Abstract
Background
Discovering patterns of cognitive domains and characterizing how these patterns associate with other risk factors and biomarkers can improve our understanding of the determinants of cognitive aging.
Objective
To discover patterns of cognitive domains using neuropsychological test results in Long Life Family Study (LLFS) and characterize how these patterns associate with aging markers.
Methods
5086 LLFS participants were administered neuropsychological tests at enrollment. We performed a cluster analysis of six baseline neuropsychological test scores and tested the association between the identified clusters and various clinical variables, biomarkers, and polygenic risk scores using generalized estimating equations and the Chi-square test. We used Cox regression to correlate the clusters with the hazard of various medical events. We investigated whether the cluster information could enhance the prediction of cognitive decline using Bayesian beta regression.
Results
We identified 12 clusters with different cognitive signatures that represent profiles of performance across multiple neuropsychological tests. These signatures significantly correlated with 26 variables including polygenic risk scores, physical and pulmonary functions, and blood biomarkers and were associated with the hazard of mortality (p-value=<0.01), CVD (p-value = 0.03), dementia (p-value = 0.01), and skin cancer (p-value = 0.03).
Conclusions
The identified cognitive signatures capture multiple domains simultaneously and provide a holistic vision of cognitive function, showing that different patterns of cognitive function can coexist in aging individuals. Such patterns can be used for clinical intervention and primary care.
Keywords: Cognition, Neuropsychology, Aging, Longevity, Cluster analysis, Survival
Introduction
Cognitive change as a normal process of aging has been well documented in the scientific literature [1, 2]. At an older age, maintaining good cognitive function is critical for functional independence and effective communication. Understanding the mechanisms and identifying the demographic, biological, and psychosocial factors of cognitive aging is important to help people maintain their cognitive health as they grow older. However, our knowledge of patterns and risk factors of cognitive aging is still limited.
While aging is generally associated with decline in processing speed, learning, and retrieval of information [3], the cognitive performance of aging individuals varies both between and within individuals [4–6]. Even when controlling for the effect of age, individuals may experience different rates of cognitive decline, and the same individual may perform dissimilarly in different cognitive domains [7, 8]. For example, some individuals may maintain an overall better cognition than others, and the same individual may experience severe decline in memory but subtle decline in executive function. The variability of the cognitive performance, which is influenced by factors such as education [9, 10], genetic factors [11–14], and psychosocial factors [15, 16], has also been associated with the progression to cognitive impairment and Alzheimer’s disease [17–19]. Therefore, it is important to understand which aspects of cognition are affected by certain risk factors and what type of cognitive patterns might suggest the onset of functional impairment and diseases.
Discovering patterns of cognitive domains and characterizing how these patterns associate with other risk factors and biological markers can improve our understanding of the determinants of cognitive aging. Moreover, identifying subgroups of participants who have distinct cognitive profiles can assist in therapeutic interventions and primary care [20]. In this study, we aimed to accomplish these goals through a cluster analysis of scores on multiple neuropsychological tests administered at enrollment in the Long Life Family Study (LLFS) [21]. We hypothesize that, while individual tests capture specific aspects of cognition, the combination of multiple neuropsychological test scores should capture the global cognitive state of individuals. Therefore, to better describe global patterns of cognitive function, we sought to discover cognitive signatures defined as profiles of performance across multiple neuropsychological tests. By associating these cognitive signatures with various aging markers, clinical variables, and medical events, the patterns of strengths and weaknesses across neuropsychological tests can inform about the underlying biological and physical contributors to cognitive aging.
Materials and Methods
Participants and neuropsychological measures
The Long Life Family Study (LLFS) is an international, multicenter study of two-generation families with longevity and healthy aging that was designed to discover genetic, environmental, and behavioral determinants of exceptional survival. A total of 583 families and 4,935 people were recruited between 2006 and 2009 based on a metric of familial longevity, and additional 151 family members were recruited during 2014–2017. Readers can refer to Wojczynski (2021) [19] for more details of the LLFS study. Cognitive function was assessed at enrollment and visit-two by administering seven tests in the National Alzheimer’s Coordinating Center Unified Data Set [20]. See descriptions of these tests in LLFS in Paola (2020) [21]. In addition to those tests, participants (except those enrolled in Denmark) are followed longitudinally with their cognitive function assessed by the Modified Telephone Interview for Cognitive Status (TICS).
Neuropsychological tests
We used six neuropsychological test scores measured at enrollment in the cluster analysis: logical memory test with immediate and delayed recall, semantic fluency, digit span forward and backward, and Digit Symbol Substitution Test (DSST). The neuropsychological scores were standardized within the strata defined by the different combinations of age, sex, and education to ensure the resulting clusters are not dominantly explained by these variables. We chose eight age categories to capture the range of ages at enrollment: <=49, 50–59, 60–69, 70–79, 80–84, 85–89, 90–94, and >=95. We also chose two education categories based on whether participants’ years-of-education exceeded 12 years or not. In the analysis, we only included participants with valid test scores, where the test validity was established by the staff who administered the tests. Among these participants, we excluded participants who had three or more missing test scores. We used the scaled Euclidean distance in clustering to account for the missing values in the remaining participants.
Statistical analysis
Using the six standardized neuropsychological scores at enrollment, we performed a hierarchical cluster analysis with scaled Euclidean distance and average linkage to discover cognitive signatures in LLFS participants. We used the dynamic tree cut method (Langfelder, Zhang, and Horvath 2008) in the R package “dynamicTreeCut 1.63.1” to detect clusters on the dendrogram generated by hierarchical clustering. The optimal number of clusters was decided by maximizing the Calinski-Harabasz (CH) index [22] (see Supplement Table 1 for the CH indices under different parameters of the dynamic tree cut method). We defined the associated cognitive signatures by the vector of the average values of the six neuropsychological test scores at enrollment in the participants of each cluster.
To examine the association between the identified clusters and various aging markers summarized in Table 1, for continuous outcomes we used generalized estimating equations method with cluster labels as categorical covariates, adjusted by age-at-enrollment and sex. We used an exchangeable correlation structure to account for within-family correlation and the Wald test to determine whether the effect of clusters on the continuous outcomes was statistically significant. These analyses were conducted using the R package “geepack 1.3–2”. For discrete outcomes, we used a χ2 test to determine their association between the clusters. Haplotype groups were defined as E2 = E2/E2 or E2/E3, E3 = E3/E3, and E4 = E2/E4, E3/E4, or E4/E4, with E3 as the reference group. We used Benjamini-Hochberg [23] adjusted p-value to adjust for multiple comparisons.
Table 1.
Variables (aging markers) that are used to characterize the identify clusters
| Variable measurement time | Variables |
|---|---|
| At visit-one (enrollment) |
|
| At visit-two |
|
| At follow-ups |
|
We used Cox proportional hazards regression to examine the association between clusters and the hazard for mortality and other medical events. All models were stratified by whether the birth year was >1935, which is the threshold that approximately separated participants into two generations (Supplementary Figure 1). We used the likelihood-ratio test to determine whether the effect of the cluster membership on mortality and medical events were significant. See supplementary files for more details of the specification of those models.
To understand whether using the cluster membership could enhance the prediction of other outcomes, e.g., cognitive change over time, we fit two Bayesian beta regression models [24, 25] to predict trajectories of TICS scores. The first model, model (a), included cluster labels as covariates and cluster-specific age effects, i.e., each cluster had its own age coefficient. The second model, model (b), included the baseline neuropsychological test scores as covariates and an overall age effect. Both models were adjusted for sex and education. We compared two models by both Mean Square Error (MSE) and Mean Square Prediction Error (MSPE) through a five-fold cross validation. Specifically, we calculated the MSE in the training set and MSPE in the test set. All analyses were conducted using R version 4.0.2.
Results
Clusters and their associated cognitive signatures
From the initial 5086 participants, we excluded 323 participants with invalid neuropsychological tests at enrollment, 151 participants enrolled at visit-two, 65 participants with three or more missing neuropsychological test scores, and seven participants with missing years-of-education. Table 2 summarizes characteristics and neuropsychological test scores at enrollment for the 4540 participants in our analysis, stratified by whether the birth year was > 1935. Figure 1 shows the dendrogram (left panel) and the size of the 12 clusters (right panel) detected by hierarchical clustering using the dynamic tree cut method.
Table 2.
Characteristics and the neuropsychological scores at enrollment of subjects in the LLFS study
| Birth year <= 1935 | Birth year > 1935 | |
|---|---|---|
| No. at enrollment | 1649 | 2891 |
| Sex, male (%) | 771 (46.76%) | 1281 (44.31%) |
| Age at enrollment | 87.00 (7.54) | 59.21 (7.15) |
| Years of education | 10.25 (3.84) | 12.63 (3.00) |
| Logical memory (immediate) | 8.82 (4.57) | 13.37 (3.91) |
| Logical memory (delayed) | 6.70 (4.69) | 12.00 (4.22) |
| Semantic fluency | 16.18 (5.37) | 22.47 (5.76) |
| Digit span forward | 7.64 (2.20) | 8.60 (2.18) |
| Digit span backward | 5.53 (2.10) | 6.83 (2.31) |
| DSST | 30.81 (12.72) | 51.50 (12.01) |
We reported counts and proportion for sex and mean and standard deviation for other continuous variables. Participants were stratified into two groups by whether birth year is > 1935, which is the threshold that approximately separates participants into two generations.
Figure 1.
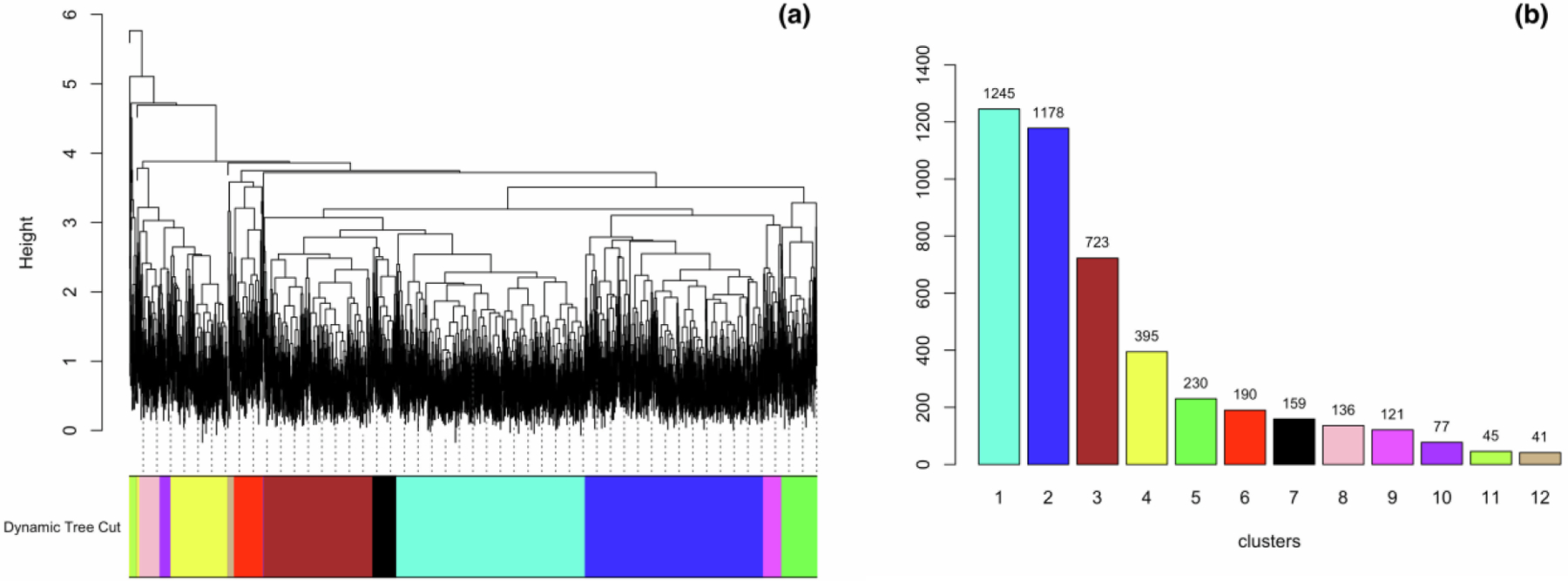
(a) The dendrogram from hierarchical clustering. (b) The size of each cluster. Panel (a) shows the dendrogram of hierarchical clustering. Using dynamic tree cut, the dendrogram was cut into different regions, represented by the different colors. Panel (b) shows the 12 clusters with corresponding colors in (a), as well as the number of participants in each cluster
The average values of the neuropsychological test scores per cluster (cognitive signatures), with the annotation of the average age-at-enrollment of the participants in each cluster, are visualized in heatmaps in Figure 2, and the summary statistics are reported in Table 3. In Figure 2a, the clusters/columns are sorted by the mean age-at-enrollment of cluster members from young (left, cluster 10, age-at-enrollment 66.68±13.45 years) to old (right, cluster 11, age-at-enrollment 74.60±15.67 years). In Figure 2b, the clusters/columns are sorted by the similarity of the cognitive signatures, from worse (left) to better (right).
Figure 2.
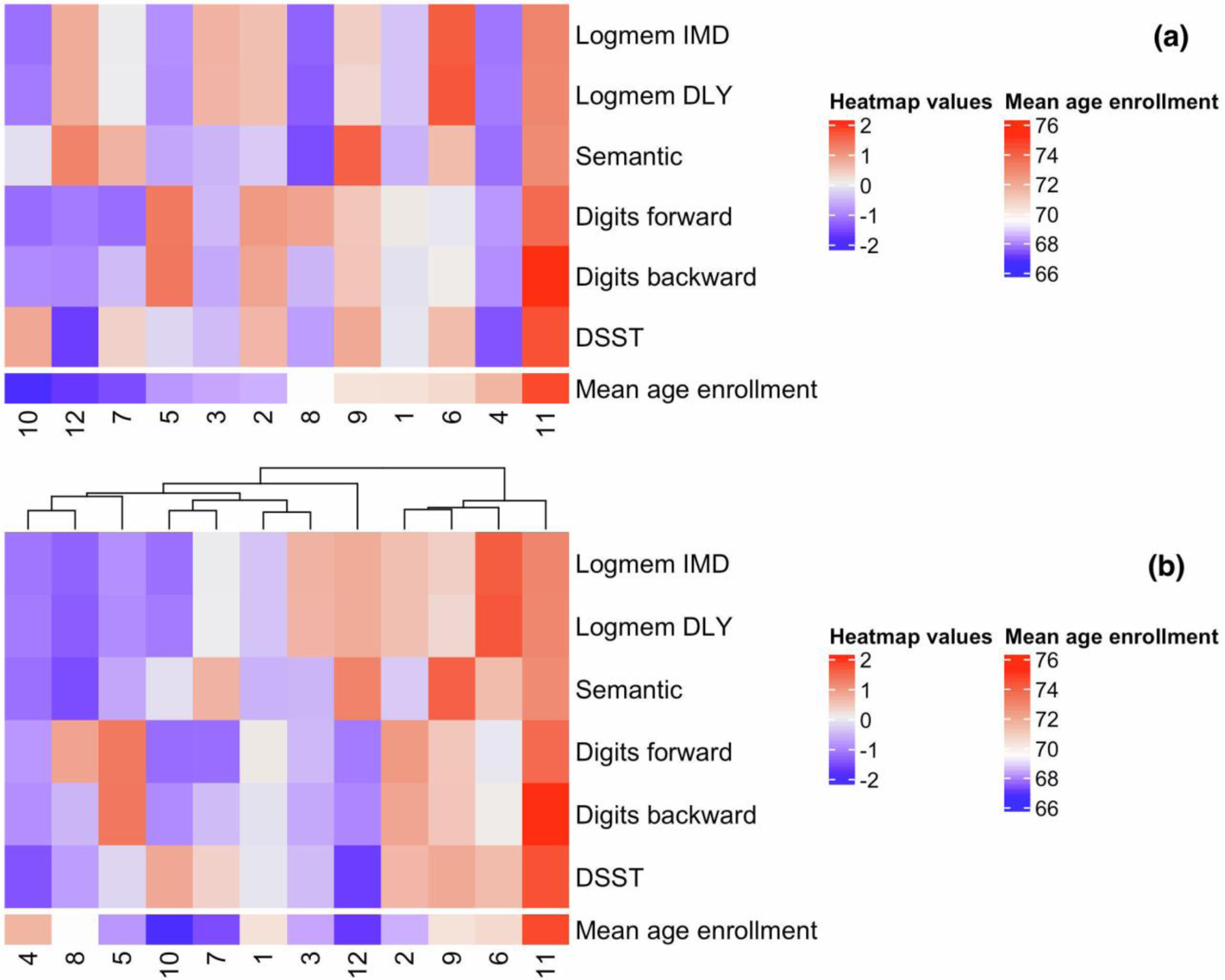
The heatmaps of cognitive signatures. Panel (a), clusters are ordered by the mean age-at-enrollment from young to old. Panel (b), clusters are ordered by the similarity of the cognitive signatures from worse to better. Both heatmaps show the average test scores in each cluster (scaled by row), where red indicates positive row-scaled values (greater than average) and blue indicates negative row-scaled values (less than average). For example, cluster 11 represents a group of individuals with greater than average scores for all tests, while cluster 4 represents a group of individuals with lower than average scores for all tests. Logmem IMD: logical memory test with immediate recall; Logmem DLY: logical memory test with delayed recall
Table 3.
The average value and standard deviation of the six neuropsychological tests at enrollment in each cluster (cognitive signatures)
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | Cluster 7 | Cluster8 | Cluster 9 | Cluster 10 | Cluster 11 | Cluster 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Logical memory (immediate) | 9.77 (3.70) | 13.95 (3.54) | 14.59 (3.39) | 6.54 (3.64) | 8.10 (3.17) | 17.78 (3.13) | 11.82 (3.31) | 6.26 (3.32) | 12.93 (3.46) | 7.51 (2.91) | 15.58 (4.34) | 15.30 (4.08) |
| Logical memory (delayed) | 7.97 (4.00) | 12.46 (3.91) | 13.09 (3.86) | 4.87 (3.71) | 6.04 (3.57) | 16.79 (3.51) | 10.23 (3.70) | 3.87 (2.88) | 10.91 (3.96) | 5.80 (3.15) | 13.96 (4.97) | 14.03 (4.69) |
| Semantic fluency | 18.73 (5.64) | 20.33 (5.48) | 19.41 (5.95) | 15.28 (6.94) | 18.84 (6.15) | 24.11 (7.42) | 25.91 (5.53) | 14.07 (4.38) | 29.91 (6.7) | 22.32 (5.37) | 25.87 (6.38) | 29.41 (6.34) |
| Digit span forward | 8.04 (1.71) | 9.99 (1.57) | 6.88 (1.56) | 6.02 (1.53) | 10.77 (1.27) | 7.56 (1.92) | 5.42 (1.33) | 9.83 (1.31) | 8.87 (1.67) | 5.47 (1.33) | 10.84 (1.36) | 5.80 (1.45) |
| Digit span backward | 5.95 (1.62) | 8.10 (2.10) | 4.89 (1.60) | 4.21 (1.53) | 9.20 (1.75) | 6.14 (1.65) | 5.35 (1.35) | 5.22 (1.79) | 7.26 (1.73) | 4.39 (1.48) | 10.44 (1.70) | 4.29 (1.23) |
| DSST | 42.53 (14.72) | 50.30 (14.15) | 41.43 (14.29) | 31.18 (14.95) | 43.19 (15.31) | 46.72 (15.25) | 48.84 (14.68) | 38.18 (14.84) | 50.03 (18.28) | 53.93 (11.55) | 54.65 (21.3) | 33.7 (13.47) |
The heatmaps in both Figure 2a and Figure 2b depict the variety of the cognitive signatures associated with the identified clusters. The signatures associated with clusters 2 and 6 represent better than average performance in four or five tests. The signatures associated with clusters 3 and 12 represent better than average performance in logical memory but lower than average DSST and Digit Span scores. The clusters 7, 10, and 5 included participants of younger age with better than average performance in at most two tests, while cluster 4 included participants of older age with worse than average performance in all tests. Moreover, the cognitive signatures suggested that the differences in cognitive function between clusters are not simply age-related. For example, cluster 11 included 45 participants with better than average performance in all six tests but with the oldest average age (age-at-enrollment: 75.6±15.67 years). In contrast, cluster 10 included 77 participants with lower than average performance in five tests but with the youngest average age (age-at-enrollment: 66.78±13.45 years).
Association of cognitive signatures with genetic and aging markers
Overall, the cognitive signatures based on the baseline neuropsychological test scores correlated with three polygenic risk scores (PRS), 10 circulating aging markers, four markers of physical and cardiovascular function at baseline, the scores of the same neuropsychological tests administered at visit-two, education, and smoking. The average values of all of these variables in each cluster, with the annotation of the average age at enrollment, are visualized in Figure 3, and the summary statistics are reported in Supplementary Table 4 and Table 5. We summarized the findings in the following paragraphs.
Figure 3.
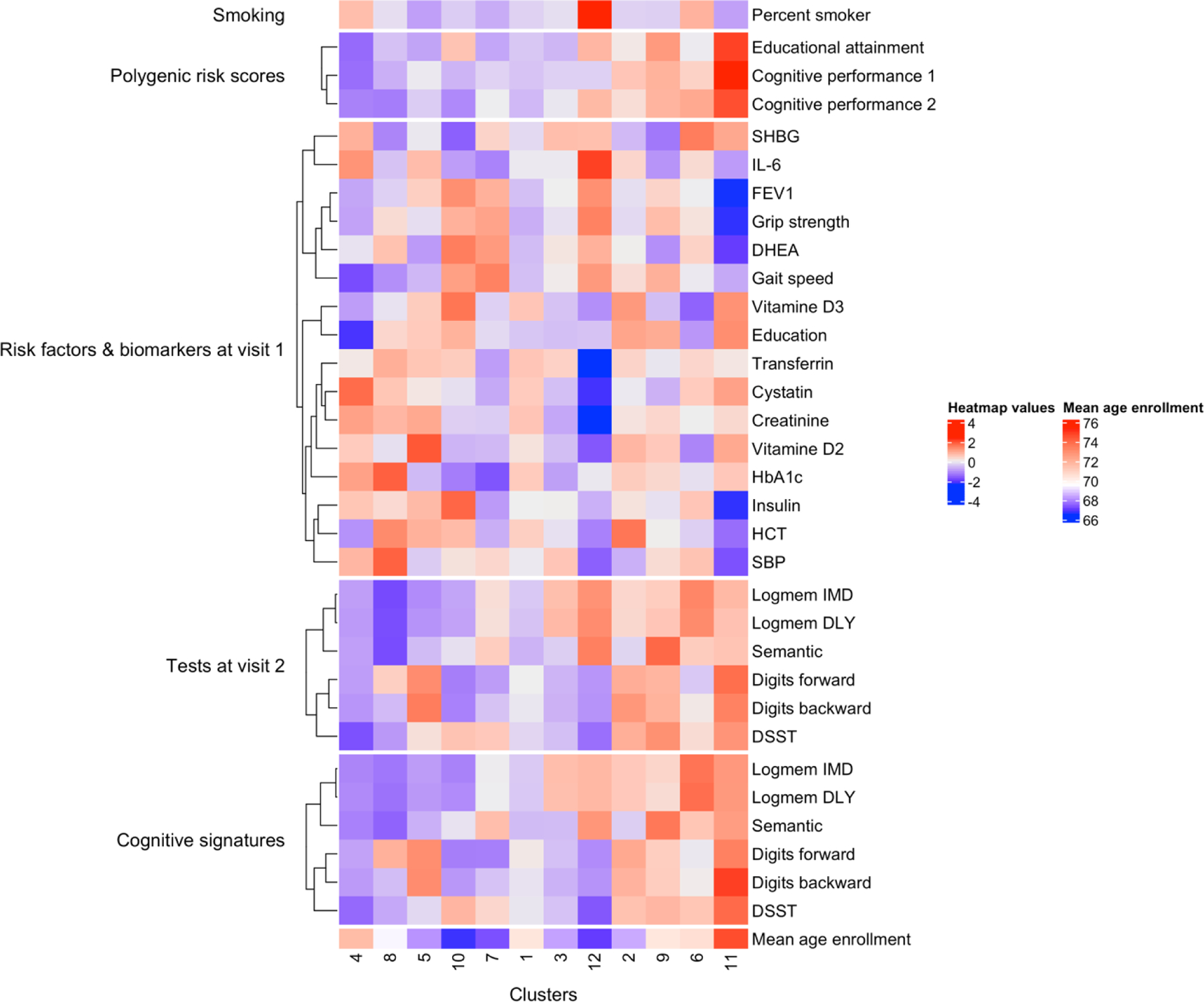
Heatmap of the average values of the aging markers that are significantly associated with the identified clusters. The values in the heatmap are scaled by row for visualization, where red indicates higher values and blue indicates lower values. Percent smoker: the percentage of participants who are smokers in each cluster; SHBG: Sex Hormone Binding Globulin; HCT: Hematocrit; SBP: systolic blood pressure
Three PRS (educational attainment and two measures of cognitive performance) were significantly associated with the cognitive signatures. The values of the three PRS appear to have a positive relationship with cognitive signatures: clusters with better cognitive signatures, such as cluster 11, have on average higher PRS, and clusters with worse cognitive signatures, for example, cluster 4, 8, and 5, have on average lower PRS. Such a relationship suggests that the substantially better than average cognitive performance of these individuals at old age may be genetically determined.
The frequencies of APOE haplotype groups (Supplementary Table 3) were significantly different across all the 12 clusters (χ2 p-value=0.04). Moreover, comparing each cluster to cluster 1, the cluster 3, which included younger participants (68.3 ± 14.7 years) with worse than average performance in four of the six tests, was significantly enriched for E4 (24% of individuals have E4 in cluster 3, χ2 p-value=0.01).
The cognitive signatures were associated with the levels of a set of circulating biomarkers including interleukin-6 (IL-6), hemoglobin A1C (HbA1c), and vitamin D2 and D3. Interestingly, lower than average IL-6 levels were associated with both excellent and poor cognitive signatures. Clusters 10, 7, 9, and 11 were associated with lower IL6 levels, but the cognitive signatures of clusters 9 and 11 described better than average cognitive function across domains, while the cognitive signatures of clusters 10 and 7 described worse than average cognitive function. Levels of vitamin D2 and D3 were also associated with the cognitive signatures with complicated relations: higher than average levels in cluster 11 and lower than average levels in cluster 6, but both clusters show excellent cognitive signatures. Both gait speed and grip strength were associated with the cognitive signatures, but the associations appear to be explained by age. Typically, most clusters with younger participants (clusters 3, 7, 10, and 12) were characterized by better than average physical function, and most clusters with older participants (clusters 1, 6, and 11) were characterized by worse than average physical function. Noticeable exceptions were cluster 5 of younger individuals with worse than average physical and cognitive function, and cluster 9 of older individuals with better than average physical and cognitive function. Our analyses indicated that individual biomarkers can show a complex association with cognitive signatures, and the combination of multiple biomarkers is needed to better characterize the profiles of cognitive function.
Though we only used neuropsychological test scores at baseline for the clustering analysis, in the identified clusters, the tests at visit-two showed a pattern like these tests at baseline. For example, at both visits, cluster 5 has better than average Digit Span scores but lower than average scores on the other tests, and cluster 12 has better than average logical memory and semantic fluency scores but lower than average Digit Span and DSST scores.
The cluster analysis identified several interesting groups of participants. For example, cluster 12, which included a small number of participants with younger age and worse than average performance in three tests, was characterized by the largest proportion of smokers (20%) and the highest average IL-6 level (98 mg/dL ± 10.5). Cluster 6 included older individuals (mean age 71 years ± 16.3) with lower than average education level (11± 3 years), but their signature described better than average cognitive function. Interestingly, this cluster also had a higher prevalence of carriers of APOE E2 (19%, Supplementary Table 4), which is reported to have a protective effect on cognitive decline [26]. Moreover, some clusters had similar profiles of biomarkers and risk factors, but their cognitive signatures were different. For example, cluster 2 and cluster 5 were similar with respect to most of the circulating biomarkers, physical function, education level, and even mean age-at-enrollment. However, cluster 2 included participants with good performance in five of six tests, whereas cluster 5 included participants with good performance only in Digit Span tests. The PRS of education and cognition in these two clusters were different, and hence the clusters’ different cognitive signatures could be attributed to genetic differences.
Hazards of medical events
The cognitive signatures were significantly correlated with mortality, CVD, dementia, and skin cancer (likelihood-ratio test p-value=<0.01, 0.03, 0.01, and 0.03, respectively). Table 4 summarizes the hazard ratios for those four outcomes in each cluster.
Table 4.
Hazard ratios for mortality, cardiovascular disease (CVD), dementia, and skin cancer in each cluster with cluster 1 as the reference group
| Mortality | CVD | Dementia | Skin Cancer | |
|---|---|---|---|---|
| Cluster 2 | 0.84 | 1.34 | 0.73 | 1.38 |
| Cluster 3 | 1.21 | 1.03 | 0.96 | 1.13 |
| Cluster 4 | 1.38 | 0.79 | 2.00 | 0.71 |
| Cluster 5 | 0.90 | 1.05 | 1.55 | 1.84 |
| Cluster 6 | 0.79 | 0.90 | 0.59 | 0.97 |
| Cluster 7 | 1.07 | 0.77 | 0.88 | 0.76 |
| Cluster 8 | 1.34 | 0.93 | 2.53 | 1.33 |
| Cluster 9 | 0.82 | 0.58 | 0.88 | 1.11 |
| Cluster 10 | 1.71 | 1.13 | NA* | 0.99 |
| Cluster 11 | 0.70 | 0.48 | 0.73 | 1.13 |
| Cluster 12 | 1.11 | 0.46 | NA* | 0.33 |
The numbers with a bold font indicate a significant association (P-value < 0.05) between the cluster and the medical event.
Participants in cluster 10 and 12 have no cases of dementia, hence the hazard ratios for these two clusters are non-available.
The participants in clusters 2, 3, 4, and 10 had statistically significant hazards for mortality compared to the reference cluster (cluster 1). Cluster 2, which included participants with better than average cognitive signatures, had a decreased hazard of mortality (HR=0.84, p-value=0.03). Participants in this cluster were slightly younger than those in cluster 1 (68.4±14.9 years vs. 70.3±15.6 years), but they had a significantly higher hazard of cardiovascular disease (HR =1.3, p-value = 0.01) and skin cancer (HR=1.4, p-value = 0.02). Cluster 3 included slightly younger people than cluster 1 (68.3±14.7 years vs. 70.3±15.6 years) with better performance on the episodic memory test (i.e., logical memory) but worse performance on tests of executive function (i.e., DSST, Digit Spans, and semantic fluency), and a 20% increased hazard of mortality. Cluster 4 included slightly older people than cluster 1 (71.6±15.7 years vs. 70.3±15.6 years) with worse performance across all cognitive domains and an almost 40% increased hazard of mortality. Interestingly, cluster 10 included the youngest participants (66.7±13.5 years) but with substantially worse performance on tests of episodic memory, attention, and working memory and a 70% increased hazard of mortality compared to cluster 1. A significantly higher hazard of dementia was found only for participants in cluster 4 (HR=2.00, p-value=0.01) and cluster 8 (HR=2.53, p-value=0.01) who showed multi-domain impairment in episodic memory, working memory, and processing speed.
Prediction of longitudinal changes of TICS scores
We compared two models predicting the trajectory of TICS: model (a) versus model (b). Model (a) included cluster labels as covariates and cluster-specific age effects. Model (b) included the baseline neuropsychological test scores as covariates and an overall age effect. For a fair comparison, we used the same data to fit both models. We excluded participants in cluster 12 due to insufficient outcomes: only three participants in this cluster were administered with TICS.
Table 5 shows the estimated coefficients and credible intervals of the age effect on TICS scores in both models as well as the predicted change of TICS scores from age 70 to age 85. In addition, the model with cluster labels produced cluster-specific rates of change of TICS scores (see the forest plot in Figure 4). For example, participants in cluster 4 with the worst cognitive signature and an increased hazard for dementia had the most rapid decline of TICS scores (−3.1 from age 70 to 85); Participants in cluster 9 with better than average cognitive signature had the slowest decline of TICS scores (−1.03 from age 70 to 85). These cluster-specific rates could be more easily interpretable for clinical prediction.
Table 5.
Estimated age effect and confidence interval of the prediction modelling of TICS scores as well as the predicted TICS score change from age 70 to 85 in each cluster
| Age effect | Estimated standardized coefficient | CI 95% | Predicted TICS score change from age 70 to 85 |
|---|---|---|---|
| Model (a): cluster 1 | −0.032 | (−0.034, −0.030) | −2.96 |
| Model (a): cluster 2 | −0.022 | (−0.024, −0.020) | −1.76 |
| Model (a): cluster 3 | −0.023 | (−0.026, −0.019) | −2.15 |
| Model (a): cluster 4 | −0.036 | (−0.041, −0.031) | −4.26 |
| Model (a): cluster 5 | −0.025 | (−0.030, −0.020) | −2.30 |
| Model (a): cluster 6 | −0.024 | (−0.030, −0.019) | −1.90 |
| Model (a): cluster 7 | −0.029 | (−0.036, −0.027) | −2.83 |
| Model (a): cluster 8 | −0.028 | (−0.034, −0.021) | −2.96 |
| Model (a): cluster 9 | −0.022 | (−0.028, −0.015) | −1.10 |
| Model (a): cluster 10 | −0.023 | (−0.032, −0.012) | −3.19 |
| Model (a): cluster 11 | −0.019 | (−0.031, −0.009) | −1.38 |
| Model (b): overall | −0.013 | (−0.015, −0.012) | −1.37 |
The first 11 rows in the table show the estimated age effect of each cluster in model (a). Cluster 12 is excluded because only 3 participants in this cluster have TICS scores measured. The last row in the table is the overall age effect in model (b).
For a better interpretation of the estimated coefficients from the beta regression, we also predicted the change of TICS score from age 70 to 85 in each cluster.
Figure 4.
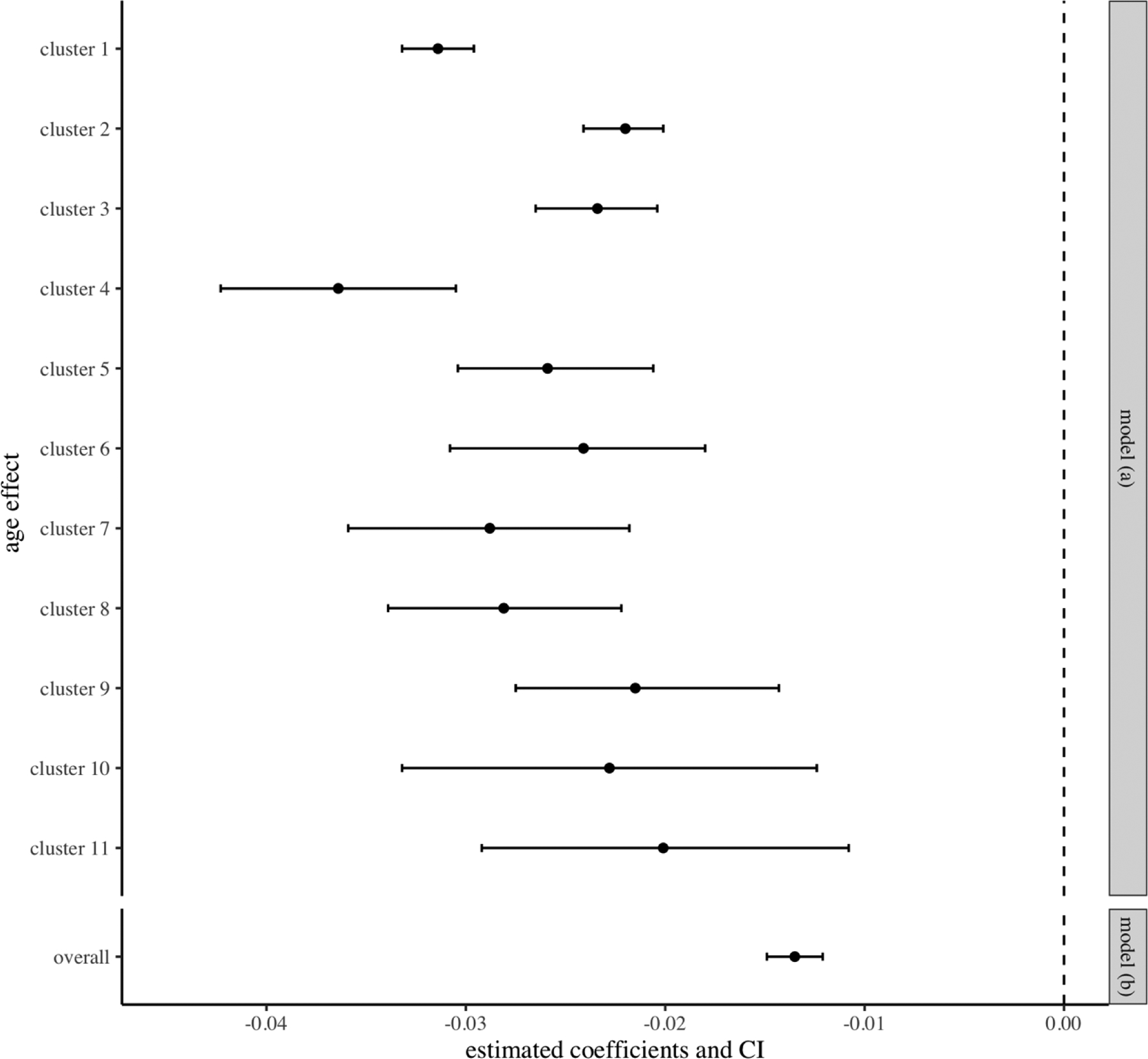
Estimated age effect and confidence interval of the prediction modeling of TICS scores. The first 11 items are the estimated age effect of each cluster in the model (a). The last item is the overall age effect in the model (b)
We performed a five-fold cross-validation to compare the two models. Model (a) had an average MSE of 5.06 and an average MSPE of 14.31, while model (b) had an average MSE of 5.17 and an average MSPE of 9.70. The results showed that the model with cluster membership produced a better fit, while the model with the original test scores produced better predictions for new observations.
Discussion
Overview
We performed a hierarchical cluster analysis of six neuropsychological test scores at enrollment and successfully identified 12 clusters of participants from the LLFS with different cognitive signatures. We found 20 variables that were significantly correlated with the signatures, including PRS, blood biomarkers, and markers of physical and pulmonary functions. In addition, the identified clusters were predictive of mortality, CVD, dementia, and skin cancer, and the cluster membership could provide a more personalized prediction of longitudinal change in TICS scores.
Main findings
The cognitive signatures that we discovered are defined as profiles of performance across multiple neuropsychological tests. Previous studies have used the term cognitive signature to describe functional profiles of brain networks when engaged with cognitive activities [27], or combinations of structural features from fMRI [27–29]. In our study, the cognitive signatures based on neuropsychological tests can provide an interesting perspective in summarizing the domains of episodic memory, attention, working memory, processing speed, and semantic fluency.
The cognitive signatures showed that different patterns of cognitive function exist in aging individuals. While a few clusters of participants had higher or lower than average cognitive function across all domains, the most common scenario was for people to perform well in certain cognitive domains but not in others (e.g., high episodic memory but low executive function or vice versa). In addition, the associations between the cognitive signatures and various aging markers and medical events showed that the interpretation of each neuropsychological test result should depend on the other test results and age. For example, participants in clusters 2 and 10 have similar DSST scores, but their profiles of biomarkers (IL6 levels, physical functions) are very different. Such different characteristics cannot be interpreted only based on the DSST scores but may be captured by the overall cognitive signatures. Therefore, the cluster analysis provides a holistic vision of cognitive domains and integrates information across cognition, metabolism, and physical function. This integration can be beneficial for understanding the relationship between cognitive performance and related risk factors and for identifying participants at high risk for cognitive decline.
The most interpretable result in our analyses is the positive correlation between PRS and cognitive signatures: better cognitive performance tracked with higher PRS for cognition and educational attainment. The results suggest that genetics is an important determinant of cognitive function and agree with the high heritability of neuropsychological test scores in LLFS [30]. Surprisingly, genome-wide association studies have failed to find many genetic correlates of neuropsychological test performance and only studies with substantial sample sizes have detected results at a genome-wide level of significance [31, 32]. It will be interesting to evaluate the heritability of the cognitive signatures and assess whether the cognitive signatures provide a more sensitive phenotype for genetic studies.
The identified cognitive signatures are correlated with aging markers in complex relations. A meta-analysis found that individuals with higher IL-6 are more likely to experience global cognitive decline at follow-up [33]. In our analysis, clusters with similar IL-6 levels have distinct cognitive signatures (cluster 10, 7, 2, and 1). Another interesting relation highlighted by our analyses was the non-linear relationship between vitamin D levels and cognitive performance. It has been shown that lower levels of vitamin D predict poor cognitive functions [34]. However, participants in cluster 6 had overall excellent cognitive performance but reduced vitamin D2 and D3 levels.
Our analysis showed that the combination of various aging markers, risk factors, as well as genetic factors may be associated with an individual’s cognitive function. Furthermore, in the prediction of trajectories of TICS scores, the model including the cluster membership produced a better fit than the model including neuropsychological test scores as individual covariates. However, the model including neuropsychological test scores as individual covariates produced better predictions. Another advantage of using cluster membership as covariate is that it can provide estimates of the cluster-specific rate of decline.
Many studies have applied cluster analysis or latent class methods on neuropsychological test scores to discover patterns of cognitive function in older individuals [6, 15, 35–40]. A study by Sebastiani et al. (2020) [41] showed the heterogeneity of cognitive decline in LLFS through Bayesian model-based clustering. However, the study was performed on each neurocognitive test separately rather than on all neurocognitive tests together. Some of the previous studies only connected the identified clusters to limited demographic/clinic variables. The novelty of our study lies in two aspects. First, LLFS had a much larger number of participants and a wider range of ages at enrollment than other studies, so our analysis identified many clusters with more specific cognitive signatures that have not been reported in previous studies. Second, our paper studied the association between the cognitive signatures with a variety of variables including circulating biomarkers, clinical variables, and even genetic information, providing a thorough characterization of the identified signatures.
Our work has some limitations. The neuropsychological assessment in the LLFS does not assess all possible cognitive domains. Alternative or more challenging tests may be informative in identifying additional or more robust cognitive signatures. We only used the scores of the neuropsychological tests administered at enrollment in the cluster analysis. While it would be very interesting to identify signatures of cognitive change by analyzing repeated test scores over time, the substantial loss at follow-up due to mortality in the oldest generation is a substantial analytical challenge that will require the development of new statistical methods. In addition, TICS is the only test that was administered longitudinally in LLFS participants to assess their cognitive state over time. It will be important to expand this analysis in other studies with a richer longitudinal assessment of cognitive function. Approximately 3900 LLFS participants were longitudinally assessed with the TICS test, and hence those with missing TICS scores had to be excluded from the modeling of TICS scores. We did not have access to additional data for independent validation of our results.
Conclusions
We discovered 12 cognitive signatures that describe the profiles of six neuropsychological test scores assessing episodic memory, attention, working memory, processing speed, and semantic fluency. The cognitive signatures and their correlated risk factors show the complexity of cognitive aging, suggesting the need for a system-based approach when investigating the effect of these risk factors and designing effective interventions.
Supplementary Material
Acknowledgement
All participants provided informed written consent approved by the field centers’ institutional review board (IRB). The study is approved by the ethics committee of Washington University at St. Louis. We thank all the participants for their time and availability.
Funding
This work was sponsored by the National Institute on Aging (K01AG057798 to Stacy L. Andersen, R01AG061844 to Paola Sebastiani, 5U19AG063893 to all LLFS investigators). There was no involvement of the funding source in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Footnotes
Conflict of interest
The authors have no conflict of interest to report.
Data availability
The datasets used and/or analyzed during the current study are available through dbGaP: dbGaP Study Accession - phs000397.v1.p1.
References
- [1].Harada CN, Natelson Love MC, Triebel KL (2013) Normal Cognitive Aging. Clin Geriatr Med 29, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murman DL (2015) The Impact of Age on Cognition. Seminars in hearing 36, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Correia R, Barroso J, Nieto A (2018) Age related cognitive changes: The importance of modulating factors. J Gerontol Geriatr 4, 1–10. [Google Scholar]
- [4].Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA (2002) Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 17, 179–193. [PubMed] [Google Scholar]
- [5].Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, Rodgers B (1999) An analysis of diversity in the cognitive performance of elderly community dwellers: Individual differences in change scores as a function of age. Psychol Aging 14, 365–379. [DOI] [PubMed] [Google Scholar]
- [6].Zammit AR, Hall CB, Lipton RB, Katz MJ, Muniz-Terrera G (2018) Identification of Heterogeneous Cognitive Subgroups in Community-Dwelling Older Adults: A Latent Class Analysis of the Einstein Aging Study. J Int Neuropsychol Soc 24, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hilborn JV, Strauss E, Hultsch DF, Hunter MA (2009) Intraindividual variability across cognitive domains: Investigation of dispersion levels and performance profiles in older adults. J Clin Exp Neuropsychol 31, 412–424. [DOI] [PubMed] [Google Scholar]
- [8].Christensen H (2001) What Cognitive Changes can be Expected with Normal Ageing? Aust N Z J Psychiatry 35, 768–775. [DOI] [PubMed] [Google Scholar]
- [9].Ardila A, Ostrosky-Solis F, Rosselli M, Gómez C (2000) Age-Related Cognitive Decline During Normal Aging: The Complex Effect of Education. Arch Clin Neuropsychol 15, 495–513. [PubMed] [Google Scholar]
- [10].Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA (2009) Educational attainment and cognitive decline in old age. Neurology 72, 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nagel I, Chicherio C, Li S-C, Von Oertzen T, Sander T, Villringer A, Heekeren H, Bäckman L, Lindenberger U (2008) Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Macaulay RK, Halpin A, Cohen AS, Calamia M, Boeve A, Zhang L, Brouillette RM, Foil HC, Bruce-Keller A, Keller JN (2020) Predictors of Heterogeneity in Cognitive Function: APOE-e4, Sex, Education, Depression, and Vascular Risk. Arch Clin Neuropsychol 35, 660–670. [DOI] [PubMed] [Google Scholar]
- [13].Rietman ML, Onland-Moret NC, Nooyens ACJ, Ibi D, van Dijk KW, Samson LD, Pennings JLA, Schipper M, Wong A, Spijkerman AMW, Dollé MET, Verschuren WMM (2022) The APOE locus is linked to decline in general cognitive function: 20-years follow-up in the Doetinchem Cohort Study. Transl Psychiatry 12, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kang DW, Wang S-M, Um YH, Kim N-Y, Lee CU, Lim HK (2022) Impact of APOE ε4 Carrier Status on Associations Between Subthreshold, Positive Amyloid-β Deposition, Brain Function, and Cognitive Performance in Cognitively Normal Older Adults: A Prospective Study. Front Aging Neurosci 14, 871323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Santos NC, Costa PS, Cunha P, Cotter J, Sampaio A, Zihl J, Almeida OFX, Cerqueira JJ, Palha JA, Sousa N (2013) Mood is a key determinant of cognitive performance in community-dwelling older adults: a cross-sectional analysis. AGE 35, 1983–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wilson R, Bennett D (2017) How Does Psychosocial Behavior Contribute to Cognitive Health in Old Age? Brain Sci 7, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cloutier S, Chertkow H, Kergoat M-J, Gauthier S, Belleville S (2015) Patterns of Cognitive Decline Prior to Dementia in Persons with Mild Cognitive Impairment. J Alzheimer’s Dis 47, 901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pourzinal D, Yang JHJ, Byrne GJ, O’Sullivan JD, Mitchell L, McMahon KL, Copland DA, Dissanayaka NN (2020) Identifying subtypes of mild cognitive impairment in Parkinson’s disease using cluster analysis. J Neurol 267, 3213–3222. [DOI] [PubMed] [Google Scholar]
- [19].Qiu Y, Jacobs DM, Messer K, Salmon DP, Feldman HH (2019) Cognitive heterogeneity in probable Alzheimer disease: Clinical and neuropathologic features. Neurology 93, e778–e790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Michelet M, Lund A, Strand BH, Engedal K, Selbaek G, Bergh S (2020) Characteristics of patients assessed for cognitive decline in primary healthcare, compared to patients assessed in specialist healthcare. Scand J Prim Health Care 38, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wojczynski MK, Jiuan Lin S, Sebastiani P, Perls TT, Lee J, Kulminski A, Newman A, Zmuda JM, Christensen K, Province MA (2021) NIA Long Life Family Study: Objectives, Design, and Heritability of Cross-Sectional and Longitudinal Phenotypes. J Gerontol Ser A 77, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Caliński T, Harabasz J (1974) A dendrite method for cluster analysis. Commun Stat 3, 1–27. [Google Scholar]
- [23].Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc Ser B (Stat Method) 57, 289–300. [Google Scholar]
- [24].Smithson M, Verkuilen J (2006) A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol Methods 11, 54–71. [DOI] [PubMed] [Google Scholar]
- [25].Xiang Q, Andersen SL, Perls TT, Sebastiani P (2021) Studying the Interplay Between Apolipoprotein E and Education on Cognitive Decline in Centenarians Using Bayesian Beta Regression. Front Genet 11, 606831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reas ET, Laughlin GA, Bergstrom J, Kritz-Silverstein D, Barrett-Connor E, McEvoy LK (2019) Effects of APOE on cognitive aging in community-dwelling older adults. Neuropsychology 33, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jung J, Visser M, Binney RJ, Lambon Ralph MA (2018) Establishing the cognitive signature of human brain networks derived from structural and functional connectivity. Brain Struct Funct 223, 4023–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hanken K, Eling P, Hildebrandt H (2015) Is there a cognitive signature for MS-related fatigue? Mult Scler 21, 376–381. [DOI] [PubMed] [Google Scholar]
- [29].Panter-Brick C, Eggerman M, Ager A, Hadfield K, Dajani R (2020) Measuring the psychosocial, biological, and cognitive signatures of profound stress in humanitarian settings: impacts, challenges, and strategies in the field. Confl Health 14, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matteini AM, Fallin MD, Kammerer CM, Schupf N, Yashin AI, Christensen K, Arbeev KG, Barr G, Mayeux R, Newman AB, Walston JD (2010) Heritability Estimates of Endophenotypes of Long and Health Life: The Long Life Family Study. J Gerontol Ser A 65A, 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, Hagenaars SP, Ritchie SJ, Marioni RE, Fawns-Ritchie C, Liewald DCM, Okely JA, Ahola-Olli AV, Barnes CLK, Bertram L, Bis JC, Burdick KE, Christoforou A, Derosse P, Djurovic S, Espeseth T, Giakoumaki S, Giddaluru S, Gustavson DE, Hayward C, Hofer E, Ikram MA, Karlsson R, Knowles E, Lahti J, Leber M, Li S, Mather KA, Melle I, Morris D, Oldmeadow C, Palviainen T, Payton A, Pazoki R, Petrovic K, Reynolds CA, Sargurupremraj M, Scholz M, Smith JA, Smith AV, Terzikhan N, Thalamuthu A, Trompet S, Van Der Lee SJ, Ware EB, Windham BG, Wright MJ, Yang J, Yu J, Ames D, Amin N, Amouyel P, Andreassen OA, Armstrong NJ, Assareh AA, Attia JR, Attix D, Avramopoulos D, Bennett DA, Böhmer AC, Boyle PA, Brodaty H, Campbell H, Cannon TD, Cirulli ET, Congdon E, Conley ED, Corley J, Cox SR, Dale AM, Dehghan A, Dick D, Dickinson D, Eriksson JG, Evangelou E, Faul JD, Ford I, Freimer NA, Gao H, Giegling I, Gillespie NA, Gordon SD, Gottesman RF, Griswold ME, Gudnason V, Harris TB, Hartmann AM, Hatzimanolis A, Heiss G, Holliday EG, Joshi PK, Kähönen M, Kardia SLR, Karlsson I, Kleineidam L, Knopman DS, Kochan NA, Konte B, Kwok JB, Le Hellard S, Lee T, Lehtimäki T, Li S-C, Lill CM, Liu T, Koini M, London E, Longstreth WT, Lopez OL, Loukola A, Luck T, Lundervold AJ, Lundquist A, Lyytikäinen L-P, Martin NG, Montgomery GW, Murray AD, Need AC, Noordam R, Nyberg L, Ollier W, Papenberg G, Pattie A, Polasek O, Poldrack RA, Psaty BM, Reppermund S, Riedel-Heller SG, Rose RJ, Rotter JI, Roussos P, Rovio SP, Saba Y, Sabb FW, Sachdev PS, Satizabal CL, Schmid M, Scott RJ, Scult MA, Simino J, Slagboom PE, Smyrnis N, Soumaré A, Stefanis NC, Stott DJ, Straub RE, Sundet K, Taylor AM, Taylor KD, Tzoulaki I, Tzourio C, Uitterlinden A, Vitart V, Voineskos AN, Kaprio J, Wagner M, Wagner H, Weinhold L, Wen KH, Widen E, Yang Q, Zhao W, Adams HHH, Arking DE, Bilder RM, Bitsios P, Boerwinkle E, Chiba-Falek O, Corvin A, De Jager PL, Debette S, Donohoe G, Elliott P, Fitzpatrick AL, Gill M, Glahn DC, Hägg S, Hansell NK, Hariri AR, Ikram MK, Jukema JW, Vuoksimaa E, Keller MC, Kremen WS, Launer L, Lindenberger U, Palotie A, Pedersen NL, Pendleton N, Porteous DJ, Räikkönen K, Raitakari OT, Ramirez A, Reinvang I, Rudan I, Dan R, Schmidt R, Schmidt H, Schofield PW, Schofield PR, Starr JM, Steen VM, Trollor JN, Turner ST, Van Duijn CM, Villringer A, Weinberger DR, Weir DR, Wilson JF, Malhotra A, McIntosh AM, Gale CR, Seshadri S, Mosley TH, Bressler J, Lencz T, Deary IJ (2018) Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun 9, 2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, De Leeuw CA, Nagel M, Awasthi S, Barr PB, Coleman JRI, Grasby KL, Hammerschlag AR, Kaminski JA, Karlsson R, Krapohl E, Lam M, Nygaard M, Reynolds CA, Trampush JW, Young H, Zabaneh D, Hägg S, Hansell NK, Karlsson IK, Linnarsson S, Montgomery GW, Muñoz-Manchado AB, Quinlan EB, Schumann G, Skene NG, Webb BT, White T, Arking DE, Avramopoulos D, Bilder RM, Bitsios P, Burdick KE, Cannon TD, Chiba-Falek O, Christoforou A, Cirulli ET, Congdon E, Corvin A, Davies G, Deary IJ, Derosse P, Dickinson D, Djurovic S, Donohoe G, Conley ED, Eriksson JG, Espeseth T, Freimer NA, Giakoumaki S, Giegling I, Gill M, Glahn DC, Hariri AR, Hatzimanolis A, Keller MC, Knowles E, Koltai D, Konte B, Lahti J, Le Hellard S, Lencz T, Liewald DC, London E, Lundervold AJ, Malhotra AK, Melle I, Morris D, Need AC, Ollier W, Palotie A, Payton A, Pendleton N, Poldrack RA, Räikkönen K, Reinvang I, Roussos P, Rujescu D, Sabb FW, Scult MA, Smeland OB, Smyrnis N, Starr JM, Steen VM, Stefanis NC, Straub RE, Sundet K, Tiemeier H, Voineskos AN, Weinberger DR, Widen E, Yu J, Abecasis G, Andreassen OA, Breen G, Christiansen L, Debrabant B, Dick DM, Heinz A, Hjerling-Leffler J, Ikram MA, Kendler KS, Martin NG, Medland SE, Pedersen NL, Plomin R, Polderman TJC, Ripke S, Van Der Sluis S, Sullivan PF, Vrieze SI, Wright MJ, Posthuma D (2018) Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet 50, 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bradburn S, Sarginson J, Murgatroyd CA (2018) Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-demented Adults: A Meta-Analysis of Prospective Studies. Front Aging Neurosci 9, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Landel V, Annweiler C, Millet P, Morello M, Féron F (2016) Vitamin D, Cognition and Alzheimer’s Disease: The Therapeutic Benefit is in the D-Tails. J Alzheimer’s Dis 53, 419–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gunstad J, Paul RH, Brickman AM, Cohen RA, Arns M, Roe D, Lawrence JJ, Gordon E (2006) Patterns of cognitive performance in middle-aged and older adults: A cluster analytic examination. J Geriatr Psychiatry Neurol 19, 59–64. [DOI] [PubMed] [Google Scholar]
- [36].Passarino G, Montesanto A, De Rango F, Garasto S, Berardelli M, Domma F, Mari V, Feraco E, Franceschi C, De Benedictis G (2007) A cluster analysis to define human aging phenotypes. Biogerontology 8, 283–290. [DOI] [PubMed] [Google Scholar]
- [37].Foss MP, Formigheri P, Speciali JG (2009) Heterogeneity of cognitive aging in Brazilian normal elderls. Dement Neuropsychol 3, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Paulo AC, Sampaio A, Santos NC, Costa PS, Cunha P, Zihl J, Cerqueira J, Palha JA, Sousa N (2011) Patterns of Cognitive Performance in Healthy Ageing in Northern Portugal: A Cross-Sectional Analysis. PLoS One 6, e24553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Damian M, Hausner L, Jekel K, Richter M, Froelich L, Almkvist O, Boada M, Bullock R, De Deyn PP, Frisoni GB, Hampel H, Jones RW, Kehoe P, Lenoir H, Minthon L, Olde Rikkert MGM, Rodriguez G, Scheltens P, Soininen H, Spiru L, Touchon J, Tsolaki M, Vellas B, Verhey FRJ, Winblad B, Wahlund LO, Wilcock G, Visser PJ (2013) Single-Domain Amnestic Mild Cognitive Impairment Identified by Cluster Analysis Predicts Alzheimer’s Disease in the European Prospective DESCRIPA Study. Dement Geriatr Cogn Disord 36, 1–19. [DOI] [PubMed] [Google Scholar]
- [40].MacAulay RK, Calamia MR, Cohen AS, Daigle K, Foil H, Brouillette R, Bruce-Keller AJ, Keller JN (2018) Understanding heterogeneity in older adults: Latent growth curve modeling of cognitive functioning. J Clin Exp Neuropsychol 40, 292–302. [DOI] [PubMed] [Google Scholar]
- [41].Sebastiani P, Andersen SL, Sweigart B, Du M, Cosentino S, Thyagarajan B, Christensen K, Schupf N, Perls TT (2020) Patterns of multi-domain cognitive aging in participants of the Long Life Family Study. GeroScience 42, 1335–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sebastiani P, Thyagarajan B, Sun F, Honig LS, Schupf N, Cosentino S, Feitosa MF, Wojczynski M, Newman AB, Montano M, Perls TT (2016) Age and Sex Distributions of Age-Related Biomarker Values in Healthy Older Adults from the Long Life Family Study. J Am Geriatr Soc 64, e189–e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gunn S, Wainberg M, Song Z, Andersen S, Boudreau R, Feitosa MF, Tan Q, Montasser ME, O’Connell JR, Stitziel N, Price N, Perls T, Schork NJ, Sebastiani P (2022) Distribution of 54 polygenic risk scores for common diseases in long lived individuals and their offspring. GeroScience 44, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available through dbGaP: dbGaP Study Accession - phs000397.v1.p1.


