Abstract
Background
Although some cancer therapies have overt and/or subclinical cardiotoxic effects that increase subsequent cardiovascular risk in breast cancer patients, we have recently shown that the breast tumor itself can also induce cardiac hypertrophy through the activation of the endothelin system to contribute to cardiovascular risk. However, the extent to which the suppression of the activation of the endothelin system could improve cardiac remodeling in breast cancer patients has yet to be investigated.
Objectives
We aimed to retrospectively assess the cardiac morphology/function in patients with breast cancer before receiving cancer chemotherapy and to investigate if the suppression of the activation of the endothelin system improves cardiac remodeling in a mouse model of breast cancer.
Methods
Our study involved 28 previously studied women with breast cancer (including 24 after tumor resection) before receiving adjuvant therapy and 17 control healthy women. In addition, we explored how the endothelin system contributed to breast cancer–induced cardiac remodeling using a mouse model of breast cancer.
Results
Our results indicate that before chemotherapy, breast cancer patients already exhibit relative cardiac remodeling and subclinical cardiac dysfunction, which was associated with the activation of the endothelin system. Importantly, our mouse data also show that the endothelin receptor blocker atrasentan significantly lessened cardiac remodeling and improved cardiac function in a preclinical model of breast cancer.
Conclusions
Although our findings should be further examined in other preclinical/clinical models, our data suggest that endothelin receptor blockers may play a role in cardiac health in individuals with breast cancer. (Understanding and Treating Heart Failure With Preserved Ejection Fraction: Novel Mechanisms, Diagnostics and Potential Therapeutics [Alberta HEART]; NCT02052804 and Multidisciplinary Team Intervention in Cardio-Oncology [TITAN]; NCT01621659)
Key Words: breast tumor, cardiac remodeling, endothelin system
Central Illustration
In recent decades, the rate of long-term survival after breast cancer diagnosis has improved as a result of advances in screening, early diagnosis, and systemic therapies.1,2 However, these patients demonstrate an increased risk of developing cardiovascular disease after their cancer treatment.3,4 Indeed, the increased risk of cardiovascular complications after chemotherapy has been considered as a main cause of death in this population.5, 6, 7, 8 Notably, this increase in cardiovascular morbidity and mortality has historically been attributed to the known cardiovascular toxicities of many of the most frequently used breast cancer therapies.6, 7, 8 However, in a recent discovery, we provided evidence that challenges this dogma and suggests that the cancer cells directly influence cardiac morphology.9
Given that: 1) breast cancer cells secrete endothelin(ET)-1 to stimulate tumor growth;10 2) endothelin receptor type A (ETAR) is highly expressed in both cancer cells and cardiomyocytes;11, 12, 13, 14 3) activation of the endothelin system is known to contribute to cardiac remodeling and heart failure pathogenesis;11, 12, 13, 14 and 4) inhibition of endothelin signaling lessens heart failure and cardiac hypertrophy,15, 16, 17 we postulated that shared signaling pathways perpetuate endothelin axis activation in both systems. Thus, although the activation of the endothelin system is vital for breast tumor proliferation and metastasis,10 we hypothesized that a tumor-mediated humoral effect is also involved in the development of cardiac remodeling in breast cancer patients. We further hypothesized that this tumor-mediated humoral factor is ET-1. Consistent with this notion, we previously reported that breast cancer cells directly signal cardiomyocytes and induce cardiac hypertrophy through the activation of the endothelin system.9 However, the suppression of the cancer cell–mediated activation of endothelin signaling and the subsequent effects on cardiac remodeling in patients with breast cancer have yet to be investigated.
Based on the previous information provided, we retrospectively reviewed left ventricular (LV) and right ventricular (RV) volumes, mass, and function as well as global and regional myocardial deformation on cardiac magnetic resonance (CMR) in a previously studied cohort of women with breast cancer.9 In addition, we investigated if suppressing the activation of the endothelin system improves cardiac remodeling or cardiac injury in a mouse model of human breast cancer.
Methods
Additional methods are provided in the Supplemental Appendix.
Experimental design and treatment protocol in mice
All animal procedures were approved by the University of Alberta Institutional Animal Care and Use Committee (Health Sciences 2 Committee; approval date: October 2020; number: AUP00001794), which conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health and the principles for biomedical research involving animals developed by the Council for International Organizations of Medical Sciences.
We purchased female athymic nude mice from Charles River Laboratories. We housed these mice at 25°C, 12:12-hour light/dark cycle with ad libitum access to food and water. At 18 weeks of age, all mice received subcutaneous estrogen implants (0.5 mg/pellet/mouse, 21-day release) at the left shoulder to promote the growth of the upcoming implanted breast cancer cells. Five days later, mice were injected into the subcutaneous flank with 5 × 106 ZR-75-1 breast cancer cells (1:1 Matrigel [Corning] to ZR-75-1 media) (n = 20) or vehicle (n = 10). Three weeks after estrogen implantation, once tumors become visible, ZR-75-1–injected mice were then randomized to receive either vehicle (n = 10) or water containing a selective ETAR blocker (atrasentan18 [10 mg/kg/d]) (n = 10) for 4 weeks.
Retrospective cohort analysis
We obtained ethics approval from the University of Alberta Biomedical Panel and the Alberta Cancer Board along with informed written consent from all participants. Our study includes data from 28 women with breast cancer before receiving systemic cancer treatment.9 We screened patients with early-stage breast cancer with planned anthracycline or trastuzumab therapy from a tertiary care cancer hospital (Cross Cancer Institute). After a comprehensive medical review, we excluded patients with diabetes, hypertension, or previous cancer therapies. Of note, 24 of our patients underwent breast cancer lumpectomy at a median of 44 days before CMR, and the remaining 4 received cancer treatment in a neoadjuvant setting. Patients receiving neoadjuvant treatment underwent CMR before receiving this therapy. Biometric data (height, weight, blood pressure, and heart rate) were recorded on the day of CMR. A cohort of age-, body mass index–, body surface area–matched healthy control women (n = 17) were included in the study (Supplemental Table 1). CMR was performed on a 1.5-T system (Aera, Siemens Healthcare). Cardiac structure and function were derived from steady-state free precession cine imaging of conventional short and long-axis views.
Statistical analysis
We used GraphPad Prism software (version 8.01, GraphPad Software, Inc) to perform our statistical analysis. Results for human data are shown as mean ± SD, whereas our results for animal data are presented as mean ± SEM. The Kolmogorov-Smirnov test was used to assess the normality of distribution of each parameter. We used 1-way analysis of variance to compare >2 groups followed by the Tukey-Kramer post hoc test for multiple pairwise comparisons or the unpaired 2-tailed t-test to compare 2 groups. Pearson’s correlation coefficient was used to determine the correlations between big ET-1 levels and RV/LV parameters. A P value <0.05 was considered statistically significant.
Results
ET-1 receptor blocker improves cardiac remodeling in a mouse model of human breast cancer
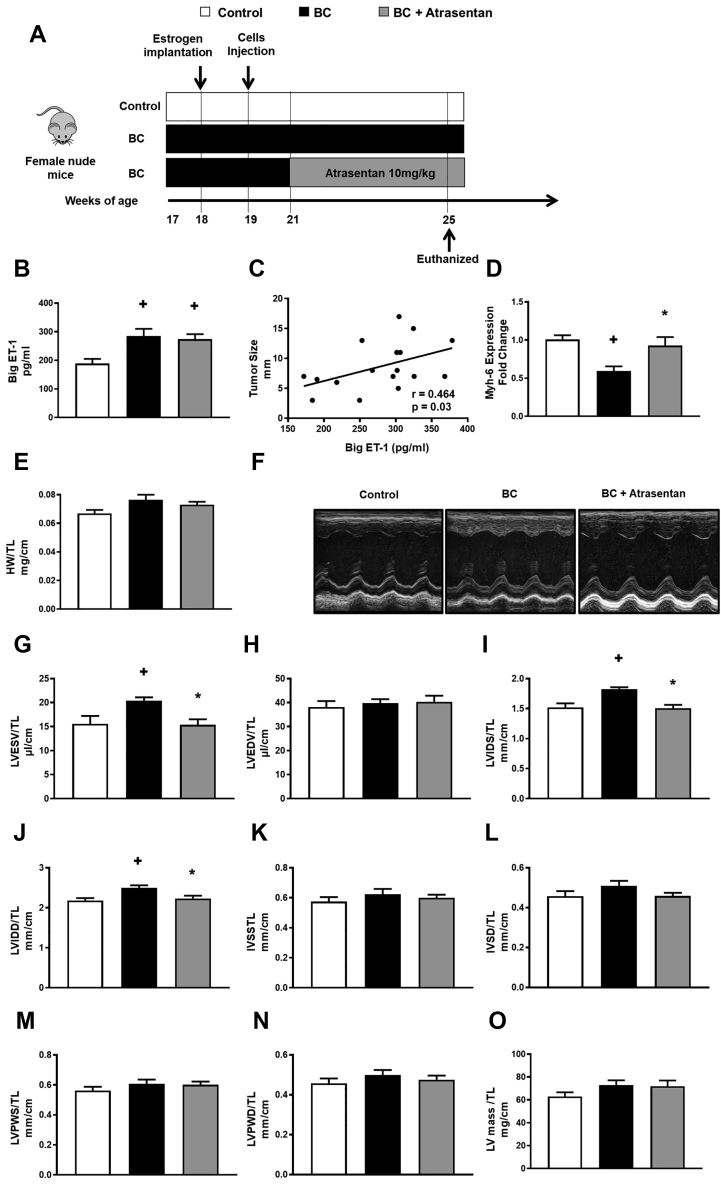
To address our hypothesis that a tumor-mediated ET-1 effect is involved in the development of cardiac remodeling in breast cancer patients, we first set out to explore this in immunocompromised mice that had human breast cancer cells injected into their subcutaneous flank (Figure 1A, Supplemental Figure 1A) to establish tumors. As predicted, our mouse model of human breast cancer was associated with high circulating levels of big ET-1 (Figure 1B). Of interest, we observed that the circulating big ET-1 positively correlated with tumor size, suggesting that the tumor is a major contributor to the activation of the endothelin system in our mouse model of breast cancer (Figure 1C). Importantly, we found that our mouse model of breast cancer demonstrated signs of cardiac remodeling at the molecular level as evidenced by a significant reduction in Myh6 gene expression19 (Figure 1D); a slight but not significant increase in heart weight/tibia length (TL); and significant increases in LV end-systolic volume/TL, LV internal diameter systole/TL, and LV internal diameter diastole/TL compared with control mice (Figures 1F, 1G, 1I, and 1J). Conversely, breast cancer–injected mice treated with the ETAR blocker atrasentan showed a significant reduction in LV end-systolic volume/TL, LV internal diameter diastole/TL, and LV internal diameter systole/TL (Figures 1G, 1I, and 1J) compared with breast cancer–injected mice administered vehicle. However, there were no significant changes in LV wall thickness or calculated LV mass demonstrating that breast cancer–injected mice treated with atrasentan did not display overt reduction in cardiac hypertrophy (Figures 1K to 1O). Also, our results showed that the aortic diameter and the pressure gradient across the aortic valve were unchanged in our mouse model of breast cancer and that atrasentan treatment did not alter these parameters, suggesting that the cardiac remodeling in our breast cancer model was independent from changes in blood pressure (Supplemental Figures 1B and 1C). Together, our findings indicate that the molecular signs of cardiac remodeling and differences in morphology in our breast cancer model can, at least in part, be credited to the activation of the endothelin system.
Figure 1.
Atrasentan Improves Cardiac Remodeling in BC–Injected Mice
(A) A schematic of the study design. (B) Cancer-injected mice also had high levels of big endothelin (ET)-1. (C) Big ET-1 positively correlated with tumor size. Also, there was a reduction in (D) Myh6 level and (E) a modest increase in heart weight (HW)/tibia length (TL). However, (F) echocardiography demonstrated atrasentan improves (G) left ventricular end-systolic volume (LVESV)/TL, (H) left ventricular internal diameter systole (LVIDS) /TL, and (I) left ventricular internal diameter diastole (LVIDD)/TL in cancer-injected mice. Nevertheless, there were no significant changes in (J) left ventricular end-diastolic volume (LVEDV)/TL, (K) interventricular septum in systole (IVSS), (L) interventricular septum in diastole (IVSD), (M) left ventricular posterior wall thickness in systole (LVPWS), (N) left ventricular posterior wall thickness in diastole (LVPWD), and (O) left ventricular (LV) mass. Results are shown as mean ± SEM (n = 6-9 per group). +P < 0.05 vs its own control group. ∗P < 0.05 vs its breast cancer (BC)-injected mice.
ET-1 receptor blocker improves signs of cardiac dysfunction in a mouse model of human breast cancer
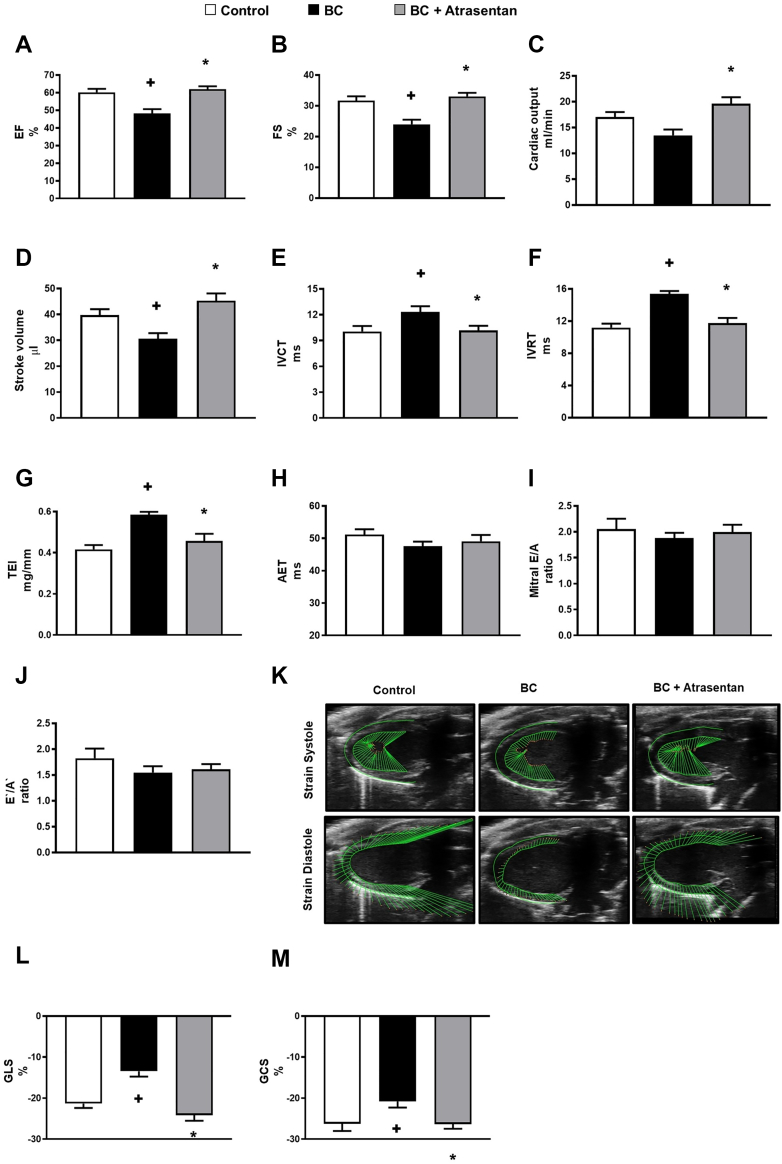
To test the hypothesis that the activation of endothelin signaling contributes to the modest cardiac dysfunction in breast cancer, we examined if atrasentan improves cardiac dysfunction in our preclinical mouse model of human breast cancer. Notably, echocardiography revealed that our mouse model of breast cancer demonstrated modest impairments in parameters of systolic function (such as ejection fraction, fractional shortening, and stroke volume) (Figures 2A to 2D) and diastolic function (such as isovolumic contraction time, isovolumic relaxation time, and Tei index) (Figures 2E to 2G). Conversely, breast cancer–injected mice treated with atrasentan showed a significant improvement in systolic and diastolic function compared with breast cancer–injected mice administered vehicle (Figure 2). In addition, we found that our mouse model of breast cancer demonstrated reduced cardiac global longitudinal strain (GLS) and global circumferential strain (GCS) compared with the controls (Figures 2K to 2M). Importantly, we observed that atrasentan significantly improved cardiac GLS and GCS in our mouse model of breast cancer compared with breast cancer–injected mice administered vehicle (Figures 2K to 2M). Collectively, our findings suggest that the cardiac dysfunction in our breast cancer model may, at least in part, be attributed to the activation of the endothelin system.
Figure 2.
Atrasentan Improves Cardiac Dysfunction in BC-Injected Mice
Four weeks after treatment of breast tumor–injected mice with vehicle or atrasentan, breast cancer (BC)-injected mice treated with atrasentan showed a significant improvement in (A) ejection fraction (EF), (B) fractional shortening (FS), (C) cardiac output, (D) stroke volume, (E) isovolumic contraction time (IVCT), (F) isovolumic relaxation time (IVRT), and (G) Tei (TEI) index. However, there were no significant changes in (H) aortic ejection time (AET), (I) mitral E-wave velocity/A-wave velocity (mitral E/A), and (J) E′/A′ in all groups. (K) Representative echocardiography of myocardial strain. Strain data demonstrated that atrasentan significantly improved (L) global longitudinal strain (GLS) and (M) global circumferential strain (GCS) in BC-injected mice. Results are shown as mean ± SEM (n = 6-9 per group). +P < 0.05 vs its own control group. ∗P < 0.05 vs its BC-injected mice treated with vehicle.
Lack of cardiac inflammation and fibrosis in a mouse model of human breast cancer
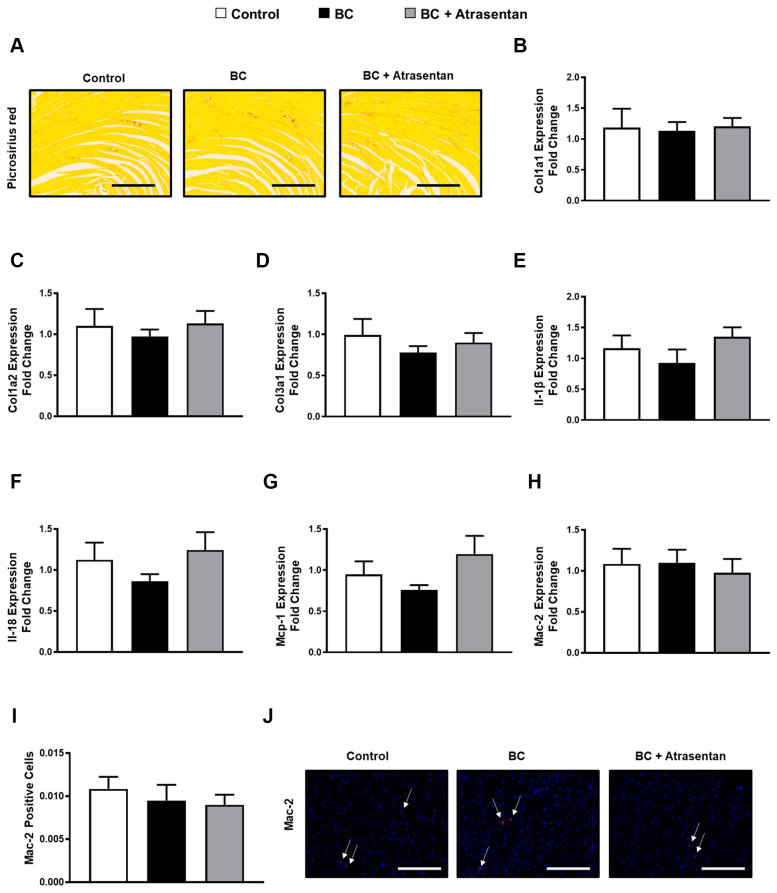
Using picrosirius red staining of histologic cardiac sections and real-time polymerase chain reaction, we found there were no significant changes in the interstitial cardiac fibrosis (Figure 3A) or the cardiac transcript levels of fibrotic markers collagen 1a1, collagen 1a2, and collagen 3a1 (Figures 3B to 3D), respectively, in our mouse model of breast cancer. Similarly, there were no significant changes in the cardiac transcript levels of the inflammatory markers interleukin 1β, interleukin 18, monocyte chemoattractant protein-1, Galectin-3 (Mac-2) (Figures 3E to 3H), or the number of Galectin-3 (Mac-2)–positive cells (Figures 3I and 3J) in all groups of our mouse model of breast cancer. Together, our data suggest that the cardiac remodeling in our preclinical breast cancer models occurs independently from cardiac inflammation and/or fibrosis.
Figure 3.
Minimal Changes in Select Inflammatory and Fibrosis Mediators in Mice
(A to J) Four weeks after treatment of tumor-injected mice with vehicle or atrasentan, there were no significant changes in cardiac fibrosis/inflammation makers in all groups. (A) Representative images of mouse heart sections stained with picrosirius red. Quantification of transcript levels (B) collagen 1a1 (Col1a1), (C) collagen 1a2 (Col1a2), (D) collagen 3a1 (Col3a1), (E) interleukin (IL)-1b, (F) Il-18, (G) monocyte chemoattractant protein (Mcp)-1, and (H) Mac-2 that were normalized to Rpl32. (I) Mac-2–positive cells. (J) Representative images of mouse heart with immunostaining for Mac-2. Results for animal data are shown as mean ± SEM (n = 6-9 per group). +P < 0.05 vs its own control group. ∗P < 0.05 vs its breast cancer (BC)-injected mice.
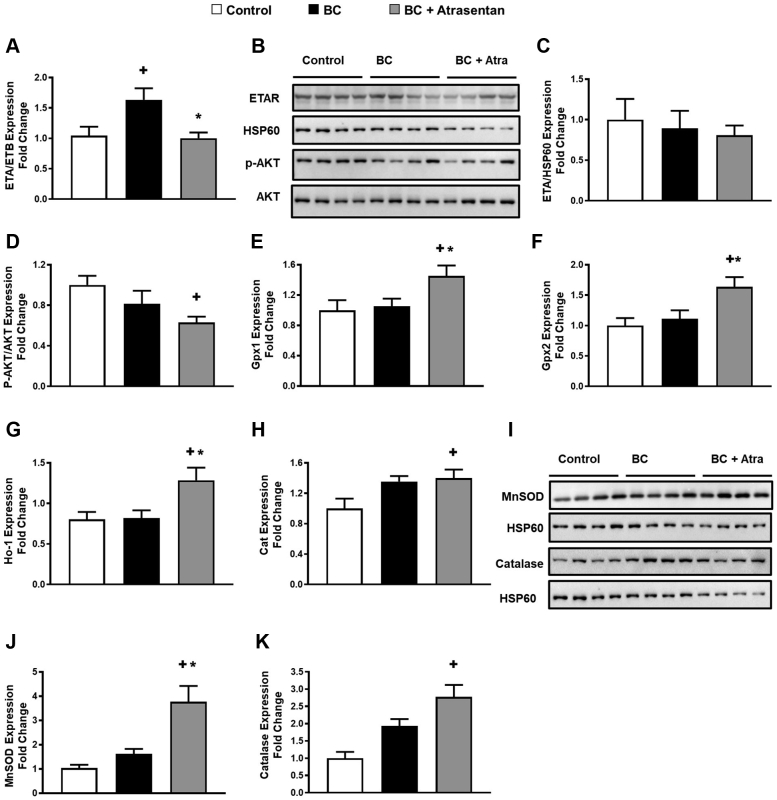
Atrasentan restores endothelin-A/endothelin-B receptor expression and upregulates antioxidant markers in the heart of a mouse model of human breast cancer
To test whether the cardioprotective effect of atrasentan is associated with a change in the endothelin-related pathway, we measured endothelin-A (ETA)/endothelin-B (ETB) receptor expression in the mouse heart tissues using quantitative reverse transcription polymerase chain reaction as well as the protein level of ETAR and phospho-AKT (p-AKT) using Western blot analysis. We found that our mouse model of breast cancer demonstrated a significant upregulation of ETA/ETB receptors compared with hearts from control mice (Figure 4A). Importantly, atrasentan significantly restored ETA/ETB receptor levels in the heart to that of the control group in our mouse model of human breast cancer (Figure 4A). However, although our mouse model of breast cancer did not demonstrate a significant change in p-AKT protein level or ETA receptor (Figures 4B and 4D), atrasentan significantly decreased p-AKT protein levels in the heart tissues (Figures 4B and 4D), suggesting that atrasentan significantly blocked the endothelin signaling in our breast cancer–injected mice. Nevertheless, the absence of elevated p-AKT in our breast cancer–injected mice administered vehicle suggested that the activation of endothelin signaling may not have occurred. Alternatively, it is possible that p-AKT was increased in hearts from vehicle-treated mice but that the levels of p-AKT normalized at the time of measurement. Because we have no direct evidence of this latter possibility, we currently cannot make any definitive conclusions about this finding.
Figure 4.
Atrasentan Downregulates p-AKT and Upregulates Antioxidants in Mice
Four weeks after treatment of breast tumor–injected mice with vehicle or atrasentan, we found that atrasentan downregulated p-AKT and upregulated antioxidant markers in the heart tissue. (A) Quantification of transcript levels of endothelin(ET)-A/ETB receptor by quantitative reverse transcription polymerase chain reaction. (B) Lysates from heart were immunoblotted with antibodies against (C) ETA receptor/HSP60 and (D) p-AKT/AKT. Quantification of cardiac transcript levels of (E) glutathione peroxidase (Gpx)-1, (F) Gpx-2, (G) heme oxygenase (Ho)-1, and (H) catalase (Cat) by quantitative reverse transcription polymerase chain reaction. (I) Lysates from heart were immunoblotted with antibodies against MnSOD/HSP60 and catalase/HSP60 in the heart tissue of control and breast cancer (BC)-injected mice. Quantification of (J) MnSOD/HSP60 and (K) catalase/HSP60, respectively. Results are shown as mean ± SEM (n = 6-9 per group). +P < 0.05 vs its own control group. ∗P < 0.05 vs its BC-injected mice treated with vehicle. Some results presented in this figure have been previously reported.9
In addition to ET receptors and p-AKT, given the antioxidant effect of ETA blockers such as atrasentan,20 we also measured the antioxidant effect of atrasentan in heart tissues. Notably, only breast cancer–injected mice treated with atrasentan showed a significant upregulation of the cardiac transcript and protein levels of antioxidant markers (Figures 4E to 4K). However, there were no significant changes in other oxidative stress markers in these same groups (Supplemental Figure 2), including ones that have previously been shown to be altered by atrasentan20 as well as ones in our previous study.21 Overall, our data suggest that atrasentan alters ET-1–related signaling in the hearts of breast cancer–injected mice. Thus, although we cannot explain the significant change in some antioxidant markers and the nonsignificant difference in oxidative stress markers, our data suggest that atrasentan still alters ET-1–related signaling in the hearts of breast cancer–injected mice.
Women with breast cancer before chemotherapy display right and left cardiac remodeling relative to healthy controls
In order to translate our findings in mice to women with breast cancer, we performed a retrospective cohort study of women with mainly resected breast cancer and healthy control participants who underwent CMR scans. We have previously shown that women with breast cancer had increased LV volumes and mass with no change in left ventricular ejection fraction (LVEF) compared with the healthy control participants (Table 1).9 In the present study, we found that breast cancer patients also demonstrated an increase in right-sided cardiac volumes with no alteration in right ventricular ejection fraction (Table 1). Given that GLS is acknowledged as a more sensitive measure to predict subclinical cardiac events and mortality compared with LVEF,22,23 we also measured GLS in our women with breast cancer and the healthy controls. Compared with the healthy control women, patients with breast cancer demonstrated a nonstatistically significant reduction in LV GLS. However, a previous study showed a significant reduction in LV GLS in a larger cohort of cancer patients before receiving chemotherapy.24 Although women with breast cancer in our study did not demonstrate any elevation in systolic and diastolic blood pressure values at the time of baseline assessment before the CMR study, heart rate was significantly increased in breast cancer patients compared with the healthy control women (Table 1). Together, our data suggest that patients with resected breast cancer exhibit left and right-sided cardiac remodeling prior to receiving chemotherapy (Table 1).
Table 1.
Clinical and CMR Characteristics in Patients With Breast Cancer and Healthy Control Women
| Healthy (n = 17) | Coefficient of Variations | Cancer (n = 28) | Coefficient of Variations | P Value | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age, y | 59 ± 8.4 | 14.27 | 52.5 ± 10.2 | 19.56 | 0.14 |
| Systolic blood pressure, mm Hg | 125.5 ± 15.2 | 12.12 | 130 ± 15 | 11.55 | 0.36 |
| Diastolic blood pressure, mm Hg | 74.4 ± 7.7 | 10.45 | 71.5 ± 9.7 | 13.65 | 0.32 |
| Heart rate, beats/min | 68.75± 10.29 | 14.97 | 79.6 ± 13.05 | 16.40 | 0.006 |
| Big endothelin-1, pg/mL | 2.9 ± 0.47 | 15.88 | 3.4 ± 0.48 | 14.49 | 0.014 |
| CMR characteristics | |||||
| Indexed cardiac volumes and mass | |||||
| LVEDV, mL/m2 | 73.9 ± 12.4 | 16.95 | 85.6 ± 19 | 22.28 | 0.031 |
| LVESV, mL/m2 | 28.4 ± 4.9 | 17.52 | 33.7 ± 9.3 | 27.77 | 0.038 |
| LV stroke volume, mL/m2 | 45.5 ± 9.3 | 20.51 | 51.9 ± 12.4 | 23.96 | 0.076 |
| LVEF, % | 61.4 ± 4.5 | 7.463 | 60.8 ± 6.4 | 10.53 | 0.73 |
| LV mass, g/m2 | 50.7 ± 7.6 | 14.99 | 61.1 ± 9.4 | 15.54 | <0.001 |
| RVEDV, mL/m2 | 68.1 ± 16.7 | 24.63 | 84 ± 22.9 | 27.29 | 0.017 |
| RVESV, mL/m2 | 28.9 ± 8.1 | 28.26 | 32 ± 11.2 | 35.13 | 0.34 |
| RV stroke volume, mL/m2 | 40.5 ± 8.1 | 19.99 | 52.01 ± 13.9 | 26.75 | 0.027 |
| RVEF, % | 58.5 ± 6.2 | 10.72 | 62.4 ± 7.08 | 11.36 | 0.069 |
| LAV, mL/m2 | 44.6 ± 12.5 | 28.11 | 50.9 ± 14.2 | 28.03 | 0.14 |
| Global longitudinal strain | |||||
| LV GLS endocardial, % | −21.8 ± 2.3 | 10.99 | −20.37 ± 2.4 | 11.80 | 0.055 |
| LV GLS midwall, % | −21.7 ± 2.2 | 10.40 | −20.38 ± 2.4 | 11.94 | 0.073 |
| LV GLS epicardial, % | −18.37 ± 2.25 | 12.26 | −17.2 ± 12.3 | 13.59 | 0.11 |
| LV GLS average, % | −20.6 ± 2.2 | 10.71 | −19.3 ± 2.34 | 12.13 | 0.068 |
Values are mean ± SD or %.
Some results presented in this Table have been previously reported.9
CMR = cardiac magnetic resonance; GLS = global longitudinal strain; LAV = left atrial volume; LV = left ventricular; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; RV = right ventricular; RVEDV = right ventricular end-diastolic volume; RVEF = right ventricular ejection fraction; RVESV = right ventricular end-systolic volume.
Relative left and right cardiac remodeling in women with breast cancer is correlated with the activation of the endothelin system
Based on our previous study,9 the increase in LV mass and volumes in women with breast cancer was associated with an elevation in big ET-1 (Figure 5). However, whether or not RV volumes as well as left atrial volume (LAV) and GLS are correlated with the activation of the endothelin system has not been previously studied. In the current study, we found that circulating levels of big ET-1, a marker of endothelin system activation,16,25 correlated positively with LAV (Figure 5A). However, there was no significant correlation between big ET-1 and RV structure and function. Overall, these data suggest that the activation of the endothelin system might contribute, at least in part, to cardiac remodeling in women with breast cancer.
Figure 5.

Cardiac Remodeling Is Correlated With Big ET-1 in Cancer Patients
Pearson’s correlation coefficient in breast cancer patients (n = 28) revealed that (A) circulating big ET-1 positively correlated with left atrial volume (LAV) and (B) LV mass. No significant correlation between big ET-1 and (C) indexed LVEDV or (D) indexed right ventricular end-diastolic volume (RVEDV). (E) Significant positive correlation between big ET-1 concentration and indexed LVESV. No significant correlation between big ET-1 and (F) indexed right ventricular end-systolic volume (RVESV), (G) indexed left ventricular stroke volume (LVSV), or (H) indexed right ventricular systolic volume (RVSV). (I) Significant negative correlation between big ET-1 concentration and left ventricular ejection fraction (LVEF). No significant correlation between big ET-1 and (J) right ventricular ejection fraction (RVEF), (K) GLS, or (L) heart rate. Some results presented in this Figure have been previously reported.9 Abbreviations as in Figures 1 and 2.
Discussion
We observed that the activation of the endothelin system in women with breast cancer was correlated with left atrial remodeling. This is a potentially important finding because LAV is considered a sensitive predictor of outcomes in patients with cardiovascular disease and is an early marker of heart failure with preserved ejection fraction.26, 27, 28 We did not find a significant correlation between LV GLS (an emerging metric of cardiac function and prognosis) and big ET-1 within the patients with breast cancer. However, the lack of correlation in our patient cohort might be attributed to our relatively small sample size. Alternatively, the majority of our patients with breast cancer (24/28) had their tumors surgically removed before the blood draw and CMR measurements. Thus, it is possible that tumor resection lessens the production of tumor-mediated big ET-1. Nevertheless, animal data suggest that increased circulating big ET-1 contributes to subclinical cardiac dysfunction (Central illustration).
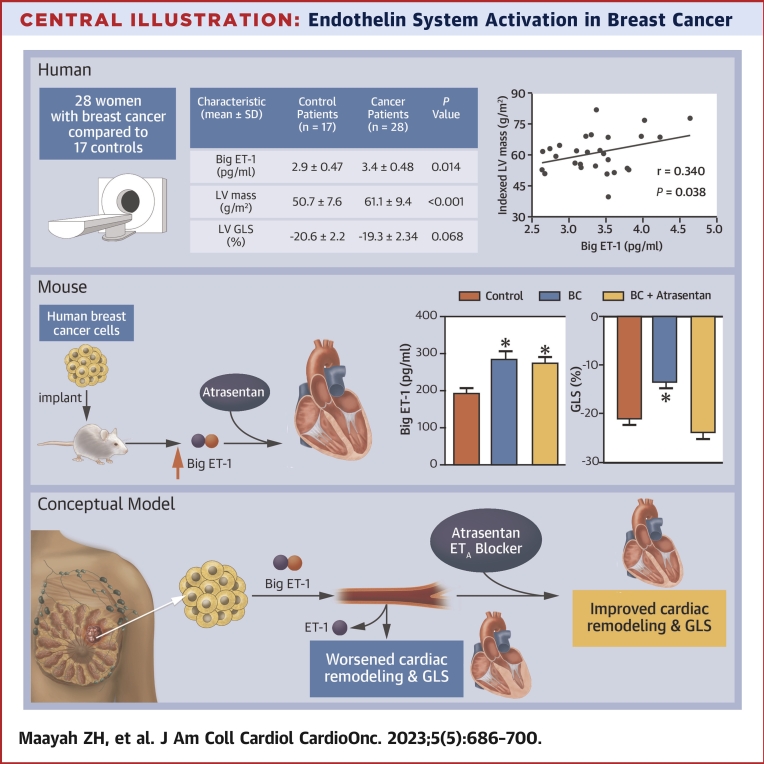
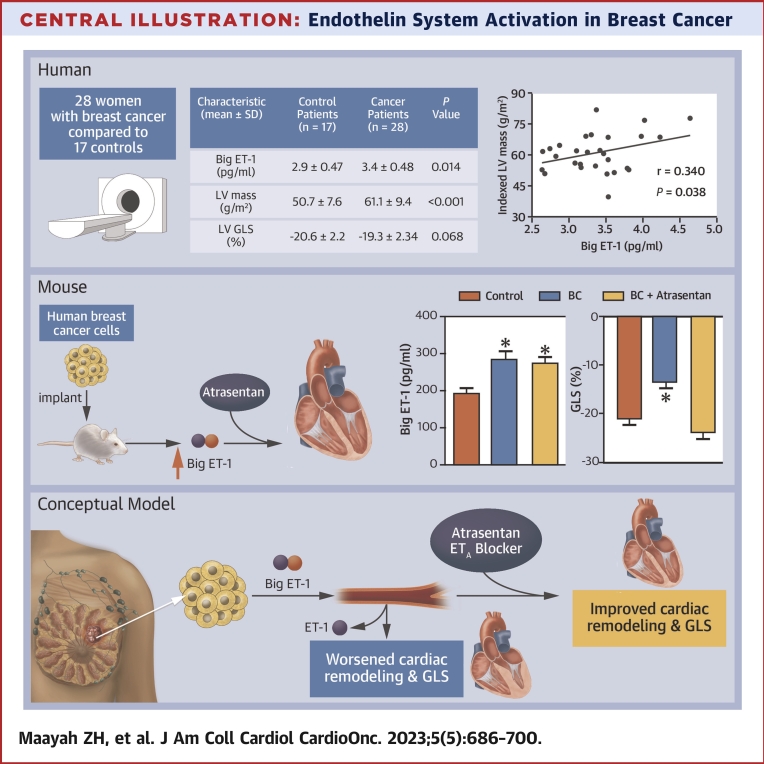
Central Illustration.
Endothelin System Activation in Breast Cancer
Women with breast cancer (BC) who underwent cardiac magnetic resonance showed significant increases in circulating big endothelin-1 (ET-1) concentrations and left ventricular (LV) mass as well as a worsening in LV global longitudinal strain (GLS) compared with healthy control women. LV mass was correlated with big ET-1 concentrations in these women. Mice injected with human breast cancer cells were used to test if ET-1 receptor blockade using the ET-1 receptor blocker atrasentan improved cardiac remodeling. Similar to humans, BC was associated with a significant increase in circulating big ET-1 levels and worsening LV GLS compared with control mice. However, ET-1 receptor blockade using atrasentan prevented the worsening in LV GLS. The conceptual model suggests that ET-1 receptor blockade may be effective in improved cardiac remodeling and GLS in women with breast cancer.
We also observed that resting heart rate was significantly increased in breast cancer patients compared with the healthy control women. This observation has been reported in other studies29, 30, 31 but has never been mechanistically explained. It is possible that many factors can affect heart rate in cancer patients such as mental stress, anxiety, and depression.32 Also, although anemia could be another important factor that can increase heart rate to compensate for the decreased oxygen-carrying capacity in patients with cancer,33 we do not expect that this is the case in our patients because they were not anemic. Notably, the elevated heart rate in our patients might be attributed to the activation of the sympathetic nervous system, potentially by ET-1. In support of this, previous studies have shown that ET-1 stimulates stress-related sympathetic nervous system via the activation of ETAR.34,35 Indeed, stimulation of ETAR increases the release of norepinephrine from sympathetic neurons, resulting in the activation of cardiac β-receptors and a subsequent elevation in heart rate.36 However, given that most of our patients had tumor resection before the heart rate and CMI measure, it is difficult to attribute this elevation in heart rate to the production of endothelin by the primary breast cancer. Indeed, healing responses postsurgery or physiologic stress can trigger the release of circulating factors such as big ET-1 and also elevate heart rate. However, given that ET-1 (∼40 seconds) and big ET-1 (∼23 minutes) have short half-lives,33,37 intermittent elevations in circulating ET-1 and big ET-1 levels produced by the primary tumor or residual cancer cells might be masked and/or the time of day collection of blood may also contribute to changes in ET-1 levels.38 Also, it is possible that there might be upregulation of ETARs in the body, making it more susceptible to effects from endogenous ET-1 such as the sympathoexcitatory effect.34 These limitations of our study have yet to be fully explored.
Based on the associations identified in our human study, we attempted to explore causality in our mouse model of human breast cancer. We show that the presence of breast cancer is associated with the activation of the endothelin system and that ET-1 receptor blockade significantly attenuated cardiac remodeling in our mouse model of human breast cancer. Although other mouse models of cancer can induce cardiac cachexia, our findings indicate that the tumor itself causes a modest cardiac hypertrophy. Although the exact reasons for the different cardiac effects in other tumor-bearing mouse models is not known, the type of cancer, the time period after cardiac study, and the differing secreted factors may play a role. Nevertheless, based on our findings, we predict that the suppression of the activation of the endothelin system could improve cardiac remodeling and the subclinical cardiac dysfunction in our breast cancer patients. Thus, given the potential synergistic effect of the ETA blocker and breast cancer chemotherapies,39 we propose that the endothelin system may constitute an important therapeutic target to mitigate cardiovascular risk in women with breast cancer. Indeed, it has already been shown that endothelin receptor blockers such as atrasentan potentiate the antiproliferative effect of breast cancer therapies such as trastuzumab in breast cancer cells,39,40 and this may be worthy of further investigation.
Considering the fact that breast cancer cells release proinflammatory factors to contribute to cancer progression,41 it is reasonable to assume that the development of cardiac remodeling in our breast cancer model might also be attributed to inflammation and subsequent cardiac fibrosis. However, although previous work has suggested that inflammation has an important role in the pathogenesis of cancer-related heart disease,29 we do not expect this is the case in our study. Indeed, the cardiac remodeling in our mouse model of breast cancer lacks cardiac inflammation and/or fibrosis. Thus, it is unlikely that the cardiac remodeling associated with breast cancer is caused by inflammation and/or fibrosis. Consistent with this notion, we have previously shown that conditioned medium of human breast cancer cells that release inflammatory factors were not able to induce cardiomyocyte hypertrophy.9
Study limitations
An important limitation of the current study is that most of our patients had their tumor removed surgically before the blood draw and CMR measure. Thus, it is plausible that the absence of tumor lessens the overall production of circulating big ET-1. Also, circulating big ET-1 levels might not accurately reflect the amount of big ET-1 located in the microenvironment surrounding the tumor or the heart. In addition, we cannot rule out potential confounding variables such as healing responses postsurgery or a stress-linked increase in catecholamines. Indeed, the increased sympathetic activity could elevate shear stress and thereby trigger the release of circulating factors such as big ET-1. Thus, the increased big ET-1, heart rate, and LV mass might be attributed to elevated sympathetic activity. In addition, we used a relatively small sample size of patients and healthy women in our study; thus, these findings should be confirmed in a larger number of patients with breast cancer and their matched control at baseline before the surgical removal of the tumor.
Another important limitation of the current study is that for our mouse studies, we injected cancer cells into the subcutaneous flank but not orthotopically in the mammary fat pad, which would have been more clinically appropriate. Also, although our findings indicate that the molecular signs of cardiac remodeling in our breast cancer mouse model can, at least in part, be credited to the activation of endothelin system, we did not confirm our finding using another breast cancer line to rule out cell-type specific findings. Furthermore, we did not provide a direct measurement of arterial pressure in our mouse model. Finally, the lack of significant changes in some cytokines as well as Mac-2 levels does not rule out the possibility that inflammation is occurring. This is especially true given that we used an athymic nude mouse; thus, it is difficult to predict how adaptive immunity may play a role in cardiac changes in cancer patients. Also, it is possible that inflammation preceded the cardiac changes, and they were missed in our experiments.
Conclusions
In summary, we show that there is a significant correlation between the activation of the endothelin system and cardiac remodeling as well as cardiac dysfunction in breast cancer patients even after tumor resection. However, whether or not the activation of the endothelin system directly causes cardiac injury and remodeling in our patients is currently unknown. Nevertheless, our data indicate that ET-1 receptor blockade significantly improved cardiac remodeling in our mouse model of human breast cancer. Thus, the presented data suggest that ET-1 receptor blockade may be important for cardiac health in individuals with breast cancer, and this should be examined further in preclinical models and clinical studies. Lastly, although we use atrasentan in our mouse studies, results from the SONAR (Study of Diabetic Nephropathy with Atrasentan) study suggest worsening heart failure with atrasentan.42 Thus, other drugs that inhibit ET-1 signaling such as empagliflozin43,44 could be considered instead of atrasentan.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Although some cancer therapies have overt and/or subclinical cardiotoxic effects that increase subsequent cardiovascular risk in breast cancer patients, women with breast cancer exhibit relative cardiac remodeling and cardiac dysfunction even before chemotherapy. We show that there is a significant correlation between the activation of the endothelin system and cardiac remodeling as well as cardiac dysfunction in breast cancer patients even after tumor resection. However, whether or not the activation of the endothelin system directly causes cardiac injury and remodeling in our patients is currently unknown.
TRANSLATIONAL OUTLOOK: Our data indicate that ET-1 receptor blockade improved cardiac remodeling in our mouse model of human breast cancer. Thus, the presented data suggest that ET-1 receptor blockade may be important for cardiac health in individuals with breast cancer, and this should be examined further in clinical and preclinical models. If confirmed, ET-1 receptor blockers may hold promise to reduce the risk of incident heart disease in cancer survivors.
Funding Support and Author Disclosures
This work was supported by a grant from the Canadian Institutes of Health Research and a grant from the generous supporters of the Lois Hole Hospital for Women through the Women and Children's Health Research Institute, University of Alberta to Dr Dyck. Dr Dyck is a Canada Research Chair in Molecular Medicine. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors acknowledge the technical assistance of Heidi Silver from the University of Alberta.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section and supplemental table and figures, please see the online version of this paper.
Appendix
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Ma J., Gaudet M.M., et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw P.T., Stevens J., Khankari N., Teitelbaum S.L., Neugut A.I., Gammon M.D. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27(1):6–13. doi: 10.1097/EDE.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson D.I., Wiebe N., Cheung W.Y., et al. Incident cardiovascular disease among adults with cancer: a population-based cohort study. J Am Coll Cardiol CardioOnc. 2022;4(1):85–94. doi: 10.1016/j.jaccao.2022.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones L.W., Haykowsky M.J., Swartz J.J., Douglas P.S., Mackey J.R. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50(15):1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Keefe D.L. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95(7):1592–1600. doi: 10.1002/cncr.10854. [DOI] [PubMed] [Google Scholar]
- 7.Ky B., Vejpongsa P., Yeh E.T., Force T., Moslehi J.J. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res. 2013;113(6):754–764. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groarke J., Tong D., Khambhati J., Cheng S., Moslehi J. Breast cancer therapies and cardiomyopathy. Med Clin North Am. 2012;96(5):1001–1019. doi: 10.1016/j.mcna.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maayah Z.H., Takahara S., Alam A.S., et al. Breast cancer diagnosis is associated with relative left ventricular hypertrophy and elevated endothelin-1 signaling. BMC Cancer. 2020;20(1):751. doi: 10.1186/s12885-020-07217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sin A., Tang W., Wen C.Y., Chung S.K., Chiu K.Y. The emerging role of endothelin-1 in the pathogenesis of subchondral bone disturbance and osteoarthritis. Osteoarthritis Cartilage. 2015;23(4):516–524. doi: 10.1016/j.joca.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Frank D., Kuhn C., Brors B., et al. Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch-specific gene program. Hypertension. 2008;51(2):309–318. doi: 10.1161/HYPERTENSIONAHA.107.098046. [DOI] [PubMed] [Google Scholar]
- 12.Sugden P.H., Clerk A. Endothelin signalling in the cardiac myocyte and its pathophysiological relevance. Curr Vasc Pharmacol. 2005;3(4):343–351. doi: 10.2174/157016105774329390. [DOI] [PubMed] [Google Scholar]
- 13.Schwebe M., Ameling S., Hammer E., et al. Protective effects of endothelin receptor A and B inhibitors against doxorubicin-induced cardiomyopathy. Biochem Pharmacol. 2015;94(2):109–129. doi: 10.1016/j.bcp.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Bien S., Riad A., Ritter C.A., et al. The endothelin receptor blocker bosentan inhibits doxorubicin-induced cardiomyopathy. Cancer Res. 2007;67(21):10428–10435. doi: 10.1158/0008-5472.CAN-07-1344. [DOI] [PubMed] [Google Scholar]
- 15.Valero-Munoz M., Li S., Wilson R.M., Boldbaatar B., Iglarz M., Sam F. Dual endothelin-A/endothelin-B receptor blockade and cardiac remodeling in heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9(11) doi: 10.1161/CIRCHEARTFAILURE.116.003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei C.M., Lerman A., Rodeheffer R.J., et al. Endothelin in human congestive heart failure. Circulation. 1994;89(4):1580–1586. doi: 10.1161/01.cir.89.4.1580. [DOI] [PubMed] [Google Scholar]
- 17.Archer C.R., Robinson E.L., Drawnel F.M., Roderick H.L. Endothelin-1 promotes hypertrophic remodelling of cardiac myocytes by activating sustained signalling and transcription downstream of endothelin type A receptors. Cell Signal. 2017;36:240–254. doi: 10.1016/j.cellsig.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opgenorth T.J., Adler A.L., Calzadilla S.V., et al. Pharmacological characterization of A-127722: an orally active and highly potent ETA-selective receptor antagonist. J Pharmacol Exp Ther. 1996;276(2):473–481. [PubMed] [Google Scholar]
- 19.Dirkx E., da Costa Martins P.A., De Windt L.J. Regulation of fetal gene expression in heart failure. Biochim Biophys Acta. 2013;1832(12):2414–2424. doi: 10.1016/j.bbadis.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Kang W.L., Xu G.S. Atrasentan increased the expression of klotho by mediating miR-199b-5p and prevented renal tubular injury in diabetic nephropathy. Sci Rep. 2016;6 doi: 10.1038/srep19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumura N., Takahara S., Maayah Z.H., et al. Resveratrol improves cardiac function and exercise performance in MI-induced heart failure through the inhibition of cardiotoxic HETE metabolites. J Mol Cell Cardiol. 2018;125:162–173. doi: 10.1016/j.yjmcc.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Verdonschot J.A.J., Merken J.J., Brunner-La Rocca H.P., et al. Value of speckle tracking-based deformation analysis in screening relatives of patients with asymptomatic dilated cardiomyopathy. J Am Coll Cardiol Img. 2020;13(2 Pt 2):549–558. doi: 10.1016/j.jcmg.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Smiseth O.A., Torp H., Opdahl A., Haugaa K.H., Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37(15):1196–1207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tadic M., Genger M., Baudisch A., et al. Left ventricular strain in chemotherapy-naive and radiotherapy-naive patients with cancer. Can J Cardiol. 2018;34(3):281–287. doi: 10.1016/j.cjca.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Pacher R., Bergler-Klein J., Globits S., et al. Plasma big endothelin-1 concentrations in congestive heart failure patients with or without systemic hypertension. Am J Cardiol. 1993;71(15):1293–1299. doi: 10.1016/0002-9149(93)90543-l. [DOI] [PubMed] [Google Scholar]
- 26.Airale L., Paini A., Ianniello E., et al. Left atrial volume indexed for height(2) is a new sensitive marker for subclinical cardiac organ damage in female hypertensive patients. Hypertens Res. 2021;44(6):692–699. doi: 10.1038/s41440-021-00614-4. [DOI] [PubMed] [Google Scholar]
- 27.Xu L., Pagano J., Chow K., et al. Cardiac remodelling predicts outcome in patients with chronic heart failure. ESC Heart Fail. 2021;8(6):5352–5362. doi: 10.1002/ehf2.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimbas R.C., Visoiu I.S., Magda S.L., et al. New insights into the potential utility of the left atrial function analysis in heart failure with preserved ejection fraction diagnosis. PLoS One. 2022;17(5) doi: 10.1371/journal.pone.0267962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labib D., Satriano A., Dykstra S., et al. Effect of active cancer on the cardiac phenotype: a cardiac magnetic resonance imaging-based study of myocardial tissue health and deformation in patients with chemotherapy-naive cancer. J Am Heart Assoc. 2021;10(9) doi: 10.1161/JAHA.120.019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavo N., Raderer M., Hulsmann M., et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101(23):1874–1880. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 31.Anker M.S., Frey M.K., Goliasch G., et al. Increased resting heart rate and prognosis in treatment-naive unselected cancer patients: results from a prospective observational study. Eur J Heart Fail. 2020;22(7):1230–1238. doi: 10.1002/ejhf.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh E.T., Tong A.T., Lenihan D.J., et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109(25):3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 33.Busti F., Marchi G., Ugolini S., Castagna A., Girelli D. Anemia and iron deficiency in cancer patients: role of iron replacement therapy. Pharmaceuticals (Basel) 2018;11(4):94. doi: 10.3390/ph11040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruno R.M., Sudano I., Ghiadoni L., Masi L., Taddei S. Interactions between sympathetic nervous system and endogenous endothelin in patients with essential hypertension. Hypertension. 2011;57(1):79–84. doi: 10.1161/HYPERTENSIONAHA.110.163584. [DOI] [PubMed] [Google Scholar]
- 35.Garrido M.R., Israel A. Role of endothelin in stress-induced hypertension. J Hum Hypertens. 2002;16(suppl 1):S29–33. doi: 10.1038/sj.jhh.1001338. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann L.H., Stanmore D.A., Backs J. The role of endothelin-1 in the sympathetic nervous system in the heart. Life Sci. 2014;118(2):165–172. doi: 10.1016/j.lfs.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C.L., Xie S., Qiao X., et al. Plasma endothelin-1-related peptides as the prognostic biomarkers for heart failure: a PRISMA-compliant meta-analysis. Medicine (Baltimore) 2017;96(50) doi: 10.1097/MD.0000000000009342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douma L.G., Barral D., Gumz M.L. Interplay of the Circadian Clock and Endothelin System. Physiology (Bethesda) 2021;36(1):35–43. doi: 10.1152/physiol.00021.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischgrabe J., Gotte M., Michels K., Kiesel L., Wulfing P. Targeting endothelin A receptor enhances anti-proliferative and anti-invasive effects of the HER2 antibody trastuzumab in HER2-overexpressing breast cancer cells. Int J Cancer. 2010;127(3):696–706. doi: 10.1002/ijc.25076. [DOI] [PubMed] [Google Scholar]
- 40.Askoxylakis V., Ferraro G.B., Badeaux M., et al. Dual endothelin receptor inhibition enhances T-DM1 efficacy in brain metastases from HER2-positive breast cancer. NPJ Breast Cancer. 2019;5:4. doi: 10.1038/s41523-018-0100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulasingam V., Diamandis E.P. Proteomics analysis of conditioned media from three breast cancer cell lines: a mine for biomarkers and therapeutic targets. Mol Cell Proteomics. 2007;6(11):1997–2011. doi: 10.1074/mcp.M600465-MCP200. [DOI] [PubMed] [Google Scholar]
- 42.Heerspink H.J.L., Parving H.H., Andress D.L., et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393(10184):1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 43.Zou R., Shi W., Qiu J., et al. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion injury through improving mitochondrial homeostasis. Cardiovasc Diabetol. 2022;21(1):106. doi: 10.1186/s12933-022-01532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pirklbauer M., Bernd M., Fuchs L., et al. Empagliflozin Inhibits basal and IL-1beta-mediated MCP-1/CCL2 and endothelin-1 expression in human proximal tubular cells. Int J Mol Sci. 2020;21(21):8189. doi: 10.3390/ijms21218189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.