Abstract
Background and Aims
Reports of outcomes after atrial fibrillation (AF) diagnosis are conflicting. The aim of this study was to investigate mortality and hospitalization rates following AF diagnosis over time, by cause and by patient features.
Methods
Individuals aged ≥16 years with a first diagnosis of AF were identified from the UK Clinical Practice Research Datalink-GOLD dataset from 1 January 2001, to 31 December 2017. The primary outcomes were all-cause and cause-specific mortality and hospitalization at 1 year following diagnosis. Poisson regression was used to calculate rate ratios (RRs) for mortality and incidence RRs (IRRs) for hospitalization and 95% confidence intervals (CIs) comparing 2001/02 and 2016/17, adjusted for age, sex, region, socio-economic status, and 18 major comorbidities.
Results
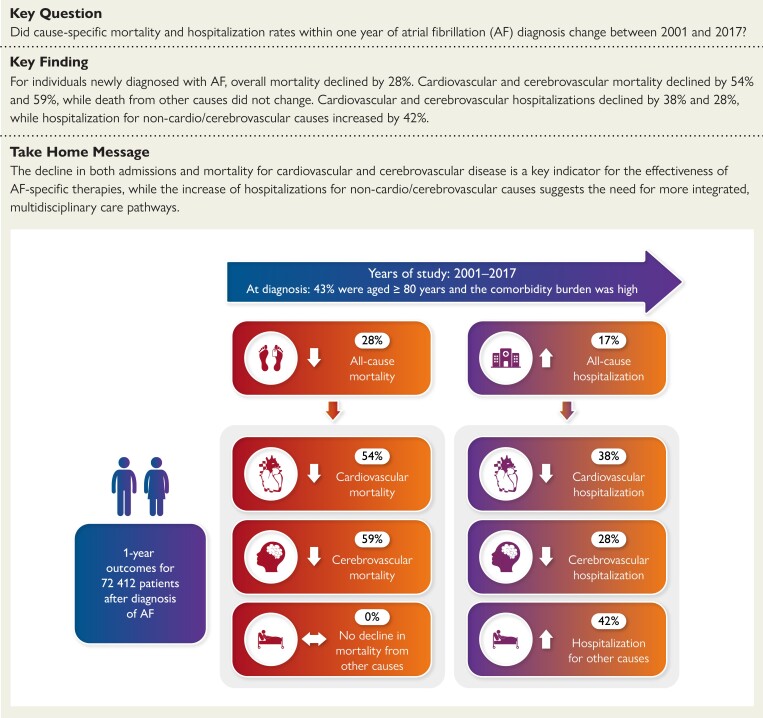
Of 72 412 participants, mean (standard deviation) age was 75.6 (12.4) years, and 44 762 (61.8%) had ≥3 comorbidities. All-cause mortality declined (RR 2016/17 vs. 2001/02 0.72; 95% CI 0.65–0.80), with large declines for cardiovascular (RR 0.46; 95% CI 0.37–0.58) and cerebrovascular mortality (RR 0.41; 95% CI 0.29–0.60) but not for non-cardio/cerebrovascular causes of death (RR 0.91; 95% CI 0.80–1.04). In 2016/17, deaths caused from dementia (67, 8.0%), outstripped deaths from acute myocardial infarction, heart failure, and acute stroke combined (56, 6.7%, P < .001). Overall hospitalization rates increased (IRR 2016/17 vs. 2001/02 1.17; 95% CI, 1.13–1.22), especially for non-cardio/cerebrovascular causes (IRR 1.42; 95% CI 1.39–1.45). Older, more deprived, and hospital-diagnosed AF patients experienced higher event rates.
Conclusions
After AF diagnosis, cardio/cerebrovascular mortality and hospitalization has declined, whilst hospitalization for non-cardio/cerebrovascular disease has increased.
Keywords: Atrial fibrillation, Mortality, Electronic health records, Hospitalization, Public health
Structured Graphical Abstract
Structured Graphical abstract.
Trends in all-cause and cause-specific mortality and hospitalization within 1 year after atrial fibrillation (AF) diagnosis between 2001 and 2017 in the UK.
See the editorial comment for this article ‘Adverse outcomes in patients with atrial fibrillation: how previous successes can unmask new challenges’, by P.B. Meyre and D. Conen, https://doi.org/10.1093/eurheartj/ehad575.
Introduction
Evaluating whether rates of mortality and hospitalization after atrial fibrillation (AF) diagnosis have changed over time is essential to understand the success or failure of the current management of AF. Recent reports of temporal trends of mortality amongst patients with AF are conflicting. Most studies demonstrate that mortality has decreased,1–4 others report that it has remained steady,5 and some suggest that it has marginally increased.6 To our knowledge, the reasons underlying the inconsistency between these reports are unknown.
Most studies have restricted analyses to all-cause mortality without investigating the underlying patterns, such as changes in mortality by subgroup or cause. In addition, though reports generally concur that hospitalization rates amongst individuals with AF have increased over time,3,7 little is known about temporal trends in cause-specific hospitalization. In-depth analysis of how patient characteristics and cause-specific mortality and hospitalization vary over time could help explain the observed trends and inform the development of more targeted therapies or public health strategies.
To address this knowledge gap, we used a large longitudinal database of electronic health records (EHRs) that links primary care, secondary care, and the national death registry from a representative sample of the UK population. We performed a detailed assessment of health outcomes in patients with a new diagnosis of AF and analysed changes in cause-specific mortality and hospitalization over time and by sex, age, socio-economic status, and diagnostic care setting.
Methods
The study findings are reported in accordance with the Reporting of Studies Conducted Using Observational Routinely Collected Health Data recommendations and CODE-EHR framework.8,9
Data source
We used EHRs from the Clinical Practice Research Datalink (CPRD) from 1 January 2001, to 30 November 2018. Clinical Practice Research Datalink is one of the largest databases of longitudinal primary care records in the world and contains anonymized patient data from ∼7% of the UK population, providing a cohort which is broadly representative of the general population in terms of age, sex, and ethnicity.10 In order to contribute to the database, general practices and other health centres must meet pre-specified standards for research quality data (‘up-to-standard’).10,11 Diagnostic coding for AF in CPRD has been shown to be consistent and valid, with a positive predictive value of 98%.12 Primary care records were linked to hospital admissions using Hospital Episodes Statistics Admitted Patient Care data and mortality data from the Office for National Statistics. Scientific approval for this study was given by the CPRD Independent Scientific Advisory Committee.
Study population
Patients eligible for inclusion were all individuals aged 16 years and older who were registered with their general practice for at least 12 months.13 To identify AF cases between 1 January 2001 and 31 December 2017, we used a list of 11 diagnostic codes from hospital (International Classification of Diseases, tenth revision) and primary care (Read) coding schemes that included both AF and atrial flutter (see Supplementary data online, Table S1). We defined a new AF diagnosis as the first record of AF in primary care or hospital admission records from any diagnostic position.13–15 We excluded all individuals who had a diagnosis of AF before the study start date or within the first 12 months of registration with their general practice.13
Study outcomes
We investigated mortality rates at 1 year following diagnosis as well as the number of hospital admissions with an overnight stay within 1 year of diagnosis (not counting the index admission for those who received their diagnosis in the hospital). The cause of death was recorded in the Medical Certificate of Cause of Death. The cause of hospitalization was defined as the primary discharge diagnosis. Causes of death and hospitalization were mapped to 9 and 11 disease categories, respectively (see Supplementary data online, Table S2).13 In subgroup analyses, disease categories were further grouped into cardiovascular, cerebrovascular, and non-cardio/cerebrovascular causes (see Supplementary data online, Table S2) to reflect that many AF-specific therapies have sought to reduce cardiovascular events and stroke.16
Baseline variables
We extracted baseline characteristics from each patient’s health record data including systolic and diastolic blood pressure, smoking status, body mass index (BMI), socio-economic status, and the prevalence of 18 common chronic conditions (see Supplementary data online).13
Statistical analyses
Baseline characteristics are presented as frequencies (percentage) for categorical data, medians and inter-quartile range for non-normally distributed continuous data, or means and standard deviation (SD) for normally distributed continuous data. We report crude mortality and hospitalization rates as well as adjusted mortality rate and adjusted incident hospitalization by calendar year of diagnosis and subgroups (age, sex, socio-economic status, and place of diagnosis). Crude mortality rates were computed as the cumulative incidence of mortality at 1 year accounting for observation time (i.e. for those who died or were censored within 1 year). Cause-specific mortality rates were computed through cumulative incidence function while taking the competing risk of death from other causes into account.17 Hospitalizations were assessed as the number of hospital admissions per patient-years of follow-up within 1 year of AF diagnosis.
To examine trends over time and by subgroup, we used Poisson regression models offset for observation time and present the resulting rate ratios (RRs) or incident RRs (IRRs) and corresponding 95% confidence intervals (CIs). All models account for the calendar year of diagnosis, age at diagnosis (as a continuous variable), sex, region (geographical division information provided by CPRD), socio-economic status, and baseline comorbidities. Follow-up time was defined as the date of AF diagnosis to the earliest of the following dates: death, de-registration from their practice, or the practice ceased contributing data and for a maximum of 1 year. To further describe observed temporal trends, we grouped the first 2 and the last 2 years of the study together and calculated adjusted RRs for mortality or IRRs for hospitalization. McNemar’s test was used to compare numbers of deaths from each cause. Statistical analyses were performed in R, version 4.0.1 (R Foundation) with statistical significance set at P < .05.
Results
Patient population
We identified 72 412 patients who received a diagnosis of AF between 2001 and 2017. For these cases, the mean age at diagnosis was 75.6 years (SD 12.4), 31 283 (43.2%) were aged 80 years or older, 34 903 (48.2%) were women, and 44 762 (61.8%) had 3 or more of the selected comorbidities. Over the study period, there was an increase in comorbidity burden—notably in the prevalence of diabetes, cancer, and chronic kidney disease (Table 1; Supplementary data online, Table S4). Just over half of cases were diagnosed during an inpatient admission (39 084, 53%), and these individuals were older with a higher comorbidity burden than individuals diagnosed in primary care (Table 1). At the time of AF diagnosis, individuals who were the most deprived had a higher prevalence of comorbidities than the most affluent (see Supplementary data online, Table S3), and women compared with men were older (78.46 vs. 72.95 years, P < .001), less frequently had prevalent ischaemic heart disease (21.0% vs. 29.4%, P < .001), and had a higher mean CHA2DS2-VASc score (3.90 vs. 2.53, P < .001).
Table 1.
Characteristics of patients at the time of diagnosis with atrial fibrillation
| All patients | Time period | Place of diagnosis | |||
|---|---|---|---|---|---|
| 2001–02 | 2016–17 | Primary care | Hospital admission | ||
| n = 72 412 | n = 6954 | n = 5119 | n = 33 328 | n = 39 084 | |
| Age (years) | 75.61 (12.42) | 75.63 (11.82) | 75.14 (12.73) | 74.35 (12.03) | 76.68 (12.64) |
| Men | 37 509 (51.8) | 3 514 (50.5) | 2 723 (53.2) | 17 984 (54.0) | 19 525 (50.0) |
| Ethnicity (White) | 67 299 (94.5) | 6 023 (88.9) | 4 703 (94.5) | 30 394 (94.5) | 36 905 (94.4) |
| Socio-economic status quintile | |||||
| 1 (least deprived) | 15 687 (21.7) | 1 329 (19.1) | 1 431 (28.0) | 7 929 (23.8) | 7 758 (19.9) |
| 2 | 15 668 (21.6) | 1 512 (21.8) | 1 054 (20.6) | 7 459 (22.4) | 8 209 (21.0) |
| 3 | 16 421 (22.7) | 1 655 (23.8) | 1 003 (19.6) | 7 606 (22.8) | 8 815 (22.6) |
| 4 | 13 712 (18.9) | 1 345 (19.4) | 914 (17.9) | 5 965 (17.9) | 7 747 (19.8) |
| 5 (most deprived) | 10 895 (15.1) | 1 108 (15.9) | 716 (14.0) | 4 357 (13.1) | 6 538 (16.7) |
| Ever smoker | 38 473 (53.1) | 1 801 (25.9) | 3 257 (63.6) | 17 171 (51.5) | 21 302 (54.5) |
| Systolic blood pressure (mm Hg) | 136.58 (16.13) | 143.49 (19.02) | 132.83 (13.98) | 137.43 (15.48) | 135.79 (16.66) |
| Diastolic blood pressure (mm Hg) | 77.26 (8.95) | 80.56 (9.42) | 75.80 (8.48) | 78.56 (8.64) | 76.05 (9.07) |
| Heart rate (b.p.m.) | 77.94 (16.40) | 80.56 (17.90) | 76.81 (15.52) | 78.80 (16.68) | 76.97 (16.02) |
| BMI (kg/m2) | 27.84 (6.29) | 26.98 (5.84) | 28.28 (6.68) | 28.35 (6.25) | 27.33 (6.30) |
| Chronic kidney disease | 13 786 (19.0) | 208 (3.0) | 1 362 (26.6) | 5 483 (16.5) | 8 303 (21.2) |
| Cancer | 15 823 (21.9) | 995 (14.3) | 1 482 (29.0) | 6 365 (19.1) | 9 458 (24.2) |
| Chronic obstructive pulmonary disease | 17 672 (24.4) | 1 631 (23.5) | 1 256 (24.5) | 7 358 (22.1) | 10 314 (26.4) |
| Diabetes | 12 206 (16.9) | 804 (11.6) | 1 207 (23.6) | 4 946 (14.8) | 7 260 (18.6) |
| Dyslipidaemia | 11 720 (16.2) | 509 (7.3) | 1 163 (22.7) | 5 369 (16.1) | 6 351 (16.2) |
| Heart failure | 13 846 (19.1) | 1 901 (27.3) | 693 (13.5) | 5 549 (16.6) | 8 297 (21.2) |
| Hypertension | 41 326 (57.1) | 3 067 (44.1) | 3 218 (62.9) | 19 170 (57.5) | 22 156 (56.7) |
| Ischaemic heart disease | 18 364 (25.4) | 1 954 (28.1) | 1 159 (22.6) | 7 042 (21.1) | 11 322 (29.0) |
| Stroke/TIA | 10 310 (14.2) | 1 057 (15.2) | 687 (13.4) | 4 260 (12.8) | 6 050 (15.5) |
| Osteoarthritis | 26 980 (37.3) | 2 181 (31.4) | 2 055 (40.1) | 12 057 (36.2) | 14 923 (38.2) |
| Thyroid disease | 7 200 (9.9) | 496 (7.1) | 586 (11.4) | 3 165 (9.5) | 4 035 (10.3) |
| Three or more comorbidities | 44 762 (61.8) | 3 497 (50.3) | 3 481 (68.0) | 19 160 (57.5) | 25 602 (65.5) |
| CHA2DS2-VASc score | 3.19 (1.66) | 3.11 (1.61) | 3.21 (1.67) | 3.01 (1.63) | 3.34 (1.66) |
Data are mean (SD) or n (%). Socio-economic status (SES) refers to Index of Multiple Deprivation 2015 quintile, with SES 1 referring to the most affluent and SES 5 to the most deprived socio-economic quintile. Number of comorbidities refers to any of the 18 conditions investigated. Category percentages refer to complete cases.
BMI, body mass index; TIA, transient ischaemic attack.
Mortality
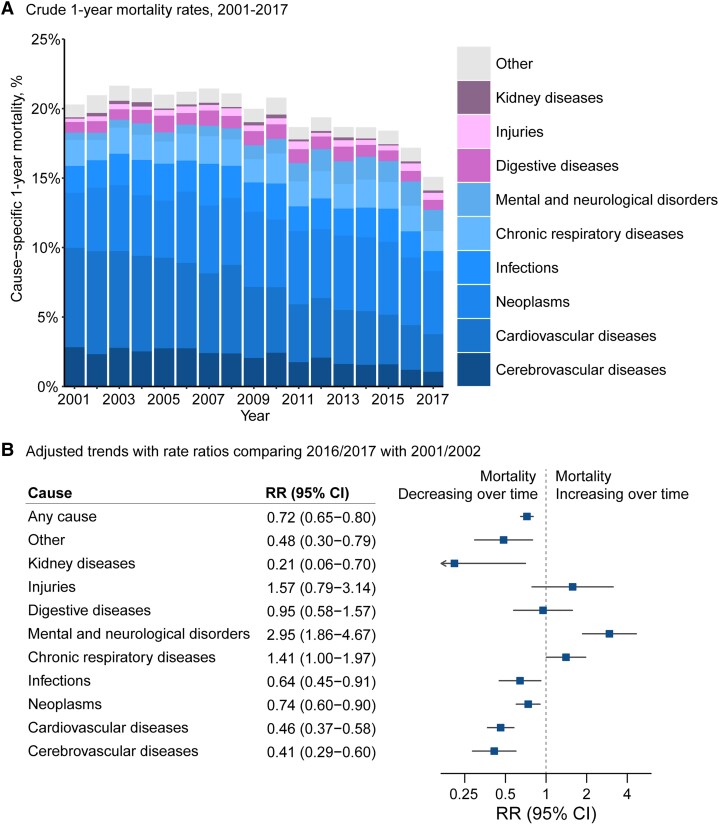
One-year mortality rates following AF were substantial (20.0%) and declined over the study period (adjusted RR per 5 years, 0.90, 95% CI 0.88–0.92; RR 2016/17 vs. 2001/02, 0.72, 95% CI 0.65–0.80; Figure 1). When all-cause mortality rates were stratified by specific causes, we observed diverging trends between cardiovascular, cerebrovascular, and other causes. One-year mortality rates from cardiovascular causes and cerebrovascular causes declined from 7.3 and 2.6% in 2001/02 to 3.0 and 1.1% in 2016/17, respectively (RR 2016/17 vs. 2001/02, cardiovascular cause RR 0.46, 95% CI 0.37–0.58; cerebrovascular cause RR 0.41, 95% CI 0.29–0.60). By contrast, 1-year mortality rates for non-cardio/cerebrovascular mortality did not change (RR 2016/17 vs. 2001/02, 0.91; 95% CI 0.80–1.04). There was an increase in mortality rates from mental and neurological disorders, from 2.5% in 2001/02 to 10.1% in 2016/17 (RR 2016/17 vs. 2001/02, 2.95; 95% CI 1.86–4.67; Figure 1B), and of these deaths, 87.2% were caused by dementia, Alzheimer’s disease, and Parkinson’s disease (see Supplementary data online, Table S5).
Figure 1.
Temporal trends in all-cause and cause-specific mortality rates at 1 year following diagnosis of atrial fibrillation. (A) Crude rate of all-cause and cause-specific mortality following diagnosis of atrial fibrillation. Labels for years 2001–17 present individual causes of death as a share of the total number of deaths at 1 year. (B) Rate ratios from multivariable Poisson regression models comparing 1-year mortality rates in 2016/2017 with 2001/2002 by first reported cause of death, adjusting for patients’ age, sex, socioeconomic status, region, and 18 baseline comorbidities. ‘Other’ refers to any other causes except the pre-defined nine disease categories for cause of death.
Amongst patients who died in 2016/17, the most frequent cause of death was cancer (241 death, 29.0% of all deaths). Infections (86, 10.4%), chronic respiratory disease (85, 10.2%), and mental and neurological disorders (84, 10.1%) accounted for more deaths than cerebrovascular disease (58, 7.0%; P < .001). Further analyses of individual causes of death revealed that more patients died from dementia in 2016/17 than the combined total of acute myocardial infarction, heart failure, and acute stroke [67 (8.0%) vs. 56 (6.7%), P < .001] (Supplementary data online, Table S5).
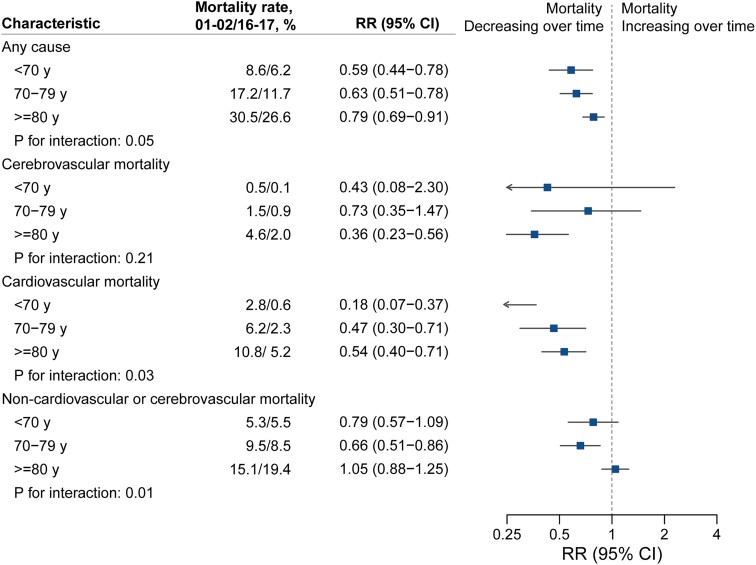
Age-stratified analyses revealed that all-cause mortality declined across all age groups (Figure 2). Cause-specific analysis showed that cardiovascular mortality declined across age groups and more steeply in younger age groups. However, amongst individuals aged 80 years or older, there was no decrease in mortality from non-cardio/cerebrovascular causes (RR 2016/17 vs. 2001/02, 1.05; 95% CI 0.88–1.25).
Figure 2.
Temporal trends in all-cause and cause-specific mortality rates at 1 year following diagnosis of atrial fibrillation by age group. Crude mortality rates by age groups alongside rate ratios, 95% confidence intervals, and interaction P-values from multivariable Poisson regression models accounting for year of diagnosis, age (as a continuous variable), sex, socio-economic status, region, and 18 baseline comorbidities. Interaction P-values refer to the interaction between age group (categorized as age < 70, age 70–79, or age ≥ 80 years) and year of diagnosis.
Over the study period, overall mortality was marginally higher in men compared with women (RR 1.06; 95% CI 1.03–1.10), but causes of death presented sex-specific patterns (see Supplementary data online, Figure S1). With increasing age, the proportion of deaths attributable to cardiovascular causes decreased in men (34.3% for age < 55 years to 26.8% for age ≥ 80 years) and increased in women (13.5% for age < 55 years to 28.1% for age ≥ 80 years).
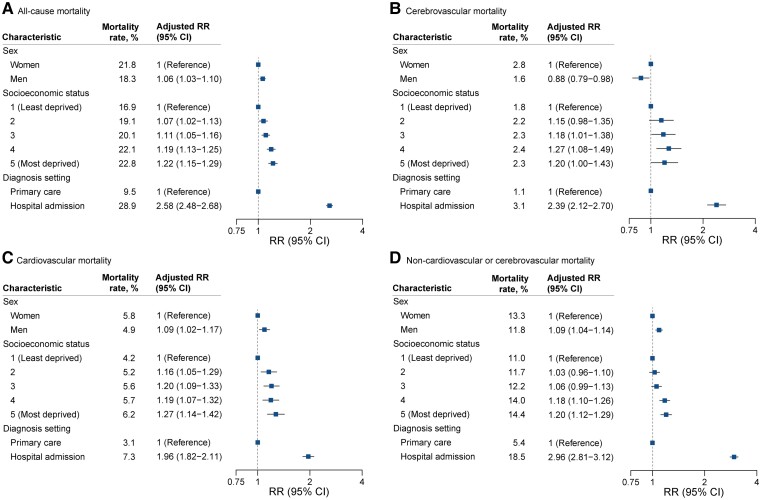
Additionally, patients’ socio-economic background and the care setting in which patients received their diagnoses were associated with differences in mortality rate over the study period. At the same age and sex, patients in the most deprived socio-economic quintile had 22% higher mortality rates within 1 year than those in the least deprived quintile (RR for most deprived vs. least deprived quintile, 1.22; 95% CI 1.15–1.29). Whilst there was a progressive socio-economic gradient for all-cause and cardiovascular mortality, rates for non-cardio/cerebrovascular mortality were only increased in patients in the two most deprived quintiles (Figure 3). One-year mortality was 2.5-fold higher in patients who received their diagnosis in hospital compared with primary care (RR for hospital diagnosis vs. primary care diagnosis, 2.58; 95% CI 2.48–2.68). Cause-specific analysis revealed that non-cardio/cerebrovascular mortality (RR 2.96; 95% CI 2.81–3.12) contributed more to this pattern than cardiovascular or cerebrovascular mortality (RR 1.96, 95% CI 1.82–2.11; RR 2.39, 95% CI 2.12–2.70, respectively).
Figure 3.
Differences in mortality rates 1 year after diagnosis of atrial fibrillation by sex, socio-economic status, and place of diagnosis. Crude mortality rates by each category of sex, socio-economic status, and diagnostic setting alongside rate ratios and 95% confidence intervals from multivariable Poisson regression models accounting for year of diagnosis, age (as a continuous variable), sex, socio-economic status, region, and 18 baseline comorbidities.
Hospitalizations
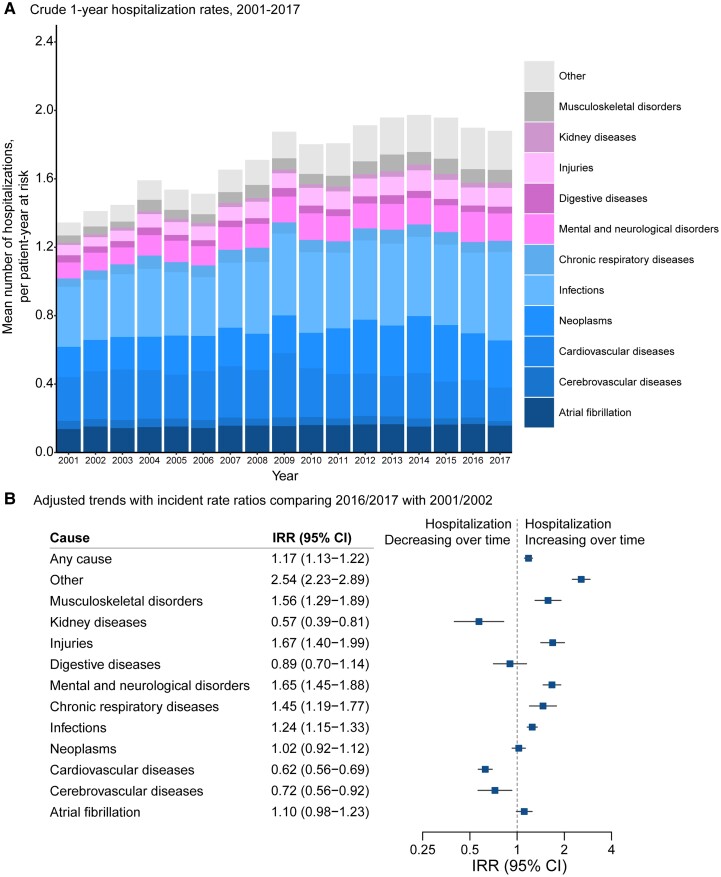
The number of hospital admissions within the first year following the index presentation with AF was high (1.72 hospitalizations per patient-year at risk) and increased over the study period (IRR per 5 years 1.05, 95% CI 1.04–1.06; IRR 2016/17 vs. 2001/02, 1.17, 95% CI 1.13–1.22; Figure 4). Admissions for AF accounted for 9.0% of hospitalizations within a year of diagnosis (0.15 hospitalizations per patient-year at risk) and remained steady over the study period (IRR per 5 years 1.02; 95% CI 0.97–1.05). Infection was the most common cause of hospitalization within a year of AF diagnosis, and its frequency increased (IRR 2016/17 vs. 2001/02, 1.24, 95% CI 1.15–1.33). Admissions for cardiovascular and cerebrovascular diseases represented less than a fifth (18.6%) of all admissions (0.32 hospitalizations per patients year at risk, cardiovascular cause 0.27; cerebrovascular cause 0.05) and decreased in frequency (IRR 2016/17 vs. 2001/02 cardiovascular cause 0.62, 95% CI 0.56–0.69; cerebrovascular cause 0.72, 95% CI 0.56–0.92). In parallel, for non-cardio/cerebrovascular disease categories, admissions rose by 42% in 2016/17 compared with 2001/02 (IRR 1.42; 95% CI 1.39–1.45). Notably, admissions for injuries and mental and neurological disorders rose by more than 50% (IRR 2016/17 vs. 2001/02, 1.67, 95% CI 1.40–1.99; 1.65, 95% CI, 1.45–1.88, respectively).
Figure 4.
Temporal trends in all-cause and cause-specific mean number of hospitalizations at 1 year following diagnosis of atrial fibrillation. (A) Crude rate of all-cause and cause-specific hospitalization following diagnosis of atrial fibrillation. Labels for years 2001–17 present individual causes of hospitalization as a share of the total number of hospitalizations at 1 year. (B) Rate ratios from multivariable Poisson regression models comparing 1-year hospitalization rates in 2016/2017 with 2001/2002 by primary cause of admission, adjusting for patients’ age, sex, socio-economic status, region, and 18 baseline comorbidities. ‘Other’ refers to any other causes except the pre-defined 11 disease categories for hospitalizations.
Over the study period, sex-stratified patterns for hospital admissions were similar to those reported for mortality (IRR for men compared with women, 1.11; 95% CI 1.07–1.15), but the frequency of hospitalization was similar between individuals in the most and least deprived socio-economic quintiles (IRR for most deprived vs. least deprived quintile, 1.02; 95% CI 0.96–1.07) (see Supplementary data online, Figure S2). Similar to mortality, patients who received their AF diagnosis in hospital compared with primary care were at increased risk of hospitalization in the first year after diagnosis (IRR for hospital diagnosis vs. primary care diagnosis, 1.41; 95% CI 1.37–1.46), and this was consistent across disease categories. Individuals aged 80 years or older experienced a greater increase in hospitalization compared with younger individuals (IRR 2016/17 vs. 2001/02, age ≥ 80 years 1.39, 95% CI 1.30–1.48; age 70–79 years 1.03, 95% CI 0.96–1.10; age < 70 years 1.15, 95% CI 1.08–1.22; see Supplementary data online, Figure S3) with a 54% increase in hospitalization for non-cardiovascular or cerebrovascular causes by the end of the study period compared with the start (IRR 2016/17 vs. 2001/02, 1.54; 95% CI 1.44–1.65).
Discussion
This population-based study provides novel insights into the shifting patterns of causes of death and hospitalizations amongst patients with AF in a high-income country. Over 16 years, the individual-level comorbidity burden at the point of AF diagnosis has increased. Substantial declines in cardiovascular and cerebrovascular mortality are contrasted with increases in the numbers of deaths from chronic respiratory disease, dementia, and injuries. From 2001 to 2017, hospitalization in the first year after AF diagnosis became more common, on account of increasing admissions from non-cardio/cerebrovascular causes, especially in older persons (Structured Graphical Abstract).
These findings build on and expand prior reports of declining mortality, and increasing hospitalization burden, over recent decades amongst patients with AF in high-income countries. However, previous reports only considered all-cause mortality1,2 and/or provided an incomplete picture of the trends for different causes by subdividing mortality or hospitalization between cardiovascular or non-cardiovascular categories4 or only investigating specific outcomes (such as heart failure and stroke).1,2,18 Other reports have described causes of death amongst randomized controlled trial populations, which may not be reflective of the real-world population with AF or routine practice.19 A previous UK-based analysis of individuals with AF including CPRD data from 1998 to 2016 suggested that mortality was predominantly related to ischaemic heart disease and stroke,20 but as the authors did not assess trends over time, it is likely that this was driven by events at the start of the study period.
We found that the relative risk of death from cardiovascular or cerebrovascular causes after diagnosis of AF declined by more than 50% over 16 years. The decline in both admission and mortality for cardiovascular and cerebrovascular disease is a key indicator for the effectiveness of these AF-specific therapies. In particular, the decrease in cerebrovascular events appears to tally with the increased utilization of oral anticoagulation (especially direct oral anticoagulants) for patients with AF and elevated stroke risk in the UK.21
The changing characteristics of patients at the time of AF diagnosis may also have contributed to the observed reduction in cardiovascular and cerebrovascular events. The prevalence of ischaemic heart disease and heart failure was lower amongst patients at the time of AF diagnosis in 2017 than 2001, which accords with reports that have shown that the standardized incidence of each has decreased in the UK population over the last two decades.15,22 Thus, the improvement in cardiovascular outcomes observed in our cohort of patients with AF may be partly the result of more general trends in improvements in primary and secondary prevention of cardiovascular disease. Moreover, patients diagnosed with AF in 2017 may also have been at a different point in their disease trajectory compared with patients diagnosed in 2001. Clinical thresholds for investigation for AF and documentation of diagnosis may have been altered by evidence since the early 1990s for effectiveness of oral anticoagulation in stroke prophylaxis,16 and reports have shown that the use of electrocardiographic recording has increased in populations at risk of AF.23 Arrhythmic burden can alter stroke risk,24 so more frequent identification of paroxysmal or (previously) sub-clinical AF may have contributed to lower rates of cerebrovascular outcomes.
For patients aged 80 years and older, who made up 43% of the cohort, the hospitalization burden for non-cardio/cerebrovascular events increased by over 50%. By the end of the study period, dementia was a more common cause of death within 1 year of AF diagnosis than acute myocardial infarction, heart failure, and stroke combined. Though this observation could be partly related to greater awareness and coding of dementia,25 it also strengthens the evidence that the relationship between AF and dementia is a pressing research priority. Randomized clinical trials are required to determine whether interventions (such as oral anticoagulation in low stroke risk patients or sub-clinical AF)26,27 can effectively prevent decline in cognitive function and avert or delay the onset of dementia.
The total number of comorbidities at the time of AF diagnosis increased over time, such that, by 2017, 7 in 10 patients had 3 or more of the investigated comorbidities at the time of AF diagnosis. Hospitalization for injuries and musculoskeletal disorders also increased by 67 and 57%, respectively, over the study period. Our findings highlight the importance of multi-morbidity, frailty, and senescence, rather than the presence of the arrhythmia itself, as important determinants of prognosis for patients after AF diagnosis.28,29 As the number of older and more comorbid individuals with AF encountered in usual care is increasing,14 non-cardio/cerebrovascular comorbidities, hospitalizations, and deaths are emerging as important potential therapeutic targets in patients with AF. The EHRA-PATHS project aims to develop inter-disciplinary, patient-centred, systematic care pathways for multi-morbid patients with AF,30 and the AFFIRMO project will provide randomized trial evidence for whether integration of the comprehensive geriatric assessment to tailor AF care can reduce the occurrence of major clinical outcomes.31
Patients diagnosed in hospital or from the most deprived group had worse outcomes compared with those diagnosed in the community or from the most affluent group. Although increased burden of comorbidities might partly explain the increased frequency of death in these groups,14 the persisting difference after full adjustment for these factors suggests that other social and health-care factors might also contribute.32 We have previously reported that the proportion of patients diagnosed with AF during a hospital admission increased in the UK over this time period (51.9% in 1998 to 58.1% in 2017)14 and that the most deprived individuals in the UK experience an AF diagnosis at a younger age than the most affluent individuals.14 The discrepancy in outcomes by diagnostic setting and deprivation warrants targeted strategies and healthcare resource planning.20
Limitations
First, research relying on routinely collected health data relies on the accuracy of clinical coding. For example, a greater proportion of individuals in the final 2 years of the study period were recorded as ever smokers than in the first 2 years, but it is also known that recording of smoking status in UK primary care records increased after 2001.33 The validity of clinical diagnoses recorded in the linked CPRD dataset across UK primary care secondary care and death certificates has been independently investigated for a range of conditions (see Supplementary data online). Second, we did not have comprehensive information on medication and procedural treatment provided to the cohort, and so we are unable to make any direct inference on the associations between utilization of guideline-directed therapy and the observed trends in deaths and hospitalizations, although this has been reported in the literature.21,34–36 Third, given the observational nature of the study, we do not assume causation, and there is the risk of residual confounding from unmeasured risk factors or comorbidities. Fourth, we included atrial flutter diagnosis codes to identify AF cases as atrial flutter also confers an elevated stroke risk, shares the same recommendations for anticoagulation as AF, and many people with atrial flutter also have AF.37 Fifth, this was a study of a cohort of patients with AF from a high-income country and a predominantly White ethnic composition, so these results may not be generalizable to settings with a different country-level income classification or ethnic composition.
Conclusions
This nationwide population-based study demonstrates major reductions in cardiovascular and cerebrovascular events for patients with AF since the turn of the millennium. In contrast, there are high and increasing rates of non-cardio/cerebrovascular events, which may represent an important target to further improve outcomes for patients after AF diagnosis.
Supplementary data
Supplementary data are available at European Heart Journal online.
Supplementary Material
Contributor Information
Jianhua Wu, Centre for Primary Care, Wolfson Institute of Population Health, Queen Mary University of London, 58 Turner Street, London E1 2AB, UK.
Ramesh Nadarajah, Leeds Institute of Data Analytics, University of Leeds, Leeds, UK; Leeds Institute for Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Yoko M Nakao, Leeds Institute of Data Analytics, University of Leeds, Leeds, UK; Leeds Institute for Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK.
Kazuhiro Nakao, Leeds Institute of Data Analytics, University of Leeds, Leeds, UK; Leeds Institute for Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK.
Chris Wilkinson, Academic Cardiovascular Unit, South Tees NHS Foundation Trust, Middlesbrough, UK; Hull York Medical School, University of York, York, UK.
J Campbell Cowan, Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
A John Camm, Molecular and Clinical Sciences Research Institute, St George’s University of London, London, UK.
Chris P Gale, Leeds Institute of Data Analytics, University of Leeds, Leeds, UK; Leeds Institute for Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Declarations
Disclosure of Interest
C.P.G. reports personal fees from AstraZeneca, Amgen, Bayer, Boehrinher-Ingelheim, Daiichi Sankyo, Vifor, Pharma, Menarini, Wondr Medical, Raisio Group, and Oxford University Press. He has received educational and research grants from BMS, Abbott Inc., the British Heart Foundation, National Institute of Health Research, Horizon 2020, and the European Society of Cardiology, outside the submitted work. A.J.C. reports personal fees from Abbott, Bayer, BMS, Sanofi, Milestone, Boston Scientific, InCarda, and Menarini. Y.M.N. reports a study grant from Bayer. All other authors declare no competing interests.
Data Availability
Patient-level data will not be made available. Data should be requested through CPRD directly.
Funding
J.W. is supported by Barts Charity (MGU0504). and R.N. is supported by the British Heart Foundation (FS/20/12/34789).
Ethical Approval
Scientific approval for this study was given by the CPRD Independent Scientific Advisory Committee (ref: 19_76).
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DDet al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham heart study: a cohort study. Lancet 2015;386:154–162. 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmidt M, Ulrichsen SP, Pedersen L, Bøtker HE, Nielsen JC, Sørensen HT. 30-year nationwide trends in incidence of atrial fibrillation in Denmark and associated 5-year risk of heart failure, stroke, and death. Int J Cardiol 2016;225:30–36. 10.1016/j.ijcard.2016.09.071 [DOI] [PubMed] [Google Scholar]
- 3. Freeman JV, Wang Y, Akar J, Desai N, Krumholz H. National trends in atrial fibrillation hospitalization, readmission, and mortality for Medicare beneficiaries, 1999–2013. Circulation 2017;135:1227–1239. 10.1161/CIRCULATIONAHA.116.022388 [DOI] [PubMed] [Google Scholar]
- 4. Vinter N, Huang Q, Fenger-Grøn M, Frost L, Benjamin EJ, Trinquart L. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham heart study): community based cohort study. BMJ 2020;370:m2724. 10.1136/bmj.m2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chamberlain AM, Gersh BJ, Alonso A, Chen LY, Berardi C, Manemann SMet al. Decade-long trends in atrial fibrillation incidence and survival: a community study. Am J Med 2015;128:260–267.e1. 10.1016/j.amjmed.2014.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams BA, Honushefsky AM, Berger PB. Temporal trends in the incidence, prevalence, and survival of patients with atrial fibrillation from 2004 to 2016. Am J Cardiol 2017;120:1961–1965. 10.1016/j.amjcard.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 7. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Seward JBet al. Changing trends of hospital utilization in patients after their first episode of atrial fibrillation. Am J Cardiol 2008;102:568–572. 10.1016/j.amjcard.2008.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen Iet al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med 2015;12:e1001885. 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kotecha D, Asselbergs FW, Achenbach S, Anker SD, Atar D, Baigent Cet al. CODE-EHR best practice framework for the use of structured electronic healthcare records in clinical research. BMJ 2022;378:e069048. 10.1136/bmj-2021-069048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, Van Staa Tet al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol 2015;44:827–836. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol 2010;69:4–14. 10.1111/j.1365-2125.2009.03537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruigómez A, Johansson S, Wallander MA, Rodríguez LA. Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc Disord 2005;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conrad N, Judge A, Canoy D, Tran J, Pinho-Gomes A-C, Millett ERet al. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86 000 individuals. JAMA Cardiol 2019;4:1102–1111. 10.1001/jamacardio.2019.3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu J, Nadarajah R, Nakao YM, Nakao K, Wilkinson C, Mamas MAet al. Temporal trends and patterns in atrial fibrillation incidence: a population-based study of 3.4 million individuals. Lancet Reg Health Eur 2022;17:100386. 10.1016/j.lanepe.2022.100386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo APet al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572–580. 10.1016/S0140-6736(17)32520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MDet al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 17. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–609. 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HLet al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol 2015;66:1000–1007. 10.1016/j.jacc.2015.06.1314 [DOI] [PubMed] [Google Scholar]
- 19. Marijon E, Le Heuzey J-Y, Connolly S, Yang S, Pogue J, Brueckmann Met al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation 2013;128:2192–2201. 10.1161/CIRCULATIONAHA.112.000491 [DOI] [PubMed] [Google Scholar]
- 20. Chung S-C, Sofat R, Acosta-Mena D, Taylor JA, Lambiase PD, Casas JPet al. Atrial fibrillation epidemiology, disparity and healthcare contacts: a population-wide study of 5.6 million individuals. Lancet Reg Health Eur 2021;7:100157. 10.1016/j.lanepe.2021.100157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cowan JC, Wu J, Hall M, Orlowski A, West RM, Gale CP. A 10 year study of hospitalized atrial fibrillation-related stroke in England and its association with uptake of oral anticoagulation. Eur Heart J 2018;39:2975–2983. 10.1093/eurheartj/ehy411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study. BMJ 2012;344:d8059. 10.1136/bmj.d8059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams BA, Chamberlain AM, Blankenship JC, Hylek EM, Voyce S. Trends in atrial fibrillation incidence rates within an integrated health care delivery system, 2006 to 2018. JAMA Netw Open 2020;3:e2014874–e2014874. 10.1001/jamanetworkopen.2020.14874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hohnloser SH, Capucci A, Fain E, Gold MR, van Gelder IC, Healey Jet al. Asymptomatic atrial fibrillation and stroke evaluation in pacemaker patients and the atrial fibrillation reduction atrial pacing trial (ASSERT). Am Heart J 2006;152:442–447. 10.1016/j.ahj.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 25. Wu Y-T, Beiser AS, Breteler MM, Fratiglioni L, Helmer C, Hendrie HCet al. The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurology 2017;13:327–339. 10.1038/nrneurol.2017.63 [DOI] [PubMed] [Google Scholar]
- 26. Rivard L, Khairy P, Talajic M, Tardif J-C, Nattel S, Bherer Let al. Blinded randomized trial of anticoagulation to prevent ischemic stroke and neurocognitive impairment in atrial fibrillation (BRAIN-AF): methods and design. Can J Cardiol 2019;35:1069–1077. 10.1016/j.cjca.2019.04.022 [DOI] [PubMed] [Google Scholar]
- 27. Chua W. Clearing the cognitive cloud: direct oral anticoagulants or vitamin K antagonists for reducing dementia risk in patients with atrial fibrillation? Heart 2021;107:1854–1855. 10.1136/heartjnl-2021-320138 [DOI] [PubMed] [Google Scholar]
- 28. Wilkinson C, Wu J, Clegg A, Nadarajah R, Rockwood K, Todd Oet al. Impact of oral anticoagulation on the association between frailty and clinical outcomes in people with atrial fibrillation: nationwide primary care records on treatment analysis. Europace 2022;24:1065–1075. 10.1093/europace/euac022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Proietti M, Romiti GF, Raparelli V, Diemberger I, Boriani G, Dalla Vecchia LAet al. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: a systematic review and meta-analysis of 1,187,000 patients. Ageing Res Rev 2022;79:101652. 10.1016/j.arr.2022.101652 [DOI] [PubMed] [Google Scholar]
- 30. Heidbuchel H, Van Gelder IC, Desteghe L, Investigators EHRA-PATHS. ESC and EHRA lead a path towards integrated care for multimorbid atrial fibrillation patients: the Horizon 2020 EHRA-PATHS project. Eur Heart J 2022;43:1450–1452. 10.1093/eurheartj/ehab672 [DOI] [PubMed] [Google Scholar]
- 31. Johnsen SP, Proietti M, Maggioni AP, Lip GY. A multinational European network to implement integrated care in elderly multimorbid atrial fibrillation patients: the AFFIRMO consortium. Eur Heart J 2022;43:2916–2918. 10.1093/eurheartj/ehac265 [DOI] [PubMed] [Google Scholar]
- 32. Lawson CA, Zaccardi F, Squire I, Ling S, Davies MJ, Lam CSet al. 20-year trends in cause-specific heart failure outcomes by sex, socioeconomic status, and place of diagnosis: a population-based study. Lancet Public Health 2019;4:e406–e420. 10.1016/S2468-2667(19)30108-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simpson CR, Hippisley-Cox J, Sheikh A. Trends in the epidemiology of smoking recorded in UK general practice. Br J Gen Pract 2010;60:e121–e127. 10.3399/bjgp10X483544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cowan C, Healicon R, Robson I, Long WR, Barrett J, Fay Met al. The use of anticoagulants in the management of atrial fibrillation among general practices in England. Heart 2013;99:1166–1172. 10.1136/heartjnl-2012-303472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu J, Alsaeed ES, Barrett J, Hall M, Cowan C, Gale CP. Prescription of oral anticoagulants and antiplatelets for stroke prophylaxis in atrial fibrillation: nationwide time series ecological analysis. Europace 2020;22:1311–1319. 10.1093/europace/euaa126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orlowski A, Gale CP, Ashton R, Petrungaro B, Slater R, Nadarajah Ret al. Clinical and budget impacts of changes in oral anticoagulation prescribing for atrial fibrillation. Heart 2021;107:47–53. 10.1136/heartjnl-2020-317006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient-level data will not be made available. Data should be requested through CPRD directly.