Abstract
Topic Importance
This review discusses the rationale for vasopressin use, summarizes the results of clinical trials evaluating vasopressin, and focuses on the timing of vasopressin initiation to provide clinicians guidance for optimal adjunctive vasopressin initiation in patients with septic shock.
Review Findings
Patients with septic shock require vasoactive agents to restore adequate tissue perfusion. After norepinephrine, vasopressin is the suggested second-line adjunctive agent in patients with persistent inadequate mean arterial pressure. Vasopressin use in practice is heterogeneous likely because of inconsistent clinical trial findings, the lack of specific recommendations for when it should be used, and the high drug acquisition cost. Despite these limitations, vasopressin has demonstrated price inelastic demand, and its use in the United States has continued to increase. However, questions remain regarding optimal vasopressin use in patients with septic shock, particularly regarding patient selection and the timing of vasopressin initiation.
Summary
Experimental studies evaluating the initiation timing of vasopressin in patients with septic shock are limited, and recent observational studies have revealed an association between vasopressin initiation at lower norepinephrine-equivalent doses or lower lactate concentrations and lower mortality.
Key Words: septic shock, vasoactive agents, vasopressin, vasopressors
Sepsis is a highly prevalent and morbid disease estimated to account for up to 20% of annual deaths globally.1 If not identified rapidly and intervened on, sepsis may progress to septic shock, which is associated with mortality rates of up to 50%.2, 3, 4 Patients with septic shock have persistent hypotension requiring vasoactive agents to augment BP despite fluid resuscitation.5 Norepinephrine is recommended as a first-line treatment by the Surviving Sepsis Campaign (SSC) guidelines for patients with septic shock.6 If mean arterial pressure (MAP) remains inadequate with norepinephrine, vasopressin is suggested as the second-line adjunctive agent.6 More than 30% of patients with septic shock in the United States receive adjunctive vasopressin, with use rising over time despite exponential increases in vasopressin cost.7 This price inelasticity of vasopressin demand, where demand does not decrease as cost rises, indicates that clinicians’ perceived benefit of vasopressin overcomes its cost barrier. Despite increased use over time, ideal patient selection and initiation timing for adjunctive vasopressin in the course of a patient’s shock are not well elucidated. The 2021 iteration of the SSC guidelines note that initiating vasopressin when the patient requires between 0.25 and 0.5 μg/kg/min of norepinephrine “seems sensible.” Yet, many questions remain regarding the timing of vasopressin initiation. This article reviews the rationale for vasopressin use in septic shock, discusses the results of clinical trials evaluating vasopressin, and focuses on the timing of its initiation, with the goal of providing clinicians guidance on the bedside use of adjunctive vasopressin in patients with septic shock. This review expands on a recent article in discussing vasopressor escalation in septic shock.8
Literature Search
A search of MEDLINE via PubMed for clinical trials, randomized controlled trials, and meta-analyses was conducted to exhaust the search of articles pertinent to this review article. The following medical subject headings were used for evaluation of pertinent articles: septic shock, vasopressins, arginine vasopressin. The search was conducted May 26, 2022, with these terms: (“shock, septic” [MeSH]) and (“vasopressins”[MeSH]) or (“arginine vasopressin”[MeSH]) not (“terlipressin” [Title/Abstract]) not (“selepressin” [Title/Abstract]).
Evidence Review
Vasopressin Physiologic Features and Rationale
Exogenously administered vasopressin exerts multiple physiologic effects mediated primarily through activity at vasopressin receptors (V1, V2, and V3 receptors).9,10 Activity at V1 receptors in the vascular smooth muscle cells results in the vasoconstrictive effects targeted when using vasopressin in patients with vasodilatory shock. Activation of V1 receptors has been shown to cause nitric oxide-mediated vasodilation of the pulmonary vasculature in rodent models.11, 12, 13 However, this effect seems to be inconsistent across animal species, with vasopressin causing pulmonary vasoconstriction in a canine animal model.14 Activation of V2 receptors in the renal collecting ducts results in antidiuretic effects and resorption of free water. Finally, activity at V3 receptors in the pituitary include stimulation of adrenocorticotropic hormone production. Additionally, exogenous administration of vasopressin in patients with septic shock may be considered hormone replacement therapy because of the findings that vasopressin concentrations appropriately rise in the early phase of septic shock then quickly decline to subtherapeutic concentrations within the first 24 h after shock onset.9,15, 16, 17
Mechanistically, vasopressin is an attractive agent in patients with septic shock receiving escalating doses of norepinephrine, as suggested by the SSC guidelines.6 Other than its use as a hormone replacement, vasopressin is used as a vasoconstrictor to limit exogenous catecholamine exposure. Catecholamines exert their hemodynamic effects through activity at adrenergic α-receptors and β-receptors. These receptors are susceptible to downregulation and desensitization in states of shock, often necessitating higher doses to sustain hemodynamic augmentation.18 High catecholamine doses (> 1 μg/kg/min) have been associated with higher mortality, but the true cause-and-effect relationship is challenging to untangle because it is difficult to overcome the confounding in these observational studies resulting from the high severity of illness that often requires high catecholamine dosages.19, 20, 21, 22, 23, 24 Catecholamines have been shown to have other adverse effects, including increased risk of arrhythmias25,26 and immunomodulation.27, 28, 29, 30 Norepinephrine enhances antiinflammatory IL-10 response and attenuates the proinflammatory cytokine tumor necrosis factor alpha, whereas vasopressin exhibits no immunomodulatory effects. Importantly, higher norepinephrine doses cause more profound proinflammatory and antiinflammatory cytokine imbalance.27 Further, the addition of vasopressin to norepinephrine decreases overall cytokine concentrations compared with norepinephrine monotherapy in patients with septic shock.31 In light of the potential dose-dependent detrimental effects associated with catecholamines, it is prudent to use noncatecholamine adjuncts like vasopressin to limit catecholamine doses.
Clinical Trials
To date, three large randomized controlled trials have evaluated the use of adjunctive vasopressin in septic shock. The Vasopressin in Septic Shock Trial (VASST) randomized patients with septic shock to receive either open-label norepinephrine with masked norepinephrine (equating to norepinephrine monotherapy; n = 382) or open-label norepinephrine with masked adjunctive vasopressin (n = 397).17 After masked study drug initiation, open-label norepinephrine requirements were significantly lower in patients randomized to vasopressin (P < .001). The rates of serious adverse effects were similar between groups, but the addition of vasopressin caused lower heart rates.32 Overall, no difference in the primary outcome of 28-day mortality was detected between trial arms (35.4% norepinephrine and vasopressin vs 39.3% norepinephrine monotherapy; absolute difference, –3.9%; 95% CI, –10.7% to 2.9%). However, in the stratum of patients with norepinephrine requirements of 5 to 14 μg/min at the time of randomization, 90-day mortality was lower in patients randomized to receive vasopressin (35.8% vs 46.1%; absolute difference, –10.4%; 95% CI, –20.3% to –0.4%; P = .10 for interaction). Similarly, in a post hoc subgroup analysis, patients with a lactate concentration of ≤ 1.4 mM at randomization receiving vasopressin showed lower rates of all-cause mortality (18.9% vs 33.8%; absolute difference, –14.9%; 95% CI, –27.9% to –1.5%; P = .04 for interaction).17
The Vasopressin vs Norepinephrine as Initial Therapy in Septic Shock (VANISH) trial randomized patients either to norepinephrine (n = 204) or to vasopressin (n = 204) as the initial vasoactive agent.33 No difference was detected between the vasopressin and norepinephrine groups in the primary outcome of kidney failure-free days or 28-day mortality. However, patients allocated to vasopressin showed a lower frequency of the use of kidney replacement therapy (absolute difference, –9.9%; 95% CI, –19.3% to –0.6%). Similar to VASST, patients randomized to receive vasopressin showed lower norepinephrine dose requirements after randomization. Although this study was intended to evaluate vasopressin as the initial vasoactive agent, 85% of patients already were receiving norepinephrine at the time of randomization, leaving a small portion of patients who received vasopressin as the initial agent. Therefore, this study is best described as evaluating catecholamine-adjunctive vasopressin.33
The third large clinical trial was published only in abstract form. This trial randomized 387 patients with septic shock receiving “low doses” of norepinephrine either to norepinephrine with adjunctive vasopressin or to norepinephrine alone and found lower rates of 28-day mortality (34.0% vs 42.3%; P = .03) in patients who received vasopressin.34 The total duration of vasopressor treatment also was lower in the vasopressin arm (37 h vs 68 h; P = .02). Although reported to be evaluating “early” vasopressin use, full details of this trial are unclear, including the norepinephrine dose at randomization (when vasopressin was initiated). One additional large randomized controlled trial, the Vasopressin versus Norepinephrine for the Management of Septic Shock in Cancer Patients trial, randomized 250 patients with cancer with septic shock to receive either vasopressin or norepinephrine as first-line vasoactive therapy and similarly did not detect a difference in 28-day mortality (absolute difference, 4.0%; 95% CI, –8.2% to 16.1%).35 It is important to note that the Vasopressin vs Norepinephrine for the Management of Septic Shock in Cancer Patients trial was designed to evaluate vasopressin as a first-line vasopressor, and it is unknown how many patients were receiving open-label norepinephrine at the time of randomization and study enrollment.
Several meta-analyses have evaluated the use of vasopressin as first-line or adjunctive therapy in patients with vasodilatory or septic shock.36, 37, 38, 39, 40, 41 An individual patient data meta-analysis of four randomized controlled trials, including VASST and VANISH, found no difference in 28-day mortality with the use of vasopressin (relative risk [RR], 0.98; 95% CI, 0.86-1.12).38 However, meta-analyses that included randomized controlled trials evaluating multiple vasopressin receptor agonists (including vasopressin, terlipressin, pituitrin, and selepressin) found reduced mortality associated with vasopressin receptor agonist use (RR, 0.91; 95% CI, 0.85-0.99).36,37 One meta-analysis that evaluated randomized controlled trials of noncatecholamine vasoactive agents as a whole, including vasopressin receptor agonists and angiotensin II, also showed improved 28-day mortality favoring noncatecholamine vasoactive agents (RR, 0.92; 95% CI, 0.86-0.99).39 In the 2021 iteration of the SSC guidelines, an internal meta-analysis of 10 randomized controlled trials found improved mortality with the use of vasopressin (RR, 0.91; 95% CI, 0.83-0.99).6 These meta-analyses also found approximately twofold to fivefold higher risk for digital ischemia with vasopressin receptor agonists, but no between-group differences in total or serious adverse effects. Overall, most of the data from these meta-analyses indicate vasopressin receptor agonists can reduce mortality in patients with vasodilatory and septic shock safely.
Vasopressin Use in Clinical Practice
Vasopressin has been discussed in each iteration of the SSC guidelines. In the initial (2004) iteration, vasopressin was noted as a consideration in patients with refractory septic shock despite fluid resuscitation and “high-dose” vasopressors.42 More recent iterations increased the strength of this recommendation from ungraded guidance to suggestions with moderate quality of evidence in support.6,43, 44, 45 The 2021 iteration is the first to introduce concepts surrounding the timing of vasopressin initiation, with a remark stating that “in our practice, vasopressin is usually started when the dose of norepinephrine is in the range of 0.25-0.5 [μg]/kg/min.” Because of insufficient evidence at the time, this “in our practice” statement was generated through surveying SSC panel members and represents the most frequent response.6 Although this is not a recommendation by the SSC for a specific threshold of catecholamine dose at which vasopressin should be initiated, this statement represents clinician interest and the need for further research on the topic. In fact, evaluating how to escalate vasopressors and when to initiate second-line and third-line agents in patients with septic shock has been identified as a research priority by the SSC Research Committee.46,47
Escalating norepinephrine dose requirements are a common consideration for bedside vasopressin decision-making. Norepinephrine dose requirements are a clinical marker of shock severity and prognosis in patients with septic shock,23 typically are used as a threshold for initiation of adjunctive agents, and as previously mentioned, are used as the marker that the SSC guidelines describe for starting vasopressin.6,48,49 Although norepinephrine dose requirements may be easy to use at the bedside, it is unknown if this is the optimal marker for vasopressin initiation decision-making. Further, the norepinephrine-equivalent dose at which vasopressin was initiated has varied substantially in both experimental and observational studies (Table 1).17,33,35,50, 51, 52, 53, 54, 55, 56, 57, 58 Although most studies reported norepinephrine-equivalent doses of between 20 and 30 μg/min (or 0.3-0.4 μg/kg/min) at the time of vasopressin initiation, norepinephrine doses exceeding 1 μg/kg/min also were reported. In addition to norepinephrine dose requirements, the timing of vasopressin initiation also can be considered in the context of other factors available at the bedside, such as the degree of hypoperfusion (represented by the lactate concentration) or the time from shock onset (described as the duration of time since initiation of vasopressors). These clinical factors also have varied greatly in observational and experimental studies at the time of vasopressin initiation (Table 1).
Table 1.
Vasopressin Initiation in Clinical Trials of Patients With Vasodilatory Shock
| Study | Methodology | No. of Patients | Year(s) | At Vasopressin Initiation |
||
|---|---|---|---|---|---|---|
| Norepinephrine Dose | Lactate Concentration, mM | Timing From Shock Onset, h | ||||
| Experimental studies | ||||||
| Russell et al17 (2008; VASST) | Multicenter, randomized, double-blind trial of patients with septic shock | 779: AVP plus norepinephrine, 397 Norepinephrine alone, 382 |
2001-2006 | 20.7 ± 22.1 μg/min 0.26 ± 0.27 μg/kg/min |
3.5 ± 3.2 | 11.9 ± 8.9a |
| Gordon et al33 (2016; VANISH) | Multicenter, randomized, double-blind trial of patients with septic shock | 409: AVP plus placebo, 205 Norepinephrine plus placebo, 204 |
2013-2015 | 0.16 (0.1-0.3) μg/kg/min | 2.3 (1.4-4.0) | 3.5 (1.8-5.2)b |
| Hajjar et al35 (2019; VANCS II) | Single-center, randomized, double-blind trial of patients with cancer and septic shock | 250: AVP, 125 Norepinephrine, 125 |
2014-2016 | 0.33 (0.31-0.37) μg/kg/minc | AVP, 2.67 (1.88-3.88) Norepinephrine, 2.77 (1.66-5.10) |
NA |
| Dunser et al54 (2003) | Single-center, randomized trial of patients with catecholamine-resistant vasodilatory shock resulting from sepsis or after cardiovascular surgery | 48: AVP plus norepinephrine, 24 Norepinephrine alone, 24 |
2001-2002 | 0.84 ± 0.55 μg/kg/min | NA | NA |
| Torgersen et al55 (2010) | Single-center, open-label trial of patients with vasodilatory shock resulting from sepsis or after cardiac surgery | 50: AVP 0.033 U/min, 25 AVP 0.067 U/min, 25 |
2008 | AVP 0.033, 0.98 ± 0.6 μg/kg/min AVP 0.067, 0.86 ± 0.34μg/kg/min |
AVP 0.033, 4.0 ± 2.9 AVP 0.067, 5.4 ± 5.0 |
NA |
| Observational studies | ||||||
| Luckner et al56 (2005) | Retrospective, single-center trial of patients with vasodilatory shock receiving vasopressin | 316 | 1999-2003 | 1.06 ± 1.25 μg/kg/min | 4.8 ± 4.2 | NA |
| Luckner et al57 (2007) | Retrospective, single-center trial of patients with vasodilatory shock receiving vasopressin | 78 | 1999-2006 | 1.07 ± 1.10 μg/kg/min | 5.1 ± 4.0 | NA |
| Sacha et al50 (2018) | Retrospective, single-center trial of patients with septic shock receiving vasopressin | 938 | 2011-2015 | 28.2 ± 19.9 μg/min 0.34 ± 0.26 μg/kg/min |
4.8 ± 4.4 | NA |
| Allen et al52 (2018) | Retrospective, multicenter trial of patients with shock receiving vasopressin | 400 | 2013-2015 | 0.4 (0.2-0.5) μg/kg/min | NA | NA |
| Dubrawka et al58 (2021) | Retrospective, single-center trial of obese patients with septic shock receiving vasopressin | 182 | 2010-2017 | Standard dose, 32.5 (21.0-50.0) μg/mind High dose, 34.0 (19.3-49.3) μg/mind |
NA | Standard dose, 15.0 (7.8-38.1) High dose, 18.6 (10.3-80.3)d |
| Sacha et al51 (2022) | Retrospective, multicenter trial of patients with septic shock receiving vasopressin | 1,610 | 2012-2017 | 25.0 (18.0-40.0) μg/min | 3.9 (2.3-7.2) | 5.3 (2.12-12.2) |
Data are presented as mean ± SD or median (interquartile range), as reported in original publication. Norepinephrine-equivalent dose, lactate concentration, and timing to vasopressin initiation are reported in the total included patient population, unless otherwise specified. AVP = arginine vasopressin; NA = not available. VANCS II = Vasopressin vs Norepinephrine for the Management of Septic Shock in Cancer Patients; VANISH = Vasopressin vs Norepinephrine as Initial Therapy in Septic Shock; VASST = Vasopressin and Septic Shock Trial.
Time from meeting inclusion criteria to initiation of study drug infusion.
Time from onset of shock to receiving first study drug.
Reported as median dose of norepinephrine on day 0.
Standard dose of vasopressin was defined as receipt of vasopressin ≤ 0.04 units/min, and high dose of vasopressin was defined as vasopressin > 0.04 units/min.
Timing of Vasopressin Initiation
Few experimental studies have investigated the clinical question of the optimal timing for vasopressin initiation in patients with septic shock. Although designed to evaluate first-line vasopressin use, most patients (85%) included in the VANISH trial already were receiving norepinephrine at a median dose of 0.16 μg/kg/min, which could have masked the beneficial effects of initial or very early vasopressin use.33 To date, no large, randomized clinical trial has sought to compare different timing thresholds of vasopressin initiation in patients with septic shock.
Three small observational studies evaluated the use of early adjunctive vasopressin compared with norepinephrine monotherapy in patients with septic shock.59, 60, 61 One prospective observational study of 50 patients with septic shock who received either norepinephrine alone (n = 30) or norepinephrine with vasopressin initiation within 24 h (n = 20) found greater improvement in organ dysfunction (median Sequential Organ Failure Assessment score change) in vasopressin recipients both at 48 h (+1 [interquartile range (IQR), –1 to 3] vs –2 [IQR, –3 to 1]; P < .001) and at 72 h after inclusion (–2 [IQR, –4 to 0] vs –3 [IQR, –5 to 0]; P = .049).59 The between-group difference in Sequential Organ Failure Assessment score over the first 72 h also was demonstrated in a repeated measures of variance analysis that accounted for baseline Sequential Organ Failure Assessment score (P = .009). In a second prospective, open-label, before-after cohort study of 82 patients with septic shock, vasopressin initiation (0.04 units/min) within 4 h of norepinephrine administration (n = 41) was compared with norepinephrine monotherapy (n = 41).60 Early vasopressin use was associated with a 2-h reduction in time to achieve and maintain MAP of 65 mm Hg (5.7 h [IQR, 1.7-10.3 h] vs 7.6 h [IQR, 3.6-16.7 h]; P = .058). Additionally, no difference was detected for any clinical outcomes evaluated. Finally, one retrospective cohort study found that patients who received vasopressin within 4 h of norepinephrine initiation (n = 48) demonstrated a shorter time to achieving the MAP target (6.2 ± 4.9 h vs 9.9 ± 9.1 h; P = .02) compared with those who received norepinephrine alone (n = 48).61 These studies showed improved MAP and organ function when vasopressin was initiated early in patients with septic shock compared with norepinephrine monotherapy. However, these studies are heterogeneous in the way vasopressin was used and lacked the methodologic rigor required to answer successfully the question of when to initiate vasopressin.
Several observational studies have evaluated vasopressin initiation timing by restricting inclusion to only vasopressin recipients and comparing groups defined by patient characteristics or clinical variables at vasopressin initiation.51,61, 62, 63, 64, 65 Most of these studies primarily evaluated the time from shock onset to vasopressin initiation, typically defined as the time of vasopressor initiation.62, 63, 64, 65 Findings were heterogeneous in these relatively small (largest n = 385) retrospective studies. Some found earlier initiation of vasopressin after shock onset was associated with faster time to shock resolution62; improvement in the composite outcome of either in-hospital mortality, organ dysfunction, or both64; and reduced frequency of new-onset arrhythmias.65 Conversely, one study did not detect an association between hemodynamic response after vasopressin initiation and the timing of vasopressin initiation.63 Additionally, a meta-analysis that included both prospective and observational studies evaluating the timing of vasopressin initiation did not detect an association between initiation of vasopressin within 6 h of shock onset and short-term mortality, new-onset arrhythmias, and ICU length of stay.66 It is important to note that these studies varied in the timing thresholds evaluated, with “early” being defined as anywhere from 3 to 7 h after shock onset, and have varied in the clinical outcomes evaluated.
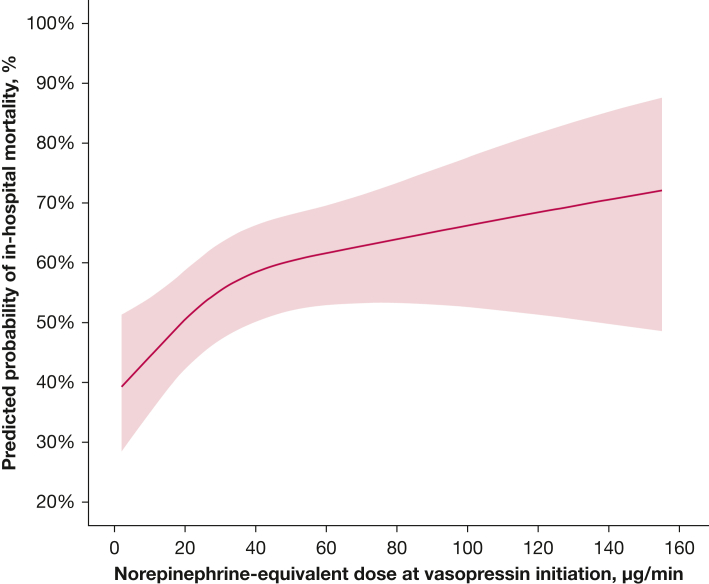
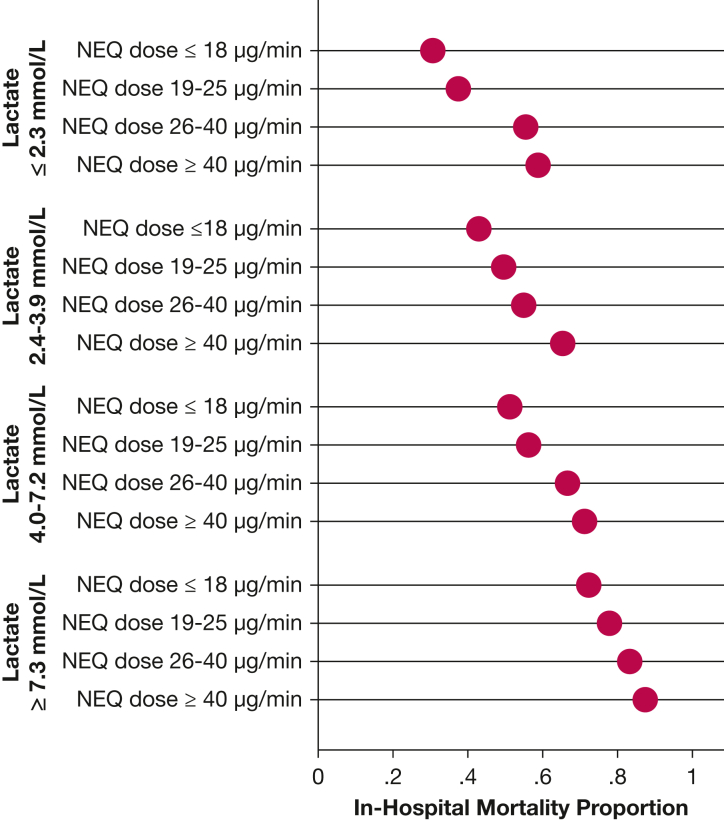
An observational study was the largest study to investigate the timing of vasopressin initiation.51 This study included 1,610 patients with septic shock and evaluated vasopressin initiation timing in three contexts: the norepinephrine-equivalent dose at vasopressin initiation, the lactate concentration at vasopressin initiation, and the time from shock onset to vasopressin initiation. After adjustment for patient severity and confounders, the odds of in-hospital mortality increased 20.7% for every 10-μg/min increase in norepinephrine-equivalent dose at vasopressin initiation, up to a norepinephrine-equivalent dose of 60 μg/min (adjusted OR, 1.21; 95% CI, 1.09-1.34) (Table 2). At norepinephrine-equivalent doses exceeding 60 μg/min at vasopressin initiation, no association was detected. To aid with translation of the findings to bedside practice, comparisons of vasopressin initiation at norepinephrine-equivalent dose thresholds also were described. Odds of in-hospital mortality were lower for vasopressin initiation at a norepinephrine-equivalent dose of 10 μg/min vs 25 μg/min or 60 μg/min (Fig 1, Table 2). This study also found an association between higher lactate concentration at vasopressin initiation and higher in-hospital mortality (Table 2). Finally, no association was detected between the time from shock onset to vasopressin initiation and in-hospital mortality.51 When outcomes were described by quartiles of both lactate concentration and norepinephrine-equivalent dose at the time of vasopressin initiation, the lowest rates of in-hospital mortality were observed in patients in whom vasopressin was initiated when the norepinephrine-equivalent dose was ≤ 18 μg/min and the lactate concentration was ≤ 2.3 mM (Fig 2). This study corroborates the subgroup findings from the VASST and suggests that patients who ultimately receive adjunctive vasopressin benefit most from its initiation earlier in the course of shock, specifically at lower norepinephrine-equivalent doses (< 15 μg/min, in agreement with the VASST subgroup analysis) and at lower lactate concentrations. The reasons for the discrepant findings related to temporal time of vasopressin initiation after shock onset between this large observational studies and other observational studies are unclear. One hypothesis is that temporal time from shock onset (or catecholamine vasopressor initiation) is related inconsistently to severity and trajectory in patients with septic shock. Indeed, it may be that a bedside indicator for septic shock severity, like norepinephrine-equivalent dose or lactate concentration, is a better clinical tool to use for determining when vasopressin should be initiated.
Table 2.
Associations Between Norepinephrine-Equivalent Dose and Lactate Concentration at Vasopressin Initiation and In-Hospital Mortality
| Timing Variable | Adjusted OR (95% CI) |
|---|---|
| NEQ, per 10 μg/mina,b | 1.21 (1.09-1.34) |
| NEQ threshold comparison, μg/minb | |
| 10 vs 25 | 0.75 (0.65-0.88) |
| 10 vs 60 | 0.39 (0.24-0.65) |
| 25 vs 60 | 0.52 (0.36-0.74) |
| Lactate, per mMc,d | |
| At 2.1 h from shock onset | 1.12 (1.06-1.18) |
| At 5.3 h from shock onset | 1.14 (1.06-1.22) |
| At 12.2 h from shock onset | 1.18 (1.07-1.32) |
NEQ = Norepinephrine-equivalent dose.
Adjusted OR presented for every 10-μg/min increase in NEQ up to 60 μg/min.
Adjusted for the following known confounders: lactate at vasopressin initiation, time from shock onset, age, weight, sex, race, immune suppression, ICU location, hydrocortisone receipt, mechanical ventilation, acute kidney injury, receipt of appropriate antibiotics, Sequential Organ Failure Assessment score, Acute Physiology and Chronic Health Evaluation III score, fluid balance, and volume fluid bolus administration.
Because of the presence of effect modification between lactate concentration and duration of time from shock onset, the association between lactate concentration at vasopressin initiation and in-hospital mortality was described by quartile of time from shock onset.
Adjusted for the following known confounders: NEQ at vasopressin initiation, age, weight, sex, race, immune suppression, ICU location, hydrocortisone receipt, mechanical ventilation, acute kidney injury, receipt of appropriate antibiotics, Sequential Organ Failure Assessment score, Acute Physiology and Chronic Health Evaluation III score, fluid balance, and volume fluid bolus administration.
Figure 1.
Line graph showing norepinephrine-equivalent doses at vasopressin initiation, modeled as a restricted cubic spline with three knots (placed by the algorithm at 10 μg/min, 25 μg/min, and 60 μg/min), were used to create a prediction model for in-hospital mortality, adjusting for severity and known confounders. The shaded red area indicates the 95% CI of the predicted probability. Initiation of vasopressin at lower norepinephrine-equivalent doses was associated with lower odds of in-hospital mortality. Initiation at 10 μg/min vs 25 μg/min: OR, 0.75 (95% CI, 0.65-0.88); initiation at 10 μg/min vs 60 μg/min: OR, 0.39 (95% CI, 0.24-0.65); and initiation at 25 μg/min vs 60 μg/min: OR, 0.52 (95% CI, 0.36-0.74). (Reprinted with permission from Sacha et al.51)
Figure 2.
Graph showing mortality by lactate concentration and NEQ (measure in micrograms per minute) at vasopressin initiation. In-hospital mortality proportion is presented in quartiles of lactate concentration at vasopressin initiation by quartile of NEE at vasopressin initiation. Both higher lactate concentration and higher NEE at vasopressin initiation were associated with higher mortality. NEQ = Norepinephrine-equivalent dose. (Reprinted with permission from Sacha et al.51)
Important disparities in results exist between experimental and observational studies of vasopressin initiation timing. The only study to show a clinical outcome benefit with early adjunctive vasopressin when compared with norepinephrine alone in clinical trials was the VANISH trial, yet an individual patient data meta-analysis did not detect an effect of vasopressin on 28-day mortality.17 No evidence for heterogeneity of treatment effect by either norepinephrine dose (> 15 μg/min or < 15 μg/min) or lactate concentration (> 2 mM or < 2 mM) at randomization was detected in the meta-analysis. A number of potential reasons exist for these differences in findings. First, in the meta-analysis, norepinephrine dose and lactate concentration were evaluated as dichotomous subgroups, a method that has a lower statistical power to detect an effect compared with analyzing these variable in their continuous form (as was carried out in the large observational study).67 Second, important differences exist in the populations of patients evaluated. The randomized trials centered on the population of patients with septic shock receiving norepinephrine to compare adjunctive vasopressin initiation with continuation of norepinephrine monotherapy.17,33 However, most of the observational studies evaluated the population of patients who received vasopressin, which is a population with more severe illness in whom first-line norepinephrine has failed as determined by the treating clinicians. Therefore, the magnitude of benefit with early vasopressin seems to be context dependent: the benefit of early vasopressin initiation lies with those who ultimately will receive vasopressin. However, currently available models cannot predict a patient’s shock trajectory or their likelihood to be initiated on vasopressin accurately based on data available at the onset of shock. As such, if a clinician incorporates into their practice the SSC suggestion to use vasopressin as a component of the hemodynamic management of septic shock, it is prudent to initiate vasopressin earlier as opposed to waiting to initiate vasopressin until refractory shock has developed in the patient.
Hemodynamic Response to Vasopressin
Hemodynamic response to vasopressin initiation occurs in almost half of patients with septic shock (40%-45%) and, when achieved, is associated with improved outcomes.50,51,68 Defined as achievement of both a reduction in norepinephrine-equivalent dose and MAP of ≥ 65 mm Hg at 6 h after vasopressin initiation, hemodynamic response after vasopressin initiation has been associated with lower ICU mortality (adjusted OR, 0.51; 95% CI, 0.35-0.76), lower 28-day mortality (adjusted hazard ratio, 0.60; 95% CI, 0.49-0.75), and improved clinical trajectory (adjusted OR, 1.63; 95% CI, 1.26-2.10).50,68 A number of factors have been identified as being associated with vasopressin responsiveness, including lower lactate concentration at vasopressin initiation, higher arterial pH at vasopressin initiation, higher left ventricular ejection fraction, and nonmedical (vs medical) ICU location.50,69,70 It is likely that other, currently unidentified, factors are associated with (and potentially causing) vasopressin responsiveness, including genetic factors, biomarkers, and endogenous vasopressin concentrations. Importantly, enrollment of a heterogeneous population of patients with septic shock in studies such as the VASST and VANISH trial instead of a targeted population in whom vasopressin would have led to a positive hemodynamic response may have masked a beneficial mortality effect with vasopressin. Until further research is conducted in this area, clinicians should consider using early hemodynamic response to vasopressin to guide adjustments in their patient’s therapeutic regimen. This includes monitoring for hemodynamic response after vasopressin is initiated and adjusting the regimen (such as increasing vasopressin dose, ceasing vasopressin, or adding agents with alternative pharmacologic agents) if hemodynamic response is not achieved within 6 h of initiation.
Future State
Many unanswered questions remain regarding optimal use of vasopressin in patients with septic shock (Table 3). One important question includes the ideal timing of vasopressin’s initiation. Specifically, indicators of ideal timing for vasopressin initiation (norepinephrine-equivalent dose vs lactate concentration vs other clinical biomarkers) should be an area of future research. It is likely that a single factor is inadequate to predict beneficial effects with vasopressin in patients with septic shock, but rather, a cluster of factors identify a treatment-responsive subphenotype for vasopressin.71,72 Clinical prediction models, potentially derived with machine learning or artificial intelligence algorithms, could be used to identify these treatment-responsive subphenotypes for adjunctive vasopressin. Vasopressin response subphenotypes then could be used as a component of a precision vasoactive drug strategy or for predictive enrichment in an adaptive clinical trial.72, 73, 74 Additionally, when discussing norepinephrine-equivalent dose as a clinical threshold for initiation of vasopressin, weight-based dosing vs non-weight-based dosing should be elucidated further. Most studies evaluating this parameter used non-weight-based dosing thresholds; however, these thresholds are difficult to translate to practice at institutions that use weight-based doses. Similarly, many countries are supplied norepinephrine as norepinephrine bitartrate, whereas others have a norepinephrine base, where 1 μg of norepinephrine bitartrate equals 0.5 μg of norepinephrine base. Future trials and publications should indicate which norepinephrine product is used at the study institution to increase applicability of results across institutions.
Table 3.
Important Research Questions for Vasopressin in Septic Shock
| Can patients who will respond to vasopressin’s initiation be identified preemptively? |
| When is the most ideal time during the course of shock to initiate vasopressin in a patient? |
| What clinical marker should be used as an indicator for vasopressin’s initiation? |
| What is the maximum safe vasopressin dose? |
| Should the vasopressin dosage be titrated? |
| What therapeutic intervention should be undertaken in individuals who do not respond to vasopressin initiation? |
| How should vasopressin be discontinued in patients in the convalescent phase? |
Future vasopressin studies in patients with septic shock are imperative. Vasopressin use continues to increase despite variable trial findings and moderate-quality evidence supporting its use in septic shock.6,7 Increasing use of vasopressin in this setting suggests that clinicians deem it a necessary therapy for patients with septic shock.7,51,75, 76, 77 Therefore, additional, high-quality evidence is needed to optimize vasopressin use. Without these data, clinical practice is unable to be advanced toward patient-centered care where the vasoactive regimen is targeted to each individual patient’s hemodynamic status and clinical course and agents are selected based on patient factors indicating that they will benefit from a drug’s use.72 As of early 2023, 16 active clinical trials are registered on ClinicalTrials.gov that include vasopressin as an intervention; however, only two of these trials are evaluating vasopressin use specifically in patients with septic shock.78,79 Because randomized controlled trials have not focused recently on this intervention and do not seem to be prevalent in the pipeline, high-quality observational studies need to be considered, including those with enhancements in methodologies for causal inference.80
Summary
Vasopressin is the recommended second-line vasoactive agent in patients with septic shock and escalating doses of norepinephrine. However, the timing of its initiation is not well elucidated in the literature or in clinical guidelines. Recent observational studies have shown that initiating vasopressin at lower norepinephrine-equivalent doses or lower lactate concentrations is associated with lower mortality. When vasopressin is used, it is reasonable to initiate it when norepinephrine-equivalent doses are < 15 μg/min. Future studies of vasopressin should focus on the timing of its initiation at various clinical thresholds and patient selection for receipt of vasopressin.
Funding/Support
Supported by the National Institute of General Medical Sciences, National Institutes of Health [Grant K08GM147806 to S. R. B.].
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: G. L. S. reports that she is a consultant for Wolters Kluwer. None declared (S. R. B.).
Acknowledgments
Role of sponsors: The funding source had no role in writing the article or the decision to submit for publication.
Disclaimer: The contents herein are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1.Rudd K.E., Johnson S.C., Agesa K.M., et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadri S.S., Rhee C., Strich J.R., et al. Estimating ten-year trends in septic shock incidence and mortality in United States Academic Medical Centers Using Clinical Data. Chest. 2017;151(2):278–285. doi: 10.1016/j.chest.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent J.L., Jones G., David S., Olariu E., Cadwell K.K. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. 2019;23(1):196. doi: 10.1186/s13054-019-2478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman T.G., Simpson S.Q., Sciarretta K.L., et al. Sepsis among Medicare beneficiaries: 1. The burdens of sepsis, 2012-2018. Crit Care Med. 2020;48(3):276–288. doi: 10.1097/CCM.0000000000004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer M., Deutschman C.S., Seymour C.W., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans L., Rhodes A., Alhazzani W., et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 7.Sacha G.L., Kiser T.H., Wright G.C., et al. Association between vasopressin rebranding and utilization in patients with septic shock. Crit Care Med. 2022;50(4):644–654. doi: 10.1097/CCM.0000000000005305. [DOI] [PubMed] [Google Scholar]
- 8.Teja B., Bosch N.A., Walkey A.J. How we escalate vasopressor and corticosteroid therapy in patients with septic shock. Chest. 2023;163(3):567–574. doi: 10.1016/j.chest.2022.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Holmes C.L., Patel B.M., Russell J.A., Walley K.R. Physiology of vasopressin relevant to management of septic shock. Chest. 2001;120(3):989–1002. doi: 10.1378/chest.120.3.989. [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration Center for Drug Evaluation and Research Vasopressin 2044850rig1s000 pharmacology review(s), April 4, 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/2044850rig1s000PharmR.pdf US Food and Drug Administration website.
- 11.Evora P.R., Pearson P.J., Schaff H.V. Arginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery. V1-receptor-mediated production of nitric oxide. Chest. 1993;103(4):1241–1245. doi: 10.1378/chest.103.4.1241. [DOI] [PubMed] [Google Scholar]
- 12.Eichinger M.R., Walker B.R. Enhanced pulmonary arterial dilation to arginine vasopressin in chronically hypoxic rats. Am J Physiol. 1994;267(6 pt 2):H2413–H2419. doi: 10.1152/ajpheart.1994.267.6.H2413. [DOI] [PubMed] [Google Scholar]
- 13.Walker B.R., Haynes J., Jr., Wang H.L., Voelkel N.F. Vasopressin-induced pulmonary vasodilation in rats. Am J Physiol. 1989;257(2 pt 2):H415–H422. doi: 10.1152/ajpheart.1989.257.2.H415. [DOI] [PubMed] [Google Scholar]
- 14.Leather H.A., Segers P., Berends N., Vandermeersch E., Wouters P.F. Effects of vasopressin on right ventricular function in an experimental model of acute pulmonary hypertension. Crit Care Med. 2002;30(11):2548–2552. doi: 10.1097/00003246-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Landry D.W., Levin H.R., Gallant E.M., et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95(5):1122–1125. doi: 10.1161/01.cir.95.5.1122. [DOI] [PubMed] [Google Scholar]
- 16.Sharshar T., Blanchard A., Paillard M., Raphael J.C., Gajdos P., Annane D. Circulating vasopressin levels in septic shock. Crit Care Med. 2003;31(6):1752–1758. doi: 10.1097/01.CCM.0000063046.82359.4A. [DOI] [PubMed] [Google Scholar]
- 17.Russell J.A., Walley K.R., Singer J., et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 18.Bangash M.N., Kong M.L., Pearse R.M. Use of inotropes and vasopressor agents in critically ill patients. Br J Pharmacol. 2012;165(7):2015–2033. doi: 10.1111/j.1476-5381.2011.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunser M.W., Ruokonen E., Pettila V., et al. Association of arterial blood pressure and vasopressor load with septic shock mortality: a post hoc analysis of a multicenter trial. Crit Care. 2009;13(6):R181. doi: 10.1186/cc8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin C., Medam S., Antonini F., et al. Norepinephrine: not too much, too long. Shock. 2015;44(4):305–309. doi: 10.1097/SHK.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 21.Brown S.M., Lanspa M.J., Jones J.P., et al. Survival after shock requiring high-dose vasopressor therapy. Chest. 2013;143(3):664–671. doi: 10.1378/chest.12-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins C.R., Gomersall C.D., Leung P., Joynt G.M. Outcome of patients receiving high dose vasopressor therapy: a retrospective cohort study. Anaesth Intensive Care. 2009;37(2):286–289. doi: 10.1177/0310057X0903700212. [DOI] [PubMed] [Google Scholar]
- 23.Sato R., Duggal A., Sacha G.L., et al. The relationship between norepinephrine equivalent dose of vasopressors within 24 hours from the onset of septic shock and in-hospital mortality rate. Chest. 2023;163(1):148–151. doi: 10.1016/j.chest.2022.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Richards-Belle A., Hylands M., Muttalib F., et al. Lower versus higher exposure to vasopressor therapy in vasodilatory hypotension: a systematic review with meta-analysis. Crit Care Med. 2023;51(2):254–266. doi: 10.1097/CCM.0000000000005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domizi R., Calcinaro S., Harris S., et al. Relationship between norepinephrine dose, tachycardia and outcome in septic shock: a multicentre evaluation. J Crit Care. 2020;57:185–190. doi: 10.1016/j.jcrc.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Wieruszewski E.D., Jones G.M., Samarin M.J., Kimmons L.A. Predictors of dysrhythmias with norepinephrine use in septic shock. J Crit Care. 2021;61:133–137. doi: 10.1016/j.jcrc.2020.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Stolk R.F., van der Pasch E., Naumann F., et al. Norepinephrine dysregulates the immune response and compromises host defense during sepsis. Am J Respir Crit Care Med. 2020;202(6):830–842. doi: 10.1164/rccm.202002-0339OC. [DOI] [PubMed] [Google Scholar]
- 28.Devi S., Alexandre Y.O., Loi J.K., et al. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity. 2021;54(6):1219–1230 e1217. doi: 10.1016/j.immuni.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Stolk R.F., van der Poll T., Angus D.C., van der Hoeven J.G., Pickkers P., Kox M. Potentially inadvertent immunomodulation: norepinephrine use in sepsis. Am J Respir Crit Care Med. 2016;194(5):550–558. doi: 10.1164/rccm.201604-0862CP. [DOI] [PubMed] [Google Scholar]
- 30.van der Poll T., Jansen J., Endert E., Sauerwein H.P., van Deventer S.J. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994;62(5):2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell J.A., Fjell C., Hsu J.L., et al. Vasopressin compared with norepinephrine augments the decline of plasma cytokine levels in septic shock. Am J Respir Crit Care Med. 2013;188(3):356–364. doi: 10.1164/rccm.201302-0355OC. [DOI] [PubMed] [Google Scholar]
- 32.Gordon A.C., Wang N., Walley K.R., Ashby D., Russell J.A. The cardiopulmonary effects of vasopressin compared with norepinephrine in septic shock. Chest. 2012;142(3):593–605. doi: 10.1378/chest.11-2604. [DOI] [PubMed] [Google Scholar]
- 33.Gordon A.C., Mason A.J., Thirunavukkarasu N., et al. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA. 2016;316(5):509–518. doi: 10.1001/jama.2016.10485. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira S., Dessa F., Rocha C., Oliveira F. Early vasopressin application in shock study. Crit Care. 2014;18(1):P158. [Google Scholar]
- 35.Hajjar L.A., Zambolim C., Belletti A., et al. Vasopressin versus norepinephrine for the management of septic shock in cancer patients: the VANCS II randomized clinical trial. Crit Care Med. 2019;47(12):1743–1750. doi: 10.1097/CCM.0000000000004023. [DOI] [PubMed] [Google Scholar]
- 36.Honarmand K., Um K.J., Belley-Cote E.P., et al. Canadian Critical Care Society clinical practice guideline: the use of vasopressin and vasopressin analogues in critically ill adults with distributive shock. Can J Anaesth. 2020;67(3):369–376. doi: 10.1007/s12630-019-01546-x. [DOI] [PubMed] [Google Scholar]
- 37.Jiang L., Sheng Y., Feng X., Wu J. The effects and safety of vasopressin receptor agonists in patients with septic shock: a meta-analysis and trial sequential analysis. Crit Care. 2019;23(1):91. doi: 10.1186/s13054-019-2362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagendran M., Russell J.A., Walley K.R., et al. Vasopressin in septic shock: an individual patient data meta-analysis of randomised controlled trials. Intensive Care Med. 2019;45(6):844–855. doi: 10.1007/s00134-019-05620-2. [DOI] [PubMed] [Google Scholar]
- 39.Zhong L., Ji X.W., Wang H.L., Zhao G.M., Zhou Q., Xie B. Non-catecholamine vasopressors in the treatment of adult patients with septic shock-evidence from meta-analysis and trial sequential analysis of randomized clinical trials. J Intensive Care. 2020;8(1):83. doi: 10.1186/s40560-020-00500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIntyre W.F., Um K.J., Alhazzani W., et al. Association of vasopressin plus catecholamine vasopressors vs catecholamines alone with atrial fibrillation in patients with distributive shock: a systematic review and meta-analysis. JAMA. 2018;319(18):1889–1900. doi: 10.1001/jama.2018.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chidambaram S., Goh E.L., Rey V.G., Khan M.A. Vasopressin vs noradrenaline: have we found the perfect recipe to improve outcome in septic shock? J Crit Care. 2019;49:99–104. doi: 10.1016/j.jcrc.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 42.Dellinger R.P., Carlet J.M., Masur H., et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 43.Dellinger R.P., Levy M.M., Carlet J.M., et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 44.Dellinger R.P., Levy M.M., Rhodes A., et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhodes A., Evans L.E., Alhazzani W., et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 46.Coopersmith C.M., De Backer D., Deutschman C.S., et al. Surviving Sepsis Campaign: research priorities for sepsis and septic shock. Crit Care Med. 2018;46(8):1334–1356. doi: 10.1097/CCM.0000000000003225. [DOI] [PubMed] [Google Scholar]
- 47.Lat I., Coopersmith C.M., De Backer D., et al. The Surviving Sepsis Campaign: fluid resuscitation and vasopressor therapy research priorities in adult patients. Crit Care Med. 2021;49(4):623–635. doi: 10.1097/CCM.0000000000004864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jentzer J.C., Vallabhajosyula S., Khanna A.K., Chawla L.S., Busse L.W., Kashani K.B. Management of refractory vasodilatory shock. Chest. 2018;154(2):416–426. doi: 10.1016/j.chest.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 49.Russell J.A. Bench-to-bedside review: vasopressin in the management of septic shock. Crit Care. 2011;15(4):226. doi: 10.1186/cc8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sacha G.L., Lam S.W., Duggal A., et al. Predictors of response to fixed-dose vasopressin in adult patients with septic shock. Ann Intensive Care. 2018;8(1):35. doi: 10.1186/s13613-018-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sacha G.L., Lam S.W., Wang L., Duggal A., Reddy A.J., Bauer S.R. Association of catecholamine dose, lactate, and shock duration at vasopressin initiation with mortality in patients with septic shock. Crit Care Med. 2022;50(4):614–623. doi: 10.1097/CCM.0000000000005317. [DOI] [PubMed] [Google Scholar]
- 52.Allen B., Kram B., Kram S., et al. Predictors of vasopressin responsiveness in critically ill adults. Ann Pharmacother. 2018;52(2):126–132. doi: 10.1177/1060028017729480. [DOI] [PubMed] [Google Scholar]
- 53.Bosch N.A., Teja B., Wunsch H., Walkey A.J. Practice patterns in the initiation of secondary vasopressors and adjunctive corticosteroids during septic shock in the United States. Ann Am Thorac Soc. 2021;18(12):2049–2057. doi: 10.1513/AnnalsATS.202102-196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunser M.W., Mayr A.J., Ulmer H., et al. Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation. 2003;107(18):2313–2319. doi: 10.1161/01.CIR.0000066692.71008.BB. [DOI] [PubMed] [Google Scholar]
- 55.Torgersen C., Dunser M.W., Wenzel V., et al. Comparing two different arginine vasopressin doses in advanced vasodilatory shock: a randomized, controlled, open-label trial. Intensive Care Med. 2010;36(1):57–65. doi: 10.1007/s00134-009-1630-1. [DOI] [PubMed] [Google Scholar]
- 56.Luckner G., Dunser M.W., Jochberger S., et al. Arginine vasopressin in 316 patients with advanced vasodilatory shock. Crit Care Med. 2005;33(11):2659–2666. doi: 10.1097/01.ccm.0000186749.34028.40. [DOI] [PubMed] [Google Scholar]
- 57.Luckner G., Mayr V.D., Jochberger S., et al. Comparison of two dose regimens of arginine vasopressin in advanced vasodilatory shock. Crit Care Med. 2007;35(10):2280–2285. doi: 10.1097/01.ccm.0000281853.50661.23. [DOI] [PubMed] [Google Scholar]
- 58.Dubrawka C.A., Betthauser K.D., Pope H.E., Gibson G.A. Effect of vasopressin dose on hemodynamic response in obese patients with septic shock: a retrospective observational study. Ann Pharmacother. 2021;55(12):1447–1454. doi: 10.1177/10600280211007213. [DOI] [PubMed] [Google Scholar]
- 59.Bihari D., Prakash S., Bersten A. Low-dose vasopressin in addition to noradrenaline may lead to faster resolution of organ failure in patients with severe sepsis/septic shock. Anaesth Intensive Care. 2014;42(5):671–674. [PubMed] [Google Scholar]
- 60.Hammond D.A., Ficek O.A., Painter J.T., et al. Prospective open-label trial of early concomitant vasopressin and norepinephrine therapy versus initial norepinephrine monotherapy in septic shock. Pharmacotherapy. 2018;38(5):531–538. doi: 10.1002/phar.2105. [DOI] [PubMed] [Google Scholar]
- 61.Hammond D.A., Cullen J., Painter J.T., et al. Efficacy and safety of the early addition of vasopressin to norepinephrine in septic shock. J Intensive Care Med. 2019;34(11-12):910–916. doi: 10.1177/0885066617725255. [DOI] [PubMed] [Google Scholar]
- 62.Brask AL, Shemanski SM, Barnes TE, and Holmes AK. Timing of vasopressin addition to norepinephrine and efficacy outcomes in patients with septic shock. Ann Pharmacother. 2023; 57(5):521-526. [DOI] [PubMed]
- 63.Jakowenko N.D., Murata J., Kopp B.J., Erstad B.L. Influence of timing and catecholamine requirements on vasopressin responsiveness in critically ill patients with septic shock. J Intensive Care Med. 2022;37(11):1512–1519. doi: 10.1177/08850666221081836. [DOI] [PubMed] [Google Scholar]
- 64.Rydz A.C., Elefritz J.L., Conroy M., et al. Early initiation of vasopressin reduces organ failure and mortality in septic shock. Shock. 2022;58(4):269–274. doi: 10.1097/SHK.0000000000001978. [DOI] [PubMed] [Google Scholar]
- 65.Reardon D.P., DeGrado J.R., Anger K.E., Szumita P.M. Early vasopressin reduces incidence of new onset arrhythmias. J Crit Care. 2014;29(4):482–485. doi: 10.1016/j.jcrc.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Huang H., Wu C., Shen Q., Xu H., Fang Y., Mao W. The effect of early vasopressin use on patients with septic shock: a systematic review and meta-analysis. Am J Emerg Med. 2021;48:203–208. doi: 10.1016/j.ajem.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Altman D.G., Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bauer S.R., Sacha G.L., Siuba M.T., et al. Vasopressin response and clinical trajectory in septic shock patients. J Intensive Care Med. 2023;38(3):273–279. doi: 10.1177/08850666221118282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bauer S.R., Sacha G.L., Siuba M.T., et al. Association of arterial pH with hemodynamic response to vasopressin in patients with septic shock: an observational cohort study. Crit Care Explor. 2022;4(2) doi: 10.1097/CCE.0000000000000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dugar S., Siuba M.T., Sacha G.L., et al. Echocardiographic profiles and hemodynamic response after vasopressin initiation in septic shock: a cross-sectional study. J Crit Care. 2023;76 doi: 10.1016/j.jcrc.2023.154298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seymour C.W., Gomez H., Chang C.H., et al. Precision medicine for all? Challenges and opportunities for a precision medicine approach to critical illness. Crit Care. 2017;21(1):257. doi: 10.1186/s13054-017-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bauer SR, Gellatly RM and Erstad BL, Precision fluid and vasoactive drug therapy for critically ill patients [published online ahead of print January 6, 2023]. Pharmacotherapy, 2023, 10.1002/phar.2763. [DOI] [PMC free article] [PubMed]
- 73.Stanski N.L., Wong H.R. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol. 2020;16(1):20–31. doi: 10.1038/s41581-019-0199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leligdowicz A., Harhay M.O., Calfee C.S. Immune modulation in sepsis, ARDS, and Covid-19: the road traveled and the road ahead. NEJM Evid. 2022;1(11) doi: 10.1056/EVIDra2200118. [DOI] [PubMed] [Google Scholar]
- 75.Hammond D.A., Rech M.A., Daley M.J., et al. Perceptions regarding vasopressin use and practices in septic shock, and cost containment strategies. J Am Coll Clin Pharm. 2019;2(3):257–267. doi: 10.1002/jac5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bitton E., Zimmerman S., Azevedo L.C.P., et al. An international survey of adherence to Surviving Sepsis Campaign Guidelines 2016 regarding fluid resuscitation and vasopressors in the initial management of septic shock. J Crit Care. 2022;68:144–154. doi: 10.1016/j.jcrc.2021.11.016. [DOI] [PubMed] [Google Scholar]
- 77.Hsu J.L., Liu V., Patterson A.J., Martin G.S., Nicolls M.R., Russell J.A. Potential for overuse of corticosteroids and vasopressin in septic shock. Crit Care. 2012;16(5):447. doi: 10.1186/cc11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.National Institutes of Health Clinical Center. Hypotension in the weaning from vasopressor drugs. NCT05506319. ClinicalTrials.gov. National Institutes of Health; 2022. Updated August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT05506319
- 79.National Institutes of Health Clinical Center. Efficacy and safety of vasopressin versus terlipressin as a second vasopressor in critically ill cirrhotics with septic shock- the VITEL-C trial. NCT005315557. ClinicalTrials.gov. National Institutes of Health; 2022. Updated April 7, 2022. https://clinicaltrials.gov/ct2/show/NCT005315557
- 80.Hernan M.A. Methods of public health research—strengthening causal inference from observational data. N Engl J Med. 2021;385(15):1345–1348. doi: 10.1056/NEJMp2113319. [DOI] [PubMed] [Google Scholar]