was also treated with esomeprazole, whereas the fourth, with a normal genotype, used esomeprazole (Supplementary Table 1, Supplemental Digital Content, http://links.lww.com/TDM/A678). Co-medication with esomeprazole may deserve additional attention, as esomeprazole may have been prescribed for reflux symptoms.34 Postoperative improvement of reflux symptoms may affect the loss of stomach acids and rapid absorption of escitalopram,24 possibly contributing to changes in the bioavailability of escitalopram.
Furthermore, in our cohort, 75% and 20% of the RYGB and the SG groups had elevated preoperative C-reactive protein (CRP) concentrations. Immunomodulatory pathways may regulate the metabolic activity of hepatic CYP enzymes,35 and the downregulation of hepatic metabolism in the inflammatory state may, at least partially, account for considerable interindividual and intra-individual pharmacokinetic variation. Therefore, future research may be needed to control for the effects of inflammatory states that are common in obesity.36
Our study has several strengths. First, we performed a full pharmacokinetic analysis under steady-state conditions, and genotyping for the main CYP isoenzyme involved in escitalopram metabolism. Using 3 time points from 1 to 12 months postoperatively provided a comprehensive overview of the short- and long-term effects of 2 different types of bariatric surgery on escitalopram pharmacokinetics, although we were unable to investigate the possible effects occurring directly after surgery. We also included patients who underwent the 2 dominant surgical techniques: SG and RYGB. Finally, our cohort was well-characterized in postoperative changes in body composition.
However, our study has some limitations. Larger sample sizes would have enabled more precise conclusions regarding a specific type of surgery and better insight into the effects of comedications that can interfere with escitalopram metabolism. In particular, owing to the intraindividual and interindividual variability of escitalopram pharmacokinetics, the number of investigated patients was rather small. Furthermore, the clinical relevance of the investigated pharmacokinetic alterations remains unclear because we did not include information on clinical outcomes. Moreover, 3 patients received esomeprazole, a potential inhibitor of CYP2C19, and because of the small number of patients, we were not able to account for the interplay between esomeprazole and bariatric surgery on escitalopram pharmacokinetics. The small cohort size did not allow us to evaluate the impact of inflammation reflected by elevated CRP levels, which was observed in one patient in the SG group and in 3 patients in the RYGB group at baseline. Inflammation may affect CYP2C19 activity in these patients37,38; however, in patients with low CRP levels, the associated impact on escitalopram pharmacokinetics should be negligible. Although escitalopram generally exerts linear pharmacokinetics, it is unknown whether the situation is the same for low-grade inflammation or during comedication with CYP2C19 inhibitors. Thus, there is a risk that applying dose-normalized escitalopram levels to subjects who use doses higher than 10 mg/d may not provide accurate concentration data.
Considering the large interindividual and intra-individual pharmacokinetic variations in escitalopram after bariatric surgery, which in some cases also cause therapeutic failure,26 integrating regular assessments of serum or plasma concentrations into postoperative care may provide guidance regarding the need for dose adjustments aimed at optimizing drug therapy.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to the The Joint Research Committee at St. Olav University Hospital and the Faculty of Medicine, Norwegian University of Science and Technology for funding this project. The authors also thank the nurses at the Centre for Obesity Research, St. Olav University Hospital, for their contributions.
Footnotes
Supported by the FFU and Joint Research Committee at St. Olav University Hospital and the Faculty of Medicine, Norwegian University of Science and Technology.
The authors have no competing interests to declare. G. Schoretsanitis has served as a consultant for HLS Therapeutics and Thermo Fisher Scientific and has received speaker fees from HLS Therapeutics. The authors have no competing interests to declare.
This study was approved by the Regional Committee for Medical and Health Research Ethics in Mid-Norway (Ref. 2016/1145).
All patients provided written informed consent before commencement of the study and all patients provided written informed consent before the study.
The dataset generated and analyzed in the current study is available from the corresponding author upon reasonable request.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.drug-monitoring.com).
O. Spigset, A. Helland, and M. Strømmen conceived and/or designed the study. M. Strømmen and H-M. Krabseth performed data collection and organization. O. Spigset, A. Helland, and G. Schoretsanitis analyzed the data and contributed models. The first draft of the manuscript was written by G. Schoretsanitis, and all authors commented on previous versions of the manuscript. All the authors have read and approved the final version of the manuscript.
Contributor Information
Magnus Strømmen, Email: magnus.strommen@stolav.no.
Hege-Merete Krabseth, Email: hege-merete.krabseth@stolav.no.
Arne Helland, Email: arne.helland@stolav.no.
Olav Spigset, Email: olav.spigset@legemidler.no.
REFERENCES
- 1.Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correll CU, Detraux J, De Lepeleire J, et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14:119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warrings B, Samanski L, Deckert J, et al. Impact of body mass index on serum concentrations of antidepressants and antipsychotics. Ther Drug Monit. 2021;43:286–291. [DOI] [PubMed] [Google Scholar]
- 5.Greenblatt DJ, Harmatz JS, Chow CR. Vortioxetine disposition in obesity: potential implications for patient safety. J Clin Psychopharmacol. 2018;38:172–179. [DOI] [PubMed] [Google Scholar]
- 6.Schoretsanitis G, Haen E, Hiemke C, et al. Sex and body weight are major determinants of venlafaxine pharmacokinetics. Int Clin Psychopharmacol. 2018;33:322–329. [DOI] [PubMed] [Google Scholar]
- 7.Coughlin JW, Steffen KJ, Sockalingam S, et al. Psychotropic medications in metabolic and bariatric surgery: research updates and clinical considerations. Curr Psychiatry Rep. 2022;24:89–98. [DOI] [PubMed] [Google Scholar]
- 8.Roerig JL, Steffen KJ, Zimmerman C, et al. A comparison of duloxetine plasma levels in postbariatric surgery patients versus matched nonsurgical control subjects. J Clin Psychopharmacol. 2013;33:479–484. [DOI] [PubMed] [Google Scholar]
- 9.Loh HH, Francis B, Lim LL, et al. Improvement in mood symptoms after post-bariatric surgery among people with obesity: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2021;37:e3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbuti M, Brancati GE, Calderone A, et al. Prevalence of mood, panic and eating disorders in obese patients referred to bariatric surgery: patterns of comorbidity and relationship with body mass index. Eat Weight Disord. 2022;27:1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sockalingam S, Leung SE, Wnuk S, et al. Psychiatric management of bariatric surgery patients: a review of psychopharmacological and psychological treatments and their impact on postoperative mental health and weight outcomes. Psychosomatics. 2020;61:498–507. [DOI] [PubMed] [Google Scholar]
- 12.American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011–2020. Newberry, FL: American Society for Metabolic and Bariatric Surgery; 2023. [Google Scholar]
- 13.Colquitt J, Clegg A, Loveman E, et al. Surgery for morbid obesity. Cochrane Database Syst Rev. 2005(4):CD003641. [DOI] [PubMed] [Google Scholar]
- 14.Paine MF, Khalighi M, Fisher JM, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther. 1997;283:1552–1562. [PubMed] [Google Scholar]
- 15.Quercia I, Dutia R, Kotler DP, et al. Gastrointestinal changes after bariatric surgery. Diabetes Metab. 2014;40:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kvitne KE, Krogstad V, Wegler C, et al. Short- and long-term effects of body weight, calorie restriction and gastric bypass on CYP1A2, CYP2C19 and CYP2C9 activity. Br J Clin Pharmacol. 2022;88:4121–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kvitne KE, Robertsen I, Skovlund E, et al. Short- and long-term effects of body weight loss following calorie restriction and gastric bypass on CYP3A-activity - a non-randomized three-armed controlled trial. Clin Transl Sci. 2022;15:221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bingham K, Hawa R, Sockalingam S. SSRI discontinuation syndrome following bariatric surgery: a case report and focused literature review. Psychosomatics. 2014;55:692–697. [DOI] [PubMed] [Google Scholar]
- 19.Eek E, van Driel M, Falk M, et al. Antidepressant use in Australia and Sweden-A cross-country comparison. Pharmacoepidemiol Drug Saf. 2021;30:409–417. [DOI] [PubMed] [Google Scholar]
- 20.Eichentopf L, Hiemke C, Conca A, et al. Systematic review and meta-analysis on the therapeutic reference range for escitalopram: blood concentrations, clinical effects and serotonin transporter occupancy. Front Psychiatry. 2022;13:972141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allergan USA Inc. Lexapro® (Escitalopram) Tablets, for Oral Use. Madison, NJ: Allergan; 2020. [Google Scholar]
- 22.Huezo-Diaz P, Perroud N, Spencer EP, et al. CYP2C19 genotype predicts steady state escitalopram concentration in GENDEP. J Psychopharmacol. 2012;26:398–407. [DOI] [PubMed] [Google Scholar]
- 23.von Moltke LL, Greenblatt DJ, Giancarlo GM, et al. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab Dispos. 2001;29:1102–1109. [PubMed] [Google Scholar]
- 24.Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46:281–290. [DOI] [PubMed] [Google Scholar]
- 25.Thelen K, Dressman JB. Cytochrome P450-mediated metabolism in the human gut wall. J Pharm Pharmacol. 2009;61:541–558. [DOI] [PubMed] [Google Scholar]
- 26.Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marzinke MA, Petrides AK, Steele K, et al. Decreased escitalopram concentrations post-Roux-en-Y gastric bypass surgery. Ther Drug Monit. 2015;37:408–412. [DOI] [PubMed] [Google Scholar]
- 28.Wallerstedt SM, Nylen K, Axelsson MAB. Serum concentrations of antidepressants, antipsychotics, and antiepileptics over the bariatric surgery procedure. Eur J Clin Pharmacol. 2021;77:1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegstad S, Havnen H, Helland A, et al. Enantiomeric separation and quantification of citalopram in serum by ultra-high performance supercritical fluid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1061-1062:103–109. [DOI] [PubMed] [Google Scholar]
- 30.Steenackers N, Vanuytsel T, Augustijns P, et al. Adaptations in gastrointestinal physiology after sleeve gastrectomy and Roux-en-Y gastric bypass. Lancet Gastroenterol Hepatol. 2021;6:225–237. [DOI] [PubMed] [Google Scholar]
- 31.Stefater MA, Wilson-Perez HE, Chambers AP, et al. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev. 2012;33:595–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lloret-Linares C, Daali Y, Abbara C, et al. CYP450 activities before and after Roux-en-Y gastric bypass: correlation with their intestinal and liver content. Surg Obes Relat Dis. 2019;15:1299–1310. [DOI] [PubMed] [Google Scholar]
- 33.Gjestad C, Westin AA, Skogvoll E, et al. Effect of proton pump inhibitors on the serum concentrations of the selective serotonin reuptake inhibitors citalopram, escitalopram, and sertraline. Ther Drug Monit. 2015;37:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hefner G, Shams ME, Unterecker S, et al. Inflammation and psychotropic drugs: the relationship between C-reactive protein and antipsychotic drug levels. Psychopharmacology (Berl). 2016;233:1695–1705. [DOI] [PubMed] [Google Scholar]
- 36.Aronson D, Bartha P, Zinder O, et al. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int J Obes Relat Metab Disord. 2004;28:674–679. [DOI] [PubMed] [Google Scholar]
- 37.Veringa A, Ter Avest M, Span LF, et al. Voriconazole metabolism is influenced by severe inflammation: a prospective study. J Antimicrob Chemother. 2017;72:261–267. [DOI] [PubMed] [Google Scholar]
- 38.van den Born DA, Martson AG, Veringa A, et al. Voriconazole exposure is influenced by inflammation: a population pharmacokinetic model. Int J Antimicrob Agents. 2023;61:106750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


