Abstract
Background
Androgen deprivation therapy is the cornerstone of treatment for patients with advanced prostate cancer. Meta-analysis of small, oncology-focused trials suggest gonadotropin-releasing hormone (GnRH) antagonists may be associated with fewer adverse cardiovascular outcomes compared with GnRH agonists.
Objectives
This study sought to determine whether GnRH antagonists were associated with fewer major adverse cardiovascular events compared with GnRH agonists.
Methods
Electronic databases were searched for all prospective, randomized trials comparing GnRH antagonists with agonists. The primary outcome was a major adverse cardiovascular event as defined by the following standardized Medical Dictionary for Regulatory Activities terms: “myocardial infarction,” “central nervous system hemorrhages and cerebrovascular conditions,” and all-cause mortality. Bayesian meta-analysis models with random effects were fitted.
Results
A total of 11 eligible studies of a maximum duration of 3 to 36 months (median = 12 months) enrolling 4,248 participants were included. Only 1 trial used a blinded, adjudicated event process, whereas potential bias persisted in all trials given their open-label design. A total of 152 patients with primary outcome events were observed, 76 of 2,655 (2.9%) in GnRH antagonist-treated participants and 76 of 1,593 (4.8%) in agonist-treated individuals. Compared with GnRH agonists, the pooled OR of GnRH antagonists for the primary endpoint was 0.57 (95% credible interval: 0.37-0.86) and 0.58 (95% credible interval: 0.32-1.08) for all-cause death.
Conclusions
Despite the addition of the largest, dedicated cardiovascular outcome trial, the volume and quality of available data to definitively answer this question remain suboptimal. Notwithstanding these limitations, the available data suggest that GnRH antagonists are associated with fewer cardiovascular events, and possibly mortality, compared with GnRH agonists.
Key Words: androgen deprivation, gonadotropin-releasing hormone antagonist, major adverse cardiovascular event, prostate cancer
Central Illustration
Cardiovascular (CV) disease is very common among men with prostate cancer and represents the leading cause of non–cancer-related death.1,2 Androgen deprivation therapy (ADT) is the cornerstone of treatment for men with advanced prostate cancer, although the extent to which it contributes to excess CV events remains controversial.3 Emerging evidence suggests that the mode of ADT may differentially impact CV,4,5 although the clinical trials to date have been unable to address this definitively.
The most contemporary meta-analyses have shown that the use of a gonadotropin-releasing hormone (GnRH) antagonist in patients with prostate cancer planned for ADT is associated with a reduced likelihood of CV events compared with the use of a GnRH agonist.6,7 However, none of the included trials in those analyses were specifically designed to answer the question of relative cardiovascular safety. Recently, the largest dedicated CV endpoint trial,8 PRONOUNCE (A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease), was terminated early because of low event rates and slower than anticipated enrollment.9
The aims of this study were to determine whether the impact of additional events from the PRONOUNCE trial affected the prevailing notion of GnRH antagonist protection and whether this varied across a spectrum of CV events with harmonized definitions and among patients with established CV disease in whom a differential CV effect may be most marked.
Methods
This systematic review was preregistered in PROSPERO (CRD42021242591) and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.
Literature search
The electronic databases (MEDLINE, Web of Science, Cochrane Library, and Scopus) were searched in August 2021 (and later updated in October 2022) for eligible studies. Initial screening was performed based on study title and abstract followed by the full-text paper review by 2 reviewers (A.N. and S.T.) independently. Disagreements were resolved by consensus. The following search terms were used: (degarelix OR relugolix OR GnRH antagonist OR LHRH [luteinizing hormone-releasing hormone] antagonist OR leuprorelin OR goserelin OR GnRH agonist OR LHRH agonist) AND prostate cancer AND (randomized controlled trial OR RCT OR prospective study).
Inclusion/exclusion criteria
All randomized clinical trials in which a GnRH antagonist was compared with a GnRH agonist for the treatment of prostate cancer were included. Nonrandomized trials, phase 1, nonhuman, and observational studies were excluded. Trials in which survival outcomes were not reported or could not be obtained in both arms were excluded.
Data extraction
The following data were extracted: publication year, sample size at randomization and by study arm, maximum trial duration (and median duration where available), methods of randomization, blinding status, treatment strategy, dose and duration of treatment, outcome definitions, adjudication status, and the number of individual CV and all-cause mortality outcomes.
Outcomes
The primary outcome was the number of patients with major adverse cardiovascular events (MACEs) as defined by the following standardized Medical Dictionary for Regulatory Activities (MedDRA) headings: “myocardial infarction,” “central nervous system hemorrhages and cerebrovascular conditions,” and all-cause mortality (Supplemental Table 1). This outcome was chosen because it was a consistent, prespecified analysis in the major trials (HERO [A Study to Evaluate the Safety and Efficacy of Relugolix in Men With Advanced Prostate Cancer] and PRONOUNCE) or could be derived from existing data sources. The secondary outcome was all-cause mortality.
Outcomes data were obtained from all publicly available sources with the following hierarchy: manuscripts, abstract presentations, and clinicaltrials.gov. Outcomes reported in the primary analysis were obtained from peer-reviewed sources (manuscripts and abstracts). When rates of the primary outcome were not specifically reported, MedDRA adverse event tables from clinicaltrials.gov were used. Source data provenance is listed in Supplemental Table 2.
Risk of bias
Two reviewers independently assessed the risk of bias of each individual study. An evaluation of the risk of bias of the included studies was performed according to the Cochrane handbook.10 Selection, performance, detection, attrition, reporting bias, and other potential sources of bias were assessed as “yes,” “no,” or “unclear” in each of the included trials.
Statistical methods
Bayesian meta-analysis models using logistic regression with random effects were fitted to estimate odds ratios of having an outcome between GnRH antagonists and GnRH agonists.11 Bayesian meta-analysis was performed to enable studies with zero events to contribute to the odds ratio estimation without data adjustment.11 Random effects were allowed to account for heterogeneity across studies. Between-study heterogeneity was measured as the SD of the log OR across studies denoted by The ORs and the associated 95% credible intervals (CrIs) are presented in forest plots.
Two additional analyses were conducted. As a sensitivity analysis, a meta-analysis of the primary endpoint was conducted without the most recent PRONOUNCE data to assess the incremental impact on the prevailing results. A trial-level subgroup analysis was also performed among patients with a history of established CV disease.
Results
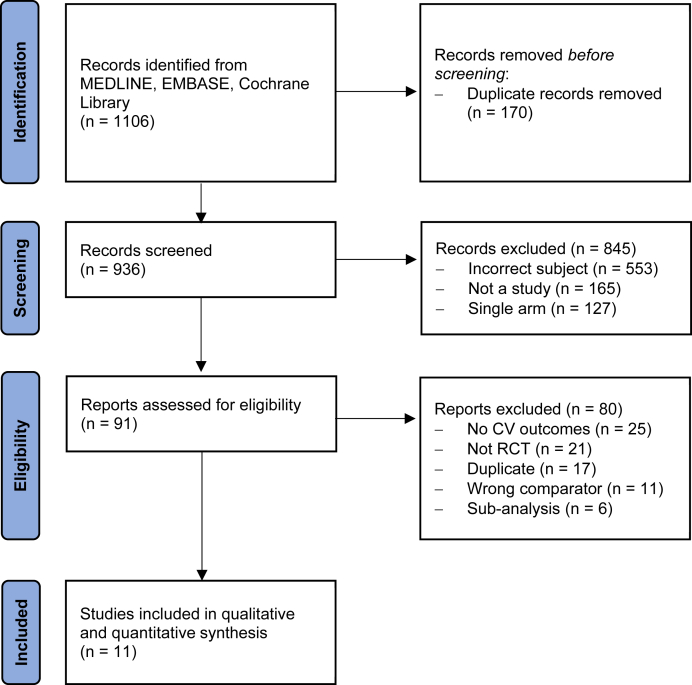
The last search was updated in October 2022. After the removal of duplicates, the search yielded 936 records. After screening titles and abstracts, we retrieved the full text of 91 articles. Of these, we excluded 80 articles for reasons outlined in Figure 1, and 11 studies were included in the meta-analysis. Data on outcome events were extracted from peer-reviewed literature for 4 trials and from clinicaltrials.gov for 7 trials (Supplemental Table 2).
Figure 1.
Included Studies in the Systematic Review
Preferred Reporting Items for Systematic Reviews and Meta-Analysis diagram representing the flow of the included studies. CV = cardiovascular; RCT = randomized controlled trial.
Study characteristics
Studies were conducted between 2006 and 2021 and published between 2008 and 2021 with 5 from Europe, 2 from North America, 1 from Asia, 1 from the Middle East, and 2 multinational (Table 1). All trials ran to completion with the exception of CS35A, which had an open-label extension that was terminated prematurely because of an insufficient number of patients enrolling, and PRONOUNCE because of lower than anticipated event numbers. All studies were open-label.
Table 1.
Characteristics of the Included Studies
| First Author | Trial Name | ClinicalTrials.gov ID Code | Study | Publication | Max Duration | Primary Endpoint | Antagonist | Agonist | Antagonist Sample |
Agonist Sample | Metastatic Disease (%) | Baseline CVD (%) | Median Age (y) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Klotz22,23 | CS21 | NCT00295750 | 2006-2007 | 2008 | 12 | Percentage of patients with testosterone level ≤0.5 ng/mL | Degarelix | Leuprolide | 409 | 201 | 20.0 | 32.1 | 73.0 |
| Anderson24 | CS28 | NCT00831233 | 2009-2010 | 2013 | 3 | Change from baseline in IPSS | Degarelix | Goserelin | 27 | 13 | 35.0 | NR | 70.0 |
| Mason25 | CS30 | NCT00833248 | 2009-2011 | 2013 | 3 | Change from baseline in prostate size | Degarelix | Goserelin | 180 | 64 | Excluded | NR | 70.7 |
| Axcrona26 | CS31 | NCT00884273 | 2009-2011 | 2012 | 3 | Change from baseline in prostate size | Degarelix | Goserelin | 84 | 98 | 29.6 | NR | 72.0 |
| Shorea | CS35A | NCT01242748 | 2009-2011 | 2015 | 13-36 | Percentage of patients with testosterone level ≤0.5 ng/mL | Degarelix | Goserelin | 565 | 282 | NR | NR | 71.6 |
| Higano27 | CS37 | NCT00928434 | 2009-2012 | 2015 | 14 | Percentage of patients with PSA level ≤4 ng/mL | Degarelix | Leuprolide | 225 | 178 | Excluded | NR | 72.0 |
| Ozono28 | Ozono | NCT01964170 | 2013-2016 | 2018 | 12 | Cumulative probability of patients with testosterone level ≤0.5 ng/mL | Degarelix | Goserelin | 117 | 117 | 18.8 | NR | 75.7 |
| Saad29 | C27002 | NCT02083185 | 2014-2017 | 2018 | 12 | Percentage of patients with testosterone level ≤0.5 ng/mL | Relugolix | Leuprolide | 110 | 24 | NR | NR | 70.9 |
| Margel30 | Margel | NCT02475057 | 2015-2019 | 2019 | 12 | Endothelial function | Degarelix | Any | 41 | 39 | 26.3 | 100 | 71.5 |
| Shore5 | HERO | NCT03085095 | 2017-2020 | 2020 | 12 | Cumulative probability of testosterone level ≤0.5 ng/mL | Relugolix | Leuprolide | 622 | 308 | 31.7 | 13.9 | 71.0 |
| Lopes9 | PRONOUNCE | NCT02663908 | 2016-2021 | 2021 | 12 | Time to first occurrence of adjudicated MACE (all-cause death, MI, or stroke) | Degarelix | Leuprolide | 275 | 269 | 20.4 | 100 | 73.0 |
CVD = cardiovascular disease; HERO = A Study to Evaluate the Safety and Efficacy of Relugolix in Men With Advanced Prostate Cancer; IPSS = International Prostate Symptom Score; MACE = major adverse cardiovascular event; MI = myocardial infarction; NR = not reported; PRONOUNCE = A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease.
Data abstracted from www.clinicaltrials.gov.
Nine studies evaluated degarelix (degarelix treated = 1,923) as the GnRH antagonist, whereas 2 studies evaluated relugolix (relugolix treated = 732). The GnRH agonist arms were goserelin in 5 trials (goserelin treated = 574), leuprolide in 5 trials (n = 980), and 1 trial did not specify a particular compound (n = 39). The median duration of the included trials was 12 months with 3 trials of a 3-month duration and 2 trials longer than 12 months. All trials had prostate cancer–focused primary outcomes except for the Margel study, which evaluated endothelial function (a marker of vascular function), and the PRONOUNCE trial, which reported a blinded and centrally adjudicated composite. The rates of the primary outcome for our analysis were reported as a prespecified safety analysis in HERO and as a secondary analysis of PRONOUNCE (unlike the adjudicated PRONOUNCE primary outcome, the secondary analysis used the same adverse event definition as HERO, censored at end of treatment [day 336]) and Margel. For all other studies, adverse event reporting tables were used.
Risk of bias
Given the varying modes of administration of these agents, blinding is challenging; therefore, all trials were open-label, introducing a potential source of bias (Supplemental Figure 1). Although many of the trials used an objective biochemical primary outcome (such as testosterone levels), the use of an adverse event CV endpoint that may not have been systematically or consistently collected is prone to ascertainment bias at the level of the patient and investigator. Although this is less likely to be the case in the Margel and HERO trials, which prespecified CV endpoints, and in PRONOUNCE whose primary endpoint was adjudicated MACEs, even a blinded committee can only mitigate variability in outcome assessment but cannot overcome upstream ascertainment bias. An assessment of incomplete outcome data is problematic because none of these other trials were designed for CV safety assessment. The Egger’s test was suggestive of potential publication (P = 0.04) bias (Supplemental Figure 2).
Patient characteristics
The trial median ages ranged from 70 to 75.7 years. The proportion of patients with metastatic prostate cancer ranged from 0% to 61.5% with 2 trials excluding these patients (CS30 and CS37) and 2 trials not reporting this characteristic (C27002 and CS35A). Traditional CV factor profiles were reported in 4 trials; an arrhythmia-focused adverse event report from CS21 included details on CV comorbidities. HERO presented them in aggregate with varying definitions of lifestyle and CV risk factors, whereas Margel and PRONOUNCE elicited these directly from a case report form. In Margel and PRONOUNCE, approximately half of the patients reported current or past cigarette smoking (53.8% [43/80] in Margel and 53.9% [293/544] in PRONOUNCE), which was significantly lower in CS21 at 13.0% (79/610). Approximately one-third of the patients in Margel (31.3%, 25/80) and PRONOUNCE (32.2%, 175/544) had diabetes, although this was not reported in CS21. Hypertension was prevalent in PRONOUNCE at 85.8% (467/544) followed by 72.5% in Margel (58/80) and 52.9% (323/610) in CS21. The rates of baseline-established CV disease were reported using standardized MedDRA terms of “myocardial infarction” and “CNS hemorrhages and cerebrovascular conditions” in HERO, revealing 13.9% (129/930). In the CV safety report from CS21, 32.1% of patients were reported as having baseline atherosclerotic disease (196/610), whereas both the Margel and PRONOUNCE studies enrolled only patients with prior myocardial infarction, stroke, or peripheral artery disease. Baseline CV medication use was only reported in Margel and PRONOUNCE. Lipid-lowering therapy was prescribed in 84.2% (458/544) of patients in PRONOUNCE and statins in 72.5% (58/80) in Margel; antiplatelet therapy was not reported in PRONOUNCE, whereas it was reported in 71.3% (57/80) of patients in Margel; and beta-blocker use was 68.8% (374/544) in PRONOUNCE and 41.3% (33/80) in Margel.
Primary and secondary outcomes
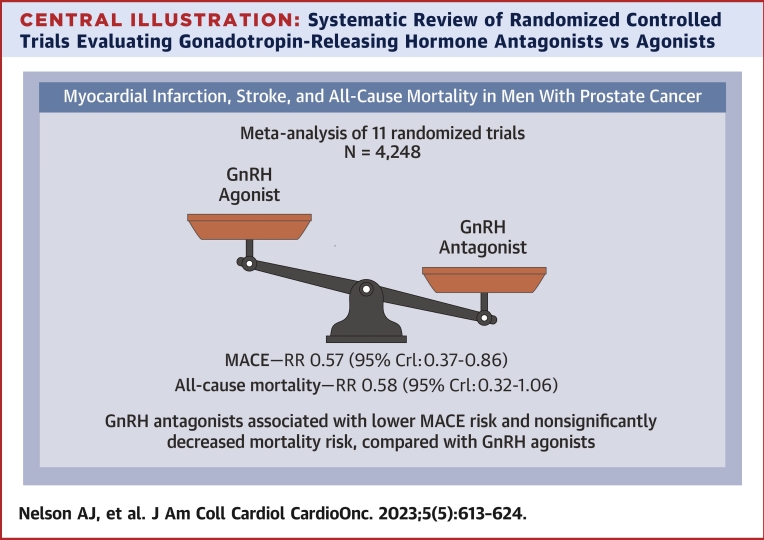
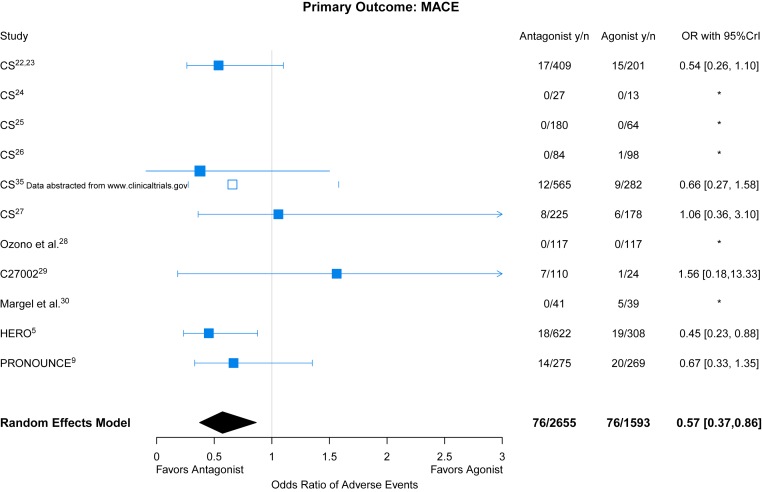
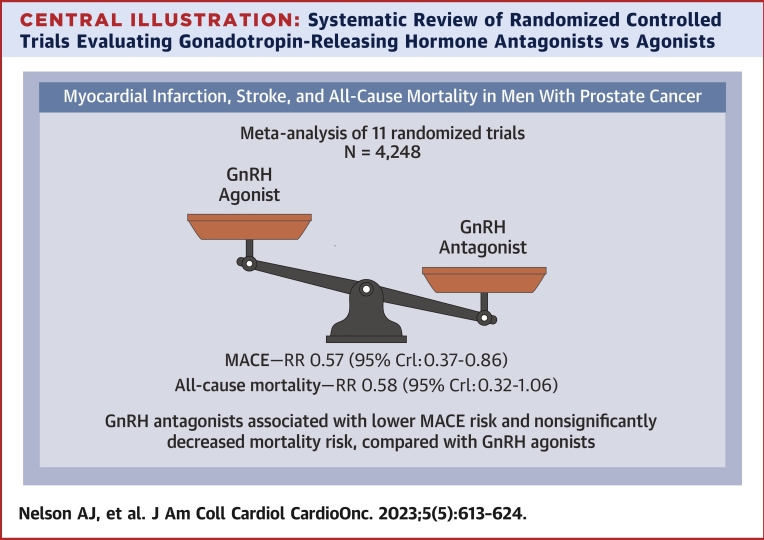
Among a total of 4,248 included patients, there were 152 patients with total primary CV outcome events (3.6%): 76 patients with events in the 2,655 GnRH antagonist-treated patients (2.9%) and 76 patients with events in the 1,593 agonist-treated patients (4.8%). These events included the following: CV, 28.9%; myocardial infarction, 26.3%; and death, 44.7%. Three studies did not have events in either treatment arm (CS28, CS30, and Ozono), and 2 studies (CS31 and Margel) did not have events in 1 of the treatment arms, but they contribute to pooled estimates in the Bayesian meta-analysis. Comparing the effect of GnRH antagonists with agonists for the primary outcome, the pooled OR was 0.57 (95% CrI: 0.37-0.86) (Figure 2, Central Illustration). Moderate between-study heterogeneity was estimated ( = 0.32).
Figure 2.
GnRH Antagonist vs Agonist and MACE
Forest plot of ORs and corresponding 95% credible interval (CrI) for gonadotropin-releasing hormone (GnRH) antagonists and major adverse cardiovascular event (MACE) compared with GnRH agonists. HERO = A Study to Evaluate the Safety and Efficacy of Relugolix in Men With Advanced Prostate Cancer; PRONOUNCE = A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease.
Central Illustration.
Systematic Review of Randomized Controlled Trials Evaluating Gonadotropin-Releasing Hormone Antagonists vs Agonists
Included randomized controlled trials evaluating gonadotropin-releasing hormone (GnRH) antagonists and agonists among patients with prostate cancer. Forest plot of odds ratios comparing GnRH antagonists vs agonists for major adverse cardiovascular events (MACEs). CrI = credible interval; RR = relative risk.
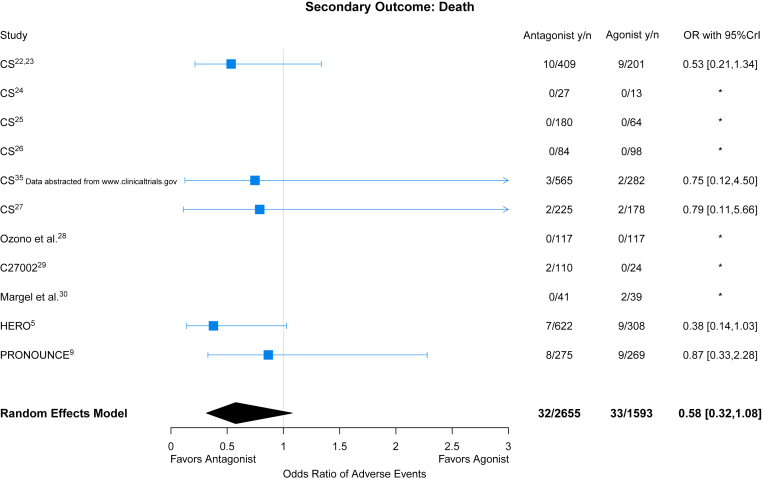
With respect to all-cause mortality, 6 studies recorded deaths during follow-up with fewer deaths reported in GnRH antagonist–treated patients compared with agonist-treated patients (OR: 0.58; 95% CrI: 0.32-1.08) (Figure 3). Between-study heterogeneity was moderate with an estimated of 0.38 for all-cause mortality.
Figure 3.
GnRH Antagonist vs Agonist on All-Cause Mortality
Forest plot of ORs and corresponding 95% CrI for GnRH antagonists and all-cause mortality when compared with GnRH agonists. Abbreviations as in Figure 2.
Sensitivity and subgroup analyses
In the sensitivity analysis without PRONOUNCE, comparing the effect of GnRH antagonists with agonists for the primary outcome, the pooled OR was 0.55 (95% CrI: 0.29-0.94) with between-study heterogeneity of 0.40 (Supplemental Figure 3). For all-cause mortality, the pooled OR was 0.51 (95% CrI: 0.24-1.14) with between-study heterogeneity of 0.42 (Supplemental Figure 4).
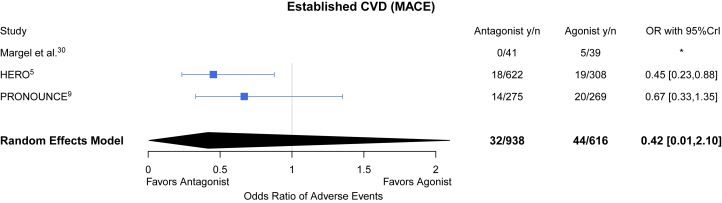
With respect to the subgroup of patients with established atherosclerotic disease, although CS21 reported the proportion of patients with underlying myocardial infarction or stroke, a breakdown of event rates by this diagnosis was not reported. Thus, among the group of patients with established atherosclerotic CV disease comprising 13% from HERO and all patients from the Margel and PRONOUNCE trials, there were 76 patients with events among 1,554 patients (4.9%). Among those with established atherosclerotic CV disease, the pooled estimate for the primary outcome was OR was 0.42 (95% CrI: 0.01-2.10) for GnRH antagonists compared with agonists (Figure 4). The between-study heterogeneity was 0.82.
Figure 4.
GnRH Antagonist vs Agonist on MACE Among Those With CVD
Forest plot of ORs and corresponding 95% CrI for GnRH antagonists and MACE when compared with GnRH agonists. CVD = cardiovascular disease; other abbreviations as in Figure 2.
Discussion
The major findings from this systematic review and meta-analysis are as follows: 1) despite being individually underpowered, the findings of PRONOUNCE remain within the prevailing estimates from prior evidence and thus do not significantly impact the overall finding that GnRH antagonists are associated with fewer CV sequelae compared with GnRH agonists, although it does provide a degree of further confidence in the finding; 2) although this analysis contributes an additional 30% more events, the CrIs remain broad and the moderate between-study heterogeneity implies persistent and considerable uncertainty about the magnitude of differential benefit between these classes; and 3) despite the enrollment of close to 4,500 patients in over a decade of trials, there is insufficient high-quality, randomized evidence to definitively answer the question of whether or not GnRH antagonist therapy is superior to GnRH agonist therapy in terms of CV safety, particularly among those with established CV disease.
Several lines of evidence suggest that GnRH antagonists may have preferable CV safety compared with GnRH agonists; whether this reflects antagonist benefit and agonist neutrality or antagonist neutrality and agonist harm is unclear. Compared with agonists, GnRH antagonists do not cause an initial surge in testosterone or follicle-stimulating hormone, with injection of the former having been uniquely associated with short-term risk of acute CV and cerebrovascular events in older adult men following receipt.12 GnRH antagonists are generally associated with greater suppression of follicle-stimulating hormone release, which may attenuate the development of the metabolic syndrome, insulin resistance, and atherosclerotic plaque formation.3,13 Finally, GnRH agonists could activate GnRH receptors located on proinflammatory T cells within an atherosclerotic plaque, which may result in cytokine production and macrophage stimulation, causing plaque destabilization.14 For this reason, we hypothesized that those with underlying CV disease may be at particular risk for an adverse event, and, thus, any differential effect between antagonists and agonists may be magnified. Unfortunately, despite the addition of PRONOUNCE and the totality of events favoring antagonist benefit, CrIs evaluating this subgroup remained broad (thus underpowered), so a conclusion cannot be drawn.
Despite varying primary outcome definitions, our results are consistent with other meta-analyses that reported relative risk (RR) reductions for antagonists compared with agonists for adverse CV events of RR = 0.57 (95% CI: 0.39-0.81),7 RR = 0.52 (95% CI: 0.34-0.80),6 and HR = 0.44 (95% CI: 0.24-0.74).4 Data from observational studies are conflicting, with some suggesting significance (HR: 0.76; 95% CI: 0.60-0.95)15 and in some cases profound associated benefits of antagonists compared with agonists (RR: 0.39; 95% CI: 0.19-0.80),16 whereas others have suggested the reverse17 or even neutrality.18 These differences in results likely reflect incomplete adjustment for CV factors and treatment status as well as confounding by indication.
Low event rates mean the observed 43% RR reduction translates to an absolute risk difference of 1.9% between arms and a number needed to treat of 53 to prevent 1 MACE. Although this represents a modest absolute effect size, longer treatment exposure and higher baseline CV risk among patients in real-world clinical practice may amplify this treatment effect. Furthermore, a strong trend toward a mortality benefit from antagonists compared with agonists was observed, as has been reported elsewhere. However, without cause-specific and adjudicated mortality, it is difficult to know whether this represents CV impact, a competing oncologic signal, or a combination of the two.
The juxtaposition of PRONOUNCE against the existing trials comparing GnRH agents highlights the challenges of aggregating studies that were not designed to assess CV safety and the ongoing knowledge gaps that exist in the field of cardio-oncology.19,20 Without preceding awareness of the potential CV signal, it is impossible to know how systematically CV adverse events were collected in oncology-focused trials and to what degree these were subject to ascertainment bias, significantly limiting the certainty of any conclusions we can make. Furthermore, oncology trials understandably did not specifically seek to characterize underlying CV risk factors nor did they focus on collecting CV concomitant medications; thus, our understanding of these patients and their CV risk and care patterns is largely unknown. Given that the span of these trials is now close to 15 years, it is conceivable that any differential safety profile of agonists compared with antagonists may have been modified by iterative treatment of CV risk among these patients. Indeed, this may be the case with PRONOUNCE in which a cardiologist was required to have reviewed each trial participant and is likely to have contributed to the higher rates of CV medication use compared with contemporary real-world data sets and may have differentially attenuated the CV event rate in the agonist arm.16, 17, 18 Unfortunately, there were insufficient events in the earlier trials to perform a subgroup analysis by period of trial conduct that may have been potentially informative in this regard.
The repurposing of oncology trial data for CV adverse event ascertainment is particularly problematic when considering trial duration. Almost all trials in this study were of 3 to 12 months’ duration but, given the potential atherosclerotic-mediated mechanisms of these GnRH agents, are not likely to be fit for purpose when one considers trials of at least 2 to 3 years are generally required to demonstrate treatment effects on atherosclerotic clinical endpoints. This is consistent with a real-world analysis from a pharmacovigilance database in which the median time from initiation of GnRH modulating therapy to an adverse CV event was 541 days.21 With aggregated evidence and a prevailing narrative that a differential effect between GnRH agonists and antagonists exists, many would consider there to be a perceived lack of equipoise to facilitate randomization of high CV risk patients to a GnRH agonist when an antagonist is now commercially and widely available. This experience offers yet another reminder that there is only a narrow window to perform high-quality, randomized studies before the development of secular trends and observational data that will subsequently inform clinical practice. Given the operational difficulties faced by PRONOUNCE in completing a large-scale, cardiovascular outcome trial, the growing field of cardio-oncology needs to continue working on defining and harmonizing key CV data elements and events in order to more rigorously determine comparative safety profiles of investigational agents in both labeling and postmarketing studies as well as registry-based clinical trials.1 With increased cancer survivorship, a more granular understanding of competing CV risk and treatment is becoming an integral component of contemporary oncologic care, and this must be reflected in clinical trial conduct. A regulatory requirement for newly approved cancer drugs to have a randomized long-term assessment of CV safety could help.
Study limitations
The results from our analysis need to be considered in the context of several limitations. Our analysis is trial level; thus, the evaluation of subgroups was limited to only those studies that reported event rates by the population of interest. In this context, pooling of participant-level data would facilitate a more robust assessment of subgroups and also allow aggregation of data from trials that only reported events in 1 treatment arm, which were excluded from our analysis. The open-label nature of trials and the potential for incomplete (and nonstandardized) CV event ascertainment leaves multiple sources for bias. The potential for publication bias was also suggested in our analysis; however, the results of the Egger’s test should be interpreted with caution given the small number of included studies. The primary outcomes were derived from adverse event reporting; thus, there exists the potential for double counting of events. Because the median follow-up for CV events was not available for all studies, a Bayesian approach was undertaken reporting ORs rather than HRs. Heterogenous and ill-defined follow-up also meant a comparison of timing of events between arms was not possible; thus, the hypothesis that early events may disproportionally occur with agonists vs antagonists remains untested and deserving of further study.
Conclusions
The available randomized controlled trial data suggest GnRH antagonist use may be associated with fewer CV events compared with GnRH agonists, although the amount and quality of data to definitively answer this question remain elusive. A better understanding of the different biological interactions of these agents might help clarify this question.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: CV endpoints were not prospectively acquired in most prostate cancer trials, thereby limiting definitive conclusions regarding relative CV safety of GnRH antagonists and agonists. Within the limits of available data, GnRH antagonists appear to have a more favorable CV profile compared with GnRH agonists among patients treated with ADT for prostate cancer.
TRANSLATIONAL OUTLOOK: The field of cardio-oncology must advocate for harmonized CV data elements and event definitions in oncology trials to enable rigorous evaluation of CV safety.
Funding Support and Author Disclosures
Dr Nelson has received grant support paid to his institution; and has received consulting fees from Bayer, Boehringer Ingelheim, AstraZeneca, and Lilly. Dr Lopes has received grant support paid to his institution; and has received consulting fees from Bayer, Bristol Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, Sanofi, Boehringer Ingelheim, Daiichi Sankyo, Merck, and Portola. Dr Slovin has received honoraria for Advisory Boards/educational programs from Novartis, Janssen, Pfizer, Merck, Sanofi-Aventis, Clovis, and Physician Education Resource; and has received research funding from Sanofi-Aventis, Novartis, Poseida Pharma, and Immunomedex. Dr Nilsson has received research grant support (eg, Steering Committee or Data and Safety Monitoring Committee) and speaker/consulting honoraria (eg, Advisory Boards) from Ferring Pharmaceuticals, AstraZeneca, Coegin AB, and Follicum AB. Dr Bhatt has served on the Advisory Boards of AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; has served on the Board of Directors of AngioWave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, and TobeSoft; has served as Chair for American Heart Association Quality Oversight Committee; has been a consultant for Broadview Ventures; has served on Data Monitoring Committees of Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute, and Rutgers University (for the National Institutes of Health-funded MINT Trial); has received honoraria from American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor, Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); has served as Deputy Editor for Clinical Cardiology; has served as Chair for NCDR-ACTION Registry Steering Committee and VA CART Research and Publications Committee; holds a patent for sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent); has received research funding from Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89Bio; has received royalties from Elsevier (Editor, Braunwald’s Heart Disease); has served as Site Co-Investigator for Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, and Vascular Solutions; is a trustee for American College of Cardiology; and has completed unfunded research for FlowCo, and Takeda. Dr Goodman has received research grant support (eg, Steering Committee or Data and Safety Monitoring Committee) and speaker/consulting honoraria (eg, Advisory Boards) from Ferring Pharmaceuticals, Tolmar Pharmaceuticals, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi-Sankyo/American Regent, Eli Lilly, Esperion, GlaxoSmithKline, HLS Therapeutics, JAMP Pharma, Janssen/Johnson and Johnson, Merck, Novartis, Novo Nordisk A/C, Pendopharm, Pfizer, Regeneron, Sanofi, Servier, and Valeo Pharma; and has received salary support/honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Center for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Center, PERFUSE Research Institute, and the TIMI Study Group (Brigham Health). Dr Evans has received research grants from Ferring, Astellas, Pfizer, and Janssen; and has received honoraria for speaking and consulting from Pfizer, Janssen, Astellas, and Myovant. Dr Clarke has received fees for consultation and lectures from AstraZeneca, Astrellas, Bayer, Janssen, Pfizer, and Novartis. Dr Shore has received fees from Amgen, Astellas, Bayer, Dendreon, Ferring, Genentech/Roche, Janssen, Medivation/Astellas, Myovant, Pfizer, and Tolmar. Dr Margel has received honoraria and research grants, paid to his institution, from Ferring Pharmaceuticals. Dr Tombal has received grants and fees from Amgen, Astellas, Bayer, Ferring, Janssen, and Sanofi-Genzyme. Dr Leong has received consulting/speaker fees from Myovant Sciences, Janssen Pharmaceuticals, Novartis, Tolmar, Sanofi, Pfizer, and Abbvie; and has served on the advisory board of Ferring. Dr Alexander has received institutional grant support from Boehringer Ingelheim, Bayer, Bristol-Myers Squibb, CryoLife, CSL Behring, Ferring, Glaxosmithkline, and Humacyte; and has received consulting fees/honoraria from AbbVie, Bayer, Bristol-Myers Squibb, CryoLife, CSL Behring, Pfizer, and Portola. Dr Higano has received grants from Aragon, Astellas, AstraZeneca, Clovis, Dendreon, eFFECTOR Therapeutics, Emergent, Ferring, Genentech, Hoffman-Laroche, Medivation, Pfizer, and Bayer; has received personal fees from AstraZeneca, Astellas, Genentech, Merck Sharp and Dohme, Myovant, Menarini, Tolmar, Vaccitech, and Verity; has received Data and Safety Monitoring Board membership fees from Advantagene, AstraZeneca, and Exelixis; and has received fees from Aptevo, Asana Aragon Pharma, Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Churchill Pharma, Clovis, Dendreon, eFFECTOR Therapeutics, Endocyte, Emergent BioSolutions, Ferring, Genentech, Hinova, Hoffman-Laroche, Janssen, Medivation, Myriad, Orion, and Pfizer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Vivek Narayan, MD, MSCE, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Leong D.P., Fradet V., Shayegan B., et al. Cardiovascular risk in men with prostate cancer: insights from the RADICAL PC study. J Urol. 2020;203:1109–1116. doi: 10.1097/JU.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 2.Sturgeon K.M., Deng L., Bluethmann S.M., et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melloni C., Nelson A. Effect of androgen deprivation therapy on metabolic complications and cardiovascular risk. J Cardiovasc Transl Res. 2020;13:451–462. doi: 10.1007/s12265-019-09942-w. [DOI] [PubMed] [Google Scholar]
- 4.Albertsen P.C., Klotz L., Tombal B., Grady J., Olesen T.K., Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565–573. doi: 10.1016/j.eururo.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Shore N.D., Saad F., Cookson M.S., et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 6.Abufaraj M., Iwata T., Kimura S., et al. Differential impact of gonadotropin-releasing hormone antagonist versus agonist on clinical safety and oncologic outcomes on patients with metastatic prostate cancer: a meta-analysis of randomized controlled trials. Eur Urol. 2021;79:44–53. doi: 10.1016/j.eururo.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Cirne F., Aghel N., Petropoulos J.A., et al. The cardiovascular effects of Gnrh antagonists in men with prostate cancer. Eur Heart J Cardiovasc Pharmacother. 2022;8(3):253–262. doi: 10.1093/ehjcvp/pvab005. [DOI] [PubMed] [Google Scholar]
- 8.Melloni C., Slovin S.F., Blemings A., et al. Cardiovascular safety of degarelix versus leuprolide for advanced prostate cancer: the PRONOUNCE trial study design. J Am Coll Cardiol CardioOnc. 2020;2:70–81. doi: 10.1016/j.jaccao.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes R.D., Higano C.S., Slovin S.F., et al. Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: the primary results of the PRONOUNCE randomized trial. Circulation. 2021;144:1295–1307. doi: 10.1161/CIRCULATIONAHA.121.056810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J.P.T., Thomas J., Chandler J., et al. Cochrane; 2022. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022)https://www.training.cochrane.org/handbook [Google Scholar]
- 11.Hong H., Wang C., Rosner G.L. Meta-analysis of rare adverse events in randomized clinical trials: Bayesian and frequentist methods. Clin Trials. 2021;18:3–16. doi: 10.1177/1740774520969136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Layton J.B., Li D., Meier C.R., Sharpless J.L., Sturmer T., Brookhart M.A. Injection testosterone and adverse cardiovascular events: a case-crossover analysis. Clin Endocrinol (Oxf) 2018;88:719–727. doi: 10.1111/cen.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford E.D., Schally A.V., Pinthus J.H., et al. The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol Oncol. 2017;35:183–191. doi: 10.1016/j.urolonc.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Tanriverdi F., Gonzalez-Martinez D., Hu Y., Kelestimur F., Bouloux P.M. GnRH-I and GnRH-II have differential modulatory effects on human peripheral blood mononuclear cell proliferation and interleukin-2 receptor gamma-chain mRNA expression in healthy males. Clin Exp Immunol. 2005;142:103–110. doi: 10.1111/j.1365-2249.2005.02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrone V., Degli Esposti L., Giacomini E., Veronesi C., Blini V., Oderda M. Cardiovascular risk profile in prostate cancer patients treated with GnRH agonists versus antagonists: an Italian real-world analysis. Ther Clin Risk Manag. 2020;16:393–401. doi: 10.2147/TCRM.S249208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davey P., Kirby M.G. Cardiovascular risk profiles of GnRH agonists and antagonists: real-world analysis from UK general practice. World J Urol. 2021;39:307–315. doi: 10.1007/s00345-020-03433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George G., Garmo H., Scailteux L.M., et al. Risk of cardiovascular disease following gonadotropin-releasing hormone agonists vs antagonists in prostate cancer: Real-world evidence from five databases. Int J Cancer. 2021;148:2203–2211. doi: 10.1002/ijc.33397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hupe M.C., Hammerer P., Ketz M., Kossack N., Colling C., Merseburger A.S. Retrospective analysis of patients with prostate cancer initiating GnRH agonists/antagonists therapy using a German claims database: epidemiological and patient outcomes. Front Oncol. 2018;8:543. doi: 10.3389/fonc.2018.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt D.L. Birth and maturation of cardio-oncology. J Am Coll Cardiol CardioOnc. 2019;1:114–116. doi: 10.1016/j.jaccao.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leiva O., AbdelHameid D., Connors J.M., Cannon C.P., Bhatt D.L. Common pathophysiology in cancer, atrial fibrillation, atherosclerosis, and thrombosis: JACC: CardioOncology state-of-the-art review. J Am Coll Cardiol CardioOnc. 2021;3:619–634. doi: 10.1016/j.jaccao.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cone E.B., Marchese M., Reese S.W., et al. Lower odds of cardiac events for gonadotrophin-releasing hormone antagonists versus agonists. BJU Int. 2020;126:9–10. doi: 10.1111/bju.15059. [DOI] [PubMed] [Google Scholar]
- 22.Klotz L., Boccon-Gibod L., Shore N.D., et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith M.R., Klotz L., Persson B.E., Olesen T.K., Wilde A.A. Cardiovascular safety of degarelix: results from a 12-month, comparative, randomized, open label, parallel group phase III trial in patients with prostate cancer. J Urol. 2010;184:2313–2319. doi: 10.1016/j.juro.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson J., Al-Ali G., Wirth M., et al. Degarelix versus goserelin (+ antiandrogen flare protection) in the relief of lower urinary tract symptoms secondary to prostate cancer: results from a phase IIIb study ( NCT00831233) Urol Int. 2013;90:321–328. doi: 10.1159/000345423. [DOI] [PubMed] [Google Scholar]
- 25.Mason M., Maldonado Pijoan X., Steidle C., et al. Neoadjuvant androgen deprivation therapy for prostate volume reduction, lower urinary tract symptom relief and quality of life improvement in men with intermediate- to high-risk prostate cancer: a randomised non-inferiority trial of degarelix versus goserelin plus bicalutamide. Clin Oncol (R Coll Radiol) 2013;25:190–196. doi: 10.1016/j.clon.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Axcrona K., Aaltomaa S., da Silva C.M., et al. Androgen deprivation therapy for volume reduction, lower urinary tract symptom relief and quality of life improvement in patients with prostate cancer: degarelix vs goserelin plus bicalutamide. BJU Int. 2012;110:1721–1728. doi: 10.1111/j.1464-410X.2012.11107.x. [DOI] [PubMed] [Google Scholar]
- 27.Higano C.S., Crawford E.D., Shore N.D., et al. Risk of cardiovascular events with degarelix versus leuprolide after biochemical relapse of prostate cancer: exploratory analysis of a randomized controlled trial. J Clin Oncol. 2015;33 151-151. [Google Scholar]
- 28.Ozono S., Tsukamoto T., Naito S., et al. Efficacy and safety of 3-month dosing regimen of degarelix in Japanese subjects with prostate cancer: a phase III study. Cancer Sci. 2018;109:1920–1929. doi: 10.1111/cas.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saad F., Bailen J.L., Pieczonka J.M., et al. Second interim analysis (IA2) results from a phase II trial of TAK-385, an oral GnRH antagonist, in prostate cancer patients (pts) J Clin Oncol. 2016;34 200-200. [Google Scholar]
- 30.Margel D., Peer A., Ber Y., et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199–1208. doi: 10.1097/JU.0000000000000384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.