Abstract
OBJECTIVES:
To determine the concordance between activated partial thromboplastin time (aPTT) and anti-factor-Xa (anti-Xa) in adults undergoing extracorporeal membrane oxygenation (ECMO) and to identify the factors associated with discordant paired aPTT/anti-Xa.
DESIGN:
Pre-planned secondary analysis of the Low-Dose Heparin in Critically Ill Patients Undergoing Extracorporeal Membrane Oxygenation pilot randomized unblinded, parallel-group controlled trial.
SETTING:
Two ICUs in two university hospitals.
PATIENTS:
Thirty-two critically ill patients who underwent ECMO and who had at least one paired aPTT and anti-Xa assay performed at the same time.
INTERVENTIONS:
We analyzed the concordance between aPTT and anti-Xa and identified factors associated with discordant paired aPTT/anti-Xa based on their respective therapeutic ranges. We also compared biological parameters between heparin resistance episode and no heparin resistance.
MEASUREMENTS AND MAIN RESULTS:
Of the 32 patients who were included in this study, 24 (75%) had at least one discordant paired aPTT/anti-Xa. Of the 581 paired aPTT/anti-Xa that were analyzed, 202 were discordant. The aPTT was relatively lower than anti-Xa in 66 cases (32.7%) or relatively higher than anti-Xa in 136 cases (67.3%). Thirty-three heparin resistance episodes were identified in six patients (19%).
CONCLUSIONS:
In these critically ill patients undergoing ECMO, one third of paired aPTT/anti-Xa measures was discordant. Coagulopathy and heparin resistance might be the reasons for discordance. Our results support the potential importance of routinely monitoring both tests in this setting.
Keywords: activated partial thromboplastin time, anticoagulation, anti-factor-Xa, extracorporeal membrane oxygenation, heparin resistance
KEY POINTS
Question: This study explores the concordance between anti-factor-Xa (anti-Xa) and activated partial thromboplastin time (aPTT) in patients undergoing extracorporeal membrane oxygenation and who receive anticoagulation with heparin.
Findings: In this pre-planned analysis of a randomized pilot study, one third of paired aPTT/anti-Xa measures were discordant. Coagulopathy and heparin resistance might be the reasons for discordance.
Meaning: Our results support the potential importance of routinely monitoring both tests in this setting.
Increasing experience and technological advances in extracorporeal membrane oxygenation (ECMO) have resulted in improved patient outcomes; however, bleeding and thrombosis remain frequent complications during ECMO with impacts on morbidity and mortality (1, 2). Blood contact with nonbiologic surfaces results in a highly procoagulant state mediated by thrombin. To address this, anticoagulation is routinely given. Although bivalirudin in comparison with heparin might achieve therapeutic anticoagulation in a shorter time and might decrease the wholistic cost of systemic anticoagulation in patients undergoing ECMO, unfractionated heparin (UFH) remains the first choice of anticoagulant due to clinicians familiarity, its relatively short half-life and reversibility (3–6). In a recent international survey, the three commonly used methods to monitor anticoagulation in adults undergoing ECMO include activated partial thromboplastin time (aPTT) (41.8%), activated clotting time (ACT) (30%), and anti-factor-Xa (anti-Xa) activity (22.7%) (3).
aPTT is a global clotting assay that measures ability of heparin-antithrombin complex to inactivate relevant coagulation factors. Many factors can affect the aPTT other than heparin effect, including increased or decreased coagulation factor levels (particularly factor II, factor VIII, and anti-thrombin), presence of inhibitors, and various preanalytical and analytical variables. In addition, there is a high variability in aPTT assays from one laboratory to another making therapeutic ranges of aPTT different between hospitals. The anti-Xa assay is a measure of the functional activity of heparin. It measures the extent to which exogenous factor Xa is inhibited by UFH-antithrombin complex. Therefore, the assay is not sensitive to changes in other coagulation factor levels, as is the case for aPTT, so it provides a more direct measure of heparin activity. Both tests are affected by antithrombin III deficit (7), which is more common in patients undergoing ECMO. Heparin resistance defined by high doses of heparin to achieve a targeted level of anticoagulation may be related to an antithrombin III deficit and would lead to discordance between aPTT and anti-Xa (8). Discordances between aPTT and anti-Xa levels have been reported in non-ECMO adults receiving UFH (9–11). Studies evaluating aPTT and anti-Xa to monitor anticoagulation in ECMO patients are sparse, mainly restricted to pediatric populations or focus on the correlation between one or the other test with the heparin dose (12–14).
Therefore, we conducted a study to first evaluate the correlation between aPTT and anti-Xa in adults undergoing ECMO and receiving UFH, second to investigate heparin resistance in this population, and third to identify potential reasons for discordance between aPTT and anti-Xa.
MATERIALS AND METHODS
Study Design and Participants
This study was a pre-planned secondary analysis of a two-center randomized unblinded, parallel-group controlled trial, the Low-Dose Heparin in Critically Ill Patients Undergoing Extracorporeal Membrane Oxygenation (HELP-ECMO) trial comparing therapeutic anticoagulation with UFH and a low dose heparin protocol (URL: http://www.ANZCTR.org.au; unique identifier: ACTRN12613001324707) (15). The trial was conducted at two university-associated hospitals: the Alfred Hospital in Melbourne and the Royal Prince Alfred Hospital (RPA) in Sydney, NSW, Australia. The study was approved by the human research ethics committees at both participating sites (in December 2013 at the Alfred Hospital—Project No 560/13 Low Dose Heparin in Critically Ill Patients Undergoing Extracorporeal Membrane Oxygenation—Feasibility Study, and in April 2015 at RPA—Project No X14-0312 Heparin Low Dose Protocol in ECMO Patients). Written informed consent from the person responsible was obtained before enrolment, and when not available a deferred consent procedure was applied at the Alfred Hospital only. The procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975.
Patients older than 16 years who underwent venovenous or venoarterial ECMO and who did not have a preexisting indication for therapeutic anticoagulation or a contraindication to heparin could be randomized unless they had been on ECMO for more than 48 hours (15). Patients randomized in the “therapeutic anticoagulation” group had targeted aPTT ranges comprised between 50 and 70 seconds, while patients randomized in the “low-dose heparin” group received up to 12,000 U/24 hr aiming for aPTT less than 45 seconds. At both participating sites, aPTT was the routine method to monitor UFH anticoagulation both in routine practice and during the randomized trial. For the clinical trial, samples for anti-Xa measurement were taken at the same time as samples for aPTT. Both aPTT and anti-Xa results were available to the treating clinicians, however, as anti-Xa assays were performed less frequently by the pathology service at both hospitals compared with aPTT, there was a delay in the reporting of anti-Xa results compared with aPTT. Heparin dose was adjusted only on aPTT results and not on anti-Xa values. Patients who had at least one paired anti-Xa and aPTT assay performed at the same time were included in this substudy.
Study Data
Data were prospectively collected, and included baseline patient characteristics, comorbidities, and illness severity scores (ICU admission Acute Physiology and Chronic Health Evaluation III [APACHE III] score and the Sequential Organ Failure Assessment [SOFA] score) before randomization (16). Characteristics of ECMO with the type and indication for ECMO also collected. Daily data on UFH dose, and results of laboratory tests, including hemoglobin level, prothrombin time (PT), international normalized ratio (INR), fibrinogen, platelet count, aPTT, and anti-Xa levels, were recorded. Daily heparin dose included bolus and infusion of heparin, and heparin used for ECMO circuit and/or cannulation. Information about time within therapeutic range was not available.
aPTT/anti-Xa Measures, Therapeutic Range, and Heparin Resistance Definition
aPTT was measured at least once a day, 6 hours after any change in anticoagulation level and when considered necessary by the ICU team. Anti-Xa was measured at the same time of aPTT when possible. Both sites used the same assay for aPTT measurement (STA-R automated coagulation instrument [Diagnostica Stago, Forest Hill, Australia] and Triniclot aPTT S reagent [Diagnostica Stago]). Anti-Xa was measured using the liquid anti-Xa reagent (Diagnostica Stago) in both sites. For this analysis, the target therapeutic range was predefined. For aPTT, therapeutic range was defined as 50–80 seconds in RPA hospital and 50–90 seconds in Alfred hospital. For anti-Xa level, therapeutic range was defined as 0.3 to 0.7 international units/mL (IU/mL). Paired aPTT/anti-Xa were classified into three groups: 1) “concordant” results when aPTT and anti-Xa were both either in, under or over their therapeutic ranges; 2) discordant “high” when aPTT was relatively higher than the anti-Xa based on the respective therapeutic ranges (i.e., aPTT higher than the therapeutic ranges and anti-Xa either in or lower than the therapeutic ranges or aPTT in the therapeutic ranges and anti-Xa lower than the therapeutic ranges); and 3) discordant “low” when aPTT was relatively lower than the anti-Xa based on the respective therapeutic ranges (i.e., aPTT in or lower than the therapeutic ranges and anti-Xa higher than the therapeutic ranges or aPTT lower than the therapeutic ranges and anti-Xa in or above the therapeutic ranges). Both, aPTT and anti-Xa were considered as continuous variables and categorized as being within or not in their therapeutic reference ranges. Heparin resistance was defined as an aPTT below 50 seconds despite a heparin daily dose (including bolus) superior to 35,000 U/d based on the literature (17).
Statistical Analysis
At patient level analysis, continuous variables were expressed as median (interquartile range [IQR]) and categorical variables were expressed as number (percentage). Continuous variables were compared with a Wilcoxon test. Categorical variables were compared with a Fisher exact test.
To account for repeated measures within an individual patient, comparison between aPTT/anti-Xa discordances and heparin resistance was performed using linear mixed modeling with time treated as a fixed effect and patient treated as a random effect. Differences were evaluated with p value, which were calculated according to Satterthwaite approximation method, assuming a violation of equal variation hypothesis in order to take into account for repeated measurement. No statistical adjustment for multiple testing was done. Statistical significance was set at p value of less than 0.05. Analyses were performed with R statistical software (Version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patients
All of the 32 patients enrolled in the study had at least one paired aPTT/anti-Xa and were included in the analysis. Twenty-three (71.9%) underwent venovenous ECMO and 9 (28.1%) venoarterial ECMO. Indications for ECMO and baseline characteristics are summarized in Table 1. Baseline APACHE III score was 57.5 (45.5–88.25) and median SOFA score at ECMO initiation was 9 (8–13) (Table 1). Twenty-four patients (75%) had at least one discordant paired aPTT/anti-Xa. In nine patients (37.5%), more than 50% of the aPTT measures were in lower ranges than those of the anti-Xa and in 15 patients (62.5%) more than 50% of aPTT measures in higher ranges than those of the anti-Xa. The median number of paired aPTT/anti-Xa per patient was 8 (5–15.3), this number was higher in patients with discordant aPTT/anti-Xa compared with patients without discordance (4.5 [2–8.25] vs. 14 [9.8–19]; p = 0.002). Patients with discordant paired aPTT/anti-Xa had a higher weight (80 kg [74.3–86 kg] vs. 62.5 kg [53.8–76.3 kg]; p = 0.03) and a longer time on ECMO than patients without discordant paired aPTT/anti-Xa (10 d [IQR, 7–16 d] vs. 6 d [2.8–8.3 d]; p = 0.03). They also received higher daily dose of heparin than patients without discordant paired aPTT/anti-Xa (22,241 U [13,936–31,445 U] vs. 9,340 U [7,450–11,500 U]; p = 0.008) (Table 1). Similarly, there was also an association between the allocation group of the original study (“low dose heparin” group and “therapeutic anticoagulation” group) and the concordance between paired anti-Xa and aPTT in this current substudy, with more discordances in the “therapeutic anticoagulation” group (Table 1).
TABLE 1.
Comparison of Characteristics of Patients With and Without Discordant Paired Activated Partial Thromboplastin Time/Anti-Factor-Xa
| Variables | Overall, n = 32 | No Discordant aPTT/Anti-Xa, n = 8 | At Least One Discordant aPTT/Anti-Xa, n = 24 | p |
|---|---|---|---|---|
| Age, yr | 38 (26.8–57.8) | 39.50 (26.3–47) | 38 (27.5–61.5) | 0.45 |
| Gender, male | 24 (75) | 6 (75) | 18 (75) | 1 |
| Weight, kg | 79 (69.8–85.3) | 62.5 (53.8–76.3) | 80 (74.3–86) | 0.03 |
| Acute Physiology and Chronic Health Evaluation III score | 57.5 (45.5–88.3) | 59 (54.5–68) | 55.5 (43.3–90) | 0.56 |
| Proportion of patients randomized in: | 0.04 | |||
| “Low-dose heparin” group | 16 (50) | 7 (87.5) | 9 (37.5) | |
| “Therapeutic anticoagulation” group | 16 (50) | 1 (12.5) | 15 (62.5) | |
| Comorbidities | ||||
| Immunosuppressed | 4 (12.5) | 1 (12.5) | 3 (12.5) | 1 |
| Respiratory failure | 3 (9.4) | 3 (37.5) | 0 (0) | 0.01 |
| Cardiovascular disease | 5 (15.6) | 0 (0) | 5 (20.8) | 0.3 |
| Renal failure | 1 (3.1) | 1 (12.5) | 0 (0) | 0.25 |
| Insulin dependent diabetes | 1 (3.1) | 0 (0) | 1 (4.2) | 1 |
| Ongoing sepsis before ECMO | 15 (46.9) | 3 (37.5) | 12 (50) | 0.69 |
| Heparin dose (U/d) | 20,476 (11,176–29,904) | 9,340 (7,450–11,500) | 22,241 (13,936–31,445) | 0.008 |
| ECMO type | ||||
| Venovenous ECMO | 23 (71.9) | 7 (87.5) | 16 (66.7) | 0.39 |
| Venoarterial ECMO | 9 (28.1) | 1 (12.5) | 8 (33.3) | 0.39 |
| Indications for venovenous ECMO | 0.07 | |||
| Asthma | 2 (6.2) | 1 (12.5) | 1 (4.2) | |
| Bacterial or viral pneumonia | 9 (28.1) | 2 (25) | 7 (29.1) | |
| Lung transplantation | 3 (9.3) | 3 (37.5) | 0 (0) | |
| Other respiratory disorders | 9 (28.1) | 1 (12.5) | 8 (33.3) | |
| Indications for venoarterial ECMO | 0.51 | |||
| Acute cardiomyopathy | 2 (6.2) | 0 (0) | 2 (8.3) | |
| Acute myocardial infarction | 1 (3.1) | 0 (0) | 1 (4.2) | |
| Heart transplantation | 3 (9.4) | 0 (0) | 3 (12.5) | |
| Myocarditis | 1 (3.1) | 1 (12.5) | 0 (0) | |
| Other | 2 (6.2) | 0 (0) | 2 (8.3) | |
| Days on ECMO | 8 (5.5–14) | 6 (2.8–8.3) | 10 (7–16) | 0.03 |
anti-Xa = anti-factor-Xa, aPTT = activated partial thromboplastin time, ECMO = extracorporeal membrane oxygenation.
Continuous variables are expressed as median (interquartile range) and categorical variables are expressed as percentage.
Discordant/Concordant Paired aPTT/Anti-Xa
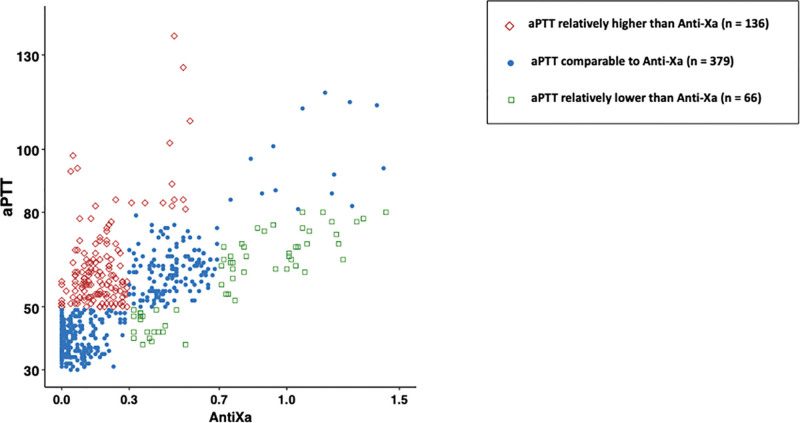
Of the 581 paired aPTT/anti-Xa available for the analysis, 379 (65.2%) were classified as “concordant”; of the 202 discordant paired aPTT/anti-Xa (34.8%), 136 (67.3%) were classified as discordant “high” with aPTT relatively higher than anti-Xa and 66 (32.7%) were classified as discordant “low” with aPTT relatively lower than anti-Xa (Fig. 1). Figures S1 and S2 (http://links.lww.com/CCX/B270) provided these descriptions in patients randomized in the "therapeutic anticoagulation" group (Fig. S1, http://links.lww.com/CCX/B270) and in the "low dose heparin" group, respectively (Fig. S2, http://links.lww.com/CCX/B270). Compared with concordant pairs, an aPTT relatively higher than the paired anti-Xa was associated with thrombocytopenia (140 G/L [77–178 G/L] vs. 174 G/L [117–241 G/L]; p < 0.001) and a lower PT (14.5 [13.5–15.8] vs. 15 [14.3–16.3]; p < 0.001) (Table 2). Compared with concordant pairs, an aPTT relatively lower than the paired anti-Xa was associated with higher platelet count (260 G/L [128–330 G/L] vs. 168 G/L [113–246 G/L]; p = 0.002) and higher daily heparin dose (43,200 [24,963–52,800] vs. 20,400 [12,000–34,600]; p = 0.003) (Table 2). Patients with mainly discordant paired with aPTT relatively lower than anti-Xa (classified discordant “low”) received significantly higher daily dose of heparin and had more heparin resistance than patients with mainly discordant paired with aPTT relatively higher than anti-Xa (Table S1, http://links.lww.com/CCX/B270).
Figure 1.
Scatter plot with the 581 paired activated partial thromboplastin time (aPTT)/anti-factor-Xa (anti-Xa). Each paired aPTT/anti-Xa is classified into three groups: concordant paired aPTT/anti-Xa (blue circle), discordant “low” (aPTT relatively lower than anti-Xa) (green square), and discordant “high” (aPTT relatively higher than anti-Xa) (red diamond).
TABLE 2.
Comparison of Daily Biological Parameters and Daily Heparin Dose on the Day of the Paired Activated Partial Thromboplastin Time/Anti-Factor-Xa Between Concordant/Discordant Paired Activated Partial Thromboplastin Time/Anti-Factor-Xa
| Variables | Concordant Pairs (Reference) (n = 379) | Discordant “Low” (n = 66) | Discordant “High” (n = 136) | p a | |
|---|---|---|---|---|---|
| Reference vs. “Low” | Reference vs. “High” | ||||
| Lowest hemoglobin (g/L) | 84 (77–92.5) | 84 (78–93.8) | 83.5 (75.8–99) | 0.61 | 0.39 |
| Highest hemoglobin (g/L) | 97 (90–106) | 94.5 (89–105) | 99.50 (92–114) | 0.72 | 0.71 |
| Highest plasma free hemoglobin (g/L) | 5 (3–8) | 4 (3–6) | 6 (4–11) | 0.43 | 0.21 |
| Lowest corrected ionized calcium (mmol/L) | 1.12 (1.04–1.22) | 1.14 (1.08–1.19) | 1.08 (1.02–1.19) | 0.34 | 0.63 |
| Highest urea (mmol/L) | 10 (8–13) | 10 (8.25–12) | 11 (7–16) | 0.45 | 0.30 |
| Highest bilirubin (µmol/L) | 12 (8–20) | 8 (5–16) | 12 (11–19.8) | 0.60 | 0.17 |
| Lowest arterial pH | 7.36 (7.31–7.42) | 7.34 (7.3–7.38) | 7.40 (7.33–7.44) | 0.12 | 0.08 |
| Highest d-dimer (mg/L) | 114 (34–184) | 146 (73–220) | 127 (56–155) | 0.18 | 0.91 |
| Highest INR | 1.2 (1.1–1.3) | 1.1 (1.1–1.2) | 1.1 (1–1.3) | 0.54 | < 0.001 |
| Lowest INR | 1.2 (1.1–1.3) | 1.2 (1–1.3) | 1.2 (1–1.3) | 0.69 | 0.35 |
| Highest fibrinogen (g/L) | 4.7 (3.5–6.3) | 5 (4.5–6.3) | 4.4 (2.3–6) | 0.19 | 0.26 |
| Lowest fibrinogen (g/L) | 4.8 (3.4–6.4) | 5.1 (4.2–6.2) | 4.7 (3.1–6) | 0.33 | 0.44 |
| Highest PT (s) | 15 (14.3–16.3) | 15.25 (14.4–16) | 14.5 (13.5–15.8) | 0.45 | < 0.001 |
| Lowest PT (s) | 15 (14–16.2) | 15.3 (14.1–16.4) | 14.8 (13.8–15.8) | 1 | 0.2 |
| Highest platelet count (G/L) | 168 (113–246) | 260 (128–330) | 139 (86–177) | 0.002 | < 0.001 |
| Lowest platelet count (G/L) | 174 (117–241) | 208.5 (111–255) | 140 (77–178) | 0.003 | < 0.001 |
| Daily heparin dose, U/24 hr | 20,400 (12,000–34,600) | 43,200 (24,963–52,800) | 13,600 (10,800–24,688) | 0.003 | 0.59 |
| Pairs in patients randomized in “the therapeutic anticoagulation group,” n (%) | 210 (55.4) | 43 (65.2) | 89 (65.4) | 0.07 | 0.06 |
INR = international normalized ratio, PT = prothrombin time.
p value was obtained with Satterthwaite approximation method derived from a linear mixed model analysis to account for repeated measures.
Discordant was considered as “low” when activated partial thromboplastin time (aPTT) was relatively lower than anti-factor-Xa (anti-Xa) and “high” when aPTT was relatively higher than anti-Xa. All variables are expressed as median (interquartile range).
Heparin Resistance
On daily screening, 33 heparin resistance episodes were identified in six patients (18.8%). Twenty-five of these episodes occurred in four patients randomized to the therapeutic anticoagulation group and seven episodes occurred in two patients randomized to low-dose group (who crossed over to therapeutic anticoagulation). Patients with heparin resistance received a higher median daily dose of heparin than patients without heparin resistance (50,100 U/d [42,900–59,200 U/d] vs. 17,175 U/d [12,000–31,200 U/d]; p = 0.02). Heparin resistance was associated with higher median d-dimer level on the day of the heparin resistant episode (180 mg/L [128–215 mg/L] vs. 121 mg/L [34–181 mg/L]; p = 0.005) (Table 3). Patient-level factors that were associated with heparin resistance included younger age (27 yr [22.3–33.3 yr] vs. 45 yr [30.5–60.8 yr]; p = 0.04) and lower APACHE III score 37.5 ([21.3–41.8] vs. 64 [53.8–91.3]; p = 0.001) (Table S2, http://links.lww.com/CCX/B270). Subgroup analysis was performed in patients randomized in "the therapeutic anticoagulation" group (Table S3, http://links.lww.com/CCX/B270).
TABLE 3.
Comparison of Biological Parameters Between Heparin Resistance Episodes and No Heparin Resistance
| Variablesa | No Heparin Resistance (n = 548) | Heparin Resistance (n = 33) | p b |
|---|---|---|---|
| aPTT (s) | 54 (42.8–64) | 42 (37–46) | < 0.001 |
| Anti-Xa (IU/L) | 0.2 (0.06–0.48) | 0.2 (0.1–0.35) | < 0.001 |
| Lowest hemoglobin (g/L) | 84 (76.8–94.3) | 89 (83–94) | 0.02 |
| Highest hemoglobin (g/L) | 97 (90–107) | 100 (94–105) | 0.06 |
| Highest plasma free hemoglobin (g/L) | 5 (3–8) | 7 (5–10) | 0.83 |
| Lowest corrected ionized calcium (mmol/L) | 1.1 (1–1.2) | 1.2 (1.1–2.3) | 0.51 |
| Highest urea (mmol/L) | 10 (7–13) | 11 (8.9–15) | 0.67 |
| Highest bilirubin (µmol/L) | 12 (8–18) | 20 (7–26.8) | 0.42 |
| Lowest arterial pH | 7.36 (7.31–7.43) | 7.37 (7.35–7.42) | 0.38 |
| Highest d-dimer (mg/L) | 121 (34–181) | 180 (128–215) | 0.005 |
| Highest INR | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 0.07 |
| Lowest INR | 1.2 (1.08–1.3) | 1.2 (1.1–1.2) | 0.58 |
| Highest fibrinogen (g/L) | 4.7 (3.2–6.1) | 5.8 (5.1–7) | 0.55 |
| Lowest fibrinogen (g/L) | 4.8 (3.3–6.2) | 5.1 (4.1–6.9) | 0.08 |
| Highest PT (s) | 14.9 (14–16.2) | 15.8 (15.2–16) | 0.13 |
| Lowest PT (s) | 14.9 (13.9–16.2) | 15.4 (14.7–15.8) | 0.83 |
| Highest platelet count (G/L) | 160 (106–245) | 158 (107–269) | 0.20 |
| Lowest platelet count (G/L) | 162 (106–235) | 158 (107–219) | 0.04 |
| Daily heparin dose, U/24 hr | 17,175 (12,000–31,200) | 50,100 (42,900–59,200) | 0.02 |
| aPTT/anti-Xa discordance, n (%) | 0.002 | ||
| Concordant aPTT/anti-Xa | 353 (64.4) | 26 (78.8) | |
| aPTT relatively lower than anti-Xa | 59 (10.8) | 7 (21.2) | |
| aPTT relatively higher than anti-Xa | 136 (24.8) | 0 (0) |
anti-Xa = anti-factor-Xa, aPTT = activated partial thromboplastin time, INR = international normalized ratio, PT = prothrombin time.
Highest and lowest values are for the day of the heparin resistance episode.
p value was obtained with Satterthwaite approximation method derived from a linear mixed model analysis to account for repeated measures within individual patients.
Continuous variables are expressed as median (interquartile range) and categorical variables are expressed as percentage.
DISCUSSION
In this secondary analysis of the HELP-ECMO randomized pilot study, we found that 34.8% of the 581 paired samples were discordant based on their respective anticoagulation therapeutic ranges. Two thirds of the discordant paired aPTT/anti-Xa corresponded to a relatively higher aPTT compared with anti-Xa, possibly because of an associated coagulopathy. Patients who experienced at least one discordant paired aPTT/anti-Xa had a higher body weight and received higher daily doses of heparin. Thirty-three heparin resistant episodes were identified in six patients. Our results suggest that aPTT and anti-Xa should both be used for monitoring heparin during ECMO, as they provide complementary information and help to identify heparin resistance.
Determining the optimal anticoagulation patterns in patients undergoing ECMO is a challenge as these patients might develop both procoagulability and high risk of bleeding concomitantly (18). Although the optimal anticoagulation monitoring method remains unknown in this setting with accumulating evidence that aPTT does not properly correlate with heparin dose, aPTT remains the method of choice of UFH monitoring in this population (3, 19). Recently, experts have suggested titration of UFH by either aPTT or anti-Xa (20), and in the case of discrepancy between methods, clinicians should only refer to anti-Xa (20).
Discordance between aPTT and anti-Xa has been reported previously in this setting. Liveris et al (12) found 44.2% of discordance between aPTT and anti-Xa in pediatric patients undergoing ECMO. Several reasons might explain these discordances. First, patients undergoing ECMO are at high risk of bleeding diathesis due to their underlying disease and/or coagulation activation because of the ECMO circuit. As a consequence, an increased aPTT does not always relate to heparin treatment and might be misinterpreted, leading to unjustified and inappropriate changes in heparin dose (19). In our study, we found more severe thrombocytopenia in cases of relatively higher aPTT than anti-Xa, suggesting a possible associated coagulopathy. Second, heparin resistance in a setting of systemic inflammation with or without deficit in anti-thrombin III causes the absence of change in aPTT (and no anticoagulation effect) while the dose of heparin increases (17). Although we did not routinely monitor anti-thrombin III, patients with heparin resistance tend to have higher inflammation biomarkers, including fibrinogen concentration, than those without heparin resistance. Discordance between aPTT and anti-Xa might also be secondary to falsely low anti-Xa assay due to high free hemoglobin or hyperbilirubinemia (19). In our study, time-varying changes in plasma free hemoglobin and bilirubin level were not significantly different between “concordant,” discordant “low,” and discordant “high” pairs. However, differences in hemostasis parameters, including platelet count and INR, between those three groups might reflect different coagulation profiles.
Regarding heparin resistance, international guidelines recommend the use of anti-Xa, rather than aPTT, to monitor heparin treatment in patients with heparin resistance to adjust heparin dose (21). aPTT integrates both coagulopathy and heparin anticoagulation level. Previous studies have highlighted the poor correlation between heparin dose and aPTT in ECMO patients (14, 22), and recent studies reported a better correlation between anti-Xa and heparin than with aPTT or ACT (23). There was an association between the group of randomization of the initial pilot study (“low-dose heparin” group and the “therapeutic anticoagulation” group) and the concordance between paired anti-Xa and aPTT in this current substudy; however, it remains unknown whether the allocation group (“low dose heparin” or “therapeutic anticoagulation”) had an impact on our study findings.
The limitations of both anti-Xa and aPTT have led to the suggestion of the use of multimodal anticoagulation monitoring approach (19, 24). There is evidence that anti-Xa is a more accurate measure of heparin effect; however, anti-Xa does not provide information on common hemostasis disorders that are relevant to patients on ECMO, such as disseminated intravascular coagulation, and therefore aPTT may provide additional complementary information.
Limitations and Strengths
This was a pre-planned secondary analysis of a randomized trial with data prospectively collected. A large number of paired aPTT and anti-Xa and clinical outcomes were analyzed. However, our study has some limitations, including the relatively small sample size. Only the cumulative daily dose of heparin was known, so it was not possible to equally correlate heparin dose and assays results (for instance heparin might have been stopped for several hours when aPTT and anti-Xa were performed). We could only compared values at a fixed time and not the time spent above or below a certain values. It was not standard of care to measure anti-Xa nor to adjust anticoagulation based on anti-Xa values in both participating sites. As a consequence, our study cannot investigate whether the use of aPTT compared with anti-Xa to titrate anticoagulation impacts on bleeding and/or clotting. The median number of paired aPTT/anti-Xa was higher in patients with discordant paired aPTT/anti-Xa compared with patients without discordant paired aPTT/anti-Xa and might have led to a bias. Antithrombin III activity level was not routinely measured and was left to the discretion of clinicians precluding any consideration of this parameter in the interpretation of our findings. Viscoelastometry assays were also not available; therefore, their values in monitoring anticoagulation in patients undergoing ECMO could not be analyzed. We included patients on both venovenous and venoarterial ECMO, which may have different risks of thrombosis and changes to coagulation profile. Finally, we could not analyze the clinical relevance of aPTT/anti-Xa discordance or heparin resistance because of the small sample size and the inability to adjust for important confounders.
Impact of Study Findings
Our study reports discrepancies between aPTT and anti-Xa in patients receiving heparin while undergoing ECMO. These results suggest that aPTT alone might not be the optimal test to monitor heparin anticoagulation in patients undergoing ECMO, adding further evidence to the available literature. Concomitant use of anti-Xa and aPTT may help to identify underlying abnormalities including both heparin resistance and coagulopathy in patients receiving heparin, although heparin resistance is primarily suspected when high dose of heparin is given without significant increase in aPTT.
Future Research
Future research should investigate the benefit of a multimodal approach to monitor heparin therapy in this population and how to integrate into anticoagulation protocols other factors including coagulopathy. A pilot randomized controlled trial has shown that it is feasible to conduct a large trial comparing viscoelastic tests with conventional coagulation measures to guide anticoagulation therapy (25). Further research is warranted to identify the optimal approach to monitoring heparin in patients on ECMO. Defining the indications for argatroban or bivalirudin in this population would also be of interest.
CONCLUSIONS
In conclusion, paired aPTT/anti-Xa were discordant in more than one third of cases possibly because of either coagulopathy or heparin resistance suggesting that aPTT alone should not be used for monitoring heparin anticoagulation in patients on ECMO. Further research should investigate the optimal anticoagulation management in patients undergoing ECMO.
Supplementary Material
Footnotes
Drs. Aubron and Chapalain have contributed equally to this work and are co-first authors.
The authors have disclosed that they do not have any potential conflicts of interest.
This study was funded by an Australian and New Zealand College of Anaesthetists Project Grant (2015/007).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Aubron C, DePuydt J, Belon F, et al. : Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care 2016; 6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nasr DM, Rabinstein AA: Neurologic complications of extracorporeal membrane oxygenation. J Clin Neurol 2015; 11:383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Protti A, Iapichino GE, Nardo MD, et al. : Anticoagulation management and antithrombin supplementation practice during veno-venous extracorporeal membrane oxygenation: A worldwide survey. Anesthesiology 2020; 132:562–570 [DOI] [PubMed] [Google Scholar]
- 4.Doyle AJ, Hunt BJ: Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front Med (Lausanne) 2018; 5:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berei TJ, Lillyblad MP, Wilson KJ, et al. : Evaluation of systemic heparin versus bivalirudin in adult patients supported by extracorporeal membrane oxygenation. ASAIO J 2018; 64:623–629 [DOI] [PubMed] [Google Scholar]
- 6.Seelhammer TG, Brokmeier HM, Hamzah M, et al. : Analysis of wholesale drug acquisition and laboratory assessment costs between heparin compared with bivalirudin-based systemic anticoagulation strategies in adult extracorporeal membrane oxygenation. Crit Care Med 2023; 51:e115–e121 [DOI] [PubMed] [Google Scholar]
- 7.Vandiver JW, Vondracek TG: Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy 2012; 32:546–558 [DOI] [PubMed] [Google Scholar]
- 8.Levy JH, Connors JM: Heparin resistance - clinical perspectives and management strategies. N Engl J Med 2021; 385:826–832 [DOI] [PubMed] [Google Scholar]
- 9.Price EA, Jin J, Nguyen HM, et al. : Discordant aPTT and anti-Xa values and outcomes in hospitalized patients treated with intravenous unfractionated heparin. Ann Pharmacother 2013; 47:151–158 [DOI] [PubMed] [Google Scholar]
- 10.Whitman-Purves E, Coons JC, Miller T, et al. : Performance of anti-factor Xa versus activated partial thromboplastin time for heparin monitoring using multiple nomograms. Clin Appl Thromb Hemost 2018; 24:310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratano D, Alberio L, Delodder F, et al. : Agreement between activated partial thromboplastin time and anti-Xa activity in critically ill patients receiving therapeutic unfractionated heparin. Thromb Res 2019; 175:53–58 [DOI] [PubMed] [Google Scholar]
- 12.Liveris A, Bello RA, Friedmann P, et al. : Anti-factor Xa assay is a superior correlate of heparin dose than activated partial thromboplastin time or activated clotting time in pediatric extracorporeal membrane oxygenation*. Pediatr Crit Care Med 2014; 15:e72–e79 [DOI] [PubMed] [Google Scholar]
- 13.Irby K, Swearingen C, Byrnes J, et al. : Unfractionated heparin activity measured by anti-factor Xa levels is associated with the need for extracorporeal membrane oxygenation circuit/membrane oxygenator change: A retrospective pediatric study. Pediatr Crit Care Med 2014; 15:e175–e182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnouk S, Altshuler D, Lewis TC, et al. : Evaluation of anti-Xa and activated partial thromboplastin time monitoring of heparin in adult patients receiving extracorporeal membrane oxygenation support. ASAIO J 2020; 66:300–306 [DOI] [PubMed] [Google Scholar]
- 15.Aubron C, McQuilten Z, Bailey M, et al. ; endorsed by the International ECMO Network (ECMONet): Low-dose versus therapeutic anticoagulation in patients on extracorporeal membrane oxygenation: A pilot randomized trial. Crit Care Med 2019; 47:e563–e571 [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 17.Levine MN, Hirsh J, Gent M, et al. : A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch Intern Med 1994; 154:49–56 [PubMed] [Google Scholar]
- 18.Murphy DA, Hockings LE, Andrews RK, et al. : Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev 2015; 29:90–101 [DOI] [PubMed] [Google Scholar]
- 19.Chlebowski MM, Baltagi S, Carlson M, et al. : Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care 2020; 24:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy JH, Staudinger T, Steiner ME: How to manage anticoagulation during extracorporeal membrane oxygenation. Intensive Care Med 2022; 48:1076–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smythe MA, Priziola J, Dobesh PP, et al. : Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis 2016; 41:165–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delmas C, Jacquemin A, Vardon-Bounes F, et al. : Anticoagulation monitoring under ECMO support: A comparative study between the activated coagulation time and the anti-Xa activity assay. J Intensive Care Med 2020; 35:679–686 [DOI] [PubMed] [Google Scholar]
- 23.Al-Jazairi A, Raslan S, Al-mehizia R, et al. : Performance assessment of a multifaceted unfractionated heparin dosing protocol in adult patients on extracorporeal membrane oxygenator. Ann Pharmacother 2021; 55:592–604 [DOI] [PubMed] [Google Scholar]
- 24.Honore PM, Barreto Gutierrez L, Kugener L, et al. : Use of multiple laboratory tests including anti-factor Xa to optimally manage anticoagulation during ECMO. Crit Care 2020; 24:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panigada M, Iapichino G E, Brioni M, et al. : Thromboelastography-based anticoagulation management during extracorporeal membrane oxygenation: A safety and feasibility pilot study. Ann Intensive Care 2018; 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.