Abstract
Over the past decade, the treatment landscape of chronic lymphocytic leukemia (CLL) has dramatically changed, shifting from cytotoxic chemotherapy to targeted therapies. Bruton’s tyrosine kinase (BTK) inhibitors have revolutionized the treatment of CLL and are increasingly applied in many other malignancies. However, ibrutinib, the first BTK inhibitor approved, is associated with serious toxicities, including atrial fibrillation in up to 38% of patients, ventricular arrhythmias, and other cardiovascular toxicities. Emerging data suggest several newer BTK inhibitors (eg, acalabrutinib, zanubrutinib) are still associated with cardiotoxic risks. This review examines the current state of evidence, including incidence rates, risk factors, mechanisms, and management strategies of cardiovascular toxicities with BTK inhibitors and other CLL therapies. We specifically focus on atrial fibrillation, ventricular arrhythmias/sudden death, hypertension, heart failure, bleeding, and stroke. We also touch on other emerging BTK therapies (eg, pirtobrutinib). Finally, we highlight key unanswered questions and future directions of research.
Key Words: acalabrutinib, atrial fibrillation, Bruton’s tyrosine kinase inhibitors, cardio-oncology, ibrutinib, sudden death, zanubrutinib
Central Illustration
Highlights
-
•
Although life-saving, BTK inhibitors are associated with an up to 3-5 fold increase in AF and other (eg, VA) events when compared with patients not exposed to BTK inhibitor therapy.
-
•
New or worsened hypertension is frequently seen with BTK inhibitor use (ibrutinib, acalabrutinib, zanubrutinib).
-
•
Although less than ibrutinib, newer BTK inhibitors still appear to link with residual cardiotoxic risks.
-
•
Development of BTK inhibitor-associated cardiovascular events (eg, AF, VAs) are associated with worse long-term cardiovascular and overall survival.
Ibrutinib is a novel first-generation Bruton’s tyrosine kinase inhibitor with dramatic efficacy in several hematologic malignancies, including chronic lymphocytic leukemia (CLL).1, 2, 3, 4, 5, 6 Due to its success, >200,000 patients are currently being treated with ibrutinib, and there are nearly 300 additional ongoing trials testing ibrutinib in CLL and other malignancies.7,8 However, ibrutinib is linked with a >4-fold increase in cardiac events compared with CLL patients not exposed to ibrutinib, including atrial fibrillation (AF), ventricular arrhythmias (VA), heart failure, and hypertension.1,9, 10, 11 The occurrence of these events presents a major dilemma for the continuation of this life-saving therapy, because currently there is limited pooled evidence to guide the determination of future or management of cardiotoxic events among patients with hematologic malignancies.4
The next-generation, more target-specific Bruton’s tyrosine kinase (BTK) inhibitor therapies (eg, acalabrutinib and zanubrutinib) are currently approved for CLL and were designed to potentially have less cardiovascular toxicity than ibrutinib.3 Available evidence suggests that these agents may have similar or improved efficacy to ibrutinib with less cardiac activity as evidenced by landmark oncologic trials.9,12,13 However, a growing number of cardiac-focused reports have suggested a residual increase in cardiovascular risk even with next-generation BTK inhibitor therapies.1,5,10,11 These observations provide serious questions for clinicians and investigators on the current state of evidence and practical considerations for the estimation and management of cardiovascular risk in this growing population.
In this review, we discuss the cardiovascular effects associated with BTK inhibitor therapy, and key practical considerations in the management of patients treated with this class of drug. We specifically discuss data and management of cardiac risk with ibrutinib and next-generation BTK inhibitor therapies. We also review additional agents within CLL, including B-cell lymphoma-2 (Bcl-2) inhibitors, and their potential cardiovascular risks.
We focus on the following common cardiovascular toxicities in this population as well as the available evidence and potential strategies to guide management:
-
•
Atrial fibrillation
-
•
Ventricular arrhythmias and sudden death
-
•
Hypertension
-
•
Heart failure
-
•
Bleeding
-
•
Stroke
History of Cardiovascular Complications With Cancer Therapies in CLL
Over the past decade, a better understanding of the key molecular pathways involved in CLL has led to revolutionary targeting of the triggers of disease. In CLL, aberrant B-cell receptor signaling is a key driver of the malignancy; and this can be selectively targeted for the control of disease. BTK is one such target. BTK is a member of the TEC kinase family and plays a key role in antigen-dependent B-cell receptor signaling responsible for B-cell proliferation. Through unchecked activation of this kinase, uncontrolled downstream signaling cascades trigger robust responses that are crucial in the development of most B-cell malignancies, including CLL. Noting this critical step in the proliferation cascade, inhibitory targeting of BTK was pivotally hypothesized to be a potential therapeutic target in the treatment of CLL and other hematologic malignancies.1
Currently, BTK inhibition is achieved by multiple means, most commonly with the introduction of small molecular inhibitors that interfere with the binding of the kinase to downstream substrates.1 Yet, these single and multitargeted kinases may be focused or display multiple downstream actions that affect cancer, cardiovascular, and other organ-system homeostasis. Although these kinase inhibitors may display dramatic anticancer effects, the unintended or unknown downstream off-target (or even on-target) effects may lead to unanticipated cardiovascular effects.1 These toxicities may manifest early or late, and the homeostatic milieu of these patients with cancer can provide unique challenges in the treatment of such effects. Given that BTK belongs to a family of enzymes with a multitude of enzymatic responses (including cardiac), inhibition of the kinase with small-molecule compounds may come with the potential for unintended cardiac side effects (Table 1).
Table 1.
Target and Indications for Approved BTK Inhibitors
| Drug | Mechanism of Action | Current Indications (or Phase of Clinical Testing) | Cardiotoxic Effects Reported |
|---|---|---|---|
| Ibrutinib | Irreversible inhibitor of BTK at C481 via covalent bond |
|
|
| Acalabrutinib | Irreversible inhibitor of BTK at C481 via covalent bond |
|
|
| Zanubrutinib | Irreversible inhibitor of BTK at C481 via covalent bond |
|
|
| Pirtobrutinib | Reversible inhibitor of BTK via noncovalent bond formation |
|
|
| Other common therapies (ie, venetoclax) | |||
| Venetoclax | Bcl-2 antagonist, thereby committing lymphoid cells to a pro-apoptotic state |
|
|
Bcl-2 = B-cell leukemia/lymphoma 2 protein; BTK = Burton’s tyrosine kinase; CLL = chronic lymphocytic leukemia.
Initially approved by the U.S. Food and Drug Administration (FDA) in 2014 for CLL, ibrutinib was originally developed as an irreversible inhibitor of BTK, a key regulator in the survival and propagation of many lymphoid cells. The observation that an inherited loss of B-cells, as seen in X-linked agammaglobulinemia, led to avoidance of CLL and other lymphoid malignancies in these patients spurred the repurposing of ibrutinib for CLL.1 Due to high success in CLL and other hematologic malignancies, and broad immunomodulatory effects, ibrutinib is now widely used as standard treatment across many malignancies. Given ibrutinib’s wide use, its adverse effect profile has been increasingly well characterized with the most notable cardiotoxic side effect of ibrutinib being AF. The development of ibrutinib-associated AF can lead to unique treatment challenges related to potential bleeding complications, and drug-drug interactions.
BTK Inhibitor-Associated AF
Incidence of AF
In early cancer-focused clinical trials, the incidence of new AF ranged from 5% to 17%, including high-grade (≥3) symptomatic events in up to 9% of patients.1,2 Retrospective studies have shown a median time to incident AF of 5 to 8 months.11 However, recent cardiac-focused surveillance data have demonstrated that 14 of 53 CLL patients treated with ibrutinib experienced AF at a median of 13 months (estimated cumulative incidence of 21% [95% CI: 7%-31%), 23% [95% CI: 9%-35%], and 38% [95% CI: 19%-53%) at 6, 12, and 24 months, respectively).14 Notably, this is the only available study in which electrocardiographic and systematic cardiac event monitoring were prospectively used among BTK inhibitor-treated patients.14 As a result, it is possible that the true AF risk (symptomatic and asymptomatic) with BTK inhibitor therapy may be substantially higher than reported in available cancer-focused trials.
Risk factors for AF
Although AF has become increasingly common in patients taking ibrutinib, the risk factors predisposing to these events remain largely unclear. In a meta-analysis of cancer trials, history of prior AF and age older than 65 years were associated with the development of (symptomatic) AF events among 1,505 ibrutinib-treated patients.15 Similar observations were noted in a preliminary acalabrutinib report.16 In cardiac-focused studies, the presence of left atrial remodeling, marked by atrial dilation, significantly increased the risk for ibrutinib-associated AF events.11,17 The presence of pre-existing cardiovascular disease (CVD) also appears to confer an increased risk of AF events.18 Furthermore, in an evaluation of 581 patients, the development of early hypertension after ibrutinib initiation was associated with a subsequent increase in AF events and other major cardiovascular events.19
Possible mechanisms
The mechanisms of ibrutinib and other BTK inhibitor-associated AF events are incompletely understood. Available data support both potential on- and off-target pathways. Inhibition of C-terminal Src kinase (CSK) may play a role in the development of AF and is a potential inhibitory target of BTK inhibitor therapy. CSK has been found to be enriched in the atria compared with other cardiomyocytes, and deletion of this enzyme has been shown to increase the incidence of AF, cardiac fibrosis, and inflammation (eg, IL-6 mediated).20 BTK-deficient mice treated with weeks of ibrutinib were found to have an increased incidence of AF, atrial fibrosis, and inflammation, with chemoproteomic profiling identifying CSK as a potential etiology of arrhythmia inducement.20 Additional proposed mechanisms of BTK inhibitor arrhythmias, particularly with ibrutinib, focus on PI3K/AKT inhibition and ion channel pathways.11,21 This includes possible enhanced Ca2+/calmodulin-dependent protein kinase II (CaMKII). In vitro studies have proposed that ibrutinib specifically may inhibit multiple currents of the cardiac action potential, including INa and Ito, possibly from the direct binding of ibrutinib to these channels.22,23 In vitro, the second-generation BTK inhibitor therapy acalabrutinib did not produce atrial arrhythmia, although these drugs still appear to confer increased AF risk in clinical practice.5,20 The mechanisms behind the residual AF risk seen with newer covalent BTK inhibitor therapy remain unexplored. Despite variations in proposed mechanisms, cardiac remodeling and fibrosis have consistently been a central downstream determinant of ibrutinib-associated AF events.23 Yet, as with the risk factors leading to arrhythmia, additional studies are needed to definitively determine the mechanism of BTK inhibitor-associated AF events.
Outcomes after AF development
Among those patients who develop AF while on a BTK inhibitor, available evidence suggests worse long-term outcomes compared with patients who do not develop AF events. In a retrospective study evaluating 280 CLL patients treated with ibrutinib, those with treatment-associated AF events had worse progression-free survival and a nearly 3-fold increase in long-term mortality.24 The reasons for this increase are not well understood but may relate to BTK inhibitor treatment disruptions, bleeding, stroke, and other AF-related complications. Alternatively, it remains unclear if dose reductions or therapeutic changes result in the worsening of the underlying malignancy.
Next-generation BTK inhibitors and AF risk
Given the propensity of ibrutinib to contribute to the formation of atrial arrhythmias, there was much excitement when the second-generation BTK inhibitor therapies began to see clinical use. These agents, including acalabrutinib and zanubrutinib, were developed to express greater selectivity toward BTK, thereby limiting off-target kinase inhibition that has been theorized to contribute to the cardiotoxic side effects of ibrutinib.
Next-generation BTK inhibitors (eg, acalabrutinib, zanubrutinib) appear to have fewer adverse cardiac effects than ibrutinib.5,6 Available cancer trials have suggested that the risk of AF may be attenuated with newer BTK inhibitor therapy (eg, 30%-45% reduction with acalabrutinib [5%-10% high-grade] and zanubrutinib [3%-6% high-grade]).5,6 In ELEVATE-RR, which compared acalabrutinib to ibrutinib in previously treated CLL, the reported incidence of AF was lower with acalabrutinib (9% vs 16%; P = 0.02).5 Pooled analysis of available clinical trials with acalabrutinib has suggested an AF incidence of 5% to 9%.13 Similarly, a phase III trial comparing zanubrutinib to ibrutinib in relapsed or refractory CLL also suggested a lower incidence of AF.4,6 Both agents link with a near 40% to 45% reduction in clinical (symptomatic) AF events. Yet, the arrhythmia monitoring strategies for these trials were heterogeneous and focused on brief oncologic office electrocardiograms (ECGs). As such, the true risk of AF remains unclear. This is supported by a preliminary report suggesting substantial residual AF risk of nearly 3-fold with acalabrutinib, and a 2- to 5-fold increase with zanubrutinib compared with traditional risk predicted rates; despite relative reduction compared with ibrutinib.25 Notably, in an evaluation of acalabrutinib-treated hematologic malignancy patients, the development of incident AF and other cardiac events is associated with worse long-term survival.16
In addition to the covalent (irreversible binding) BTK inhibitor therapies previously discussed, there is ongoing development of noncovalent (reversible binding) BTK inhibitor including recently FDA-approved pirtobrutinib (Table 2). Noncovalent BTK inhibitors show differences in the duration of kinase inhibition, which has been hypothesized to be associated with lower cardiac and noncardiac toxicity susceptibilities.26,27 In theory, this may decrease potential on-target mediated adverse events. Although still in early phase testing, initial data suggest AF event rates of <5%.26,27 However, given the observations of higher rates with more rigorous cardiac evaluation or real-world use of covalent BTK inhibitor than seen in initial anticancer efficacy-focused trials, further investigation is needed to better determine the cardiotoxicity risk of reversible BTK inhibitor therapies.
Table 2.
Cardiovascular Toxicities Associated With BTK Inhibitors and Other Therapies in Chronic Lymphocytic Leukemia Patients
| Drug | Atrial Fibrillation | Ventricular Arrhythmias | Hypertension | Heart Failure | Bleeding | Stroke |
|---|---|---|---|---|---|---|
| FDA-approved drugs | ||||||
| Ibrutinib | +++ | ++ | +++ | + | +++ | + |
| Acalabrutinib | ++ | ++ | ++ | ? | ++ | − |
| Zanubrutinib | ++ | ? | ++ | ? | ++ | − |
| Pirtobrutinib | + | ? | ? | ? | ++ | − |
| Other BTK inhibitors in early phase testing | ||||||
| Other (reversible) BTK inhibitors | + | ? | ? | ? | ++ | ? |
| Other drug therapiesa | ||||||
| Venetoclax | − | − | − | − | + | − |
| Idelalisib | + | − | ++ | + | − | − |
| Rituximab | − | − | − | − | − | − |
? indicates areas where systematic cardiac data are not widely available. ∗More emphasis placed on comprehensive cardiovascular studies where adjudication methodology is well known.
BTK = Bruton’s tyrosine kinase; FDA = Food and Drug Administration.
More common drug therapies.
Management of Cardiotoxic AF
The management of BTK inhibitor-associated AF events poses unique challenges caused by higher risks of bleeding, and potential therapeutic drug-drug interactions with standard antiarrhythmics. Before initiation of treatment with a BTK inhibitor, we suggest patients undergo a basic cardiac assessment, inclusive of cardiovascular history, measurement of blood pressure, and 12-lead ECG. Among those with more than 1 CVD risk factor (eg, age >50 years, prior cardiac arrhythmia, hypertension, heart failure, moderate or greater valvular disease, prior cardiotoxic therapy administration), a baseline echocardiogram is also recommended.14,28
Those with pre-existing AF can, in most instances, still be safely treated with BTK inhibitor therapy, although it is important to take into consideration pre-existing cardiovascular comorbidities before making a decision regarding therapy. In patients with pre-existing AF, second-generation BTK inhibitors are preferred given their lower incidence of arrhythmic events when compared with ibrutinib; although the risk of AF is still there, albeit lower. Alternatively, consider other classes of medications, such as Bcl-2 antagonists (eg, venetoclax), given their overall safer cardiac profile.
For patients that develop AF events after BTK inhibitor-initiation, we recommend management in collaboration with a multi-disciplinary cardio-oncology team, and if cardio-oncology is not available, general cardiology could be considered. As AF confers an elevated risk of stroke, calculation of a patient’s risk factors for stroke using the CHA2DS2-VASc scoring system is recommended, and those with an identified increased risk of stroke should be considered for anticoagulation. The consensus surrounding anticoagulation varies in this population, but our experience suggests direct oral anticoagulants (DOACs) are preferred in those with higher stroke risk. These agents offer easier administration, fewer drug-drug interactions, and more reliable pharmacologic effects. However, bleeding risk must also be considered as BTK inhibitor therapies are associated with increased bleeding risk that worsens while on anticoagulation.1,2,13,29 For this reason, a more conservative anticoagulation strategy (ie, only those patients with significantly increased stroke risk receive anticoagulation) can be used in patients with substantial bleeding risk.11 Warfarin was not tested in concurrent use with BTK inhibitors, and is relatively contraindicated because of high safety risk. It is currently not recommended to proceed with aspirin as a thromboembolic preventive strategy.
Furthermore, while any DOAC may be used, Xa inhibitors may be preferred over dabigatran caused by adjustments required if a patient has impaired renal function in addition to the need for a common P-glycoprotein (P-gp) inhibitor such as amiodarone. It is noted in the prescribing information that ibrutinib and zanubrutinib likely inhibit P-gp, although the clinical significance of this is uncertain. For Xa inhibitors, interactions with P-gp inhibitors only require adjustment if combined with a moderate or strong CYP3A inhibitor, which would require adjustment of the BTK inhibitor as well. Overall, some experts prefer apixaban based on retrospective and indirect comparisons that suggest favorable efficacy and safety, as well as its comparatively low reliance on renal clearance.30,31 For patients in whom twice-daily administration is a barrier to adherence, only daily DOACs (such as rivaroxaban or edoxaban) may be considered.
Management of symptomatic AF itself can be challenging, although most providers rely on a strategy of rate control initially with beta-blockers, such as metoprolol, as first-line agents. Other atrioventricular nodal blocking agents, including nondihydropyridine calcium-channel blockers, can be efficacious and considered, but their therapeutic levels can become challenging to predict given the effect of BTK inhibitors on the CYP3A4 metabolism pathway. Finally, there is extremely limited data on the utility of rhythm control (eg, cardioversion, ablation) in these patients, and consideration of these options should be weighed on an individual basis. If rhythm control is desired, the rhythm control approach in AF can be informed by the AF pattern—whether patients are in the so-called staccato vs legato pattern of AF—because the former would require regular antiarrhythmic therapy while the latter can be controlled with cardioversion as needed (electrically or pill in pocket). This may be a useful approach to reduce the antiarrhythmic therapy burden in people with paroxysmal AF events on BTK inhibitor.
BTK Inhibitor-Associated VAs
Incidence of VAs
Although not originally noted in the initial studies of ibrutinib and other BTK inhibitor therapies, there is increasing evidence to suggest that treatment with BTK inhibitor may lead to an increased risk of potentially serious VAs, including ventricular tachycardia and sudden death (ie, ventricular fibrillation). In 2 large clinical analyses published in 2017 and 2018 of hematologic malignancy patients taking ibrutinib, the reported incidence of VA and/or sudden cardiac death was observed to approach 6.0 to 7.9 per 1,000 person-years, a value >10-fold greater than expected (Figure 1).32,33 Sudden cardiac deaths were rare, occurring at 1 in 1,134 person-years of ibrutinib treatment (eg, <0.1% per year).12,33 Among these populations, there did not appear to be an association with prior CVD in those who developed these arrhythmias.32,33 Consistent with this, a recent analysis from the manufacturer of ibrutinib noted an incidence of 1% for deaths caused by cardiac causes or sudden death amongst nearly 5,000 patients treated in clinical trials.34
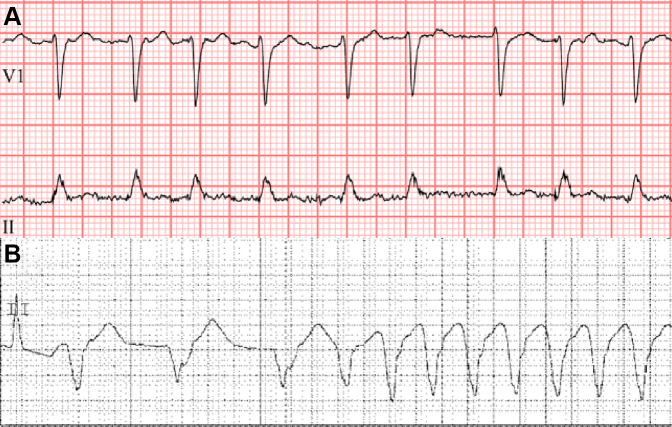
Figure 1.
Representative Electrocardiograms From Patient(s) With BTK Inhibitor-Associated Arrhythmias
(A) A patient with the development of atrial fibrillation shortly after Bruton’s tyrosine kinase inhibitor (here ibrutinib) initiation. (B) A patient with ventricular arrhythmia development after Bruton’s tyrosine kinase inhibitor therapy.
Risk Factors for VAs
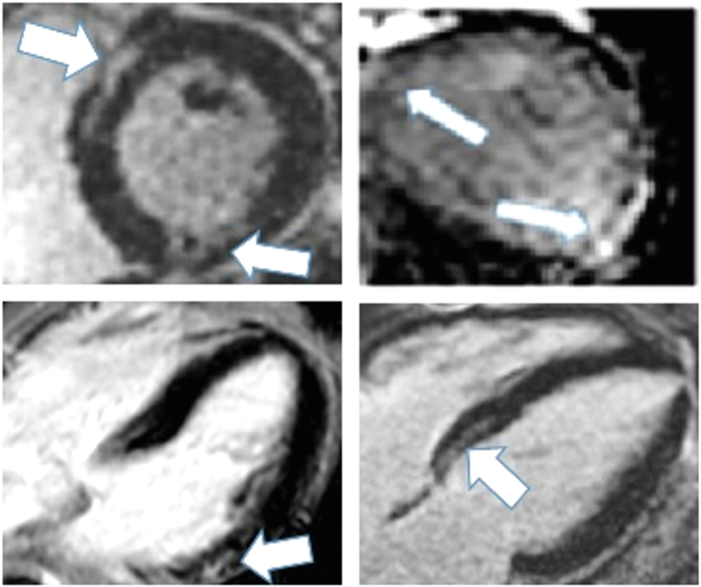
The predictors of VAs with BTK inhibitor use are largely unknown. Available data suggest that older age can increase risk.12 In a recent analysis of patients with suspected ibrutinib-associated cardiotoxicity, inclusive of VAs, myocardial fibrosis and edema were prevalent.35 Among 33 ibrutinib-treated patients who underwent cardiac magnetic resonance imaging, late gadolinium enhancement, a marker of discrete myocardial fibrosis, was present in 54.8% of patients (Figure 2). Notably, the presence of abnormal cardiovascular magnetic resonance (CMR) measures was most strongly correlated with definite ibrutinib-associated cardiotoxicity, and its presence predicted future major cardiac events among those undergoing treatment.35
Figure 2.
Cardiac Magnetic Resonance Images From a Patient With Ibrutinib-Associated Cardiotoxicity
Representative late gadolinium enhancement (white arrows) cardiac magnetic resonance images from hematologic malignancy patients with suspected cardiotoxicity (paroxysms of atrial fibrillation and nonsustained ventricular tachycardia) after ibrutinib initiation.32
Probable mechanisms for VAs with BTK inhibitors
Mechanisms of VAs remain incompletely understood. Previously proposed intracellular processes include enhanced automaticity of late sodium channels or propagation of fibrosis through aforementioned pathways associated with AF.9,20,33 Notably, ibrutinib and other BTK inhibitor therapies do not considerably contribute to the prolongation of the QT interval, and thus the formation of torsades de pointes is an unlikely explanation of VAs in this population.36 Preclinical murine data suggest a potential shared role for impairment of insulin-like growth factor 1 dependent activation of intracellular calcium handling in VA susceptibility with both ibrutinib and acalabrutinib (next-generation BTK inhibitor) therapies.37 Despite the lack of definitive human mechanistic data, in a preliminary report, increased ventricular ectopy was observed with direct ibrutinib application to ventricular human-induced pluripotent stem cell-derived ventricular cardiomyocytes, suggesting at least some direct (rather than solely indirect) myocardial mechanism of ventricular dysrhythmia with BTK inhibition.9 These observations raise concern that ventricular arrhythmogenicity may be a class effect of BTK inhibitors; however, additional studies are needed.
Next-generation BTK inhibitors and VA risk
Although lower than that seen with ibrutinib, acalabrutinib has also been associated with a significantly increased risk in VA risk when compared with the general population.12 In a pooled analysis of early cancer treatment trials, VAs were not initially reported.13 However, a large single-center cardiac-focused analysis of nearly 300 patients taking acalabrutinib reported a nearly 8-fold increase in VAs, including a case of sudden death.12 In this study, the majority of these cases were observed in patients without a history of structural heart disease (including coronary artery disease and heart failure). Sudden cardiac death was rare, occurring in <0.1% per person-years of treatment; with symptomatic VAs occurring at 0.6% per person-year.12 Further, no specific cardiac or electrocardiographic variables were associated with the development of these arrhythmias beyond older age.12 The median time to VA event was just over 14 months.12 The observed risk of ventricular events was approximately one-third lower with acalabrutinib compared with ibrutinib. Recently, separate cases of unexplained ventricular events with zanubrutinib have similarly been reported.9 Notably, as with early ibrutinib trials, no ventricular events were reported in early cancer trials using these novel agents. Yet, with zanubrutinib and the new noncovalent/reversible next-generation BTK inhibitors, more rigorous cardiac-focused electrocardiographic studies are needed.
Management of VAs with BTK inhibitors
Practically, based on our experience and the current available evidence, we suggest assessment by ECG of all patients initiating BTK inhibitor therapy, with serial interim ECG assessments at least quarterly over the initial 12 to 18 months.12,33 Similarly, a baseline echocardiogram for the exclusion of structural heart disease, including depressed left ventricular ejection fraction (LVEF), should be considered for all patients with at least 1 risk factor at the time of treatment initiation. Assessment of other baseline cardiovascular risk factors (eg, heart failure, prior atrial arrhythmia or VA, uncontrolled hypertension, myocarditis) should also be performed before initiation of BTK inhibitor therapy. Clinicians should have a low threshold to inquire about symptoms of arrhythmias, such as palpitations, shortness of breath, chest pain, and syncope, and consider the possibility of VAs when initiating and during the course of BTK inhibitor therapy.
For those patients with a previous history of symptomatic VAs, uncontrolled or continued heart failure (eg, LVEF <40%), or sudden cardiac death, we suggest, with careful multidisciplinary collaboration, alternative therapy (eg, Bcl-2 antagonist, venetoclax) to be strongly considered, given the uncertain risk associated with these agents. In those patients who develop symptomatic VAs during treatment, we suggest holding (or discontinuing) therapy, and further evaluation with imaging such as echocardiogram and/or cardiac magnetic resonance imaging, and cardiology referral. Similarly, in those with repetitive or prolonged runs of ventricular tachycardia, temporary cessation of BTK inhibitor should be considered. In these and other clinical arrhythmic scenarios, the application of CMR may prove particularly useful in prognosticating future and recurrent cardiac event risk based on the currently available evidence.35 Yet, because these events remain rare, additional prospective studies are needed to determine the exact thresholds or duration of VA most predictive of sudden death.
BTK Inhibitor-Associated Hypertension
Hypertension, both worsening and treatment-emergent, is a well-documented adverse effect of BTK inhibitor therapy. Within hematologic malignancy populations, long-term outcomes are less clearly delineated, because prior anecdotal observations of increased susceptibility to hypotension have led to conflicting understandings of the effects of blood pressure on outcomes.38 However, given the documented increase in incident hypertension amongst those taking BTK inhibitors and its association with future cardiotoxic event risk, increased attention is warranted.
Incidence of hypertension
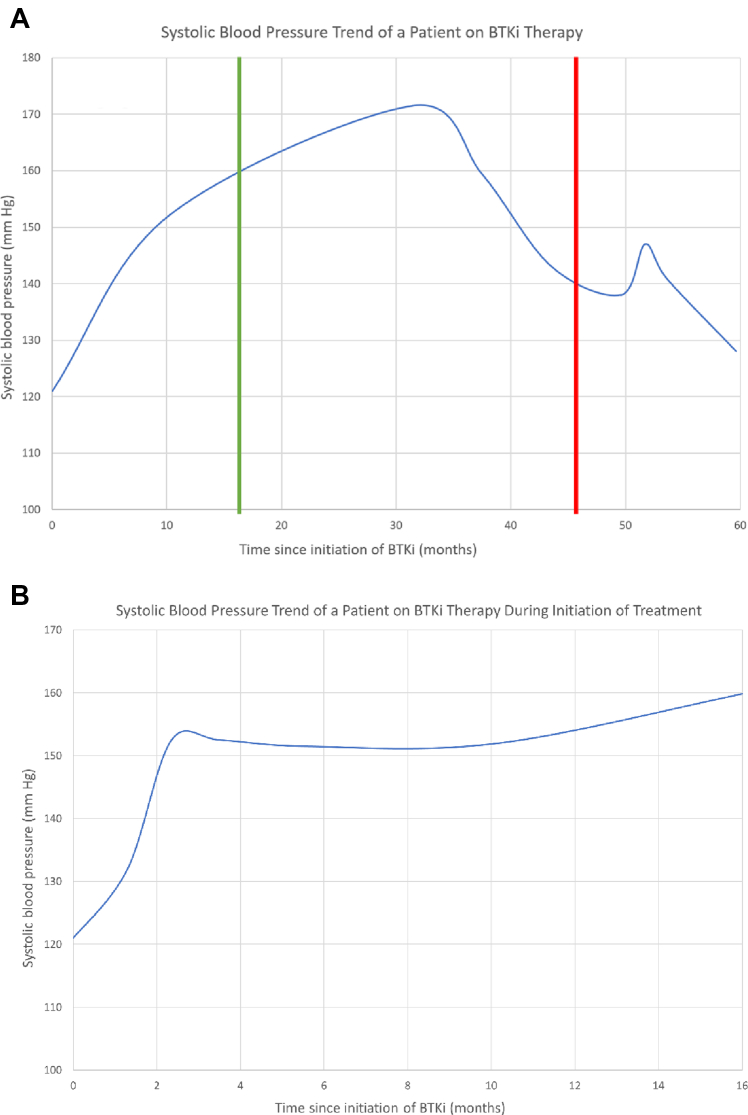
In early ibrutinib clinical trials, grade ≥3 hypertension was reported in 4% of patients, whereas any grade hypertension was seen in up to 14% of patients treated with ibrutinib monotherapy.39,40 Longer-term analysis has demonstrated that the incidence may have been under-recognized, with pooled hematologic malignancy trial data demonstrating grade ≥3 hypertension in 28% of patients treated with ibrutinib.41 In a large meta-analysis of ibrutinib clinical trials, ibrutinib exposure had a relative risk of 2.82 (95% CI: 1.52-5.23) for the development of hypertension.42 In a large single-center hypertension-focused study of 562 patients treated with ibrutinib, 78.3% of ibrutinib-treated patients developed new or worsening hypertension, with a median time to hypertension development of nearly 6 months.19 Notably, this was >10-fold higher than Framingham Heart Study predicted rates.19 The extent of worsening blood pressure increase may be particularly important, with 10% of patients observing a 50-mm Hg increase in their systolic blood pressure (Figure 3).19 Notably, the development of ibrutinib-associated hypertension was associated with a >2-fold increase in risk for the development of other major cardiac events (eg, AF, VA, heart failure).42 Other studies have similarly observed an increase in blood pressure with ibrutinib treatment.43,44 In a recent evaluation of acalabrutinib-treated patients, similar (yet less profound) increases in blood pressure were observed.5,10,45 Yet, here too, the development of early blood pressure increase was associated with major cardiac events.10 Although more rigorous cardiac-focused data with zanubrutinib are not yet available, in a landmark phase trial comparing zanubrutinib with ibrutinib, the rates of significant (>140/90 mm Hg) hypertension were similar.29 Together, these observations suggest hypertension may be a class effect of BTK inhibition.
Figure 3.
Change in Blood Pressure From a Patient Initiated on BTKi Therapy
Increase in systolic blood pressure in a patient treated with ibrutinib over a period of several years (A). The left green vertical line represents the initiation of antihypertensive therapy, and the right red vertical line represents the time of ibrutinib discontinuation because of subsequent symptomatic atrial fibrillation development. (B) The rapid onset of hypertension in the same patient in the months after starting ibrutinib. BTKi = Bruton’s tyrosine kinase inhibitor.
Risk factors for development of BTK inhibitor-related hypertension
Available data suggest baseline systolic blood pressure and the current use of CYP3A4 inhibitors are associated with ibrutinib-associated hypertension development.19 With acalabrutinib, baseline history of prior arrhythmia as well as Black race are associated with new hypertension development.10 However, additional investigation of the predictors of new or worsening hypertension with BTK inhibitor treatment is needed.
Possible mechanisms of BTK inhibitor-related hypertension
Several possible mechanisms have been proposed to contribute to the worsening or development of hypertension associated with BTK inhibitors, particularly ibrutinib. Similar to BTK-related AF, inhibition of the PI3K pathway may contribute to cellular remodeling mechanisms that possibly result in vascular tissue fibrosis.21 However, there appear to be additional processes at play, because similar rates of hypertension have not been demonstrated in more potent direct inhibitors of PI3K, namely idelalisib.46,47 Additional proposed mechanisms include the down-regulation of nitric oxide formation, resulting in dysregulation of vascular tone, which is also a pathway shared with second-generation BTK inhibitor therapies including acalabrutinib.48 It remains unclear whether the degree of hypertension observed in BTK inhibitors is caused by off-target kinase inhibition or systemic inflammatory changes that predispose to the development of hypertension. Because acalabrutinib, a second-generation BTK inhibitor with greater BTK selectivity still demonstrates a significant incidence of hypertension, this may support the mechanism inherent to BTK inhibition. Further evaluation is ongoing into further mechanisms that may predispose patients to the development of hypertension while undergoing treatment with BTK inhibitors.
Outcomes of BTK inhibitor-associated hypertension
In a large retrospective analysis of patients with lymphoid malignancies treated with ibrutinib, there was an increased risk of development of major adverse cardiovascular events (MACE).19 Nearly 20% of patients in those with new or worsening hypertension experienced MACE, as compared with <10% of those with stable or no hypertension.19 Cardiovascular complications were most manifested as AF (13%) and heart failure.19 Although the incidence of hypertension was lower in patients treated with acalabrutinib rather than ibrutinib, there remains a considerable hypertensive burden within this population. Patients with the early development of hypertension signaled an increased incidence of MACE, with these events identified in 18% of patients treated with acalabrutinib demonstrating new or worsening hypertension.10 Similar to ibrutinib, AF was the most common MACE identified, seen in 8% of acalabrutinib patients. In this analysis, the degree of systolic blood pressure elevation significantly corresponded to the subsequent risk of developing AF, as well as MACE.10,19 This suggests that the development of hypertension while undergoing BTK inhibitor therapy, particularly with a more profound systolic blood pressure increase, may predispose toward the development of further major cardiovascular events.
Next-generation BTK inhibitors and risk of hypertension
Concerning hypertension, a wide variation in reported hypertension rates among initial prospective clinical data have been noted, ranging from 9% to 40% developing any grade hypertension with next-generation therapies (eg, acalabrutinib, zanubrutinib). For example, longer-term retrospective data appear to signal an increased risk of hypertension with acalabrutinib, although overall less incident than ibrutinib.13 Among 280 consecutive patients treated with acalabrutinib, 59% of patients developed new or worsening hypertension within 1 year of acalabrutinib initiation.10 This was still nearly 8-fold greater than Framingham Heart Study predicted rates.10 Initial hematologic malignancy trial data with zanubrutinib demonstrated a 5% incidence of grade ≥3 hypertension.4 However, in a recent phase III cancer-outcome-focused trial comparing zanubrutinib with ibrutinib, the rates of significant hypertension (>140/90 mm Hg) were similar.6 This is notable, because this suggests hypertension may be common even with zanubrutinib.
At this time, there are limited data regarding the development of hypertension for other next-generation BTK inhibitor therapies.35 Early pirtobrutinib cancer trial data suggested a 1% incidence of any grade treatment-related hypertension (>140/90 mm Hg), and no cases of severe grade ≥3 hypertension (>160/110 mm Hg).26 Given the noncontinuous inhibition of BTK mechanistically, this may result in a substantial reduction in cardiotoxic blood pressure changes. Yet, as with the irreversible BTK inhibitors, further long-term comprehensive (ie, use of standard blood pressure measures) studies are needed.
Management of BTK inhibitor-associated hypertension
Patients with a pre-existing history of hypertension should not be precluded from initiation of BTK inhibitor therapy. Many individuals with hypertension can be successfully treated with BTK inhibitors but may require closer monitoring and a pretreatment comprehensive evaluation to determine the ongoing risk of worsening hypertension. As treatment has been associated with hypertension onset even after several months of treatment, clinicians must remain diligent toward this adverse effect. Initial screening may consist of biweekly blood pressure checks in the office or at home, and close communication for any changes from pretreatment baseline.
For patients who develop hypertension while undergoing BTK inhibitor therapy, early intervention with guideline-directed antihypertensive medication remains the standard of care.19 Further, patients should utilize at-home blood pressure monitoring devices to observe trends in blood pressure. Currently, there is no first-line recommended medication for the treatment of BTK inhibitor-associated hypertension. Options consist of renin-angiotensin-aldosterone-system (RAAS) antagonists, including angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; dihydropyridine calcium-channel blockers, including amlodipine or nifedipine; or thiazide-based diuretic agents. However, given the comorbidities that many patients with lymphoid malignancies possess, many clinicians will prefer to use dihydropyridine calcium-channel blockers as first-line agents, given relatively minimal side effects. Medications that inhibit or interact within the CYP3A4 metabolism pathway, namely diltiazem and verapamil, should be limited as treatment options for hypertension, because their inhibition of CYP3A4 may lead to increased therapeutic levels of the BTK inhibitor. In many patients, given the degree of blood pressure elevation, multiple agents may be necessary to attain a blood pressure target that is consistent with currently published guidelines.
Rarely does hypertension lead to discontinuation of BTK inhibitor therapy. In the event that grade ≥3 (symptomatic) hypertension does occur, BTK inhibitor interruption is a reasonable first step in our view, followed by potential dose reduction upon return to a reasonably controlled blood pressure. If recurrent severe episodes occur on ibrutinib therapy, a transition to an alternative BTK inhibitor such as acalabrutinib is reasonable, although some residual hypertension risk may still be present. In patients who develop treatment-emergent or worsening hypertension, clinicians should maintain a suspicion for additional cardiotoxicities, because the development of hypertension appears to be strongly associated with the development of other BTK inhibitor-associated toxicities, namely AF.10,19
BTK Inhibitor-Associated Heart Failure
Heart failure has been a well-documented adverse effect of many anticancer therapies, particularly systemic chemotherapies that cause direct myocardial damage. BTK inhibitor therapies have also been associated with the development of new-onset heart failure.
Incidence of heart failure
In initial clinical trials, an appreciable signal for heart failure was not seen. However, recent data from pooled long-term follow-up of several later-phase ibrutinib trials suggest a potential increase in the risk of heart failure. In these analyses, heart failure was noted in up to 5% of patients, often developing years after treatment initiation.49,50 In a large retrospective analysis of 860 patients treated with ibrutinib for CLL, a significantly increased risk of developing heart failure was observed when compared with treatment with chemotherapy.51 In this study, nearly 3 years into treatment, the risk of heart failure was 7.7%, compared with 3.6% in the control group. Similarly, a pharmacovigilance database study of ibrutinib suggested a higher reporting likelihood of heart failure, at over 3 times the reported odds compared with all other drugs throughout the entire database.52
Risk factors for development of BTK inhibitor-related heart failure
Other cardiotoxic effects of BTK inhibitor therapies can also predispose to or directly cause the development of heart failure. Available evidence suggests that pre-existing AF may increase the risk of developing heart failure, especially among those patients taking ibrutinib.51 Similarly, the presence of myocardial fibrosis on CMR imaging may also be seen in a high proportion of patients taking ibrutinib.35 Although these imaging characteristics are frequently encountered in other causes of cardiomyopathy, the presence of such findings in patients on BTK inhibitor therapies remains unclear as a predictive measure toward the development of future incidence of heart failure.
Proposed mechanisms and outcomes of heart failure
Proposed etiologies for the development of heart failure among patients taking BTK inhibitors tend to focus on off-target kinase inhibition and subsequent negative effects on the myocardium. The PI3K-Akt pathway, previously discussed as a possible contributory mechanism for the development of BTK inhibitor-related AF, has known importance in the regulation of cardiac myocyte homeostasis, particularly under times of stress. As a major point in the antiapoptosis pathway of cardiomyocytes, inhibition of PI3K-Akt by off-target kinase inhibition can lead to myocardial cell disarray, fibrosis, disruptions in calcium signaling, and death leading to the development of heart failure.21,23,52,53 Other pathways, inclusive of immunomodulation and nitric oxide metabolism alterations, as well as hypertension-mediated cardiac remodeling (with diastolic dysfunction), have been hypothesized to contribute to this cardiotoxic risk. However, the long-term effects of heart failure development with BTK inhibitors on survival outcomes are largely unclear.
Heart failure in patients on next-generation BTK inhibitor therapy
There are currently limited long-term data on the risk of heart failure with newer BTK inhibitor therapies. At this time, the largest evidence exists for acalabrutinib. In a pooled analysis of 760 patients taking acalabrutinib, <1% of patients developed any grade of (symptomatic) congestive heart failure.13 Evidence regarding heart failure in other next-generation agents, including reversible BTK inhibitors, is extremely limited, because this outcome was frequently not reported.
Management of BTK inhibitor-associated heart failure
We generally suggest that patients with decompensated heart failure are not immediately initiated on BTK inhibitor therapy when possible. There are no data on the safety of a standardized cutoff in baseline LVEF for BTK inhibitor initiation, because most trials did not include patients with known baseline heart failure. In those with a previous history of heart failure, we recommend a next-generation agent to minimize the risk of additional cardiotoxic effects such as AF or uncontrolled hypertension, which are often poorly tolerated in patients with considerable heart failure and could lead to further decompensation. Alternative therapies should be considered, as clinically indicated for malignancy control. It should be noted that patients on Bcl-2 antagonist therapy may require aggressive intravenous fluids for mitigation of tumor lysis syndrome risk, a well-documented complication of this therapy, which could provoke heart failure exacerbation. For those with pre-existing cardiomyopathy, discussion of the optimal regimen may be best handled through close collaboration between oncology and cardiology (or by cardio-oncology specialists, if available).
Before initiating therapy, we suggest a screening echocardiogram for those patients 50 years of age or older, baseline ECG for all patients, and consideration of ambulatory heart rate monitoring to determine if there are any baseline arrhythmias. In those undergoing therapy with BTK inhibitors, patients with prior heart failure should be advised to strictly monitor their weight and blood pressure more frequently. Given the chronic nature of medication administration, it is worthwhile to consider interval systolic function assessments with either repeat transthoracic echocardiography or CMR (Figure 4). Medication management of these patients should be in accordance with currently published guidelines, consisting of adequate management of hypertension, heart rate, and fluid status.54
Figure 4.
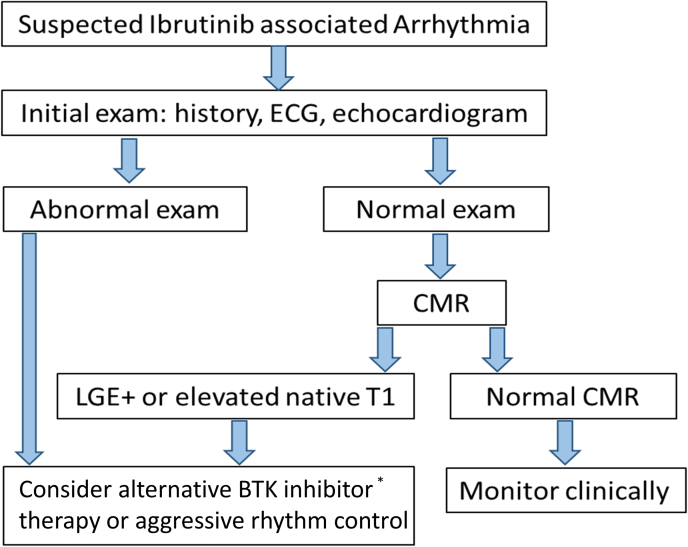
Proposed Algorithm for Risk Stratifying and Managing Ibrutinib-Associated Arrhythmias
Among patients with suspected ibrutinib-associated cardiotoxicity, the presence of late gadolinium enhancement (LGE) or elevated native T1 is associated with a higher risk of future or recurrent cardiotoxic events. ∗Additional cardiac data on the safety of newer Bruton’s tyrosine kinase (BTK) inhibitors is still needed. CMR = cardiac magnetic resonance; ECG = electrocardiogram.
For those patients with new or worsened symptomatic (high-grade) heart failure with depressed LVEF while undergoing BTK inhibitor therapy, we suggest discontinuation of BTK inhibitor and initiation of guideline-directed medical therapy as soon as possible. Prioritization should be given to prompt control of blood pressure with RAAS antagonists as well as control of volume status with diuretic agents as indicated. Once safe to do so, the initiation of beta-blockers should be prioritized. After stabilization, discussion amongst an interdisciplinary team (hematology/oncology and cardio-oncology) can be helpful to stratify the subsequent risk of continuing BTK inhibitor therapy vs switching to an alternative approach.
BTK inhibitor-associated bleeding
Bleeding-related events are not uncommon with BTK inhibitor therapy. Although most are low-grade events typically consisting of ecchymoses or petechiae, these events have the potential to transform into life-threatening hemorrhagic events that require hospitalization and intensive management.
Incidence of major bleeding
Major bleeding, defined as needing transfusion or hospitalization, was seen in nearly 10% of patients taking ibrutinib in original phase III trials.39,40,55 Subsequent longer-term pooled analyses have suggested ranges from 30% to 38% for any bleeding event, and high-grade events observed in around 3% of patients.56,57 It should be noted that in many of these trials, patients receiving warfarin or other vitamin K antagonists were typically excluded, thereby supporting some degree of inherent bleeding risk attributed to BTK inhibitor therapy rather than external agents.
Risk factors for BTK inhibitor-associated bleeding
Several studies have attempted to characterize potential risk factors that may signify the development of bleeding events while on BTK inhibitor therapy. One group identified the prolongation of collagen/epinephrine membrane closure time, a marker of platelet function, as a potential risk factor for the development of low-grade bleeding events.58 Additional risk factors identified for an increased bleeding risk, include those with a prior history of bleeding, increased age, and treatment with antiplatelet or anticoagulant therapy.59, 60, 61, 62
Possible mechanisms of BTK inhibitor-related bleeding
Although not clearly defined, the proposed mechanisms of BTK inhibitor-associated bleeding appear to result from a combination of both on- and off-target kinase inhibition, particularly in platelet activation and function. In human platelets, BTK is a key regulator of downstream signaling crucial for platelet activation, including signal transduction within the prothrombotic glycogen VI collagen activation pathway.63, 64, 65 BTK inhibitors tested in vitro reduced platelet adhesion and granule secretion in response to collagen-induced aggregation signals known to trigger platelet activation.58,65,66 Further, the off-target enzyme TEC appears to be implicated in the pathogenesis of platelet-mediated bleeding and may help explain why less selective BTK inhibitor therapies, such as ibrutinib, are associated with a somewhat greater inherent bleeding risk when compared to more selective BTK inhibitors.63
Outcomes
Currently, there is limited available evidence on the long-term ramifications of major bleeding events treated with BTK inhibitors. Most bleeding events are low-grade and self-resolving, which do not typically confer an increased risk of mortality amongst patients. Further, most patients remain able to continue therapy through these episodes.
Bleeding with next-generation BTK inhibitors
Initial data on next-generation BTK inhibitor therapies suggest no or modest decrease in bleeding risk compared with ibrutinib. In the initial phase 3 trial of acalabrutinib for patients with CLL, low-grade bleeding was seen in 38% of patients, and high-grade bleeding in 4.5% of patients.5 In this trial, rates of high-grade bleeding were comparable to those rates seen in ibrutinib. Further, in a single-center retrospective analysis of 289 patients treated with acalabrutinib, 83% experienced at least 1 bleeding event, with 3% of patients exhibiting a grade 3 or greater bleeding event.67 Based on these data, acalabrutinib appears to still possess significant bleeding risk. Although the majority of bleeds appear minor and may be interpreted as clinically nonrelevant.
With zanubrutinib, pooled safety analysis from initial clinical trials indicated that bleeding or bruising events were seen in 55% of patients treated with zanubrutinib. As seen with the other BTK inhibitors, most of these were minor; 4% of patients reported major hemorrhagic events, with 1 bleeding-related fatality noted.29 Ongoing postmarket analysis will be crucial to determine the ongoing bleeding risk for this medication as it becomes more commonly used.
Management of BTK inhibitor-related bleeding
Although bleeding events are common adverse effects noted with BTK inhibitor therapy, the majority of these will not require treatment discontinuation. Minor events, including bruising and petechiae, do not suggest underlying hemorrhage, and therapy may be sustained without dose adjustments or discontinuation.68 Ibrutinib may be held in the case of noticeable or major bleeding events, although without hemodynamic consequence. Because bleeding appears to be primarily mediated through a platelet-inhibitory process, patients with major hemorrhage should be aggressively resuscitated with platelet transfusion, regardless of platelet count. Although not specifically studied in this population, some clinicians may also elect for the administration of tranexamic acid.69 BTK inhibitor therapy should be held indefinitely in the event of such a bleed, and discussion of ongoing usage should be a part of the discussion with the primary oncology team. Because the risk of major bleeding appears to increase with the use of both antiplatelet agents and anticoagulants, it may be worth considering alternative anticancer-specific therapies in those with a very high risk of serious bleeding and need for long-term antiplatelet or anticoagulant therapy. In our experience, it is reasonable in the perioperative period to hold BTK inhibitor therapy for 3 days with minor procedures or 7 days with major procedures both before and after a planned procedure, given the heightened bleeding risk with BTK inhibitor therapy.62 Nevertheless, the given risks and benefits of holding BTK inhibitor-treatment for some (less urgent) procedures should be discussed with the patient depending on their disease control and clinical status.
BTK Inhibitor-Related Stroke
With a known association of AF, it follows that BTK inhibitors also may be associated with an increased risk of stroke. Whereas traditional management of AF focuses on minimizing the future risk of stroke, BTK inhibitor-related AF typically poses a therapeutic challenge given the unique patient population taking these medications, as well as the inherent bleeding risks that have been associated with BTK inhibition.
Incidence of stroke
In the original ibrutinib trials for CLL, ischemic stroke was noted in one of over 500 patients treated with ibrutinib therapy.39,40,55 A single-center retrospective analysis of more than 580 patients evaluated the incidence of stroke or transient ischemia attack in a population of patients treated with ibrutinib. In this analysis, only 1 patient among those who had developed AF while on ibrutinib therapy had a stroke or transient ischemia attack event.11 Of note, this patient was noted to have a CHADS2 score of 1 and was on high-dose aspirin therapy for stroke prophylaxis. Similarly, ischemic stroke was found to be a rare complication in an even larger cohort of patients on ibrutinib, with a 3-year cumulative incidence of 1.6% in ibrutinib-treated individuals, which did not reach significance when compared with population-matched control subjects.52 However, in a recent SEER-Medicare study (median age 77 years), ibrutinib was associated with a 1.9-fold increase in stroke risk among older patients and was related to the presence of AF.70
Risk factors, mechanisms, and outcomes of stroke
The risk factors of stroke, beyond AF in older patients, remain unclear. However, the additive effect of AF and proinflammatory states seen in hematologic malignancies (and possibly upregulated with BTK inhibition) may serve as a key nidus for the occurrence of these events.48,71 As with arrhythmias, more long-term mechanistic and outcome studies are needed.
Next-generation BTK inhibitors and stroke risk
Currently, stroke has not been identified as a major adverse effect of the next-generation BTK inhibitors, including acalabrutinib or zanubrutinib. To our knowledge, there has not been an established signal for increased neurovascular complications among early-phase trials for the reversible BTK inhibitor therapies.
Management/prevention of BTK inhibitor-related stroke
Prevention of stroke in patients undergoing BTK inhibitor therapy primarily centers around those patients who either have a history of AF or have developed new AF while on a BTK inhibitor. Determination of a patient’s stroke risk is performed using standardized risk-assessment tools including the CHA2DS2-VASc calculator. For those patients with an elevated risk of stroke using this tool, it is recommended that anticoagulation be pursued barring any contraindications such as coagulopathy or profound thrombocytopenia.72 Further, optimization of additional stroke risk factors, including hypertension, hyperlipidemia, and diabetes, should be aggressively managed.
Anticoagulation remains the mainstay of stroke prevention in patients with AF and should be considered in patients with hematologic malignancies treated with a BTK inhibitor. We suggest systemic anticoagulation with a DOAC for those with elevated CHA2DS2-VASc scores, in line with current general population guidelines.73 We also note that additional studies focused on the optimal CHA2DS2-VASc thresholds in BTK inhibitor and cardio-oncology patients are needed. If oral anticoagulation is not deemed appropriate or safe, low-molecular-weight heparin products can be considered as a therapeutic option. Although vitamin K antagonists do remain an option for systemic anticoagulation, generally these are not first-line agents if DOACs are a viable option, given their requirements for frequent laboratory monitoring and dose-adjustments.
Alternative Therapies in CLL Beyond BTK Inhibitors
Perhaps the most common non-BTK therapy is venetoclax, an oral inhibitor of B-cell lymphoma-2—another crucial regulator of B cell homeostasis. It is a commonly used therapy in CLL, often in combination with anti-CD 20 monoclonal antibody.74,75 As opposed to BTK inhibitors, which are taken daily for many years until treatment resistance or side effects develop, venetoclax is given for a fixed duration of time (typically 2 years or less) with the intent of disease eradication rather than disease control. Given its significant efficacy in inducing apoptosis within B-cell populations, it currently has approved usage within acute myeloid leukemias, as well as untreated or relapsed CLL.76 Major known adverse effects associated with venetoclax administration include neutropenia, thrombocytopenia, and the possibility of tumor lysis syndrome.77 Cardiotoxicity does not appear to be as prevalent among those treated with venetoclax. In a pooled analysis of 350 patients with CLL treated with venetoclax monotherapy as part of initial clinical trials, there were no reported adverse cardiac events including arrhythmias, hypertension, or heart failure.78 Although there are no comprehensive cardiac-focused systematic studies, larger phase III trials of CLL patients undergoing treatment with venetoclax have not demonstrated any signal toward cardiotoxicity.79, 80, 81, 82 With the available evidence, this suggests that venetoclax may be a viable option for some patients wherein high concern for limiting cardiotoxicity persists.
Similarly, available preliminary evidence suggests a lower burden of cardiotoxicity with previous (less common) treatments, such as idelalisib. In multicenter cancer trials, idelalisib was associated with a lower risk of AF. In an FDA-reporting analysis in 2018, there were 63 cases of AF and 24 cases of heart failure reported with idelalisib use. Yet, as with BTK inhibitor therapies, the investigators concluded more prospective studies focused on cardiovascular disease risk were needed.83
Pretreatment and On-Treatment Evaluations
Based on the aforementioned and emerging studies and our multidisciplinary experience, we suggest a systematic approach to cardiac consideration before BTK inhibitor treatment initiation.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 This specifically includes obtaining a clear cardiovascular history, including screening for any evidence of baseline palpitations, dyspnea, exertional chest pain, edema, unexplained syncope, or overt arrhythmias. If there is a concern for arrhythmia or prior electrocardiographic evidence of AF, next-generation BTK inhibitor therapies can be considered. Similarly, blood pressure and pulse should be recorded. We generally consider baseline echocardiogram to assess for depressed LVEF or significant atrial enlargement, particularly among patients >50 years of age.14 Additionally, several standard antiarrhythmic agents may produce potential drug-drug interactions with BTK inhibitors, including diltiazem, verapamil, and dronedarone (Supplemental Table 1). For patients on these therapies, consideration of alternative cardiac (or anticancer) therapies should be given.
Following BTK inhibitor initiation, if there is no evidence of arrhythmia, we suggest consideration for quarterly ECGs (or at least biannually) in alignment with common frequencies of oncologic visits during the first year of BTK inhibitor treatment for CLL or other hematologic malignancies. We also suggest asking patients about cardiac symptoms during these visits (eg, the same previously mentioned items), to allow for assessment for potential cardiac toxicities. During these assessments, a basic cardiovascular history and evaluation (eg, ECG and blood pressure monitoring) should be considered (Supplemental Table 2) based on expert opinion. Given these aforementioned cardiac risks, shared, multidisciplinary decision-making on the risks and benefits of these potent anticancer therapies should be weighed throughout the course of treatment.
Diagnostic Algorithm and Treatment for Suspected Cardiotoxicity
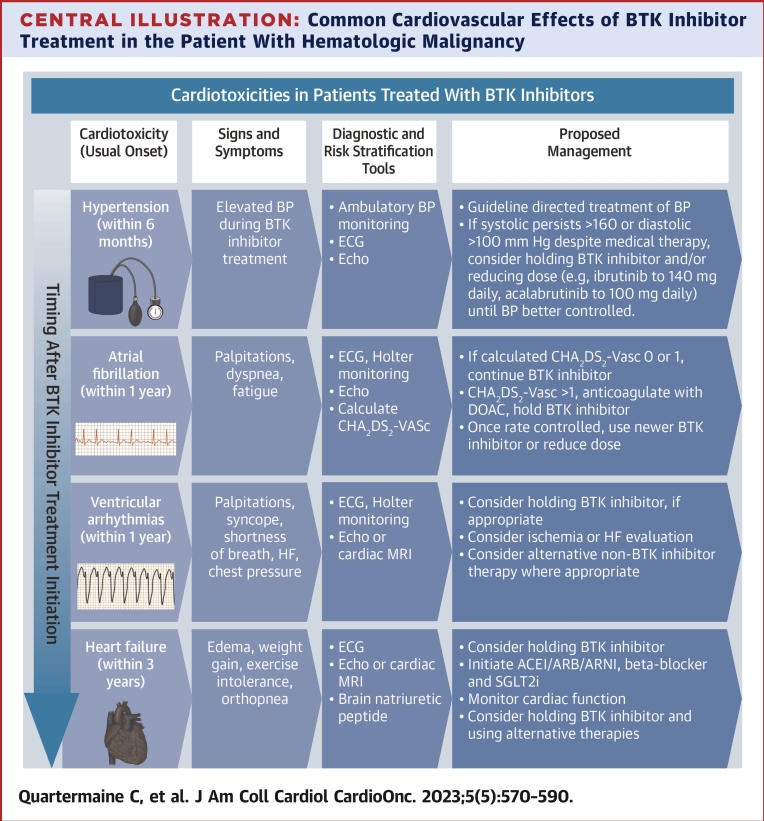
We suggest a practical diagnostic algorithm, including noninvasive and clinical examinations, that could be routinely be applied in clinical care to confirm and manage BTK inhibitor-associated cardiotoxicity (Central Illustration). Among patients on BTK inhibitor therapy or recent BTK inhibitor treatment, cardiotoxicity must always be considered in the setting of new cardiac signs or symptoms. Initial evaluation includes obtaining a clinical history of palpitations, shortness of breath, chest pain, edema, and syncope. This is followed by a 12-lead ECG assessment and transthoracic echocardiogram, and extended (eg, 3-7 days) Holter monitoring in patients without overt arrhythmia seen on standard 10-second ECG. These are generally widely available in centers where BTK inhibitors are being prescribed. Among patients with symptomatic VAs, in whom sudden death events are observed, or where further myocardial characterization is needed, we suggest obtaining a CMR. If the CMR or echocardiogram is abnormal or a high burden of arrhythmia is seen on rhythm monitoring, dose reduction, temporary hold, or alternative therapies should be considered (Figures 4 and 5). Similarly, we suggest transthoracic echocardiogram and ECG monitoring in patients with suspected heart failure. Blood pressure should be assessed at each routine office visit during the first year of BTK inhibitor treatment initiation. In patients with elevated blood pressures on 2 or more visits (by home or ambulatory assessment) after BTK inhibitor initiation, we generally recommend early initiation of antihypertensive therapy targeting a blood pressure <130/80 mm Hg (and <140/90 mm Hg in those >75 years of age or with diabetes). Generally, beta-blockers or renin-aldosterone modulators may be effective, with fewer drug-drug interactions among patients treated with these anticancer agents. Considerations for stroke prevention and bleeding are also outlined in Table 3.
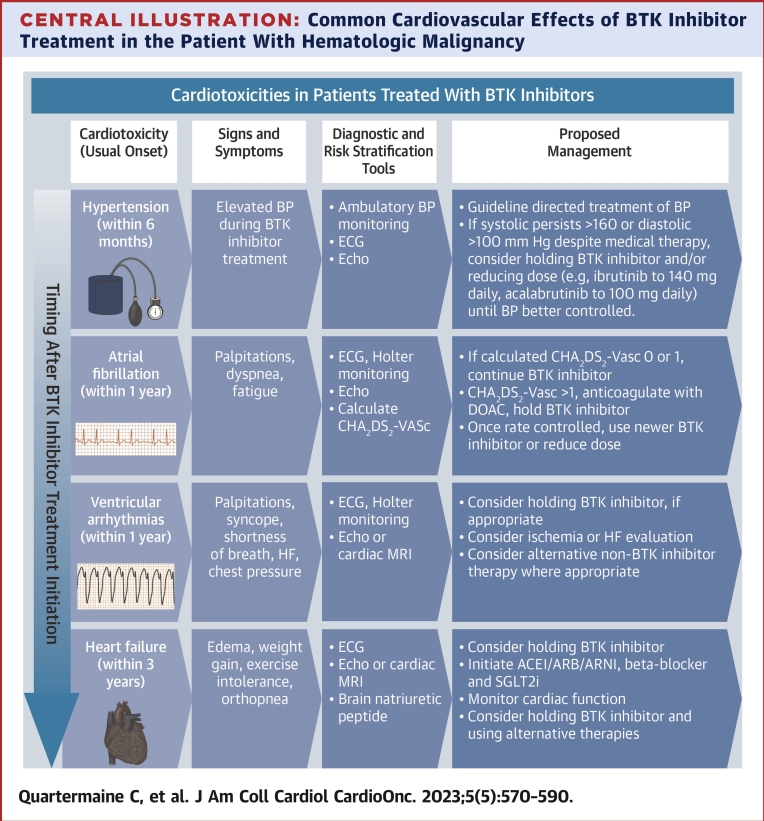
Central Illustration.
Common Cardiovascular Effects of BTK Inhibitor Treatment in the Patient With Hematologic Malignancy
∗Symptom (eg, palpitations, shortness of breath, chest pain) triggered Holter monitoring strategy is suggested for patients treated with BTK inhibitor therapies. ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; BP = blood pressure; BTK = Bruton’s tyrosine kinase; DOAC = direct oral anticoagulant; HF = heart failure; SGLT2i = sodium/glucose cotransporter-2 inhibitor.
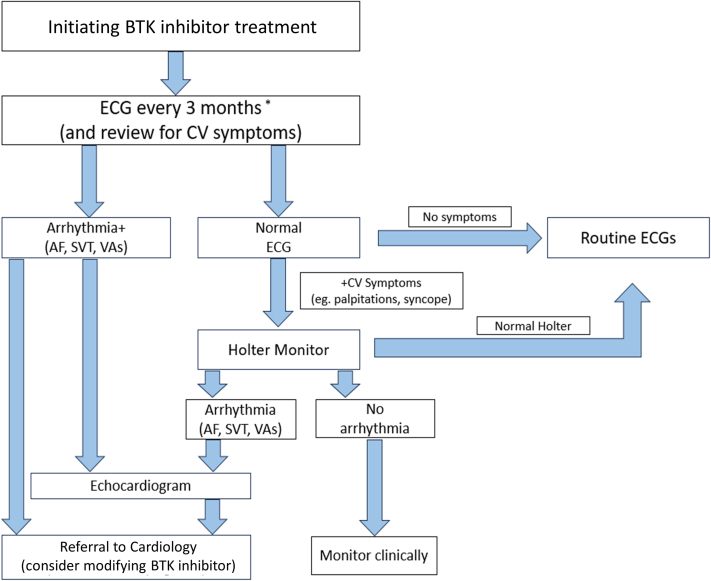
Figure 5.
Proposed Algorithm for Routine Cardiac Monitoring for Patients Initiating BTK Inhibitor Therapy
We suggest a screening ECG as part of each office visit (or at least every quarter during the first year of treatment) when initiating any BTK inhibitor therapy, and further assessment with longer-term Holter monitoring in those with symptoms (eg, palpitations). If arrhythmia is detected on screening ECG, an echocardiogram and potential referral to cardiology (or cardio-oncology referral, if available) is suggested, with potential modification of BTK inhibitor therapy. ∗ECG every 3-6 months after the first year of BTK inhibitor treatment; cardio-oncology referral, if available, otherwise general cardiology. AF = atrial fibrillation; CV = cardiovascular disease; SVT = supraventricular tachycardia; VA = ventricular arrhythmia; other abbreviations as in Figure 4.
Table 3.
Summary of Key Symptoms, Testing, Risk Stratification, and Suggested Management for BTK Inhibitor Associated Cardiotoxicities
| Atrial fibrillation | |
| Symptoms | Palpitations, shortness of breath, chest pain |
| Testing | Obtain 12-lead ECG, transthoracic echocardiogram; consider extended Holter monitoring |
| Risk-stratification | Atrial dilation, depressed LVEF, myocardial fibrosis on CMR, CHA2DS2-VASc >1 |
| Treatment | Anticoagulation (consider if CHA2DS2-VASc >1), rate control with beta-blockers, consider switching to alternative BTK inhibitor or dose reduction |
| Hypertension | |
| Symptoms | Elevated blood pressure >130/80 mm Hg in a continued pattern |
| Testing | In-office and ambulatory blood pressure monitoring, 12-lead ECG, transthoracic echocardiogram |
| Risk-stratification | Elevated blood pressure on 2 or more readings in office or at home over 3 months, LVH on echocardiography or CMR, or LVH on ECG |
| Treatment | Target blood pressure of <130/80 mm Hg giving priority to RAAS agents, beta-blockers |
| Ventricular arrhythmias | |
| Symptoms | Palpitations, chest pain, syncope, signs of heart failure |
| Testing | Obtain 12-lead ECG, transthoracic echocardiogram; consider extended Holter monitoring. If VA noted or clinical suspicion is high, obtain CMR |
| Risk-stratification | Evidence of late-gadolinium enhancement or abnormal T1/T2 on CMR, depressed LVEF on echocardiography or CMR |
| Treatment | Consider holding BTK inhibitor (immediately if prolonged VT or cardiac arrest); resume with caution or consider alternative therapy |
| Heart failure | |
| Symptoms | Dyspnea, exertional intolerance, edema, orthopnea |
| Testing | Obtain 12-lead ECG, transthoracic echocardiogram, consider BNP and CMR |
| Risk-stratification | Reduced LVEF (<50%) on functional cardiac assessment |
| Treatment | Consider holding BTK inhibitor. Initiate RAAS agents, beta-blockers, SGLT2 inhibitors as able. Periodic functional assessment every 6-12 mo with echocardiography or CMR |
| Stroke | |
| Recommendations | Anticoagulation with DOAC for those patients with AF and elevated CHA2DS2-VASc score (eg, >1) in whom bleeding risk is not prohibitive (eg, no prior issues with bleeding) |
| Bleeding | |
| Recommendations | Majority of bleeding events are minor, and most patients can continue therapy. However, if a patient has a history of major hemorrhage, this may warrant further risk-benefit analysis if anticoagulation is to be considered for patients with AF on BTK inhibitor |
Note: Shared decision-making with patients should be considered where appropriate.
AF = atrial fibrillation; BNP = B-type natriuretic peptide; BTK = Bruton’s tyrosine kinase; CMR = cardiac magnetic resonance imaging; DOAC = direct oral anticoagulant; LVEF = left ventricular ejection fraction; LVH = left ventricular hypertrophy; RAAS = renin-angiotensin-aldosterone-system; SGLT2 = sodium-glucose cotransporter-2; VA = ventricular arrhythmia; VT = ventricular tachycardia.
Finally, the risk-benefit ratio should be considered for the continuation or resumption of BTK inhibitors in patients with suspected or definite cardiotoxicity. Although cardiovascular data are limited, it is reasonable to consider a next-generation BTK inhibitor in patients with ibrutinib-associated cardiotoxicity. In 2 small trials, transition to a next-generation drug, such as acalabrutinib, appeared to be linked with lower (symptomatic) AF burden.84,85 Generally, the occurrence of extended ventricular tachycardia or sudden cardiac death should trigger consideration of immediate discontinuation. With other cardiovascular adverse events, resuming BTK inhibitor treatment should include multidisciplinary discussion (oncology, cardiology, pharmacy) when possible. However, additional cardiac-focused prospective studies are needed to confirm these findings and inform the optimal strategies for rechallenge among patients with serious cardiotoxicity. Key areas for future investigation are summarized in Table 4.
Table 4.
Summary Take-Home Points and Selected Common Issues That Remain to Be Addressed With BTK Inhibitor Therapies
| Key take home points |
| BTK inhibitors dramatically improve survival outcomes in CLL and other malignancy populations |
| BTK inhibitors are associated with a >4-fold increase in AF and other arrhythmiasa when compared with patients not exposed to BTK inhibitor therapy, with some unique cardiotoxic mechanisms |
| New or worsened hypertension is common with BTK inhibitor use (ibrutinib, acalabrutinib, zanubrutinib) |
| Other cardiovascular events (eg, heart failure, ventricular arrhythmias) are increased with BTK inhibitor use |
| Although less than ibrutinib, newer BTK inhibitors still appear to link with increased cardiotoxic risk |
| Early hypertension and older age appear to disproportionately increase the risk of AF and other cardiotoxic events with BTK inhibitor therapy among CLL patients |
| Cardiovascular events (eg, AF, ventricular arrhythmias) influence long-term cardiovascular and overall survival after BTK inhibitor treatment |
| Caution should be used when interpreting clinical trial data for BTK inhibitor cardiotoxic risk assessment, because many trials do not systematically quantify or ascertain subclinical CVD events |
| Evidence gaps and future research directions |
| Specific predictive factors of long-term cardiotoxic risk with BTK inhibitor therapies in CLL and other hematologic malignancy populations |
| Mechanisms underlying increased cardiotoxic risk, beyond selectivity (given emerging data suggesting residual cardiac risk with selective BTK inhibitors) |
| Comparison of cardiac toxicities profiles of newer BTK inhibitors (eg, acalabrutinib vs zanubrutinib) |
| True burden of arrhythmia(s) or hypertension using systematic ECG or blood pressure monitoring with BTK inhibitor treatment |
| True incidence, predictive factors, and preventative strategies for potentially fatal ventricular arrhythmias |
| Role of subclinical remodeling (eg, fibrosis) and early hypertension in major cardiotoxic event susceptibility |
| Optimal strategy for preventing (and/or controlling) hypertension and other cardiovascular events with BTK inhibitor therapy; and for BTK inhibitor rechallenge |
| Personalized cardioprotection strategies/plan (eg, integrating biologic, genetic, and imaging markers) |
Note: Shared decision-making with patients should be considered where appropriate.
CLL = chronic lymphocytic leukemia; CVD = cardiovascular disease; ECG = electrocardiogram; other abbreviations as in Table 3.
Conclusions
BTK inhibitor therapies have revolutionized the treatment of CLL and many hematologic malignancies. Yet, even in the era of next-generation BTK inhibitors, cardiotoxic events remain a potential limitation to the effective application of these therapies. Understanding the effects of established and emerging therapies will facilitate the targeted control and prevention of these adverse events. A multidisciplinary strategy, inclusive of cardio-oncologic assessments (eg, ECG, imaging, clinical assessment) and shared decision-making with patients and the cardiovascular and oncologic teams, is needed for optimal application of BTK inhibitors and other CLL therapies. Despite the gaps in current evidence and risk assessment, emerging data may provide a basis for future investigations focused on defining the true incidence and burden of cardiovascular disease among CLL patients treated with contemporary therapies. Together, these provide compelling impetus for more rigorous investigations of the predictive factors, mechanisms, and preventive strategies to reduce cardiotoxic risk in a growing population of patients taking BTK inhibitor therapies.
Funding Support and Author Disclosures
This work was supported in part by a National Institutes of Health P50-CA140158 grant. Dr Awan has received research funding from Innate Pharma and Pharmacyclics; has provided consulting services to Gilead Sciences, Pharmacyclics, Inc, Janssen, Abbvie, Sunesis, AstraZeneca, Genentech, and Novartis Oncology; and has served on the Speakers Bureau of Abbvie and AstraZeneca. Dr Fradley has received grant support from Medtronic and AstraZeneca; and provides consulting services to Abbvie, AstraZeneca, Johnson and Johnson, Myovant, Pfizer, and Zoll. Dr Kittai is supported by the ASCO Career Development Award; receives research funding from AstraZeneca; and has consulted for AstraZeneca, Abbvie, Beigene, Bristol Myers Squibb, Eli Lilly, Janssen, and KITE. Dr Rogers has received research funding from Genentech, AbbVie, Janssen, and Novartis; has consulted for AstraZeneca, Janssen, Pharmacyclics, AbbVie, Genentech, Beigene, and LOXO@Lilly; and is a scholar in clinical research of the Leukemia and Lymphoma Society (CDP 2331-20). Dr Woyach was supported by R01-CA197870, R01-CA250503, R01-CA177292, R01-CA192928, and scholar in clinical research grants from the Leukemia and Lymphoma Society (CDP 2331-20); has received research funding from Abbvie, Pharmacyclics, Janssen, Acerta, Loxo, Karyopharm, and Morphosys; and has consulted for Janssen and Pharmacyclics. Dr Addison is supported by National Institutes of Health grant numbers K23-HL155890 and R01HL170038, and an American Heart Association-Robert Wood Johnson Foundation Program (Harold Amos) grant. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Husam Abdel-Qadir MD, PhD, served as Guest Associate Editor for this paper. Paaladinesh Thavendiranathan, MD, MSc, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Byrd J.C., Furman R.R., Coutre S.E., et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woyach J.A., Ruppert A.S., Heerema N.A., et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–2528. doi: 10.1056/NEJMoa1812836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd J.C., Harrington B., O’Brien S., et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tam C.S., Opat S., D’sa S., et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136:2038–2050. doi: 10.1182/blood.2020006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd J.C., Hillmen P., Ghia P., et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39:3441–3452. doi: 10.1200/JCO.21.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown J.R., Eichhorst B., Hillmen P., et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2022;388(4):319–332. doi: 10.1056/NEJMoa2211582. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA A Cancer J Clinicians. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 8.Search of: ibrutinib - list results. ClinicalTrials.gov. https://clinicaltrials.gov/search?term=ibrutinib
- 9.Fazal M., Gomez S., Cheng P., Rhee J.-W., Baykaner T. Tyrosine kinase inhibitor associated polymorphic ventricular tachycardia. J Am Coll Cardiol CardioOnc. 2022;4:S4–S5. [Google Scholar]
- 10.Chen S.T., Azali L., Rosen L., et al. Hypertension and incident cardiovascular events after next-generation BTKi therapy initiation. J Hematol Oncol. 2022;15(1):92. doi: 10.1186/s13045-022-01302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiczer T.E., Levine L.B., Brumbaugh J., et al. Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv. 2017;1:1739–1748. doi: 10.1182/bloodadvances.2017009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhat S.A., Gambril J., Azali L., et al. Ventricular arrhythmias and sudden death events following acalabrutinib initiation. Blood. 2022;140:2142–2145. doi: 10.1182/blood.2022016953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown J.R., Byrd J.C., Ghia P., et al. Cardiovascular adverse events in patients with chronic lymphocytic leukemia receiving acalabrutinib monotherapy: pooled analysis of 762 patients. Haematologica. 2022;107:1335–1346. doi: 10.3324/haematol.2021.278901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baptiste F., Cautela J., Ancedy Y., et al. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart. 2019;6 doi: 10.1136/openhrt-2019-001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown J.R., Moslehi J., O’Brien S., et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102:1796–1805. doi: 10.3324/haematol.2017.171041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azali L., Hazelden L., Wiczer T., et al. Evaluation of the incidence and risk factors associated with major cardiovascular events in patients receiving acalabrutinib therapy. Blood. 2020;136:29–30. [Google Scholar]
- 17.Lentz R., Feinglass J., Ma S., Akhter N. Leukemia & lymphoma risk factors for the development of atrial fibrillation on ibrutinib treatment risk factors for the development of atrial fibrillation on ibrutinib treatment. Leuk Lymphoma. 2019;60(6):1447–1453. doi: 10.1080/10428194.2018.1533129. [DOI] [PubMed] [Google Scholar]
- 18.Avalon J.C., Fuqua J., Miller T., et al. Pre-existing cardiovascular disease increases risk of atrial arrhythmia and mortality in cancer patients treated with Ibrutinib. Cardio-Oncology. 2021;7:38. doi: 10.1186/s40959-021-00125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickerson T., Wiczer T., Waller A., et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134:1919–1928. doi: 10.1182/blood.2019000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao L., Salem J.-E., Clauss S., et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src kinase. Circulation. 2020;142:2443–2455. doi: 10.1161/CIRCULATIONAHA.120.049210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pretorius L., Du X.J., Woodcock E.A., et al. Reduced phosphoinositide 3-kinase (p110α) activation increases the susceptibility to atrial fibrillation. Am J Pathol. 2009;175:998–1009. doi: 10.2353/ajpath.2009.090126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuomi J.M., Bohne L.J., Dorey T.W., et al. Distinct effects of ibrutinib and acalabrutinib on mouse atrial and sinoatrial node electrophysiology and arrhythmogenesis. J Am Heart Assoc. 2021;10(22) doi: 10.1161/JAHA.121.022369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L., Li L., Ruan Y., et al. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16:1374–1382. doi: 10.1016/j.hrthm.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Archibald W.J., Rabe K.G., Kabat B.F., et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib: risk prediction, management, and clinical outcomes. Ann Hematol. 2021;100:143–155. doi: 10.1007/s00277-020-04094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buck B., Poliner M., Ghazi S., Chen S.T. Risks of serious cardiovascular events with next generation BTK inhibitors. J Am Coll Cardiol CardioOnc. 2023;(1_Sup) [Google Scholar]
- 26.Mato A.R., Shah N.N., Jurczak W., et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397:892–901. doi: 10.1016/S0140-6736(21)00224-5. [DOI] [PubMed] [Google Scholar]
- 27.Mato A.R., Pagel J.M., Coombs C.C., et al. Pirtobrutinib, a next generation, highly selective, non-covalent BTK inhibitor in previously treated CLL/SLL: updated results from the Phase 1/2 BRUIN Study. Blood. 2021;138 391-391. [Google Scholar]
- 28.Singh A., El Hangouche N., McGee K., et al. Utilizing left atrial strain to identify patients at risk for atrial fibrillation on ibrutinib. Echocardiography. 2021;38:81–88. doi: 10.1111/echo.14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam C.S., Dimopoulos M., Garcia-Sanz R., et al. Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv. 2022;6:1296–1308. doi: 10.1182/bloodadvances.2021005621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau W.C.Y., Torre C.O., Man K.K.C., et al. Comparative effectiveness and safety between apixaban, dabigatran, edoxaban, and rivaroxaban among patients with atrial fibrillation: a multinational population-based cohort study. Ann Intern Med. 2022;175:1515–1524. doi: 10.7326/M22-0511. [DOI] [PubMed] [Google Scholar]
- 31.Yao X., Abraham N.S., Sangaralingham L.R., et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. JAHA. 2016;5 doi: 10.1161/JAHA.116.003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lampson B.L., Yu L., Glynn R.J., et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129:2581–2584. doi: 10.1182/blood-2016-10-742437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guha A., Derbala M.H., Zhao Q., et al. Ventricular arrhythmias following ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol. 2018;72:697–698. doi: 10.1016/j.jacc.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen Updates Warnings Section of Imbruvica Label. 2022. https://www.formularywatch.com/view/janssen-updates-warnings-section-of-imbruvica-label. An access date, if required would be August 1, 2023.
- 35.Buck B., Chum A.P., Patel M., et al. Cardiovascular magnetic resonance imaging in patients with ibrutinib-associated cardiotoxicity. JAMA Oncol. 2023;9(4):552–555. doi: 10.1001/jamaoncol.2022.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Jong J., Hellemans P., Jiao J.J., et al. Ibrutinib does not prolong the corrected QT interval in healthy subjects: results from a thorough QT study. Cancer Chemother Pharmacol. 2017;80:1227–1237. doi: 10.1007/s00280-017-3471-x. [DOI] [PubMed] [Google Scholar]
- 37.Tarnowski D., Feder A.-L., Trum M., et al. Ibrutinib impairs IGF-1-dependent activation of intracellular Ca handling in isolated mouse ventricular myocytes. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1190099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen J.B., Geara A.S., Hogan J.J., Townsend R.R. Hypertension in cancer patients and survivors. J Am Coll Cardiol CardioOnc. 2019;1:238–251. doi: 10.1016/j.jaccao.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burger J.A., Tedeschi A., Barr P.M., et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrd J.C., Brown J.R., O’Brien S., et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrd J.C., Furman R.R., Coutre S.E., et al. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC-1102 study. Clin Cancer Res. 2020;26:3918–3927. doi: 10.1158/1078-0432.CCR-19-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caldeira D., Alves D., Costa J., Ferreira J.J., Pinto F.J. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PloS One. 2019;14 doi: 10.1371/journal.pone.0211228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee D.H., Hawk F., Seok K., et al. Association between ibrutinib treatment and hypertension. Heart. 2022;108:445–450. doi: 10.1136/heartjnl-2021-319110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roeker L.E., Sarraf Yazdy M., Rhodes J., et al. Hypertension in patients treated with ibrutinib for chronic lymphocytic leukemia. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woyach J.A., Blachly J.S., Rogers K.A., et al. Acalabrutinib plus obinutuzumab in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia. Cancer Discov. 2020;10:394–405. doi: 10.1158/2159-8290.CD-19-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gopal A.K., Kahl B.S., de Vos S., et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furman R.R., Sharman J.P., Coutre S.E., et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Natarajan G., Terrazas C., Oghumu S., et al. Ibrutinib enhances IL-17 response by modulating the function of bone marrow derived dendritic cells. OncoImmunology. 2016;5 doi: 10.1080/2162402X.2015.1057385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munir T., Brown J.R., O’Brien S., et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94:1353–1363. doi: 10.1002/ajh.25638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barr P.M., Owen C., Robak T., et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:3440–3450. doi: 10.1182/bloodadvances.2021006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdel-Qadir H., Sabrie N., Leong D., et al. Cardiovascular risk associated with ibrutinib use in chronic lymphocytic leukemia: a population-based cohort study. J Clin Oncol. 2021;39:3453–3462. doi: 10.1200/JCO.21.00693. [DOI] [PubMed] [Google Scholar]
- 52.Salem J.-E., Manouchehri A., Bretagne M., et al. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;74:1667–1678. doi: 10.1016/j.jacc.2019.07.056. [DOI] [PubMed] [Google Scholar]
- 53.Chen M.H., Kerkelä R., Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118:84–95. doi: 10.1161/CIRCULATIONAHA.108.776831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Shanafelt T.D., Wang X.V., Kay N.E., et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–443. doi: 10.1056/NEJMoa1817073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J., Zhao A., Zhou H., Zhu J., Niu T. Risk of bleeding associated with ibrutinib in patients with B-cell malignancies: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.580622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Brien S., Hillmen P., Coutre S., et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18:648–657.e15. doi: 10.1016/j.clml.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 58.Lipsky A.H., Farooqui M.Z.H., Tian X., et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100:1571–1578. doi: 10.3324/haematol.2015.126672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kunk P.R., Mock J., Devitt M.E., et al. Major bleeding with ibrutinib: more than expected. Blood. 2016;128 3229-3229. [Google Scholar]
- 60.Mock J., Kunk P.R., Palkimas S., et al. Risk of major bleeding with ibrutinib. Clin Lymphoma Myeloma Leuk. 2018;18:755–761. doi: 10.1016/j.clml.2018.07.287. [DOI] [PubMed] [Google Scholar]
- 61.Raz M.A., Arnason J., Bairey O., et al. The risk of bleeding in patients receiving ibrutinib combined with novel direct oral anticoagulants. Br J Haematol. 2020;189(2):e31–e33. doi: 10.1111/bjh.16422. [DOI] [PubMed] [Google Scholar]
- 62.Kumar P.S., Wiczer T., Rosen L., et al. Evaluation of bleeding events in patients receiving acalabrutinib therapy. Leukemia. 2023;37(7):1554–1557. doi: 10.1038/s41375-023-01869-1. [DOI] [PubMed] [Google Scholar]
- 63.Atkinson B.T., Ellmeier W., Watson S.P. Tec regulates platelet activation by GPVI in the absence of Btk. Blood. 2003;102:3592–3599. doi: 10.1182/blood-2003-04-1142. [DOI] [PubMed] [Google Scholar]
- 64.Rayes J., Watson S.P., Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest. 2019;129:12–23. doi: 10.1172/JCI122955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng T.J., Lofurno E.R., Melrose A.R., et al. Assessment of the effects of Syk and BTK inhibitors on GPVI-mediated platelet signaling and function. Am J Physiol Cell Physiol. 2021;320:C902–C915. doi: 10.1152/ajpcell.00296.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamel S., Horton L., Ysebaert L., et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29:783–787. doi: 10.1038/leu.2014.247. [DOI] [PubMed] [Google Scholar]
- 67.Kumar P.S., Wiczer T., Rosen L., et al. Evaluation of the incidence and risk factors associated with bleeding events in patients receiving acalabrutinib therapy. Blood. 2021;138 3729-3729. [Google Scholar]
- 68.Lipsky A., Lamanna N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematology. 2020;2020:336–345. doi: 10.1182/hematology.2020000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boriani G., Corradini P., Cuneo A., et al. Practical management of ibrutinib in the real life: Focus on atrial fibrillation and bleeding. Hematol Onc. 2018;36:624–632. doi: 10.1002/hon.2503. [DOI] [PubMed] [Google Scholar]
- 70.Diamond A., Bensken W.P., Vu L., Dong W., Koroukian S.M., Caimi P. Ibrutinib is associated with increased cardiovascular events and major bleeding in older CLL patients. J Am Coll Cardiol CardioOnc. 2023;5:233–243. doi: 10.1016/j.jaccao.2023.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solman I.G., Blum L.K., Hoh H.Y., et al. Ibrutinib restores immune cell numbers and function in first-line and relapsed/refractory chronic lymphocytic leukemia. Leukemia Res. 2020;97 doi: 10.1016/j.leukres.2020.106432. [DOI] [PubMed] [Google Scholar]
- 72.Kleindorfer D.O., Towfighi A., Chaturvedi S., et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021:52. doi: 10.1161/STR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 73.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J Am Coll Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 74.Molica S. Venetoclax: a real game changer in treatment of chronic lymphocytic leukemia. Int J Hematol Oncol. 2020;9:IJH31. doi: 10.2217/ijh-2020-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tam C.S., Allan J.N., Siddiqi T., et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: primary analysis of the CAPTIVATE FD cohort. Blood. 2022;139:3278–3289. doi: 10.1182/blood.2021014488. [DOI] [PubMed] [Google Scholar]
- 76.Thompson P.A., Wang Y., Keating M.J., et al. Venetoclax consolidation in patients with high-risk CLL who have been on ibrutinib more than a year achieves a high rate of undetectable minimal residual disease. Blood. 2021;138 3723-3723. [Google Scholar]
- 77.Lew T.E., Seymour J.F. Clinical experiences with venetoclax and other pro-apoptotic agents in lymphoid malignancies: lessons from monotherapy and chemotherapy combination. J Hematol Oncol. 2022;15:75. doi: 10.1186/s13045-022-01295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davids M.S., Hallek M., Wierda W., et al. Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res. 2018;24:4371–4379. doi: 10.1158/1078-0432.CCR-17-3761. [DOI] [PubMed] [Google Scholar]
- 79.Seymour J.F., Kipps T.J., Eichhorst B., et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107–1120. doi: 10.1056/NEJMoa1713976. [DOI] [PubMed] [Google Scholar]
- 80.Kater A.P., Wu J.Q., Kipps T., et al. Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO Phase III study. J Clin Oncol. 2020;38:4042–4054. doi: 10.1200/JCO.20.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Sawaf O., Zhang C., Tandon M., et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21:1188–1200. doi: 10.1016/S1470-2045(20)30443-5. [DOI] [PubMed] [Google Scholar]
- 82.Fischer K., Al-Sawaf O., Bahlo J., et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225–2236. doi: 10.1056/NEJMoa1815281. [DOI] [PubMed] [Google Scholar]
- 83.Mahida H., Gharia B., Ugoeke N., Maludum O., Asif A., Calderon D. Abstract 11835: evaluation of cardiovascular adverse events associated with ibrutinib, venetoclax and idelalisib used in treatment of chronic lymphocytic leukemia. Circulation. 2018;138 [Google Scholar]
- 84.Rogers K.A., Thompson P.A., Allan J.N., et al. Phase II study of acalabrutinib in ibrutinib-intolerant patients with relapsed/refractory chronic lymphocytic leukemia. Haematol. 2021;106:2364–2373. doi: 10.3324/haematol.2020.272500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Awan F.T., Schuh A., Brown J.R., et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3:1553–1562. doi: 10.1182/bloodadvances.2018030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.