Abstract
Background:
Psychosocial interventions have emerged as an important component of a comprehensive therapeutic approach in early-onset schizophrenia, typically representing a more severe form of the disorder. Despite the feasibility and efficacy of Theory of Mind (ToM) psychotherapy for schizophrenia, relatively little is known regarding the neural mechanism underlying its effect on early-onset schizophrenia.
Methods:
We performed a randomized, active controlled trial in patients with early-onset schizophrenia, who were randomly allocated into either an intervention (ToM psychotherapy) or an active control (health education) group. Diffusion tensor imaging data were collected to construct brain structural networks, with both global and regional topological properties measured using graph theory.
Results:
We enrolled 28 patients with early-onset schizophrenia in our study. After 5 weeks of treatment, both the intervention and active control groups showed significant improvement in psychotic symptoms, yet the improvement was greater in the intervention group. Importantly, in contrast with no brain structural network change after treatment in the active control group, the intervention group showed increased nodal centrality of the left insula that was associated with psychotic symptom improvement.
Limitations:
We did not collect important information concerning the participants’ cognitive abilities, particularly ToM performance.
Conclusion:
These findings suggest a potential neural mechanism by which ToM psychotherapy exerts a beneficial effect on early-onset schizophrenia via strengthening the coordination capacity of the insula in brain structural networks, which may provide a clinically translatable biomarker for monitoring or predicting responses to ToM psychotherapy.
Clinical trial registration: NCT05577338; ClinicalTrials.gov
Introduction
Schizophrenia is a chronic mental disorder expressed as a complex behavioural syndrome with a heterogeneous combination of psychotic symptoms (e.g., delusions, hallucinations and disorganization) and cognitive dysfunctions, which may be attributable to genetic and/or environmental disruption of brain development.1,2 Early-onset schizophrenia, defined as onset of schizophrenia before the age of 18 years, occurs in a substantial proportion of patients with schizophrenia. 3–5 Despite evidence for a similar profile of clinical manifestations and neurobiological abnormalities between early- and adult-onset schizophrenia, extensive research has shown that those with early onset have more severe premorbid deficits, higher levels of psychotic symptoms, worse cognitive abilities, poorer outcomes and greater genetic predisposition than those with adult onset,3,5–18 jointly supporting a plausible hypothesis that early-onset schizophrenia represents a more severe form of the disorder. As such, studying early-onset schizophrenia might provide important insights into the neurodevelopmental origins of the disorder and the complexity by which genetic and environmental factors interact to modulate disease phenotype.
Cognitive impairments are a prominent and debilitating feature of early-onset schizophrenia that predicts illness chronicity and contributes to poor functional outcomes.13,19 Given that pharmacological treatment alone has yielded limited clinical benefits in improving cognitive dysfunctions in patients with early-onset schizophrenia,20 there is an urgent need for effective therapeutic interventions for cognitive deficits in this population.20,21 Under such circumstances, psychosocial interventions (e.g., cognitive remediation) have emerged as an important component of a comprehensive therapeutic approach in early-onset schizophrenia.22,23 It is now increasingly recognized that cognitive remediation therapy for schizophrenia is a behavioural training–based intervention that aims to improve cognition and functional outcome with the goal of durability and generalization. 24 An expanding body of evidence indicates that cognitive remediation therapy benefits patients with early-onset schizophrenia.25 Theory of Mind (ToM) refers to the ability to consider various viewpoints in the process of attributing mental states to others and plays a central role in regulating social interactions.26 It is well documented that patients with schizophrenia show impairments in ToM,27 which influence real-world social functioning and are strongly associated with community outcomes.28,29 Targeted ToM interventions are specifically designed to improve patients’ ability to attribute mental states to others. Prior data have established the feasibility and efficacy of cognitive remediation targeting ToM functions for schizophrenia.26,30–34 However, relatively little is known regarding the neural mechanism underlying the effect of ToM psychotherapy on early-onset schizophrenia.
The human brain is a complex, integrated system of highly interconnected regions via white matter fibres, typically referred to as brain structural networks. Modern neuroimaging techniques, and diffusion MRI in particular, have made it feasible to reconstruct comprehensive maps of white matter structural connections in vivo.35,36 Leveraging diffusion MRI, a large number of studies have found structural connectivity damage in early- and adult-onset schizophrenia, characterized by alterations in multiple diffusion measures (e.g., fractional anisotropy [FA]) reflecting white matter microstructural integrity.37–46 In parallel, advances in network science and graph theory have improved our ability to study the topological organization of brain structural networks that exists above and beyond lower-order structural connectivity. 47–49 In this framework, there is strong empirical evidence for structural network topological disruptions in schizophrenia, manifested as changes in global and regional network properties.50–57 Prior work focusing exclusively on early-onset schizophrenia has also reported abnormal structural network topology in this condition.58 Collectively, these findings suggest that brain structural network measures derived from a combination of diffusion MRI and graph theory may serve as useful imaging biomarkers for early-onset schizophrenia and its treatment.
In this study, we performed a randomized, active controlled trial to investigate the neural mechanism underlying the effect of ToM psychotherapy on patients with early-onset schizophrenia, by integrating diffusion tensor imaging (DTI) data with graph theoretical analysis. In light of the aforementioned evidence from the literature, we hypothesized that ToM psychotherapy would lead to alterations in brain structural networks through which ToM psychotherapy exerts its beneficial effect on patients with early-onset schizophrenia.
Methods
Participants
This randomized controlled trial was registered with ClinicalTrials. gov (NCT05577338). We recruited right-handed adolescent patients with early-onset schizophrenia from Anhui Mental Health Center. Written informed consent was obtained from all participants, and the study was approved by the ethics committee of The First Affiliated Hospital of Anhui Medical University (PJ2022–10–37). Diagnosis of schizophrenia was determined by the consensus of 2 psychiatrists using the MINI-International Neuro-psychiatric Interview in accordance with the International Classification of Diseases (ICD-10) criteria. Right handedness was determined using the Edinburgh handedness inventory. 59 The other inclusion criteria were age 13–18 years; a capacity for understanding, judging and expressing; and an ability to complete the experiment independently. The exclusion criteria were present or any history of neurologic or major physical diseases, history of head injury with loss of consciousness, history of substance abuse, history of electroconvulsive therapy, mental retardation and MRI contraindications. For all patients, the Positive and Negative Syndrome Scale (PANSS)60 was used to assess the severity of psychotic symptoms.
Study design
Following successful completion of screening, half the patients were randomly assigned to the intervention group and half to the active control group, controlling for equal distribution of female and male participants between the groups. Patients in the intervention group underwent semi-structured, multidomain, tailored group psychotherapy for 5 weeks (a 2-hour session twice a week) using an appropriately adapted ToM intervention strategy.30 In brief, the ToM intervention was conducted by a well-trained psychiatrist on groups of 10 members using comic strips and cartoons depicting human social interactions. Patients were instructed to note the relevant details, to decipher meaningful information (i.e., place, time, emotions, characters’ actions and physical features), to read the verbal part of the comic strips and to identify their literal meaning. They were then asked to interpret hidden meaning using all the information collected and to hypothesize interpretations of scenes on the basis of expressed emotions, relationships between characters, implicit motivations and mental states. The active control group received group health education about the disease (e.g., epidemiology, diagnosis, clinical features, treatment), the duration of which was identical to that of psychotherapy. Both groups received their regular antipsychotic medication as prescribed by the attending psychiatrists during this study. All patients completed 2 study visits: baseline (before psychotherapy or health education) and follow-up (after 5 weeks of psychotherapy or health education). Clinical assessment and MRI examination occurred at both baseline and follow-up.
Image acquisition
The MRI data were collected on a 3 T MR system (Discovery MR750w, General Electric) with a 24-channel head coil. During scanning, tight but comfortable foam and earplugs were used to minimize head movement and scanner noise. All participants were instructed to relax, keep their eyes closed but not fall asleep, think of nothing in particular and move as little as possible. Structural images were obtained using a high-resolution 3-dimensional T1-weighted brain volume (BRAVO) sequence with the following parameters: repetition time (TR) 8.5 ms; echo time (TE) 3.2 ms; inversion time (TI) 450 ms; flip angle 12°; field of view (FOV) 256 mm × 256 mm; matrix size 256 × 256; slice thickness 1 mm, no gap; voxel size 1 mm × 1 mm × 1 mm; 188 sagittal slices; and acquisition time 296 s. The DTI data were acquired using a spin-echo single-shot echo planar imaging (SE-SS-EPI) sequence with the following parameters: TR 10 000 ms; TE 74 ms; flip angle 90°; FOV 256 mm × 256 mm; matrix 128 × 128; slice thickness 3 mm, no gap; 50 axial slices; 64 diffusion gradient directions (b = 1000 s/mm2 plus 5 b = 0 reference images); and acquisition time 700 s. Routine T2-weighted images were also obtained to exclude any organic brain abnormality. All images were visually inspected to ensure that only images without visible artifacts were included in subsequent analyses.
DTI data processing
The software packages FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl),61 Diffusion Toolkit (DTK; http://trackvis.org/dtk) and Pipeline for Analyzing braiN Diffusion imAges (PANDA; http://www.nitrc.org/projects/panda)62 were used for DTI data processing. Eddy currents in the gradient coils induce stretches and shears in the diffusion-weighted images. These distortions are different for different gradient directions. Eddy current correction implemented in FSL was adopted to correct for these distortions and simple head motions by registering the diffusion-weighted images to a reference volume (i.e., the first b0 image) using affine transformations. Correspondingly, the diffusion gradient direction of each diffusion-weighted image was rotated according to the resultant affine transformation information.63 Then, brain tissues were extracted using the FSL Brain Extraction Tool (http://www.fmrib.ox.ac.uk/fsl/bet2). Next, the 3-dimensional maps of the diffusion tensor and FA were calculated using the DTIFIT toolbox. Finally, a deterministic streamline tracking algorithm, Fiber Assignment by Continuous Tracking (FACT), was performed to obtain the whole-brain fibre tractography.64–66 The fibre tracking procedure started from the deep white matter regions and ended at a voxel with a turning angle greater than 45° or with an FA less than 0.2.
Brain structural network construction
Because brain structural networks were constructed in native diffusion space, the automated anatomic labelling (AAL) template67 in the Montreal Neurological Institute (MNI) space was initially transformed to individual native diffusion space. Briefly, individual structural images were first coregistered to their b0 images using a linear transformation. Then, the coregistered structural images were normalized to the MNI space using a nonlinear transformation. The derived deformation parameters were inverted and used to transform the AAL template from the MNI space to individual native diffusion space. Nodes and edges are 2 basic elements of a brain network. Here, 90 cortical and subcortical regions within the AAL template were defined as nodes. To define edges, we calculated the number of fibres (with end points located in both nodes during the fibre tracking) between any pairs of nodes, yielding a 90 × 90 fibre number (FN) matrix for each participant. To balance the sensitivity and specificity, a threshold of 3 fibres was applied to all FN matrices; that is, 2 nodes were considered connected if the FN between them was greater than or equal to 3.68,69 Finally, each FN matrix was thresholded and converted into a binary matrix.
Structural network analysis
Graph theoretical analysis was carried out on the resulting structural networks using GRETNA software (http://www.nitrc.org/projects/gretna).70 Both global and regional network properties were measured. For global property, we calculated 2 network efficiency measures: global efficiency (reflecting the capability of parallel information transfer over the network) and local efficiency (representing the fault tolerance of the network that indicates how well the information is communicated within the neighbours of a given node when this node is eliminated).71–73 For regional property, we focused primarily on betweenness centrality, a commonly used measure to describe the importance of individual nodes within a network.74 Betweenness centrality is defined as the fraction of all shortest paths in the network that pass through a given node. Brain regions with higher betweenness centrality are considered hubs that are assumed to play a pivotal role in global brain communication because of their central embedding in the overall network. The calculation procedure of these global and regional network measures is detailed in the previous literature.47
Statistical analysis
The statistical analyses of demographic and clinical data were conducted using the SPSS 23.0 software package (SPSS Inc.). For cross-sectional analyses of baseline data, we compared age, education, illness duration and psychotic symptoms (PANSS scores) between the intervention and active control groups using 2-sample t tests. The Pearson χ2 test was adopted to test group difference in gender. For longitudinal analyses of PANSS scores, we used 2-way mixed analyses of variance (ANOVA), with group as a between-subjects factor (intervention v. active control) and time as a within-subjects factor (baseline v. follow-up). We focused our analysis principally on group × time interactions, followed by post hoc paired t tests for comparing time points within each group separately. The significance level was set at p < 0.05.
In terms of neuroimaging data, we made use of the above-mentioned 2-way mixed ANOVA to examine group × time interactions on structural network measures, followed by post hoc paired t tests. For global and local efficiency, the significance threshold was set at p < 0.05. For betweenness centrality of 90 nodes, correction for multiple comparisons was performed using a false-positive correction (i.e., p < [1/90] = 0.011), which is not as conservative as a Bonferroni or false discovery rate correction and thus does not yield strong type I error control for exploratory analysis at a regional level of network organization.75 In addition, we calculated longitudinal changes (follow-up – baseline) in network measures and PANSS scores, followed by Pearson correlation analyses to evaluate their associations within each group.
Results
Demographic and clinical characteristics
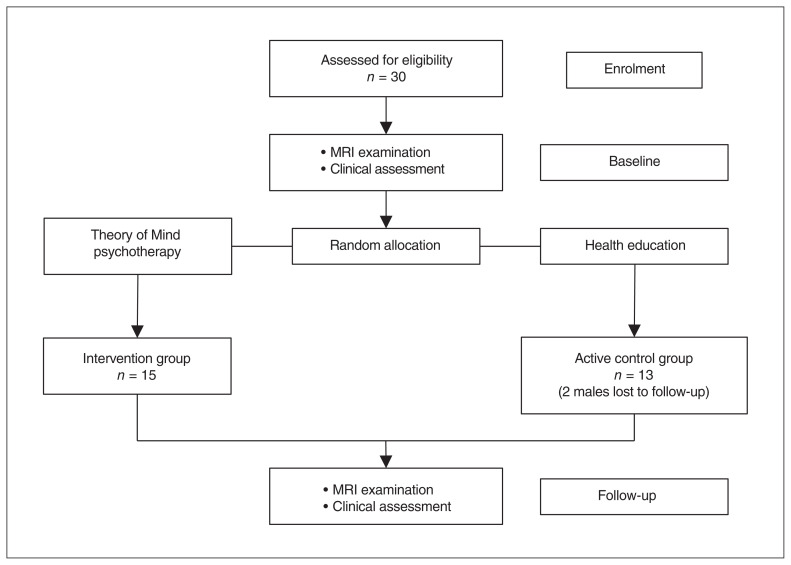
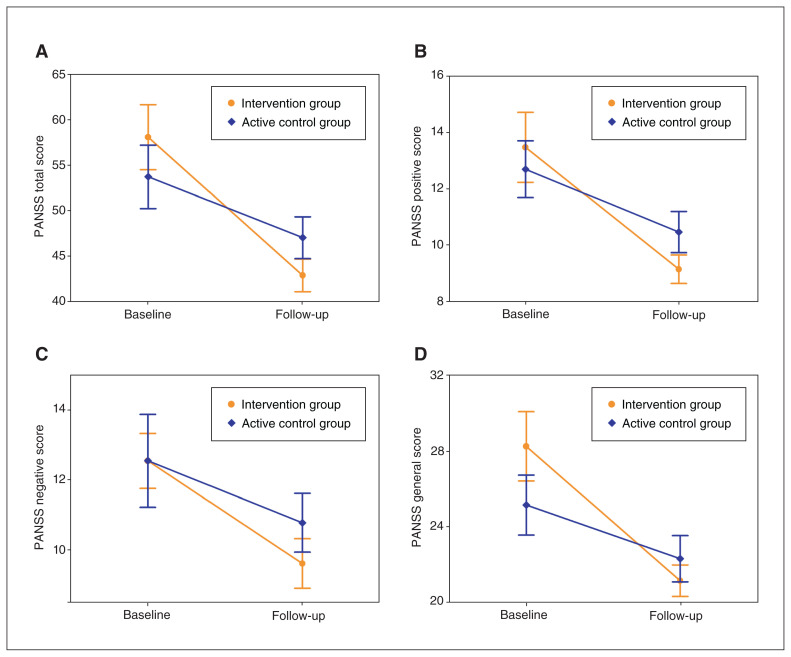
We recruited 30 participants; 15 were assigned to the intervention group and 15 to the active control group. Two male participants in the active control group were lost to follow-up and were not included in the analysis, leaving a final sample of 28 patients (Figure 1). Demographic and clinical data of the patients at baseline are presented in Table 1. There were no significant differences in gender, age, education, illness duration or PANSS scores between the intervention and active control groups. Two-way mixed ANOVA showed significant group × time interaction effects on PANSS total (F = 7.93, p = 0.010), positive (F = 4.45, p = 0.047) and general (F = 7.17, p = 0.014) scores. Post hoc analyses revealed decreased PANSS total, positive and general scores from baseline to follow-up in both groups, but a greater decrease in the intervention group (Figure 2 and Table 2). For PANSS negative score, we found a significant decrease from baseline to follow-up in both groups, but a marginally significant group × time interaction indicating a trend toward a greater decrease in the intervention group (F = 3.46, p = 0.076; Figure 2 and Table 2).
Figure 1.
Flow of participants through the study. MRI = magnetic resonance imaging.
Table 1.
Demographic and clinical characteristics of the patients with early-onset schizophrenia at baseline
| Characteristic | Group, mean ± SD* | Statistical test | p value | |
|---|---|---|---|---|
|
| ||||
| Intervention, n = 15 | Active control, n = 13 | |||
| Gender, F/M | 9/6 | 9/4 | χ2 = 0.258 | 0.705 |
| Age, yr | 16.20 ± 1.32 | 16.54 ± 1.39 | t = −0.657 | 0.517 |
| Education, yr | 10.00 ± 1.41 | 10.23 ± 1.59 | t = −0.407 | 0.688 |
| Illness duration, mo | 20.13 ± 15.91 | 20.85 ± 18.28 | t = −0.110 | 0.913 |
| PANSS score | ||||
| Total | 58.07 ± 13.88 | 53.69 ± 12.57 | t = 0.868 | 0.393 |
| Positive | 13.47 ± 4.81 | 12.69 ± 3.64 | t = 0.474 | 0.639 |
| Negative | 12.53 ± 3.04 | 12.54 ± 4.79 | t = −0.003 | 0.997 |
| General | 28.27 ± 7.12 | 25.15 ± 5.73 | t = 1.261 | 0.218 |
F = female; M = male; PANSS = Positive and Negative Syndrome Scale; SD = standard deviation.
Unless indicated otherwise.
Figure 2.
Longitudinal changes in psychotic symptoms. PANSS = Positive and Negative Syndrome Scale.
Table 2.
Longitudinal changes in psychotic symptoms of patients with early-onset schizophrenia
| PANSS score | Intervention group | Active control group | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Change,* mean ± SD | paired t test | p value | Change,* mean ± SD | paired t test | p value | |
| Total | −15.20 ± 8.86 | −6.646 | 1.10 × 10−5 | −6.69 ± 7.62 | −3.167 | 0.008 |
| Positive | −4.33 ± 3.44 | −4.884 | 2.42 × 10−4 | −2.23 ± 2.20 | −3.649 | 0.003 |
| Negative | −2.93 ± 1.16 | −9.769 | 1.25 × 10−7 | −1.77 ± 2.86 | −2.229 | 0.046 |
| General | −7.13 ± 4.85 | −5.693 | 5.60 × 10−5 | −2.85 ± 3.29 | −3.122 | 0.009 |
PANSS = Positive and Negative Syndrome Scale.
Change = follow-up – baseline.
Brain structural network changes
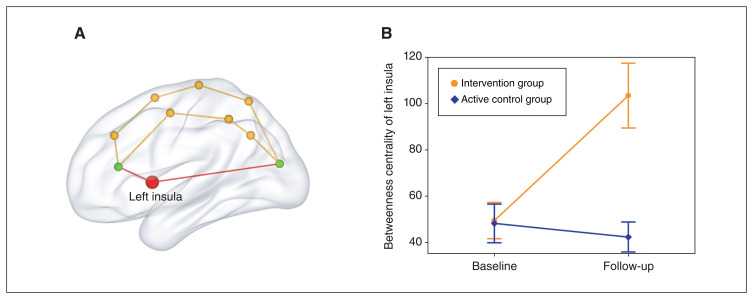
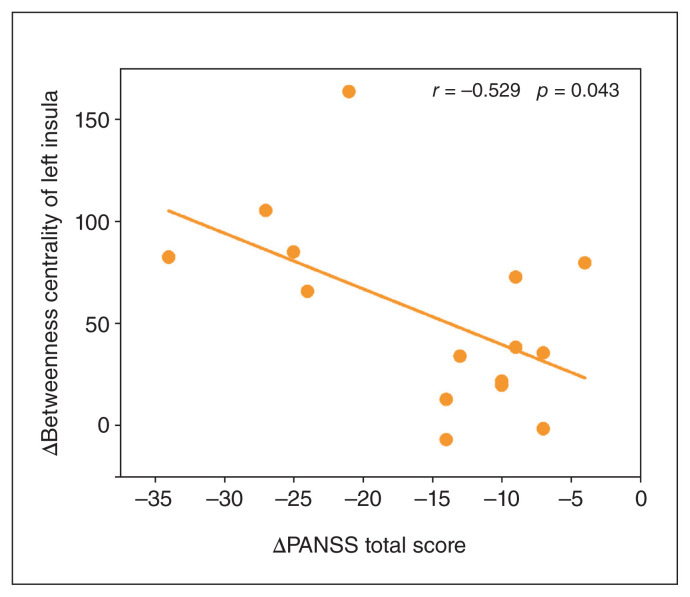
With respect to global network property, 2-way mixed ANOVA showed no significant group × time interactions for global (F = 1.98, p = 0.171) or local (F = 0.97, p = 0.333) efficiency. As to regional network property, we found a significant group × time interaction effect on betweenness centrality of the left insula (F = 15.22, p = 6.04 × 10−4) that survived a false-positive correction. Post hoc analyses showed increased betweenness centrality of the left insula from baseline to follow-up in the intervention group (t = 4.58, p = 4.28 × 10−4), but no change in the active control group (t = −0.63, p = 0.540) (Figure 3). The group × time interaction results for other brain regions are shown in Appendix 1, Table S1, available at https://www.jpn.ca/lookup/doi/10.1503/jpn.230049/tab-related-content. For completeness, we conducted the same analyses using other regional network measures (degree centrality and nodal efficiency), but found no significant results (Appendix 1, Tables S2 and S3). Moreover, we observed a significant PANSS total score change × group interaction effect on betweenness centrality change of the left insula (F = 19.57, p = 0.002), which was driven by a significant negative correlation between PANSS total score change and betweenness centrality change of the left insula in the intervention group (r = −0.529, p = 0.043) (Figure 4) and a nonsignificant correlation in the active control group (r = 0.41, p = 0.163).
Figure 3.
Longitudinal change in betweenness centrality of the left insula. Betweenness centrality is defined as the fraction of all shortest paths in the network that pass through a given node. The red path passing through the left insula (red node) represents the shortest path between the 2 green nodes.
Figure 4.
Scatter plot of the negative correlation between PANSS total score change and betweenness centrality change of the left insula in the intervention group. Change = follow-up – baseline. PANSS = Positive and Negative Syndrome Scale.
Discussion
By applying graph theoretical analysis to DTI data, we performed, to our knowledge, the first randomized, active controlled trial to examine the neural mechanism underlying the effect of ToM psychotherapy on patients with early-onset schizophrenia. After 5 weeks of treatment, both the intervention (ToM psychotherapy + antipsychotics) and active control (health education + antipsychotics) groups showed significant improvement in psychotic symptoms; yet, the improvement was greater in the intervention group. In contrast with the active control group, which showed no change in brain structural network after treatment, the intervention group showed increased nodal centrality of the left insula that was associated with improvement of psychotic symptoms. These findings suggest that ToM psychotherapy may contribute to alterations in brain structural networks, exerting a beneficial effect on patients with early-onset schizophrenia.
It is well established that psychotherapy holds promise as an effective treatment in adults with schizophrenia.76,77 There is also recent evidence in favour of its potential usefulness in adolescents with early-onset schizophrenia.22,23 For example, a pioneering study suggested that cognitive behavioural therapy adapted to the needs of adolescents with early-onset schizophrenia was a safe and tolerable treatment approach for improving negative symptoms, global functioning and quality of life.78 Puig and colleagues reported that cognitive remediation therapy induced significant, reliable, medium-to-large cognitive improvements and significant functional gains in adolescents with early-onset schizophrenia.25 Complementing the pilot work, our data showed that ToM psychotherapy could help alleviate psychotic symptoms, especially positive symptoms and general psychopathology, in patients with early-onset schizophrenia. Our findings, taken with those from the earlier reports, indicate that a combination of psychotherapy and antipsychotics may be a better intervention strategy for early-onset schizophrenia.
Extending the behavioural evidence, our neuroimaging analysis further showed that ToM psychotherapy led to increased nodal centrality of the left insula that was associated with improvement of psychotic symptoms in patients with early-onset schizophrenia. It is generally accepted that the insula acts as a key cortical hub engaged in a wide variety of functions ranging from lower-order sensorimotor processes to higher-order cognition and emotion,79–84 deficits of which have been frequently reported in schizophrenia.85–87 Moreover, numerous clinical neuroimaging studies have documented structural and functional abnormalities of the insula in schizophrenia including early-onset schizophrenia,87–98 highlighting its prominent role in the disease neuropathology. 87 From a large-scale brain organization perspective, the insula-anchored salience network has been suggested to play a central role in the psychopathology and cognitive dysfunction in schizophrenia,99 which is often referred to as a salience dysregulation disorder.100 In parallel, the involvement of the insula in ToM functions has been evident,101,102 implying its potential contribution to the neural effect of ToM psychotherapy. That said, increased nodal centrality of the insula may reflect its strengthened role in coordinating whole-brain structural networks, presumably in response to ToM intervention. Combined, these data invite us to speculate that ToM psychotherapy might induce intervention-dependent neuroplasticity in the insula, expressed as its elevated importance in global information integration, which may in turn give rise to improvement of psychotic symptoms in patients affected by early-onset schizophrenia. Nevertheless, given the lack of a healthy control group, it is not clear whether the nodal centrality increase represents normalization or compensation. Note that the lateralization of the effect observed in the left insula is consistent with previous findings showing a pattern of grey matter abnormalities in the left hemisphere in individuals with early-onset schizophrenia.103,104 Furthermore, a recent coordinate-based meta-analysis showed left-lateralized insular grey matter reduction in individuals with recently diagnosed schizophrenia, which appeared to be bilateral in those with chronic schizophrenia.105 From the perspective of core psychotic symptoms, the left-lateralized grey matter abnormality of the insula has been thought to contribute significantly to auditory hallucinations in individuals with schizophrenia.106–108
Limitations
Our study has a few limitations. First, while our small sample size is commonplace for within-subject neuroimaging study designs, it limits the statistical power and the generalizability of the findings. This is a well-known challenge in early-onset schizophrenia research, as larger samples are difficult to recruit because of the uncommon early onset of schizophrenia and the moderate compliance of patients. Second, the patients with early-onset schizophrenia were receiving their regular antipsychotic medication during the study. The confounding effect of antipsychotics on our results merits further investigation but was beyond the scope of this research. Third, the intervention duration of 5 weeks was relatively short and may have resulted in transient and subtle neuroimaging changes. Extending the duration of the intervention would help detect and estimate the stability and reliability of the treatment effects. Fourth, we did not collect important information concerning the participants’ cognitive abilities, particularly ToM, which is a key outcome to assess when investigating the impact of a ToM intervention. Further analysis of this information may help in the interpretation of our findings. Fifth, considering our small sample size, a lenient false-positive correction was used to adjust for multiple comparisons. Application of a more rigorous Bonferroni or false discovery rate correction to a large data set is warranted to validate our preliminary results. Sixth, our sample included more females than males, in contrast with a higher prevalence of schizophrenia among males. This may have led to gender bias, which should be addressed in future studies. Finally, neither the participants nor the individuals conducting the analysis were blinded to the study conditions, which could have introduced biases.
Conclusion
Our randomized, active controlled trial in patients with early-onset schizophrenia suggests a potential neural mechanism by which ToM psychotherapy exerts its antipsychotic efficacy via strengthening the coordination capacity of the insula in brain structural networks. More generally, our findings may provide a clinically translatable biomarker for monitoring or predicting responses to ToM psychotherapy and expose the insula as a promising anatomic target for novel interventions (e.g., transcranial magnetic stimulation) in patients with early-onset schizophrenia.
Supplementary Material
Acknowledgement
The authors are grateful to study participants for volunteering their time.
Footnotes
Competing interests: None declared.
Contributors: Y.-B. Jia and J. Zhu designed the study. H. Zhong and H. Cai acquired the data, which S. Liu and Y. Qian analyzed. S. Liu wrote the article. All of the authors critically revised it for important intellectual content and gave final approval of the version to be published.
Funding: The study was supported by the National Natural Science Foundation of China (grant no. 82071905), the Outstanding Youth Support Project of Anhui Province Universities (grant no. gxyqZD2022026), the Scientific Research Key Project of Anhui Province Universities (grant no. 2022AH051135), the Scientific Research Foundation of Anhui Medical University (grant no. 2022xkj143) and the Anhui University Collaborative Innovation Project (grant no. GXXT-2021-065).
Data sharing: Data that support the findings of this study are publicly available in the study’s Open Science Framework repository (https://osf.io/j9fc2/).
References
- 1.Carpenter WT, Jr, Buchanan RW. Schizophrenia. N Engl J Med 1994; 330:681–90. [DOI] [PubMed] [Google Scholar]
- 2.Hemmings G. Schizophrenia. Lancet 2004;364:1312–3. [DOI] [PubMed] [Google Scholar]
- 3.Kumra S, Charles Schulz S. Editorial: research progress in early-onset schizophrenia. Schizophr Bull 2008;34:15–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernal DL, Boldsen SK, Lauritsen MB, et al. Long-term outcome of early-onset compared to adult-onset schizophrenia: a nationwide Danish register study. Schizophr Res 2020;220:123–9. [DOI] [PubMed] [Google Scholar]
- 5.Remschmidt H, Theisen F. Early-onset schizophrenia. Neuropsychobiology 2012;66:63–9. [DOI] [PubMed] [Google Scholar]
- 6.Vourdas A, Pipe R, Corrigall R, et al. Increased developmental deviance and premorbid dysfunction in early onset schizophrenia. Schizophr Res 2003;62:13–22. [DOI] [PubMed] [Google Scholar]
- 7.Hollis C. Child and adolescent (juvenile onset) schizophrenia. A case control study of premorbid developmental impairments. Br J Psychiatry 1995;166:489–95. [DOI] [PubMed] [Google Scholar]
- 8.Nicolson R, Lenane M, Singaracharlu S, et al. Premorbid speech and language impairments in childhood-onset schizophrenia: association with risk factors. Am J Psychiatry 2000;157:794–800. [DOI] [PubMed] [Google Scholar]
- 9.Hoff AL, Harris D, Faustman WO, et al. A neuropsychological study of early onset schizophrenia. Schizophr Res 1996;20:21–8. [DOI] [PubMed] [Google Scholar]
- 10.Hollis C. Adult outcomes of child- and adolescent-onset schizophrenia: diagnostic stability and predictive validity. Am J Psychiatry 2000;157:1652–9. [DOI] [PubMed] [Google Scholar]
- 11.Holmén A, Juuhl-Langseth M, Thormodsen R, et al. Executive function in early- and adult onset schizophrenia. Schizophr Res 2012;142:177–82. [DOI] [PubMed] [Google Scholar]
- 12.Li ZT, Li SB, Wen JF, et al. Early-onset schizophrenia showed similar but more severe olfactory identification impairment than adult-onset schizophrenia. Front Psychiatry 2020;11:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oie M, Sundet K, Ueland T. Neurocognition and functional outcome in early-onset schizophrenia and attention-deficit/hyperactivity disorder: a 13-year follow-up. Neuropsychology 2011;25:25–35. [DOI] [PubMed] [Google Scholar]
- 14.McClellan J, Stock SAmerican Academy of C, Adolescent Psychiatry Committee on Quality I. Practice parameter for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry 2013;52:976–90. [DOI] [PubMed] [Google Scholar]
- 15.Vyas NS, Patel NH, Puri BK. Neurobiology and phenotypic expression in early onset schizophrenia. Early Interv Psychiatry 2011;5:3–14. [DOI] [PubMed] [Google Scholar]
- 16.Kendall T, Hollis C, Stafford M, et al. Guideline Development G. Recognition and management of psychosis and schizophrenia in children and young people: summary of NICE guidance. BMJ 2013;346:f150. [DOI] [PubMed] [Google Scholar]
- 17.Frangou S. Cognitive function in early onset schizophrenia: a selective review. Front Hum Neurosci 2010;3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De la Serna E, Puig O, Mezquida G, et al. Relationship between cognition and age at onset of first-episode psychosis: comparative study between adolescents, young adults, and adults. Eur Child Adolesc Psychiatry 2023;32:639–49. [DOI] [PubMed] [Google Scholar]
- 19.Nieto RG, Castellanos FX. A meta-analysis of neuropsychological functioning in patients with early onset schizophrenia and pediatric bipolar disorder. J Clin Child Adolesc Psychol 2011;40:266–80. [DOI] [PubMed] [Google Scholar]
- 20.Frazier JA, Giuliano AJ, Johnson JL, et al. Neurocognitive outcomes in the Treatment of Early-Onset Schizophrenia Spectrum Disorders study. J Am Acad Child Adolesc Psychiatry 2012;51:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachman P, Jalbrzikowski M, Bearden CE. The voices go, but the song remains the same: how can we rescue cognition in early-onset schizophrenia? J Am Acad Child Adolesc Psychiatry 2012;51:464–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armando M, Pontillo M, Vicari S. Psychosocial interventions for very early and early-onset schizophrenia: a review of treatment efficacy. Curr Opin Psychiatry 2015;28:312–23. [DOI] [PubMed] [Google Scholar]
- 23.Schimmelmann BG, Schmidt SJ, Carbon M, et al. Treatment of adolescents with early-onset schizophrenia spectrum disorders: in search of a rational, evidence-informed approach. Curr Opin Psychiatry 2013;26:219–30. [DOI] [PubMed] [Google Scholar]
- 24.Wykes T, Huddy V, Cellard C, et al. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry 2011;168:472–85. [DOI] [PubMed] [Google Scholar]
- 25.Puig O, Penades R, Baeza I, et al. Cognitive remediation therapy in adolescents with early-onset schizophrenia: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry 2014;53:859–68. [DOI] [PubMed] [Google Scholar]
- 26.Vass E, Fekete Z, Simon V, et al. Interventions for the treatment of theory of mind deficits in schizophrenia: Systematic literature review. Psychiatry Res 2018;267:37–47. [DOI] [PubMed] [Google Scholar]
- 27.Bora E. Theory of mind and schizotypy: a meta-analysis. Schizophr Res 2020;222:97–103. [DOI] [PubMed] [Google Scholar]
- 28.Weng Y, Lin J, Ahorsu DK, et al. Neuropathways of theory of mind in schizophrenia: a systematic review and meta-analysis. Neurosci Biobehav Rev 2022;137:104625. [DOI] [PubMed] [Google Scholar]
- 29.Savla GN, Vella L, Armstrong CC, et al. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull 2013;39:979–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bechi M, Spangaro M, Bosia M, et al. Theory of Mind intervention for outpatients with schizophrenia. Neuropsychol Rehabil 2013;23:383–400. [DOI] [PubMed] [Google Scholar]
- 31.Gürcan MB, Yildiz M, Patir K, et al. The effects of narrative and movie therapy on the theory of mind and social functioning of patients with schizophrenia. Noro Psikiyatr Ars 2021;58:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bechi M, Riccaboni R, Ali S, et al. Theory of mind and emotion processing training for patients with schizophrenia: preliminary findings. Psychiatry Res 2012;198:371–7. [DOI] [PubMed] [Google Scholar]
- 33.Bechi M, Agostoni G, Buonocore M, et al. The association of autistic traits with Theory of Mind and its training efficacy in patients with schizophrenia. Schizophr Res Cogn 2020;19:100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vass E, Simon V, Fekete Z, et al. A novel virtual reality-based theory of mind intervention for outpatients with schizophrenia: a proof-of-concept pilot study. Clin Psychol Psychother 2021;28:727–38. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Toga AW. Connectome imaging for mapping human brain pathways. Mol Psychiatry 2017;22:1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winston GP. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quanti Imaging Med Surg 2012;2:254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Zhuo C, Qin W, et al. Performances of diffusion kurtosis imaging and diffusion tensor imaging in detecting white matter abnormality in schizophrenia. Neuroimage Clin 2015;7:170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J, Zhuo C, Liu F, et al. Neural substrates underlying delusions in schizophrenia. Sci Rep 2016;6:33857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamnes CK, Agartz I. White matter microstructure in early-onset schizophrenia: a systematic review of diffusion tensor imaging studies. J Am Acad Child Adolesc Psychiatry 2016;55:269–79. [DOI] [PubMed] [Google Scholar]
- 40.Carreira Figueiredo I, Borgan F, Pasternak O, et al. White-matter free-water diffusion MRI in schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology 2022;47:1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao Y, Sun H, Shi S, et al. White matter abnormalities in never-treated patients with long-term schizophrenia. Am J Psychiatry 2018;175:1129–36. [DOI] [PubMed] [Google Scholar]
- 42.Voineskos AN, Lobaugh NJ, Bouix S, et al. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain 2010;133:1494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraguljac NV, Anthony T, Skidmore FM, et al. Micro- and macro-structural white matter integrity in never-treated and currently unmedicated patients with schizophrenia and effects of short-term antipsychotic treatment. Biol Psychiatry Cogn Neurosci Neuroimaging 2019;4:462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunimatsu N, Aoki S, Kunimatsu A, et al. Tract-specific analysis of white matter integrity disruption in schizophrenia. Psychiatry Res 2012;201:136–43. [DOI] [PubMed] [Google Scholar]
- 45.Fujino J, Takahashi H, Miyata J, et al. Impaired empathic abilities and reduced white matter integrity in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2014;48:117–23. [DOI] [PubMed] [Google Scholar]
- 46.Oestreich LKL, Lyall AE, Pasternak O, et al. Characterizing white matter changes in chronic schizophrenia: a free-water imaging multi-site study. Schizophr Res 2017;189:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–69. [DOI] [PubMed] [Google Scholar]
- 48.Fornito A, Zalesky A, Breakspear M. Graph analysis of the human connectome: promise, progress, and pitfalls. Neuroimage 2013; 80: 426–44. [DOI] [PubMed] [Google Scholar]
- 49.Liao X, Vasilakos AV, He Y. Small-world human brain networks: Perspectives and challenges. Neurosci Biobehav Rev 2017;77:286–300. [DOI] [PubMed] [Google Scholar]
- 50.Zhu J, Wang C, Liu F, et al. Alterations of functional and structural networks in schizophrenia patients with auditory verbal hallucinations. Front Hum Neurosci 2016;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu J, Qian Y, Zhang B, et al. Abnormal synchronization of functional and structural networks in schizophrenia. Brain Imaging Behav 2020;14:2232–41. [DOI] [PubMed] [Google Scholar]
- 52.Micheloyannis S. Graph-based network analysis in schizophrenia. World J Psychiatry 2012;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Narr KL, Leaver AM. Connectome and schizophrenia. Curr Opin Psychiatry 2015;28:229–35. [DOI] [PubMed] [Google Scholar]
- 54.van den Heuvel MP, Fornito A. Brain networks in schizophrenia. Neuropsychol Rev 2014;24:32–48. [DOI] [PubMed] [Google Scholar]
- 55.Luo C, Lencer R, Hu N, et al. Characteristics of white matter structural networks in chronic schizophrenia treated with clozapine or risperidone and those never treated. Int J Neuropsychopharmacol 2020;23:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li F, Lui S, Yao L, et al. Altered white matter connectivity within and between networks in antipsychotic-naive first-episode schizophrenia. Schizophr Bull 2018;44:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Biase MA, Cropley VL, Baune BT, et al. White matter connectivity disruptions in early and chronic schizophrenia. Psychol Med 2017;47:2797–810. [DOI] [PubMed] [Google Scholar]
- 58.Cabral J, Fernandes HM, Van Hartevelt TJ, et al. Structural connectivity in schizophrenia and its impact on the dynamics of spontaneous functional networks. Chaos 2013;23:046111. [DOI] [PubMed] [Google Scholar]
- 59.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 60.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261–76. [DOI] [PubMed] [Google Scholar]
- 61.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–19. [DOI] [PubMed] [Google Scholar]
- 62.Cui Z, Zhong S, Xu P, et al. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci 2013;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 2009;61:1336–49. [DOI] [PubMed] [Google Scholar]
- 64.Zhao W, Zhu D, Zhang Y, et al. Relationship between illness duration, corpus callosum changes, and sustained attention dysfunction in major depressive disorder. Quant Imaging Med Surg 2021;11:2980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Li X, Zhang C, et al. Selective micro-structural integrity impairment of the isthmus subregion of the corpus callosum in alcohol-dependent males. BMC Psychiatry 2019;19:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang B, Zhu DM, Zhao W, et al. Selective microstructural integrity impairments of the anterior corpus callosum are associated with cognitive deficits in obstructive sleep apnea. Brain Behav 2019;9:e01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–89. [DOI] [PubMed] [Google Scholar]
- 68.Zalesky A, Fornito A, Cocchi L, et al. Connectome sensitivity or specificity: which is more important? Neuroimage 2016;142:407–20. [DOI] [PubMed] [Google Scholar]
- 69.de Reus MA, van den Heuvel MP. Estimating false positives and negatives in brain networks. Neuroimage 2013;70:402–9. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Wang X, Xia M, et al. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 2015;9:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu J, Zhuo C, Liu F, et al. Distinct disruptions of resting-state functional brain networks in familial and sporadic schizophrenia. Sci Rep 2016;6:23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol 2007;3:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett 2001;87:198701. [DOI] [PubMed] [Google Scholar]
- 74.Mirakyan M. ABCDE: Approximating Betweenness-Centrality ranking with progressive-DropEdge. PeerJ Comput Science 2021;7:e699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci 2010;30:9477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaebel W, Zielasek J. Schizophrenia in 2020: trends in diagnosis and therapy. Psychiatry Clin Neurosci 2015;69:661–73. [DOI] [PubMed] [Google Scholar]
- 77.Bighelli I, Rodolico A, García-Mieres H, et al. Psychosocial and psychological interventions for relapse prevention in schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry 2021;8:969–80. [DOI] [PubMed] [Google Scholar]
- 78.Muller H, Kommescher M, Guttgemanns J, et al. Cognitive behavioral therapy in adolescents with early-onset psychosis: a randomized controlled pilot study. Eur Child Adolesc Psychiatry 2020;29:1011–22. [DOI] [PubMed] [Google Scholar]
- 79.Wang R, Mo F, Shen Y, et al. Functional connectivity gradients of the insula to different cerebral systems. Hum Brain Mapp 2022;44:790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gasquoine PG. Contributions of the insula to cognition and emotion. Neuropsychol Rev 2014;24:77–87. [DOI] [PubMed] [Google Scholar]
- 81.Gogolla N. The insular cortex. Curr Biol 2017;27:R580-r6. [DOI] [PubMed] [Google Scholar]
- 82.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010;214:655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molnar-Szakacs I, Uddin LQ. Anterior insula as a gatekeeper of executive control. Neurosci Biobehav Rev 2022;139:104736. [DOI] [PubMed] [Google Scholar]
- 84.Uddin LQ, Nomi JS, Hébert-Seropian B, et al. Structure and function of the human insula. J Clin Neurophysiol 2017;34:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yasuda Y, Okada N, Nemoto K, et al. Brain morphological and functional features in cognitive subgroups of schizophrenia. Psychiatry Clin Neurosci 2020;74:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sheffield JM, Huang AS, Rogers BP, et al. Insula sub-regions across the psychosis spectrum: morphology and clinical correlates. Transl Psychiatry 2021;11:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res 2010;123:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tian Y, Zalesky A, Bousman C, et al. Insula functional connectivity in schizophrenia: subregions, gradients, and symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging 2019;4:399–408. [DOI] [PubMed] [Google Scholar]
- 89.Li XB, Wang LB, Xiong YB, et al. Altered resting-state functional connectivity of the insula in individuals with clinical high-risk and patients with first-episode schizophrenia. Psychiatry Res 2019;282: 112608. [DOI] [PubMed] [Google Scholar]
- 90.Moran ME, Weisinger B, Ludovici K, et al. At the boundary of the self: the insular cortex in patients with childhood-onset schizophrenia, their healthy siblings, and normal volunteers. Int J Dev Neurosci 2014;32:58–63. [DOI] [PubMed] [Google Scholar]
- 91.Zhu J, Zhang S, Cai H, et al. Common and distinct functional stability abnormalities across three major psychiatric disorders. Neuroimage Clin 2020;27:102352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu J, Zhu DM, Qian Y, et al. Altered spatial and temporal concordance among intrinsic brain activity measures in schizophrenia. J Psychiatr Res 2018;106:91–8. [DOI] [PubMed] [Google Scholar]
- 93.Zhu J, Zhuo C, Qin W, et al. Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res 2015;63:28–35. [DOI] [PubMed] [Google Scholar]
- 94.Zhu J, Zhuo C, Xu L, et al. Altered coupling between resting-state cerebral blood flow and functional connectivity in schizophrenia. Schizophr Bull 2017;43:1363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhuo C, Zhu J, Qin W, et al. Cerebral blood flow alterations specific to auditory verbal hallucinations in schizophrenia. Br J Psychiatry 2017;210:209–15. [DOI] [PubMed] [Google Scholar]
- 96.Egashira K, Matsuo K, Mihara T, et al. Different and shared brain volume abnormalities in late- and early-onset schizophrenia. Neuropsychobiology 2014;70:142–51. [DOI] [PubMed] [Google Scholar]
- 97.Shi LJ, Zhou HY, Wang Y, et al. Altered empathy-related resting-state functional connectivity in adolescents with early-onset schizophrenia and autism spectrum disorders. Asian J Psychiatr 2020;53:102167. [DOI] [PubMed] [Google Scholar]
- 98.Hilland E, Johannessen C, Jonassen R, et al. Aberrant default mode connectivity in adolescents with early-onset psychosis: a resting state fMRI study. Neuroimage Clin 2022;33:102881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Menon V, Palaniyappan L, Supekar K. Integrative brain network and salience models of psychopathology and cognitive dysfunction in schizophrenia. Biol Psychiatry 2023;94:108–20. [DOI] [PubMed] [Google Scholar]
- 100.van Os J. A salience dysregulation syndrome. Br J Psychiatry 2018;194:101–3. [DOI] [PubMed] [Google Scholar]
- 101.Corradi-Dell’Acqua C, Ronchi R, Thomasson M, et al. Deficits in cognitive and affective theory of mind relate to dissociated lesion patterns in prefrontal and insular cortex. Cortex 2020;128:218–33. [DOI] [PubMed] [Google Scholar]
- 102.Tholen MG, Trautwein FM, Böckler A, et al. Functional magnetic resonance imaging (fMRI) item analysis of empathy and theory of mind. Hum Brain Mapp 2020;41:2611–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paillère-Martinot M, Caclin A, Artiges E, et al. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res 2001;50:19–26. [DOI] [PubMed] [Google Scholar]
- 104.Palaniyappan L, Crow TJ, Hough M, et al. Gyrification of Broca’s region is anomalously lateralized at onset of schizophrenia in adolescence and regresses at 2 year follow-up. Schizophr Res 2013; 147:39–45. [DOI] [PubMed] [Google Scholar]
- 105.Liloia D, Brasso C, Cauda F, et al. Updating and characterizing neuroanatomical markers in high-risk subjects, recently diagnosed and chronic patients with schizophrenia: a revised coordinate-based meta-analysis. Neurosci Biobehav Rev 2021;123:83–103. [DOI] [PubMed] [Google Scholar]
- 106.Romeo Z, Spironelli C. Hearing voices in the head: two meta-analyses on structural correlates of auditory hallucinations in schizophrenia. Neuroimage Clin 2022;36:103241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palaniyappan L, Balain V, Radua J, et al. Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr Res 2012;137:169–73. [DOI] [PubMed] [Google Scholar]
- 108.Shapleske J, Rossell SL, Chitnis XA, et al. A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cereb Cortex 2002;12:1331–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.