Abstract
Cancer treatment–induced cardiotoxicities are an ongoing concern throughout the cancer care continuum from treatment initiation to survivorship. Several “standard-of-care” primary, secondary, and tertiary prevention strategies are available to prevent the development or further progression of cancer treatment–induced cardiotoxicities and their risk factors. Despite exercise’s established benefits on the cardiovascular system, it has not been widely adopted as a nonpharmacologic cardioprotective strategy within cardio-oncology care. In this state-of-the-art review, the authors discuss cancer treatment–induced cardiotoxicities, review the existing evidence supporting the role of exercise in preventing and managing these sequelae in at-risk and affected individuals living after cancer diagnoses, and propose considerations for implementing exercise-based services in cardio-oncology practice.
Key Words: cancer, cardio-oncology, cardiotoxicities, exercise
Central Illustration
Highlights
-
•
Exercise training is an underused prevention strategy for cardiotoxicities.
-
•
Exercise improves functional capacity, cardiac function, and cardiac biomarkers.
-
•
Implementation of exercise services into cardio-oncology has several challenges.
The spectrum of adverse effects that cancer treatments have on the cardiovascular system is vast. These sequelae are collectively referred to as cardiovascular toxicities and can be classified according to broad clinical categories, including cardiac dysfunction and heart failure, myocarditis, arrhythmias and QT interval prolongation, hypertension, and vascular toxicity (Central Illustration).1 However, in this review we focus on cardiac vs vascular cardiotoxicities given that cardiomyopathies are the most prevalent and best studied to date.2 Cardiotoxicities may present at any point along the cancer care continuum, including acutely (eg, within 2 weeks of treatment), early (eg, between 2 weeks and 1 year of treatment), and late (eg, >1-year post-treatment). Although there are several strategies to lower the risk for cardiotoxicity development, such as reduced cardiotoxic cancer treatment dose and pharmacologic interventions, these strategies often come with side effects and risks.1 Given the myriad points at which reduced cardiac health occurs across the cancer care continuum, an understanding of effective nonpharmacologic strategies for reducing cardiotoxicity risk is needed. Such strategies include preventing: 1) the onset and progression of cardiovascular disease (CVD) risk factors or subclinical CVD in otherwise healthy populations (ie, primary prevention); 2) the worsening of CVD risk factors or development of overt CVD in populations with increased CVD risk (ie, secondary prevention); and 3) the progression of established CVD and future CVD events (ie, tertiary prevention).3, 4, 5 Importantly, primary, secondary, and tertiary strategies may be implemented at any point along the cancer survivorship continuum (ie, before, during, or after treatment) according to patient risk.
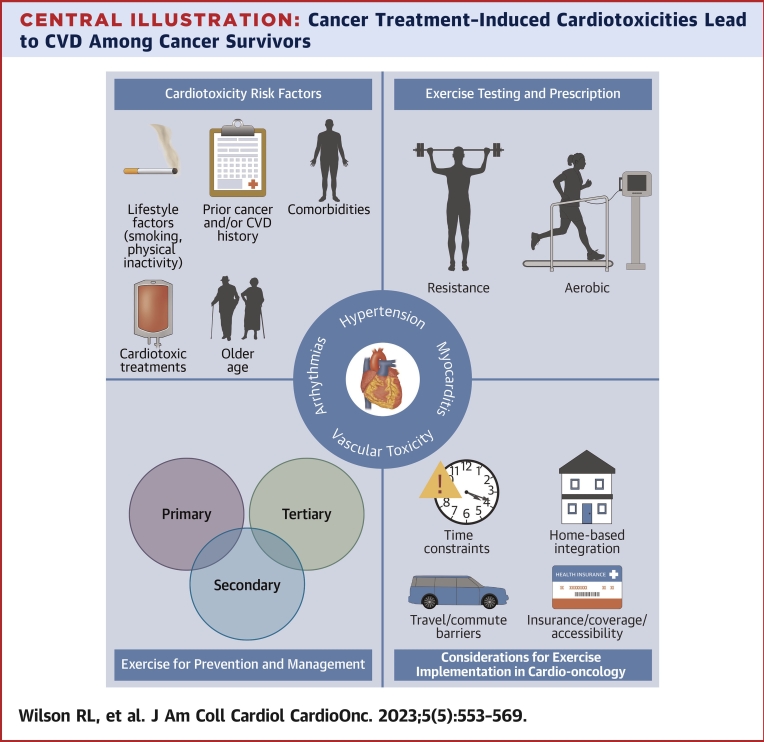
Central Illustration.
Cancer Treatment–Induced Cardiotoxicities Lead to CVD Among Cancer Survivors
Risk for cardiotoxicity development is increased by receipt of cardiotoxic treatments (eg, anthracyclines) and the presence of other characteristics, such as older age and comorbidities. Exercise may be used as a strategy to prevent and/or manage in the primary, tertiary, or secondary setting, with cardio-oncology facilities being the ideal place to implement such strategies. CVD = cardiovascular disease.
Exercise training is a well-established nonpharmacologic strategy that has cardiac health benefits at the primary, secondary, and tertiary levels of prevention within noncancer cardiology settings3, 4, 5; the American Heart Association presented its first exercise guidelines for cardiac exercise rehabilitation in 1975.6 In contrast, the implementation of exercise into cardio-oncology care is not as well established,7 with low to moderate research quality and moderate to high risk for bias reported with this paradigm.8 However, there is a strong rationale to include exercise given its multisystem benefits.7 Exercise has been deemed safe, tolerable, and effective for alleviating fatigue and improving cardiorespiratory fitness, body composition, physical function, strength, and quality of life among patients with cancer9; however, the evidence indicating if, how, and when exercise may specifically benefit the cardiac health of patients with cancer who receive cardiotoxic treatment is still in its infancy. Thus, only cancer survivors with confirmed diagnoses of CVDs are considered eligible for referral to exercise-based cardiology rehabilitation (ie, only tertiary prevention strategies) within the current cardio-oncology care model.
In this state-of-the-art review, we: 1) provide a brief overview of the prevalence of cancer treatment–induced cardiotoxicity; 2) discuss fundamental concepts of exercise testing and prescription; 3) review the existing evidence supporting the role of exercise in preventing and managing cancer treatment–induced cardiotoxicities; and 4) discuss considerations for implementing exercise-based services in cardio-oncology practice.
Statement of the Problem: Cancer Treatment–Induced Cardiotoxicities
The prevalence of cancer treatment–induced cardiotoxicities varies widely depending on the definitions used (eg, Common Terminology Criteria for Adverse Events vs International Classification of Diseases medical billing codes), study setting and design (eg, clinical trial vs community cohort study), population and cancer type studied (eg, pediatric vs adult), cancer treatment used, and duration of follow-up, among other factors.10 Unfortunately, long lag times between inciting treatment exposures and the occurrence of symptomatic events, complex interactions and effects of multimodal and multiagent treatment regimens, and the variable impact of pre-existing or emerging CVD risk factors may all hinder precise risk modeling and subsequent patient care planning.
The severity and incidence of cancer treatment–induced cardiotoxicities vary widely and depend on the cancer, patient characteristics, dose and duration of therapy, and type and combination of therapies. For example, among 865 patients receiving various cardiotoxic treatments for primarily breast and hematological malignancies, myocardial dysfunction or cardiotoxicity presence was reported to be mild in 92% and 32% of patients, moderate in 4% and 3%, and severe in 3% and 3%, respectively.11 Furthermore, individuals reporting severe cardiotoxicities were 15.8 times more likely to die compared with individuals without. Conversely, among the 10,724 participants of the CCSS (Childhood Cancer Survivor Study), the cumulative incidence rates of coronary artery disease and heart failure by 45 years of age were reported to be 5.3% and 4.8%, respectively.12 Nonetheless, our current understanding of cardiotoxicity has come largely from studies in patients who received anthracycline and/or trastuzumab for hematological and breast malignancies, with the use of cardiac imaging driven by these specific oncology therapies, not cardiovascular risk profile.13 Data from population cohorts point to higher CVD burden among survivors of other cancers, such as lung and colorectal cancers, compared with general population,14 suggesting the need to broaden cardiovascular stratification and assessment strategies beyond patients receiving known cardiotoxic agents such as anthracyclines.
Highlights
-
•
Cancer treatment–induced cardiotoxicity prevalence varies by cancer type and treatment.
-
•
Current understanding of cardiotoxicities comes from patients with hematological or breast malignancies receiving anthracycline and trastuzumab chemotherapies.
Testing and Prescription Principles
Exercise testing and training are cornerstones of chronic disease management in numerous clinical settings. In this section, we expand upon the content of Table 1 and Supplemental Figure 1 by discussing the role of exercise testing and training across primary, secondary, and tertiary prevention settings to help facilitate decision making surrounding implementing exercise within cardio-oncology practice.
Table 1.
Overview of Commonly Used Aerobic Exercise Testing Modalities
| CPET | Maximal and Submaximal | 6MWT | 1RM | Multiple-Repetition Maximum | Hand-Grip Dynamometry | |
|---|---|---|---|---|---|---|
| Test description | 8- to 12-min exercise test on a bicycle or treadmill in which the load or speed is progressively increased until maximal criteria are reached, coupled with automated gas exchange systems to obtain Vo2peak | 3- to 12-min exercise test on a bicycle or treadmill in which the load or speed is progressively increased until maximal criteria are reached (or 85% of age-predicted maximum heart rate for submaximal test) METs or Vo2peak is estimated using prediction equations based on achieved treadmill speed/grade and duration or the peak cycle ergometer workload (watts). |
Measures distance covered during 6 min of walking in a 100-ft hallway103 | Maximal amount of weight lifted in one repetition following a series of sets to progress the weight to the maximal capacity | Maximal amount of weight lifted a predefined number of repetitions (eg, 8, 10) with good technique Maximal strength estimated using prediction equations |
Maximal amount of force or average maximum force generated bilaterally over three trials |
| Outcome | Linear metric from <15 mL O2/kg/min (poor) to >95 ml O2/kg/min (endurance trained) | Linear metric from 1 MET (poor) to >16 METs (endurance trained) | Linear metric from <100 m (poor) to >700 m (excellent) | Linear metric from 0 to >500 lb depending on muscle group and exercise performed | Linear metric from 0 to >500 lb depending on muscle group and exercise performed | Linear metric from 0 to >200 lb (age- and sex-based norm-defined ratings) |
| Limitations | Requires advanced equipment and trained personnel | Maximal: requires advanced equipment and trained personnel Submaximal: estimated Vo2peak equations derived from healthy, nononcologic populations |
Ceiling effects | Requires advanced equipment and trained personnel | Requires advanced equipment and trained personnel | Requires specialized equipment |
| Reliability and validity | ++ (cancer: prostate)104 | − | ++ (noncancer)34 | +++ (noncancer)105,106 | + (cancer: breast)38 | +++ (noncancer)107 + (cancer: breast)108 |
| Proof of concept | +++ (noncancer)109 + (cancer: lung)110 |
Maximal: ++ (cancer: mixed)17 Submaximal: + |
++ (cancer: lung)31 | + (cancer: cachexia)111 | − | +++ (noncancer)40 + (cancer: advanced, malnourished)112,113 |
| Prospective validation | ++ (cancer: lung, colorectal, Hodgkin’s)21,22 | − | ++ (cancer: glioma)114 | + (cancer: mixed)36 | − | +++ (noncancer)40 ++ (cancer: GI, lung, cachexia, advanced)41, 42, 43, 44,115,116 |
| Incremental value | ++ (cancer: breast, colorectal)21,110,117 | − | + (cancer: glioma)118 | + (cancer: mixed)36 | − | +++ (noncancer: all-cause mortality, CVD mortality, CVD incidence)119 ± (lung)42,120 |
| Clinical utility | ++ (lung)121 − (other) |
− | − | − | − | − |
| Modifiable | +++ (cancer: mixed, lung)46,122 | ++ (cancer: mixed)46 | ++ (cancer: mixed)123 | +++ (cancer: mixed)45 | +++ (cancer: mixed)45 | +++ (cancer: mixed)45 |
| Ease of use | − (noncancer)25 | Maximal: + Submaximal: ++ |
+++ | − | − | +++ |
| Normative values | +++ (noncancer)16 | +++ (noncancer)103 | +++ (noncancer)103 | + (noncancer)37 | − | + (cancer: advanced)39 |
| Setting | Primary, secondary, tertiary | Primary, secondary, tertiary | Tertiary, certain primary and secondary | Primary, secondary, tertiary | Primary, secondary, tertiary | Primary, secondary, tertiary |
| Patient population | Any | Any | Inoperable Undergoing treatment Frail, elderly Skeletal myopathy Respiratory limitation |
Any | Any | Any |
| Test purpose | Prognostication, guiding exercise training | Prognostication, guiding exercise training | Prognostication | Prognostication, guiding exercise training | Prognostication, guiding exercise training | Prognostication |
Table adapted from Wagner et al.124 Reliability and validity: Has the test demonstrated reliability and validity? Proof of concept: Does the variable differ between patients with and without adverse outcomes? Prospective validation: Can the variable predict future outcomes in a prospective cohort? Incremental value: Does the variable add predictive value over and above standard, clinically established risk markers? Clinical utility: Does the variable change predicted risk sufficiently to modify recommended therapy? Modifiable: Does the variable change with intervention? Ease of use: Is the assessment widely applicable? Reference values: Are published reference values available?
1RM = 1-repetition maximum; 6MWT = six-minute walk test; CPET = cardiopulmonary exercise testing; CVD = cardiovascular disease; GI = gastrointestinal; Vo2peak = maximal oxygen consumption; + = minimal requirements of criteria met, small evidence base; ++ = criteria met, moderate evidence base; +++ = criteria fully met, large evidence base; − = criteria not met or no evidence.
Exercise testing in cardio-oncology practice
Over the past 3 decades, exercise testing has increasingly been used in cancer as an objective assessment of functional capacity to help guide risk stratification, monitor toxicities, individualize exercise prescriptions, and assess intervention efficacy.15 Here, we provide a brief overview of commonly used aerobic and resistance-based exercise testing modalities to objectively evaluate cardiorespiratory fitness and muscular strength, as well as why and when they may be appropriate to use within the cardio-oncology setting.
Aerobic exercise testing
Cardiorespiratory fitness, as measured by maximal oxygen consumption (Vo2peak), is the gold-standard assessment of aerobic exercise capacity when maximal cardiopulmonary exercise testing (CPET) is coupled with automated gas exchange assessment, with or without parallel invasive hemodynamic studies.16 Additionally, in cardiology settings Vo2peak is used as a prognostic measure, as it is strongly associated with all-cause and cardiovascular-specific mortality.17 Poor cardiorespiratory fitness has been associated with a higher prevalence of treatment-related toxicities18, 19, 20, 21, 22 and postoperative complications,23 as well as all-cause, cardiovascular, and cancer mortality17 and therefore has been used for risk stratification across the cancer continuum. Evaluation of cardiorespiratory fitness via CPET also allows the identification of metabolic thresholds that can be used to guide aerobic exercise training.24 CPET is the ideal method of assessing cardiorespiratory fitness across prevention settings (ie, primary to tertiary). It is important to note, however, that the widespread adoption of CPET across care settings is limited because of the need for specialized equipment and trained personnel to conduct and interpret CPET results.25
Incremental (sub)maximal exercise testing is an alternative tool to estimate cardiorespiratory fitness that can be used across prevention settings. The major benefit of estimating cardiorespiratory fitness via submaximal tests is that it does not require sophisticated metabolic cart equipment or specially trained personnel, and heart rate and work load levels (eg, treadmill speed, cycle ergometer watts) can still be used to inform aerobic exercise training prescription and monitoring.16 However, many estimation equations are specific to the predetermined groups (eg, healthy White men, athletes, military professionals) they were developed for, and the difference between actual and estimated Vo2peak using these equations in patients with cancer has been shown to exceed 30%.26 Fortunately, (sub)maximal aerobic exercise testing protocols and prediction equations have been developed and validated for cancer survivors.27 Moreover, a recent study in patients with breast cancer revealed that including cancer treatment history in an estimated equation reduced the difference between measured and estimated Vo2peak to <1%.26
The 6-minute walk test (6MWT) is another method of estimating cardiorespiratory fitness that has high reliability (coefficient of variation ∼ 3%)28,29 and has been used for risk stratification. Compared with a 6MWT distance of <350 m, a distance of >400 m is associated with an adjusted reduced risk for all-cause mortality of about 50% in patients with advanced cancer.30,31 Advantages of the 6MWT are that there is no need for sophisticated equipment, and it can therefore be performed by majority of patients in most clinical and community settings. Nevertheless, because maximum walking speed is about 4.5 mph even in healthy populations, the 6MWT is not sensitive enough to discriminate between patients who can complete >500 m.32,33 In addition, the 6MWT does not allow a careful evaluation regarding the pathogenetic and clinical mechanisms involved in dyspnea and fatigue sensation compared with CPET-derived variables.34 Given its limitations, use of the 6MWT in cardio-oncology practice is restricted primarily to screening for functional impairments and prognostication in patients within tertiary prevention settings.
Resistance exercise testing
The gold standard for resistance exercise testing is the 1-repetition maximum (1RM), which determines the greatest resistance that can be moved through a complete range of motion with proper technique 1 time.35 The test-retest reliability of 1RM is very high, and importantly, higher levels of muscular strength evaluated using 1RM are associated with lower cancer mortality risk, independent of clinically established measures of overall and central adiposity and other potential confounders.36 Normative values are available for certain lower and upper body exercises in noncancer settings.37 Finally, this test is the preferred testing modality for exercise prescription because it objectively quantifies peak load and uses the same patterns undertaken during resistance exercise training.
However, 1RM testing may not be safe, well tolerated, or feasible for some patients. Alternatively, 10- to 15-repetition maximum (ie, the maximum weight that can be moved 10 to 15 times) protocols may be used to estimate the strength of patients at risk for, or living with, chronic diseases such as CVD, pulmonary diseases, and metabolic diseases. The test-retest reliability for multiple-repetition maximum testing is high38; however, its prognostic importance is not well defined in patients with cancer. This type of testing uses the same equipment as 1RM testing and therefore requires access to specialized equipment and well-trained personnel to administer the test, resources that may not be widely available across most hospitals.
Surrogate strength tests, such as isometric grip strength, have been validated to estimate upper extremity strength in patients with cancer and survivors.39 Importantly, lower grip strength is associated with poor outcomes in numerous cancer settings40 (eg, lung,41,42 gastrointestinal,43 and advanced or metastatic cancer44), normative values are available to facilitate test interpretation,39 and resistance training is associated with improved grip strength during chemotherapy.45 Nonetheless, grip strength does not accurately reflect whole-body strength and is limited to the maximal strength of the hand and arm muscles. Therefore, this modality is for prognostic purposes only.
Exercise training in cardio-oncology practice
In 2019, the American Heart Association proposed Cardio-Oncology Rehabilitation (CORE), a multidisciplinary approach to the cardiovascular rehabilitation of cancer survivors drawing upon established cardiac rehabilitation programs for cardiology patients without cancer.7 CORE recommends individualized aerobic and resistance exercise on the basis of the guidelines of the American College of Sports Medicine (ie, 150 min/wk of moderate-intensity or 75 min/wk of vigorous-intensity aerobic exercise and 2 strength training sessions)9 for cancer survivors identified at increased risk for cardiotoxicity development. Here we describe some considerations for exercise prescription for patients with cancer identified at high risk for or with established cardiotoxicities.
Aerobic exercise is the most commonly used training modality in cardiovascular care and may include activities such as walking, cycling, dancing, and swimming.35 To date, most aerobic exercise training programs in oncology have tested moderate-intensity continuous exercise (ie, ∼60%-75% of measured or estimated Vo2peak involving longer durations of activity causing noticeable but not excessive increases in heart rate and breathing rate) or high-intensity interval training (HIIT) (involving shorter durations of exercise that oscillate between vigorous [>75% Vo2peak] and lower [∼40%-50% Vo2peak] intensities) completed 2 or 3 days per week for 20 to 60 minutes per session over 12 to 15 weeks.46 The available evidence suggests that both moderate-intensity continuous exercise and HIIT are effective at preventing and treating CVD and its risk factors,47 are safe, and are well tolerated by most patients with cancer.48,49
Resistance exercise also plays a critical role in CVD prevention and treatment via its effects on muscular strength, power, hypertrophy, and endurance.35 Most resistance exercise training programs in oncology have tested moderate-intensity prescriptions using 2 or 3 sets of 8 to 12 repetitions at 60% to 70% of 1RM or a rating of perceived exertion of 5 or 6 on a 10-point scale, performed 2 or 3 days a week for 30 to 60 minutes per session over 12 to 16 weeks using weighted equipment, resistance bands, or body weight.50,51 Resistance exercise has been shown to be effective at improving muscular strength, muscle mass, and physical function, as well as being safe and well tolerated for most patients with cancer.45
Aerobic and resistance exercise approaches are often combined to improve the functional capacity and health of patients given their synergistic effects on maximizing improvements in cardiorespiratory fitness, muscular strength, physical function, and body composition.52,53 Ideally, across primary, secondary, and tertiary settings, the intensity, duration, and frequency of training sessions are gradually progressed across the entire program, and training intensity is sequenced in a nonlinear fashion whereby higher intensity or higher volume training is followed by lower intensity (recovery) training and rest days to optimize adaptation (ie, the principle of rest and recovery).54 Additionally, selection of equipment as well as intensity is typically tailored to each patient’s ability and exercise goals. Appropriate patient supervision strategies during exercise training should be informed by patients’ health status and use a combination of monitoring heart rate and respiratory responses, ratings of perceived exertion or effort, oxygen saturation, and active observation for signs of exercise-related adverse events (eg, arthralgia).
Safety considerations for exercise testing and training in cardio-oncology practice
Exercise may be limited if safety issues exist, such as weakness, bone defects, cognitive dysfunction, or musculoskeletal deficits associated with cancer treatment. Patient preparedness and cardiopulmonary safety should be assessed using a CPET before initiating exercise (Table 1) or a (sub)maximal alternative in the event that resources are limited.28,33 The American Heart Association recommends performing CPET during the initial patient assessment visit in a CORE program.7 Additionally, regular CPET to reassess heart rate at a given intensity throughout an exercise program should also occur when heart rate is used to monitor and prescribe exercise intensity. This is particularly important for patients with autonomic dysfunction, or treatments known to induce this, as there is a risk for exercise overdosing if exercise intensity is prescribed using only age-predicted maximum heart rate.55 Overall, the widely used American College of Sports Medicine absolute and relative contraindications for exercise should be used as the foundation of safety considerations for cardio-oncology patients considered for exercise programs.35
Highlights
-
•
Aerobic and resistance-based exercise tests may be used risk-stratify patients, monitor toxicities, individualize exercise prescriptions, and assess exercise efficacy.
-
•
CORE is a multidisciplinary approach to the cardiovascular rehabilitation of cancer survivors drawing upon established noncancer cardiology rehabilitation programs.
Response to the Problem: The Role of Exercise Training
In this section we review exemplar trials supporting the role of exercise as a primary, secondary, and tertiary prevention strategy for cancer treatment–induced cardiotoxicities (Supplemental Table 1). We focus on exercise training trials with cardiovascular endpoints including Vo2peak, cardiac function, and biomarkers of cardiac injury.
Exercise as a primary prevention strategy
Exercise can be used as a primary prevention strategy to prevent the development of treatment-induced cardiotoxicities and risk factors. At least 8 studies56, 57, 58, 59, 60, 61, 62, 63, 64, 65 have examined the effect of exercise on cardiac health among patients with cancer receiving cardiotoxic treatment in the primary prevention setting.
Cardiorespiratory fitness
Ma et al66 conducted a meta-analysis examining patients with breast cancer receiving anthracyclines and/or trastuzumab and reported aerobic exercise therapy to be associated with a 5.6 mL/kg/min increase in Vo2peak. However, this analysis was not limited to randomized controlled trials (RCTs), nor is it generalizable to other cancer types, treatments, or nonaerobic exercise interventions. RCTs examining combined aerobic and resistance exercise among patients with breast cancer receiving cardiotoxic treatments have reported mixed results, with an attenuation of treatment-induced declines in Vo2peak compared with nonexercising control subjects65 or no effect.61,64 Importantly, findings from 2 trials in women initiating breast cancer treatment indicate that exercise both during and after chemotherapy is associated with greater improvements in Vo2peak, suggesting that exercise should be initiated early during treatment and continue in the post-treatment setting.67,68 The attenuation of significant Vo2peak decline may be related to exercise volume, irrespective of mode; Kirkham et al62 and Haykowsky et al56 reported higher attendance (patients attended ≥67% and 55% of sessions, respectively) to have more favorable (maintained or improved) Vo2peak adaptations. Future research should focus on manipulations of frequency, intensity, time, and type to assess for a potential threshold for maintenance or improvement in Vo2peak while on cardiotoxic treatments.
Cardiac function
Although Vo2peak is an accepted prognosis outcome within cardiology, its implementation within cardio-oncology has not been validated.69 However, echocardiography is recommended as part of standard for care for patients with cancer considered at high risk for cardiotoxicities.1 Ma et al66 assessed left ventricular ejection fraction (LVEF), global longitudinal strain (GLS), and E/A ratio in a meta-analysis, but results for improvements in diastolic function were unclear, with a significant effect of exercise only on E/A ratio. Similar evidence is presented within the RCTs described in Supplemental Table 1; some combined aerobic and resistance exercise studies have revealed a trend in the attenuation of cancer treatment–induced LVEF and GLS decreases compared with nonexercising control subjects,63,64 but most studies showed no effect.58,59,65 However, these studies report only resting echocardiographic measures. There is some argument that measures of exercising cardiac function are more sensitive to cardiac changes and therefore more informative concerning cardiotoxicity risk.70 Foulkes et al65 demonstrated this among patients with breast cancer receiving cardiotoxic treatments: peak, but not rest, cardiac function measures were the driving force for Vo2peak changes after a combined aerobic and resistance exercise intervention.
Biomarkers of cardiac injury
Assessment of cardiac injury–related biomarkers, such as natriuretic peptides, is also recommended in patients considered at high risk for developing cardiotoxicities.1 The majority of studies showed no exercise effect in attenuating treatment-induced changes in biomarkers of cardiac injury.59,60,66 However, Foulkes et al65 reported that patients with breast cancer undertaking a combined aerobic and resistance exercise intervention had attenuated treatment-induced increases in troponin compared with nonexercising control subjects. Furthermore, Ansund et al71 conducted a 1-year follow-up of a 16-week exercise study completed while patients were on chemotherapy and reported, irrespective of exercise mode completed during treatment (aerobic only or combined aerobic and resistance training), that both exercise groups had significantly lower N-terminal pro–brain natriuretic peptide levels at 1-year follow-up compared with usual-care control subjects despite an absence of group differences at baseline and immediately after the 16-week intervention.
Summary
There is some indication that the use of exercise as a primary prevention strategy may mitigate significant declines in cardiovascular health compared with a nonexercising control group as assessed by outcomes of cardiorespiratory fitness, cardiac function, and biomarkers of cardiac injury (Table 2). However, the current evidence is heterogenous, with the majority of studies having major limitations (eg, no randomization, low-dose interventions, cardiovascular outcomes assessed as secondary outcomes). Specifically, the necessary exercise stimulus to improve or maintain baseline cardiovascular health is unclear given that some studies indicate that exercise volume may play a critical role in favorable cardiovascular-related adaptations.56,62 Furthermore, the appropriate modes of exercise are also unclear, because of heterogeneous exercise prescriptions across studies with respect to the aerobic and resistance components, as well as differences in frequency, intensity, and time. It may be that while on cardiotoxic treatment, cardiovascular health declines are inevitable, and until treatment is stopped, the body does not have the ability to favorably adapt to exercise; as such, the role of exercise at this stage of prevention may simply be to maintain or prevent substantial treatment-induced detrimental changes.
Table 2.
Potential Therapeutic Effects of Exercise Within Each Level of Prevention
| Vo2peak | Cardiac Function | Cardiac Injury Biomarkers | |
|---|---|---|---|
| Primary prevention | Possible prevention of substantial treatment-induced declines if exercise volume is sufficient | Possible prevention of substantial treatment-induced detrimental changes in LVEF, GLS, cardiac output, and stroke volume | Possible prevention of substantial treatment-induced detrimental changes |
| Secondary prevention | Improvement, but how much improvement may be influenced by cancer diagnosis and time out from treatment | Possible improvement of some measures of cardiac function (eg, resting heart rate) | Unknown |
| Tertiary prevention | Possible improvement | Unknown | Unknown |
GLS = global longitudinal strain; LVEF = left ventricular ejection fraction; Vo2peak = maximal oxygen consumption.
Exercise as a secondary prevention strategy
Approximately 6 studies have been conducted in the secondary prevention setting among patients with cancer with evidence of cardiac dysfunction or reduced cardiorespiratory fitness in absence of symptoms or clinically overt CVD.62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78
Cardiorespiratory fitness
Targeting survivors of childhood cancer, Bourdon et al79 conducted a meta-analysis to assess the impact of aerobic exercise on cardiorespiratory fitness and reported a significant improvement in Vo2peak of 1.4 mL/kg/min compared with nonexercising control subjects. Interestingly, this analysis suggested that exercise may be less effective on cardiorespiratory fitness for survivors of childhood cancer compared with survivors with adult-onset cancer, with meta-analyses by Scott et al46 and Beaudry et al80 reporting 2.8 and 3.1 mL/kg/min increases in Vo2peak in favor of exercise. However, these analyses in adult-onset cancers included mixed intervention modalities and are not directly comparable with the meta-analysis of Bourdon et al,79 who examined only aerobic exercise. The reasons for the divergent findings between survivors of childhood and adult cancers are not known but could be due to differences in age, exercise volume, exercise adherence, or the disrupted normal physiological growth and development that result from the cardiotoxic therapies received during childhood, causing direct damage to the heart, lungs, and surrounding vessels and leading to oxidative stress and exercise intolerance.81 Nonetheless, not all the studies included in these meta-analyses were off treatment (ie, not secondary prevention interventions), with subanalyses indicating a greater exercise effect post-treatment82 or no effect.79,80
Most studies presented in Supplemental Table 1 showed improvements in Vo2peak compared with baseline or nonexercising control subjects.22,72,74,75,78, However, it is of note that only 2 of these studies were RCTs75,78; as such, caution is warranted in the interpretation of the effect of exercise on Vo2peak. Both Adams et al75 and Smith et al74 reported clinically significant improvements in Vo2peak (ie, >3.5 mL/kg/min or ∼10%), which represents the reversal of a decade’s worth of cardiorespiratory aging within 12 weeks of HIIT or combined aerobic and resistance training among patients with testicular cancer and survivors of childhood cancer, respectively. Although other studies did not reveal improvements in Vo2peak with exercise,76,77 Grote et al83 completed further analysis indicating that time away from treatment may be a critical factor for adaptations to exercise, with increases in Vo2peak more likely in those ≥5 years out from treatment compared with <5 years.
Cardiac function
Despite their being a measure used within standard of care, few studies have assessed echocardiographic parameters in exercise secondary prevention studies,72, 73, 74,78, and only one of these studies was an RCT.78 Following the cessation of cardiotoxic cancer treatment, therapy-induced declines in cardiac function do not appear to improve with usual care,61 so intervention in the post-treatment phase is critical. Exercise did not improve LVEF and/or GLS in survivors of childhood acute lymphoblastic leukemia72,73, or breast cancer and leiomyosarcoma,78 while Smith et al74 reported an improvement in LVEF following the exercise intervention in a case series of 5 patients. Furthermore, 5 studies72, 73, 74, 75, 76 evaluated systolic blood pressure, but no study revealed evidence of improvement following exercise.72, 73, 74, 75, 76 Additionally, there were mixed effects for the impact of exercise on changes in resting heart rate, with 2 studies showing significant reductions in resting heart rate following exercise75,77 and 1 study showing no change.76
Biomarkers of cardiac injury
Biomarkers of cardiac injury such as troponin and N-terminal pro–brain natriuretic peptide have not been readily examined in exercise interventions conducted post-treatment. Kerrigan et al78 reported no change in high-sensitivity troponin after a 10-week aerobic-only exercise intervention among patients with breast cancer and leiomyosarcoma with subclinical cardiotoxicities. However, they did report changes in cardiometabolic biomarkers, with improvements in high sensitivity C-reactive protein and low-density lipoproteins in favor of the exercise group.
Summary
The presented studies using exercise as secondary prevention produced mixed results across common cardiovascular health outcomes, including Vo2peak, cardiac function, and biomarkers of cardiac injury (Supplemental Table 1, Table 2). In contrast to the impact of exercise as a primary prevention strategy, whereby outcomes, at best, are likely maintained, exercise as a secondary prevention strategy may induce improvements in Vo2peak, but the exercise stimulus required to improve cardiac function and cardiac injury biomarkers is currently under-researched. Nonetheless, adaptations to exercise may be dependent on the time of cancer diagnosis (eg, child or adult onset, as well as time since treatment).68,77,79
Exercise as a tertiary prevention strategy
To date, only 2 trials have assessed exercise as a tertiary prevention strategy of cancer treatment–induced cardiotoxicities. Bonsignore et al84 completed a retrospective analysis comparing patients with breast cancer with noncancer control subjects with coronary artery disease who both completed an exercise cardiac rehabilitation program, and Tsai et al85 primarily assessed the feasibility of exercise among cancer survivors. Both studies reported exercise either in the cardiac rehabilitation setting or home- or clinic-based settings to be feasible and effective as a result of attendance or no adverse events and exercise having a significant effect on Vo2peak,84,85 although no changes in LVEF were observed.85 This improvement in Vo2peak is in line with the results of a meta-analysis examining the effect of cardiac rehabilitation for cancer survivors, which reported a 2.6 mL/kg/min increase in favor of exercise, but not all included studies targeted patients with established cardiotoxicities.8 Nonetheless, although Tsai et al85 and Bonsignore et al84 suggested that exercise is safe and effective for cardiorespiratory fitness improvement in patients with cancer with treatment-induced heart failure, this is contrary to another study by Jones et al,86 who reported the incidence of cardiovascular mortality or hospitalization to be higher among those completing an aerobic exercise intervention. Therefore, caution is warranted when using exercise in this vulnerable cohort.
Summary
Although evidence for exercise in the tertiary level of prevention is limited, there has been promising work from in vitro studies and preclinical models suggesting that exercise may have cardioprotective effects after treatment-induced cardiotoxicity.87,88 The clinical evidence among patients with cancer, however, is limited, with much of the current understanding of the effect of exercise on established cardiotoxicities drawn from cardiology patients without cancer. Future research should continue to expand on this research gap, targeting patients with cancer and survivors with existing cardiovascular conditions.
Research gaps
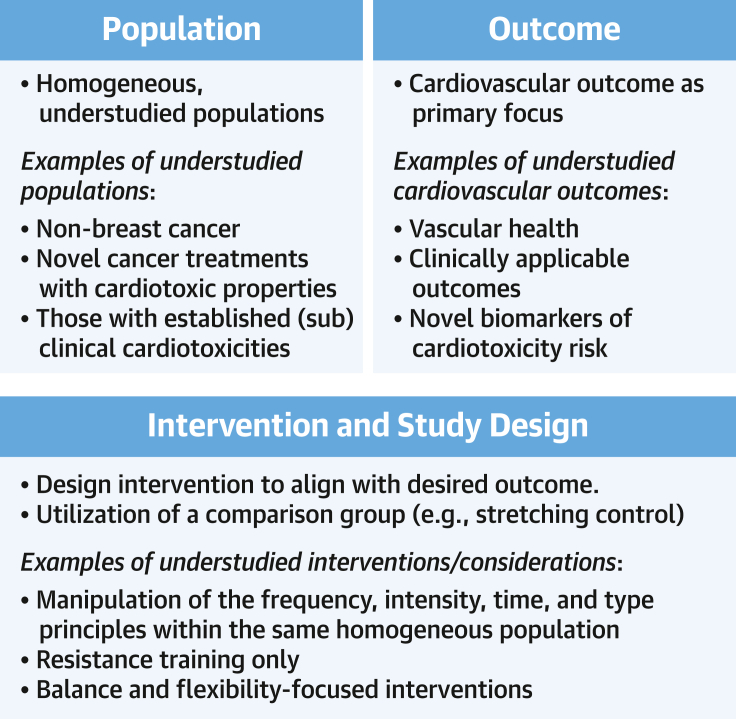
A joint statement was recently published by the American College of Cardiology and the American Heart Association, emphasizing the need for exercise intervention investigations targeting heart failure, which includes cancer survivors who develop cardiotoxicity.89 Given that the exercise cardio-oncology paradigm is still developing, for this field to reach the next level of evidence, researchers should consider the quality of study design as their top priority, with particular focus on the use of the population, intervention, comparator, and outcome criteria90 (Figure 1).
Figure 1.
Knowledge Gaps and High Priorities for Future Research Directions
Exercise cardio-oncology is a developing paradigm. Future research should focus on producing high-quality research on the basis of the population, intervention, comparison, and outcome criteria to improve the future evidence base.
Population and outcome
Future research should target homogeneous populations, with suggested areas of focus that have very few, if any, high-quality studies including patients with cancer with established subclinical or clinically diagnosed cardiotoxicities, cancer types beyond breast cancer, and novel cancer treatments with cardiotoxic properties (eg, immune checkpoint inhibitors, mitogen-activated protein kinase inhibitors) (Figure 1).1 Furthermore, the classification of cardiotoxicities is complex, and new treatments create a clear need for continued revision and definition of clinically relevant cardiotoxicity outcomes, as well as the identification of novel markers of risk and their incorporation in prognostic models. As such, future exercise research should primarily target outcomes of clinical applicability (Figure 1).
Intervention and comparator
There is sufficient evidence to suggest that the general cancer exercise guidelines are beneficial for patients and survivors.9 However, evidence for the individualization of exercise (ie, precision exercise medicine: effectively tailoring exercise to specific patients and their cancer types, treatments, etc) is limited, particularly within exercise cardio-oncology. Therefore, future research should look to compare various manipulations of the frequency, intensity, time, and type principles across different homogeneous populations to best understand what intervention design is ideally prescribed for which population and outcome. This in turn will add to the growing literature of key considerations for exercise prescription on the basis of a patient’s unique profile, resulting in safe, evidence-based, prescription of individualized exercise (Figure 1). Furthermore, long-term follow-up can provide valuable insight into the incidence of cardiotoxicities on the basis of interventions implemented in the primary and secondary levels of prevention.
Highlights
-
•
Exercise as a primary prevention strategy may mitigate significant declines in cardiorespiratory fitness, cardiac function, and biomarkers of cardiac injury.
-
•
Exercise as a secondary prevention strategy may induce improvements in Vo2peak.
-
•
The clinical evidence for the benefits of exercise as a tertiary prevention strategy among patients with cancer is limited, with much of the current understanding drawn from cardiology patients without cancer.
Considerations and Opportunities for Implementing Exercise in Cardio-Oncology Practice
Compared with traditional cardiac rehabilitation, exercise cardio-oncology is significantly more complex and nuanced because of multiple extracardiac factors, including but not exclusive to: 1) the patient’s baseline cardiovascular risk factor profile prior to cancer treatment; 2) the stage and type of malignancy and prognosis; 3) pre-existing comorbidities and factors related to frailty before, during, and after treatment; and 4) anticipated benefits on the basis of limited current evidence unique to each cancer group. Here we discuss considerations and opportunities to implement a cardio-oncology program (Figure 2).
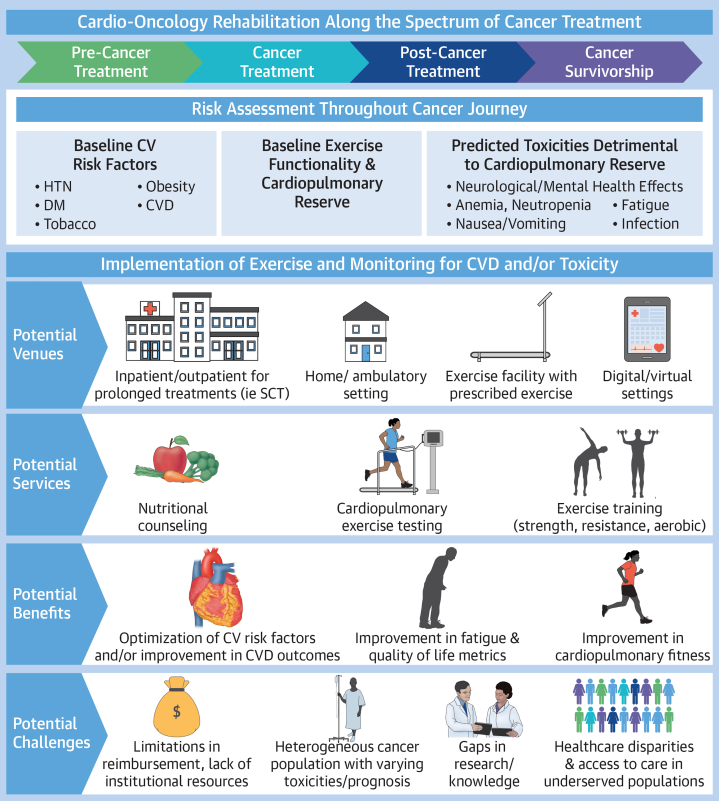
Figure 2.
Implementation of Exercise Within a Cardio-Oncology Program
Along the cancer care continuum, regular assessment of cardiotoxicity risk should be completed. Upon identifying patients at risk, it is recommended to refer them to cardio-oncology exercise programs, which may be offered through a number of different venues with various services depending on patient needs; however, this process may be challenging given current underrepresentation of the needs of such services within global standard of care. CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; HTN = hypertension; SCT = stem cell transplantation.
Elements of an exercise cardio-oncology practice
A successful CORE program stems from a cardio-oncology program that is structured to accommodate timely cardiac testing and exercise needs for a heterogeneous spectrum of malignancies and cardiotoxicities at various stages of cancer diagnosis and treatment. This ability to meet the needs of a heterogeneous population can add to the multidisciplinary strength of being able to identify all patients that can benefit from a CORE program. Therefore, cardio-oncology programs should have the necessary resources to risk-stratify all patients so that they may be appropriately triaged and allocated to the applicable CORE-related resources afforded at each institution or referred elsewhere as required. In addition, as many cancer treatments are time sensitive, CORE initiation and referrals may need to originate from oncology for patients thought to benefit from exercise rehabilitation before (ie, “prehabilitation”), during, or after treatments depending on pre-existing CVD risk factors, baseline cardiopulmonary functional capacity, and the potential for toxicities that may cause significant decline in short- and long-term cardiovascular health. Furthermore, the foundations of an effective exercise rehabilitation program require the multidisciplinary expertise of clinicians, including physicians, nurses, advanced practice providers, exercise physiologists, nutritionists, and psychologists with cardiovascular and oncologic specialty backgrounds.
Variability in patient health profiles
As cancer therapies range in duration and intensity with varying toxicity profiles that occur either acutely or in the long term, treatments may cause myriad effects leading to increased fatigue, decreased exercise tolerance, and poor adherence to ideal diet and evidence-based therapies in cardiovascular primary, secondary, and tertiary prevention. This may include various effects such as anemia, neutropenia, thrombocytopenia, infections, gastrointestinal effects leading to weight loss, decreased appetite, and increased frailty. Psychosocial distress is also a major contributor; available institutional resources should proactively help identify and provide a supportive atmosphere and refer as appropriate to social work and mental health professionals for treatment. Such factors may also deter patients from participating in exercise programs. For instance, patients with breast cancer who declined participation in an exercise-based trial cited time of recruitment, information overload, symptoms and side effects, and the hospital setting as major reasons for not wanting to participate.91 Thus, access to support systems (ie, mental health) are needed, in addition to providing convenient times and venues, if possible, to minimize these barriers during patients’ cancer journeys to optimize exercise therapy both during treatment and into survivorship.
Access to exercise cardio-oncology programs
Home- and ambulatory-based rehabilitation strategies have been historically studied for cardiovascular patients in various health care systems.92,93 With the advent of technologies providing virtual telemedicine visits during the COVID-19 pandemic, along with a rise in smartphone applications focused on fitness, the opportunities to provide CORE beyond the inpatient and outpatient settings has increased. However, strategies for how to deliver effective care in this technology-based environment require further study. Studies looking at implementing remote or unsupervised exercise programs in a variety of cancer populations have been proposed and/or are ongoing.85,94, 95, 96, 97
In addition, efforts are needed to provide equitable CORE care to patients of all socioeconomic backgrounds, as patients who come from under-represented and under-resourced groups may lack access, which may detrimentally affect their cardiovascular and/or oncologic prognoses.98 For instance, in the adolescent and young adult cancer survivor population, lower amounts of moderate to vigorous physical activity were associated with Black race, lower household income, education less than high school level, and CVD risk factors.99 As links of pre-existing CVD and risk factors have been established to higher risks for cardiotoxicity during cancer treatment, certain groups that may have higher CVD disease burden may subsequently fare worse, with higher cardiovascular event rates during or after cancer treatments, and may potentially benefit from CORE care.
Institutional and financial support for exercise cardio-oncology programs
To effectively build a CORE wing of a cardio-oncology program, approaching institutional leadership to identify resources is needed to supply a wide range of health care professionals involved in CORE care in addition to the physical facilities needed for these exercise interventions. Hiring and recruiting CORE staff members and establishing the physical facilities needed to provide these services are additional challenges. However, innovative strategies can potentially be deployed in settings in which prolonged inpatient stays are warranted (ie, stem cell transplantation, chimeric antigen T-cell therapy) and where physical activity can be implemented as tolerated during various stages of conditioning, treatment, and post-treatment, particularly as such populations may be at higher risk for short- and long-term CVD complications.100 Algorithms for referral for CORE services should be designed for each section of oncology on the basis of institutional needs and particular strengths and higher volumes of certain cancer populations; this can help prioritize which patients require CORE services and ensure steady referrals and volume. Ongoing quality initiative projects should identify areas for improvement in the referral and CORE service process, with ongoing tracking of cardiovascular and oncologic outcomes.
Although there are ongoing research efforts in evaluating CORE-based exercise interventions in a variety of cancer states, settings, and strategies, a major obstacle remains a lack of reimbursement. Similar challenges were also recently noted in a 2023 American College of Cardiology/American Heart Association scientific statement on supervised exercise testing in heart failure with preserved ejection fraction.89 Traditional cardiac rehabilitation is covered by Medicare and other major insurance plans in patients: 1) with documented diagnoses of acute myocardial infarction within the preceding 12 months; 2) who underwent coronary bypass surgery; 3) with stable angina pectoris; 4) who underwent heart valve repair or replacement; 5) who underwent percutaneous transluminal coronary angioplasty or coronary stenting; 6) who underwent heart or heart and lung transplantation; or 7) with stable, chronic heart failure, defined as patients with LVEFs of 35% or less and NYHA functional class II to IV symptoms despite being on optimal heart failure therapy for at least 6 weeks.101,102 There is no direct exercise rehabilitation program similar to cardiac rehabilitation available to the cancer population unless a patient develops any of the aforementioned cardiac conditions or undergoes any of the procedures or operations. Thus, institutional support, or other forms of support (ie, private or public grants to support research) to provide such exercise interventions is paramount in providing the foundations for an effective CORE program.
On a national scale, advocacy is needed to provide support, guidelines, and reimbursement for cancer centers to provide CORE services. However, as alluded to earlier in this review, exercise interventions reported in the literature have varying strengths of quality supporting their efficacy for preventing and managing cancer treatment–induced cardiotoxicities. In addition, a successful program requires staffing by a diverse group of health care professionals with experience in cardiovascular and cancer care. As many of these individuals may already function within other rehabilitation and cancer support–based programs, their roles may be “hybridized” to include CORE services, but institutional support and buy-in are necessary for this to occur to provide appropriate compensation and protected time to provide these services.
Highlights
-
•
A successful CORE program accommodates heterogeneous malignancies, treatments, and cardiotoxicities and has resources to risk-stratify patients, a strong referral process, and a multidisciplinary team.
-
•
Program accessibility and financial support are critical elements to consider for the potential outreach of a cardio-oncology program.
Conclusions
The personal and societal burdens of cancer treatment–induced cardiovascular toxicities are becoming increasingly well recognized and are likely to increase with the regular emergence of new therapies that adversely affect the cardiovascular system. Aerobic and resistance exercise training is a potent strategy for preventing and managing cancer-related cardiovascular sequelae; however, the current cardio-oncology care model lacks the referral, testing, and intervention resources and infrastructure needed to widely implement them. A coordinated effort from medical, patient advocacy, research, and clinical care groups is urgently needed to raise awareness of these issues and generate the evidence required to demonstrate the safety, tolerability, and efficacy of exercise-based therapies across prevention settings and facilitate higher level, health care economics–related decision making to support the widespread implementation of CORE.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Patients exposed to cardiotoxic cancer treatments are at increased risk of developing cardiotoxicities throughout treatment and survivorship. Exercise is a known strategy to improve cardiovascular disease risk, however, only cancer survivors with confirmed diagnoses of cardiovascular diseases are considered eligible for referral to exercise-based cardiology rehabilitation within the current cardio-oncology care model.
TRANSLATIONAL SKILLS: Future research should continue to explore cardiology and general exercise oncology paradigms to inform research and practice of implementing exercise within cardio-oncology clinical care.
Funding Support and Author Disclosures
This work was supported by a Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748) to Dr Scott. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank Kathy Siestema, MD, for her assistance and interpretation of the clinical cases in this review.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figure, tables, and references as well as the literature search strategy, please see the online version of this paper.
Appendix
References
- 1.Herrmann J., Lenihan D., Armenian S., et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottinor W., Chow E.J. Mitigating, monitoring, and managing long-term chemotherapy- and radiation-induced cardiac toxicity. Hematology Am Soc Hematol Educ Program. 2022;2022:251–258. doi: 10.1182/hematology.2022000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kisling L.A., M Das J. StatPearls. StatPearls Publishing; Treasure Island, FL: 2023. Prevention strategies. [Google Scholar]
- 4.Alves A.J., Viana J.L., Cavalcante S.L., et al. Physical activity in primary and secondary prevention of cardiovascular disease: overview updated. World J Cardiol. 2016;8:575–583. doi: 10.4330/wjc.v8.i10.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad K. Current status of primary, secondary, and tertiary prevention of coronary artery disease. Int J Angiol. 2021;30:177–186. doi: 10.1055/s-0041-1731273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Heart Association . American Heart Association; Chicago: 1975. Exercise Testing and Training of Individuals With Heart Disease or a High Risk Rate for Its Development: A Handbook for Physicians. [Google Scholar]
- 7.Gilchrist S.C., Barac A., Ades P.A., et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997–e1012. doi: 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakhraei R., Peck B.S.S., Abdel-Qadir H., et al. Research quality and impact of cardiac rehabilitation in cancer survivors: a systematic review and meta-analysis. J Am Coll Cardiol CardioOnc. 2022;4:195–206. doi: 10.1016/j.jaccao.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell K.L., Winters-Stone K.M., Wiskemann J., et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shelburne N., Adhikari B., Brell J., et al. Cancer treatment-related cardiotoxicity: current state of knowledge and future research priorities. J Natl Cancer Inst. 2014;106:dju232. doi: 10.1093/jnci/dju232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Sendón J., Álvarez-Ortega C., Zamora Auñon P., et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41:1720–1729. doi: 10.1093/eurheartj/ehaa006. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong G.T., Oeffinger K.C., Chen Y., et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barac A., Isaacs C., N M.S., et al. Trends in the use of cardiac imaging for women with newly diagnosed breast cancer. J Cardiovasc Transl Res. 2020;13:478–489. doi: 10.1007/s12265-020-10023-6. [DOI] [PubMed] [Google Scholar]
- 14.Florido R., Daya N.R., Ndumele C.E., et al. Cardiovascular disease risk among cancer survivors: the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2022;80:22–32. doi: 10.1016/j.jacc.2022.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott J.M., Stene G., Edvardsen E., Jones L.W. Performance status in cancer: not broken, but time for an upgrade? J Clin Oncol. 2020;38:2824–2829. doi: 10.1200/JCO.20.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross R., Blair S.N., Arena R., et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 17.Groarke J.D., Payne D.L., Claggett B., et al. Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes. 2020;6:315–322. doi: 10.1093/ehjqcco/qcaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones L.W., Haykowsky M., Pituskin E.N., et al. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor--positive operable breast cancer. Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 19.West M.A., Parry M.G., Lythgoe D., et al. Cardiopulmonary exercise testing for the prediction of morbidity risk after rectal cancer surgery. Br J Surg. 2014;101:1166–1172. doi: 10.1002/bjs.9551. [DOI] [PubMed] [Google Scholar]
- 20.West M.A., Lythgoe D., Barben C.P., et al. Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: a prospective blinded observational study. Br J Anaesth. 2014;112:665–671. doi: 10.1093/bja/aet408. [DOI] [PubMed] [Google Scholar]
- 21.West M.A., Asher R., Browning M., et al. Validation of preoperative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery. Br J Surg. 2016;103:744–752. doi: 10.1002/bjs.10112. [DOI] [PubMed] [Google Scholar]
- 22.Adams M.J., Lipsitz S.R., Colan S.D., et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 23.Marshall C.H., Al-Mallah M.H., Dardari Z., et al. Cardiorespiratory fitness and incident lung and colorectal cancer in men and women: results from the Henry Ford Exercise Testing (FIT) cohort. Cancer. 2019;125:2594–2601. doi: 10.1002/cncr.32085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasso J.P., Eves N.D., Christensen J.F., Koelwyn G.J., Scott J., Jones L.W. A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle. 2015;6:115–124. doi: 10.1002/jcsm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers J., Forman D.E., Balady G.J., et al. Supervision of exercise testing by nonphysicians: a scientific statement from the American Heart Association. Circulation. 2014;130:1014–1027. doi: 10.1161/CIR.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalski M., Rowed K., Lavery J.A., et al. Validity of estimated cardiorespiratory fitness in patients with primary breast cancer. J Am Coll Cardiol CardioOnc. 2022;4:210–219. doi: 10.1016/j.jaccao.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kee Shackelford D.Y., Brown J.M., Peterson B.M., Schaffer J., Hayward R. The University of Northern Colorado Cancer Rehabilitation Institute treadmill protocol accurately measures Vo2peak in cancer survivors. Int J Phys Med Rehabil. 2017;5:2. [Google Scholar]
- 28.Schmidt K., Vogt L., Thiel C., Jager E., Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34:631–636. doi: 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 29.Wonders K.Y., Gnau K., Schmitz K.H. Measuring the feasibility and effectiveness of an individualized exercise program delivered virtually to cancer survivors. Curr Sports Med Rep. 2021;20:271–276. doi: 10.1249/JSR.0000000000000846. [DOI] [PubMed] [Google Scholar]
- 30.Kasymjanova G., Correa J.A., Kreisman H., et al. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:602–607. doi: 10.1097/JTO.0b013e31819e77e8. [DOI] [PubMed] [Google Scholar]
- 31.Jones L.W., Hornsby W.E., Goetzinger A., et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76:248–252. doi: 10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland A.E., Spruit M.A., Troosters T., et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 33.Jones L.W., Eves N.D., Haykowsky M., Joy A.A., Douglas P.S. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 34.Brooks D., Solway S., Gibbons W.J. ATS statement on six-minute walk test. Am J Respir Crit Care Med. 2003;167:1287. doi: 10.1164/ajrccm.167.9.950. [DOI] [PubMed] [Google Scholar]
- 35.American College of Sports Medicine . Lippincott Williams & Wilkins; Philadelphia: 2013. ACSM’s Guidelines for Exercise Testing and Prescription. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz J.R., Sui X., Lobelo F., et al. Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer Epidemiol Biomarkers Prev. 2009;18:1468–1476. doi: 10.1158/1055-9965.EPI-08-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parrino R.L., Strand K.L., Hockman A.C., Signorile J.F. Leg press and chest press strength normative values by half-decades in older persons. Exp Gerontol. 2021;150 doi: 10.1016/j.exger.2021.111401. [DOI] [PubMed] [Google Scholar]
- 38.Dos Santos W.D.N., Siqueira G.D.J., Martins W.R., et al. Reliability and agreement of the 10-repetition maximum test in breast cancer survivors. Front Oncol. 2019;9:918. doi: 10.3389/fonc.2019.00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiegert E.V.M., da Silva N.F., de Oliveira L.C., Calixto-Lima L. Reference values for handgrip strength and their association with survival in patients with incurable cancer. Eur J Clin Nutr. 2022;76:93–102. doi: 10.1038/s41430-021-00921-6. [DOI] [PubMed] [Google Scholar]
- 40.Lee J. Associations between handgrip strength and disease-specific mortality including cancer, cardiovascular, and respiratory diseases in older adults: a meta-analysis. J Aging Phys Act. 2019;28:320–331. doi: 10.1123/japa.2018-0348. [DOI] [PubMed] [Google Scholar]
- 41.Yin L., Zhang L., Li N., et al. Comparison of the AWGS and optimal stratification-defined handgrip strength thresholds for predicting survival in patients with lung cancer. Nutrition. 2021;90 doi: 10.1016/j.nut.2021.111258. [DOI] [PubMed] [Google Scholar]
- 42.Burtin C., Bezuidenhout J., Sanders K.J.C., et al. Handgrip weakness, low fat-free mass, and overall survival in non-small cell lung cancer treated with curative-intent radiotherapy. J Cachexia Sarcopenia Muscle. 2020;11:424–431. doi: 10.1002/jcsm.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong Q.-T., Cai H.-Y., Zhang Z., et al. Influence of body composition, muscle strength, and physical performance on the postoperative complications and survival after radical gastrectomy for gastric cancer: a comprehensive analysis from a large-scale prospective study. Clin Nutr. 2021;40:3360–3369. doi: 10.1016/j.clnu.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Versteeg K.S., Blauwhoff-Buskermolen S., Buffart L.M., et al. Higher muscle strength is associated with prolonged survival in older patients with advanced cancer. Oncologist. 2018;23:580–585. doi: 10.1634/theoncologist.2017-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGovern A., Mahony N., Mockler D., Fleming N. Efficacy of resistance training during adjuvant chemotherapy and radiation therapy in cancer care: a systematic review and meta-analysis. Support Care Cancer. 2022;30:3701–3719. doi: 10.1007/s00520-021-06708-6. [DOI] [PubMed] [Google Scholar]
- 46.Scott J.M., Zabor E.C., Schwitzer E., et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36:2297–2305. doi: 10.1200/JCO.2017.77.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haykowsky M.J., Liang Y., Pechter D., Jones L.W., McAlister F.A., Clark A.M. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49:2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 48.Tsuji K., Matsuoka Y.J., Ochi E. High-intensity interval training in breast cancer survivors: a systematic review. BMC Cancer. 2021;21:1–11. doi: 10.1186/s12885-021-07804-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heredia-Ciuró A., Fernández-Sánchez M., Martín-Núñez J., et al. High-intensity interval training effects in cardiorespiratory fitness of lung cancer survivors: a systematic review and meta-analysis. Support Care Cancer. 2022;30:3017–3027. doi: 10.1007/s00520-021-06647-2. [DOI] [PubMed] [Google Scholar]
- 50.Lee J. The effects of resistance training on muscular strength and hypertrophy in elderly cancer patients: a systematic review and meta-analysis. J Sport Health Sci. 2022;11:194–201. doi: 10.1016/j.jshs.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gebruers N., Camberlin M., Theunissen F., et al. The effect of training interventions on physical performance, quality of life, and fatigue in patients receiving breast cancer treatment: a systematic review. Support Care Cancer. 2019;27:109–122. doi: 10.1007/s00520-018-4490-9. [DOI] [PubMed] [Google Scholar]
- 52.Beckers P.J., Denollet J., Possemiers N.M., Wuyts F.L., Vrints C.J., Conraads V.M. Combined endurance-resistance training vs. endurance training in patients with chronic heart failure: a prospective randomized study. Eur Heart J. 2008;29:1858–1866. doi: 10.1093/eurheartj/ehn222. [DOI] [PubMed] [Google Scholar]
- 53.Mandic S., Tymchak W., Kim D., et al. Effects of aerobic or aerobic and resistance training on cardiorespiratory and skeletal muscle function in heart failure: a randomized controlled pilot trial. Clin Rehabil. 2009;23:207–216. doi: 10.1177/0269215508095362. [DOI] [PubMed] [Google Scholar]
- 54.Scott J.M., Thomas S.M., Peppercorn J.M., et al. Effects of exercise therapy dosing schedule on impaired cardiorespiratory fitness in patients with primary breast cancer: a randomized controlled trial. Circulation. 2020;141:560–570. doi: 10.1161/CIRCULATIONAHA.119.043483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmerman A., Planek M.I.C., Chu C., et al. Exercise, cancer and cardiovascular disease: what should clinicians advise? Cardiovasc Endocrinol Metab. 2021;10:62–71. doi: 10.1097/XCE.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haykowsky M.J., Mackey J.R., Thompson R.B., Jones L.W., Paterson D.I. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. 2009;15:4963–4967. doi: 10.1158/1078-0432.CCR-09-0628. [DOI] [PubMed] [Google Scholar]
- 57.Jones L.W., Fels D.R., West M., et al. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res (Phila) 2013;6:925–937. doi: 10.1158/1940-6207.CAPR-12-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hornsby W.E., Douglas P.S., West M.J., et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53:65–74. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- 59.Kirkham A.A., Eves N.D., Shave R.E., et al. The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: a RCT. Breast Cancer Res Treat. 2018;167:719–729. doi: 10.1007/s10549-017-4554-4. [DOI] [PubMed] [Google Scholar]
- 60.Howden E.J., Bigaran A., Beaudry R., et al. Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol. 2019;26:305–315. doi: 10.1177/2047487318811181. [DOI] [PubMed] [Google Scholar]
- 61.Foulkes S.J., Howden E.J., Bigaran A., et al. Persistent impairment in cardiopulmonary fitness after breast cancer chemotherapy. Med Sci Sports Exerc. 2019;51:1573–1581. doi: 10.1249/MSS.0000000000001970. [DOI] [PubMed] [Google Scholar]
- 62.Kirkham A.A., Virani S.A., Bland K.A., et al. Exercise training affects hemodynamics not cardiac function during anthracycline-based chemotherapy. Breast Cancer Res Treat. 2020;184:75–85. doi: 10.1007/s10549-020-05824-x. [DOI] [PubMed] [Google Scholar]
- 63.Hojan K., Procyk D., Horyńska-Kęstowicz D., Leporowska E., Litwiniuk M. The Preventive role of regular physical training in ventricular remodeling, serum cardiac markers, and exercise performance changes in breast cancer in women undergoing trastuzumab therapy—an REH-HER study. J Clin Med. 2020;9:1379. doi: 10.3390/jcm9051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung W.P., Yang H.L., Hsu Y.T., et al. Real-time exercise reduces impaired cardiac function in breast cancer patients undergoing chemotherapy: a randomized controlled trial. Ann Phys Rehabil Med. 2022;65 doi: 10.1016/j.rehab.2021.101485. [DOI] [PubMed] [Google Scholar]
- 65.Foulkes S.J., Howden E.J., Haykowsky M.J., et al. Exercise for the prevention of anthracycline-induced functional disability and cardiac dysfunction: the BREXIT study. Circulation. 2023;147:532–545. doi: 10.1161/CIRCULATIONAHA.122.062814. [DOI] [PubMed] [Google Scholar]
- 66.Ma Z.Y., Yao S.S., Shi Y.Y., Lu N.N., Cheng F. Effect of aerobic exercise on cardiotoxic outcomes in women with breast cancer undergoing anthracycline or trastuzumab treatment: a systematic review and meta-analysis. Support Care Cancer. 2022;30:10323–10334. doi: 10.1007/s00520-022-07368-w. [DOI] [PubMed] [Google Scholar]
- 67.Scott JM, Lee J, Herndon JE, et al. Timing of exercise therapy when initiating adjuvant chemotherapy for breast cancer: a randomized trial. Eur Heart J. Published online February 23, 2023. https://doi.org/10.1093/eurheartj/ehad085. [DOI] [PMC free article] [PubMed]
- 68.van der Schoot G.G.F., Ormel H.L., Westerink N.L., et al. Optimal timing of a physical exercise intervention to improve cardiorespiratory fitness: during or after chemotherapy. J Am Coll Cardiol CardioOnc. 2022;4:491–503. doi: 10.1016/j.jaccao.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Psotka M.A., Abraham W.T., Fiuzat M., et al. Functional and symptomatic clinical trial endpoints: the HFC-ARC scientific expert panel. J Am Coll Cardiol HF. 2022;10:889–901. doi: 10.1016/j.jchf.2022.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Foulkes S., Costello B.T., Howden E.J., et al. Exercise cardiovascular magnetic resonance reveals reduced cardiac reserve in pediatric cancer survivors with impaired cardiopulmonary fitness. J Cardiovasc Magn Reson. 2020;22:64. doi: 10.1186/s12968-020-00658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ansund J., Mijwel S., Bolam K.A., et al. High intensity exercise during breast cancer chemotherapy—effects on long-term myocardial damage and physical capacity—data from the OptiTrain RCT. Cardiooncology. 2021;7:7. doi: 10.1186/s40959-021-00091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Järvelä L.S., Kemppainen J., Niinikoski H., et al. Effects of a home-based exercise program on metabolic risk factors and fitness in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59:155–160. doi: 10.1002/pbc.24049. [DOI] [PubMed] [Google Scholar]
- 73.Järvelä L.S., Saraste M., Niinikoski H., et al. Home-based exercise training improves left ventricle diastolic function in survivors of childhood ALL: a tissue Doppler and velocity vector imaging study. Pediatr Blood Cancer. 2016;63:1629–1635. doi: 10.1002/pbc.26051. [DOI] [PubMed] [Google Scholar]
- 74.Smith W.A., Ness K.K., Joshi V., Hudson M.M., Robison L.L., Green D.M. Exercise training in childhood cancer survivors with subclinical cardiomyopathy who were treated with anthracyclines. Pediatr Blood Cancer. 2014;61:942–945. doi: 10.1002/pbc.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adams S.C., Delorey D.S., Davenport M.H., et al. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: a phase 2 randomized controlled trial. Cancer. 2017;123:4057–4065. doi: 10.1002/cncr.30859. [DOI] [PubMed] [Google Scholar]
- 76.Long T.M., Rath S.R., Wallman K.E., et al. Exercise training improves vascular function and secondary health measures in survivors of pediatric oncology related cerebral insult. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grote S., Ricci J.M., Dehom S., Modeste N., Sealy D.A., Tarleton H.P. Heart rate variability and cardiovascular adaptations among cancer-survivors following a 26-week exercise intervention. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420969816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerrigan D.J., Reddy M., Walker E.M., et al. Cardiac rehabilitation improves fitness in patients with subclinical markers of cardiotoxicity while receiving chemotherapy: a randomized controlled study. J Cardiopulm Rehabil Prev. 2023;43:129–134. doi: 10.1097/HCR.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 79.Bourdon A., Grandy S.A., Keats M.R. Aerobic exercise and cardiopulmonary fitness in childhood cancer survivors treated with a cardiotoxic agent: a meta-analysis. Support Care Cancer. 2018;26:2113–2123. doi: 10.1007/s00520-018-4208-z. [DOI] [PubMed] [Google Scholar]
- 80.Beaudry R., Kruger C., Liang Y., Parliament M., Haykowsky M., McNeely M. Effect of supervised exercise on aerobic capacity in cancer survivors: adherence and workload predict variance in effect. World J Metaanal. 2015;3:43–53. [Google Scholar]
- 81.Gilliam L.A., St Clair D.K. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal. 2011;15:2543–2563. doi: 10.1089/ars.2011.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones L.W., Liang Y., Pituskin E.N., et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grote S., Almstedt H.C., Tarleton H.P. Cardiometabolic health among cancer survivors: a 13-week pilot study of a combined aerobic and resistance training program. Oncol Nurs Forum. 2016;43:306–315. doi: 10.1188/16.ONF.306-315. [DOI] [PubMed] [Google Scholar]
- 84.Bonsignore A., Marzolini S., Oh P. Cardiac rehabilitation for women with breast cancer and treatment-related heart failure compared with coronary artery disease: a retrospective study. J Rehabil Med. 2017;49:277–281. doi: 10.2340/16501977-2203. [DOI] [PubMed] [Google Scholar]
- 85.Tsai E., Mouhayar E., Lenihan D., et al. Feasibility and outcomes of an exercise intervention for chemotherapy-induced heart failure. J Cardiopulm Rehabil Prev. 2019;39:199–203. doi: 10.1097/HCR.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones L.W., Douglas P.S., Khouri M.G., et al. Safety and efficacy of aerobic training in patients with cancer who have heart failure: an analysis of the HF-ACTION randomized trial. J Clin Oncol. 2014;32:2496–2502. doi: 10.1200/JCO.2013.53.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghignatti P.V.D.C., Nogueira L.J., Lehnen A.M., Leguisamo N.M. Cardioprotective effects of exercise training on doxorubicin-induced cardiomyopathy: a systematic review with meta-analysis of preclinical studies. Sci Rep. 2021;11:6330. doi: 10.1038/s41598-021-83877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naaktgeboren W.R., Binyam D., Stuiver M.M., et al. Efficacy of physical exercise to offset anthracycline-induced cardiotoxicity: a systematic review and meta-analysis of clinical and preclinical studies. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.021580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sachdev V., Sharma K., Keteyian S.J., et al. Supervised exercise training for chronic heart failure with preserved ejection fraction: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2023;147:e699–e715. doi: 10.1161/CIR.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 90.Richardson W.S., Wilson M.C., Nishikawa J., Hayward R.S. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12–A13. [PubMed] [Google Scholar]
- 91.Andersen HH, Mikkelsen MK, Obarzanek CE, Paludan C, Nielsen D. Reasons for declining participation in an exercise-based trial among older women with breast cancer receiving systemic anti-cancer treatment—a qualitative interview study. Physiother Theory Pract. Published online March 9, 2023. https://doi.org/10.1080/09593985.2023.2187675. [DOI] [PubMed]
- 92.DeBusk R.F., Miller N.H., Superko H.R., et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120:721–729. doi: 10.7326/0003-4819-120-9-199405010-00001. [DOI] [PubMed] [Google Scholar]
- 93.Ma J., Berra K., Haskell W.L., et al. Case management to reduce risk of cardiovascular disease in a county health care system. Arch Intern Med. 2009;169:1988–1995. doi: 10.1001/archinternmed.2009.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pelosi A.C., Rostirola G.C., Pereira J.S., et al. Remote and unsupervised exercise strategies for improving the physical activity of colorectal cancer patients: a meta-analysis. Healthcare (Basel) 2023;11:723. doi: 10.3390/healthcare11050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lavín-Pérez A.M., Collado-Mateo D., Hinojo González C., et al. High-intensity exercise prescription guided by heart rate variability in breast cancer patients: a study protocol for a randomized controlled trial. BMC Sports Sci Med Rehabil. 2023;15:28. doi: 10.1186/s13102-023-00634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ryu J., Lee E.Y., Min J., et al. Effect of a 1-year tailored exercise program according to cancer trajectories in patients with breast cancer: study protocol for a randomized controlled trial. BMC Cancer. 2023;23:200. doi: 10.1186/s12885-023-10664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Campanini I., Ligabue M.B., Bò M.C., Bassi M.C., Lusuardi M., Merlo A. Self-managed physical activity in cancer survivors for the management of cancer-related fatigue: a scoping review. PLoS One. 2022;17 doi: 10.1371/journal.pone.0279375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohman R.E., Yang E.H., Abel M.L. Inequity in cardio-oncology: identifying disparities in cardiotoxicity and links to cardiac and cancer outcomes. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.023852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berkman A.M., Andersen C.R., Tang K., Gilchrist S.C., Roth M.E. Disparities in physical activity in adolescent and young adult cancer survivors. J Cancer Surviv. 2023;17:848–858. doi: 10.1007/s11764-022-01264-2. [DOI] [PubMed] [Google Scholar]
- 100.Tuzovic M., Mead M., Young P.A., Schiller G., Yang E.H. Cardiac complications in the adult bone marrow transplant patient. Curr Oncol Rep. 2019;21:28. doi: 10.1007/s11912-019-0774-6. [DOI] [PubMed] [Google Scholar]
- 101.Centers for Medicare and Medicaid Services Cardiac rehabilitation programs. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?ncdid=36&ncdver=1&doctype=all&year=2023&sortBy=title&bc=14
- 102.Centers for Medicare and Medicaid Services Cardiac rehabilitation (CR) programs—chronic heart failure. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=270
- 103.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 104.Scott J.M., Hornsby W.E., Lane A., Kenjale A.A., Eves N.D., Jones L.W. Reliability of maximal cardiopulmonary exercise testing in men with prostate cancer. Med Sci Sports Exerc. 2015;47:27–32. doi: 10.1249/MSS.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grgic J., Lazinica B., Schoenfeld B.J., Pedisic Z. Test–retest reliability of the one-repetition maximum (1RM) strength assessment: a systematic review. Sports Med Open. 2020;6:31. doi: 10.1186/s40798-020-00260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barbalho M., Gentil P., Raiol R., Del Vecchio F., Ramirez-Campillo R., Coswig V. High 1RM tests reproducibility and validity are not dependent on training experience, muscle group tested or strength level in older women. Sports. 2018;6:171. doi: 10.3390/sports6040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bobos P., Nazari G., Lu Z., MacDermid J.C. Measurement properties of the hand grip strength assessment: a systematic review with meta-analysis. Arch Phys Med Rehabil. 2020;101:553–565. doi: 10.1016/j.apmr.2019.10.183. [DOI] [PubMed] [Google Scholar]
- 108.Rogers B.H., Brown J.C., Gater D.R., Schmitz K.H. Association between maximal bench press strength and isometric handgrip strength among breast cancer survivors. Archives of Arch Phys Med Rehabil. 2017;98:264–269. doi: 10.1016/j.apmr.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmid D., Leitzmann M.F. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol. 2015;26:272–278. doi: 10.1093/annonc/mdu250. [DOI] [PubMed] [Google Scholar]
- 110.Jones L.W., Watson D., Herndon J.E., II, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116:4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anderson L.J., Lee J., Mallen M.C., et al. Evaluation of physical function and its association with body composition, quality of life and biomarkers in cancer cachexia patients. Clin Nutr. 2021;40:978–986. doi: 10.1016/j.clnu.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kilgour R.D., Vigano A., Trutschnigg B., Lucar E., Borod M., Morais J.A. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer. 2013;21:3261–3270. doi: 10.1007/s00520-013-1894-4. [DOI] [PubMed] [Google Scholar]
- 113.Tribolet P., Kaegi-Braun N., Gressies C., et al. Handgrip strength values depend on tumor entity and predict 180-day mortality in malnourished cancer patients. Nutrients. 2022;14:2173. doi: 10.3390/nu14102173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jones L.W., Cohen R.R., Mabe S.K., et al. Assessment of physical functioning in recurrent glioma: preliminary comparison of performance status to functional capacity testing. J Neurooncol. 2009;94:79–85. doi: 10.1007/s11060-009-9803-x. [DOI] [PubMed] [Google Scholar]
- 115.Xie H., Ruan G., Zhang Q., et al. Combination of nutritional risk index and handgrip strength on the survival of patients with cancer cachexia: a multi-center cohort study. J Inflamm Res. 2022;15:1005–1015. doi: 10.2147/JIR.S352250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bland K.A., Neil-Sztramko S.E., Zadravec K., et al. Attention to principles of exercise training: an updated systematic review of randomized controlled trials in cancers other than breast and prostate. BMC Cancer. 2021;21:1179. doi: 10.1186/s12885-021-08701-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jones L.W., Courneya K.S., Mackey J.R., et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ruden E., Reardon D.A., Coan A.D., et al. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011;29:2918–2923. doi: 10.1200/JCO.2011.34.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Celis-Morales C.A., Welsh P., Lyall D.M., et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651. doi: 10.1136/bmj.k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bentsen K.K., Hansen O., Ryg J., Giger A.-K.W., Jeppesen S.S. Combination of the G-8 screening tool and hand-grip strength to predict long-term overall survival in non-small cell lung cancer patients undergoing stereotactic body radiotherapy. Cancers. 2021;13:3363. doi: 10.3390/cancers13133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Postmus P.E., Kerr K.M., Oudkerk M., et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 122.Sebio Garcia R., Yanez Brage M.I., Gimenez Moolhuyzen E., Granger C.L., Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23:486–497. doi: 10.1093/icvts/ivw152. [DOI] [PubMed] [Google Scholar]
- 123.Michael C.M., Lehrer E.J., Schmitz K.H., Zaorsky N.G. Prehabilitation exercise therapy for cancer: a systematic review and meta-analysis. Cancer Med. 2021;10:4195–4205. doi: 10.1002/cam4.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wagner J., Agostoni P., Arena R., et al. The role of gas exchange variables in cardiopulmonary exercise testing for risk stratification and management of heart failure with reduced ejection fraction. Am Heart J. 2018;202:116–126. doi: 10.1016/j.ahj.2018.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.