Abstract
Background
Little is known about patients with cancer presenting with acute chest discomfort to the emergency department (ED).
Objectives
The aim of this study was to assess the prevalence of acute myocardial infarction (AMI), outcomes, and the diagnostic utility of recommended diagnostic tools in this population.
Methods
Patients presenting with chest pain to the ED were prospectively enrolled in an international multicenter diagnostic study with central adjudication. Cancer status was assessed prospectively and additional cancer details retrospectively. Findings were externally validated in an independent multicenter cohort.
Results
Among 8,267 patients, 711 (8.6%) had cancer. Patients with cancer had a higher burden of cardiovascular risk factors and pre-existing cardiac disease. Total length of stay in the ED (5.2 hours vs 4.3 hours) and hospitalization rate (49.8% vs 34.3%) were both increased in patients with cancer (P < 0.001 for both). Among 8,093 patients eligible for the AMI analyses, those with cancer more often had final diagnoses of AMI (184 of 686 with cancer [26.8%] vs 1,561 of 7,407 without cancer [21.1%]; P < 0.001). In patients with cancer, high-sensitivity cardiac troponin T (hs-cTnT) but not high sensitivity cardiac troponin I (hs-cTnI) concentration had lower diagnostic accuracy for non–ST-segment elevation myocardial infarction (for hs-cTnT, area under the curve: 0.89 [95% CI: 0.86-0.92] vs 0.94 [95% CI: 0.93-0.94] [P < 0.001]; for hs-cTnI, area under the curve: 0.93 [95% CI: 0.91-0.95] vs 0.95 [95% CI: 0.94-0.95] [P = 0.10]). In patients with cancer, the European Society of Cardiology 0/1-hour hs-cTnT and hs-cTnI algorithms maintained very high safety but had lower efficacy, with twice the number of patients remaining in the observe zone. Similar findings were obtained in the external validation cohort.
Conclusions
Patients with cancer have a substantially higher prevalence of AMI as the cause of chest pain. Length of ED stay and hospitalization rates are increased. The diagnostic performance of hs-cTnT and the efficacy of both the European Society of Cardiology 0/1-hour hs-cTnT and hs-cTnI algorithms is reduced. (Advantageous Predictors of Acute Coronary Syndromes Evaluation [APACE] Study; NCT00470587)
Key Words: acute coronary syndrome, biomarkers, cancer survivorship, coronary artery disease, diagnosis, outcomes
Central Illustration
Cancer and acute myocardial infarction (AMI) are the leading causes of death in both high-income and low- and middle-income countries.1 Thanks to advances in cancer screening and cancer therapy, cancer survival has significantly improved in recent decades.2,3 Growing evidence suggests that patients with cancer and cancer survivors are at increased risk for acute cardiovascular events, including AMI.4,5 Reasons for the increased risk include, first, that cancer and AMI share common risk factors, such as age, smoking, diabetes, obesity, and a sedentary lifestyle.6 Second, anticancer therapy, including chemotherapeutic agents, targeted therapies, and chest radiotherapy, can cause acute and chronic coronary damage.7 Third, with an aging population and improved cancer survival, the remaining life span during which AMI may occur in patients with cancer has increased.
As cancer may also lead to multiple noncardiac causes of acute chest pain, such as musculoskeletal pain due to bone metastasis, pulmonary embolism, pneumonia, and pleuritis in patients with lung cancer or pulmonary metastasis, it is unknown whether patients with cancer presenting with acute chest pain to the emergency department (ED) ultimately have a lower or higher likelihood of having AMI as the cause of their acute chest pain.7,8
Also, uncertainties remain regarding the accuracy of established diagnostic pillars for AMI, including chest pain characteristics (CPCs), electrocardiographic (ECG) signatures, and high-sensitivity cardiac troponin concentration in patients with cancer.9, 10, 11 Cancer-related pain, chronic opioid analgesic therapy, epigastric pain after vomiting or during mucositis, and neuropathy induced by oncologic therapies could mask or modify typical ischemia symptoms.7,12, 13, 14 Cardiac damage related to cancer, anticancer therapy, or cardiac comorbidities associated with aging such as hypertensive heart disease may result in ECG changes similar to those characterizing AMI. Similarly, high-sensitivity cardiac troponin T (hs-cTnT) and high sensitivity cardiac troponin I (hs-cTnI) concentrations may more often be chronically elevated because of these cardiac comorbidities.
To address these knowledge gaps, we aimed to evaluate: 1) the prevalence of AMI among consecutive patients with histories of cancer presenting with acute chest pain to the ED; 2) time to discharge from the ED and hospitalization rate as measures of the complexity of diagnostic evaluation and management efficiency; 3) the diagnostic accuracy of 34 predefined CPCs and ECG signatures; 4) the diagnostic accuracy of hs-cTnT and hs-cTnI concentrations; and 5) the performance of the European Society of Cardiology (ESC) 0/1-hour hs-cTnT and hs-cTnI algorithms.10
Methods
Study design and Population
This was a secondary analysis from the APACE (Advantageous Predictors of Acute Coronary Syndromes Evaluation) study. APACE is a multicenter, international, prospective diagnostic study (NCT00470587),15, 16, 17, 18, 19, 20, 21, 22 conducted in 12 EDs in 5 different European countries, enrolling patients >18 years of age presenting to EDs with nontraumatic acute chest pain as the main symptom. Patients were excluded if they had had prehospital cardiopulmonary resuscitation, were in shock, or had terminal kidney failure on long-term hemodialysis. The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees. Written informed consent was obtained from all participants. Reporting is in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement (Supplemental Table 1).23
External validation cohort
The results were externally validated in an independent, prospective, multicenter, international cohort, the TRAPID-AMI (High-Sensitivity Cardiac Troponin T Assay for Rapid Rule-Out of AMI) study, to further test their generalizability.24 Additional information for the TRAPID-AMI study can be found in the Supplemental Methods.
Cancer-related variables
Cancer status (present or absent, active or inactive) was prospectively assessed for all patients. The following cancer-specific variables were extracted retrospectively to complement the existing APACE dataset: primary site of origin, stage as advanced or localized, and administered treatment (surgery, chest radiotherapy, and chemotherapy). In most cases, cancer had been or was being managed and treated at the same hospital to which the patient initially presented with chest pain. Therefore, the corresponding medical records were available and could be reviewed. If the patient had been treated at a different hospital, we contacted the corresponding physician and requested the medical charts relevant to the cancer event. Nonmelanoma skin cancers as well as in situ carcinomas and monoclonal gammopathy of undetermined significance were not considered malignant cancer. Patients with cancer included those with active or past cancer.
Cancer was defined as “active” if it was diagnosed or treated within the previous 6 months and if it was recurrent, regionally advanced or metastatic, or not in complete remission (in case of hematological cancers), according to the definition of the Haemostasis and Cancer Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis.25 A solid malignancy was defined as “advanced” if at any time point it was staged according to the TNM classification as ≥T3 (extension to perivisceral structures) or ≥N1 (lymph node involvement) or ≥M1 (1 or more metastasis).26
Cardiotoxic cancer therapy was defined as treatment with at least 1 drug that was linked to any kind of cardiac damage through prior research, while cancer therapy with drugs related to acute coronary syndrome (ACS) was defined as treatment with at least 1 drug that was linked to ACS by prior research.7
CPCs, ECG criteria, and time to ED discharge
A total of 34 CPCs were predefined and prospectively recorded in the ED during the patient interview by trained physicians and nurses, blinded to 12-lead ECG findings and cardiac troponin levels, on a standardized case report.16 See the Supplemental Methods for details. Four predefined ECG criteria for the diagnosis of AMI were evaluated: ST-segment elevation, ST-segment depression, T-wave inversion, and previously unknown left bundle brunch block. Time to discharge from the ED was defined as the time interval between presentation to the ED and discharge home or to the ward, the catheterization laboratory, the intensive care unit, or the ED observation ward.
Adjudication of the final diagnosis
Adjudication of the final diagnosis was performed centrally in the core laboratory according to current guidelines and the fourth universal definition of myocardial infarction,10,11,27 as previously reported in detail.15,18,28 To address the uncommon but previously described phenomenon of discrepant results for hs-cTnT and hs-cTnI and the corresponding possible underestimation of the true performance of hs-cTnI-based algorithms using an adjudication based partly on serial hs-cTnT measurements, we performed a second adjudication using serial hs-cTnI blood concentrations from study samples, which was used for all hs-cTnI-based analyses. The most common reasons for missing samples after 1 hour or later were: 1) very early discharge; 2) early transfer to the catheterization laboratory or coronary care unit; 3) diagnostic procedures around the time window that precluded blood draws; and 4) patient refusal to undergo further blood sampling for the study. Additional information regarding the central diagnostic adjudication, blood samplings, laboratory methods, and follow-up can be found in the Supplemental Methods.
Clinical endpoints
The primary diagnostic endpoint was AMI (ST-segment elevation myocardial infarction [STEMI] or non–ST-segment elevation myocardial infarction [NSTEMI]) at presentation to the ED, stratified by history of cancer. When assessing the diagnostic performance of hs-cTnT and hs-cTnI and the ESC 0/1-hour hs-cTnT and hs-cTnI algorithms, the diagnostic endpoint was NSTEMI (types 1 and 2) at presentation to the ED, as in the case of STEMI, hs-cTnT and hs-cTnI concentrations are usually not required for its early diagnosis.
Statistical analysis
Categorical variables are reported as count (percentage), and groups were compared using the chi-square test or Fisher exact test, as appropriate. Continuous variables are reported as median (IQR) and were compared using the Mann-Whitney U test. Ninety-five percent CIs were calculated using Wilson’s method if not otherwise specified.29
Positive likelihood ratios (LR+) with 95% CIs were calculated to assess the value of each CPC and ECG finding for the diagnosis of AMI in patients with and without cancer.
Standardized prevalence ratios were calculated as the ratio of observed to expected cases of each of the most frequent cancer types.30 The number of expected cases was extracted from previous studies.31,32
Kaplan-Meier curves were used to plot 5-year all-cause mortality, and the log-rank test was used to assess differences between the 2 groups (patients with and without cancer). For 5-year cardiovascular mortality and 5-year future AMI, competing risk analysis using the Fine-Gray method was used, and cumulative incidence function plots were constructed.
The diagnostic accuracy of hs-cTnT and hs-cTnI concentrations for NSTEMI in patients with and without cancer was assessed using receiver-operating characteristic curves and their subtended area under the curve (AUC). CIs for AUCs and P values for comparison of AUCs were calculated according to DeLong et al.33 Additionally, to assess discrimination, a multivariable model was fitted including age, sex, glomerular filtration rate, ECG findings, history of coronary artery disease, hypertension, diabetes mellitus, and active smoking. Subgroup analyses were planned a priori for solid vs hematologic malignancies, active or advanced vs inactive or localized, chemotherapy vs no chemotherapy, ongoing vs past chemotherapy vs ongoing chemotherapy with drugs related to ACS vs cardiotoxic chemotherapy, and time since cancer diagnosis.
To evaluate the performance of the ESC 0/1-hour hs-cTnT and hs-cTnI algorithms, safety was assessed as the sensitivity and negative predictive value for the triage toward rule-out of index NSTEMI and accuracy as the specificity and positive predictive value for the rule-in toward index NSTEMI, and efficacy was quantified as the proportion of patients triaged toward rule-out or rule-in within 1 hour. The diagnostic performance measures for the ESC 0/1-hour hs-cTnT and hs-cTnI algorithms were compared using binomial exact test and the Pearson chi-square test (95% CIs were calculated using the Agresti-Coull method).
All hypothesis testing was 2 tailed, and P values <0.05 were considered to indicate statistical significance. All statistical analyses were performed using R version 4.1.1 (R Foundation for Statistical Computing). The list of R packages used can be found in the Supplemental Appendix.
Results
Baseline characteristics
The main cohort included 8,267 patients presenting to the ED with acute chest pain, of whom 711 (8.6%) had active or past cancer (Supplemental Figure 1). Patients with active or past cancer were older and more often had cardiovascular risk factors and pre-existing cardiac disorders than those without cancer. Consequently, long-term cardiovascular medications at the index visit were more frequently taken by patients with cancer than those without (Table 1).
Table 1.
Baseline Characteristics of Patients With and Those Without Cancer
| Overall (N = 8,267) |
No Cancer (n = 7,556) |
Cancer (n = 711) |
P Value | |
|---|---|---|---|---|
| Age, y | 61.0 (49.0-74.0) | 60.0 (48.0-72.0) | 75.0 (66.0-81.0) | <0.001 |
| Male | 5,482 (66.3) | 4,997 (66.1) | 485 (68.2) | 0.26 |
| Time from chest pain onset to ED presentation | 5.5 (2.3-18.0) | 5.5 (2.0-17.5) | 6.5 (3.0-19.0) | <0.001 |
| Time from chest pain maximum to ED presentation | 3.0 (1.5-6.5) | 3.0 (1.5-6.0) | 3.6 (2.0-7.5) | <0.001 |
| Pre-existing cardiac disease | ||||
| Coronary artery disease | 2,599 (31.4) | 2,302 (30.5) | 297 (41.8) | <0.001 |
| Myocardial infarction | 1,860 (22.5) | 1,652 (21.9) | 208 (29.3) | <0.001 |
| Coronary intervention (PCI or CABG) | 176 (26.3) | 1,952 (25.8) | 224 (31.5) | 0.001 |
| Cardiovascular risk factors | ||||
| Hypertension | 4,909 (59.4) | 4,365 (57.8) | 544 (76.5) | <0.001 |
| Dyslipidemia | 4,000 (48.4) | 3,586 (47.5) | 414 (58.2) | <0.001 |
| Diabetes | 1,467 (17.8) | 1,288 (17.1) | 179 (25.2) | <0.001 |
| Current smoking | 2,058 (25.0) | 1,954 (25.9) | 104 (14.7) | <0.001 |
| Medical history | ||||
| Cerebrovascular disease | 429 (5.2) | 369 (4.9) | 60 (8.4) | <0.001 |
| Peripheral artery disease | 418 (5.1) | 359 (4.8) | 59 (8.3) | <0.001 |
| Obstructive lung disease | 798 (9.7) | 686 (9.1) | 112 (15.8) | <0.001 |
| Chronic kidney disease | 789 (9.5) | 639 (8.5) | 150 (21.1) | <0.001 |
| Pulmonary embolism | 205 (2.5) | 173 (2.3) | 32 (4.5) | <0.001 |
| Chronic cardiovascular pharmacologic therapy at index visit | ||||
| Antiplatelet medications | 3,081 (37.3) | 2,736 (36.2) | 345 (48.5) | <0.001 |
| Anticoagulant agents | 805 (9.7) | 698 (9.2) | 107 (15.0) | <0.001 |
| Beta-blocker | 2,715 (32.8) | 2,397 (31.7) | 318 (44.7) | <0.001 |
| Calcium antagonist agents | 1,245 (15.1) | 1,097 (14.5) | 148 (20.8) | <0.001 |
| Angiotensin inhibitorsa | 3,225 (39.0) | 2,852 (37.7) | 373 (52.5) | <0.001 |
| Nitrates | 767 (9.3) | 651 (8.6) | 116 (16.3) | <0.001 |
| Statins | 2,843 (34.4) | 2,518 (33.3) | 325 (45.7) | <0.001 |
| Hemoglobin concentration and renal function at ED arrival | ||||
| Hemoglobin, g/L | 143.0 (132.0-153.0) | 143.0 (133.0-153.0) | 134.0 (121.0-146.0) | <0.001 |
| eGFR, mL/min/1.73 m2 | 83.3 (67.8-99.2) | 84.4 (69.1-100) | 71.1 (55.5-86.7) | <0.001 |
Values are median (IQR) or n (%).
CABG = coronary artery bypass grafting; ED = emergency department; eGFR = estimated glomerular filtration rate according to the Modification of Diet in Renal Disease formula; PCI = percutaneous coronary intervention.
Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
Among the 711 patients with cancer, 592 (83.3%) had solid subtypes, 98 (13.8%) had hematologic subtypes, and 13 (1.8%) had both. For 8 patients, data on subtypes were not available, and they were not considered for subgroup analysis. Baseline characteristics of patients with solid vs hematologic cancers are shown in Supplemental Table 2. A total of 246 patients with cancer (35%) received at least 1 cancer therapy (Supplemental Table 3), while 67 patients (9.4%) received chest radiotherapy, among whom 16 had active cancer on admission to the ED. The most common cancers receiving chest radiotherapy were breast cancer (n = 40 [59.7%]), lung cancer (n = 6 [9.0%]), non-Hodgkin lymphoma (n = 6 [9.0%]), esophageal cancer (n = 5 [7.4%]), and Hodgkin lymphoma (n = 5 [7.5%]). The baseline characteristics of the entire cohort, stratified according to a final adjudicated diagnosis of AMI, are listed in Supplemental Table 4. The proportion of each cancer characteristic (eg, active, advanced, previous chemotherapy, time since cancer diagnosis) did not differ between the AMI and non-AMI cancer groups (Supplemental Table 5).
Final adjudicated diagnosis
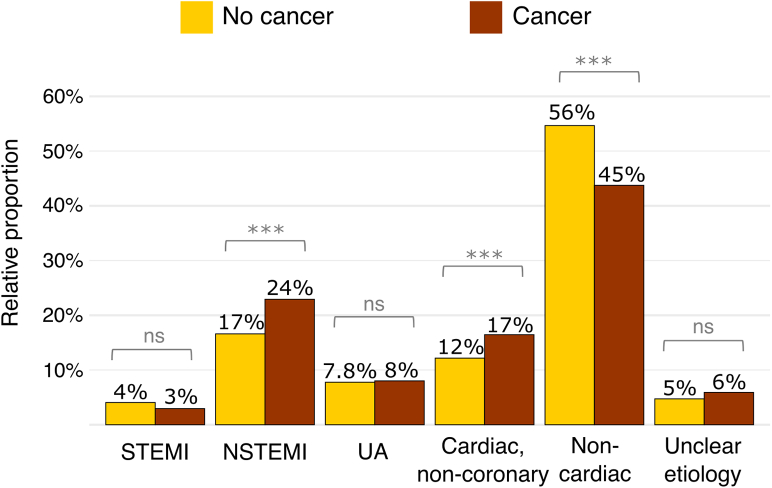
After excluding patients on long-term dialysis and those with unclear final adjudicated diagnoses in conjunction with hs-cTnT >14 ng/L, a total of 8,093 patients (of whom 686 had active or past cancer) were eligible for the diagnostic analyses. Final diagnoses of AMI (STEMI and NSTEMI) were adjudicated in 184 patients (26.8%) with cancer vs 1,561 patients (21.1%) without cancer (P < 0.001). This difference was due largely to a significantly higher proportion of NSTEMIs in patients with cancer (23.8% vs 16.9%; P < 0.001) (Figure 1, Central Illustration). Type 1 NSTEMIs were diagnosed in 126 patients with cancer (18.4%) vs 983 patients without cancer (13.3%) (P < 0.001) and type 2 NSTEMIs in 37 (5.4%) vs 271 (3.7%) patients, respectively (P = 0.03). Among type 2 NSTEMI triggers, anemia was more frequent in patients with vs those without cancer, while tachycardia was less prevalent in patients with cancer compared with those without cancer (Supplemental Table 6). In a subgroup analysis of patients with type 2 NSTEMI stratified according to cancer therapy timing, there were higher proportions of anemia and bradycardia as triggers in those with ongoing cancer therapy (Supplemental Table 7). Unstable angina was diagnosed in 57 patients with cancer (8.0%) vs 587 patients without cancer (7.8%) (P = 0.78). Among non-AMI diagnoses, non–coronary disease–related cardiac pain was more frequent in patients with cancer (n = 116 [16.9%]) than in those without cancer (n = 919 [12.4%]) (P < 0.001); in contrast, noncardiac pain was less frequent in patients with cancer (n = 311 [45.3%]) than in those without cancer (n = 4,129 [55.7%]) (P < 0.001) (Supplemental Table 8, Central Illustration). Of note, takotsubo cardiomyopathy had a comparable incidence in patients with (0 of 686 [0%]) and without (17 of 7,407 [0.23%]) cancer (P = 0.39). Similar results were obtained when adjudicating the potential AMI diagnosis with hs-cTnI (Supplemental Figure 2).
Figure 1.
Bar Plot Depicting Final Adjudicated Diagnosis According to Cancer Status
The prevalence of non–ST-segment elevation myocardial infarction (NSTEMI) and noncoronary cardiac pain was higher in patients with cancer, while the prevalence of noncardiac pain was lower in patients with cancer. ∗∗∗P < 0.001. ns = non significant; STEMI = ST-segment elevation myocardial infarction; UA = unstable angina.
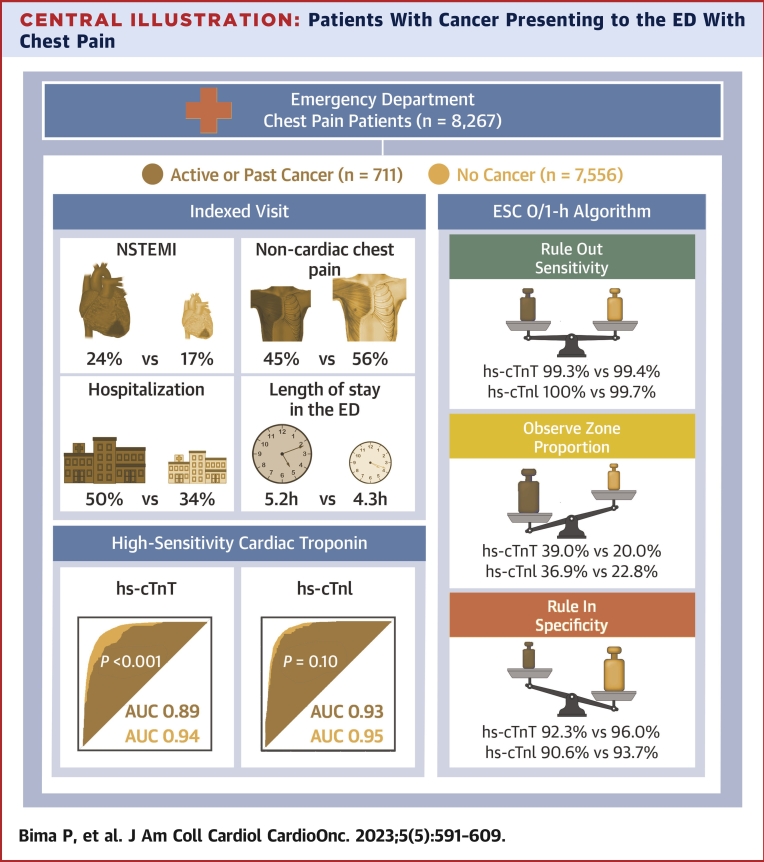
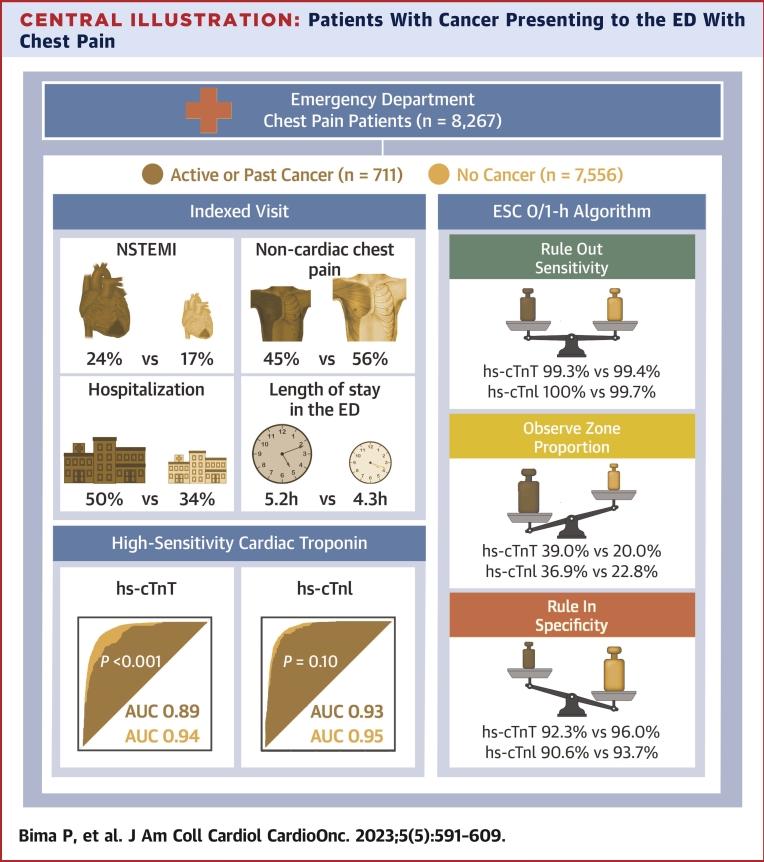
Central Illustration.
Patients With Cancer Presenting to the ED With Chest Pain
Among 8,267 patients, 711 (8.6%) had active or past cancer. The prevalence of index non–ST-segment elevation myocardial infarction (NSTEMI) was higher and the prevalence of noncardiac chest pain was lower in patients with cancer compared with those without cancer. Hospitalization rate and length of stay in the emergency department (ED) were also higher in patients with cancer. The diagnostic performance of high-sensitivity cardiac troponin T (hs-cTnT), but not that of high-sensitivity cardiac troponin I (hs-cTnI), was reduced in patients with cancer. The European Society of Cardiology (ESC) 0/1-hour algorithms for both hs-cTnT and hs-cTnI maintained very high safety for rule-out, although the observe-zone proportion was higher, and specificity for rule-in was lower in patients with cancer. AUC = area under the curve.
Length of stay in the ED and disposition decision
The total length of stay in the ED was longer in patients with cancer (median time 5.2 hours; IQR: 3.1-7.8 hours) than in those without cancer (median time 4.3 hours; IQR: 2.8-6.7 hours) (P < 0.001) (Supplemental Figure 3). This difference remained consistent in patients with final adjudicated diagnoses of AMI (4.5 hours [IQR: 2.2-7.1 hours] vs 3.2 hours [IQR: 1.3-6.4 hours]; P < 0.001) and non-AMI (5.3 hours [IQR: 3.3-7.9 hours] vs 4.5 hours [IQR: 3.0-6.7 hours]; P < 0.001). The length of stay in the ED was comparable in patients with solid versus hematologic cancer subtypes. Hospitalization rate was higher in patients with cancer (49.8%) than in those without cancer (34.3%) (P < 0.001) (Central Illustration), driven mainly by non-AMI diagnoses (36.3% vs 21.3%; P < 0.001).
In an age-adjusted analysis, the length of stay in the ED remained longer in patients with cancer younger than 50 and older than 65 years (Supplemental Figure 4), while it was comparable in the age group between 50 and 65 years. In an age-adjusted hospitalization rate analysis, the hospitalization rate of patients with cancer remained higher across all age groups, irrespective of the final adjudicated diagnosis (Supplemental Table 9).
Diagnostic and therapeutic interventions for ACS
Among patients presenting with ACS (n = 2,389), those with cancer were treated with fewer invasive procedures compared with those without cancer (diagnostic coronary angiography [67.6% vs 74.7%, respectively; P = 0.017] and percutaneous coronary intervention [46.9% vs 55.1%, respectively; P = 0.016]). The frequency of stress testing was not different between patients with and those without cancer (21.2% vs 21.0%, respectively). No difference was detected in the frequency of anti-ischemic medications at discharge, although numerically fewer patients with cancer were treated with antiplatelet medications at discharge (P = 0.073) (Supplemental Table 10).
Cardiovascular biomarkers
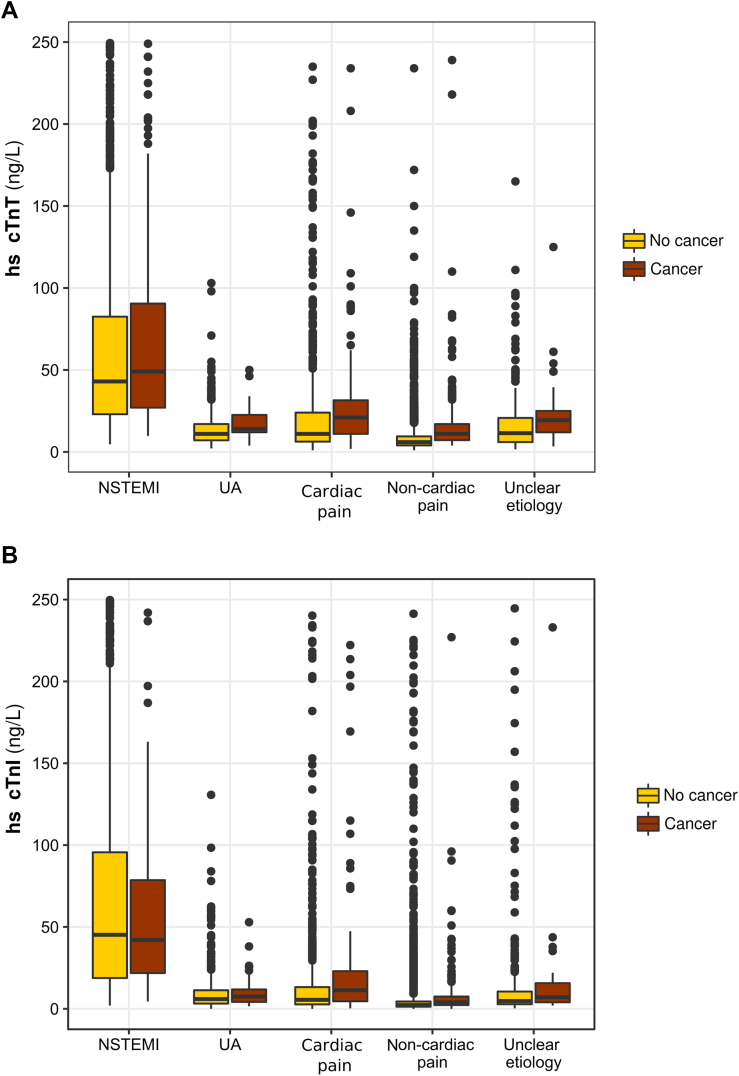
As shown in Table 2, concentrations of both 0- and 1-hour hs-cTnT, hs-cTnI, N-terminal pro–brain natriuretic peptide, and high-sensitivity C-reactive protein were significantly higher in patients with cancer than in those without. Moreover, 0-hour hs-cTnT and hs-cTnI concentrations were higher in patients with cancer with non-AMI causes of acute chest pain than in patients without cancer (Figures 2A and 2B).
Table 2.
Cardiovascular Biomarkers in Patients With and Those Without Cancer
| Overall (n = 8,267) |
No Cancer (n = 7,556) |
Cancer (n = 711) |
P Value | |
|---|---|---|---|---|
| hs-cTnT at 0 h, ng/L | 9 (4-23) | 8 (4-21) | 17 (9-36) | <0.001 |
| hs-cTnT at 1 h, ng/L | 9 (4-23) | 8 (4-22) | 17 (9-37) | <0.001 |
| Mean absolute hs-cTnT 0- to 1-h change, ng/L | 17 ± 217 | 17 ± 221 | 15 ± 160 | <0.001 |
| Median absolute hs-cTnT 0- to 1-h change, ng/L | 1 (0-2) | 1 (0-2) | 1 (0-3) | <0.001 |
| hs-cTnI at 0 h, ng/L | 5 (2-19) | 4 (2-18) | 9 (4-31) | <0.001 |
| hs-cTnI at 1 h, ng/L | 4 (2-19) | 4 (2-18) | 9 (4-34) | <0.001 |
| Mean absolute hs-cTnI 0- to 1-h change, ng/L | 134 ± 1,280 | 127 ± 1,167 | 208 ± 2,149 | <0.001 |
| Median absolute hs-cTnI 0- to 1-h change, ng/L | 1 (0-3) | 1 (0-3) | 1 (0-5) | <0.001 |
| NT-proBNP, pg/mL (n = 5,072) | 629 (246-1,866) | 571 (230-1,633) | 1,772 (687-4,006) | <0.001 |
| hs-CRP, mg/L (n = 5,018) | 2 (1-5) | 2 (1-5) | 3 (1-11) | <0.001 |
Values are median (IQR) or mean ± SD. For readability, no decimal figures are presented.
hs-CRP = high-sensitivity C-reactive protein; hs-cTnI = high-sensitivity cardiac troponin I; hs-cTnT = high-sensitivity cardiac troponin T; NT-proBNP = N-terminal pro–brain natriuretic peptide.
Figure 2.
hs-cTnT and hs-cTnI Concentrations in Different Diagnostic Groups
(A) Boxplot showing high-sensitivity cardiac troponin T (hs-cTnT) concentrations in the different final adjudicated diagnosis groups according to cancer status. NSTEMI, P = 0.57; UA, P < 0.001; noncoronary cardiac pain, P < 0.001; noncardiac pain, P < 0.001; unclear etiology, P = 0.001. (B) Boxplot showing high-sensitivity cardiac troponin I (hs-cTnI) concentrations in the different final adjudicated diagnosis groups according to cancer status. NSTEMI, P = 0.37; UA, P = 0.04; cardiac pain, P < 0.001; noncardiac pain, P < 0.001; unclear etiology, P = 0.006. Abbreviations as in Figure 1.
Standardized prevalence ratios of cancer subtypes
As shown in Supplemental Figure 5, the prevalence rates, assessed using the standardized prevalence ratio, of esophageal, lung, and testicular cancers and of myelodysplastic syndromes were higher in this ED chest-pain cohort than expected according to the reference prevalence. In contrast, melanoma, brain and thyroid cancers, Hodgkin lymphoma, and myeloproliferative syndromes were less frequent than expected.
Diagnostic accuracy of CPCs, ECG findings, and hs-cTnT and hs-cTnI concentrations
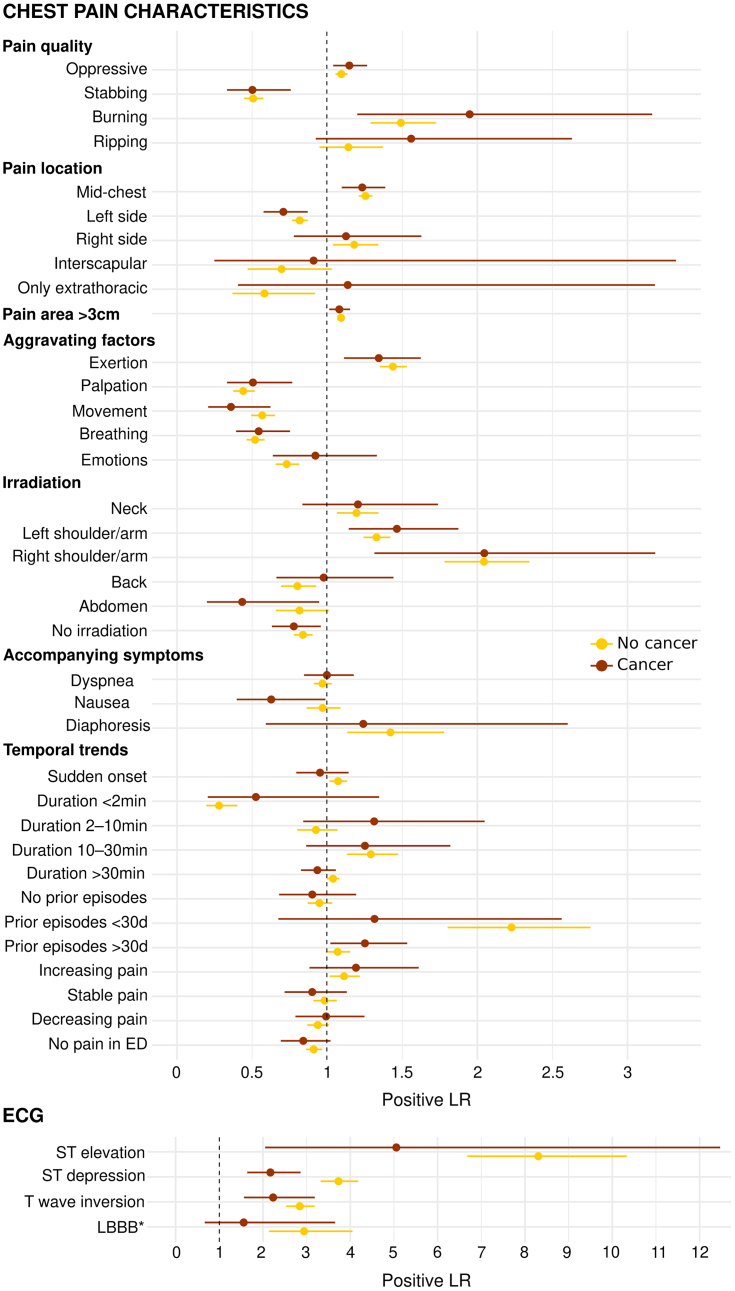
Overall, the diagnostic accuracy for AMI of predefined binary CPCs and ECG findings as quantified by the LR+ were similar in patients with and those without histories of cancer (Figure 3, Supplemental Table 11). In contrast, ST-segment depression showed lower diagnostic accuracy (P for interaction = 0.008) in patients with cancer (LR+ = 2.2; 95% CI: 1.6-2.9) compared with patients without (LR+ = 3.7; 95% CI: 3.3-4.2). Analogous results were obtained in the subgroup analysis of patients with active cancer (Supplemental Table 12, Supplemental Figure 6).
Figure 3.
Diagnostic Accuracy for AMI of CPCs and ECG Findings According to Cancer Status
Positive likelihood ratios (LRs) (and their 95% CIs) of predefined chest pain characteristics and findings on electrocardiography (ECG) for the diagnosis of acute myocardial infarction according to cancer status. The P value for interaction was statistically significant only for ST-segment depression (P = 0.008). Patients with cancer, n = 686; patients without cancer, n = 7,407. ∗Previously unknown left bundle branch block (LBBB). ED = emergency department.
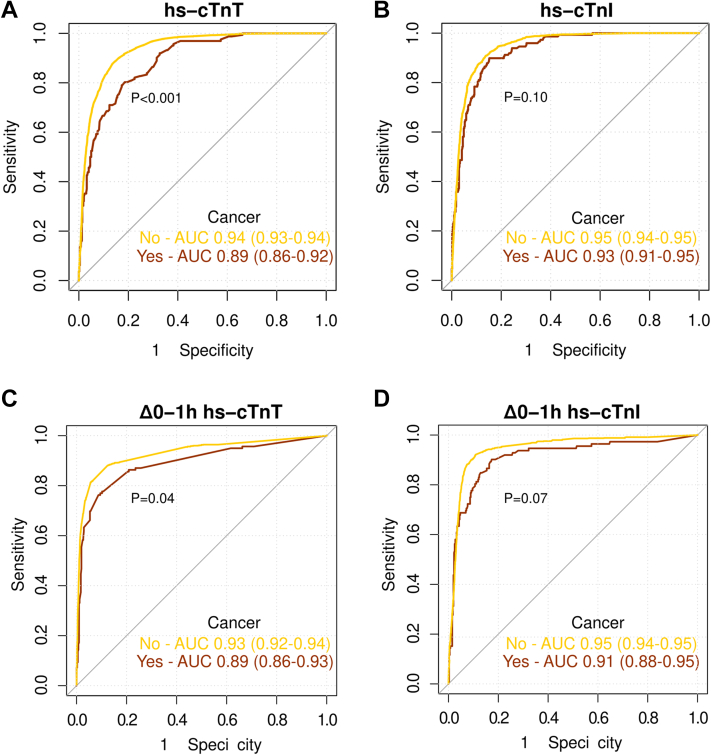
The diagnostic accuracy of hs-cTnT at ED presentation for the diagnosis of NSTEMI, although still high, was significantly lower in patients with histories of cancer compared with those without cancer (AUC: 0.89 [95% CI: 0.86-0.92] vs 0.94 [95% CI: 0.93-0.94]; P < 0.001). In contrast, the diagnostic accuracy of hs-cTnI concentrations at ED presentation for the diagnosis of NSTEMI was comparable in patients with histories of cancer compared with those without cancer (AUC: 0.93 [95% CI: 0.91-0.95] vs 0.95 [95% CI: 0.94-0.95]; P = 0.10) (Figure 4, Central Illustration). These results were consistent when controlling for other important diagnostic predictors of NSTEMI (age, sex, glomerular filtration rate, ECG findings, history of coronary artery disease, hypertension, diabetes mellitus, and active smoking) in multivariable models in patients with vs those without cancer (for hs-cTnT, AUC: 0.90 [95% CI: 0.88-0.93] vs 0.94 [95% CI: 0.94-0.95] [P = 0.002]; for hs-cTnI, AUC: 0.93 [95% CI: 0.91-0.95] vs 0.95 [95% CI: 0.94-0.90] [P = 0.17]). The diagnostic accuracy of 0/1-hour absolute changes in both hs-cTnT and hs-cTnI for NSTEMI was high in patients with cancer but slightly lower compared with that in patients without cancer.
Figure 4.
Diagnostic Performance of hs-cTnT and hs-cTnI in Patients With and Those Without Cancer
Receiver-operating characteristic curves of baseline hs-cTnT (A) and hs-cTnI (B) and 0-hour (C) and 1-hour (D) absolute change (Δ0-1h) values for the diagnosis of non–ST-segment elevation myocardial infarction according to cancer status. AUC = area under the curve; other abbreviations as in Figure 2.
Subgroup analyses showed that overall, these findings were consistent among subgroups (active or inactive, advanced or early stage, solid or hematologic malignancy, any cancer therapy or no cancer therapy, and previous cancer therapy or ongoing cancer therapy) (Supplemental Figure 7).
When evaluating the performance of 2 clinically relevant assay-specific cutoffs (upper reference limit and ESC 0/1-hour rule-in) in patients with cancer, the specificity of hs-cTnT, but not hs-cTnI, was markedly reduced. In contrast, the sensitivity of hs-cTnT, but not hs-cTnI, increased in patients with cancer (Supplemental Table 13).
Performance of the ESC 0/1-hour hs-cTnT and hs-cTnI algorithms in patients with cancer
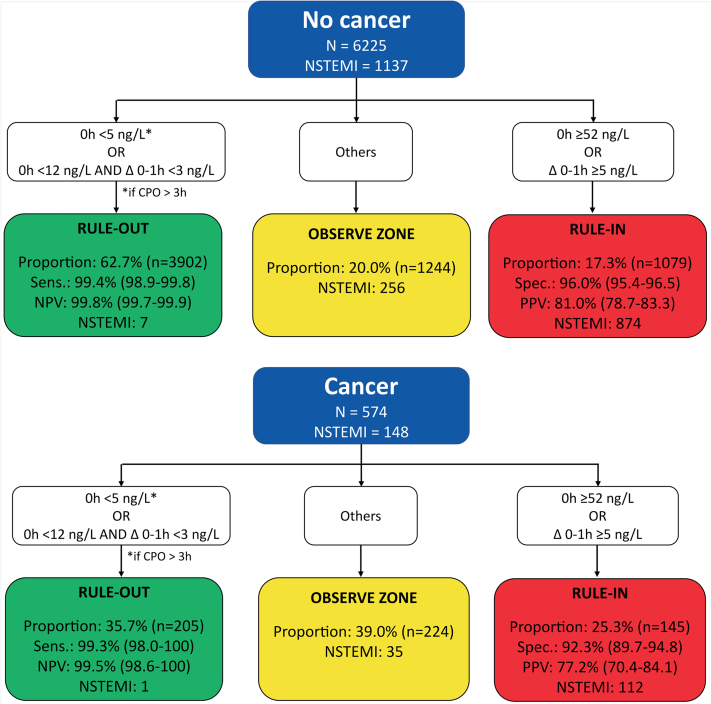
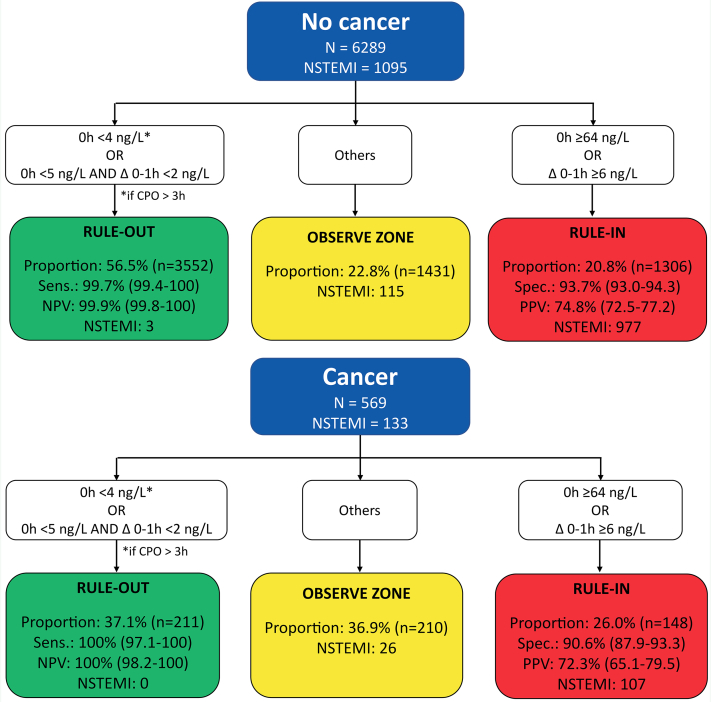
In patients with cancer, the ESC 0/1-hour hs-cTnT algorithm maintained very high safety for the rule-out of NSTEMI, comparable with that in patients without cancer (sensitivity, 99.3% [95% CI: 98.0%-100%] vs 99.4% [95% CI: 98.9%-99.8%], respectively [P > 0.99]; negative predictive value, 99.5% [95% CI: 98.6%-100%] vs 99.8% [95% CI: 99.7%-99.9%], respectively [P = 0.87]) (Figure 5, Central Illustration). However, rule-out efficacy was significantly lower in patients with cancer (35.7% vs 62.7%; P < 0.001). Also, rule-in accuracy was lower in patients with cancer compared with those without cancer (specificity, 92.3% [95% CI: 89.7%-94.8%] vs 96.0% [95% CI: 95.4%-96.5%] [P < 0.001]; positive predictive value, 77.2% [95% CI: 70.4%-84.1%] vs 81.0% [95% CI: 78.7%-83.3%] [P = 0.34], respectively), triaging more patients to rule-in (25.3% vs 17.3%, respectively; P < 0.001). Overall, the efficacy of the ESC 0/1-hour hs-cTnT algorithm was lower in patients with cancer versus those without cancer (61.0% vs 80.0%; P < 0.001), as reflected by the higher proportion of patients with cancer remaining in the observe zone (39.0% vs 20.0%; P < 0.001). Similar results were obtained when applying the ESC 0/1-hour hs-cTnI algorithm (Figure 6).
Figure 5.
Performance of the European Society of Cardiology 0/1-Hour Algorithm With High-Sensitivity Cardiac Troponin T in Patients With and Those Without Cancer
Diagnostic performance measures for the 3 European Society of Cardiology triage groups in patients with and those without cancer. The observe-zone proportion was higher and the specificity was lower in patients with cancer. CPO = chest pain onset; NPV = negative predictive value; NSTEMI = non–ST-segment elevation myocardial infarction; PPV = positive predictive value; Sens. = sensitivity; Spec. = specificity.
Figure 6.
Performance of the European Society of Cardiology 0/1-Hour Algorithm With High-Sensitivity Cardiac Troponin I in Patients With and Those Without Cancer
Overall efficacy in patients without cancer was 77.2% and in patients with cancer was 63.1% (P < 0.001). Abbreviations as in Figure 5.
In patients with cancer triaged toward the observe zone, noncardiac pain was the most common final adjudicated diagnosis (97 [43.3%] for hs-cTnT and 92 [43.8%] for hs-cTnI), while 35 patients (15.6%) for hs-cTnT and 26 (12.4%) for hs-cTnI had NSTEMI.
Long-term outcomes
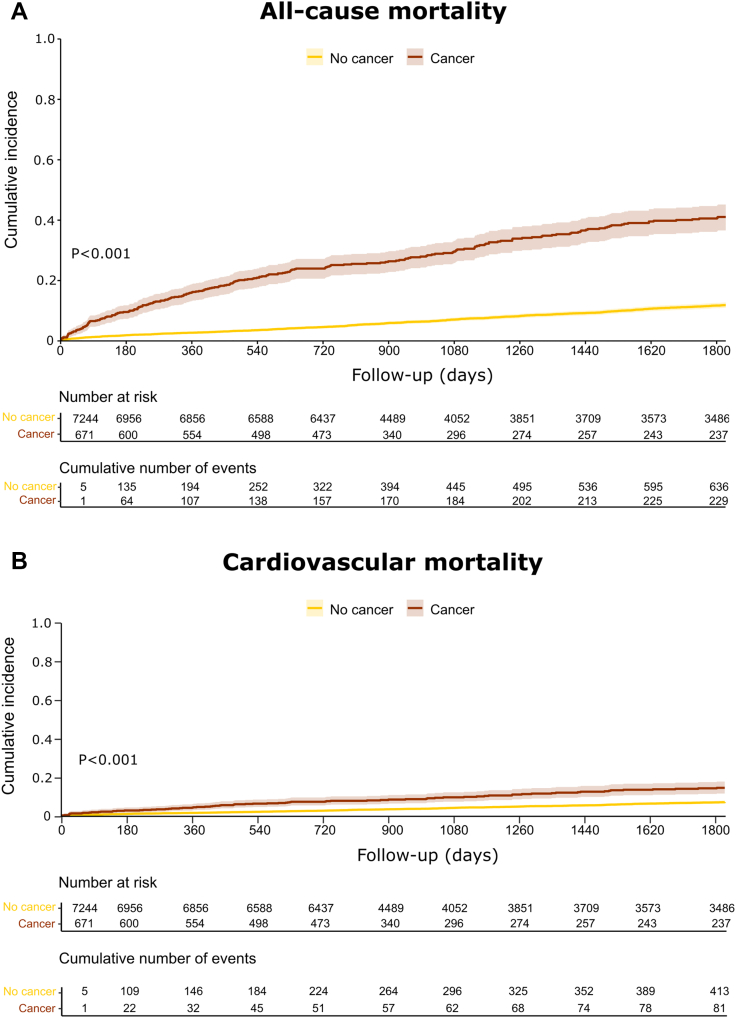
The median duration of follow-up was 1,449 days (IQR: 754-2,539 days). All-cause mortality at 5 years was 34.4% in patients with cancer versus 8.9% in those without cancer (P < 0.001, log-rank test) (Figure 7A). The 5-year cumulative incidence of cardiovascular mortality in patients with cancer was 15.0% versus 7.6% in those without cancer (P < 0.001, Gray’s test) (Figure 7B). The cumulative incidence of future AMI at 5 years was 8.1% in patients with cancer versus 6.5% in those without cancer (P = 0.20, Gray’s test) (Supplemental Figure 8).
Figure 7.
Outcomes of Patients With Cancer Presenting to the Emergency Department With Chest Pain
Kaplan-Meier plot showing 5-year all-cause mortality (A) and cumulative incidence function plot showing 5-year cardiovascular mortality (B) in patients with and without cancer.
External validation in the TRAPID-AMI study
Among 1,246 patients eligible for externally validation of the primary study endpoints (Supplemental Figure 9), 131 (10.5%) had active or past cancer. Overall, findings in the external validation cohort were comparable with those in the main cohort. Patients with cancer were older (72 years vs 60 years) and had more cardiovascular risk factors and a higher burden of disease (Supplemental Table 14). The concentrations of cardiovascular biomarkers (hs-cTnT and N-terminal pro–brain natriuretic peptide) were also higher in patients with cancer at ED presentation versus patients without cancer (Supplemental Table 15). A final adjudicated diagnosis of NSTEMI was more common in patients with cancer than those without cancer (20.6% vs 14.3%; P = 0.050) (Supplemental Table 15).
The LR+ for AMI of 20 CPCs and 4 ECG criteria were similar in patients with versus without histories of cancer (Supplemental Table 16, Supplemental Figure 10). The diagnostic accuracy of hs-cTnT concentration at ED presentation for NSTEMI was lower in patients with cancer versus those without cancer (AUC: 0.87 [95% CI: 0.79-0.95] vs 0.92 [95% CI: 0.90-0.94]) (Supplemental Figure 11). In patients with active or past cancer, the ESC 0/1-hour hs-cTnT algorithm maintained very high safety and high accuracy but had lower efficacy, with more patients remaining in the observe zone compared with those without cancer (36.2% vs 20.5%, respectively; P < 0.001) (Supplemental Figure 12).
Discussion
This secondary analysis of a large prospective, multicenter diagnostic cohort represents to the best of our knowledge the first study evaluating the prevalence of AMI, time to discharge from the ED, and long-term outcomes among consecutive patients with active or past cancer presenting with acute chest pain to the ED, as well as the diagnostic performance of the 3 main pillars in the early diagnosis of AMI in these patients: CPCs, ECG findings, and hs-cTnT and hs-cTnI.10 We report 7 major findings.
First, patients with active or past cancer presenting to the ED with acute chest pain had about 1.5 times the prevalence of a final adjudicated diagnosis of NSTEMI compared with patients without cancer. This was true for both type 1 and type 2 NSTEMI. Older age, higher prevalence of cardiovascular risk factors, and the effects of cancer and cancer therapies (eg, prothrombotic effects, vasospasm, critical anemia, previous mediastinal radiotherapy) may have contributed to this difference.7,34 Tachycardia remained the most frequent type 2 NSTEMI trigger, although anemia was more frequent in patients with cancer than in those without. In our cohort, a greater proportion of patients with cancer were treated with long-term cardiovascular medications at presentation compared with those without cancer. This contrasts with other data suggesting that patients with cancer are undertreated with regard to cardiovascular medications.35 Two reasons may in part explain these findings. First, in our chest pain cohort, patients with cancer were on average 15 years older than those without cancer, with more cardiovascular risk factors and pre-existing cardiac disorders. Second, the cohort (patients with chest pain, men and women, median age 75 years), setting (EDs) and health care systems (Europe) differed from those in other studies. For example, Sun et al36 investigated the management of cardiovascular risk factors among U.S. veterans (median age 66 years) with prostate cancer. Klimis et al35 also investigated risk factor control in patients with prostate cancer (mean age 68 years) from 24 sites in Canada, Israel, Brazil, and Australia (all non-European health care systems). In contrast to long-term cardiovascular medications at presentation, when assessing management in patients who were diagnosed with ACS, patients with cancer had lower rates of diagnostic coronary angiography and percutaneous coronary intervention compared with those without cancer. These results extend previous findings showing that in patients with STEMI with histories of cancer, percutaneous coronary intervention was underused.37,38 However, differences in management among patients with ACS seemed to be specific only for invasive diagnostic procedures and invasive treatments. There were no differences in the numbers of noninvasive diagnostic tests nor in anti-ischemic medications at discharge.
Second, the length of stay in the ED was increased by about 1 hour in patients with cancer versus patients without cancer, suggesting a more difficult and therefore longer diagnostic evaluation. Moreover, patients with cancer had a higher hospitalization rate, even if AMI had been ruled out.
Third, some cancer subtypes were more (or less) likely to present with chest pain than others: thoracic (such as esophageal and lung cancer) and testicular cancers were more prevalent in this cohort than expected, whereas thyroid cancer and melanoma were less prevalent. This is consistent with a previous observation suggesting that the adjusted HRs for AMI are increased for thoracic cancers and decreased for melanoma and endocrine cancers.5,39 Regarding hematological malignancies, myelodysplastic syndromes were more prevalent, whereas Hodgkin lymphoma and myeloproliferative syndromes were less prevalent.
Fourth, all CPCs and most ECG findings showed a similar diagnostic accuracy for AMI regardless of cancer status, and these results remained consistent also in the subgroup analysis with patients with active cancer. Only ST-segment depression had a lower, but still high, LR+ for AMI in patients with cancer. It remains controversial whether and to what extent patients with cancer may present with more atypical AMI symptoms and whether cancer could mask ischemic symptoms.7,12 Two recent single-center studies that were based on retrospective analyses of 201 and 456 patients with AMI and cancer suggested as much.12,13 In contrast, this study, based on 2 large prospective multicenter cohorts recruiting unselected patients with any kind of acute chest discomfort, demonstrated that all CPCs showed similar diagnostic accuracy for AMI regardless of cancer status and that these results remained consistent also in the subgroup analysis with patients with active cancer. Limitations of this study as well as the prior studies highlight the need for future research. In this study we could not exactly quantify the prevalence of AMI with atypical presentations or even asymptomatic AMI in patients with cancer, as some form of acute chest discomfort was required for enrollment. The lack of standardized assessment in a large number of patients presenting with a very broad range of symptoms possibly representing AMI, including asymptomatic individuals, and the lack of central adjudication of AMI by 2 independent cardiologists according to the universal definition of myocardial infarction was the reason in the prior studies. Despite the remaining uncertainty, physicians should be aware of the possibility of atypical presentation of AMI in patients with cancer.40
Fifth, the diagnostic accuracy of hs-cTnT concentrations for the diagnosis of NSTEMI, considering either the 0-hour or the absolute 1-hour change concentrations, although still high (AUC: 0.89), was lower in patients with cancer versus patients without cancer. Analogous findings were obtained in the TRAPID-AMI cohort. In contrast, this reduction in diagnostic accuracy was not seen for hs-cTnI. This discordance may suggest that chronic cardiac diseases, including cardiac effects of cancer and cardiotoxic therapies in patients with cancer, result in a more pronounced release of hs-cTnT versus hs-cTnI. Moreover, a recent translational study suggested that also active chronic skeletal muscle disease may contribute to increased hs-cTnT concentrations in patients without AMI.41 Further research is needed to better understand the release pattern between the T and I troponin subunits, as discrimination for hs-cTnI was higher than for hs-cTnT.
Sixth, in patients with active or past cancer, the ESC 0/1-hour algorithm maintained very high safety for rule-out of NSTEMI, comparable with patients without cancer, for using either hs-cTnT or hs-cTnI. However, overall, the efficacy of the ESC 0/1-hour algorithm was lower in patients with cancer versus patients without cancer, as the proportion of patients remaining in the observe zone nearly doubled. This was similar for hs-cTnT and hs-cTnI, despite slightly better discrimination for hs-cTnI than hs-cTnT in the receiver-operating characteristic curve analysis. Future studies trying to derive and validate ESC 0/1-hour hs-cTnT and hs-cTnI algorithm cutoffs optimized for use in patients with cancer are warranted.42
Seventh, 5-year all-cause and cardiovascular mortality were about 4 times and about 2 times higher in patients with cancer versus patients without cancer, respectively. The lower rates of diagnostic coronary angiography and percutaneous coronary intervention may have contributed to the higher 5-year all-cause and cardiovascular mortality in patients with cancer versus patients without cancer. This corroborates and extends prior findings in studies involving both the general population and patients undergoing percutaneous coronary intervention.5,43,44 In a single-center percutaneous coronary intervention cohort, a composite of all-cause death, AMI, and revascularization occurred in 48.6% patients with cancer and 33% of patients without cancer within 5 years. In a population-based retrospective cohort study conducted among 4,519,243 adults residing in Alberta, Canada, patients with cancer had HRs of 1.33 (95% CI: 1.29-1.37) for cardiovascular mortality and 1.01 (95% CI: 0.97-1.05) for myocardial infarction compared with subjects without cancer. This evidence might suggest that patients with active or past cancer should be considered at higher risk for cardiovascular events, and thus, optimization of cardiovascular risk factor therapies (eg, hypertension, diabetes, dyslipidemia, obesity) in this population is of utmost importance.45,46
The findings of this large multicenter diagnostic study have important clinical consequences. First, high awareness for the presence of AMI is needed in patients with active or past cancer presenting with acute chest pain or discomfort to the ED, as the prevalence of AMI is substantially higher compared with patients without cancer. Second, the initial diagnostic work-up should continue to be based on the integration of the information obtained from the 3 main diagnostic pillars: clinical characteristics including CPCs, 12-lead electrocardiography, and serial hs-cTnT and hs-cTnI concentrations.7,8,10,47 CPCs, ECG changes suggestive of AMI, and serial hs-cTnT and hs-cTnI concentrations are useful also in patients with cancer. Third, ED physicians and cardiologists should, however, be aware of the lower specificity of hs-cTnT and hs-cTnI elevations for AMI because of the confounding effects of age, pre-existing cardiac disease, cancer, and cancer therapies, particularly ongoing therapy with anthracyclines.34,48 Fourth, application of the ESC hs-cTnT and hs-cTnI algorithms continues to provide very high safety for the rule-out of AMI, high positive predictive value for the rule-in of AMI, but reduced efficacy. Therefore, the proportion of patients requiring invasive or noninvasive coronary imaging for accurate diagnosis will be higher compared with patients without cancer. Fifth, future studies should investigate whether diagnostic algorithms optimized for patients with cancer would be able to mitigate the efficacy and specificity deficit of the current ESC 0/1-hour hs-cTnT and hs-cTnI algorithms. Importantly, these findings corroborate and extend suggestions made in a recent consensus document targeting the acute cardiovascular care in patients with cancer.7 Given the descriptive nature of our study and the lack of multivariable adjustment in most of our comparisons, causal interpretations should not be drawn (eg, that cancer causes the decreased diagnostic performance of hs-cTnT). Future studies will need to elucidate whether the observed differences between patients with and those without cancer are due to cancer or a result of other risk factors rather than cancer, as patients with cancer were older, had more cardiovascular risk factors and lower renal function, and a subgroup received cardiotoxic chemotherapeutic agents, all known factors of cardiac damage and thus hs-cTnT and hs-cTnI elevation. Causal inference methods (eg, propensity score matching and confounder adjustment) might help explain why there were observed differences in biomarkers performance in the 2 populations of interest.
Study limitations
First, because of its descriptive nature, in the present study we could not determine causality. Second, the findings presented were obtained from 2 prospective diagnostic studies. Implementation studies prospectively applying the ESC 0/1-hour hs-cTnT and hs-cTnI algorithms in patients with cancer for clinical decision making are warranted.
Third, this was a secondary analysis from the APACE study, and consequently no specific sample size calculation was performed. Although this secondary analysis is the largest diagnostic study to date in patients with active or past cancer, it still may have been underpowered for some comparisons.
Fourth, the findings cannot be generalized to patients undergoing long-term dialysis, as they were excluded from the study. Fifth, not all patients in APACE had 1-hour high-sensitivity cardiac troponin samples, possibly introducing a small selection bias for this analysis (eg, adjudicated final diagnosis of NSTEMI was 13.7% in patients without 1-hour sample vs 18.9% in those with 1-hour samples). As a result, calculated sensitivities and specificities of the ESC 0/1-hour algorithm would not have been affected; however, prevalence-dependent measures such as negative predictive value would likely be slightly higher (eg, higher safety for rule-out) than reported, whereas the positive predictive value would likely by slightly lower (eg, lower accuracy for rule-in).
Sixth, “active” cancer was defined according to the definition of the Haemostasis and Cancer Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Despite this, the definition of active cancer is not univocal and varies according to different scientific societies, as a patient with a diagnosis of cancer of >6 months’ duration may still have active cancer.49
Conclusions
Patients with current or past cancer have a substantially higher prevalence of AMI when presenting with acute chest discomfort to the ED compared with patients without cancer. Longer length of stay in the ED and higher rate of hospitalization further document the increased complexity of their work-up. Higher burden of chronic cardiac disease related or unrelated to cancer and cancer therapy increased the prevalence of cardiomyocyte injury and thereby reduced the efficacy, but not the safety, of the ESC 0/1-hour algorithm.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients with active or past cancer presenting with chest pain have a high likelihood of NSTEMI. The diagnostic performance of hs-cTnT, but not hs-cTnI, was reduced. Nonetheless, the efficacy of both the ESC 0/1-hour hs-cTnT and hs-cTnI algorithms is reduced compared with those in patients without cancer.
TRANSLATIONAL OUTLOOK: The benefits of adjusting the ESC algorithm cutoffs in patients with cancer is unknown and requires further investigation. Strict control and therapy optimization of cardiovascular risk factors in all patients with cancer undergoing cancer therapies, starting from baseline, during the overall pathway of care are important.
Funding Support and Author Disclosures
APACE was supported by research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the European Union, the Cardiovascular Research Foundation Basel, the University Hospital Basel, the University of Basel, Abbott, Beckman Coulter, Roche, Ortho Clinical Diagnostics, Quidel, Siemens, and Singulex. Dr Lopez-Ayala has received research grants from the Swiss Heart Foundation (FF20079 and FF21103); and has received speaker honoraria from Quidel, paid to the institution, outside the submitted work. Dr Nestelberger has received research support from the Swiss National Science Foundation (P400PM_191037/1), the Prof Dr Max Cloëtta Foundation, Margarete und Walter Lichtenstein-Stiftung (3MS1038), and the University Hospital Basel; and has received speaker honoraria and consulting honoraria from B. Braun, Siemens, Beckman Coulter, Bayer, Ortho Clinical Diagnostics, and Orion Pharma, outside the submitted work. Dr Boeddinghaus has received research grants from the University of Basel and the Division of Internal Medicine, the Swiss Academy of Medical Sciences, and the Gottfried and Julia Bangerter-Rhyner-Foundation; and has received speaker honoraria from Siemens, outside the submitted work. Dr Koechlin has received research grants from the University of Basel, the Swiss Academy of Medical Sciences, the Gottfried and Julia Bangerter-Rhyner Foundation, and Freiwillige Akademische Gesellschaft Basel; and has received speaker honoraria from Roche Diagnostics, Abbott, and Siemens, outside the submitted work. Dr Rubini Gimenez has received research grants from the Swiss Heart Foundation and the Swiss National Science Foundation (P400PM_180828); and has received speaker and consulting honoraria from Abbott, Ortho Clinical Diagnostics, Roche, and Siemens, outside the submitted work. Dr Martin-Sanchez has received speaker, advisory, or consulting fees from Novartis, Merck Sharpe & Dohme, Bristol Myers Squibb, Pfizer, The Medicines Company, Otsuka, Thermo Fisher Scientific, Cardiorentis, and Sanofi; and has received research grants from the Spanish Ministry of Health and FEDER, Mapfre, Novartis, Bayer, Merck Sharpe & Dohme, Abbott, and Orion-Pharma, outside the submitted work. Dr Mueller has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the University Hospital Basel, the University of Basel, Beckman Coulter, Biomerieux, Brahms, Mitsubishi, Novartis, Ortho Clinical, QuAcuidel, Roche, Siemens, Singulex, and Sphingotec; and has received speaker honoraria and consulting honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Osler, Novartis, Roche, Siemens, SpinChip, and Singulex, outside the submitted work, and all paid to the institution. Dr Wildi has received funding from Julia und Gottfried Bangerter Stiftung, the University of Basel, the Wesley Medical Research Foundation, the Prince Charles Hospital Foundation, and the University of Queensland, all outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. The investigated high-sensitivity cardiac troponin assays were donated by the manufacturer, who had no role in the design of the study, the analysis of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
Acknowledgments
The authors thank the patients who participated in the study and the ED staff as well as the laboratory technicians of all participating sites for their most valuable efforts.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables, figures, a list of R packages used for the analysis, and additional APACE and TRAPID-AMI investigators and contributors, please see the online version of this paper.
Appendix
References
- 1.Dagenais G.R., Leong D.P., Rangarajan S., et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020;395:785–794. doi: 10.1016/S0140-6736(19)32007-0. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Rutherford M.J., Bardot A., et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 4.Navi B.B., Reiner A.S., Kamel H., et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson D.I., Wiebe N., Cheung W.Y., et al. Incident cardiovascular disease among adults with cancer: a population-based cohort study. J Am Coll Cardiol CardioOnc. 2022;4:85–94. doi: 10.1016/j.jaccao.2022.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gevaert S.A., Halvorsen S., Sinnaeve P.R., et al. Evaluation and management of cancer patients presenting with acute cardiovascular disease: a consensus document of the Acute CardioVascular Care (ACVC) Association and the ESC Council of Cardio-Oncology—part 1: acute coronary syndromes and acute pericardial diseases. Eur Heart J Acute Cardiovasc Care. 2021;10:947–959. doi: 10.1093/ehjacc/zuab056. [DOI] [PubMed] [Google Scholar]
- 8.Gallaway M.S., Idaikkadar N., Tai E., et al. Emergency department visits among people with cancer: Frequency, symptoms, and characteristics. J Am Coll Emerg Physicians Open. 2021;2 doi: 10.1002/emp2.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morello F., Bima P., Ferreri E., et al. After the first wave and beyond lockdown: long-lasting changes in emergency department visit number, characteristics, diagnoses, and hospital admissions. Intern Emerg Med. 2021;16:1683–1690. doi: 10.1007/s11739-021-02667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collet J.P., Thiele H., Barbato E., et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 11.Ibanez B., James S., Agewall S., et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2017;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S.W., Daraban N., Abbasi N., Lei X., Durand J.B., Daher I.N. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol. 2012;35:443–450. doi: 10.1002/clc.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balanescu D.V., Donisan T., Deswal A., et al. Acute myocardial infarction in a high-risk cancer population: outcomes following conservative versus invasive management. Int J Cardiol. 2020;313:1–8. doi: 10.1016/j.ijcard.2020.04.050. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hawwas M., Tsitlakidou D., Gupta N., Iliescu C., Cilingiroglu M., Marmagkiolis K. Acute coronary syndrome management in cancer patients. Curr Oncol Rep. 2018;20:78. doi: 10.1007/s11912-018-0724-8. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Ayala P., Nestelberger T., Boeddinghaus J., et al. Novel criteria for the observe-zone of the ESC 0/1h-hs-cTnT algorithm. Circulation. 2021;144:773–787. doi: 10.1161/CIRCULATIONAHA.120.052982. [DOI] [PubMed] [Google Scholar]
- 16.Koechlin L., Boeddinghaus J., Nestelberger T., et al. Clinical presentation of patients with prior coronary artery bypass grafting and suspected acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2021;10:746–755. doi: 10.1093/ehjacc/zuaa020. [DOI] [PubMed] [Google Scholar]
- 17.Coscia T., Nestelberger T., Boeddinghaus J., et al. Characteristics and outcomes of type 2 myocardial infarction. JAMA Cardiol. 2022;7:427–434. doi: 10.1001/jamacardio.2022.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boeddinghaus J., Nestelberger T., Lopez-Ayala P., et al. Diagnostic performance of the European Society of Cardiology 0/1-h algorithms in late presenters. J Am Coll Cardiol. 2021;77:1264–1267. doi: 10.1016/j.jacc.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Boeddinghaus J., Nestelberger T., Koechlin L., et al. Early diagnosis of myocardial infarction with point-of-care high-sensitivity cardiac troponin I. J Am Coll Cardiol. 2020;75:1111–1124. doi: 10.1016/j.jacc.2019.12.065. [DOI] [PubMed] [Google Scholar]
- 20.Twerenbold R., Costabel J.P., Nestelberger T., et al. Outcome of applying the ESC 0/1-hour algorithm in patients with suspected myocardial infarction. J Am Coll Cardiol. 2019;74:483–494. doi: 10.1016/j.jacc.2019.05.046. [DOI] [PubMed] [Google Scholar]
- 21.Nestelberger T., Boeddinghaus J., Lopez-Ayala P., et al. Cardiovascular biomarkers in the early discrimination of type 2 myocardial infarction. JAMA Cardiol. 2021;6:771–780. doi: 10.1001/jamacardio.2021.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratmann P.D., Boeddinghaus J., Nestelberger T., et al. Extending the no objective testing rules to patients triaged by the European Society of Cardiology 0/1-hour algorithms. Eur Heart J Acute Cardiovasc Care. 2022;11(11):834–840. doi: 10.1093/ehjacc/zuac120. [DOI] [PubMed] [Google Scholar]
- 23.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Mueller C., Giannitsis E., Christ M., et al. Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T. Ann Emerg Med. 2016;68:76–87.e4. doi: 10.1016/j.annemergmed.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Khorana A.A., Noble S., Lee A.Y.Y., et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the. J Thromb Haemost. 2018;16:1891–1894. doi: 10.1111/jth.14219. [DOI] [PubMed] [Google Scholar]
- 26.Amin M., Edge S., Greene F., et al. 8th ed. Springer; New York: 2017. AJCC Cancer Staging Manual. [Google Scholar]
- 27.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 28.Nestelberger T., Boeddinghaus J., Wussler D., et al. Predicting major adverse events in patients with acute myocardial infarction. J Am Coll Cardiol. 2019;74:842–854. doi: 10.1016/j.jacc.2019.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Brown L.D., Cai T.T., DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16:101–133. [Google Scholar]
- 30.Ohsawa M., Tanno K., Okamura T., et al. Standardized prevalence ratios for atrial fibrillation in adult dialysis patients in Japan. J Epidemiol. 2016;26:272–276. doi: 10.2188/jea.JE20150077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Smith A., Crouch S., Oliver S., Roman E. Estimating the prevalence of hematological malignancies and precursor conditions using data from Haematological Malignancy Research Network (HMRN) Cancer Causes Control. 2016;27:1019–1026. doi: 10.1007/s10552-016-0780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 34.Shobayo F., Bajwa M., Koutroumpakis E., et al. Acute coronary syndrome in patients with cancer. Exp Rev Cardiovasc Ther. 2022;20:275–290. doi: 10.1080/14779072.2022.2063840. [DOI] [PubMed] [Google Scholar]
- 35.Klimis H., Pinthus J.H., Aghel N., et al. The burden of uncontrolled cardiovascular risk factors in men with prostate cancer. J Am Coll Cardiol CardioOnc. 2023;5:70–81. doi: 10.1016/j.jaccao.2022.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun L., Parikh R.B., Hubbard R.A., et al. Assessment and management of cardiovascular risk factors among US veterans with prostate cancer. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohamed M.O., Van Spall H.G.C., Kontopantelis E., et al. Effect of primary percutaneous coronary intervention on in-hospital outcomes among active cancer patients presenting with ST-elevation myocardial infarction: a propensity score matching analysis. Eur Heart J Acute Cardiovasc Care. 2021;10:829–839. doi: 10.1093/ehjacc/zuaa032. [DOI] [PubMed] [Google Scholar]
- 38.Bharadwaj A., Potts J., Mohamed M.O., et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J. 2020;41:2183–2193. doi: 10.1093/eurheartj/ehz851. [DOI] [PubMed] [Google Scholar]
- 39.Grilz E., Posch F., Nopp S., et al. Relative risk of arterial and venous thromboembolism in persons with cancer vs. persons without cancer—a nationwide analysis. Eur Heart J. 2021;42:2299–2307. doi: 10.1093/eurheartj/ehab171. [DOI] [PubMed] [Google Scholar]
- 40.Bisceglia I., Canale M.L., Lestuzzi C., et al. Acute coronary syndromes in cancer patients. J Cardiovasc Med. 2020;21:944–952. doi: 10.2459/JCM.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 41.du Fay de Lavallaz J., Prepoudis A., Wendebourg M.J., et al. Skeletal muscle disorders: a noncardiac source of cardiac troponin T. Circulation. 2022;145:1764–1779. doi: 10.1161/CIRCULATIONAHA.121.058489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Twerenbold R., Badertscher P., Boeddinghaus J., et al. 0/1-Hour triage algorithm for myocardial infarction in patients with renal dysfunction. Circulation. 2018;137:436–451. doi: 10.1161/CIRCULATIONAHA.117.028901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strongman H., Gadd S., Matthews A.A., et al. Does cardiovascular mortality overtake cancer mortality during cancer survivorship? An English retrospective cohort study. J Am Coll Cardiol CardioOnc. 2022;4:113–123. doi: 10.1016/j.jaccao.2022.01.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo W., Fan X., Lewis B.R., et al. Cancer patients have a higher risk of thrombotic and ischemic events after percutaneous coronary intervention. J Am Coll Cardiol Intv. 2021;14:1094–1105. doi: 10.1016/j.jcin.2021.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tawfiq E., Selak V., Elwood J.M., et al. Performance of cardiovascular disease risk prediction equations in more than 14 000 survivors of cancer in New Zealand primary care: a validation study. Lancet. 2023;401:357–365. doi: 10.1016/S0140-6736(22)02405-9. [DOI] [PubMed] [Google Scholar]
- 46.Lyon A.R., López-Fernández T., Couch L.S., et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 47.Writing Committee Members. Gulati M., Levy P.D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):e187–e285. doi: 10.1016/j.jacc.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 48.Michel L., Mincu R.I., Mahabadi A.A., et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22:350–361. doi: 10.1002/ejhf.1631. [DOI] [PubMed] [Google Scholar]
- 49.Nouhravesh N., Strange J.E., Tønnesen J., et al. Prognosis of acute coronary syndrome stratified by cancer type and status—a nationwide cohort study. Am Heart J. 2023;256:13–24. doi: 10.1016/j.ahj.2022.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.