Keywords: collagen architecture, fibrosis, muscle matrix, muscle mechanics, perimysium
Abstract
The muscle extracellular matrix (ECM) forms a complex network of collagens, proteoglycans, and other proteins that produce a favorable environment for muscle regeneration, protect the sarcolemma from contraction-induced damage, and provide a pathway for the lateral transmission of contractile force. In each of these functions, the structure and organization of the muscle ECM play an important role. Many aspects of collagen architecture, including collagen alignment, cross linking, and packing density affect the regenerative capacity, passive mechanical properties, and contractile force transmission pathways of skeletal muscle. The balance between fortifying the muscle ECM and maintaining ECM turnover and compliance is highly dependent on the integrated organization, or architecture, of the muscle matrix, especially related to collagen. While muscle ECM remodeling patterns in response to exercise and disease are similar, in that collagen synthesis can increase in both cases, one outcome leads to a stronger muscle and the other leads to fibrosis. In this review, we provide a comprehensive analysis of the architectural features of each layer of muscle ECM: epimysium, perimysium, and endomysium. Further, we detail the importance of muscle ECM architecture to biomechanical function in the context of exercise or fibrosis, including disease, injury, and aging. We describe how collagen architecture is linked to active and passive muscle biomechanics and which architectural features are acutely dynamic and adapt over time. Future studies should investigate the significance of collagen architecture in muscle stiffness, ECM turnover, and lateral force transmission in the context of health and fibrosis.
INTRODUCTION
The extracellular matrix (ECM) is a dynamic tissue that contributes to the active and passive biomechanical function of skeletal muscle. While fibrillar collagens are the main component of the skeletal muscle ECM and dominate the biomechanical functions of the matrix (1, 2), other collagenous and noncollagenous components play significant roles in regulating the architecture, or organization, and function of the muscle matrix (3–6). The architecture of the muscle ECM is defined by macro- and micro-organizational properties of each ECM material and how they are arranged within the space of the muscle organ. Thus matrix architecture depends not just on how much of each collagenous and noncollagenous component exists in the ECM, it also matters where in the muscle each component resides, which components it interacts with, and how it is oriented within the ECM. Much like one can expect to struggle to pull apart a thick rope that has been straightened out, a collagen fibril exhibits greater resistance to tensile strain if it has been stretched out beyond its crimped state. For this reason and many others, the architecture of the ECM has profound effects on the biomechanical properties of muscle (7–12). In this review, we present an overview of the integrative aspects of ECM organization in skeletal muscle with a specific focus on fibrillar collagen architecture. We provide a brief summary of the biochemical composition of the ECM in terms of its contents of collagens, proteoglycans, and other structural components. Although matricellular proteins play an important role in cell signaling and the adaptations of the muscle ECM, we have focused this review on the structural proteins within the muscle matrix. Next, we detail the different architectural niches within each layer of muscle ECM from the basement membrane to the epimysium. Finally, we describe how matrix architecture adapts in response to exercise, injury, aging, and disease. Throughout this review, we highlight the importance of collagen architecture to the dynamic biochemical and biomechanical properties of the skeletal muscle ECM.
SKELETAL MUSCLE ECM STRUCTURAL COMPOSITION
Collagens
The ECM accounts for up to 10% of skeletal muscle mass (1). The primary component of the muscle ECM is type I collagen (1, 2, 13), which is made of a triple helix of polypeptide chains that can pack together to form collagen fibrils and further assemble with other collagenous and noncollagenous components to form collagen fibers (14). In addition to type I collagen, there are other fibril-forming collagens in the muscle ECM; these include types III, V, IX, and XI (15). Types III and V collagen are incorporated in the fibrous networks of the muscle ECM, albeit at lesser amounts than type I (1, 16–18). Types IV and VI collagen are not fibril forming but can form networks that are important to the structural integrity of the muscle fibers and their interaction with the surrounding ECM (1, 19–22). The muscle ECM also contains fibril-associated collagens with interrupted triple helices (FACITs) and membrane-associated collagens with interrupted triple helices (MACITs), which can interact with collagenous and noncollagenous components; these include types XII, XIV, XXII (FACITs) and type XIII (MACIT) (10, 23–25). While there are many types of collagen present in skeletal muscle, this review will primarily focus on types I, III, and IV collagen. Other reviews provide extensive detail on the structure and function of other collagens in muscle and other tissues (17, 20, 23, 26).
Collagen Cross Links
Fibrillar collagen often contains cross links, which are covalent bonds between collagen molecules that strengthen fibrils and provide resistance to enzymatic degradation (3, 4, 9, 27, 28). Collagen cross links can be enzymatically or nonenzymatically formed, and each type of cross link has different biomechanical properties (3, 4, 29). Enzymatic cross linking in muscle is facilitated by the enzymes lysyl hydroxylase, lysyl oxidase (LOX), and lysyl oxidase-like 1–4 (LOXL1-4) (3, 30). Lysine residues in the telopeptide region are hydroxylated by lysine hydroxylase 2 (LH2) and are converted to hydroxylysine aldehydes by LOX and LOXL enzymes (3). Downstream these residues can bind to lysine or hydroxylysine residues in the helical region of another collagen molecule to form immature, divalent cross links dehydro-hydroxylysinonorleucine (deH-HLNL) or dehydro-dihydroxylysinonorleucine (deH-DHLNL), respectively (3). These immature cross links can become mature, trivalent cross links by reacting with hydroxylysine aldehydes in the telopeptide region of a third collagen molecule (3). These cross links are pyridinoline cross links and are classified as either lysyl-pyridinoline (LP) or hydroxylysyl-pyridinoline (HLP) if the precursor divalent cross link was deH-HLNL or deH-DHLNL, respectively (3). Pyridinoline cross links are thought to provide increased mechanical properties compared to divalent cross links (4). Additionally, both types of enzymatic cross links are regulated in response to exercise, injury, disease, and aging (7, 8, 31–39). Nonenzymatic cross links, also known as advanced glycation end products (AGEs), are formed in response to long-term exposure to monosaccharides, which leads to a bond between the sugar and the collagen molecule and a series of reactions resulting in a nonenzymatic cross link like pentosidine (27–29, 40). AGEs increase the stiffness and strength of collagen fibrils and can contribute to tissue brittleness (41, 42). AGEs also increase in response to aging and in certain diseases such as desmin knockout mice (27, 28, 31). Overall, collagen cross links can alter ECM architecture to affect a range of biomechanical and biochemical properties.
Proteoglycans
Besides collagens the muscle ECM contains a range of proteoglycans, which are composed of a core protein with covalently linked glycosaminoglycan (GAG) chains (5). The negatively charged GAGs play an important role in tissue hydration, which can directly influence mechanics by regulating hydrostatic pressure (43). Some of the proteoglycans within muscle include short leucine-rich proteoglycans (SLRPs) and large proteoglycans. Some examples of SLRPs are decorin, biglycan, fibromodulin, and lumican. One pertinent role of SLRPs is the regulation of collagen fibrillogenesis and organization, which is influential in ECM remodeling (44–46). Decorin and biglycan levels are increased in Duchenne muscular dystrophy (DMD) muscle compared to healthy muscle (47, 48). Knockout of decorin in mice results in random orientation of collagen within the periodontal ligament (44, 46). Knockout of biglycan leads to flaws in collagen fibril formation (44). Deficiency of fibromodulin results in abnormal collagen fibrils in the tendon (44, 49), while lumican deficiency leads to abnormally thick, loosely packed collagen fibrils with disrupted lamellar organization in the skin and eye (44, 50, 51). Although there is limited information on the role of SLRPs in muscle, they seem to be important in the formation of new collagen and aligning it properly within the tissue. Their function could be relevant to changes in collagen alignment observed in fibrotic and exercised muscles (7, 8, 52). Even less is known about large proteoglycans in muscle, which bind growth factors within the ECM and include the heparan sulfate proteoglycans perlecan, agrin, and type XVIII collagen (16, 53–55). Hyaluronic acid is a large polysaccharide that forms a core structure capable of linking many proteoglycans. Hyaluronic acid is thus an important component in muscle ECM with reported values in the human lower extremity of ∼35 mg/g wet weight, albeit with large individual variation (56). Hyaluronic acid has been described as an important lubricant in muscle (57); however, breaking down hyaluronic acid with hyaluronidase has also been proposed to increase muscle compliance (58). These key structural components of the ECM transmit forces as well as serve as a scaffold for cells within muscle.
Other Structural Proteins
Within the skeletal muscle ECM, there are other proteins that contribute to the structural integrity of the matrix and its interaction with muscle fibers. Fibrillin-1 and elastin form fibrillar networks within the muscle ECM (59). Fibrillin-1 makes up microfibrils that can bind transforming growth factor-β (TGF-β) and guide the formation of elastic fibers from elastin (60). Elastin increases the elasticity of the ECM and can play roles in cell adhesion, migration, and signaling (60). Fibronectin is another important component of the muscle ECM as it can connect cell surface receptors, such as integrins, to structural components with the matrix such as collagens and proteoglycans (60, 61). Fibronectin also helps form the microfibrillar network containing fibrillin-1 (60, 62). In addition, the interface of the skeletal muscle ECM and the muscle fiber is host to proteins that are essential to establishing a mechanical link across the sarcolemma, basal lamina, and fibrous endomysium, which enables the transmission of contractile force to adjacent muscle fibers. Between the basal lamina and the sarcolemma are costameres, transmembrane complexes that link the cytoskeleton to the ECM (63). The two main types of costameres are integrins and the dystroglycan/sarcoglycan complex (63). Both types can bind laminins, which are proteins that form networks within the basal lamina (19, 63). Laminin can bind to collagen IV through interactions with nidogen or directly (19, 63, 64). These components are important in enabling shear forces between adjacent muscle fibers.
DIFFERENCES IN ARCHITECTURE ACROSS MUSCLE ECM LAYERS
The skeletal muscle ECM is traditionally organized into three main layers: endomysium, perimysium, and epimysium (15, 16, 65); the endomysium forms a continuous sheath around each individual muscle fiber, the perimysium forms a network around fascicles and bundles of muscle fibers, and the epimysium forms a matrix surrounding the entire muscle organ (15, 16, 65). However, there is clear structural and mechanical continuity between these layers such that biomechanical forces at the endomysium can be transmitted to the perimysium and epimysium (16, 66–68). This continuity is largely facilitated by the fibrous networks of type I and III collagens in each layer, which are viscoelastic and contribute to nonlinear scaling of mechanical properties from the level of myofibers to the whole muscle. Even so, there is variability in the architecture of the collagen networks between the layers of muscle ECM. This variability in both components and organization relates to the biomechanical function of each layer (Fig. 1).
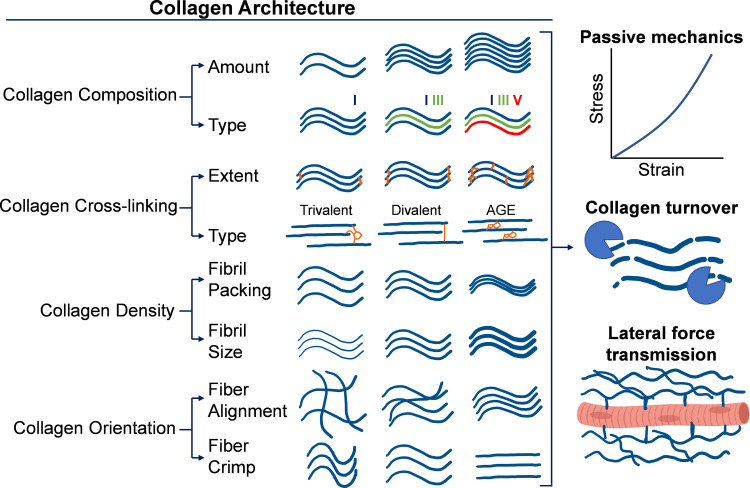
Figure 1.
Schematic of collagen architectural features within the skeletal muscle extracellular matrix (ECM). The muscle ECM contains a significant amount of fibrillar collagen, which has a complex architecture that dictates many functional properties of muscle. Composition of collagens relates to their total content and distribution among types of collagens. Cross linking relates to the degree of collagen cross links, both enzymatic and nonenzymatic, that exist between collagen molecules and fibrils. Density relates to how tightly collagen fibrils and fibers are packed together within the matrix. Orientation of collagen fibers and fibrils relates to their directional alignment and their crimp. These properties of collagen architecture relate to passive mechanics, collagen turnover, and lateral force transmission. AGE, advanced glycation end product. Figure made using BioRender.com.
Endomysium
The endomysium accounts for 0.1–1.2% of skeletal muscle dry mass (1, 2, 69, 70). The endomysium is composed of a fibrous and reticular layer (11). The fibrous layer forms a continuous sheath around each individual myofiber that appears as a network of endomysial tunnels in decellularized muscle (1, 10, 11, 16). The primary components within the fibrous endomysium are type I and III collagens, which are organized into a feltwork of fibrils that align circumferentially to the long axis of muscle fibers (11, 71). The fibrous endomysium is estimated to have a ratio of mature to immature cross links of between 2.6 and 6.6 and a ratio of deH-DHLNL to deH-HLNL cross links of 6.0 (2, 69). Thus the endomysium is primarily made of mature collagen and has more immature cross links on the biochemical pathway toward forming HLP cross links compared to LP cross links. This preference toward HLP cross links over LP is supported by a study in rat whole muscle (37) and another in desmin/nesprin knockout mice muscle bundles (31), but the ratio of HLP to LP seems to be closer in muscle bundles from healthy mice (31) and children who are typically developing or have cerebral palsy (33). These conflicts could be due to differences in mature cross link concentrations within the perimysium of the muscle bundles and the epimysium around the whole muscles; however, this is yet to be determined.
While original accounts of the endomysium asserted that the alignment of the collagen fibrils in the fibrous layer was random (71), further study revealed their circumferential orientation and sensitivity to changes in muscle length (11, 66). At resting length, the collagen fibrils of the endomysium are aligned circumferentially and are compliant to changes in muscle length (1, 11, 66); however, when muscles are shortened or lengthened the endomysial collagen fibers realign along the direction of strain (10, 11, 66). Thus, when the muscle is stretched, endomysial collagen fibers become more aligned with the axis of stretch, which is usually the long axis of the muscle. When the muscle is shortened, endomysial collagen fibers become less aligned with the axis of stretch and generally more aligned at ±90° to the long axis of the muscle. As endomysial collagen fibers become more aligned during a stretch, they increase their tensile stiffness as kinks in the fibrils and molecules begin to straighten (4, 10, 11, 72). Even so, there is evidence that the endomysium does not play a large role in the passive tensile mechanics of muscle compared to the perimysium and epimysium (73–75). This evidence is largely based on the differences in stiffness between muscle fibers, which contain endomysium, and muscle bundles and fascicles, which contain endomysium and perimysium. Essentially, larger groups of muscle tissue, i.e., whole muscle and fascicles, have greater passive mechanical properties than smaller groups of muscle tissue, i.e., bundles of myofibers and single myofibers (73, 75). Additionally, if myofibers are individually dissected and then regrouped into a bundle, their stiffness is significantly less than a bundle that was dissected intact (74). Nevertheless, the endomysium is highly important to the transmission of active contractile force from the muscle fibers to the tendons via shear force between adjacent muscle fibers (10–12, 66, 76–81), which is closely dependent on the reticular layer of the endomysium.
The reticular layer of the endomysium, also known as the basal lamina, is primarily composed of type IV collagen and laminin. Collagen IV forms a network attached to the sarcolemma of each muscle fiber (1, 19, 22, 82). The type IV collagen network is associated with a network of laminin through direct binding and via nidogen/entactin proteins (19, 63, 64, 83, 84). Laminin can also bind to dystroglycan and integrins, which help anchor the collagen IV network to the sarcolemma and provide a mechanical linkage to the cytoskeleton (19, 85, 86). The collagen IV network is also mechanically linked to the fibrous endomysium via a microfibrillar network of type VI collagen (20, 87). In this way, the reticular layer of the endomysium is mechanically linked to both the cytoskeleton and the fibrous endomysium. This detailed organization of extracellular components facilitates lateral force transmission, but mutations to these components can disrupt muscle contractile ability (87, 88).
Perimysium
The perimysium accounts for 0.4–7.0% of dry mass in skeletal muscle, with a large variability between types of muscles (1, 2, 69, 70). The perimysium is situated around fascicles and bundles of muscle fibers and supports larger vessels and the nerve supply to the muscle (10, 16, 66). Hyaluronic acid distribution is heterogeneous through the endomysium and the perimysium (56). Perimysial collagen fibers (or cables) are prominent components of the perimysium that are not found in the endomysium. These larger collagen fibers are composed mostly of type I collagen but also contain type III collagen and a small amount of type V collagen (1, 2, 16, 65, 69). The perimysium is estimated to have a ratio of mature to immature cross links of between 1.3 and 2.6 and a ratio of deH-DHLNL to deH-HLNL of 2.5 (2, 69). These values are lower compared to the endomysium, which indicate that the perimysium is made of less mature collagen than the endomysium and likely has a closer ratio of HLP to LP cross links based on the relative amounts of deH-DHLNL and deH-HLNL cross links. Further, based on the lower ratio of mature to immature cross links in the perimysium, it stands to reason that the perimysium is more susceptible to turnover than the endomysium.
Early accounts of the perimysium describe it as collagenous sheets that contain collagen fibers with a shorter crimp pattern than tendons (71, 89). These collagen fibers have been reported to be arranged in a criss-cross pattern oriented at angles of approximately ±55° to the long axis of muscle (66, 67, 71, 89). However, high-resolution images taken with second-harmonic generation microscopy show a more longitudinal alignment of perimysial collagen (7, 8). At the myotendinous junctions, the perimysial collagen fibers have a stronger alignment and are interconnected to the tendons (16, 66, 90). Throughout the muscle ECM, the perimysium connects to the endomysium via perimysial junction plates (PJPs) (67). These PJPs are thought to transmit force to the sarcolemma via integrins, which can translate mechanical forces to the cytoskeleton (66, 67, 91). Although it does not transmit contractile force using shear like the endomysium, the continuity of the perimysium with the epimysium, endomysium, and tendons enables it to participate in lateral force transmission to some extent (1, 10, 12, 16, 66, 92). Partial tenotomy experiments show that the perimysial collagen fibers take on load in tenonectomized regions of muscle and become more aligned for force transmission (10, 12, 92). Thus the realignment of collagen fibers within the perimysium has an impact on their contribution to muscle mechanical properties.
Perimysial architecture plays a significant role in the tensile mechanical properties of muscle (16, 73, 74, 93). Collagen fibers within the perimysium become more longitudinally aligned and reduce their crimp as the muscle is stretched (10, 66, 70, 93, 94). While it was originally hypothesized that the time-dependent realignment of collagen fibers was responsible for the viscous component of perimysial mechanical properties, it was shown that intrafibrillar molecular sliding drives the viscous component (10, 95, 96). Nevertheless, the reorientation of collagen fibers within the perimysium is likely related to its nonlinear mechanical properties. When perimysial collagen fibers are strained, they first reorient themselves along the axis of strain and lose their crimp (4, 10, 93, 97). Further strain causes the kinks in collagen fibrils to straighten out followed by the straightening of kinks in the collagen molecules within the fibrils (4, 10, 98). Thus, if perimysial collagen fibers are already highly aligned when the muscle is at resting length, the typical stress-strain curve would be shifted left from a standard muscle and could potentially lead to a higher stiffness at lower strains as well as a lower failure strain compared to standard muscles (7). In cases where the perimysium is strained biaxially, such as in the diaphragm, collagen fibers could undergo variable reorientation across the whole muscle; this could lead to a more complex relationship between collagen fiber alignment and stiffness that is not replicated in muscles that primarily undergo uniaxial tensile strains or in experiments that only test mechanical properties along one axis.
Epimysium
The epimysium generally accounts for 0.1–1.2% of skeletal muscle dry mass but can have wide variation between muscles. The epimysium can account for as much as 7.3% of muscle dry mass in the bovine gastrocnemius (2, 69), and it would follow that muscles with higher surface area-to-volume ratios, such as the diaphragm, would have proportionally more epimysial tissue. The primary collagens within the epimysium are types I and III (2, 16, 69). The epimysium is estimated to have a ratio of mature to immature cross links of between 1.0 and 3.8 and a ratio of deH-DHLNL to deH-HLNL of around 2.5 (2, 69). These values are similar to the perimysium, which indicate that both the epimysium and perimysium have a greater proportion of immature collagen and a smaller ratio of HLP to LP cross links than the endomysium. Taking into account the differences in HLP and LP cross links between muscles and diseases/exercise states, future studies that assess the regional turnover of collagen and cross links within the muscle ECM could provide a large benefit in identifying the architectural shifts that occur in the muscle ECM in response to disease and exercise.
The epimysium encases the whole muscle and appears as a lattice of collagen fibers that can run anywhere between parallel and perpendicular to the long axis of muscle (10, 16, 82, 89, 99, 100). These collagen fibers have a crimped pattern that is susceptible to alteration in dystrophic and diseased muscle (16, 72, 100). The epimysium consists of multiple layers of collagen fibers, which range from highly organized in the outer layer to disorganized in the middle and inner layers (68, 100). The epimysium is thought to help with transmitting tensional forces (66, 101). The epimysium is the least studied layer of muscle ECM by a large margin, but given its apparent adaptations in matrix architecture and mechanical properties in disease (99), aging (52, 100), and exercise (52), there is a great incentive to study the epimysial architecture and mechanical function in health and disease.
ECM ARCHITECTURE ADAPTATIONS AND PLASTICITY
The skeletal muscle ECM is a dynamic tissue, which turns over ∼0.5–2% of its collagen every day (102–104). Collagen fibers and other components of the ECM can adapt in response to exercise, injury, aging, and disease. There are similarities between the adaptations to these different stimuli, one being an increase in collagen synthesis. Nevertheless, the modality and intensity of the stimulus can lead to healthy adaptations, like increased contractile force transmission, or pathological adaptations, like fibrosis.
ECM Synthesis and Turnover
Multiple cell types within skeletal muscle synthesize matrix proteins and contribute to matrix remodeling. Fibro-adipogenic progenitors (FAPs) and activated fibroblasts produce most of the ECM’s structural components including collagens, proteoglycans, and fibronectin (16, 105–111). FAPs and fibroblasts also express matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPs), which break down collagen and inhibit MMPs, respectively, demonstrating their importance to ECM remodeling and adaptation (110, 112). Muscle cell progenitors are capable of expressing collagens and MMPs, which are important to matrix remodeling and proper muscle regeneration (16, 110, 113). In injured and diseased muscle, macrophages can regulate the apoptosis of FAPs or support the production of ECM through secretion of TNF-α or TGF-β, respectively (110, 114, 115). The integrated functions of multiple cell types within muscle determine the composition of the ECM by balancing the synthesis and breakdown of the structural elements. Depending on the balance between synthesis and breakdown, the muscle ECM may be remodeled in an adaptive or maladaptive fashion.
Given the critical role of the ECM, there are specific enzymes responsible for breaking components of the ECM and are tightly regulated. The matrix metalloproteinases (MMPs) are a class of enzymes that are typically secreted in a pro-state, become activated extracellularly, and target ECM proteins for degradation (6). The most prominently expressed MMPs in skeletal muscle are gelatinases (MMP2 and MMP9), which break down denatured collagen and collagen IV within the basal lamina (6). However, other MMPs act as collagenases by acting on intact collagen I and III fibers. While MMP1 and MMP13 are interstitial collagenases (116), MMP8 is mainly secreted by neutrophils (15). Some MMPs are membrane bound, such as MMP14, which plays a critical role in activating MMP2 and breaking down laminin and fibronectin (117). Importantly, during phases of matrix remodeling expression of ECM components is increased along with the expression of enzymes that break down those same ECM components. Thus blocking expression of expression of MMP2, MMP9, or MMP14 has been shown to impair muscle regeneration (118, 119). Adding MMP1 supports myoblast migration and enlargement of muscle fibers (120) while knockouts of MMP13 have reduced myoblast migration and fiber hypertrophy (113). Besides MMPs, other enzymes such as a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) and hyaluronidase are also capable of targeting ECM components for degradation (6).
The activity of enzymes that degrade ECM is tightly regulated with another class of enzymes, tissue inhibitors of metalloproteinase (TIMPs) that largely block the MMPs (6). In skeletal muscle, TIMPs have shown a variety of roles in muscle regeneration and ECM maintenance (6). Importantly, the ECM architecture itself is known to influence matrix turnover (121). While not shown specifically in skeletal muscle, the extent of collagen and type of collagen cross linking can block the susceptibility of collagen to MMP degradation (122, 123). Additionally, placing collagen fibrils under strain has also been shown to limit the susceptibility to enzymatic cleavage of collagen (124). This is purported through the exposure of MMP cleavage sites at low strains that become inaccessible at larger strains (125). Furthermore, the type of collagen also influences the degradation rate with homotrimers of type I being more resistant to degradation than type III collagen homotrimers or type I heterotrimers (with two α1-chains and one α2-chain) (126). Together these features demonstrate that the composition and architecture of the matrix are important factors of muscle ECM turnover in both healthy and diseased conditions.
Exercise
Exercise generally leads to increased collagen synthesis and decreased numbers of mature collagen cross links. When rats are exercised using treadmill running, they tend to increase collagen IV synthesis within the basal lamina surrounding the myofibers (1, 127). This increases the thickness of the reticular endomysium containing the collagen IV network. Acute eccentric exercise is sufficient to induce increases in type IV collagen content while not changing total collagen content (1, 128, 129). Synergist ablation by Achilles tendon tenotomy in rats is also sufficient to induce a large increase in collagen IV expression, which is paralleled by a 37–63% increase in myofiber cross-sectional area (130). In humans, collagen IV intensity in cross sections of muscle is increased at 22 days following an intense bout of eccentric contractions to the knee extensors (131). The increase in collagen IV synthesis could be adaptive in remodeling for larger muscle fibers and improving the lateral transmission of contractile force. Thus it is no surprise that collagen IV synthesis is robustly upregulated in response to different types of exercise.
Endurance treadmill running in rats shows a consistent trend of increased collagen synthesis and decreased mature collagen cross links in older age groups. Three days of moderate-intensity treadmill running is sufficient to increase the synthesis of collagen in mice, but total collagen content does not change (1, 132). Similarly, 4 wk of moderate treadmill running in rats is sufficient to induce collagen biosynthesis but not a change in collagen content (1, 133, 134). An acute bout of treadmill running for 9 h in mice was sufficient to increase collagen content at 20 days following the exercise, although this stimulus was severe and is not representative of a typical exercise bout (1, 135). Young rats that underwent 8 wk of treadmill running with progressively increased intensity demonstrated increased collagen content and decreased pyridinoline cross links in the gastrocnemius and soleus muscles compared to sedentary controls (37). Ten weeks of moderate treadmill running led to no change in collagen content but reduced pyridinoline cross links in middle-aged and old rat solei (38, 39). Trained old rat solei also show a drop in passive stiffness compared to trained young rats and sedentary old rats, which likely relates to the decrease in mature, pyridinoline cross links (39). Jumping exercise for 15 wk in rabbits has a similar effect to endurance training in rats such that collagen content generally increases with jump training (36). Overall, moderate endurance exercise tends to increase collagen synthesis but does not always change collagen content due to the rapid turnover and degradation of collagen (102–104). However, collagen content tends to increase following acute bouts of severe endurance exercise or in response to chronic endurance training. The number of mature cross links is generally reduced in middle-aged and older animals with endurance exercise and sometimes in young animals if the exercise intensity is high enough.
Resistance training and functional overload of skeletal muscle can also lead to adaptations to ECM architecture. In rats that underwent synergist ablation via Achilles tendon tenotomy, extensive ECM remodeling in the plantaris took place as evidenced by increases in the expression of collagens, MMPs, TIMPs, LOX, and other ECM-related factors (130). The plantaris muscle exhibited increases in total pyridinoline content and pyridinoline-to-hydroxyproline ratio between 7 and 28 days following tenotomy (130). Hydroxyproline concentration significantly decreased between 3 and 28 days following synergist ablation, but given the increase in muscle mass over the 28-day period, total hydroxyproline content was approximately equal at the initial and 28-day time points (130). In another study, young (3 mo) and old (21 mo) rats underwent weighted ladder climbing three times per week for 12 wk to induce muscle hypertrophy in the gastrocnemius and soleus muscles. Masson’s trichrome staining showed a decrease in the percentage of connective tissue area with exercise in both young and old rats (136). This was in contrast to increased expression of Col1A1 and Col3A1 in both young and old exercised rats (136). It is possible that the connective tissue was packed more densely in the exercised rats, which could lead to an increase in ECM components while exhibiting a decrease in connective tissue area in a muscle cross section. In any case, resistance training and functional overload induce changes in gene expression that lead to ECM remodeling dependent on the intensity of the overload stimulus.
Less extensively studied topics related to exercise and muscle ECM architecture include the response of collagen fiber alignment to exercise. One study looking at muscle fascia, which seems to include the epimysium, showed that voluntary wheel running in mice led to an increase in collagen fiber alignment at 45° to the muscle long axis compared to sedentary mice (52). Essentially, while sedentary mice had collagen fibers aligned mainly along the long axis of muscle, exercised mice had collagen that was reoriented at 45° to the long axis. This architectural change might be related to adaptations in lateral force transmission or other biomechanical functions, although more research needs to be done in this area.
Injury
Skeletal muscle can be injured in many different ways, so it is no surprise that there are several models of muscle injury studied in the context of fibrosis. These models of injury include the intramuscular injection of noxious substances, volumetric muscle loss, contusion, ischemia, and burn or freeze injuries. Although the extent of injury varies between these models, there are similarities in the ECM remodeling response following the initial insult. Generally, in the days and weeks following a severe injury, synthesis of collagens I, III, IV, and VI tends to increase (35, 137, 138), and there is typically an increase in interstitial area, also called fibrotic area, which is the region of tissue between muscle fibers (139). There is evidence that the amount of pyridinoline cross links is decreased following injury, along with an increase in denatured collagen (35). These indicate a remodeling response postinjury that involves the removal of mature, cross-linked collagen and the production of new, immature collagen to rebuild the damaged ECM.
The extent of ECM remodeling is dependent on the severity of the injury. Intramuscular injection of cardiotoxin (CTX) in mice is sufficient to induce an increase in fibrotic area at 4 to 7 days postinjection while glycerol injection maintains increased fibrotic area past day 14 postinjection (139). Notexin injection and freeze injury induce similar effects as CTX in mice, but after 1 mo the amount of fibrotic area is not different from uninjured mice (140). Other models of injury seem to induce more severe or robust ECM remodeling. Burn injury induced by muscle exposure to 97°C water for 10 s leads to an increase in collagen I and IV content by 14 days and an increase in collagen I, III, and IV content at 28 days postinjury (35). Additionally, burn injury reduces pyridinoline cross links by 7 days and out to 28 days, and there is a trend for higher amounts of denatured collagen starting at 7 days postburn (35). These changes indicate significant ECM remodeling as fibrillar and basement membrane collagens are increased following the injury, combined with a decline in mature collagen cross links. Contusion and ischemia injuries also induce severe ECM remodeling in mice as there is increased fibrotic area and densely packed collagen at 28 days postinjury by combined contusion and ischemia (141). However, volumetric muscle loss (VML) seems to induce the most extensive ECM remodeling with considerable variation between the injured and uninjured regions. In pigs, VML results in an increase in hydroxyproline content 30 days after injury (142). In rats, VML leads to an increase in the percentage of densely packed collagen fibers by 7 days in all regions of the muscle (137). However, in the defect area and the surrounding border, there is far more densely and loosely packed collagen than in the uninjured muscle from 7 to 48 days (137). Even so, the ratio between densely and loosely packed collagen decreased in the defect area following injury (137). By 48 days following VML in rats, collagens I, III, IV, and VI were all increased compared to before the injury, likely due to the replacement of previous contractile tissue with scar tissue (137). Thus, it is evident that more severe injuries produce more long-lasting changes to collagen content, cross linking, and packing density compared to less severe injuries. Nevertheless, the cycle of ECM remodeling that involves the turnover of the mature collagen matrix and replacement with new matrix seems to be consistent between the aforementioned models of injury.
Aging
Collagen content tends to increase with aging. In aged mice (20–24 mo), Sirius red staining showed an increase in ECM area compared to younger mice (40, 143). In human vastus lateralis, a similar effect of aging is observed such that ECM area is higher in elderly muscles and correlates positively with age across young and old subjects (144). Quantifying collagen content by hydroxyproline trends in the same direction as 20- to 24-mo-old mice show increased quadriceps and gastrocnemius collagen content compared to younger mice (40, 145). Using immunohistochemistry, another study found that collagen VI and laminin area were higher in aged mouse gastrocnemius than adult muscles (146). This same study performed proteomics analysis to show that collagen VI, collagen IV, and several laminin subunits were upregulated in the aged mouse gastrocnemius compared to adult but type I collagen was not significantly different between age groups (146). These findings were paired with an increase in basement membrane thickness within aged gastrocnemius muscles compared to adult muscles (146). These data suggest that total collagen content increases with aging, with a specific increase in matrix proteins within the basement membrane.
Aged muscles show changes in collagen architecture, specifically in cross linking and orientation of collagen fibrils. Muscles from older mice tend to have an increase in collagen cross links (8, 29, 40, 147, 148). While enzymatic collagen cross links can change readily in response to a variety of external stimuli, during aging there is a robust increase in advanced glycation end products (AGEs) that is not typically observed in response to exercise, injury, or disease (28, 29, 40). AGEs can stiffen the aged muscle ECM and make it more difficult to turnover (41, 42, 149). Thus AGEs can contribute to the accumulation of collagen within the muscle ECM and hinder muscle biomechanical properties in older muscles. Besides increased cross linking, aged muscle contains large collagen fibril bundles with greater alignment than in younger muscle (146, 150). However, there is some conflicting evidence as to whether the straightness of collagen fibers is increased or decreased in aged muscle as one study using electron microscopy reported a decrease in straightness (146) while another study using second harmonic generation microscopy showed an increase in straightness (150). Overall, the aged muscle ECM tends to accumulate collagen cross links and increase collagen alignment, similar to fibrotic muscle, which may reflect the pathology of aged muscle.
Disease
Many muscle diseases result in fibrosis, the accumulation of extracellular matrix components, which takes the place of contractile tissues and can increase tissue stiffness. In fibrosis, there are many changes to the ECM outside of general increases in matrix materials. While collagen content does increase in fibrosis, other aspects of ECM architecture such as collagen cross links and collagen fiber alignment are increased and affect muscle mechanical properties. Also, increased collagen cross linking, tension on collagen fibrils, and collagen fiber packing density can contribute to resistance of the ECM to degradation (3, 9, 121, 122, 151, 152). Clearly, the ECM remodeling that muscle undergoes during the development of fibrosis leads to pathological changes to matrix architecture that result in dysregulated muscle biomechanical properties.
In Duchenne muscular dystrophy (DMD), there is a lack of dystrophin which leads to muscle fibrosis and early mortality. In models of DMD, namely the D2.mdx and C57.mdx mice, there are large increases in collagen content and cross links across multiple muscles compared to wild-type mice (7–9, 32, 153, 154). Collagen cross links increase in D2.mdx extensor digitorum longus (EDL) and diaphragm muscles (7, 154). This trend is consistent in the C57.mdx diaphragm, which has higher amounts of cross-linked collagen than wild type and gets progressively more cross links with age (32, 155). Collagen cross links are associated with decreased specific tension in wild-type and D2.mdx muscles, and scale with elastic stiffness in D2.mdx muscles (7, 8). Collagen cross links also confer resistance to matrix turnover (3, 122, 151, 156). Similarly, increased collagen packing density in dystrophic muscles is thought to increase the resistance of the ECM to enzymatic digestion (9, 154). Overall, the increased collagen cross links in dystrophic muscle are related to muscle stiffening and promote the progression of fibrosis by making the ECM resistant to turnover.
The collagen networks within dystrophic muscle also have altered alignment in multiple layers of ECM. Perimysial collagen fiber alignment is increased at optimum muscle length in D2.mdx muscle compared to wild type (7, 8). Additionally, the alignment of perimysial collagen fibers as well as the amount of area they occupy is associated with passive stiffness in dystrophic muscle (7, 8). However, the alignment of perimysial collagen fibers can scale with stiffness differently between muscles (8). In the EDL, perimysial collagen alignment generally scales positively with passive stiffness, while in the diaphragm the opposite trend is observed such that alignment scales negatively with stiffness along the myofiber angle (longitudinal stiffness) (7, 8). This may be related to the complex muscle architecture of the diaphragm, which undergoes multiple axes of passive strain. Essentially, the alignment of perimysial collagen within the diaphragm may be oriented in a manner that is more related to its transverse stiffness rather than longitudinal stiffness. This orientation is markedly different from what is observed in the epimysial collagen fibers, which are oriented close to 40° from the myofibers in wild-type diaphragm and nearly perpendicular to the myofibers in the D2.mdx diaphragm (Fig. 2). In C57.mdx mice, the diaphragm epimysium has greater collagen fiber straightness and collagen alignment than wild type (99). Higher collagen fiber straightness relates to a reduction in collagen crimp in dystrophic diaphragm epimysial collagen fibers. This reduction in collagen crimp at resting lengths in the diaphragm epimysium could lead to a shift in the stress-strain relationship such that these epimysial collagen fibers would reach the linear region of their stereotypical mechanical behavior at lower strains than in wild-type animals, which could produce a higher stiffness and possibly a lower failure strain. The model used in Ref. 99 also predicts a higher ratio of transverse to longitudinal stiffness in the dystrophic diaphragm based on the alignment of epimysial collagen. Further, given the high surface area to volume ratio of the diaphragm, the epimysium may have a proportionally larger impact on passive mechanics in the diaphragm muscle compared to the epimysium in other muscles. Thus, taken with the findings from Ref. 8, these results suggest that increased perimysial collagen alignment in the diaphragm could point to increased stiffness in the transverse plane rather than along the myofiber angle (longitudinally), which could relate to differences in the relationship between perimysial collagen fiber alignment and longitudinal tensile stiffness between limb muscles and the diaphragm.
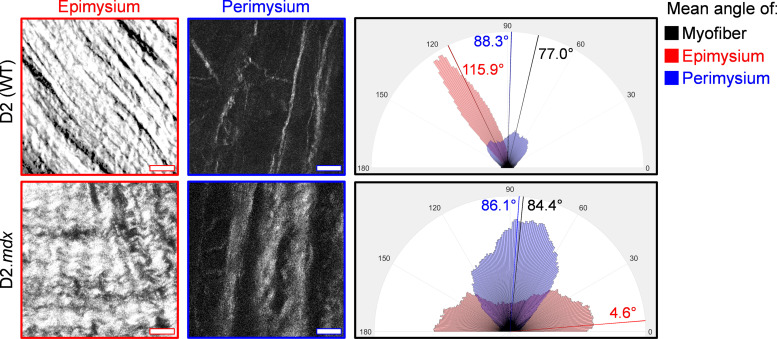
Figure 2.
Collagen alignment within the epimysium and perimysium in the D2 wild-type and D2.mdx diaphragms. Images taken using second harmonic generation microscopy show collagen fiber organization in decellularized D2 wild-type (WT) and D2.mdx whole diaphragms. The top layer of extracellular matrix (ECM), the epimysium, is oriented approximately 40° to the myofibers in the WT and 80° to the myofibers in the D2.mdx diaphragm. However, the perimysium is closely aligned along the myofiber angle in both WT and D2.mdx diaphragms. These images highlight the difference in diaphragm collagen architecture between ECM layers and genotypes. The rose plots show the distribution of collagen orientations within the representative images. Scale bars = 25 μm.
Other mutations to muscle structural proteins can lead to fibrosis. Without functional desmin, an intermediate filament that provides structural integrity to myofibers, muscles from aged mice become fibrotic (31, 94, 157). Desmin null muscles show a greater number of perimysial collagen cables and increased pyridinoline cross links compared to wild-type controls (31, 94). While desmin null muscles are stiffer than wild types, the amount of collagen cross links does not predict stiffness within the group of desmin null muscles (31, 94, 157, 158). However, cross links are useful in distinguishing stiffness between desmin null and wild-type muscles, since wild-type muscles have fewer cross links and are less stiff than desmin null muscles (31). In other forms of muscular dystrophy, there are similar levels of fibrosis that may alter ECM architecture in parallel to that observed in desmin null and dystrophic muscles (159–161).
In cerebral palsy (CP), an injury to the motor cortex during pre- or postnatal development leads to downstream fibrosis in affected skeletal muscles due to irregular sensory and motor input. It is thought that the ECM contributes to increased intrinsic stiffness in CP muscles compared to typically developing (TD) muscles (33, 162, 163). Patients with CP have increased collagen content, cross links, and passive stiffness in affected muscles compared to TD patients (33, 162, 164, 165). Hydroxylysyl- and lysyl-pyridinoline cross links are higher in CP muscles compared to TD muscles, but there is no difference in pentosidine AGE cross links (33). CP muscles also have increased levels of decorin but decreased biglycan compared to TD muscles (33). As muscle stiffness is directly responsible for fixed contractures in children with CP, methods to reduce stiffness by targeting the ECM have been proposed (58). A case series involving the local injection of hyaluronidase into upper limb muscles showed an increased range of motion indicating reduced muscle stiffness within 2 wk (166). Direct injection of collagenase into excessively stiff contractured muscle has also been proposed for use in CP, similar to its use in Dupuytren’s contracture of nonmuscle connective tissue (167). Ex vivo collagenase treatment of muscle bundles from children with CP led to decreases in passive stiffness, providing proof of concept for the use of collagenase to reduce muscle stiffness in CP (163).
Skeletal muscle fibrosis is also present in diseases that stem outside of the neuromuscular system. In type II diabetes mellitus, there are increased numbers of FAPs and enhanced collagen expression that contribute to greater ECM content within skeletal muscle compared to nondiabetics (168). It is also likely that AGEs are upregulated in the skeletal muscle of type II diabetics since AGE concentrations are increased in the kidneys, skin, and vasculature in diabetic animal models (169). Patients with chronic kidney disease (CKD) also exhibit muscle fibrosis as evidenced by higher ECM and collagen 1 area fraction and increased hydroxyproline concentration (170, 171). Muscle in CKD also has a higher amount of densely packed collagen visualized by Sirius red imaging under polarized light (170). In-depth characterization of the muscle collagen architectural changes in these diseases could help clarify the best treatments for improving muscle and whole body function.
Overall, in muscle diseases characterized by fibrosis, the architecture of the matrix changes in ways that make it stiffer and more robust (Fig. 3). While collagen content is a common marker for the extent of fibrosis, collagen cross links consistently increase in many models of muscle disease and in patients with DMD or CP. These cross links enable the progression of fibrosis by making the collagen networks harder to turn over, which is paralleled by the increased packing density of collagen. Collagen fiber alignment can also change during fibrosis, and an increase in alignment may reflect a higher degree of tension on collagen fibers, which increases their resistance to degradation. These architectural changes and others make the muscle ECM become increasingly fibrotic through a positive feedback loop of increased stiffening and reduced degradability. This also highlights that aspects of ECM architecture can also serve as targets for therapeutic interventions to block excessive muscle stiffness in a new generation of potential therapeutics.
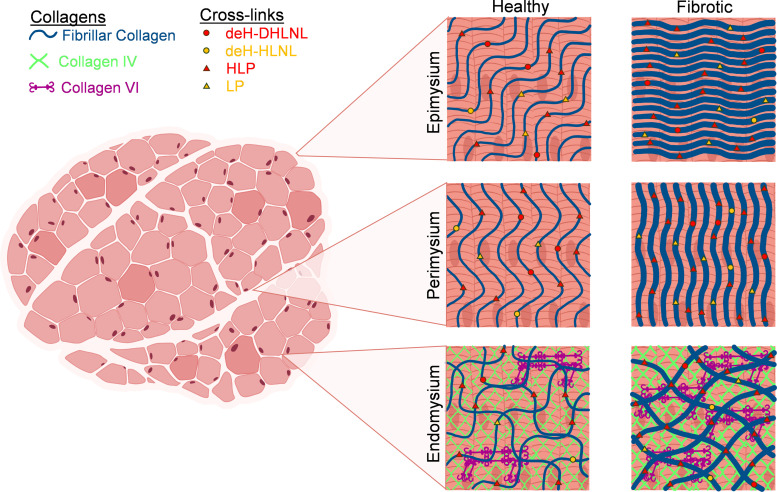
Figure 3.
Healthy and fibrotic collagen architecture across the layers of skeletal muscle extracellular matrix (ECM). Fibrotic skeletal muscle has visibly altered collagen architecture compared to healthy muscle. In general, fibrosis is accompanied by increases in collagen content, cross links, and fiber alignment. The healthy epimysium contains collagen fibers that run at a steeper angle to the myofibers than in the perimysium, and in fibrosis, the collagen may run transversely to the myofiber angle. In the perimysium, collagen fibers run in a direction similar to the myofibers, and in fibrosis, the collagen fibers become more aligned and straighter (less crimped). The endomysial collagen matrix is composed of the fibrous layer, which is mostly fibrillar collagen, and the reticular layer, which is largely collagen IV with some collagen VI that acts to link the fibrous and reticular layers. Fibrillar collagen within the endomysium forms a sheath that runs circumferentially around each myofiber. In fibrosis, there is an increase in collagens in both the reticular and fibrous layers and an increase in cross links in the fibrous layer. deH-DHLNL, dehydro-dihydroxylysinonorleucine; deH-HLNL, dehydro-hydroxylysinonorleucine; HLP, hydroxylysyl pyridinoline; LP, lysyl pyridinoline. Figure assembled using BioRender.com.
SUMMARY
From the surface of the muscle fiber to the outer edges of the muscle organ, the architecture of the muscle ECM is dynamic and contributes to many aspects of muscle biomechanics. The collagen IV network within the reticular layer of the endomysium provides a mechanical connection between the sarcolemma and the surrounding fibrous ECM via laminins, integrins, and collagen VI, among other proteins. This collagen IV network is adaptable in response to exercise to accommodate increasing sizes of myofibers and improve lateral force transmission. Surrounding the reticular endomysium is the fibrous endomysium that forms a matrix of collagens and extracellular proteins that both encompass each individual muscle fiber and connect adjacent muscle fibers using a continuous network of endomysial sheaths. The network of type I and III collagens within the fibrous endomysium can alter their orientation in response to muscle length changes and transmit contractile force to adjacent myofibers through shear interactions. In cases of disease and injury, the fibrous endomysium can hypertrophy with increased fibrillar collagens that may provide stability to the myofibers but can take the place of contractile tissue. Outside the endomysium lies the perimysium, which is mechanically linked to the endomysial sheaths by perimysial junctional plates and can assist in lateral force transmission in cases where the tendons or aponeuroses are compromised. The perimysial collagen fibers, or collagen cables, show great capacity for incurring tensile load and acutely realign during a muscular stretch or chronically realign in fibrotic muscle. The perimysium collagen network is largely responsible for the increase in mechanical properties in fascicles and bundles of muscle fibers compared to individual and regrouped muscle fibers. The perimysium is also continuous with both the tendons and the epimysium, providing a mechanical link for transferring active contractile force. Throughout all layers of the muscle ECM, collagen cross links provide stability to mature collagens, but a highly cross-linked ECM can be permissive to fibrosis through increased resistance to enzymatic degradation by MMPs. Similarly, the packing density of collagen fibers and the tension placed on them are other factors that could reduce the turnover of ECM throughout the endo-, peri-, and epimysium. Overall, the multifaceted architecture of the skeletal muscle ECM plays several biomechanical roles and is highly adaptable to a range of stimuli. Future studies that target ECM architecture therapeutically to reduce stiffness of improve collagen turnover could prove useful in many diseases characterized by fibrosis.
GRANTS
This work was supported by grants from the Hartwell Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR079545) to L. R. Smith.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.P.W., S.E.B., and L.R.S. drafted manuscript; R.P.W., S.E.B., and L.R.S. edited and revised manuscript; R.P.W., S.E.B., and L.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the members of the MyoMatrix Lab whose discussions helped to organize and develop this review.
REFERENCES
- 1. Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 2. Light N, Champion AE. Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem J 219: 1017–1026, 1984. doi: 10.1042/bj2191017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piersma B, Bank RA. Collagen cross-linking mediated by lysyl hydroxylase 2: an enzymatic battlefield to combat fibrosis. Essays Biochem 63: 377–387, 2019. doi: 10.1042/EBC20180051. [DOI] [PubMed] [Google Scholar]
- 4. Depalle B, Qin Z, Shefelbine SJ, Buehler MJ. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J Mech Behav Biomed Mater 52: 1–13, 2015. doi: 10.1016/j.jmbbm.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brandan E, Gutierrez J. Role of proteoglycans in the regulation of the skeletal muscle fibrotic response. FEBS J 280: 4109–4117, 2013. doi: 10.1111/FEBS.12278. [DOI] [PubMed] [Google Scholar]
- 6. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3: a005058, 2011. doi: 10.1101/CSHPERSPECT.A005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brashear SE, Wohlgemuth RP, Gonzalez G, Smith LR. Passive stiffness of fibrotic skeletal muscle in mdx mice relates to collagen architecture. J Physiol 599: 943–962, 2021. doi: 10.1113/JP280656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brashear SE, Wohlgemuth RP, Hu LY, Jbeily EH, Christiansen BA, Smith LR. Collagen cross-links scale with passive stiffness in dystrophic mouse muscles, but are not altered with administration of lysyl oxidase inhibitor. PLoS One 17: e0271776, 2022. doi: 10.1371/journal.pone.0271776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wohlgemuth RP, Feitzinger RM, Henricson KE, Dinh DT, Brashear SE, Smith LR. The extracellular matrix of dystrophic mouse diaphragm accounts for the majority of its passive stiffness and is resistant to collagenase digestion. Matrix Biol Plus 18: 100131, 2023. doi: 10.1016/J.MBPLUS.2023.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Purslow PP. The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Mol Integr Physiol 133: 947–966, 2002. doi: 10.1016/S1095-6433(02)00141-1. [DOI] [PubMed] [Google Scholar]
- 11. Purslow PP, Trotter JA. The morphology and mechanical properties endomysium in series-fibred muscles: variations with muscle length of. J Muscle Res Cell Motil 15: 299–308, 1994. doi: 10.1007/BF00123482. [DOI] [PubMed] [Google Scholar]
- 12. Huijing PA. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech 32: 329–345, 1999. doi: 10.1016/S0021-9290(98)00186-9. [DOI] [PubMed] [Google Scholar]
- 13. Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev 122: 735–755, 2001. doi: 10.1016/S0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 14. Varma S, Orgel JP, Schieber JD. Nanomechanics of type I collagen. Biophys J 111: 50–56, 2016. doi: 10.1016/J.BPJ.2016.05.038.doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Csapo R, Gumpenberger M, Wessner B. Skeletal muscle extracellular matrix – what do we know about its composition, regulation, and physiological roles? A narrative review. Front Physiol 11: 253, 2020. doi: 10.3389/fphys.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44: 318–331, 2011. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collins M, Posthumus M. Type V collagen genotype and exercise-related phenotype relationships: a novel hypothesis. Exerc Sport Sci Rev 39: 191–198, 2011. doi: 10.1097/JES.0B013E318224E853. [DOI] [PubMed] [Google Scholar]
- 18. Kovanen V. Intramuscular extracellular matrix: complex environment of muscle cells. Exerc Sport Sci Rev 30: 20–25, 2002. doi: 10.1097/00003677-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 19. Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem 278: 12601–12604, 2003. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 20. Bushby KM, Collins J, Hicks D. Collagen type VI myopathies. Adv Exp Med Biol 802: 185–199, 2014. doi: 10.1007/978-94-007-7893-1_12. [DOI] [PubMed] [Google Scholar]
- 21. Engvall E, Hessle H, Klier G. Molecular assembly, secretion, and matrix deposition of type VI collagen. J Cell Biol 102: 703–710, 1986. doi: 10.1083/JCB.102.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulmes DJ. Collagen diversity, synthesis and assembly. In: Collagen, edited by Fratzl P., Boston MA: Springer, 2008, p. 15–47. [Google Scholar]
- 23. Heikkinen A, Härönen H, Norman O, Pihlajaniemi T. Collagen XIII and other ECM components in the assembly and disease of the neuromuscular junction. Anatomical Record 303: 1653–1663, 2020. doi: 10.1002/AR.24092. [DOI] [PubMed] [Google Scholar]
- 24. Tu H, Huhtala P, Lee HM, Adams JC, Pihlajaniemi T. Membrane-associated collagens with interrupted triple-helices (MACITs): Evolution from a bilaterian common ancestor and functional conservation in C. elegans. BMC Evol Biol 15: 1–21, 2015. doi: 10.1186/s12862-015-0554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jakobsen JR, Mackey AL, Knudsen AB, Koch M, Kjær M, Krogsgaard MR. Composition and adaptation of human myotendinous junction and neighboring muscle fibers to heavy resistance training. Scandinavian Med Sci Sports 27: 1547–1559, 2017. doi: 10.1111/SMS.12794. [DOI] [PubMed] [Google Scholar]
- 26. Chiquet M, Birk DE, Bönnemann CG, Koch M. Collagen XII: protecting bone and muscle integrity by organizing collagen fibrils. Int J Biochem Cell Biol 53: 51–54, 2014. doi: 10.1016/J.BIOCEL.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suzuki A, Yabu A, Nakamura H. Advanced glycation end products in musculoskeletal system and disorders. Methods 203: 179–186, 2022. doi: 10.1016/J.YMETH.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 28. Olson LC, Redden JT, Schwartz Z, Cohen DJ, McClure MJ. Advanced glycation end-products in skeletal muscle aging. Bioengineering 8: 168, 2021. doi: 10.3390/BIOENGINEERING8110168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103: 2068–2076, 2007. doi: 10.1152/japplphysiol.00670.2007.-We. [DOI] [PubMed] [Google Scholar]
- 30. Yamauchi M, Shiiba M. Lysine hydroxylation and cross-linking of collagen. Methods Mol Biol 446: 95–108, 2008. doi: 10.1007/978-1-60327-084-7_7. [DOI] [PubMed] [Google Scholar]
- 31. Chapman MA, Pichika R, Lieber RL. Collagen crosslinking does not dictate stiffness in a transgenic mouse model of skeletal muscle fibrosis. J Biomech 48: 375–378, 2015. doi: 10.1016/j.jbiomech.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith LR, Hammers DW, Lee Sweeney H, Barton ER. Increased collagen cross-linking is a signature of dystrophin-deficient muscle. Muscle Nerve 54: 71–78, 2016. doi: 10.1002/mus.24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith LR, Pichika R, Meza RC, Gillies AR, Baliki MN, Chambers HG, Lieber RL. Contribution of extracellular matrix components to the stiffness of skeletal muscle contractures in patients with cerebral palsy. Connect Tissue Res 62: 287–298, 2021. doi: 10.1080/03008207.2019.1694011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petrosino JM, Leask A, Accornero F. Genetic manipulation of CCN2/CTGF unveils cell-specific ECM-remodeling effects in injured skeletal muscle. FASEB J 33: 2047–2057, 2019. doi: 10.1096/fj.201800622RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brightwell CR, Hanson ME, Ayadi AE, Prasai A, Wang Y, Finnerty CC, Fry CS. Thermal injury initiates pervasive fibrogenesis in skeletal muscle. Am J Physiol Cell Physiol 319: C277–C287, 2020. doi: 10.1152/ajpcell.00337.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ducomps C, Mauriège P, Darche B, Combes S, Lebas F, Doutreloux JP. Effects of jump training on passive mechanical stress and stiffness in rabbit skeletal muscle: role of collagen. Acta Physiol Scand 178: 215–224, 2003. doi: 10.1046/j.1365-201X.2003.01109.x. [DOI] [PubMed] [Google Scholar]
- 37. Carroll CC, Martineau K, Arthur KA, Huynh RT, Volper BD, Broderick TL. The effect of chronic treadmill exercise and acetaminophen on collagen and cross-linking in rat skeletal muscle and heart. Am J Physiol Regul Integr Comp Physiol 308: R294–R299, 2015. doi: 10.1152/ajpregu.00374.2014. [DOI] [PubMed] [Google Scholar]
- 38. Zimmerman SD, McCormick RJ, Vadlamudi RK, Thomas DP. Age and training alter collagen characteristics in fast- and slow-twitch rat limb muscle. J Appl Physiol 75: 1670–1674, 1993. doi: 10.1152/jappl.1993.75.4.1670. [DOI] [PubMed] [Google Scholar]
- 39. Gosselin LE, Adams C, Cotter TA, Mccormick RJ, Thomas DP. Effect of exercise training on passive stiffness in locomotor skeletal muscle: role of extracellular matrix. J Appl Physiol (1985) 85: 1011–1016, 1998. doi: 10.1152/jappl.1998.85.3.1011. [DOI] [PubMed] [Google Scholar]
- 40. Olson LC, Nguyen TM, Heise RL, Boyan BD, Schwartz Z, McClure MJ. Advanced glycation end products are retained in decellularized muscle matrix derived from aged skeletal muscle. Int J Mol Sci 22: 8832, 2021. doi: 10.3390/ijms22168832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamml J, Ke CY, Acevedo C, Kammer DS. The influence of AGEs and enzymatic cross-links on the mechanical properties of collagen fibrils. J Mech Behav Biomed Mater 143: 105870, 2023. doi: 10.1016/j.jmbbm.2023.105870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Svensson RB, Smith ST, Moyer PJ, Magnusson SP. Effects of maturation and advanced glycation on tensile mechanics of collagen fibrils from rat tail and Achilles tendons. Acta Biomater 70: 270–280, 2018. doi: 10.1016/j.actbio.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 43. Yanagishita M. Function of proteoglycans in the extracellular matrix. Acta Pathol Jpn 43: 283–293, 1993. doi: 10.1111/J.1440-1827.1993.TB02569.X. [DOI] [PubMed] [Google Scholar]
- 44. Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology 12: 107R–116R, 2002. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 45. Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem 271: 31767–31770, 1996. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- 46. Häkkinen L, Strassburger S, Kähäri VM, Scott PG, Eichstetter I, Iozzo RV, Larjava H. A role for decorin in the structural organization of periodontal ligament. Lab Invest 2000 80:12 80: 1869–1880, 2000. doi: 10.1038/labinvest.3780197. [DOI] [PubMed] [Google Scholar]
- 47. Bowe MA, Mendis DB, Fallon JR. The small leucine-rich repeat proteoglycan biglycan binds to-dystroglycan and is upregulated in dystrophic muscle. J Cell Biol 148: 801–810, 2000. doi: 10.1083/jcb.148.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fadic R, Mezzano V, Alvarez K, Cabrera D, Holmgren J, Brandan E. Increase in decorin and biglycan in Duchenne muscular dystrophy: role of fibroblasts as cell source of these proteoglycans in the disease. J Cellular Mol Med 10: 758–769, 2006. doi: 10.1111/j.1582-4934.2006.tb00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Svensson L, Aszódi A, Reinholt FP, Fässler R, Heinegård D, Oldberg Å. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem 274: 9636–9647, 1999. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 50. Quantock AJ, Meek KM, Chakravarti S. An X-ray diffraction investigation of corneal structure in lumican-deficient mice. Invest Ophthalmol Vis Sci 42: 1750–1756, 2001. [PubMed] [Google Scholar]
- 51. Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol 141: 1277–1286, 1998. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chaweewannakorn C, Harada T, Nyasha MR, Koide M, Shikama Y, Hagiwara Y, Sasaki K, Kanzaki M, Tsuchiya M. Imaging of muscle activity-induced morphometric changes in fibril network of myofascia by two-photon microscopy. J Anat 238: 515–526, 2021. doi: 10.1111/joa.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brandan E, Inestrosa NC. Isolation of the heparan sulfate proteoglycans from the extracellular matrix of rat skeletal muscle. J Neurobiol 18: 271–282, 1987. doi: 10.1002/neu.480180303. [DOI] [PubMed] [Google Scholar]
- 54. Iozzo RV, Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest 108: 349,–355, 2001. doi: 10.1172/JCI200113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mundhenke C, Meyer K, Drew S, Friedl A. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 receptor binding in breast carcinomas. Am J Pathol 160: 185–194, 2002. doi: 10.1016/S0002-9440(10)64362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Piehl-Aulin K, Laurent C, Engstrom-Laurent A, Hellstrom S, Henriksson J. Hyaluronan in human skeletal muscle of lower extremity: concentration, distribution, and effect of exercise. J Appl Physiol (1985) 71: 2493–2498, 1991. doi: 10.1152/jappl.1991.71.6.2493. [DOI] [PubMed] [Google Scholar]
- 57. Stecco C, Fede C, Macchi V, Porzionato A, Petrelli L, Biz C, Stern R, Caro D. R. The fasciacytes: a new cell devoted to fascial gliding regulation. Clin Anat 31: 667–676, 2018. doi: 10.1002/ca.23072. [DOI] [PubMed] [Google Scholar]
- 58. Raghavan P. Emerging therapies for spastic movement disorders. Phys Med Rehabil Clin N Am 29: 633–644, 2018. doi: 10.1016/j.pmr.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maier A, Mcdaniels CN, Mayne R. Fibrillin and elastin networks in extrafusal tissue and muscle spindles of bovine extraocular muscles. Invest Ophthalmol Vis Sci 35: 3103–3110, 1994. [PubMed] [Google Scholar]
- 60. Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol 802: 31–47, 2014. doi: 10.1007/978-94-007-7893-1_3. [DOI] [PubMed] [Google Scholar]
- 61. Singh P, Schwarzbauer JE. Fibronectin and stem cell differentiation - lessons from chondrogenesis. J Cell Sci 125: 3703–3712, 2012. doi: 10.1242/JCS.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dallas SL, Chen Q, Sivakumar P. Dynamics of assembly and reorganization of extracellular matrix proteins. Curr Top Dev Biol 75: 1–24, 2006. doi: 10.1016/S0070-2153(06)75001-3. [DOI] [PubMed] [Google Scholar]
- 63. Grounds MD, Sorokin L, White J. Strength at the extracellular matrix–muscle interface. Scand J Med Sci Sports 15: 381–391, 2005. doi: 10.1111/j.1600-0838.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 64. Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann Timpl KR, Krieg T, Engel J, Chu ML. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J 10: 3137–3146, 1991. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Purslow PP. The structure and role of intramuscular connective tissue in muscle function. Front Physiol 11: 495, 2020. doi: 10.3389/fphys.2020.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Scarr G. Fascial hierarchies and the relevance of crossed-helical arrangements of collagen to changes in the shape of muscles. J Bodyw Mov Ther 20: 377–387, 2016. doi: 10.1016/j.jbmt.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 67. Passerieux E, Rossignol R, Chopard A, Carnino A, Marini JF, Letellier T, Delage JP. Structural organization of the perimysium in bovine skeletal muscle: Junctional plates and associated intracellular subdomains. J Struct Biol 154: 206–216, 2006. doi: 10.1016/j.jsb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 68. Sleboda DA, Stover KK, Roberts TJ. Diversity of extracellular matrix morphology in vertebrate skeletal muscle. J Morphol 281: 160,–169, 2020. doi: 10.1002/jmor.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Light N, Champion AE, Voyle C, Bailey AJ. The role of epimysial, perimysial and endomysial collagen in determining texture in six bovine muscles. Meat Sci 13: 137–149, 1985. doi: 10.1016/0309-1740(85)90054-3. [DOI] [PubMed] [Google Scholar]
- 70. Purslow PP. The intramuscular connective tissue matrix and cell-matrix interaction in relation to meat toughness. In: Proceedings of the International Congress for Meat Science and Technology. Yokohama, Japan: 45th International Congress of Meat Science and Technology (ICoMST), 1999, p. 210–219. [Google Scholar]
- 71. Rowe RW. Morphology of perimysial and endomysial connective tissue in skeletal muscle. Tissue Cell 13: 681–690, 1981. doi: 10.1016/S0040-8166(81)80005-5. [DOI] [PubMed] [Google Scholar]
- 72. Wess T. Collagen fibrillar structure and hierarchies. In: Collagen, edited by Fratzl P. Boston, MA: Springer, 2008, p. 49–80. [Google Scholar]
- 73. Ward SR, Winters TM, O’Connor SM, Lieber RL. Non-linear scaling of passive mechanical properties in fibers, bundles, fascicles and whole rabbit muscles. Front Physiol 11: 211, 2020. doi: 10.3389/fphys.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meyer GA, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J Biomech 44: 771–773, 2011. doi: 10.1016/j.jbiomech.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Meyer G, Lieber RL. Muscle fibers bear a larger fraction of passive muscle tension in frogs compared with mice. J Exp Biol 221: jeb182089, 2018. doi: 10.1242/jeb.182089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jaspers RT, Brunner R, Pel JJ, Huijing PA. Acute effects of intramuscular aponeurotomy on rat gastrocnemius medialis: force transmission, muscle force and sarcomere length. J Biomech 32: 71–79, 1999. doi: 10.1016/S0021-9290(98)00159-6. [DOI] [PubMed] [Google Scholar]
- 77. Maas H, Jaspers RT, Baan GC, Huijing PA. Myofascial force transmission between a single muscle head and adjacent tissues: length effects of head III of rat EDL. J Appl Physiol 95: 2004–2013, 2003. doi: 10.1152/japplphysiol.00220.2003. [DOI] [PubMed] [Google Scholar]
- 78. Maas H, Baan GC, Huijing PA. Intermuscular interaction via myofascial force transmission: effects of tibialis anterior and extensor hallucis longus length on force transmission from rat extensor digitorum longus muscle. J Biomech 34: 927–940, 2001. doi: 10.1016/S0021-9290(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 79. Magid A, Law DJ. Myofibrils bear most of the resting tension in frog skeletal muscle. Science (1979) 230: 1280–1282, 1985. doi: 10.1126/SCIENCE.4071053. [DOI] [PubMed] [Google Scholar]
- 80. Monti RJ, Roy RR, Hodgson JA, Reggie Edgerton V. Transmission of forces within mammalian skeletal muscles. J Biomech 32: 371–380, 1999. doi: 10.1016/S0021-9290(98)00189-4. [DOI] [PubMed] [Google Scholar]
- 81. Trotter JA, Purslow PP. Functional morphology of the endomysium in series fibered muscles. J Morphol 212: 109–122, 1992. doi: 10.1002/jmor.1052120203. [DOI] [PubMed] [Google Scholar]
- 82. Purslow P. The extracellular matrix of skeletal and cardiac muscle. In: Collagen, edited by Fratzl P. Boston, MA: Springer, 2008, p. 325–357. [Google Scholar]
- 83. Aumailley M, Battaglia C, Mayer U, Reinhardt D, Nischt R, Timpl R, Fox JW. Nidogen mediates the formation of ternary complexes of basement membrane components. Kidney Int 43: 7–12, 1993. doi: 10.1038/ki.1993.3. [DOI] [PubMed] [Google Scholar]
- 84. Timpl R, Brown JC. Supramolecular assembly of basement membranes. BioEssays 18: 123–132, 1996. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 85. Mayer U. Integrins: Redundant or important players in skeletal muscle? J Biol Chem 278: 14587–14590, 2003. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- 86. Michele DE, Campbell KP. Dystrophin-glycoprotein complex: post-translational processing and dystroglycan function. J Biol Chem 278: 15457–15460, 2003. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- 87. Jobsis GJ, Keizers H, Vreijling JP, De Visser M, Speer MC, Wolterman RA, Baas F, Bolhuis PA. Type VI collagen mutations in Bethlem myopathy, an autosomal dominant myopathy with contractures. Nat Genet 14: 113–115, 1996. doi: 10.1038/ng0996-113. [DOI] [PubMed] [Google Scholar]
- 88. Zambon AA, Muntoni F. Congenital muscular dystrophies: what is new? Neuromuscular Disorders 31: 931–942, 2021. doi: 10.1016/j.nmd.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 89. Rowe RW. Collagen fibre arrangement in intramuscular connective tissue. Changes associated with muscle shortening and their possible relevance to raw meat toughness measurements. Int J Food Sci Technol 9: 501–508, 2007. doi: 10.1111/J.1365-2621.1974.TB01799.X. [DOI] [Google Scholar]
- 90. Passerieux E, Rossignol R, Letellier T, Delage JP. Physical continuity of the perimysium from myofibers to tendons: involvement in lateral force transmission in skeletal muscle. J Struct Biol 159: 19–28, 2007. doi: 10.1016/j.jsb.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 91. Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 10: 75–82, 2009. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 92. Huijing PA, Baan GC, Rebel GT. Non-myotendinous force transmission in rat extensor digitorum longus muscle. J Exp Biol 201: 683–691, 1998. doi: 10.1242/jeb.201.5.683. [DOI] [PubMed] [Google Scholar]
- 93. Purslow PP. Strain-induced reorientation of an intramuscular connective tissue network: Implications for passive muscle elasticity. J Biomech 22: 21–31, 1989. doi: 10.1016/0021-9290(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 94. Gillies AR, Chapman MA, Bushong EA, Deerinck TJ, Ellisman MH, Lieber RL. High resolution three-dimensional reconstruction of fibrotic skeletal muscle extracellular matrix. J Physiol 595: 1159–1171, 2017. doi: 10.1113/JP273376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S, Purslow P, Fratzl P. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Phil Trans R Soc Lond B 357: 191–197, 2002. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Silver FH, Landis WJ. Viscoelasticity, energy storage and transmission and dissipation by extracellular matrices in vertebrates. In: Collagen, edited by Fratzl P. Boston, MA: Springer, 2008, p. 133–154. [Google Scholar]
- 97. Mohammadkhah M, Murphy P, Simms CK. Collagen fibril organization in chicken and porcine skeletal muscle perimysium under applied tension and compression. J Mech Behav Biomed Mater 77: 734–744, 2018. doi: 10.1016/j.jmbbm.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 98. Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S. Fibrillar structure and mechanical properties of collagen. J Struct Biol 122: 119–122, 1998. doi: 10.1006/jsbi.1998.3966. [DOI] [PubMed] [Google Scholar]
- 99. Sahani R, Wallace CH, Jones BK, Blemker SS. Diaphragm muscle fibrosis involves changes in collagen organization with mechanical implications in Duchenne muscular dystrophy. J Appl Physiol (1985) 132: 653–672, 2022. doi: 10.1152/japplphysiol.00248.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech 41: 465–469, 2008. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Turrina A, Martínez-González MA, Stecco C. The muscular force transmission system: Role of the intramuscular connective tissue. J Bodyw Mov Ther 17: 95–102, 2013. doi: 10.1016/j.jbmt.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 102. Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab 288: E1153–E1159, 2005. doi: 10.1152/ajpendo.00387.2004.-We. [DOI] [PubMed] [Google Scholar]
- 103. Babraj JA, Cuthbertson DJ, Smith K, Langberg H, Miller B, Krogsgaard MR, Kjaer M, Rennie MJ. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab 289: E864–E869, 2005. doi: 10.1152/AJPENDO.00243.2005. [DOI] [PubMed] [Google Scholar]
- 104. Holwerda AM, Van Loon LJ. The impact of collagen protein ingestion on musculoskeletal connective tissue remodeling: a narrative review. Nutr Rev 80: 1497–1514, 2022. doi: 10.1093/nutrit/nuab083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gatchalian CL, Schachner M, Sanes JR. Fibroblasts that proliferate near denervated synaptic sites in skeletal muscle synthesize the adhesive molecules tenascin(J1), N-CAM, fibronectin, and a heparan sulfate proteoglycan. J Cell Biol 108: 1873–1890, 1989. doi: 10.1083/jcb.108.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kühl U, Öcalan M, Timpl R, Mayne R, Hay E, von der Mark K. Role of muscle fibroblasts in the deposition of type-IV collagen in the basal lamina of myotubes. Differentiation 28: 164–172, 1984. doi: 10.1111/j.1432-0436.1984.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 107. Riso EM, Kaasik P, Seene T. Remodelling of skeletal muscle extracellular matrix: effect of unloading and reloading. In: Composition and Function of the Extracellular Matrix in the Human Body, edited by Travascio F. IntechOpen, 2016. doi: 10.5772/61601. [DOI] [Google Scholar]
- 108. Giordani L, He GJ, Negroni E, Sakai H, Law JY, Siu MM, Wan R, Corneau A, Tajbakhsh S, Cheung TH, Le Grand F. High-dimensional single-cell cartography reveals novel skeletal muscle-resident cell populations. Mol Cell 74: 609–621, 2019. doi: 10.1016/j.molcel.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 109. Ely S, Tippett J, Moxon ER. Identification and characterization of a serotype b-specific segment of the Haemophilus influenzae genome. Infect Immun 57: 2926–2928, 1989. doi: 10.1128/iai.57.9.2926-2928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Smith LR, Barton ER. Regulation of fibrosis in muscular dystrophy. Matrix Biol 68-69: 602–615, 2018. doi: 10.1016/J.MATBIO.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Loomis T, Smith LR. Thrown for a loop: fibro-adipogenic progenitors in skeletal muscle fibrosis. Am J Physiol Cell Physiol. In press. doi: 10.1152/ajpcell.00245.2023. [DOI] [PubMed] [Google Scholar]
- 112. Wang X, Chen J, Homma ST, Wang Y, Smith GR, Ruf-Zamojski F, Sealfon SC, Zhou L. Diverse effector and regulatory functions of fibro/adipogenic progenitors during skeletal muscle fibrosis in muscular dystrophy. iScience 26: 105775, 2023. doi: 10.1016/J.ISCI.2022.105775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Smith LR, Kok HJ, Zhang B, Chung D, Spradlin RA, Rakoczy KD, Lei H, Boesze-Battaglia K, Barton ER. Matrix metalloproteinase 13 from satellite cells is required for efficient muscle growth and regeneration. Cell Physiol Biochem 54: 333–353, 2020. doi: 10.33594/000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FMV. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21: 786–794, 2015. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 115. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44: 450–462, 2016. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adh Migr 3: 337–341, 2009. doi: 10.4161/cam.3.4.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370: 61–65, 1994. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 118. Zimowska M, Brzoska E, Swierczynska M, Streminska W, Moraczewski J. Distinct patterns of MMP-9 and MMP-2 activity in slow and fast twitch skeletal muscle regeneration in vivo. Int J Dev Biol 52: 307–314, 2008. doi: 10.1387/ijdb.072331mz. [DOI] [PubMed] [Google Scholar]
- 119. Snyman C, Niesler CU. MMP-14 in skeletal muscle repair. J Muscle Res Cell Motil 36: 215–225, 2015. doi: 10.1007/s10974-015-9414-4. [DOI] [PubMed] [Google Scholar]
- 120. Wang W, Pan H, Murray K, Jefferson BS, Li Y. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am J Pathol 174: 541–549, 2009. doi: 10.2353/ajpath.2009.080509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Saini K, Cho S, Dooling LJ, Discher DE. Tension in fibrils suppresses their enzymatic degradation – A molecular mechanism for ‘use it or lose it’. Matrix Biol 85-86: 34–46, 2020. doi: 10.1016/J.MATBIO.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Van Der Slot-Verhoeven AJ, Van Dura EA, Attema J, Blauw B, DeGroot J, Huizinga TW, Zuurmond AM, Bank RA. The type of collagen cross-link determines the reversibility of experimental skin fibrosis. Biochim Biophys Acta Mol Basis Dis 1740: 60–67, 2005. doi: 10.1016/j.bbadis.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 123. Afratis NA, Klepfish M, Karamanos NK, Sagi I. The apparent competitive action of ECM proteases and cross-linking enzymes during fibrosis: applications to drug discovery. Adv Drug Deliv Rev 129: 4–15, 2018. doi: 10.1016/j.addr.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 124. Dittmore A, Silver J, Sarkar SK, Marmer B, Goldberg GI, Neuman KC. Internal strain drives spontaneous periodic buckling in collagen and regulates remodeling. Proc Natl Acad Sci U S A 113: 8436–8441, 2016. doi: 10.1073/pnas.1523228113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Flynn BP, Bhole AP, Saeidi N, Liles M, Dimarzio CA, Ruberti JW. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS One 5: e12337, 2010. doi: 10.1371/journal.pone.0012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mekkat A, Poppleton E, An B, Visse R, Nagase H, Kaplan DL, Brodsky B, Lin YS. Effects of flexibility of the α2 chain of type I collagen on collagenase cleavage. J Struct Biol 203: 247–254, 2018. doi: 10.1016/j.jsb.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kanazawa Y, Nagano M, Koinuma S, Sugiyo S, Shigeyoshi Y. Effects of endurance exercise on basement membrane in the soleus muscle of aged rats. Acta Histochem Cytochem 54: 167–175, 2021. doi: 10.1267/ahc.21-00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Koskinen SO, Ahtikoski AM, Komulainen J, Hesselink MK, Drost MR, Takala TE. Short-term effects of forced eccentric contractions on collagen synthesis and degradation in rat skeletal muscle. Pflugers Arch 444: 59–72, 2002. doi: 10.1007/s00424-002-0792-2. [DOI] [PubMed] [Google Scholar]
- 129. Koskinen SO, Wang W, Ahtikoski AM, Kjaer M, Han XY, Komulainen J, Kovanen V, Takala TE. Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am J Physiol Regul Integr Comp Physiol 280: R1292–R1300, 2001. doi: 10.1152/ajpregu.2001.280.5.R1292. [DOI] [PubMed] [Google Scholar]
- 130. Mendias CL, Schwartz AJ, Grekin JA, Gumucio JP, Sugg KB. Changes in muscle fiber contractility and extracellular matrix production during skeletal muscle hypertrophy. J Appl Physiol 122: 571–579, 2017. doi: 10.1152/japplphysiol.00719.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mackey AL, Donnelly AE, Turpeenniemi-Hujanen T, Roper HP. Skeletal muscle collagen content in humans after high-force eccentric contractions. J Appl Physiol (1985) 97: 197–203, 2004. doi: 10.1152/japplphysiol.01174.2003. [DOI] [PubMed] [Google Scholar]
- 132. Takala TE, Myllylä R, Salminen A, Anttinen H, Vihko V. Increased activities of prolyl 4-hydroxylase and galactosylhydroxylysyl glucosyltransferase, enzymes of collagen biosynthesis, in skeletal muscle of endurance-trained mice. Pflugers Arch 399: 271–274, 1983. doi: 10.1007/BF00652751. [DOI] [PubMed] [Google Scholar]
- 133. Kovanen V, Suominen H, Heikkinen E. Connective tissue of “fast” and “slow” skeletal muscle in rats–effects of endurance training. Acta Physiol Scand 108: 173–180, 1980. doi: 10.1111/j.1748-1716.1980.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 134. Kovanen V, Suominen H, Heikkinen E. Collagen of slow twitch and fast twitch muscle fibres in different types of rat skeletal muscle. Eur J Appl Physiol Occup Physiol 52: 235–242, 1984. doi: 10.1007/BF00433399. [DOI] [PubMed] [Google Scholar]
- 135. Myllylä R, Salminen A, Peltonen L, Takala TE, Vihko V. Collagen metabolism of mouse skeletal muscle during the repair of exercise injuries. Pflugers Arch 407: 64–70, 1986. doi: 10.1007/BF00580722. [DOI] [PubMed] [Google Scholar]
- 136. Guzzoni V, Ribeiro MB, Lopes GN, Marqueti R de C, de Andrade RV, Selistre-de-Araujo HS, Durigan JL. Effect of resistance training on extracellular matrix adaptations in skeletal muscle of older rats. Front Physiol 9: 374, 2018. doi: 10.3389/fphys.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hoffman DB, Raymond-Pope CJ, Sorensen JR, Corona BT, Greising SM. Temporal changes in the muscle extracellular matrix due to volumetric muscle loss injury. Connect Tissue Res 63: 124–137, 2022. doi: 10.1080/03008207.2021.1886285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kim JT, Kasukonis BM, Brown LA, Washington TA, Wolchok JC. Recovery from volumetric muscle loss injury: a comparison between young and aged rats. Exp Gerontol 83: 37–46, 2016. doi: 10.1016/j.exger.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mahdy MA, Lei HY, Wakamatsu JI, Hosaka YZ, Nishimura T. Comparative study of muscle regeneration following cardiotoxin and glycerol injury. Ann Anat 202: 18–27, 2015. doi: 10.1016/j.aanat.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 140. Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thépenier C, Pascal Q, Guguin A, Gayraud-Morel B, Cavaillon JM, Tajbakhsh S, Rocheteau P, Chrétien F. Comparative study of injury models for studying muscle regeneration in mice. PLoS One 11: e0147198, 2016. doi: 10.1371/journal.pone.0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Deng P, Qiu S, Liao F, Jiang Y, Zheng C, Zhu Q. Contusion concomitant with ischemia injury aggravates skeletal muscle necrosis and hinders muscle functional recovery. Exp Biol Med (Maywood) 247: 1577–1590, 2022. doi: 10.1177/15353702221102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Corona BT, Rivera JC, Dalske KA, Wenke JC, Greising SM. Pharmacological mitigation of fibrosis in a porcine model of volumetric muscle loss injury. Tissue Eng Part A 26: 636–646, 2020. doi: 10.1089/ten.tea.2019.0272. [DOI] [PubMed] [Google Scholar]
- 143. Chen WJ, Lin IH, Lee CW, Chen YF. Aged skeletal muscle retains the ability to remodel extracellular matrix for degradation of collagen deposition after muscle injury. Int J Mol Sci 22: 1–14, 2021. doi: 10.3390/IJMS22042123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pavan P, Monti E, Bondí M, Fan C, Stecco C, Narici M, Reggiani C, Marcucci L. Alterations of extracellular matrix mechanical properties contribute to age-related functional impairment of human skeletal muscles. Int J Mol Sci 21: 3992, 2020. doi: 10.3390/ijms21113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lacraz G, Rouleau AJ, Couture V, Söllrald T, Drouin G, Veillette N, Grandbois M, Grenier G. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS One 10: e0136217, 2015. doi: 10.1371/journal.pone.0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lofaro FD, Cisterna B, Lacavalla MA, Boschi F, Malatesta M, Quaglino D, Zancanaro C, Boraldi F. Age-related changes in the matrisome of the mouse skeletal muscle. Int J Mol Sci 22: 10564, 2021. doi: 10.3390/ijms221910564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gosselin LE, Martinez DA, Vailas AC, Sieck GC. Passive length-force properties of senescent diaphragm: relationship with collagen characteristics. J Appl Physiol (1985) 76: 2680–2685, 1994. doi: 10.1152/jappl.1994.76.6.2680. [DOI] [PubMed] [Google Scholar]
- 148. Palokangas H, Kovanen V, Duncan R, Robins SP. Age-related changes in the concentration of hydroxypyridinium crosslinks in functionally different skeletal muscles. Matrix 12: 291–296, 1992. doi: 10.1016/S0934-8832(11)80081-8. [DOI] [PubMed] [Google Scholar]
- 149. Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 275: 39027–39031, 2000. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 150. Stearns-Reider KM, D’Amore A, Beezhold K, Rothrauff B, Cavalli L, Wagner WR, Vorp DA, Tsamis A, Shinde S, Zhang C, Barchowsky A, Rando TA, Tuan RS, Ambrosio F. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 16: 518–528, 2017. doi: 10.1111/acel.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Vater CA, Harris ED, Siegel RC. Native cross-links in collagen fibrils induce resistance to human synovial collagenase. Biochem J 181: 639–645, 1979. doi: 10.1042/bj1810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Philp CJ, Siebeke I, Clements D, Miller S, Habgood A, John AE, Navaratnam V, Hubbard RB, Jenkins G, Johnson SR. Extracellular matrix cross-linking enhances fibroblast growth and protects against matrix proteolysis in lung fibrosis. Am J Respir Cell Mol Biol 58: 594–603, 2018. doi: 10.1165/rcmb.2016-0379OC. [DOI] [PubMed] [Google Scholar]
- 153. Hammers DW, Hart CC, Matheny MK, Wright LA, Armellini M, Barton ER, Sweeney HL. The D2.mdx mouse as a preclinical model of the skeletal muscle pathology associated with Duchenne muscular dystrophy. Sci Rep 10: 14070, 2020. doi: 10.1038/s41598-020-70987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am J Physiol Cell Physiol 306: C889–C898, 2014. doi: 10.1152/ajpcell.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hartel JV, Granchelli JA, Hudecki MS, Pollina CM, Gosselin LE. Impact of prednisone on TGF-B1 and collagen in diaphragm muscle from mdx mice. Muscle Nerve 24: 428–432, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 156. Robins SP, Milne G, Duncan A, Davies C, Butt R, Greiling D, James IT. Increased skin collagen extractability and proportions of collagen type iii are not normalized after 6 months healing of human excisional wounds. J Invest Dermatol 121: 267–272, 2003. doi: 10.1046/j.1523-1747.2003.12373.x. [DOI] [PubMed] [Google Scholar]
- 157. Meyer GA, Lieber RL. Skeletal muscle fibrosis develops in response to desmin deletion. Am J Physiol Cell Physiol 302: C1609–C1620, 2012. doi: 10.1152/AJPCELL.00441.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Anderson J, Li Z, Goubel F. Passive stiffness is increased in soleus muscle of desmin knockout mouse. Muscle Nerve 24: 1090–1092, 2001. doi: 10.1002/mus.1115. [DOI] [PubMed] [Google Scholar]
- 159. Gallardo E, Saenz A, Illa I. Limb-girdle muscular dystrophy 2A. Handb Clin Neurol 101: 97–110, 2011. doi: 10.1016/B978-0-08-045031-5.00006-2. [DOI] [PubMed] [Google Scholar]
- 160. Alonso-Pérez J, Carrasco-Rozas A, Borrell-Pages M, Fernández-Simón E, Piñol-Jurado P, Badimon L, Wollin L, Lleixà C, Gallardo E, Olivé M, Díaz-Manera J, Suárez-Calvet X. Nintedanib reduces muscle fibrosis and improves muscle function of the alpha-sarcoglycan-deficient mice. Biomedicines 10: 2629, 2022. doi: 10.3390/biomedicines10102629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. McKee KK, Yurchenco PD. Dual transgene amelioration of Lama2-null muscular dystrophy. Matrix Biol 118: 1–15, 2023. doi: 10.1016/j.matbio.2023.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol 589: 2625–2639, 2011. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Howard JJ, Joumaa V, Robinson KG, Lee SK, Akins RE, Syed F, Shrader MW, Huntley JS, Graham HK, Leonard T, Herzog W. Collagenase treatment decreases muscle stiffness in cerebral palsy: A preclinical ex vivo biomechanical analysis of hip adductor muscle fiber bundles. Dev Med Child Neurol. In press. doi: 10.1111/DMCN.15637. [DOI] [PubMed] [Google Scholar]
- 164. Booth CM, Cortina-Borja MJ, Theologis TN. Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Dev Med Child Neurol 43: 314–320, 2001. doi: 10.1017/S0012162201000597. [DOI] [PubMed] [Google Scholar]
- 165. De Bruin M, Smeulders MJ, Kreulen M, Huijing PA, Jaspers RT. Intramuscular connective tissue differences in spastic and control muscle: a mechanical and histological study. PLoS One 9: e101038, 2014. doi: 10.1371/journal.pone.0101038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Raghavan P, Lu Y, Mirchandani M, Stecco A. Human recombinant hyaluronidase injections for upper limb muscle stiffness in individuals with cerebral injury: a case series. EBioMedicine 9: 306–313, 2016. doi: 10.1016/j.ebiom.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Howard JJ, Huntley JS, Graham HK, Herzog WL. Intramuscular injection of collagenase clostridium histolyticum may decrease spastic muscle contracture for children with cerebral palsy. Med Hypotheses 122: 126–128, 2019. doi: 10.1016/j.mehy.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 168. Farup J, Just J, de Paoli F, Lin L, Jensen JB, Billeskov T, Roman IS, Cömert C, Møller AB, Madaro L, Groppa E, Fred RG, Kampmann U, Gormsen LC, Pedersen SB, Bross P, Stevnsner T, Eldrup N, Pers TH, Rossi FM, Puri PL, Jessen N. Human skeletal muscle CD90+ fibro-adipogenic progenitors are associated with muscle degeneration in type 2 diabetic patients. Cell Metab 33: 2201–2214, 2021. doi: 10.1016/j.cmet.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia 44: 129–146, 2001. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 170. Brightwell CR, Kulkarni AS, Paredes W, Zhang K, Perkins JB, Gatlin KJ, Custodio M, Farooq H, Zaidi B, Pai R, Buttar RS, Tang Y, Melamed ML, Hostetter TH, Pessin JE, Hawkins M, Fry CS, Abramowitz MK. Muscle fibrosis and maladaptation occur progressively in CKD and are rescued by dialysis. JCI Insight 6: e150112, 2021. doi: 10.1172/jci.insight.150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Abramowitz MK, Paredes W, Zhang K, Brightwell CR, Newsom JN, Kwon HJ, Custodio M, Buttar RS, Farooq H, Zaidi B, Pai R, Pessin JE, Hawkins M, Fry CS. Skeletal muscle fibrosis is associated with decreased muscle inflammation and weakness in patients with chronic kidney disease. Am J Physiol Renal Physiol 315: F1658–F1669, 2018. doi: 10.1152/ajprenal.00314.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]