Abstract
Background
Coronaviruses, known for their crown-like appearance, cause mild gastrointestinal and respiratory diseases. Some cause outbreaks of respiratory diseases, most recently, SARS-CoV-2, the coronavirus disease 2019 (COVID-19). Individuals with COVID-19 are reported to be in both arterial and venous prothrombotic states. In addition to a lipid-lowering effect, statin also has an anti-inflammatory effect, which addresses one of the underlying causes of thrombosis. An in-silico study revealed that statins could directly interact with the main protease enzyme of SARS-CoV-2 and prevent infectivity. Due to these pleiotropic properties, statins may positively impact the outcome of hospitalized patients with COVID-19 infections.
Methods
A total of 26 445 acute COVID-19-infected patients were included in this study. Patients were stratified based on home statin use status: no statins, high-intensity statins (atorvastatin 40–80 mg daily and rosuvastatin 20–40 mg daily), and low-to-moderate intensity statins (all other statins). A multivariate generalized linear model and logistic regression were used to predict the hospital length of stay and inpatient mortality, respectively.
Results
The hospital length of stay was compared between low-intensity and high-intensity statin use against no statin therapy. The length of stay was 3.88 days (95% CI, 3.56–4.20; P < .0001) longer among patients with low-dose statin therapy compared to patients without. The length of stay was 4.77 days (95% CI, 4.42–5.13; P <.0001) longer among patients with high-intensity statin therapy than those without. The odds of in-hospital mortality decreased by 24% (OR, 0.76; 95% CI, 0.76–0.97) among those with high-dose statin therapy compared to patients without (P = .02). There was no statistical significance between the low-dose statin group and the no statin group for inpatient mortality.
Conclusion
Hospitalized COVID-19 patients on statin therapy, regardless of intensity, are more likely to have a longer length of stay. There may be a mortality benefit in using high-intensity statin in acute COVID-19-infected patients. The results of this study are insufficient to recommend statin therapy for inpatient COVID-19 treatment. However, patients with significant cardiovascular comorbidities, where statins are indicated, should be on these medications, especially amidst the COVID-19 pandemic. Randomized controlled trials are needed to assess the potential in-hospital benefit of statin therapy on COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, thrombophilia, statins, hydroxymethylglutaryl-CoA reductase inhibitors, length of stay, hospital mortality
Introduction
The pandemic of coronavirus disease 2019 (COVID-19) started with a novel coronavirus identified in Wuhan, China, causing a cluster of pneumonia cases. The virus is particularly contagious and rapidly created an epidemic in China, which progressed into a global pandemic. Coronaviruses, enveloped positive-strand RNA viruses, are known for their crown-like appearance under the microscope and for causing mild gastrointestinal and respiratory diseases.1 Genome sequencing and analysis showed that the coronavirus that causes COVID-19 is a beta-coronavirus in the same subgenus as the severe acute respiratory syndrome (SARS) virus (as well as several bat coronaviruses) but in a different clade.2 The route of transmission of coronavirus is direct person-person contact, which occurs mainly via respiratory particles. It is transmitted via respiratory droplets when an infected individual talks, coughs, or sneezes.3
Besides respiratory complications, COVID-19-infected patients are at higher risk for both venous and arterial thromboembolism.4 A study by De Luca et al suggested that SARS-CoV-2 positivity is associated with higher mortality, in-stent thrombosis, heart failure, higher use of thrombectomy, and use of GP IIb/IIIa.5 Patients with COVID-positivity and an elevated troponin level have an increased mortality rate,5 likely from acute coronary events.
Statin, a lipid-lowering medication, works by competitively inhibiting HMG-CoA reductase, the rate-limiting step in cholesterol synthesis. The major role of statins is for the primary and secondary prevention of coronary artery disease and myocardial infarction in a patient. Additionally, statins are demonstrated to have potent anti-inflammatory properties; they help stabilize the plaque and prevent further inflammation and progression of acute coronary syndrome.6 In a 12-month, open-label, randomized, multicenter trial, in addition to lowering LDL-C level, atorvastatin was demonstrated to decrease mean absolute plaque volume compared to placebo as well as a more stabilized plaque composition under intravascular ultrasound.7
Previous studies have shown the correlation between COVID-19 positivity and the incidence of coronary syndromes and the benefit of statins in such cases.8 This study explores the potential relationship between statin therapy and hospital length of stay and inpatient mortality in acute COVID-19-infected patients.
Methods
Chart data was obtained from 15 HCA Healthcare hospitals with a combined 4000 beds, 25 emergency room sites, and 14 ambulatory surgical center sites from January 1, 2020, to August 31, 2021, for all COVID-19 cases of individuals age 18 and older. A total of 26 445 acute COVID-19-infected patients were included in this study. Patients were stratified based on home statin use status: no statins, high-intensity statins (atorvastatin 40–80 mg daily and rosuvastatin 20–40 mg daily), and low-to-moderate intensity statins (all other statins). The home medication is self-reported by patients to nurses and providers. Hospital length of stay and in-hospital mortality were analyzed using a linear model and logistic regression to predict the odds ratio, respectively. Predictor variables were determined a priori based on previous literature and biological plausibility. Factors controlled for were age, sex, race, body mass index (BMI), heart disease, kidney disease, lung disease, type 2 diabetes mellitus, remdesivir use, tocilizumab use, hydroxychloroquine use, D-dimer level, platelet count, and C-reactive protein (CRP) level. This study was approved as exempt by the Institutional Review Board due to the nature of it being retrospective with de-identified data.
Statistical Analysis
All exploratory and statistical analyses were conducted in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The predictors of hospital length of stay were assessed with multivariate linear regression with a normal distribution utilizing the generalized linear model procedure to report coefficients (β) and 95% confidence intervals (CIs). The predictors of inpatient mortality were evaluated using univariate and multivariate binary logistic regression to report odds ratio (OR) and 95% CIs. R-square statistics were used to assess the overall goodness of fit for regression models. P values less than .05 denoted statistical significance.
Results
Our study included 47.5% male, 52.5% female, 69.5% Whites, 16.5% Blacks, and 14.0% “Other” races (Table 1). Comorbidities within the sample included: 17.8% heart disease, 10.0% type 2 diabetes, 8.7% kidney disease, and 21.4% lung disease (Table 1).
Table 1.
Relevant Patient Attributes
| Patient characteristics | Total number (%) |
|---|---|
| Male | 12 553 (47.5%) |
| Female | 13 892 (52.5%) |
| White | 18 379 (69.5%) |
| Black | 4371 (16.5%) |
| Other races | 3695 (14.0%) |
| Statin use | 4835 (18.2%) |
| Heart disease | 4671 (17.8%) |
| Type 2 diabetes | 2630 (10.0%) |
| Kidney disease | 2265 (8.7%) |
| Lung disease | 5659 (21.4%) |
| Tocilizumab use | 424 (1.1%) |
| Remdesivir use | 5294 (20%) |
| Hydroxychloroquin use | 210 (0.79%) |
Length of Stay
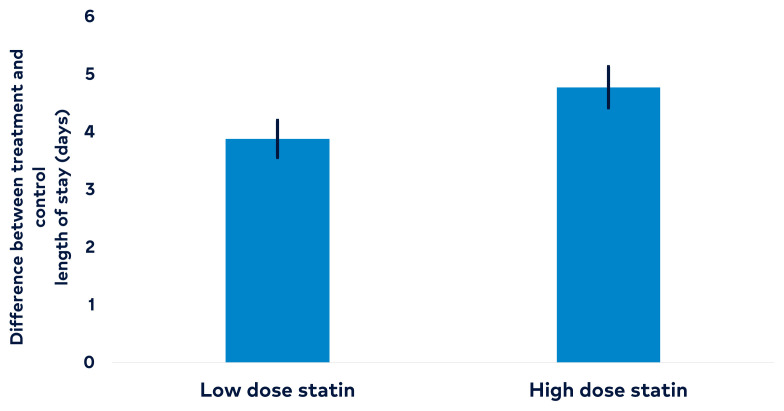
The hospital length of stay was 3.88 days longer (95% CI, 3.56–4.20) among COVID-19 patients with low-dose statin therapy compared to patients without statin therapy (P < .001) (Figure 1 and Table 2). Similarly, the length of stay was 4.77 days (95% CI, 4.42–5.13) longer among patients with high-dose statin therapy compared to patients without statin therapy (P < .001) (Table 3). Length of stay was 0.09 (95% CI, 0.09–0.10) days longer for every 1-year increase in age, assuming all other variables are held constant (P < .001). Length of stay was 0.41 days (95% CI, 0.22–0.59) longer among males compared to females, assuming all other variables are held constant (P < .001). The length of stay was 0.41 days (95% CI, 0.14–0.67) shorter among patients who were Black compared to patients who were White, assuming all other variables are held constant (P = .003). In addition, the length of stay was 0.35 days (95% CI, 0.073–0.62) shorter among patients who identified as “Other” race compared to patients who were White, assuming all other variables are held constant (P = .013). Length of stay was 0.03 days (95% CI, 0.022–0.047) longer for every 1-unit increase in BMI, assuming all other variables are held constant (P < .001).
Figure 1.
Length of stay was compared between low-intensity statin and high-intensity statin against no statin therapy. The error bar represents a 95% confidence interval. Among patients with COVID-19, the length of stay was 3.88 days longer among patients with low-dose statin therapy (95% CI, 3.56–4.20, P < .001) compared to patients without. Among patients with COVID-19, the length of stay was 4.7 days longer among patients with high-intensity statin therapy (95% CI, 4.42–5.13, P < .001) compared to patients without.
Table 2.
The Effect of Statins on In-Patient (Total) Length of Stay
| Variables | Estimated difference (days) | 95% confidence interval | P value |
|---|---|---|---|
| Low-dose statins therapy vs control | 3.88 | 3.56–4.20 | < .001 |
| High-dose statins therapy vs control | 4.77 | 4.42–5.13 | < .001 |
Table 3.
Predictor of Length of Stay in Inpatient COVID-19 Patients
| Variables | Length of stay (95% CI) | P value |
|---|---|---|
| Low-dose statins therapy | 3.88 (3.56–4.20) | <.001 |
| High-dose statins therapy | 4.77 (4.42 – 5.13) | <.001 |
| No statin therapy | Reference | Reference |
| Age | 0.09 (0.09–0.10) | <.001 |
| Male (vs Female) | 0.41 (0.22 – 0.59) | <.001 |
| BMI | 0.03 (0.022 – 0.047) | <.001 |
| Race (Black vs White) | −0.41 (−0.67 – −0.14) | .003 |
| Race (Other vs White) | −0.35 (−0.62 – −0.073) | .013 |
Inpatient Mortality
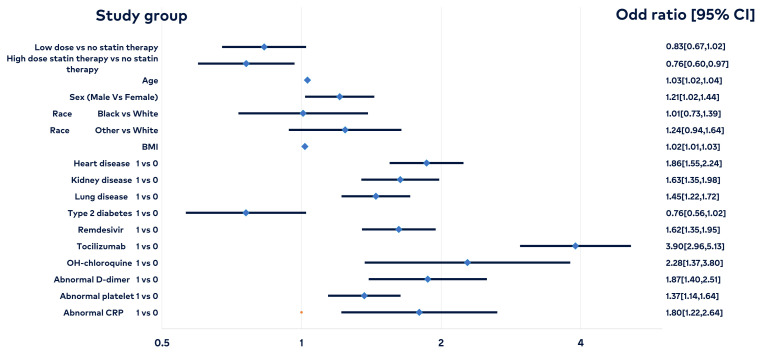
Among patients with COVID-19, the odds of in-hospital mortality were 0.76 times as likely among patients with high-dose statin therapy compared to patients without statin therapy, assuming all other variables are held constant (P = .02, 95% CI, 0.60–0.97). This means that the odds of in-hospital mortality decreased 24% among those with high-dose statin therapy compared to patients without statin therapy, assuming all other variables were held constant. There was no statistical significance between the low-dose statin group and the control for inpatient mortality (Figure 2 and Table 4). There was a statistical significance in increased mortality in patients with comorbidities, such as old age, male sex, high BMI, heart disease, kidney disease, and lung disease. There was no significant difference in race (Black vs. White vs. “Other”) or type 2 diabetes mellitus.
Figure 2.
Among patients with COVID-19, the odds of in-hospital mortality were 0.76 times as likely among patients with high-dose statin therapy compared to patients without statin therapy, assuming all other variables are held constant (P = .0249; 95% CI, 0.60–0.97). This means that the odds of in-hospital mortality decreased 24% among those with high-dose statin therapy compared to patients without statin therapy, assuming all other variables are held constant. There was no statistical significance between the low-dose statin group and the control for in-patient mortality. There was a statistical significance in increased mortality in patients with comorbidities, such as old age, male sex, high BMI, heart disease, kidney disease, and lung disease. There was no significant difference in race (Black vs White and “Other” vs White) or type 2 diabetes mellitus.
Table 4.
Odds Ratio Evaluating the Effect of Statins and Other Comorbidities in COVID-19 Inpatient Mortality
| Variables | Estimated odds ratio | 95% confidence intervals | P value |
|---|---|---|---|
| Low-dose statin therapy vs no statin therapy | 0.83 | 0.67–1.02 | .081 |
| High-dose statin therapy vs no statin therapy | 0.76 | 0.60–0.97 | .025 |
| Age (years) | 1.03 | 1.02–1.04 | <.001 |
| Sex ( Male vs Female ) | 1.21 | 1.02–1.44 | .030 |
| Race (Black vs White ) | 1.01 | 0.73–1.39 | .960 |
| Race (Other vs White) | 1.24 | 0.94–1.64 | .129 |
| Body mass index (BMI) | 1.02 | 1.01–1.03 | .007 |
| Heart disease | 1.86 | 1.55–2.24 | <.001 |
| Kidney disease | 1.63 | 1.35–1.98 | <.001 |
| Lung disease | 1.45 | 1.22–1.72 | <.001 |
| Type 2 diabetes | 0.76 | 0.56–1.02 | .070 |
Discussion
The COVID-19 pandemic brought up many concerns in the healthcare environment, particularly with the associated cardiovascular and pulmonary mortality. Statin use has been at the forefront of coronary artery disease. Our study focused on over 26 000 patients with an acute COVID-19 illness and whether statin use correlated with length of stay and inpatient mortality. The factors used in our study included age, BMI, heart disease, kidney disease, lung disease, type 2 diabetes mellitus, race, sex, and various treatments for COVID-19.
Previous studies have shown that the elderly population has had many factors leading to statin use, including an aging immune system, inflammation, epigenetic changes, and existing comorbidities.8 Very few studies have evaluated the correlation between high-intensity statin use, mortality, and length of stay in the hospital for these patients.
In general, there is a benefit for statin use in COVID-19 patients, primarily to prevent and treat cardiovascular disease. Patients who take statins are known to have lower thromboembolic episodes due to the effect statins have on the coagulation cascade and their immune-inflammatory properties, thus lowering mortality in these patients.8 It is inferred that for these patients the higher the statin dose the higher the likelihood of an improvement in mortality. However, patients with increased comorbidities are usually on a higher dose of statin and, therefore, have more complex medical problems leading to a more extended hospital stay. Given the complex nature of our patient population, there are many questions that should be answered on how statins played a role in this pandemic.
The hospital length of stay of patients who were on statins prior to COVID-19 infection was statistically longer compared to the no statin group (Table 2 and Figure 1). The increased length is potentially confounded by cardiovascular comorbidities that may present in these patients. The longer length of stay in the statins therapy arm could also be explained by the reduction in mortality, especially amongst high-dose statin therapy. Patients with cardiovascular comorbidities with no statin therapy might have succumbed to the disease earlier during their hospital stay.
The high-dose statin therapy group in COVID-19 patients showed a statistically significant reduction of 24% in mortality compared with the no statin therapy patient group (Table 4 and Figure 2). Acute COVID-19 infection can cause significant cardiovascular and pulmonary complications, which include myocardial infarction, heart failure, venous thromboembolic events, etc. A recent study by Katsoularis et al linked COVID-19 infection to myocardial events where the OR for myocardial infarction within 2 weeks of diagnosis of COVID-19 was 6.61.9 Many well-designed, randomized controlled trials describe the mortality benefit of statins in cardiovascular disease, which may explain the reduction in mortality in long-term statin users who present for acute COVID-19 infection despite their cardiovascular comorbidities.10,11
A meta-analysis in 2021 demonstrated a mortality benefit of statins in COVID-19 patients. Chow et al suggested that statin’s anti-inflammatory effect may reduce the cytokine storm in severe COVID-19 pneumonia.12 This study demonstrated that individuals who used statins before their COVID-19 hospitalization had a similar mortality risk to those who did not, and patients who were administered statins after their COVID-19 diagnosis were at lower risk of mortality.12 While our study agrees that there is a mortality benefit in COVID-19-infected patients who are taking statins, our study design did not investigate patients who were not on a statin and were started on a statin after COVID-19 diagnosis. Our study differentiated statin intensity, and the results suggested a mortality benefit in patients who reported taking high-intensity statins at home or during a hospital stay. We also established an all-cause mortality benefit for patients who used high-intensity statins before COVID-19 hospitalization.
The inpatient mortality benefits of statins can be explained by 2 effects. Firstly, long-term statin therapy reduces the incidence of cardiovascular events, and, therefore, reduces mortality and hospital length of stay in COVID-19 patients by reducing cardiovascular events. Additionally, COVID-19 symptoms are the result of a pro-inflammatory state from the disproportionate response of the immune system, known as cytokine release syndrome,12 which may lead to systemic severe diseases, such as pneumonia, gastroenteritis, acute coronary syndrome, and other lethal conditions. Statins have been previously studied for their anti-inflammatory effects.13 This effect makes statins a great medication for COVID-19 patients to reduce cardiovascular complications and potentially help with extra-cardiovascular symptoms by decreasing systemic inflammation and improving morbidity and mortality in these patients.
A limitation of this study is that we used the patient’s home medication list data. This list was self-reported by patients to staff members during admission. Our study assumed that a patient’s reported medication list was accurate and that patients had adhered to their prescribed medication. Additionally, we assumed that patients who were on statins at home were continued on their home regimen during hospitalization without assessing for potential reasons for stopping these medications, such as liver function tests or nil per oral status for procedures. As a result, our study underestimated the potential mortality benefit of statins in COVID-19 patients.
Conclusion
While our study found a mortality benefit in COVID-19-infected patients, it was not sufficient to recommend starting a statin for treatment in this patient population. Our study suggests continuing statins in COVID-19-positive patients unless absolute contraindications exist. Future randomized controlled trials are needed to establish a causal relationship between statins and mortality benefit in COVID-19 patients. Additionally, more studies are needed to assess the effect of statins on the incidence of cardiovascular complications post-COVID-19 hospitalization.
Funding Statement
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity.
Footnotes
Conflicts of Interest
The authors declare they have no conflicts of interest.
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
References
- 1. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morawska L, Milton DK. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020;71(9):2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Luca G, Debel N, Cercek M, et al. Impact of SARS-CoV-2 positivity on clinical outcome among STEMI patients undergoing mechanical reperfusion: insights from the ISACS STEMI COVID 19 registry. Atherosclerosis. 2021;332:48–54. doi: 10.1016/j.atherosclerosis.2021.06.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lefer DJ. Statins as potent antiinflammatory drugs. Circulation. 2002;106(16):2041–2042. doi: 10.1161/01.cir.0000033635.42612.88. [DOI] [PubMed] [Google Scholar]
- 7. Schartl M, Bocksch W, Koschyk DH, et al. Use of intravascular ultrasound to compare effects of different strategies of lipid-lowering therapy on plaque volume and composition in patients with coronary artery disease. Circulation. 2001;104(4):387–392. doi: 10.1161/hc2901.093188. [DOI] [PubMed] [Google Scholar]
- 8. Umakanthan S, Senthil S, John S, et al. The protective role of statins in COVID-19 patients: a retrospective observational study. Transl Med Commun. 2021;6(1):22. doi: 10.1186/s41231-021-00102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 11. Cholesterol Treatment Trialists’ (CTT) Collaboration. Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chow R, Im J, Chiu N, et al. The protective association between statins use and adverse outcomes among COVID-19 patients: a systematic review and meta-analysis. PLoS One. 2021;16(6):e0253576. doi: 10.1371/journal.pone.0253576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Darif D, Hammi I, Kihel A, El Idrissi Saik I, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog. 2021;153:104799. doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]