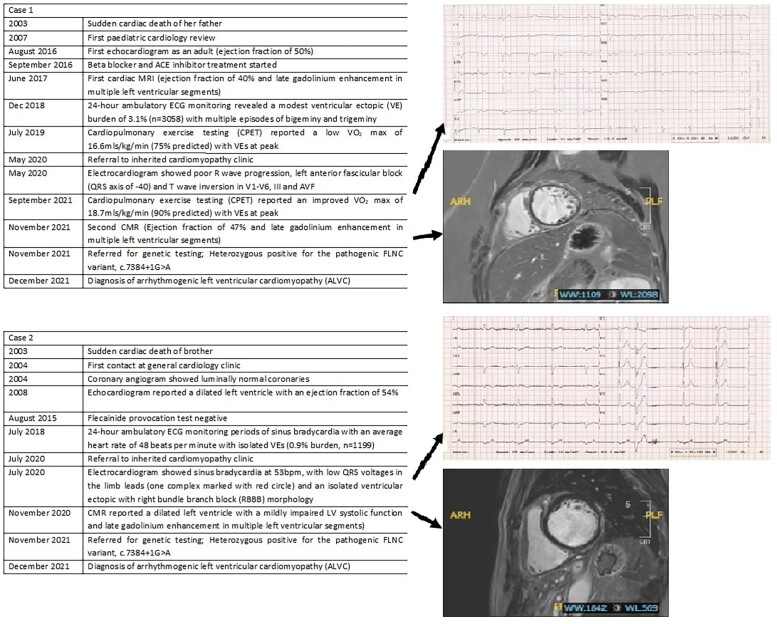
Abstract
Background
Arrhythmogenic left ventricular cardiomyopathy (ALVC) is a left ventricle–dominant arrhythmogenic cardiomyopathy (ACM) subtype often associated with malignant ventricular arrhythmias, left ventricular (LV) scar and sudden cardiac death. Awareness about LV involvement is now on the rise. The diagnosis relies on structural abnormalities on cardiac magnetic resonance (CMR) imaging and known ACM-causing genetic mutations.
Case summary
A 28-year-old lady (Case 1) was referred for cardiac screening after her father passed away suddenly. Her paternal uncle (Case 2) had been diagnosed with supposed dilated cardiomyopathy prior to referral. Both cases were worked up extensively with an electrocardiogram (ECG), 24-h ambulatory ECG monitor, exercise testing, and CMR imaging. Investigations of Case 1 showed T-wave inversion in the infero-lateral leads and a ventricular ectopic burden of 3% on ambulatory monitoring. Cardiac magnetic resonance imaging revealed moderately reduced LV systolic function (ejection fraction of 40%) with circumferential macroscopic fibrosis. Her uncle (Case 2) also had an impaired and dilated ventricle with extensive scar on CMR. Following the recent introduction of a cardiogenetic service in our unit, both were heterozygous for a pathogenic Filamin-C variant (c.7384+1G>A). Based on CMR findings and genetic results, the diagnosis of both patients was deemed to be ALVC. After years of surveillance, Patient 1 now has an implantable cardioverter defibrillator (ICD) indication.
Discussion
The importance of diagnosing patients with ACM lies in the predisposition to sudden cardiac death. Gene-specific treatment algorithms in ACM may alter management strategies, including ICD implantation as primary prevention. An in-depth multidisciplinary discussion and respecting patient autonomy are key factors in any decision pertaining to ICD implantation.
Keywords: Arrhythmogenic cardiomyopathy, Arrhythmogenic left cardiomyopathy (ALVC), Sudden cardiac death, Genetic testing, Inherited cardiomyopathies, Filamin-C, Case series
Learning points.
Arrhythmogenic cardiomyopathy (ACM) is a myocardial disorder that is not due to ischaemic, valvular heart, or hypertensive disease and has a predisposition to arrhythmias and sudden cardiac death.
Left ventricular involvement is a common finding in ACM, linked to several genetic mutations.
A cardiogenetic service is crucial in the management of inherited cardiac conditions as it confirms a genetic diagnosis, is useful for phenotypic characterization, may help evaluate cardiovascular risk, and alters therapeutic strategies. Filamin-C is a typical example, with lower thresholds for ICD implantation.
Introduction
Arrhythmogenic cardiomyopathy (ACM) is an arrhythmogenic condition of the myocardium that is not due to ischaemic, valvular heart, or hypertensive disease.1 Arrhythmogenic left ventricular cardiomyopathy (ALVC) is a left ventricular (LV)–dominant ACM subtype that has been increasingly recognized as a distinct phenotype over the past years. The diagnosis of ALVC is clinically challenging. The recently published Padua criteria have provided an approach towards identifying the condition that involves LV structural myocardial abnormalities on cardiac magnetic resonance (CMR) imaging and the presence of ACM-causing gene mutations.2 Several gene mutations have been linked to ALVC. Mutations in Filamin-C (FLNC) have particularly been shown to confer substantial arrhythmic risk, hence the lower thresholds for implantable cardioverter defibrillator (ICD) implantation.3
We present two FLNC variant positive ALVC cases from the same family diagnosed following the unexpected death of a previously healthy 36-year-old relative in his sleep. Post-mortem examination revealed a previously undiagnosed cardiomyopathy with biventricular hypertrophy.
Summary figure
Case 1
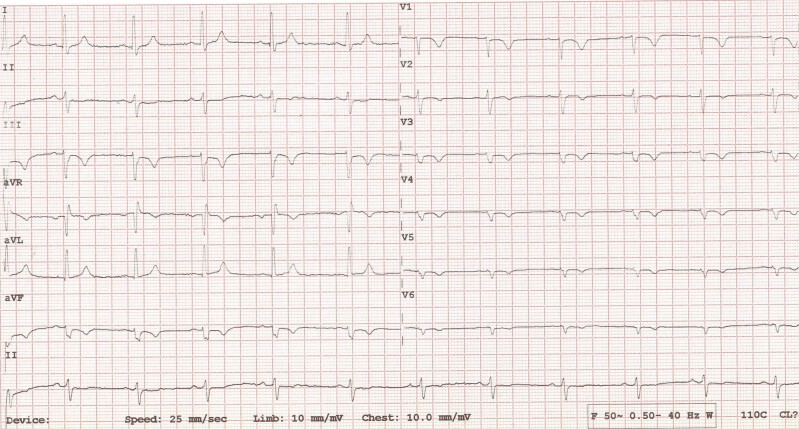
A 28-year-old female was referred to the inherited cardiomyopathy clinic for screening following the unexpected passing of her father. Cardiac genetic testing was not available at the time of the deceased passing, making it harder to establish a genotype–phenotype correlation. She was initially labelled with a diagnosis of a hypokinetic non-dilated cardiomyopathy (DCM) from the age of 23 years. The diagnosis was based on investigations including electrocardiogram (ECG), transthoracic echocardiogram (TTE), 24-h ambulatory ECG, CMR, and cardiopulmonary exercise test (CPET). Electrocardiogram showed poor R-wave progression, left anterior fascicular block (QRS axis of −40), low QRS voltages in the precordial leads, and T-wave inversion in V1–V6, III, and AVF (Figure 1). Her first TTE showed mildly reduced LV systolic function of 46% (based on Simpson’s biplane). A 24-h ambulatory ECG monitor recorded a modest ventricular ectopic (VE) burden of 3.1% (n = 3058), without any malignant arrhythmias. Cardiac magnetic resonance imaging reported LV systolic dysfunction with an ejection fraction (EF) of 40% and normal LV wall thickness of 8 mm. There was circumferential mid-wall and subepicardial late gadolinium enhancement (LGE) in the basal to mid-ventricular LV free wall segments, consistent with a non-ischaemic aetiology. Coronary angiography was not performed in this case, and the presence of fibrosis in a non-ischaemic distribution effectively ruled out coronary artery disease as a plausible cause for LV dysfunction in this clinical scenario. Cardiopulmonary exercise test reported a low VO2 max of 16.6 mL/kg/min [75% of predicted maximum for age, body surface area (BSA), and ethnicity] with VEs at peak (including two couplets).
Figure 1.
Electrocardiogram of Patient 1 showing poor R-wave progression, left anterior fascicular block (QRS axis of −40), and T-wave inversion in V1–V6, III, and AVF.
During her first visit in the clinic, the patient had a New York Heart Association (NYHA) score of 1 and did not complain of any cardiac symptoms. The patient was leading an active lifestyle, engaging in frequent exercise routines such as Zumba and circuit training with no symptoms of note. She was employed in accounting and was predominantly sedentary during her working hours. Examination was unremarkable, with normal vesicular breath sounds, normal first and second heart sounds with no added sounds, and no lower limb oedema. She has a regular pulse at 80 b.p.m. with a non-invasive blood pressure of 110/70 mmHg. Her body mass index (BMI) was 39 kg/m2 categorizing her within the obese range. She was being optimized on anti-heart failure therapy including enalapril 10 mg at night, carvedilol 12.5 mg twice daily, and empagliflozin 10 mg daily, which subsequently led to significant improvement in LVEF (50%) on TTE. Further up-titration was limited due to hypotension. Her N-terminal pro-brain natriuretic peptide (NT-proBNP) was 76 pg/mL (normal range: 5–125 pg/mL). A repeat CPET and CMR and referral for genetic testing were organized at a follow-up visit as a cardiogenetic service was unfortunately unavailable during her initial evaluation in the preceding years.
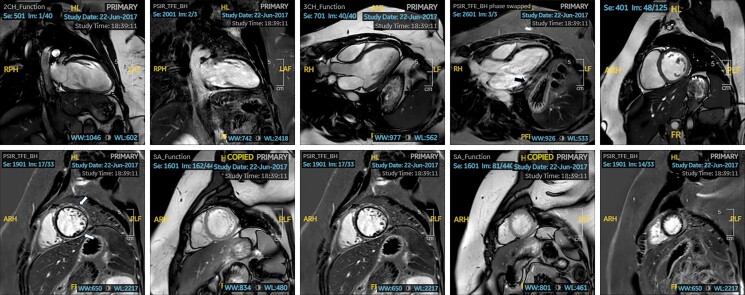
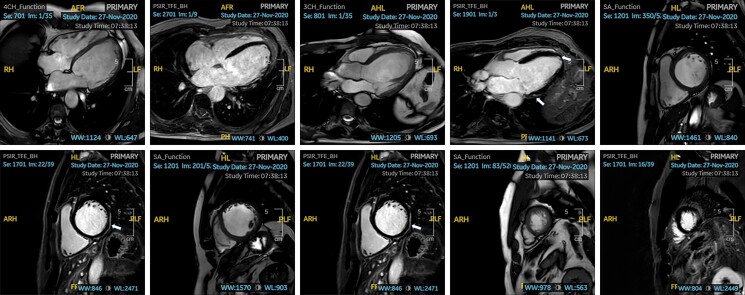
Repeat CMR reported normal LV dimensions with an improved systolic function (LVEF 47%). Thin subepicardial to mid-myocardial linear LGE was demonstrated in all LV basal segments on CMR, as well as the mid-ventricular anterior, anterolateral, and inferior walls (Figure 2A–J). The degree of scar was similar to the previous CMR. Right ventricular (RV) size and global systolic function were normal. Cardiopulmonary exercise test showed an improved VO2 max of 18.7 mL/kg/min (90% of predicted maximum for age, BSA, and ethnicity) with some isolated VEs at peak exercise.
Figure 2.
(A–J) Cine imaging and phase-sensitive inversion recovery cardiac magnetic resonance imaging sequence of Patient 1 in various planes, demonstrating thin subepicardial to mid-myocardial linear late gadolinium enhancement in all left ventricle basal segments (arrows).
Informed consent for genotyping was acquired. The GeneStudio S5™ system was used to sequence the DNA libraries, and 195 genes were included (see Supplementary material online, Appendix S1). Variants were interpreted according to the latest American College of Medical Genetics and Genomics (ACMG) guidelines.4 The patient was found to harbour a pathogenic splice site variant in FLNC, c.7384+1G>A (heterozygous). This was a novel variant, and studies to assess the functional impact of this variant were thus not available. The amino acid change was however expected to disrupt RNA splicing, hence resulting in an altered protein-coding sequence (PVS1: very strong). In silico analyses also resulted in pathogenic computational verdicts (PP3: supporting). The case was subsequently discussed at our monthly cardiogenetic multidisciplinary team meeting. All members agreed that there were sufficient grounds to label the proband with a FLNC ALVC phenotype.
After years of surveillance, the patient now has a FLNC cardiomyopathy diagnosis. Typical ECG changes, diffuse scar on CMR, absence of overt LV dysfunction, and family history of sudden cardiac death (SCD) lead to an ALVC diagnosis, instead of DCM. She now fulfils criteria for an ICD insertion, especially in the context of a family history of SCD.2 There is strong evidence to support exercise restriction in such patients.1,5 Individuals who engage in high-intensity physical activity may manifest more overt disease expressivity, and disease progression may also progress at a quicker rate. She was thus advised to refrain from these activities and to limit herself to mild–moderate aerobic training. Family planning and the possibility of disease progression during pregnancy were discussed at length. First-degree family members have been invited to undergo clinical and genetic screening. She was referred for ICD implantation, as per the latest European Society of Cardiology recommendations (2022) and the Heart Rhythm Society (HRS) consensus document for Arrhythmogenic Cardiomyopathy (2019) (Class IIA Recommendation).1,6 She was also made aware of psychology facilities should she want to avail of the services. She presented with non-anginal chest pain in the interim, with a long non-sustained ventricular tachycardia (NSVT) episode picked up incidentally on ambulatory monitoring. Implantable cardioverter defibrillator implantation was expedited, and she is now undergoing follow-up in clinic. Only short NSVTs have been noted on ICD interrogations over a 1-year follow-up, with none requiring any ICD therapies. The patient remained well in NYHA Class 1.
Case 2
A 61-year-old gentleman was referred for cardiac screening as his brother had died suddenly at the age of 36. The referral coincided with the referral of Case 1, the current case’s niece. This patient had been extensively evaluated prior to referral in a general cardiology clinic. He had been diagnosed with probable DCM 12 years prior to the index referral. Regular TTEs demonstrated a stable EF of 45–50% over the years. He had luminally normal coronaries and a negative flecainide challenge test. A 24-h ambulatory ECG monitor had revealed prolonged periods of sinus bradycardia with an average heart rate of 48 b.p.m. with isolated VEs (0.9% burden, n = 1199) harbouring a right bundle branch block (RBBB) morphology.
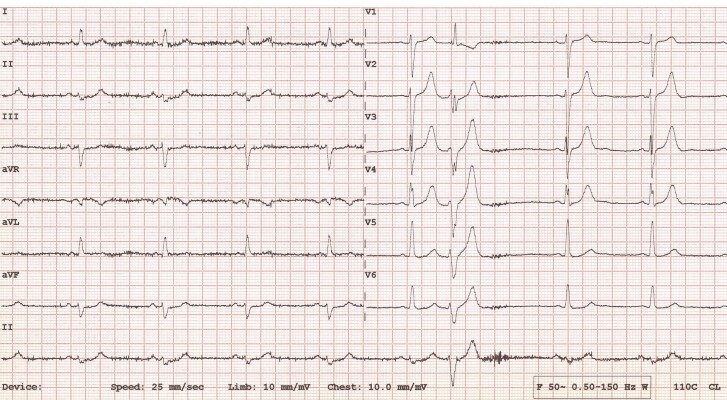
At the time of referral, the patient was on perindopril 4 mg twice daily, carvedilol 6.25 mg twice daily, and simvastatin 20 mg at night. He was asymptomatic and in NYHA functional Class 1. He had no history of syncope, palpitations, or dizziness. He worked in an office, with a moderately active lifestyle. Physical examination was normal, with clear breath sounds, no added heart sounds, and no signs of lower limb oedema. He had a regular pulse at 70 b.p.m. with a normal non-invasive blood pressure of 128/78 mmHg. N-terminal pro-brain natriuretic peptide was within normal limits (60 pg/mL, normal range 5–125 pg/mL), and an ECG showed sinus bradycardia at 53 b.p.m., with low QRS voltages in the limb leads and an isolated VE with RBBB morphology (Figure 3).
Figure 3.
Electrocardiogram of Patient 2 showing sinus bradycardia at 53 b.p.m., with low QRS voltages in the limb leads and an isolated ventricular ectopic with right bundle branch block (RBBB) morphology.
A CMR showed a dilated LV (indexed end-diastolic volume 118 mL/m2) with a mildly impaired LV systolic function (LVEF 50%). Right ventricular size and systolic function were normal. Fairly extensive mid-myocardial and subepicardial LGE in the basal to mid-inferior and infero-lateral segments of the LV was reported (Figure 4A–J). The patient was referred for genetic testing and tested heterozygous positive for the same pathogenic FLNC variant as his niece (Case 1).
Figure 4.
(A–J) Cine imaging and phase-sensitive inversion recovery cardiac magnetic resonance imaging sequence of Patient 2 in various planes demonstrating fairly extensive mid-myocardial and subepicardial late gadolinium enhancement in the basal to mid-inferior and infero-lateral segments of the left ventricle (arrows).
As per the HRS consensus published in 2019, Case 2 did not fulfil criteria for ICD implantation at the time, although he has one major risk factor (SCD in the proband).1,2 He was offered a programmed electrical stimulation or an ICD as per current guidelines,6 but he regretfully declined both. He also turned down an implantable loop recorder. A 7-day ambulatory ECG monitor and CPET were requested. Aerobic exercise capacity was normal on CPET (VO2 max 25.7 mL/kg/min, representing 87% of predicted maximum for age, BSA, and ethnicity) with an increased VE burden at peak exercise, including isolated VEs and two triplets. Repeat ambulatory monitor is currently pending, and the patient is presently under follow-up in clinic for over 1 year. The patient remains asymptomatic from cardiology point of view, with a good exercise tolerance in NYHA Class 1.
Discussion
We report a FLNC cardiomyopathy family with a strong history of SCD. Previously presumed to be medically optimized, genetic testing has now led to a clinical and genetic diagnosis. As per current recommendations, Case 1 now fulfilled criteria for ICD implantation, after years of surveillance.1,6
Arrhythmogenic cardiomyopathy is an umbrella term that refers to an arrhythmogenic condition of the myocardium that is not due to ischaemic, valvular heart, or hypertensive disease, as described by the HRS.1 Arrhythmogenic cardiomyopathy encompasses a number of disorder subtypes, including systemic, infectious, inflammatory, or genetic, and at times, there may be an overlap with other cardiomyopathies, especially DCM. Ventricular arrhythmias were considered to be a direct consequence of LV systolic dysfunction. An arrhythmogenic phenotype is now often preferred in cardiomyopathy phenotypes that present with low QRS voltages on ECG, pathological T-wave inversion, mildly impaired LV dysfunction, overt chamber dilatation, malignant arrhythmias, and diffuse myocardial fibrosis on CMR.7 This subset of DCM patients overlaps with ALVC. Patients are often gene positive for mutations in LMNA, SCN5A, FLNC, TTN, or RBM20. The abnormal ECG, mildly dilated and impaired LV function on TTE, arrhythmias during exercise on CPET, diffuse scar on CMR, positive family history, and FLNC genotype were the main factors that led to an ALVC diagnosis in this family.8
Arrhythmogenic right ventricular cardiomyopathy (ARVC) was the first reported ACM subtype, hence why it is the most cited ACM. Arrhythmogenic cardiomyopathy is now no longer regarded as a disease affecting just the right ventricle. Awareness about LV involvement is on the rise, present in up to 87% of cases (70% biventricular involvement and 17% isolated LV involvement).9Arrhythmogenic left ventricular cardiomyopathy is an uncommon cause of ACM that primarily involves fibrofatty replacement of the LV myocardium, leading to scar-related malignant arrhythmias and SCD.9 Fatty infiltration has traditionally also been used to distinguish ALVC from DCM, though this distinction is difficult in practice. Some prefer referring to ACM in cases presenting with fatty infiltration in the context of a desmosomal mutations. Thankfully, this distinction does not really impact the management strategy. The latest recommendations however reinforce the need to refer patients for genetic testing, moving towards a more personalized treatment strategy that is gene dependent.6 A genome-specific algorithm is now currently in place.
Extensive criteria are present for the diagnosis of ARVC given this is the earliest known ACM reported. Criteria for ALVC are, on the other hand, less clear. The evaluation should begin with a full history as well as baseline investigations including an ECG, TTE, ambulatory ECG monitoring, and CMR.10 Further tests may include an exercise stress test (or CPET), pharmacological testing, endomyocardial biopsy, or an electrophysiological study. Patients with ALVC may present to clinic with palpitations, syncope, following a cardiac arrest, or referral following the unexpected death of a relative. The recently proposed Padua criteria (2020) include a new classification for the diagnosis of ACM, as well as ALVC.2 The criteria for diagnosis of ACM involve six categories, each having major and minor criteria. The six categories include structural and functional abnormalities on imaging, depolarization and repolarization abnormalities, family background, and ventricular arrhythmias. In the case of ALVC, there are only two major criteria within the structural myocardial abnormalities and genetics background categories that are required for the diagnosis. The diagnosis may be made in the presence of LV structural abnormalities and a positive gene mutation for ACM, in the absence of RV involvement.2 The structural criterion involves the finding of LV LGE on CMR in more than one LV segment (based on the bull’s-eye plot) in at least two orthogonal views and must involve the free wall (subepicardial or midmyocardial), septum, or both. The LGE burden has been found to be directly related to the extent of LV dysfunction.11,12 The CMR images of both cases presented in this manuscript are in keeping with the major criterion with LGE across multiple segments. Decreased global or regional LV systolic function is considered as minor criteria. The ECG abnormalities encompassing minor criteria include T-wave inversion in left precordial leads, low-voltage QRS morphology in limb leads or >500 VEs of RBBB morphology over 24 h. Case 1 demonstrated T wave inversion in all precordial leads and a 24-h VE load of 3058 VEs, while Case 2 demonstrated low-voltage QRS morphology in the limb leads, as well as a VE load of 0.9% (1199 VEs) on 24-h ambulatory testing. Both satisfy Padua criteria for an ALVC diagnosis.
The ESC has issued new guidelines in 2023 for the management of cardiomyopathies in which they describe a diagnostic approach based primarily on the predominant cardiac phenotype.13 They have described five phenotypes including hypertrophic cardiomyopathy (HCM), DCM, non-dilated LV cardiomyopathy (NDLVC), ARVC, and restrictive cardiomyopathy (RCM). One of the major changes encompasses the term ACM, removing ALVC from the cardiomyopathy classification. While highlighting the importance of malignant arrhythmias as a red flag among patients with cardiomyopathies, the ESC did not maintain the term ACM as a distinct phenotype. Rather, it emphasized the importance of the description of the morphological and functional phenotype along with a description of the aetiology. The revised ESC classification highlights the heterogeneity in disease expressivity, shifting away from rigid nomenclature. A NDLVC phenotype may inadvertently better suite this case series, yet the practical nature of these new recommendations when dealing with overlapping phenotypes remains to be seen.13
Inhomogeneity in disease expressivity is well established in cardiomyopathies, nicely depicted in the presented case series. The penetrance in these phenotypes is often age related with various degrees of expression. Onset is often observed in the third to fifth decade of life. Certain variables may be specific to families. Different mutations may confer specific risks (malignant arrhythmias, conduction disease, and heart failure).1 Probands often have a more aggressive phenotype when compared with family members.14 A third of asymptomatic carriers in familial cardiomyopathy were given a cardiomyopathy diagnosis at the first evaluation, highlighting the importance of cascade genetic screening when a gene is identified.15 Gene-positive relatives may be clinically unaffected at baseline, have borderline findings not fulfilling clinical diagnostic criteria (incomplete penetrance), or else harbour the disease. The role for genetic testing is extensively discussed in the HRS consensus document. There are several genes implicated in the pathogenesis of ACM. Full coverage of known ACM-related genes is recommended during genotyping patients with a suspected ACM. Some of the well-established genes include Desmoplakin (DSP), Desmoglein-2 (DSG2), Plakophilin (PKP2), BLC2-Associated Athanogene 3 (BAG3), and FLNC.1,4,6,16,17 FLNC is a protein involved in myocyte integrity and cell signalling, an important gene strongly linked with ALVC.3,18 Apart from assisting in the diagnosis of an ACM, identification of likely pathogenic or pathogenic genetic variants (as per American College of Medical Genetics and Genomics 2015 classification) may alter the management of cases. Predictive testing in family members is also recommended.19,20
The strong relationship between high-intensity physical activity and adverse outcomes in ACM is now well established.1,8,21,22 The phenomenon of exercise-induced ACM also supports this phenomenon. Several cohorts consistently show that gene-positive individuals, who continue to exercise often present earlier, have a higher rate of ventricular arrhythmias and increased risk of SCD.5,23,24 Gene-positive individuals with a family history of ACM are not advised to engage in competitive sports.25 Data on the role of exercise in the pathophysiology of ACM often target families with desmosomal variants, and the science supporting exercise restriction in FLNC ACM is unfortunately not robust. Exercise recommendations also fail to make this important distinction. A shared decision approach is preferable, with a personalized exercise prescription tailored according to the patient’s phenotype. Individuals with ACM should be encouraged to undergo 150 min of physical activity weekly, at low–moderate intensity depending on symptoms, family history of SCD, degree of LV involvement, and arrhythmias.25 Disciplines that offer a low–moderate static and dynamic component would be preferable. We advised light–moderate-intensity exercise in Patient 1, advising against high-intensity physical activity because of the extensive LV involvement and positive family history. This case series nicely illustrates how genetic testing can alter a patient’s therapeutic strategy. Access to genetic testing is now at an all-time high. A paradigm shift towards genome-specific management strategies has led to the development of comprehensive cardiogenetic services in several institutions.6 One must however acknowledge the complexities involved in setting up and maintaining such a service. A multidisciplinary collaboration with cardiomyopathy experts, cardiac imagers, electrophysiologists, geneticists, and psychologists is paramount. Shared decision-making with patients should also take a prominent role. Most consensus documents and guidelines now strongly advocate this approach.6,25
Conclusion
An ACM diagnosis has several clinical implications. A comprehensive evaluation is carried out in all patients with suspected ACM. Genetic testing is certainly an important player in phenotypic characterization and risk stratification. Certain mutations have been shown to confer a higher SCD risk, hence why international recommendations have included genetics in treatment algorithms. As has been presented in both cases, referral for genetic testing years after being followed up in clinic had substantial implications for long-term prevention against SCD.
Supplementary Material
Contributor Information
Mark Abela, Department of Cardiology, Mater Dei Hospital, Triq Dun Karm, Birkirkara, Msida, MSD 2090, Malta.
Neil Grech, Department of Cardiology, Mater Dei Hospital, Triq Dun Karm, Birkirkara, Msida, MSD 2090, Malta.
Jessica Debattista, Department of Molecular Genetics, University of Malta, Msida, MSD 2090, Malta.
Tiziana Felice, Department of Cardiology, Mater Dei Hospital, Triq Dun Karm, Birkirkara, Msida, MSD 2090, Malta.
Lead author biography
 Dr Mark Abela is a cardiology registrar practising at Mater Dei Hospital. He has finished specialty training in cardiology and has undergone a fellowship in Sports Cardiology and Inherited Cardiac Conditions at St George’s Hospital in London. His main academic and clinical interests are athletic cardiac adaptation, cardiac screening, inherited cardiac conditions, and cardiac rehabilitation. He is a University of Malta graduate, having obtained his MD in 2011. He is a member of the Royal College of Physicians of London and is also an MSc graduate from the University of Edinburgh. He read for an MSc in Internal Medicine (University of Edinburgh) and an MSc in Sports Cardiology (St George’s University, London). He is the lead clinical investigator for BEAT-IT, a national cardiac screening programme in Maltese adolescents (athletes and non-athletes). He has been appointed as the official content reviewer of the 2020 ESC Position Paper on Sports Cardiology on behalf of the Maltese Cardiac Society. He has also recently been appointed a deputy editor on the European Heart Journal of Case Reports editorial board. He sits various medical committees, notably the Malta Football Association, Malta Aquatic Association, and the Maltese Olympic Committee. He also plays a very active role on an international level. He had been elected as a Sports Cardiology Nucleus member within the ESC committee for Sports Cardiology and Exercise. He is also a young ambassador for preventive cardiology in Malta. He has presented at a number of local and international conferences and also has a number of papers in international journals. He is also reading for a PhD in Medicine with the University of Malta, kindly supported by Malta Research Innovation and Development Trust (RIDT). He has been awarded a research scholarship sponsored by ‘Beating Hearts’. He is very confident that the outcomes of this research will contribute significantly towards the understanding of heart disease in young individuals. It will hopefully also pave the way to more research initiatives pertaining to inherited cardiac conditions in Malta. He looks forward to contributing more to this niche in the years to come.
Dr Mark Abela is a cardiology registrar practising at Mater Dei Hospital. He has finished specialty training in cardiology and has undergone a fellowship in Sports Cardiology and Inherited Cardiac Conditions at St George’s Hospital in London. His main academic and clinical interests are athletic cardiac adaptation, cardiac screening, inherited cardiac conditions, and cardiac rehabilitation. He is a University of Malta graduate, having obtained his MD in 2011. He is a member of the Royal College of Physicians of London and is also an MSc graduate from the University of Edinburgh. He read for an MSc in Internal Medicine (University of Edinburgh) and an MSc in Sports Cardiology (St George’s University, London). He is the lead clinical investigator for BEAT-IT, a national cardiac screening programme in Maltese adolescents (athletes and non-athletes). He has been appointed as the official content reviewer of the 2020 ESC Position Paper on Sports Cardiology on behalf of the Maltese Cardiac Society. He has also recently been appointed a deputy editor on the European Heart Journal of Case Reports editorial board. He sits various medical committees, notably the Malta Football Association, Malta Aquatic Association, and the Maltese Olympic Committee. He also plays a very active role on an international level. He had been elected as a Sports Cardiology Nucleus member within the ESC committee for Sports Cardiology and Exercise. He is also a young ambassador for preventive cardiology in Malta. He has presented at a number of local and international conferences and also has a number of papers in international journals. He is also reading for a PhD in Medicine with the University of Malta, kindly supported by Malta Research Innovation and Development Trust (RIDT). He has been awarded a research scholarship sponsored by ‘Beating Hearts’. He is very confident that the outcomes of this research will contribute significantly towards the understanding of heart disease in young individuals. It will hopefully also pave the way to more research initiatives pertaining to inherited cardiac conditions in Malta. He looks forward to contributing more to this niche in the years to come.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Funding: None declared.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals of the case series.
References
- 1. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019;16:e301–e372. [DOI] [PubMed] [Google Scholar]
- 2. Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari M, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020;319:106–114. [DOI] [PubMed] [Google Scholar]
- 3. Ortiz-Genga MF, Cuenca S, Dal Ferro M, Zorio E, Salgado-Aranda R, Climent V, et al. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol 2016;68:2440–2451. [DOI] [PubMed] [Google Scholar]
- 4. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruwald AC, Marcus F, Estes NA III, Link M, McNitt S, Polonsky B, et al. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2015;36:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 7. Celeghin R, Cipriani A, Bariani R, Bueno Marinas M, Cason M, Bevilacqua M, et al. Filamin-C variant-associated cardiomyopathy: a pooled analysis of individual patient data to evaluate the clinical profile and risk of sudden cardiac death. Heart Rhythm 2022;19:235–243. [DOI] [PubMed] [Google Scholar]
- 8. Miles C, Finocchiaro G, Papadakis M, Gray B, Westaby J, Ensam B, et al. Sudden death and left ventricular involvement in arrhythmogenic cardiomyopathy. Circulation 2019;139:1786–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, et al. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol 2008;52:2175–2187. [DOI] [PubMed] [Google Scholar]
- 10. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Norman M, Simpson M, Mogensen J, Shaw A, Hughes S, Syrris P, et al. Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation 2005;112:636–642. [DOI] [PubMed] [Google Scholar]
- 12. Rehm HL, Berg JS, Brooks LD, Bustamante CD, Evans JP, Landrum MJ, et al. Clingen—the clinical genome resource. N Engl J Med 2015;372:2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J 2023;44:3503–3626. [DOI] [PubMed] [Google Scholar]
- 14. Groeneweg JA, Bhonsale A, James CA, te Riele AS, Dooijes D, Tichnell C, et al. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet 2015;8:437–446. [DOI] [PubMed] [Google Scholar]
- 15. Mahmood A, Morris-Rosendahl D, Edwards M, Fleming A, Homfray T, Mason S, et al. 10 disease penetrance in asymptomatic carriers of familial cardiomyopathy variants. Heart 2022;108:A9.1–A9A9. [Google Scholar]
- 16. Kandhari N, Khoury S, Behr ER, Miles C. Cardiac arrest as first presentation of arrhythmogenic left ventricular cardiomyopathy due to Filamin C mutation: a case report. Eur Heart J Case Rep 2021;5:ytab422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Awad MM, Dalal D, Cho E, Amat-Alarcon N, James C, Tichnell C, et al. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Hum Genet 2006;79:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, et al. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat 2008;29:1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arbustini E, Behr ER, Carrier L, van Duijn C, Evans P, Favalli V, et al. Interpretation and actionability of genetic variants in cardiomyopathies: a position statement from the European Society of Cardiology Council on cardiovascular genomics. Eur Heart J 2022;43:1901–1916. [DOI] [PubMed] [Google Scholar]
- 20. Wilde AAM, Semsarian C, Márquez MF, Sepehri Shamloo A, Ackerman MJ, Ashley EA, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. Heart Rhythm 2022;19:e1–e60. [DOI] [PubMed] [Google Scholar]
- 21. Kübler J, Burgstahler C, Brendel JM, Gassenmaier S, Hagen F, Klingel K, et al. Cardiac MRI findings to differentiate athlete’s heart from hypertrophic (HCM), arrhythmogenic right ventricular (ARVC) and dilated (DCM) cardiomyopathy. Int J Cardiovasc Imaging 2021;37:2501–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zorzi A, Rigato I, Pilichou K, Perazzolo Marra M, Migliore F, Mazzotti E, et al. Phenotypic expression is a prerequisite for malignant arrhythmic events and sudden cardiac death in arrhythmogenic right ventricular cardiomyopathy. Europace 2016;18:1086–1094. [DOI] [PubMed] [Google Scholar]
- 23. La Gerche A, Claessen G, Dymarkowski S, Voigt JU, De Buck F, Vanhees L, et al. Exercise-induced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur Heart J 2015;36:1998–2010. [DOI] [PubMed] [Google Scholar]
- 24. Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith HJ, et al. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals of the case series.