Abstract
Background
Cancer treatment increases cardiovascular disease risk, but physical activity (PA) may prevent cardiovascular disease.
Objectives
This study examined whether greater PA was associated with better submaximal exercise capacity and cardiac function during cancer therapy.
Methods
Participants included 223 women with stage I to III breast cancer (BC) before and 3 months after undergoing treatment and 126 control participants. Leisure-time PA (LTPA) was reported using the Godin-Shephard LTPA questionnaire. Cardiac function was assessed by cardiac magnetic resonance. Submaximal exercise capacity was determined by 6-minute walk distance.
Results
BC participants reported similar baseline LTPA scores (24.7; 95% CI: 21.7-28.0) as control participants (29.4; 95% CI: 25.0-34.2). The BC group declined to 16.9 (95% CI: 14.4-19.6) at 3 months relative to 30.8 (95% CI: 26.2-35.8) in control participants. Among BC participants, more LTPA was related to better exercise capacity (β ± SE: 7.1 ± 1.6; 95% CI: 4.0-10.1) and left ventricular (LV) circumferential strain (−0.16 ± 0.07; 95% CI: −0.29 to −0.02). Increased LTPA over the 3 months was associated with decreased likelihood of treatment-induced cardiac dysfunction according to LV circumferential strain classifications (OR: 0.98; 95% CI: 0.97-0.998). BC participants reporting insufficient LTPA according to PA guidelines exhibited deteriorations in exercise capacity (adjusted mean difference ± SE: −29 ± 10 m; P = 0.029), LV end-systolic volume (5.8 ± 1.3 mL; P < 0.001), LV ejection fraction (−3.2% ± 0.8%; P = 0.002), and LV circumferential strain (2.5% ± 0.5%; P < 0.001), but BC participants meeting LTPA guidelines did not exhibit these adverse changes.
Conclusions
PA declined during BC therapy; however, PA participation was associated with attenuated declines in exercise capacity and cardiac function that are often observed in this population. (Understanding and Predicting Breast Cancer Events After Treatment [WF97415 UPBEAT]; NCT02791581)
Key Words: breast neoplasm, cardiotoxicity, heart function, lifestyle
Central Illustration
Substantial advancements in detection and treatment for women diagnosed with breast cancer (BC) have improved 5-year cancer-related survival to 90%.1 However, commonly used BC therapies, such as anthracycline-based chemotherapy (anthra-bC) and chest radiotherapy, have cardiotoxic side effects including injury to cardiac myocytes and other cells, increased oxidative stress, and reductions in left ventricular (LV) ejection fraction (LVEF), thereby accelerating the risk for cardiovascular (CV) disease.2, 3, 4, 5 CV events are now a leading cause of morbidity and mortality among women diagnosed with stage I to III BC.6,7 The improved survival rates of BC and growing number of BC survivors at increased risk of CV dysfunction has motivated the investigation of factors associated with the onset and progression of CV complications to inform intervention strategies for reducing CV events.
Engaging in regular physical activity (PA) participation may protect against CV complications in cancer populations. Strong evidence in noncancer populations demonstrates higher PA levels are associated with a lower CV disease risk and cancer incidence.8,9 In women with BC, higher levels of self-reported PA before, during, and after completing treatment are related to lower cancer and CV mortality risk.10 There is a need to investigate whether the cardioprotective benefits of PA may impact the onset and progression of CV complications in BC populations.
Often-observed decreases in exercise capacity and cardiac function in women treated for BC may be key factors contributing to increased CV disease risk.2,11, 12, 13 Although evidence suggests PA participation may improve exercise capacity and cardiac function,14 the few studies investigating these factors in cancer populations primarily take place after completion of cancer therapy and do not distinguish PA intensity level.15,16 One study reported modest, nonsignificant associations between higher self-reported PA and attenuated reductions of LVEF in women after completing BC therapy.16 In a 5-year prospective study, more physically active women had fewer symptoms of heart failure than physically inactive women following anthra-bC.15 Preclinical work also demonstrates cardioprotective benefits of exercise in BC models.11 It is hypothesized that PA participation may protect against CV complications. However, to better inform intervention efforts, there is a need to define longitudinal relationships between PA, exercise capacity, and cardiac function during BC treatment, and whether total activity or activity intensity are associated with these measures.
The primary aims of this study were to describe self-reported PA levels among noncancer control participants and women with BC at baseline and after 3 months of therapy, and determine whether these changes among women with BC were associated with submaximal exercise capacity and measures of cardiac function.
Methods
Population
Women enrolled in the UPBEAT (Understanding and Predicting Fatigue, Cardiovascular Decline, and Events After Breast Cancer; NCT02791581 study were included in this secondary analysis. UPBEAT is a multicenter prospective cohort of women diagnosed with stage I to III BC and is conducted through the Wake Forest National Cancer Institute Community Oncology Research Program (NCORP) Research Base under an National Cancer Institute CIRB-approved protocol. Study design and eligibility criteria were described previously.17 Briefly, inclusion criteria for both groups were ≥18 years old with independent ambulatory status. All eligible women with BC could identify family or friends as noncancer participants. Additional BC group inclusion criteria were a diagnosis of stage I to III BC, scheduled to receive cancer treatment, and an Eastern Cooperative Oncology Group performance status between 0 and 2. Exclusion criteria included contraindications for undergoing cardiovascular magnetic resonance (CMR) imaging, an LVEF <50% if previously assessed, current pregnancy or lactation, or uncontrolled metabolic or CV diseases. Women in the control group had no history of cancer, breast surgery, or chemotherapy. The current study includes data from baseline visits conducted before initiation of therapy and 3-month follow-up data.
Clinical Data Collection
BC stage was determined according to the 8th edition of the American Joint Committee on Cancer Staging Manual,18 and medical treatment data were retrieved from patients’ medical records. Participant medical history was ascertained by medical chart review to document diagnosis of type 2 diabetes, hypertension, hyperlipidemia, and coronary artery disease.
Physical Activity Assessment
The Godin-Shephard Leisure-Time Physical Activity Questionnaire assessed self-reported leisure-time PA (LTPA) participation.19,20 Ascertained measures included a continuous measure of total weekly LTPA (leisure score index units), and activity groups where participants were categorized as active (ie, meeting LTPA moderate-strenuous recommendations), moderately active, or insufficiently active. See the Supplemental Appendix for further details.
Submaximal Exercise Capacity Assessment
Participants’ submaximal exercise capacity was determined by completing a 6-minute walk test according to established guidelines21 and measuring the 6-minute walk distance (6MWD). The assessment was conducted on an indoor track or open corridor with no obstructions, and participants were instructed to cover as much distance as they could within six minutes at their own pace. All tests were directly observed by a trained study coordinator and distances recorded in meters.
Assessment of Cardiac Function
The primary measures of cardiac function included LVEF (%), LV end-systolic volume (LVESV, mL), LV end-diastolic volume (LVEDV, mL), and LV mean myocardial circumferential strain (LV circumferential strain, %). All participants underwent baseline assessments, but 3-month measures were assessed only in women with BC because no changes are expected for cardiac function within 3 months in healthy individuals without intervention. Participants underwent a noncontrast 10- to 15-minute rapid CMR examination following previously published methods.22, 23, 24, 25 The typical protocol settings for the real-time cardiac-triggered cine stacks included a 128 × 102 matrix, a 40 × 32-cm field of view, minimized echo time, maximized flip angle (45° to 70°), 8-mm slice thickness with 2-mm slice gap, 45-ms repetition time, and 22-ms temporal resolution. Each CMR assessment was analyzed in a core laboratory by CMR analysts blinded to patient identifiers, exam number, and prior exams. Previous work has demonstrated the high accuracy and reproducibility of CMR evaluations.23,26,27
Participants were categorized as having cancer therapy–related cardiac dysfunction (CTRCD) if they exhibited a decline in LVEF from baseline to 3 months of at least 10 percentage points to an LVEF below 50%.28 In addition, study participants were categorized as having subclinical LV dysfunction if they exhibited a 15% increase in LV circumferential strain over the study period.29
Statistical Analyses
Baseline characteristics are presented as mean ± SD for continuous variables or count (%) for categorical variables. The primary measures of leisure score index (ie, total weekly LTPA), exercise capacity, and cardiac function were modeled as continuous outcomes. The square root transformation of leisure score index was used to account for a non-normal distribution. LTPA was also categorized as active, moderately active, or insufficiently active.19 To estimate total weekly LTPA at study timepoints, a longitudinal linear mixed model was used with leisure score index as the outcome. Cancer status (BC vs healthy control), age, body mass index (BMI), and visit were included as main effects, and an interaction term between cancer status and visit tested for a difference in change over time between groups. A patient-level random effect was also included in the model. Differences in proportions of activity groups by visit were examined by chi-square tests. Effect size (d) was calculated to determine the magnitude of differences between groups at 3 months using Cohen's method following adjustment for age and BMI.
Separate longitudinal linear mixed-effects models were used to examine relationships between LTPA at baseline and 3 months with measures of CV health (6MWD) and cardiac size and function (LVESV, LVEDV, LVEF, and LV circumferential strain) among BC participants over the same time period. For each model, cancer stage, age, BMI, anthra-bC receipt (yes/no), and visit were included as main effects with a patient-level random effect. Interaction terms between total LTPA and activity groups with visit tested if change in LTPA measures over time was related to CV health outcomes. Interaction terms between cancer stage and visit, and anthracycline treatment and visit were also tested. Model results are presented as β estimate ± SE and 95% CI in the Results text and tables. For activity group analyses, post hoc contrasts tested for mean differences between the activity groups. Lastly, multiple logistic regression was used to test whether change in LTPA was associated with CTRCD/subclinical LV dysfunction, adjusting for age, BMI, cancer stage, anthracycline treatment, and baseline LV circumferential strain in corresponding analyses. Logistic regression results were reported as OR (95% CI). Statistical significance for main effects was based on a significance level of 0.05, and interaction effects were deemed significant and left in the model at 0.10. Analyses were conducted in JMP Pro 15 (SAS Institute).
Results
BC participants had a higher average age than control participants (P < 0.001) (Table 1). Race/ethnicity between groups was similar (P = 0.80) with White women constituting most of the cohort for the BC (77%) and control groups (80%). The average BMI for both groups was within the overweight category. Most women with BC were diagnosed with stage II BC (n = 115, 52%). Of 223 women in the BC group, 109 (49%) received anthra-bC. There were no differences between groups in the prevalence of any chronic comorbid conditions (all P > 0.05).
Table 1.
Baseline Demographic and Clinical Characteristics
| Breast Cancer (n = 223) | Control (n = 126) | P Value | |
|---|---|---|---|
| Age at baseline, y | 55.6 ± 10.9 | 50.6 ± 14.1 | <0.001 |
| Race | 0.73 | ||
| White | 172 (77) | 101 (80) | |
| Black | 38 (17) | 18 (14) | |
| Other, Asian, Native Hawaiian, or not reported | 13 (6) | 7 (6) | |
| Ethnicity | 0.80 | ||
| Hispanic or LatinX | 2 (1) | 2 (2) | |
| Stage at breast cancer diagnosis | |||
| I | 85 (38) | ||
| II | 115 (52) | ||
| III | 23 (10) | ||
| Anthracyclines | 109 (49) | ||
| Body mass index, kg/m2 | 29.4 ± 6.2 | 28.4 ± 6.8 | 0.19 |
| History of hypertension | 61 (31) | 18 (21) | 0.13 |
| History of hyperlipidemia | 66 (33) | 27 (31) | 0.57 |
| History of coronary artery disease | 1 (1) | 1 (1) | 0.67 |
| History of diabetes | 16 (8) | 5 (6) | 0.63 |
Values are the mean ± SD or n (%).
Medical history included 199 participants in the breast cancer group and 88 participants in the control group.
Comparisons of baseline and 3-month LTPA between BC and control groups
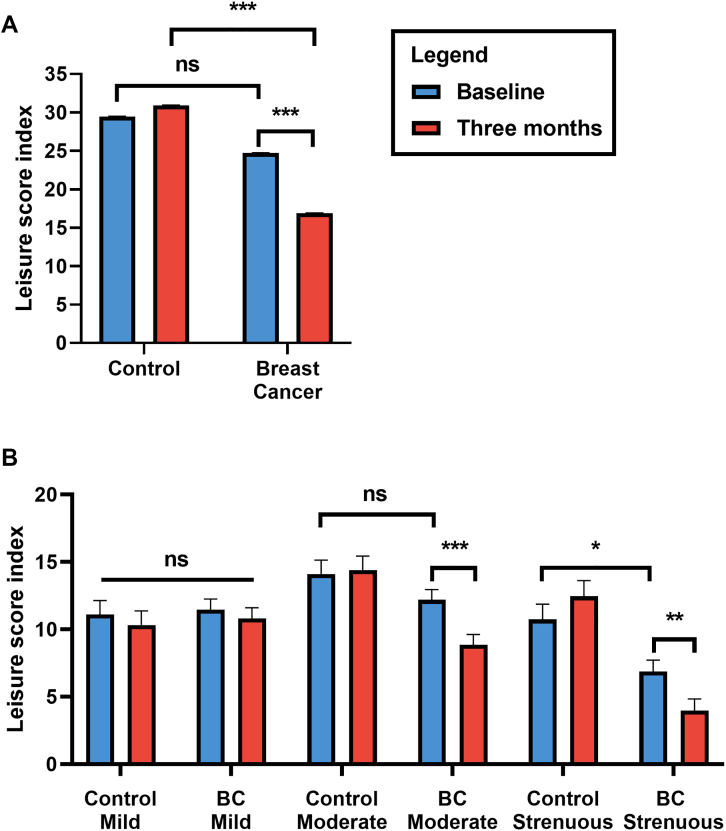
LTPA levels at baseline and 3-month data are shown in Figure 1. There was a statistically significant change in total LTPA over time by study group (β estimate ± SE: 0.25 ± 0.10; 95% CI: 0.12-0.39). At baseline, the BC and control groups reported similar total LTPA scores (24.7 ± 0.03 vs 29.4 ± 0.05; P = 0.16), but total LTPA scores declined to 16.9 ± 0.03 in the BC group vs 30.9 ± 0.05 in control participants at 3 months (P < 0.001; d = 0.53). BC participants reported similar levels of moderate activity at baseline compared to control participants (12.2 ± 0.7 vs 14.1 ± 1.0), but 3-month levels declined to 8.9 ± 0.8 (d = 0.47). BC participants reported lower strenuous LTPA levels at baseline than control participants (6.9 ± 0.9 vs 10.7 ± 1.1), and levels decreased by 3 months (4.0 ± 0.9 vs 12.5 ± 1.2; P < 0.001; d = 0.69). Control participants reported similar levels of all activity intensities at baseline and 3 months (all P > 0.05).
Figure 1.
LTPA Levels at Baseline and 3 Months
(A) The total weekly leisure time physical activity (LTPA), measured as the leisure score index (LSI), comparing control participants and breast cancer (BC). (B) Weekly LTPA separated by intensity (mild, moderate, strenuous) comparing control participants and BC participants. BC participants experienced a decline in LTPA at 3 months compared with baseline, specifically moderate-strenuous intensity levels, and compared with control participants with lower baseline strenuous-intensity LTPA in the BC group. Blue bars = baseline; red bars = 3 months. This indicates BC participants experienced reductions in LTPA at 3 months into treatment compared with control participants primarily due to moderate-strenuous LTPA declines. ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001. ns = non-significant.
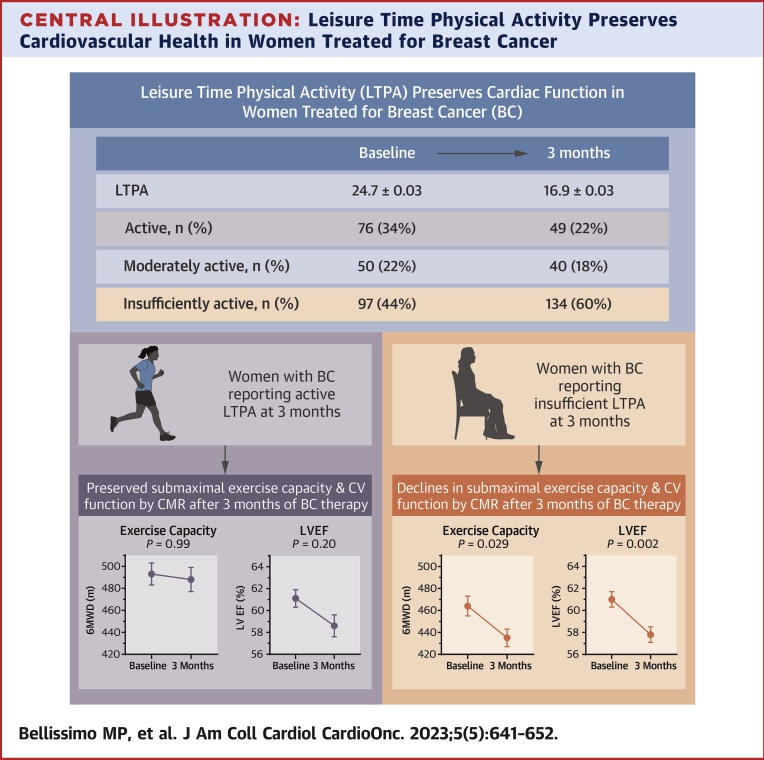
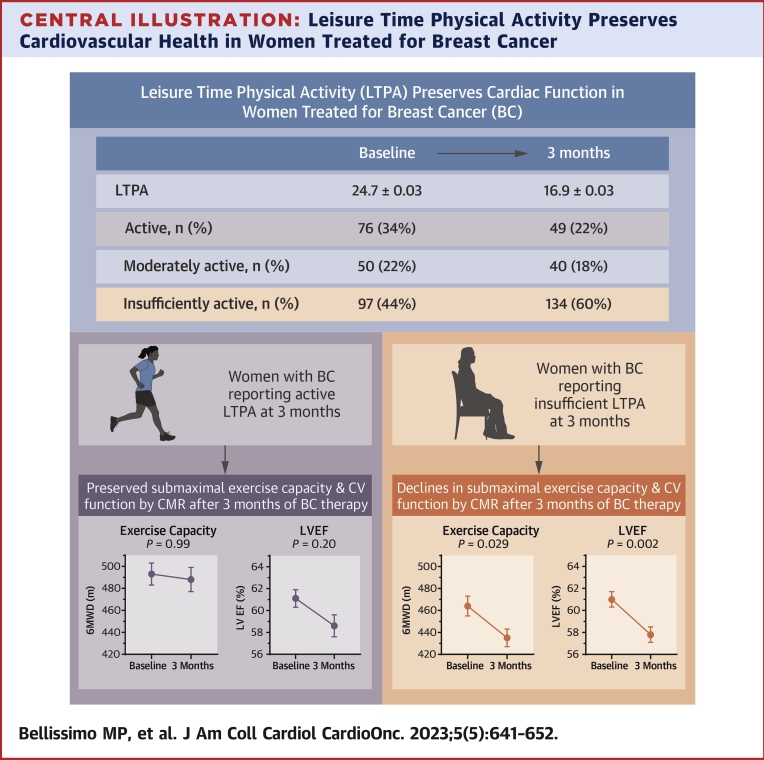
Participants were also grouped as active, moderately active, and insufficiently active. Between BC and control participants, the proportion of women in each activity category differed at baseline (P = 0.017; d = 0.22) and 3 months (P < 0.001; d = 0.76). At baseline among BC participants, 76 (34%) were active, 50 (22%) moderately active, and 97 (44%) insufficiently active. Among control participants, 61 (48%) were active, 17 (25%) moderately active, and 48 (38%) insufficiently active. By 3 months in the BC group, the proportion of women in each activity group changed (P = 0.002) with 49 (22%) considered active, 40 (18%) moderately active, and 134 (60%) insufficiently active compared with 66 (52%) active, 26 (21%) moderately active, and 34 (27%) insufficiently active in the control group.
Associations between LTPA and submaximal exercise capacity in BC participants
Fully adjusted means and 95% CI of exercise capacity are shown in Table 2 at baseline and 3 months into treatment, with results of the mixed-effects models in Table 3. Exercise capacity declined over the study period from 473 m (459-486 m) at baseline to 454 m (441-468 m) at 3 months. The interaction term between total weekly LTPA and study visit was not significant (P > 0.10) and was removed from the model. Greater total weekly LTPA levels were associated with higher submaximal exercise capacity (7.1 ± 1.6 m; 95% CI: 4.0-10.1 m).
Table 2.
Exercise Capacity and Cardiac Function Measures at Baseline and 3 Months in Breast Cancer Participants
| Baseline (n = 223) | 3 Months (n = 223) | |
|---|---|---|
| Exercise capacity, m | 473 (459 to 486) | 454 (441 to 468) |
| LV end-diastolic volume, mL | 120.7 (117.1 to 124.3) | 125.1 (121.4 to 128.8) |
| LV end-systolic volume, mL | 47.3 (45.2 to 49.3) | 52.5 (50.4 to 54.7) |
| LV ejection fraction, % | 61.0 (59.9 to 62.1) | 58.2 (57.1 to 59.3) |
| LV circumferential strain, % | −20.1 (−20.8 to −19.4) | −17.8 (−18.5 to −17.1) |
Values are the mean (95% CI) according to the fully adjusted models presented in Table 3.
LV = left ventricular.
Table 3.
Total LTPA (Baseline to 3 Months) Related to Cardiovascular Health Measures in BC Participants
| Exercise Capacity, m | LV End-Diastolic Volume, mL | LV End-Systolic Volume, mL | |
|---|---|---|---|
| 3-mo visit | −9.1 ± 3.5(−16 to −2.2) | 2.2 ± 0.8(0.5 to 3.8) | 2.6 ± 0.5(1.7 to 3.6) |
| Stage III BC | 14.3 ± 10.1 (−5.6 to 34.2) | 5.7 ± 2.9 (−0.1 to 11.4) | 2.0 ± 1.7 (−1.3 to 5.3) |
| Stage II BC | −8.8 ± 7.0 (−22.7 to 5.0) | −6.1 ± 2(−10 to −2.1) | −2.4 ± 1.2(−4.6 to -0.1) |
| 3-mo visit ∗ stage III BC | −5.5 ± 6.0 (−17.3 to 6.4) | 3.6 ± 1.4(0.8 to 6.4) | 2.6 ± 0.8(1.0 to 4.3) |
| 3-mo visit ∗ stage II BC | −7.3 ± 4.1 (−15.4 to 0.8) | −3.1 ± 1(−5.0 to −1.1) | −1.0 ± 0.6 (−2.1 to 0.2) |
| Age, y | −2.3 ± 0.4(−3.2 to −1.5) | −0.7 ± 0.1(−0.9 to −0.4) | −0.4 ± 0.1(−0.6 to -0.3) |
| Body mass index, kg/m2 | −3.9 ± 0.7(−5.3 to −2.4) | 1.3 ± 0.2(0.9 to 1.7) | 0.4 ± 0.1(0.2 to 0.7) |
| Total LTPAa | 7.1 ± 1.6(4.0 to 10.1) | 1.2 ± 0.4(0.4 to 2.0) | 0.3 ± 0.2 (−0.2 to 0.7) |
| Total LTPAa ∗ 3-mo visit | — | — | — |
| Anthracycline | −0.02 ± 5.1 (−10.0 to 10.0) | −1.3 ± 1.5 (−4.1 to 1.6) | −0.7 ± 0.8 (−2.4 to 0.9) |
| Anthracycline ∗ 3-mo visit | — | — | — |
| LV Ejection Fraction, % | LV Circumferential Strain, % | |
|---|---|---|
| 3-mo visit | −1.4 ± 0.3(−2.0 to −0.8) | 1.2 ± 0.2(0.8 to 1.6) |
| Stage III BC | 0.4 ± 0.8 (−1.2 to 2.0) | −0.39 ± 0.49 (−1.36 to 0.58) |
| Stage II BC | −0.2 ± 0.6 (−1.3 to 0.9) | 0.32 ± 0.35 (−0.36 to 1.0) |
| 3-mo visit ∗ Stage III BC | −1.0 ± 0.5 (−2.0 to 0.1) | 0.57 ± 0.35 (−0.12 to 1.25) |
| 3-mo visit ∗ Stage II BC | −0.3 ± 0.4 (−1.0 to 0.4) | 0.003 ± 0.24 (−0.48 to 0.48) |
| Age, y | 0.1 ± 0.04(0.1 to 0.2) | −0.07 ± 0.02(−0.11 to −0.03) |
| Body mass index, kg/m2 | 0.1 ± 0.1 (−0.1 to 0.2) | 0.05 ± 0.04 (−0.02 to 0.12) |
| Total LTPAa | 0.2 ± 0.1 (−0.1 to 0.4) | 0.05 ± 0.08 (−0.12 to 0.21) |
| Total LTPAa ∗ 3-mo visit | — | −0.16 ± 0.07(−0.29 to −0.02) |
| Anthracycline | 0.2 ± 0.4 (−0.6 to 1.0) | 0.44 ± 0.25 (−0.05 to 0.92) |
| Anthracycline ∗ 3-mo visit | — | −0.62 ± 0.17(−0.96 to −0.28) |
Values are beta ± SE (95% CI). All models include a patient level random effect. Bold values indicate statistically significant (P < 0.05) findings regarding leisure-time physical activity (LTPA).
— = not statistically significant and removed from the model; BC = breast cancer; LV = left ventricular.
Square root transformed. Visit variable compares 3-month visit to baseline visit. Anthracycline variable compares no treatment to receiving treatment.
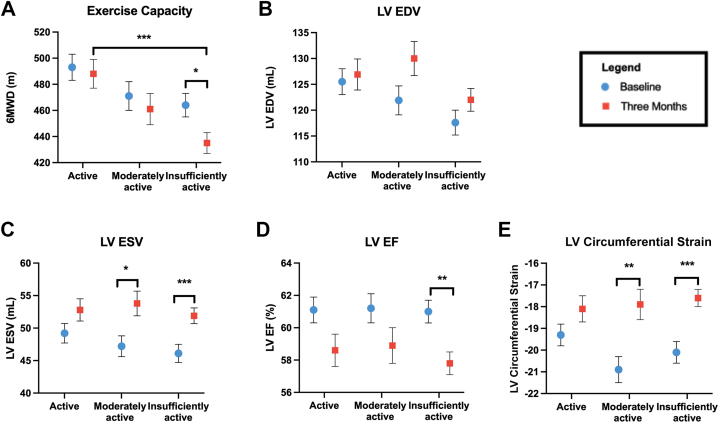
Submaximal exercise capacity for each BC LTPA group is presented in Figure 2 with group mean differences (±SE) from baseline to 3 months shown in Table 4. The active group exhibited a higher exercise capacity relative to the insufficiently active group at 3 months. The insufficiently active group was the only group to exhibit a statistically significant decline in exercise capacity from baseline to 3 months (−29 ± 10 m; P = 0.029) (Table 4). Model results for activity groups and exercise capacity are shown in Supplemental Table 1.
Figure 2.
Cardiovascular Health Measures Among BC Participants by Activity Group
Comparisons of exercise capacity (A), left ventricular (LV) end-diastolic volume (LVEDV) (B), LV end-systolic volume (LVESV) (C), LV ejection fraction (LVEF) (D), LV circumferential strain (E) by LTPA group at baseline and 3 months. Adjusted mean and standard error graphed. Blue circles = baseline; red squares = 3 months. Exercise capacity (6-minute walk distance [6MWD]), was significantly lower in the insufficiently active group at 3 months compared with the active group. LVESV and LV strain significantly increased in the moderate and insufficiently active groups, whereas LVEF decreased in the insufficiently active group. This indicates declines in cardiovascular health occurred at 3 months into treatment in those with lower LTPA levels. ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001. Abbreviations as in Figure 1.
Table 4.
Mean Differences in Cardiovascular Health Measures Among Physical Activity Groups in Breast Cancer Participants From Baseline to 3 Months
| Exercise Capacity, m |
LV End-Diastolic Volume, mL |
LV End-Systolic Volume, mL |
LV Ejection Fraction, % |
LV Circumferential Strain, % |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Difference ± SE | P Value | Mean Difference ± SE | P Value | Mean Difference ± SE | P Value | Mean Difference ± SE | P Value | Mean Difference ± SE | P Value | |
| Active | −7 ± 12 | 0.99 | 2.3 ± 2.9 | 0.97 | 3.9 ± 1.7 | 0.23 | −2.4 ± 1.0 | 0.20 | 1.2 ± 0.7 | 0.52 |
| Moderately active | −10 ± 15 | 0.99 | 8.0 ± 3.4 | 0.23 | 6.5 ± 2.1 | 0.030 | −2.3 ± 1.3 | 0.45 | 3.0 ± 0.9 | 0.008 |
| Insufficiently active | −29 ± 10 | 0.029 | 4.4 ± 2.3 | 0.37 | 5.8 ± 1.3 | <0.001 | −3.2 ± 0.8 | 0.002 | 2.5 ± 0.5 | <0.001 |
LV = left ventricular.
Associations between LTPA and cardiac function in breast cancer participants
Fully adjusted means and 95% CI of cardiac measures are shown in Table 2 with relationships between total weekly LTPA and cardiac function presented in Table 3. There were no significant interaction terms between total LTPA and study visit for models with LVEDV, LVESV, and LVEF as the outcomes. From baseline to 3 months, LVEDV increased (2.2 ± 0.8 mL; 95% CI: 0.5-3.8 mL), with an average increase of 0.7%. Higher LTPA over the study period was related to a higher LVEDV (1.2 ± 0.4 mL; 95% CI: 0.4-2.0 mL). LVESV increased over the study period (2.6 ± 0.5; 1.7-3.6) with an average increase of 8.7%. Total LTPA was not related to LVESV (0.3 ± 0.2 mL; 95% CI: −0.2 to 0.7 mL). LVEF declined (−1.4% ± 0.3%; 95% CI: −2.0% to −0.8%) by 3 months and was not related to total LTPA (0.2% ± 0.1%; 95% CI: −0.1 to 0.4). LV circumferential strain increased (ie, worsened) at 3 months (1.2% ± 0.2%, 95% CI: 0.8-1.6), although women who did not receive anthra-bC experienced less deterioration in LV circumferential strain (0.62 ± 0.17%; 95% CI: −0.96% to −0.28%) relative to women who received anthra-bC. There was a statistically significant relationship with total LTPA over time (−0.16 ± 0.07; 95% CI: −0.29 to −0.02) such that women with BC who were more active experienced a preserved LV circumferential strain over the study period.
Results by activity group are shown in Figure 2 and Table 4. The active group exhibited the smallest absolute mean change in LVEDV. LVESV values were maintained in the active group while both the moderately active (P = 0.030) and insufficiently active groups (P < 0.001) exhibited significant increases in LVESV. Active and moderately active groups maintained similar LVEF from baseline to 3 months, whereas the insufficiently active group experienced a decline in LVEF (P = 0.002). Adverse changes in LV circumferential strain were observed for the moderately active (P = 0.008) and insufficiently active groups (P < 0.001), whereas the active group did not have a significant change in LV circumferential strain over the study. Model estimates are shown in Supplemental Table 1.
LTPA and CTRCD
Of 181 BC participants with CMR data at both visits, 10 (5%) met clinical criteria for CTRCD according to LVEF. Change in total LTPA levels over the study period was not related to likelihood of meeting cardiac dysfunction criteria by LVEF (−0.01 ± 0.01; P = 0.31).
According to subclinical LV dysfunction classification by change in LV circumferential strain, 63 BC participants (37%) exhibited deteriorations of 15% or greater. Change in LTPA over the study period was significantly related to likelihood of being classified as having subclinical LV dysfunction (−0.02 ± 0.01; P = 0.027), suggesting that for every unit increase in LTPA over the study period, the likelihood of meeting subclinical LV dysfunction criteria decreased by 2% (OR: 0.98; 95% CI: 0.97-0.998).
Discussion
This study reported LTPA levels before and 3 months after beginning BC therapy and compared longitudinal associations between LTPA and submaximal exercise capacity and cardiac function. Control and BC participants reported similar levels of LTPA at baseline, but LTPA declined in the first 3 months of BC treatment, with 60% of the BC group reporting insufficient levels of LTPA at 3 months. Results also suggest a dose–response relationship of maintaining greater LTPA participation, especially moderate-strenuous intensity activity. Participants reporting higher levels of LTPA exhibited preserved cardiovascular health (Central Illustration), thereby providing supportive evidence that LTPA participation during cancer treatment may reduce CV dysfunction.
Central Illustration.
Leisure Time Physical Activity Preserves Cardiovascular Health in Women Treated for Breast Cancer
Women with breast cancer (BC) who reported meeting leisure time physical activity (LTPA) recommendations demonstrated preserved measures of cardiovascular (CV) health (exercise capacity, left ventricular function), whereas those reporting insufficient LTPA exhibited declines in these measures. 6MWD = 6-minute walk distance; CMR = cardiovascular magnetic resonance; LVEF = left ventricular ejection fraction.
In line with other reports, we observed a decline in LTPA from pretreatment through 3 months of treatment.16,20,30,31 At baseline, BC participants reported an average weekly LTPA score of 24.8, which was higher than a recently reported pretreatment median of 9.0 from a cohort of women with BC.16 Total LTPA decreased to 16.9 at 3 months, which was attributed to a decrease in moderate-strenuous activity. Among the BC group, 34% were categorized as active at baseline, which declined to 22% at 3 months, and 60% were insufficiently active at 3 months. Given the strong evidence of the broad-reaching beneficial effects of PA, including improvements in fatigue, physical function, and CV and cancer outcomes,10,32 increasing PA in women with BC represents an important target for primary and secondary prevention of CV disease and recurrent malignancies.
Maintaining higher levels of total LTPA from baseline to 3 months was associated with a greater exercise capacity, and women who were active at 3 months had superior exercise capacity relative to insufficiently active women. Baseline 6MWD values were similar to the average of BC survivors in a recent meta-analysis.33 A cross-sectional study of women spanning the BC survivorship continuum showed women with BC often exhibit a lower exercise capacity than control participants and experience significant declines during cancer treatment.11,34 Prospective data presented here suggest a dose–response relationship with PA levels to preserve exercise capacity during cancer treatment. On average, 6MWD declined by 22.5 m among BC participants, which is considered a major decline in heart failure patients35 and meets criteria for a clinically meaningful change.36 However, women in the active LTPA group did not exhibit declines in exercise capacity. Because exercise capacity is an integrated measure of oxygen delivery and uptake,37 these findings suggest PA participation is acting on 1 or more of these elements to preserve exercise capacity. Previous work shows increased PA during BC therapy improved exercise capacity measured by 6MWD.38 Together with previous work demonstrating positive effects of PA on physical functioning,32 psychosocial measures,39 and physical fitness,38 findings here contribute evidence that maintaining PA levels during cancer therapy helps to retain exercise capacity, an independent predictor of mortality.40
Similar to studies detailing acute changes in cardiac function with BC therapy,23,27,41, 42, 43 findings herein demonstrate declines in LV systolic function within the first 3 months of cancer therapy. Decreases in LVEF were predominantly attributed to increases in LVESV with concomitant small increases in LVEDV. Total LTPA was not related to LVEF or LVESV; however, differences were observed by level of activity. Active women experienced the greatest benefits for mitigating declines in cardiac function, whereas those reporting insufficient LTPA exhibited adverse changes in LVEF and LVESV. Active women were the only group to maintain LVESV from baseline to 3 months and did not exhibit significant declines in LVEF. These results suggest that greater moderate-strenuous PA participation helps to retain cardiac function.
Individuals who continued PA participation through the first 3 months of treatment experienced an attenuated deterioration in LV circumferential strain. The active group demonstrated preserved LV circumferential strain values and were less likely to meet criteria for subclinical LV dysfunction. LV circumferential strain is a sensitive indicator of subclinical LV dysfunction and may increase secondary to increased LVESV or decreased LVEDV.41,44 Relative to evaluations of LVEF, LV circumferential strain may provide earlier detection of LV dysfunction, which has important implications for guiding clinical decisions regarding administration of cancer and/or cardioprotective therapies.45 Consequently, compared with 5% of BC participants who met CTRCD criteria by LVEF, LV circumferential strain changes identified 37% of BC participants with subclinical LV dysfunction. Findings also support that greater total LTPA and meeting moderate-strenuous PA recommendations may help to preserve LV function via protection of LV circumferential strain during the first 3 months of treatment.
Findings regarding LV circumferential strain, LVEF, and LVESV are novel in humans receiving cancer treatment and support previous preclinical work demonstrating a cardioprotective effect of exercise.11 Preclinical studies, using an anthracycline-induced model of cardiomyopathy, demonstrated benefits of exercise such as improvements in LV function.11 One prospective study in humans linked higher baseline PA to a positive change in LVEF over cancer treatment.16 An intervention in BC women undergoing trastuzumab treatment reported a decline in LVEF despite participation in aerobic training in the first 4 months of treatment.46 Two recent, pilot randomized controlled trials reported attenuated decline in cardiac function with a bout of exercise performed before receiving doxorubicin,47 and improved LVEF in the exercising group relative to control participants.48 Together, preclinical and limited clinical work suggests exercise may be an effective strategy to prevent or mitigate adverse effects of cancer treatment on cardiac function, and findings reported here corroborate this evidence.
Study Limitations
Strengths of this study include the prospective design, which allows for establishing temporality between exposures and outcomes. This study used cardiac function assessed by CMR, a gold-standard technique due to its high spatial and temporal resolution, noninvasive nature, and lack of ionizing radiation. Study imitations include use of self-reported PA data. Despite the survey being validated in BC populations against accelerometers,49 overestimation of PA participation may have occurred. Use of accelerometry data would provide an objective measure of PA dose and frequency. Future studies would benefit from use of peak oxygen consumption to measure exercise capacity. Participants were enrolled shortly after receiving a BC diagnosis, potentially resulting in selection bias of healthier women and may not be reflective of all BC patients.
Conclusions
These findings may have important clinical implications providing evidence that LTPA may preserve exercise capacity and reduce cardiac dysfunction among women undergoing BC treatment. On average, self-reported LTPA declined through the first 3 months of treatment; however, maintaining higher LTPA was associated with greater exercise capacity and attenuated reductions in cardiac function in the first 3 months of treatment for stage I to III BC. Women who reported low levels of LTPA experienced significant adverse changes in both exercise capacity and cardiac function. Continued study will determine whether these relationships persist throughout the entirety of treatment for BC.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In women undergoing treatment for breast cancer, higher levels of leisure time physical activity were associated with attenuated declines in exercise capacity and cardiac function, which are often observed during breast cancer therapy.
TRANSLATIONAL OUTLOOK: Assessing physical activity is feasible in clinical settings and engaging in physical activity may help preserve key measures of cardiovascular health in women receiving breast cancer treatment. Randomized trials are needed to determine whether leisure time physical activity participation can attenuate losses of exercise capacity and cardiac function.
Funding Support and Author Disclosures
Data collection was funded by the Wake Forest NCI Community Oncology Research Program (NCORP) Research Base grant 2UG1CA189824 with support of the NCORP. Additional funding for this study was provided by National Institutes of Health, National Cancer Institute grants R01CA199167 and T32CA093423. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors gratefully acknowledge the study participants and study staff for their time and efforts that made this study possible. They would like to acknowledge the following NCORP sites for their participation: Aurora NCORP, Beaumont NCORP, Cancer Research Consortium of West Michigan NCORP, Metro Minnesota Community Oncology Research Consortium, NCORP of the Carolinas (Greenville Health System NCORP), Southeast Clinical Oncology Research Consortium NCORP, VCU Massey Cancer Center Minority Underserved NCORP, Wake Forest NCORP Research Base, ECOG-ACRIN NCORP Research Base, National Capital Area Minority Underserved NCORP. Additionally, they thank Wake Forest NCORP Research Base staff members Karen Craver, William Stanfield, Renee Glenn, and Cheyenne Wagi. And finally they would like to thank Tonya Calhoun for her efforts as Site Coordinator.
Footnotes
Jennifer A. Ligibel, MD, served as Guest Associate Editor for this paper. Kathryn J. Ruddy, MD, MPH, served as Guest Editor in Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a list of the UPBEAT Study Group, an expanded Methods section, and a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Narayan H.K., Finkelman B., French B., et al. Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation. 2017;135(15):1397–1412. doi: 10.1161/circulationaha.116.023463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinder M.C., Duan Z., Goodwin J.S., Hortobagyi G.N., Giordano S.H. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25(25):3808–3815. doi: 10.1200/jco.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 4.Saiki H., Petersen I.A., Scott C.G., et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135(15):1388–1396. doi: 10.1161/circulationaha.116.025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenlee H., Iribarren C., Rana J.S., et al. Risk of cardiovascular disease in women with and without breast cancer: the Pathways Heart Study. J Clin Oncol. 2022;40(15):1647–1658. doi: 10.1200/jco.21.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armenian S.H., Lacchetti C., Barac A., et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893–911. doi: 10.1200/jco.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw P.T., Stevens J., Khankari N., Teitelbaum S.L., Neugut A.I., Gammon M.D. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27(1):6–13. doi: 10.1097/ede.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lear S.A., Hu W., Rangarajan S., et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643–2654. doi: 10.1016/s0140-6736(17)31634-3. [DOI] [PubMed] [Google Scholar]
- 9.Eijsvogels T.M., Molossi S., Lee D.C., Emery M.S., Thompson P.D. Exercise at the extremes: the amount of exercise to reduce cardiovascular events. J Am Coll Cardiol. 2016;67(3):316–329. doi: 10.1016/j.jacc.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Irwin M.L., Smith A.W., McTiernan A., et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–3964. doi: 10.1200/jco.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu A.F., Jones L.W. Breast cancer treatment-associated cardiovascular toxicity and effects of exercise countermeasures. Cardiooncology. 2016;2:1. doi: 10.1186/s40959-016-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Upshaw J.N., Finkelman B., Hubbard R.A., et al. Comprehensive assessment of changes in left ventricular diastolic function with contemporary breast cancer therapy. J Am Coll Cardiol Img. 2020;13(1 Pt 2):198–210. doi: 10.1016/j.jcmg.2019.07.018. [DOI] [Google Scholar]
- 13.Jones L.W., Habel L.A., Weltzien E., et al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol. 2016;34(23):2743–2749. doi: 10.1200/jco.2015.65.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hambrecht R., Gielen S., Linke A., et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283(23):3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 15.Nagy A.C., Gulacsi-Bardos P., Cserep Z., Hangody L., Forster T. Late cardiac effect of anthracycline therapy in physically active breast cancer survivors - a prospective study. Neoplasma. 2017;64(1):92–100. doi: 10.4149/neo_2017_111. [DOI] [PubMed] [Google Scholar]
- 16.Upshaw J.N., Hubbard R.A., Hu J., et al. Physical activity during and after breast cancer therapy and associations of baseline physical activity with changes in cardiac function by echocardiography. Cancer Med. 2020;9(17):6122–6131. doi: 10.1002/cam4.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan J.H., D'Agostino R.B., Jr., Avis N.E., et al. Fatigue, cardiovascular decline, and events after breast cancer treatment: rationale and design of UPBEAT study. J Am Coll Cardiol CardioOnc. 2020;2(1):114–118. doi: 10.1016/j.jaccao.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin M.B., Greene F.L., Edge S.B., et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 19.Godin G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Health Fit J Can. 2011;4(4):18–22. [Google Scholar]
- 20.Amireault S., Godin G., Lacombe J., Sabiston C.M. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60. doi: 10.1186/s12874-015-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Heckbert S.R., Post W., Pearson G.D., et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48(11):2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolly M.P., Jordan J.H., Meléndez G.C., McNeal G.R., D'Agostino R.B., Jr., Hundley W.G. Automated assessments of circumferential strain from cine CMR correlate with LVEF declines in cancer patients early after receipt of cardio-toxic chemotherapy. J Cardiovasc Magn Reson. 2017;19(1):59. doi: 10.1186/s12968-017-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spahillari A., Talegawkar S., Correa A., et al. Ideal cardiovascular health, cardiovascular remodeling, and heart failure in blacks: the Jackson Heart Study. Circ Heart Fail. 2017;10(2) doi: 10.1161/circheartfailure.116.003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suerken C.K., D'Agostino R.B., Jr., Jordan J.H., et al. Simultaneous left ventricular volume and strain changes during chemotherapy associate with 2-year postchemotherapy measures of left ventricular ejection fraction. J Am Heart Assoc. 2020;9(2) doi: 10.1161/jaha.119.015400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grothues F., Smith G.C., Moon J.C., et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90(1):29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 27.Jordan J.H., Sukpraphrute B., Meléndez G.C., Jolly M.P., D'Agostino R.B., Jr., Hundley W.G. Early myocardial strain changes during potentially cardiotoxic chemotherapy may occur as a result of reductions in left ventricular end-diastolic volume: the need to interpret left ventricular strain with volumes. Circulation. 2017;135(25):2575–2577. doi: 10.1161/circulationaha.117.027930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyon A.R., López-Fernández T., Couch L.S., et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 29.Houbois C.P., Nolan M., Somerset E., et al. Serial cardiovascular magnetic resonance strain measurements to identify cardiotoxicity in breast cancer: comparison with echocardiography. J Am Coll Cardiol Img. 2021;14(5):962–974. doi: 10.1016/j.jcmg.2020.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Milne H.M., Gordon S., Guilfoyle A., Wallman K.E., Courneya K.S. Association between physical activity and quality of life among Western Australian breast cancer survivors. Psychooncology. 2007;16(12):1059–1068. doi: 10.1002/pon.1211. [DOI] [PubMed] [Google Scholar]
- 31.Valenti M., Porzio G., Aielli F., et al. Physical exercise and quality of life in breast cancer survivors. Int J Med Sci. 2008;5(1):24–28. doi: 10.7150/ijms.5.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juvet L.K., Thune I., Elvsaas I., et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166–177. doi: 10.1016/j.breast.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 33.But-Hadzic J., Dervisevic M., Karpljuk D., et al. Six-minute walk distance in breast cancer survivors-a systematic review with meta-analysis. Int J Environ Res Public Health. 2021;18(5):2591. doi: 10.3390/ijerph18052591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones L.W., Courneya K.S., Mackey J.R., et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–2537. doi: 10.1200/jco.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain S.S., Cohen D.J., Zhang Z., et al. Defining a clinically important change in 6-minute walk distance in patients with heart failure and mitral valve disease. Circ Heart Fail. 2021;14(3) doi: 10.1161/circheartfailure.120.007564. [DOI] [PubMed] [Google Scholar]
- 36.Bohannon R.W., Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23(2):377–381. doi: 10.1111/jep.12629. [DOI] [PubMed] [Google Scholar]
- 37.Acierno L.J. Adolph Fick: mathematician, physicist, physiologist. Clin Cardiol. 2000;23(5):390–391. doi: 10.1002/clc.4960230519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincent F., Labourey J.L., Leobon S., Antonini M.T., Lavau-Denes S., Tubiana-Mathieu N. Effects of a home-based walking training program on cardiorespiratory fitness in breast cancer patients receiving adjuvant chemotherapy: a pilot study. Eur J Phys Rehabil Med. 2013;49(3):319–329. [PubMed] [Google Scholar]
- 39.Aguiñaga S., Ehlers D.K., Cosman J., Severson J., Kramer A.F., McAuley E. Effects of physical activity on psychological well-being outcomes in breast cancer survivors from prediagnosis to posttreatment survivorship. Psychooncology. 2018;27(8):1987–1994. doi: 10.1002/pon.4755. [DOI] [PubMed] [Google Scholar]
- 40.Forman D.E., Fleg J.L., Kitzman D.W., et al. 6-Min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012;60(25):2653–2661. doi: 10.1016/j.jacc.2012.08.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drafts B.C., Twomley K.M., D'Agostino R., Jr., et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. J Am Coll Cardiol Img. 2013;6(8):877–885. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakano S., Takahashi M., Kimura F., et al. Cardiac magnetic resonance imaging-based myocardial strain study for evaluation of cardiotoxicity in breast cancer patients treated with trastuzumab: a pilot study to evaluate the feasibility of the method. Cardiol J. 2016;23(3):270–280. doi: 10.5603/CJ.a2016.0023. [DOI] [PubMed] [Google Scholar]
- 43.Ong G., Brezden-Masley C., Dhir V., et al. Myocardial strain imaging by cardiac magnetic resonance for detection of subclinical myocardial dysfunction in breast cancer patients receiving trastuzumab and chemotherapy. Int J Cardiol. 2018;261:228–233. doi: 10.1016/j.ijcard.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 44.Negishi T., Thavendiranathan P., Negishi K., Marwick T.H. Rationale and design of the strain surveillance of chemotherapy for improving cardiovascular outcomes: the SUCCOUR trial. J Am Coll Cardiol Img. 2018;11(8):1098–1105. doi: 10.1016/j.jcmg.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Thavendiranathan P., Negishi T., Somerset E., et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol. 2021;77(4):392–401. doi: 10.1016/j.jacc.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 46.Haykowsky M.J., Mackey J.R., Thompson R.B., Jones L.W., Paterson D.I. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. 2009;15(15):4963–4967. doi: 10.1158/1078-0432.Ccr-09-0628. [DOI] [PubMed] [Google Scholar]
- 47.Kirkham A.A., Shave R.E., Bland K.A., et al. Protective effects of acute exercise prior to doxorubicin on cardiac function of breast cancer patients: a proof-of-concept RCT. Int J Cardiol. 2017;245:263–270. doi: 10.1016/j.ijcard.2017.07.037. [DOI] [PubMed] [Google Scholar]
- 48.Chung W.P., Yang H.L., Hsu Y.T., et al. Real-time exercise reduces impaired cardiac function in breast cancer patients undergoing chemotherapy: a randomized controlled trial. Ann Phys Rehabil Med. 2022;65(2) doi: 10.1016/j.rehab.2021.101485. [DOI] [PubMed] [Google Scholar]
- 49.Amireault S., Godin G., Lacombe J., Sabiston C.M. Validation of the Godin-Shephard Leisure-Time Physical Activity Questionnaire classification coding system using accelerometer assessment among breast cancer survivors. J Cancer Surviv. 2015;9(3):532–540. doi: 10.1007/s11764-015-0430-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.