Abstract
Spinal anesthesia (SA) is gaining recognition as a safe and efficacious regional alternative to general anesthesia for elective lumbar surgery. However, unfamiliarity with management issues related to its use has limited the adoption of awake spine surgery, despite its benefits. Few centers in the United States routinely offer SA for elective lumbar surgery, and a comprehensive workflow to standardize SA for lumbar surgery is lacking. In this article, we examine recent literature on the use of SA in lumbar surgery, review the experience of our institution with SA in lumbar surgery, and provide a cohesive outline to streamline the implementation of SA from the perspective of the anesthesiologist. We review the critical features of SA in contemporary lumbar surgery, including selection of patients, methods of SA, intraoperative sedation, and management of several important technical considerations. We aimed to flatten the learning curve to improve the availability and accessibility of the technique for eligible patients.
Keywords: Intrathecal injections, Spinal anesthesia, Spine
INTRODUCTION
A significant increase in lumbar surgery utilization, especially in older adults, has been observed over the last two decades [1]. As increasingly elderly and ill patients progressively undergo more procedures, corresponding efforts have been made to optimize perioperative protocols to reduce complications and expedite recovery. The Enhanced Recovery After Surgery (ERAS) protocol for spinal surgery recommends various protocols, including the use of regional anesthesia when possible [2]. Additionally, the American Society of Anesthesiologists concludes that "Patient outcomes may be improved when regional anesthetic techniques are made available and accessible to all patients equitably” [3]. Spinal anesthesia (SA) fulfills several ERAS goals since, compared to general anesthesia, it is a regional technique that reduces time spent in the operating room and pain scores, increases early ambulation, and decreases perioperative polypharmacy [4,5]. Postoperative urinary retention (POUR) is generally more common with SA; however, a lower incidence of POUR has been shown when SA is specifically used in spine surgery [6,7]. Additionally, older patients who undergo non-cardiac surgery experience high rates of postoperative cognitive dysfunction but this complication is demonstrably reduced when SA is used in orthopedic surgery [8,9]. Although the benefits of SA are well described in literature on obstetric and joint arthroplasty, SA remains infrequently used for patients undergoing lumbar surgery. The delayed adoption of SA in lumbar surgery is likely related to physician unfamiliarity and concerns regarding the technical aspects of the modality when applied to the lumbar spine. A study has suggested that the learning curve for adopting SA in neurosurgical spine procedures becomes less daunting when both the anesthesia and surgical teams are informed of the most common clinical considerations for the technique [10]. Therefore, this study aimed to review the current strategies, considerations, and complications related to administering SA in lumbar surgery, in an effort to encourage its use in capable surgical centers.
SPINAL ANESTHESIA
Injection of local anesthetics into the subarachnoid space generally provides sufficient analgesia and motor blockade for common lumbar procedures. A review of spinal anesthetics has detailed various agents, including bupivacaine as the most common agent, and their use for procedures lasting longer than 60 min [11,12]. There are several formulations of intrathecal bupivacaine, but the 0.5% formulation is the most common because higher concentrations may precipitate when exposed to the pH level and ionic concentrations of the cerebrospinal fluid (CSF) [13]. The average dose of intrathecal bupivacaine used for non-cesarean surgery is reported to be 15 mg; therefore, 15 mg of 0.5% isobaric bupivacaine is most commonly used to induce neuraxial anesthesia [14]. However, it should be noted that several dosing algorithms for spinal bupivacaine exist, commonly based on height and weight; therefore, a range of spinal doses has been reported for spine surgery specifically (Table 1) [15-18]. A target sensory block of T6 is common, as this level generally provides sufficient anesthesia for surgery while reducing the risk of high SA [19]. The risk factors for an inadequate initial dose of SA are currently being investigated and may be related to the size of the lumbar cistern, among other anatomic considerations. However, preliminary results have been conflicting [20]. Dose-adjusting algorithms based on these factors are also being developed to prevent inadequate dosing of SA. Baricity of the anesthetic is a major determinant of diffusion within the CSF [21,22]. Although isobaric formulations are most commonly utilized, hyperbaric formulations are preferred by some practitioners. Several large meta-analyses have compared isobaric and hyperbaric bupivacaine in supine procedures, suggesting that the failure and complication rates between the solutions are comparable [20,21]. It must be noted, however, that lumbar surgeries are typically performed with the patient in a prone position. Positioning is an important consideration as studies of intrathecal fluid dynamics have affirmed that it affects the sensory block level when injecting hyper- or isobaric solutions of bupivacaine [23]. Moreover, a study has shown that maneuvering patients from the supine to lateral to prone position after injecting isobaric bupivacaine can raise the block by two to three levels [24]. Therefore, the effects of baricity in lumbar surgery may differ from those in supine procedures. We postulated that, in the prone position, the arch of normal lumbar lordosis is the lowest point of the CSF-filled spinal canal. Although not yet specifically evaluated, isobaric solutions of bupivacaine are generally preferred because the injected volume of the anesthetic will theoretically remain close to the injected level, rather than sinking in the lumbar cistern, as with hyperbaric solutions, or traveling rostrally, as with hypobaric solutions. Other considerations include the volume of the injected anesthetic and rate of injection. The volume of injected bupivacaine does not greatly influence intrathecal spread, and the speed of injection does not affect post-injection diffusion [25,26]. In summary, current evidence suggests that 10–15 mg of 0.5% bupivacaine is the optimal dose for SA and that isobaric solutions provide the most predictable block for lumbar spine surgery specifically. Patient-derived formulas and algorithms for adjusting intrathecal bupivacaine dosing based on the volume of the lumbar cistern and other anatomical characteristics are currently being developed.
Table 1.
Recent Articles on Utilization of Spinal Anesthesia for Lumbar Surgery
| Study | Procedure type | SA induction | SA maintenance | Other medications | Complications | Operative time | Patient satisfaction |
|---|---|---|---|---|---|---|---|
| De Cassai et al. [16] | Laminectomy and discectomy | 2–4 ml hyperbaric bupivacaine ± fentanyl | NR | Meperidine and morphine | Higher PONV rates with GEA | NR | Patients prefer SA; surgeons prefer GEA |
| Perez-Roman et al. [17] | Laminectomy and discectomy | 3–4 ml hyper/isobaric bupivacaine or ropivacaine ± fentanyl | Some light sedation with propofol ± midazolam ± fentanyl | NR | Overall lower complication rates with SA | 14 min shorter with SA | Lower VAS scores with SA |
| Waguia et al. [18] | Laminectomy and discectomy | 12.5–15 mg isobaric bupivacaine | Propofol titrated to effect | Preoperative acetaminophen, dexamethasone, and meloxicam | Overall lower complication rates with SA | NR | Higher patient satisfaction with SA |
SA: spinal anesthesia, NR: not reported, PONV: postoperative nausea and vomiting, GEA: general endotracheal anesthesia, VAS: visual analog scale.
PATIENT SELECTION
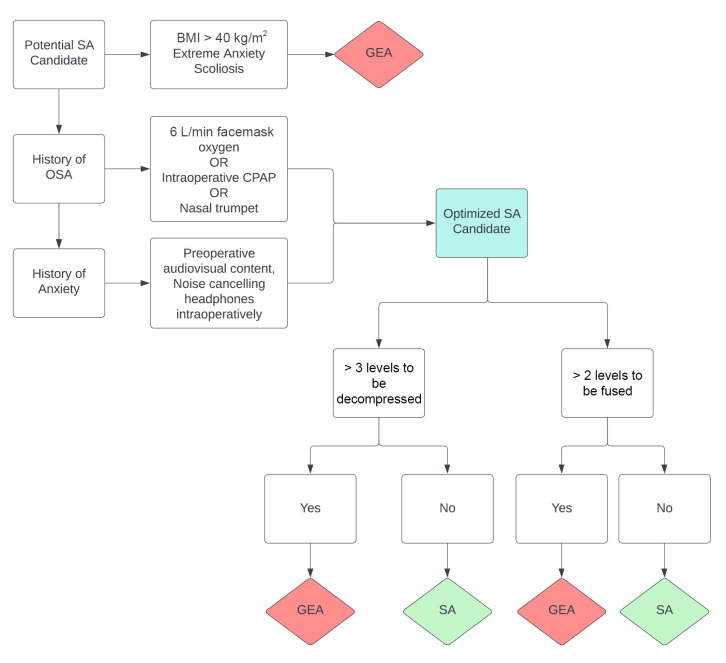
The practicality of utilizing SA for lumbar surgery should be assessed during the initial surgical consultation. Management of patient expectations and concordance between patient and physician expectations regarding surgical outcomes in lumbar surgery are directly related to patient satisfaction [27]. Therefore, a thorough discussion about the risks and benefits of SA and the anticipated patient experience is vital. An algorithm that was developed to guide the selection of eligible patients has suggested that certain demographic and surgical characteristics may preclude the use of SA for lumbar surgery. These include a history of scoliosis, anxiety, high body mass index (BMI), history of obstructive sleep apnea (OSA), ≥ 3 levels to be fused, and ≥ 4 levels to be decompressed (Fig. 1) [28,29]. Uncorrected or corrected scoliosis may complicate the initiation and spread of SA, with high complication rates associated with several methods of neuraxial anesthesia [29]. The relationship between a long-standing history of anxiety and the feasibility of SA in lumbar surgery has not been systematically explored; however, a prospective multicenter study has suggested that preoperative anxiety regarding SA is reduced when audiovisual content from the Internet is provided to the patient in addition to resources directly provided by the surgeon and anesthesiologist. Comprehensive discussion, which involves the provision of multimedia educational resources, between the patient and anesthesiologist is especially helpful for patients with preoperative anxiety. In one case, noise-cancelling headphones were shown to improve the patient experience with SA, albeit without demonstrating a significant pre- or postoperative anxiolytic effect [30]. Regarding obesity, a report has shown the feasibility of SA in patients with a body mass index > 30 kg/m2; however, the feasibility of a successful spinal injection should be left to the discretion of the anesthesiologist [31]. Meanwhile, a randomized trial has explored the efficacy of continuous positive airway pressure (CPAP) in high-risk OSA patients who underwent SA for urologic procedures and showed that CPAP may lower the incidence of apnea–hypopnea events during sedation with propofol but does not increase hemodynamic stability compared to simple facemask oxygenation [31]. Concern for airway and hemodynamic stability is an obvious consideration for the anesthesia team; therefore, applying a facemask with 6 L of oxygen per min or CPAP during the procedure may improve clinical confidence in providing SA to patients with OSA. Consequently, these management strategies may facilitate the optimization of SA in patients who may not have been candidates for SA because of precluding comorbidities (Fig. 1). The number of surgical levels to be fused or decompressed remains a surgeon-dependent metric of SA eligibility. In general, the anticipated operative time should be < 3 h, and the number of surgical levels that can be completed within this time constraint is largely at the discretion of the primary surgeon (Fig. 1). In summary, many concerns regarding patient eligibility for SA for lumbar surgery may be appropriately managed, and these supportive measures may increase confidence in selecting patients for SA and managing associated comorbidities.
Fig. 1.
Patient selection and optimization algorithm for SA. SA: spinal anesthesia, BMI: body mass index, GEA: general endotracheal anesthesia, OSA: obstructive sleep apnea, CPAP: continuous positive airway pressure.
INTRAOPERATIVE SEDATION
Although not strictly necessary, most patients who undergo lumbar surgery under SA opt for intraprocedural intravenous (IV) sedation. The most common sedatives used for SA are midazolam, propofol, and dexmedetomidine [33]. Among these, dexmedetomidine has multiple benefits in the setting of monitored SA, including reduced tachycardia, hypotension, and respiratory depression. It may also be titrated to a level of sedation that facilitates waking of patients to allow their cooperation [34,35]. Moreover, dexmedetomidine has been associated with a lower incidence of postoperative delirium in patients under SA than propofol, as demonstrated in a randomized controlled trial and matched-cohort study [36,37]. Compared to placebo, dexmedetomidine may also be used to deepen the sensory and motor blocks of SA without increasing postoperative sedation [38]. Comfortable sedation using dexmedetomidine in procedures involving SA is achieved with a maintenance infusion of 0.5 mcg/kg/h, and initial boluses of dexmedetomidine or midazolam do not improve sedation when administered at the beginning of the surgery [39]. Intravenous sedation should not be initiated until anesthesia of the surgical area is confirmed, typically by the surgical team prior to incision. A single maintenance infusion of dexmedetomidine is typically sufficient to provide adequate sedation for most patients undergoing SA. Meanwhile, a recent cohort study has suggested that remimazolam may provide moderate sedation in cases of SA and also causes decreased respiratory depression in the prone position [40,41]. We advocate for strong communication between the patient and provider during the procedure, with fine adjustments in dexmedetomidine infusion titration rather than the administration of benzodiazepine, narcotic, or propofol boluses to deepen sedation when patients express discomfort.
CONSIDERATIONS FOR PRONE POSITIONING
Unlike obstetric and orthopedic procedures performed with the patient under SA, lumbar surgery generally requires prone positioning. Here, we discuss three important considerations in the management and care of neurosurgical patients undergoing lumbar surgery in the prone position with the use of SA.
Airway management in the prone position
Appropriate intraoperative airway management is imperative in ensuring a safe and effective surgical procedure. In our experience with more than 400 cases of SA for lumbar surgery, our institution has not acutely lost an airway, which may be supported by the precautions outlined here. Nevertheless, careful contingency planning is crucial, given the life-threatening nature of intraprocedural airway loss. Mild to moderate airway obstruction during sedation, often seen in high-risk airways (e.g., patients with OSA), may be successfully managed with a nasal trumpet if there is heightened concern for airway loss (Fig. 1) [42]. For all SA cases, the OR should always be equipped with a supraglottic device, such as a laryngeal mask airway (LMA) [43,44]. The LMA is a suitable rescue airway for patients who experience acute airway loss while in the prone position, and it can facilitate fiberoptic intubation [45-47]. In general, maintenance of a high-risk airway should be performed using either CPAP or an oxygen facemask during the procedure. Additionally, a nasal trumpet should be considered for patients with signs of airway obstruction, an LMA should be readily available, and the operating room should be equipped with a fiberoptic bronchoscope, if necessary.
Patient movement and agitation in the operating room
Careful patient selection, patient education on positioning, communication with the anesthesia team, and thorough discussion regarding the expectations with SA are known to reduce anxiety and increase patient satisfaction [48]. Although IV midazolam is commonly used as a preoperative anxiolytic, its routine use may increase the risk of postoperative cognitive dysfunction and dementia in older adults [49]. Preoperative midazolam may also limit the ability of the patient to confirm adequate analgesia before the procedure begins and hinder cooperation of the patient with the anesthesia and surgical teams. We continue to emphasize the significance of communication between the anesthesia provider and the patient, which typically alleviates feelings of anxiety or discomfort, thereby reducing unnecessary administration of additional sedatives. Following the injection of SA, we advocate for a minimum 10–15 min waiting period for the intrathecal anesthetic to reach its full effect before administering analgesic or hypnotic medications.
Operative considerations
Frequently cited operative concerns regarding SA include the impact of unintended durotomy, use of intraoperative neuromonitoring, use of intraoperative computed tomography (CT), and intraoperative loss of spinal block if the surgery lasts longer than anticipated. Intraoperative durotomy does not appear to affect the efficacy of SA. Comparative studies have shown that the risk of durotomy is not significantly different between patients under SA and GEA [50]. Moreover, for cases in which durotomy has occurred, SA failure has not been reported [50]. Contemporary spinal surgeons frequently utilize intraoperative neuromonitoring, which is a potential barrier to SA use. However, triggered electromyography, a common modality, has been found to remain feasible and efficacious for patients under SA during pedicle screw placement [51]. Intraoperative three-dimensional CT, which is also frequently performed in contemporary spine surgery, has likewise been shown to be feasible under SA [52]. Lastly, intraoperative loss of effect is possible, particularly if the procedure continues longer than intended. However, IV ketamine infusion may be useful in such situations. When injected in combination with a spinal anesthetic, ketamine acts as an N-methyl-d-aspartate receptor antagonist and acts on calcium channels and opioid monoamine receptors to potentiate the effects of intrathecal bupivacaine [53]. A randomized trial has shown that preoperative IV ketamine serves as a useful adjunct to reduce postoperative pain and opioid consumption in patients under SA [54]. IV ketamine infusion may be used synergistically with intrathecal bupivacaine if the initial spinal dose is patchy or if the dose begins to decline during the surgery [50].
Inadequate initial dosing of SA significantly interferes with the surgical workflow and may dissuade surgeons from transitioning to SA. This problem may be associated with a younger patient age and the size of the lumbar cistern, among other features; however, studies on this topic are still in progress [55].
CONCLUSION
Regional anesthesia is generally recommended when possible, and SA is a safe and effective regional anesthetic technique for lumbar surgery. Prone positioning and the requirement for close injection of the anesthetic agent to the surgical area are unique considerations that entail communication and contingency planning between the surgical and anesthesia teams. Here, we presented a review of current practices and provided recommendations to facilitate the adoption of SA for elective lumbar spine surgery.
Footnotes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
This manuscript did not utilize datasets, or contain public datasets. Data sharing is not applicable.
AUTHOR CONTRIBUTIONS
Conceptualization: Nicholas S. Hernandez, Ron I. Riesenburger, James T. Kryzanski, Penny Liu.
Data curation: Nicholas S. Hernandez. Formal analysis: Nicholas S. Hernandez. Methodology: Ron I. Riesenburger, James T. Kryzanski, Penny Liu. Project administration: Ron I. Riesenburger, James T. Kryzanski, Penny Liu. Writing - original draft: Nicholas S. Hernandez, Benayas Begashaw. Writing - review & editing: Nicholas S. Hernandez, Benayas Begashaw, Ron I. Riesenburger, James T. Kryzanski, Penny Liu. Supervision: Ron I. Riesenburger, James T. Kryzanski, Penny Liu.
REFERENCES
- 1.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–65. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz N, Sharma M, Adams S, Alhourani A, Ugiliweneza B, Wang D, et al. Enhanced recovery after surgery (ERAS) for spine surgery: a systematic review. World Neurosurg. 2019;130:415–26. doi: 10.1016/j.wneu.2019.06.181. [DOI] [PubMed] [Google Scholar]
- 3. Hernandez NS, Rogers JL, Pham MH. Brachioradial pruritus caused by cervical disc herniation precipitated by trauma treated with anterior cervical discectomy and fusion: report of two cases and review of the literature. Asian J Neurosurg 2003. doi: 10.1055/s-0043-1772760. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 4.De Biase G, Gruenbaum SE, West JL, Chen S, Bojaxhi E, Kryzanski J, et al. Spinal versus general anesthesia for minimally invasive transforaminal lumbar interbody fusion: implications on operating room time, pain, and ambulation. Neurosurg Focus. 2021;51:E3. doi: 10.3171/2021.9.FOCUS21265. [DOI] [PubMed] [Google Scholar]
- 5.Olmos M, Hernandez NS, Kanter M, Liu P, Riesenburger RI, Kryzanski J. Periprocedural polypharmacy in lumbar fusions performed under spinal anesthesia compared with general anesthesia. Neurosurgery. 2023;92:632–8. doi: 10.1227/neu.0000000000002259. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez NS, Wang AY, Kanter M, Olmos M, Ahsan T, Liu P, et al. Assessing the impact of spinal versus general anesthesia on postoperative urinary retention in elective spinal surgery patients. Clin Neurol Neurosurg. 2022;222:107454. doi: 10.1016/j.clineuro.2022.107454. [DOI] [PubMed] [Google Scholar]
- 7.Brouwer TA, Van Roon EN, Rosier P, Kalkman CJ, Veeger N. Postoperative urinary retention: risk factors, bladder filling rate and time to catheterization: an observational study as part of a randomized controlled trial. Perioper Med (Lond) 2021;10:2. doi: 10.1186/s13741-020-00167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monk TG, Weldon BC, Garvan CW, Dede DE, Van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein SM, Poultsides L, Baaklini LR, Mörwald EE, Cozowicz C, Saleh JN, et al. Postoperative delirium in total knee and hip arthroplasty patients: a study of perioperative modifiable risk factors. Br J Anaesth. 2018;120:999–1008. doi: 10.1016/j.bja.2017.12.046. [DOI] [PubMed] [Google Scholar]
- 10.West JL, De Biase G, Bydon M, Bojaxhi E, Mendhi M, Quiñones-Hinojosa A, et al. What is the learning curve for lumbar spine surgery under spinal anesthesia? World Neurosurg. 2022;158:e310–e6. doi: 10.1016/j.wneu.2021.10.172. [DOI] [PubMed] [Google Scholar]
- 11.Stewart J, Gasanova I, Joshi GP. Spinal anesthesia for ambulatory surgery: current controversies and concerns. Curr Opin Anaesthesiol. 2020;33:746–52. doi: 10.1097/ACO.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 12.Rattenberry W, Hertling A, Erskine R. Spinal anaesthesia for ambulatory surgery. BJA Educ. 2019;19:321–8. doi: 10.1016/j.bjae.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolte H, Schikor K, Gergs P, Meyer J, Stark P. [On spinal anaesthesia with isobaric bupivacaine 0,5% (author's transl)] Anaesthesist. 1977;26:33–7. German. [PubMed] [Google Scholar]
- 14.Uppal V, Retter S, Shanthanna H, Prabhakar C, McKeen DM. Hyperbaric versus isobaric bupivacaine for spinal anesthesia: systematic review and meta-analysis for adult patients undergoing noncesarean delivery surgery. Anesth Analg. 2017;125:1627–37. doi: 10.1213/ANE.0000000000002254. [DOI] [PubMed] [Google Scholar]
- 15.Schnider TW, Minto CF, Bruckert H, Mandema JW. Population pharmacodynamic modeling and covariate detection for central neural blockade. Anesthesiology. 1996;85:502–12. doi: 10.1097/00000542-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 16.De Cassai A, Geraldini F, Boscolo A, Pasin L, Pettenuzzo T, Persona P, et al. General Anesthesia Compared to Spinal Anesthesia for Patients Undergoing Lumbar Vertebral Surgery: A Meta-Analysis of Randomized Controlled Trials. J Clin Med. 2020;10:102. doi: 10.3390/jcm10010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Roman RJ, Govindarajan V, Bryant JP, Wang MY. Spinal anesthesia in awake surgical procedures of the lumbar spine: a systematic review and meta-analysis of 3709 patients. Neurosurg Focus. 2021;51:E7. doi: 10.3171/2021.9.FOCUS21464. [DOI] [PubMed] [Google Scholar]
- 18.Waguia R, Touko EK, Sykes DAW, Kelly-Hedrick M, Hijji FY, Sharan AD, et al. How to start an awake spine program: Protocol and illustrative cases. IBRO Neurosci Rep. 2022;13:69–77. doi: 10.1016/j.ibneur.2022.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, He L, Ni JX. Level of sensory block after spinal anesthesia as a predictor of hypotension in parturient. Medicine (Baltimore) 2017;96:e7184. doi: 10.1097/MD.0000000000007184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Białowolska K, Horosz B, Sękowska A, Malec-Milewska M. Fixed dose versus height-adjusted conventional dose of intrathecal hyperbaric bupivacaine for caesarean delivery: a prospective, double-blinded randomised trial. J Clin Med. 2020;9:3600. doi: 10.3390/jcm9113600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sng BL, Han NLR, Leong WL, Sultana R, Siddiqui FJ, Assam PN, et al. Hyperbaric vs. isobaric bupivacaine for spinal anaesthesia for elective caesarean section: a Cochrane systematic review. Anaesthesia. 2018;73:499–511. doi: 10.1111/anae.14084. [DOI] [PubMed] [Google Scholar]
- 22.Helmi M, Uyun Y, Suwondo BS, Widodo U. Comparison of intrathecal use of isobaric and hyperbaric bupivacaine during lower abdomen surgery. Journal of Anesthesiology. 2014;2014:141324. [Google Scholar]
- 23.Tecklenburg-Weier E, Quest F, Nolte H, Meyer J. [The effect of patient positioning on the spread of sensory blockade in hyperbaric and isobaric spinal anesthesia using bupivacaine] Reg Anaesth. 1990;13:163–7. German. [PubMed] [Google Scholar]
- 24.Russell IF. Posture and isobaric subarachnoid anaesthesia. The influence on spread of spinal anaesthesia with 'isobaric' 0.5% bupivacaine plain. Anaesthesia. 1984;39:865–7. doi: 10.1111/j.1365-2044.1984.tb06571.x. [DOI] [PubMed] [Google Scholar]
- 25.Hocking G, Wildsmith JA. Intrathecal drug spread. Br J Anaesth. 2004;93:568–78. doi: 10.1093/bja/aeh204. [DOI] [PubMed] [Google Scholar]
- 26.Stienstra R, Van Poorten F. Speed of injection does not affect subarachnoid distribution of plain bupivacaine 0.5% Reg Anesth. 1990;15:208–10. [PubMed] [Google Scholar]
- 27.Mancuso CA, Duculan R, Cammisa FP, Sama AA, Hughes AP, Lebl DR, et al. Concordance Between Patients' and Surgeons' Expectations of Lumbar Surgery. Spine (Phila Pa 1976) 2021;46:249–58. doi: 10.1097/BRS.0000000000003775. [DOI] [PubMed] [Google Scholar]
- 28.Letchuman V, Agarwal N, Mummaneni VP, Wang MY, Shabani S, Patel A, et al. Awake spinal surgery: simplifying the learning curve with a patient selection algorithm. Neurosurg Focus. 2021;51:E2. doi: 10.3171/2021.9.FOCUS21433. [DOI] [PubMed] [Google Scholar]
- 29.Ko JY, Leffert LR. Clinical implications of neuraxial anesthesia in the parturient with scoliosis. Anesth Analg. 2009;109:1930–4. doi: 10.1213/ANE.0b013e3181bc3584. [DOI] [PubMed] [Google Scholar]
- 30.Tharion JG, Kale S. Patient satisfaction through an immersive experience using a Mobile Phone-Based Head-Mounted Display During Arthroscopic Knee Surgery Under spinal anesthesia: a randomized clinical trial. Anesth Analg. 2021;133:940–8. doi: 10.1213/ANE.0000000000005666. [DOI] [PubMed] [Google Scholar]
- 31.Lamon AM, Einhorn LM, Cooter M, Habib AS. The impact of body mass index on the risk of high spinal block in parturients undergoing cesarean delivery: a retrospective cohort study. J Anesth. 2017;31:552–8. doi: 10.1007/s00540-017-2352-0. [DOI] [PubMed] [Google Scholar]
- 32.Lim H, Oh M, Chung YH, Ki H, Lee JJ. Effects of continuous positive airway pressure in patients at high risk of obstructive sleep apnea during propofol sedation after spinal anesthesia. J Clin Monit Comput. 2019;33:657–63. doi: 10.1007/s10877-018-0202-8. [DOI] [PubMed] [Google Scholar]
- 33.Practice guidelines for moderate procedural sedation and analgesia 2018: a report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology. 2018;28:437–79. doi: 10.1097/ALN.0000000000002043. [DOI] [PubMed] [Google Scholar]
- 34.Arain SR, Ebert TJ. The Efficacy, Side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–6. doi: 10.1097/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 35.Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY, et al. Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trial. Anesth Analg. 2010;110:47–56. doi: 10.1213/ane.0b013e3181ae0856. [DOI] [PubMed] [Google Scholar]
- 36.Shin HJ, Woo Nam S, Kim H, Yim S, Han SH, Hwang JW, et al. Postoperative delirium after dexmedetomidine versus propofol sedation in healthy older adults undergoing orthopedic lower limb surgery with spinal anesthesia: a randomized controlled trial. Anesthesiology. 2023;138:164–71. doi: 10.1097/ALN.0000000000004438. [DOI] [PubMed] [Google Scholar]
- 37.Park JW, Kim EK, Lee HT, Park S, Do SH. The effects of propofol or dexmedetomidine sedation on postoperative recovery in elderly patients receiving lower limb surgery under spinal anesthesia: a retrospective propensity score-matched analysis. J Clin Med. 2021;10:135. doi: 10.3390/jcm10010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdallah FW, Abrishami A, Brull R. The facilitatory effects of intravenous dexmedetomidine on the duration of spinal anesthesia: a systematic review and meta-analysis. Anesth Analg. 2013;117:271–8. doi: 10.1213/ANE.0b013e318290c566. [DOI] [PubMed] [Google Scholar]
- 39.Jeong J, Jin SH, Kim DY, Cho S, Lee H, Han JI. Effects of various methods of dexmedetomidine administration for sedation in elderly patients undergoing spinal anesthesia: a randomized controlled study. Anesth Pain Med (Seoul) 2020;15:297–304. doi: 10.17085/apm.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao TY, Chen D, Sun H, Xu ZX, Lyu S, Wang T, et al. Moderate sedation with single-dose remimazolam tosilate in elderly male patients undergoing transurethral resection of the prostate with spinal anesthesia: a prospective, single-arm, single-centre clinical trial. BMC Anesthesiol. 2022;22:247. doi: 10.1186/s12871-022-01788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021;12:690875. doi: 10.3389/fphar.2021.690875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar AR, Guilleminault C, Certal V, Li D, Capasso R, Camacho M. Nasopharyngeal airway stenting devices for obstructive sleep apnoea: a systematic review and meta-analysis. J Laryngol Otol. 2015;129:2–10. doi: 10.1017/S0022215114003119. [DOI] [PubMed] [Google Scholar]
- 43.Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, et al. 2022 American Society of Anesthesiologists Practice Guidelines for management of the fifficult airway. Anesthesiology. 2022;136:31–81. doi: 10.1097/ALN.0000000000004002. [DOI] [PubMed] [Google Scholar]
- 44.Olsen KS, Petersen JT, Pedersen NA, Rovsing L. Self-positioning followed by induction of anaesthesia and insertion of a laryngeal mask airway versus endotracheal intubation and subsequent positioning for spinal surgery in the prone position: a randomised clinical trial. Eur J Anaesthesiol. 2014;31:259–65. doi: 10.1097/EJA.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 45.Abrishami A, Zilberman P, Chung F. Brief review: airway rescue with insertion of laryngeal mask airway devices with patients in the prone position. Can J Anaesth. 2010;57:1014–20. doi: 10.1007/s12630-010-9378-1. [DOI] [PubMed] [Google Scholar]
- 46.Hung MH, Fan SZ, Lin CP, Hsu YC, Shih PY, Lee TS. Emergency airway management with fiberoptic intubation in the prone position with a fixed flexed neck. Anesth Analg. 2008;107:1704–6. doi: 10.1213/ane.0b013e3181831e2e. [DOI] [PubMed] [Google Scholar]
- 47.Kramer DC, Lo JC, Gilad R, Jenkins A. Fiberoptic scope as a rescue device in an anesthetized patient in the prone position. Anesth Analg. 2007;105:890. doi: 10.1213/01.ane.0000269690.05759.eb. [DOI] [PubMed] [Google Scholar]
- 48.Southerland WA, Tollinche LE, Shapiro FE. Decision aids: the role of the patient in perioperative safety. Int Anesthesiol Clin. 2019;57:4–11. doi: 10.1097/AIA.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcantonio ER, Juarez G, Goldman L, Mangione CM, Ludwig LE, Lind L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272:1518–22. [PubMed] [Google Scholar]
- 50.Breton JM, Ludwig CG, Yang MJ, Nail TJ, Riesenburger RI, Liu P, et al. Spinal anesthesia in contemporary and complex lumbar spine surgery: experience with 343 cases. J Neurosurg Spine. 2022;36:534–41. doi: 10.3171/2021.7.SPINE21847. [DOI] [PubMed] [Google Scholar]
- 51.Kanter M, Hernandez NS, Olmos M, Karimi H, Riesenburger RI, Kryzanski JT. Intraoperative triggered electromyography for pedicle screw placement under spinal anesthesia: a preliminary report. Oper Neurosurg (Hagerstown) 2023;24:651–5. doi: 10.1227/ons.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 52.Yang MJ, Riesenburger RI, Kryzanski JT. The use of intra-operative navigation during complex lumbar spine surgery under spinal anesthesia. Clin Neurol Neurosurg. 2022;215:107186. doi: 10.1016/j.clineuro.2022.107186. [DOI] [PubMed] [Google Scholar]
- 53.Kathirvel S, Sadhasivam S, Saxena A, Kannan TR, Ganjoo P. Effects of intrathecal ketamine added to bupivacaine for spinal anaesthesia. Anaesthesia. 2000;55:899–904. doi: 10.1046/j.1365-2044.2000.01472.x. [DOI] [PubMed] [Google Scholar]
- 54.Adhikari P, Subedi A, Sah BP, Pokharel K. Analgesic effects of intravenous ketamine after spinal anaesthesia for non-elective caesarean delivery: a randomised controlled trial. BMJ Open. 2021;11:e044168. doi: 10.1136/bmjopen-2020-044168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang AY, Olmos M, Ahsan T, Kanter M, Liu P, Balonov K, et al. A second prone dose algorithm for patients undergoing spinal anesthesia during thoracolumbar surgeries. Oper Neurosurg (Hagerstown) 2023;24:283–90. doi: 10.1227/ons.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]