Abstract
Background
Malignant pleural mesotheliomas (MPMs) are aggressive and often unresectable. In the past, chemotherapy was the standard for palliative treatment. However, immunotherapy with nivolumab+ipilimumab has recently received marketing approval.
Objectives
This study evaluated the cost effectiveness of nivolumab+ipilimumab versus pemetrexed+platinum (with/without bevacizumab) for Swiss patients with unresectable MPM, overall and by histological subtype.
Methods
We developed a three-state Markov cohort model with a cycle length of 1 month, a 30-year time horizon, and a discount rate of 3% per year for costs and benefits. The model included the updated survival and treatment-dependent utility results from the Checkmate-743 and MAPS registration trials. A Swiss statutory health insurance perspective was considered with unit costs for 2022 from publicly available and real-world sources. We assumed a willingness-to-pay (WTP) threshold of CHF100,000/QALY. Model robustness was explored in sensitivity and scenario analyses.
Results
Compared with chemotherapy, nivolumab+ipilimumab incurred additional costs of CHF109,115 and 0.57 additional quality-adjusted life-years (QALYs), yielding an incremental cost-effectiveness ratio (ICER) of CHF192,585/QALY (i.e. USD201,829/QALY) gained. Relative to their 2022 list price, nivolumab+ipilimumab may be cost effective if priced at 48% across all histologies. Assuming cisplatin-based instead of carboplatin-based chemotherapy reduced the ICER to CHF158,911/QALY (i.e. USD166,539/QALY). For the non-epithelioid subtype, nivolumab+ipilimumab was cost effective compared with chemotherapy (ICER of CHF97,894/QALY, i.e. USD102,593/QALY). Chemotherapy+bevacizumab was often a dominated strategy or would require a bevacizumab cost reduction to 28%.
Conclusions
Our model projected nivolumab+ipilimumab to be cost effective for the non-epithelioid subtype but not for all histologies. Substantial discounts for nivolumab+ipilimumab would be necessary to achieve cost effectiveness for all histologies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-023-01305-3.
Key Points
| Nivolumab plus ipilimumab have been approved for the treatment of unresectable malignant pleural mesothelioma (MPM). We assessed whether ipilimumab plus nivolumab is cost effective in comparison to chemotherapy with and without bevacizumab from a Swiss statutory health insurance perspective. |
| Ipilimumab and nivolumab resulted in increased costs of CHF 109,115 (USD 114,353) and 0.57 additional quality-adjusted life years (QALYs) compared with chemotherapy, yielding an incremental cost-effectiveness ratio (ICER) of CHF 192,585 (USD 201,829) per QALY gained. Only for the subgroup of patients with non-epithelioid MPM, we found ipilimumab plus nivolumab to be cost effective in comparison to chemotherapy at current list prices. |
| Substantial rebates (to 48% of their current list prices) would be necessary to make nivolumab plus ipilimumab cost effective for all MPM histologies in Switzerland. A rebate strategy was chosen by regulatory authorities in the UK and Canada. Other strategies consisted in limiting reimbursement to non-epithelioid MPM (German authority), or to non-epithelioid MPM or PD-L1 expression ≥ 1% (Swiss authority). Closer collaboration between decision makers could avoid differing labels and hidden rebates. |
Introduction
Malignant pleural mesothelioma (MPM) is a rare and highly aggressive cancer which affects the mesothelial cells of the pleura. Other types of mesothelioma occurring in the abdomen (peritoneum), heart (pericardium), or testicles (tunica vaginalis) are less common. MPM is often caused by occupational asbestos exposure. Latency is between 20 and 50 years [1]. Therefore, MPM mostly affects elderly, frail, and medically inoperable patients [2].
MPM can be categorized into different histological subtypes: epithelioid (60%) and non-epithelioid (40%), the latter incorporating sarcomatoid/desmoplastic, mixed/biphasic, and the unspecified type [3, 4]. Non-epithelioid MPM is less responsive to chemotherapy than epithelioid MPM [5].
In 2020, 30,870 new cases and 26,278 deaths related to MPM were reported worldwide. The highest incidences were seen in Europe (incidence of 44.2%), Asia (incidence of 31.5%) and Northern America (incidence of 13.3%) [6]. In Switzerland, the peak incidence is projected for 2030, with approximately 170 new cases per year (in 8.7 million inhabitants) [7]. Common symptoms include breathlessness, chest pain, coughing, fatigue, fever, weight loss, and night sweats [1, 3].
The burden of MPM to patients and their families is high [8]. Patients have poor prognosis, with a mortality of 93% at 5 years from initial diagnosis [9]. For patients with unresectable MPM, no curative treatment options exist [3].
Traditional standard treatment for unresectable MPM is palliative chemotherapy with pemetrexed and either cisplatin or carboplatin [10, 11]. Cisplatin was preferred in many clinical trials, but it requires hydration and must therefore be given within an inpatient setting in many centres. Outside of clinical protocols, many oncologists prefer carboplatin, because it can be administered in an outpatient setting, does not require hydration, and is less emetogenic.
Bevacizumab, a monoclonal antibody against the vascular endothelial growth factor, enhances the activity of chemotherapy against some solid tumours, including MPM. In the Mesothelioma Avastin Cisplatin Pemetrexed (MAPS) trial [12], bevacizumab plus chemotherapy (pemetrexed+platin+bevacizumab) showed superior overall survival (OS) and progression-free survival (PFS). It was recommended by the European Society for Medical Oncology (ESMO) for the treatment of MPM [11]. Although bevacizumab is not approved for this indication in Switzerland, it is used off-label in some centres, based on the results of the MAPS trial [12].
Nivolumab and ipilimumab are immune checkpoint inhibitors, targeting programmed death receptor ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4, respectively. Nivolumab plus ipilimumab (nivolumab+ipilimumab) was compared with standard chemotherapy in the phase III randomized controlled CheckMate743 (CM-743) trial [5]. It prolonged OS, at the expense of higher drug costs and more treatment-related serious adverse events (AEs) (15.3% vs 6%) [5]. The most common AEs were diarrhoea and pruritis, while chemotherapy was associated with more nausea, vomiting, stomatitis, alopecia, myelosuppression, and infection [5, 12, 13].
It was observed that PD-L1 expression (PD-L1 ≥1, PD-L1 <1) affects MPM prognosis [1]. In the CM-743 trial, nivolumab+ipilimumab was associated with better OS in patients with tumour expression of PD-L1 ≥1% compared with PD-L1 <1% [2, 13–15]. However, the trial was not powered for this subgroup analysis. PD-L1 remains controversial as a predictive marker [4].
Regardless of histology and PD-L1, nivolumab+ipilimumab received marketing authorization for MPM by the European Medicines Agency (EMA) and the US Food and Drug Administration with positive drug reimbursement recommendations, for example, from the Canadian Drug and Health Technology Agency (CADTH) and the National Institute for Health and Care Excellence (NICE) in the United Kingdom (UK) [1, 2, 14, 16]. In contrast, the German Institute for Quality and Efficiency in Health Care confirmed a considerable additional benefit only for non-epithelioid MPM [17], and the Swiss regulatory agency Swissmedic limited its label to non-epithelioid or PD-L1 positive MPM.
With rising cancer drug prices, independent cost-effectiveness analyses (CEAs) are crucial. Switzerland has the third most expensive health spending per capita in the world, after the US and Germany [18]. Drug prices in Switzerland, defined by the Federal Office of Public Health, are mainly based on reference prices from other countries and for similar drugs. Unlike other authorities, Swiss reimbursement authorities do not systematically conduct independent CEAs, and there is no official willingness-to-pay (WTP) threshold in Switzerland. Next to the abovementioned analyses within the framework of national approval applications, four former CEAs were published by academic groups on first-line treatments for unresectable MPM. Two from the United States (US) payers’ perspective compared nivolumab+ipilimumab with chemotherapy [19, 20]. Two other CEAs compared chemotherapy+bevacizumab with chemotherapy: one from a Chinese and the other from a US payers’ perspective [21, 22]. Out of the four CEAs, only the latter one conducted in 2016 reported that the intervention is cost effective, more precisely the addition of bevacizumab to chemotherapy. We are not aware of any CEA for MPM in Switzerland, nor of any analysis comparing all three treatments simultaneously. Therefore, our objective was to evaluate the costs and benefits of all three first-line treatment options (nivolumab+ipilimumab, pemetrexed+platin, pemetrexed+platin+bevacizumab) for patients with unresectable MPM and relevant subgroups, and to determine their cost effectiveness and 5-year budget impact, based on the most recent results from both the CM-743 and MAPS trials, from a Swiss statutory health insurance perspective.
Methods
Model Overview and Main Outcomes
We developed a 3-state Markov cohort model with mutually exclusive health states of progression-free disease (PFD), progressed disease (PD) and death (D). State membership at the end of each model cycle was estimated by applying transition probabilities to the state membership at the end of the previous cycle. In our base case, we assumed that all patients with unresectable MPM start in the PFD state and show tumour progression before they die. This is in line with clinical observations. We based the calculation of the transition probability from PD to death on the OS curve. Since published OS curves are a weighted average of OS curves of both healthy and sick persons [23], we increased the transition probability of death with the help of the proportion of patients being alive but having progressed (Supplementary Fig. 1 and Supplementary Chapter 1, see electronic supplementary material [ESM]).
In a first scenario analysis for the Markov model, we allowed patients to die before tumour progression. We assumed the same transition probabilities to death from both the PFD and the PD state (Supplementary Fig. 2, see ESM). In a second scenario analysis, we developed a partitioned survival model (PFD, PD, D).
We compared three treatment strategies: the intervention strategy of nivolumab+ipilimumab and the comparators pemetrexed+platin and pemetrexed+platin+bevacizumab (including bevacizumab as maintenance therapy). Patients who progressed after receiving first-line treatment entered the PD state and received one or two further lines of treatment followed by late-stage palliative care, or directly late-stage palliative care. In the Markov models, the additional lines of treatment were not represented by separate health states but covered ‘within’ the PD state using on/off treatment tunnel states assuming that a certain percentage of the patients obtain further lines of treatment as outlined in Sect. 2.5.2 and Supplementary Chapter 6 (see ESM).
For the base-case analysis, we preferred a state transition model over a partitioned survival model because it is more transparent and also more flexible, allowing us to model a certain percentage of the patients to receive a second and even third-line treatment after entering the progressed state during a certain period of time. Also, it allowed us the use of on- and off-treatment utilities during the PD health state. For the partitioned survival model, further-line treatment costs could only be included as one-off costs to patients upon their direct transition from the PFD to the PD state.
In the base-case analysis, we used a time horizon of 30 years to represent the lifetime of patients, a cycle length of 1 month (30.5 days), and an annual discount rate of 3% for costs and quality-adjusted life-years (QALYs). We did not apply half-cycle correction due to the short cycle length of 1 month. All analyses were carried out from the Swiss statutory health insurance perspective (including direct medical care costs and follow-up treatment costs) using a price year of 2022. All CEA models were programmed in the TreeAge® software (Version Pro 2023) [24].
For the budget impact analysis (BIA), we developed a 5-year budget impact model in Excel (Microsoft Office Professional Plus 2019) and based it on undiscounted cost estimates from the CEA for the first 5 years (more information is provided in Sect. 2.5.7).
Population
Similar to the patients in the CM-743 trial, we assumed a hypothetical patient population with the following criteria: male and female adults with a median age of 69 years, advanced, untreated, and unresectable MPM, not amenable to curative therapy (surgery with or without chemotherapy), no prior systemic therapy, and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1. In CM-743, patients had been stratified by tumour histology (epithelioid versus non-epithelioid) and sex. The study had mainly been carried out in Europe (58.2%), but also in Asia (10.7%), North America (9.8%), and rest of the world (21.3%).
Since the French MAPS trial population did not completely match the CM-743 population, we modelled the CM-743 population. The patient population of the MAPS trial was similar, but restricted patients to those aged <75 years, who have a life expectancy of >12 weeks, and also included patients with ECOG PS of 2 (which, however, represented only 3% of patients) [12].
Intervention and Comparator Strategies
Intervention treatment in CM-743 with first-line immunotherapy consisted of nivolumab (3 mg/kg every 3 weeks [Q3W]) plus ipilimumab (1 mg/kg Q6W), both administered intravenously until disease progression, unacceptable toxicity, or a maximum treatment duration of 2 years. Since in reality patients do not always reach the maximum treatment duration even in the absence of progression or death (e.g. due to intolerance), we restricted the maximum possible nivolumab+ipilimumab treatment duration to 14 months. By proceeding like this, we obtained a modelled mean nivolumab+ipilimumab treatment duration of 8 months for the base case, which matches the published CM-743 mean (median) first-line nivolumab+ipilimumab duration of 7.9 months (5.6 months) [5] (this duration led to the trial-observed OS and PFS). For nivolumab, we assumed the weight-based dosing scheme with a 2-week frequency and decided against a flat intravenous infusion of 360 mg every 3 weeks. The flat nivolumab dose is not yet approved in Switzerland and would have meant off-label use. While it is likely to have similar efficacy and safety, this is not proven yet. In line with the CM-743 trial, we added vitamin B12 once (intramuscular injection 1000 μg) and oral vitamin B9 folic acid (400 μg per day) during the first week to nivolumab+ipilimumab.
The first comparator treatment strategy was intravenous pemetrexed 500 mg/m2 Q3W plus either carboplatin (area under the curve of 5 mg/mL/min) or cisplatin 75 mg/m2, administered in the registration trial CM-743 [5] on Day (D) 1 of a 21-day cycle for a maximum of six cycles. With the same reasoning as in the case of nivolumab+ipilimumab, we restricted the maximum pemetrexed+platin treatment duration to 3 months, resulting in a mean overall pemetrexed+platin duration of 2.9 months (the closest possible to the reported mean of 3 months) [5]. In a scenario analysis, we investigated the cost impact of 24 months of nivolumab+ipilimumab and six cycles of pemetrexed+platin without the possibility of early termination.
As concomitant medication for the first comparator, we assumed vitamin B12 supplement (at baseline, the 3rd and 6th cycle), oral vitamin B9 folic acid (7 days before the first cycle, ending 3 weeks after the last pemetrexed-based cycle), palonosetron (0.25 mg intravenous for Q3W, for a maximum of six cycles), and oral dexamethasone (4-mg tablets, 3 times at the beginning of every cycle) to mitigate the side effects of chemotherapy.
For the second comparator treatment, pemetrexed+platin+bevacizumab, we modelled bevacizumab 15 mg/kg (D1 of Q3W cycles) in addition to pemetrexed+platin. Bevacizumab was not restricted to six cycles but administered until progression, with all comedications administered as described previously.
Assuming clinical equivalence of pemetrexed+carboplatin and pemetrexed+cisplatin as widely accepted by UK clinical experts [25], only pemetrexed+carboplatin was modelled in the base-case analysis since it is the preferred treatment option in Switzerland. The cost of pemetrexed+cisplatin was considered in a scenario analysis.
A further chemotherapy option would have been raltitrexed (for people for whom treatment with pemetrexed is unsuitable). However, it is not approved for MPM in Switzerland, and also not used off-label [26, 27]. For this reason, it was not included as a comparator in this model.
Main Outcomes
The model assessed costs (overall, by resource type), life-years (LYs), QALYs, as well as incremental cost-effectiveness ratios (ICERs), expressed as costs in Swiss Francs (CHF) per QALY gained. To help international comparison, we also converted main ICERs from CHF into United States Dollars (USD), applying an average 2022 exchange rate of USD1.048 for CHF1 [28]. We used a standard rational choice approach to rank and compare all three strategies simultaneously. The resulting ICERs were compared with a WTP threshold of CHF100,000 per QALY gained, which is sometimes considered in analyses for Switzerland [29–33]. The threshold falls within threshold ranges recommended by both a recent study (0.5–1.5 times the gross domestic product [GDP] per capita) [34] and the World Health Organization (WHO) (1–3 times the GDP per capita). The wider WHO recommendation would lead to a WTP threshold range of CHF84,055–CHF252,165/QALY for Switzerland based on a GDP per capita of CHF84,055 in 2021 (latest available) [35, 36].
Model Inputs
Modelling of Survival and Progression of Disease
We did not have access to individual patient data (IPD) and therefore digitalized (using Software DigitizeIt [37]) published Kaplan-Meier OS and PFS curves from both MAPS (median follow-up [FU] of 39.4 months) [12] and CM-743 (4-year OS [15], 3-year PFS, 3-year subgroup results; minimum FU of 35.5 months [13]) (Supplementary Figs 3–6, see ESM).
Pseudo IPD was recreated in R software (version 4.2.2 [38]) according to the method of Guyot et al. [39]. The best distributional selection to fit and extrapolate the data within and beyond the trial FU periods was guided by expected clinical longer-term results and statistical fit. The latter incorporated lowest Akaike and Bayesian information criterion during the FU period (Supplementary Sects. 3.2 and 3.3), and a smoothed hazard function as in MAPS and CM-743 that initially increased before decreasing over time (Supplementary Figs. 7 and 8 [see ESM] and company submission of nivolumab+ipilimumab to NICE [2, 14]). We investigated the fit of several standard parametric distributions (exponential, Weibull, lognormal, log-logistic, Gompertz, Gamma, Generalized gamma) but also of spline functions (hazard, odds, and normal scales with 1 and 2 knots each) to the Kaplan-Meier curves. Taking all selection criteria into account, we selected spline normal 1-knot functions for both OS treatment curves (Supplementary Fig. 13, see ESM), a spline hazard 2 knots for PFS under pemetrexed+platin, and a spline normal 1-knot function for PFS under immunotherapy (Supplementary Fig. 18, Supplementary Sects. 3.4, 3.5 [see ESM], the latter presenting results of histologic subtypes).
Log-cumulative hazards plots, Schoenfeld residual plots, test of Schoenfeld residuals against log(time), and visual inspection of the OS and PFS figures allowed us to conclude non-proportional hazards for OS and PFS in CM-743 (Supplementary Chapter 4, see ESM). According to standard practice, we therefore independently fitted the OS and PFS curves for each treatment arm [2]. For the MAPS trial, we considered the proportional hazards assumption to approximately hold for both OS and PFS. In general, this allows the modelling of an intervention survival curve by using the hazard ratio (HR) to adjust the comparator curve. Since the patient inclusion criteria of both trials differed, we did not pool the recreated chemotherapy survival data of both studies but modelled the chemotherapy+bevacizumab estimates with the HR from MAPS adjusted to the chemotherapy estimates of the CM-743 trial (adjusted HRs of original graphs: 0.73 [0.61, 0.87] for OS, 0.77 [0.62, 0.95] for PFS) (Supplementary Figs. 13, 18, 30–33, see ESM).
In general, estimated survival curves were converted into transition probabilities, calculated as one minus the ratio of the survivor function at the end and beginning of a cycle.
Further-Line Treatment
We modelled a second line of treatment with nivolumab monotherapy (3 mg/kg IV Q2W) for 4 months for 62% of the patients previously treated with chemotherapy. Following first-line immunotherapy, we assumed second-line pemetrexed+carboplatin for 3 months (as reported for first-line treatment) for 65% of the patients. Of the patients still alive at the end of the second line, third-line gemcitabine 1000 mg/m2 (together with dexamethasone) and intravenous vinorelbine 30 mg/m2, each 50% on D1 and D8 of a 21-day cycle, were assumed. In contrast to first-line treatment, we only modelled pemetrexed+carboplatin and not pemetrexed+cisplatin in the second line. More details including sources are given in Supplementary Chapter 6 (see ESM).
Utilities
Swiss utility values for MPM patients were not available. Instead, we used treatment-strategy-dependent and treatment (on/off)-dependent utilities that were assessed in the NICE Single Technology Appraisal for nivolumab+ipilimumab for untreated unresectable MPM (Supplementary Table 6 in the ESM) [2]. Utilities were based on within-trial EQ-5D-3L data collected in the CM-743 trial [2]. All model inputs are summarized in Supplementary Table 8 (see ESM).
We assumed that any utility decrement associated with adverse events was already captured in the treatment-specific utilities. Therefore, no further utility decrements were applied.
Adverse Events
All treatment-related AEs of grades 3–4 in CH-743 and MAPS occurring in ≥2% of patients in at least one of the treatment arms were considered (Supplementary Table 9, see ESM). Since AEs associated with carboplatin and cisplatin are different, we used the corresponding AEs depending on whether carboplatin (base case) or cisplatin (scenario analysis) were being analysed. However, for the chemotherapy+bevacizumab strategy, we only had data available from the MAPS trial, and therefore could only include AE probabilities related to cisplatin and not carboplatin.
Resource Use
Physician Visits Including Laboratory Tests
We assumed joint visits for nivolumab (Q3W) and ipilimumab (Q6W) every 6 weeks. During each of the six cycles of Q3W chemotherapy administration, an additional intermediate oncologist visit, including small laboratory testing after 2 weeks, was added to reflect standard practice in Switzerland. We generally assumed FU visits every 3 months after the end of treatment and during progression (Supplementary Table 10, see ESM).
Imaging
In line with Swiss standard of care, our model included the following imaging resource utilization: an inpatient screening biopsy, an outpatient computed tomography (CT) chest plus abdomen scan every 6 weeks during the first year, every 3 months thereafter until progression/death, and also post-progression. Assuming that the biopsy and CT scans are performed in separate visits, we also incorporated their costs if cisplatin was given during an inpatient stay. Electrocardiogram costs were considered negligible for this analysis.
Late-Stage Palliative Care
After the end of the last treatment line, we modelled late-stage palliative care with pain medication (morphine 30 mg/day, paracetamol 3*1 g/day) and 3-monthly oncologist physician visits including laboratory tests (hemogram V, chemistry I and II).
Costs
We considered drug costs from the Swiss specialty list [40]. The amount of drug was calculated for an average Swiss patient, with costs based on the cheapest available generic of the total smallest pack/bottle required. Based on the observed distribution of male and female patients from CM-743 and Swiss demographic (weight and height) data, we derived an average Swiss weight (77.1 kg) and a body surface area of 1.93 m2 [41, 42]. These data were used for the weight-based dosing schemes for immunotherapies in line with the national standard of care. We estimated hospital inpatient costs in multiplying the cost weights of relevant diagnosis related group (DRG) codes of the SwissDRG 11.0 system [43] with the averaged Swiss Cantonal DRG base weights. For inpatient cisplatin administration, we added official one-off hospital supplementary charges for pemetrexed (CHF2,417.28) and bevacizumab (CHF3,084.09) for each stay [43]. Outpatient costs for physician visits, drug administration, laboratory testing, imaging procedures (screening biopsy, CT scan thorax abdomen), and late-stage palliative care were based on the Swiss tariff system for outpatient physician services (TARMED 1.09) [44] and the Swiss Analysis List [45]. If available, resource use and costs were coupled and compared with aggregated real-life data of mesothelioma patients of the Cantonal Hospital of Lucerne, Switzerland. For example, mean hospital inpatient terminal care costs until death for 162 patients from the oncology/palliative care unit of the Cantonal Hospital of Lucerne (derived from the latest available fourth quarter in 2021) were estimated as CHF27,427. The end of life/terminal care costs were applied to all patients who died as one-off costs when entering the death state. With regard to AE costs, we calculated one-off costs for all first-line AEs depending on their expected duration (Supplementary Table 9, see ESM) and applied them to each treatment arm at model cycle 1 adjusted by the percentages of patients receiving these.
Budget Impact Analysis Inputs and Assumptions
We performed a 5-year budget impact analysis for the years 2022 to 2026 for three scenarios: (1) a scenario without the use of nivolumab+ipilimumab, (2) a scenario for the Swissmedic MPM label for nivolumab+ipilimumab (non-epithelioid histology or PD-L1 expression ≥1%), and (3) a scenario targeting nivolumab+ipilimumab for all MPM regardless of histology or PD-L1 (as recommended by ESMO, for example [11]).
We did not integrate the previous years 2018–2021 into the BIA per se, but included costs of patients who had started chemotherapy (with/without bevacizumab) treatment during these years and continued their original treatments during 2022–2025.
Information on Swiss market shares for nivolumab+ipilimumab was not publicly available. We estimated current and projected future market shares for the three distinct scenarios of interest (Supplementary Table 11, see ESM). Separately for each treatment strategy, undiscounted mean cost results from the CEA for the first to fifth year were then multiplied by the market-share-adjusted number of MPM patients in each of the years 2022–2026.
We derived the number of Swiss patients with unresectable MPM, as well as subgroup sample sizes, from publicly available sources [46, 47], and also received information from the Foundation Swiss National Institute for Cancer Epidemiology and Registration (NICER) [48] (Supplementary Table 12, see ESM). The latter provided us with the MPM incidence rate for the latest available year (2019; 2.25 per 100,000 population per year) and the percentage of patients with the non-epithelioid subtype (58.2%). We further assumed 43.7% of patients have PD-L1 ≥1% within the epithelioid subtype [49]. To estimate the MPM incidence for the subsequent years 2020–2026, we added a yearly increase of 2.6 persons per year to the 2019 MPM incidence rate as derived from Suva accident statistics (UVG 2020 report [7]).
Uncertainty Analyses
To investigate the robustness of the base-case results, we performed deterministic and probabilistic analyses (PA) and a large series of scenario analyses.
In the deterministic analyses (DA), available 95% confidence intervals (CIs) were used as the maximum and minimum boundaries, or the 2.5% and 97.5% percentiles of assigned distributions (next paragraph). If both could not be determined, we varied the base-case parameter value by ±30%. Drug costs were generally assumed to be fixed and not varied in the DA with the exception of nivolumab, ipilimumab, bevacizumab, and supplementary inpatient hospital charges for pemetrexed and bevacizumab. We presented the results in Tornado diagrams and showed the ten most influential parameters.
For the PA, we assigned gamma distributions to unit cost parameters and beta distributions to utilities and probabilities. Drug costs were assumed to be fixed in the PA. Distribution parameters for the OS and PFS curves were assigned normal distributions, and HRs were assigned log-normal distributions. Where standard error estimates or 95% CIs were not available, we assumed standard errors to be 20% of the base case parameter values for costs and probabilities, and 10% for utilities. We performed 10,000 simulation runs and showed the 10,000 simulated ICER results in a cost-effectiveness plane. The probabilities of strategies being cost effective at varying WTP thresholds were additionally illustrated in cost-effectiveness acceptability curves (CEACs). With the help of the expected value of perfect information (EVPI), we also assessed whether further clinical research to reduce parameter uncertainty may be worthwhile. EVPI for an individual patient was calculated in TreeAge when running regular PA with a single parameter sampling loop [24]. We also calculated the EVPI for the Swiss population assuming an effective lifetime of treatments of 5 years (2022–2026) and using the estimated MPM incidences from the BIA (Section 2.5.7, Supplementary Table 8 in the ESM). Future benefits were again discounted annually at 3%.
In a first scenario analysis, we allowed patients to die before tumour progression and explored the impact if the same transition probability to death from both the PFD and the PD Markov state is applied. In a second scenario analysis, we developed a partitioned survival model. As previously mentioned, in the partitioned survival model, further-line treatment costs for an estimated period of time could only be included as one-off costs for patients upon their direct transition from the PFD to the PD state. However, such a procedure potentially overestimates further-line treatment costs as it does not integrate patients dying in the months following disease progression into this cost calculation. For this reason, we reduced the duration of the further lines of treatment by 1 month compared with the Markov model, resulting in similar mean 2L/3L treatment costs. Model inputs deviating from the Markov model inputs are shown in Supplementary Tables 5 and 7 in the ESM.
In a third scenario analysis, we assumed the impact of inpatient pemetrexed+cisplatin rather than outpatient pemetrexed+carboplatin costs, taking into account the different AE pattern, but assuming the same OS and PFS. In further scenario analyses, we also investigated different histologic subtypes, extended costs to a maximum 24-month treatment duration for nivolumab+ipilimumab and six cycles for pembrolizumab+platin, varied discount rates (0%, 5%), and restricted the time horizon to 10 years.
For the BIA, we examined in a scenario analysis the budget impact when only new MPM patients from 2022 onwards were included.
Results
Cost-Effectiveness Analysis Results
Over a 30-year lifetime horizon and after discounting, the nivolumab+ipilimumab strategy showed the highest mean benefit of 1.78 QALYs and the highest mean total costs of CHF184,060. For the chemotherapy+bevacizumab strategy, we estimated a mean of 1.59 QALYs and mean costs of CHF173,243; and for the pemetrexed+carbo strategy a mean of 1.21 QALYs and mean costs of CHF74,945 (Table 1). We found that pemetrexed+carbo+bevacizumab was extendedly dominated when compared with pemetrexed+carbo and nivolumab+ipilimumab. Nivolumab+ipilimumab relative to pemetrexed+carboplatin was projected to generate 0.57 mean incremental QALYs (0.73 LYs discounted) and mean incremental costs of CHF109,115, resulting in an ICER of CHF192,585 (i.e. USD201,829) per QALY gained. The high costs of the ipilimumab+nivolumab strategy were largely driven by the total drug costs, which represent 64% of the total costs (24% of the total costs for pemetrexed+carboplatin, 59% for pemetrexed+carbo+bevacizumab).
Table 1.
Base case ICER results (probabilistic analysis)
| Treatment | Cost (CHF) | QALY | LYa | ΔCost (CHF) | ΔQALY | ICERb (CHF/QALY gained) |
|---|---|---|---|---|---|---|
| Pem+Platin | 74,945 | 1.21 | 1.72 | |||
| Pem+Platin+Beva | 173,243 | 1.59 | 2.23 | 98,297 | 0.38 | Ext. dominated |
| Nivo+Ipi | 184,060 | 1.78 | 2.45 | 109,115 | 0.57 | 192,585 |
Beva bevacizumab, CHF Swiss Francs, ext extendedly, ICER incremental cost-effectiveness ratio, ipi ipilimumab, LYs life-years, nivo nivolumab, pem pemetrexed, QALY quality-adjusted life-year
aDiscounted
bOf non-dominated strategies
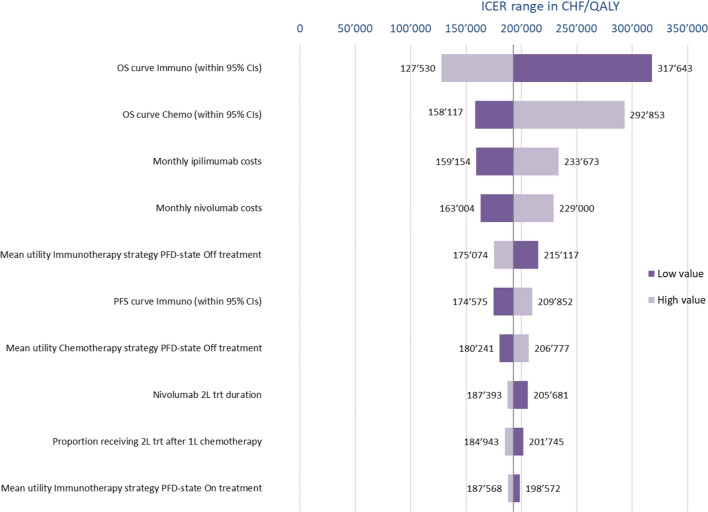
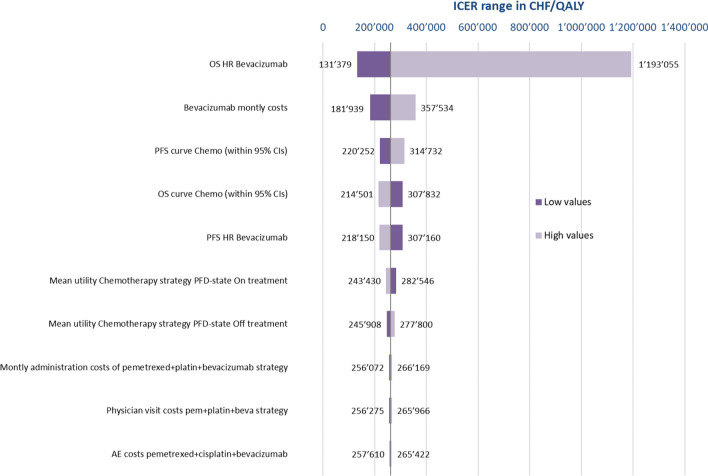
DAs (Fig. 1) show that for the nivolumab+ipilimumab versus pemetrexed+platin comparison, the ICER was most sensitive to changes in the parameter values of the OS immuno- and chemotherapy curves, but also to nivolumab and ipilimumab drug cost variations. As for the comparison of chemotherapy versus chemotherapy+bevacizumab (Fig. 2), the ICER was very sensitive to changes in the OS HR related to the addition of bevacizumab, but changes in bevacizumab costs, in the OS and PFS chemotherapy curves, and in the PFS HR also had a moderate impact.
Fig. 1.
One-way sensitivity analysis of nivolumab plus ipilimumab versus chemotherapy. CHF Swiss Francs, CIs confidence intervals, ICER incremental cost-effectiveness ratio, OS overall survival, PFD progression-free disease, PFS progression-free survival, QALY quality-adjusted life-year, trt treatment, 1L first-line, 2L second-line
Fig. 2.
One-way sensitivity analysis of chemotherapy versus chemotherapy with bevacizumab. AE adverse event, beva bevacizumab, Chemo chemotherapy, CIs confidence intervals, CHF Swiss Francs, HR hazard ratio, ICER incremental cost-effectiveness ratio, OS overall survival, pem pemetrexed, PFD progression-free disease, PFS progression-free survival, QALY quality-adjusted life-year
Across the ranges assessed, the ICER of nivolumab+ipilimumab versus pemetrexed+carboplatin was always above CHF100,000 (Fig. 1). For the comparison of pemetrexed+carboplatin and pemetrexed+carboplatin+bevacizumab, the ICER always exceeded CHF100,000/QALY and only for fell below a CHF200,000/QALY threshold for lower values of the OS HR and bevacizumab costs (Fig. 2).
In the first scenario analysis, assuming an equal probability of death from both the PFD and from the PD state, the nivolumab+ipilimumab strategy showed again the highest mean benefit of 1.69 QALYs and the highest mean total cost of CHF175,693. We estimated for the pemetrexed+platin+bevacizumab strategy a mean of 1.59 QALYs and mean cost of CHF164,268; and for the pemetrexed+platin strategy a mean of 1.21 QALYs and mean cost of CHF71,777 (Supplementary Table 13, see ESM). Again pemetrexed+platin+bevacizumab was extendedly dominated when compared with pemetrexed+platin and nivolumab+ipilimumab. Nivolumab+ipilimumab relative to pemetrexed+platin was projected to generate 0.48 mean incremental QALYs (0.60 LYs discounted) and a mean incremental cost of CHF103,916, resulting in an ICER of CHF216,905 (i.e. USD227,316) per QALY gained.
The partitioned survival model (scenario 2) projected an ICER of CHF178,094 (i.e. USD186,643) per QALY gained for nivolumab+ipilimumab relative to pemetrexed+platin. This scenario led to lower QALYs for all treatment strategies and lower cost for nivolumab+ipilimumab. Pemetrexed+platin+bevacizumab was absolutely dominated.
Assuming the use of cisplatin (inpatient treatment) instead of carboplatin (outpatient treatment) resulted in higher costs for the chemotherapies. The model projected an ICER of CHF158,911 (i.e. USD166,539) per QALY gained for nivolumab+ipilimumab versus pemetrexed+carboplatin. Pemetrexed+carboplatin+bevacizumab resulted in higher costs and lower QALYs than nivolumab+ipilimumab and was hence absolutely dominated.
Maximum treatment durations (less if progression/death was estimated to occur) resulted in increased treatment costs, particularly for the nivolumab+ipilimumab arm. Consequently, the base case ICER increased from CHF192,797 (DA) to CHF251,714/QALY gained.
Regarding histologic subtypes, QALYs for chemotherapy decreased in patients with non-epithelioid histology due to the well-known lack of effectiveness of chemotherapy in the sarcomatoid subtype. This resulted in an improved ICER of CHF97,894/QALY (USD102,593/QALY) gained for nivolumab+ipilimumab versus pemetrexed+carboplatin. In contrast, for the epithelioid subtype, where the effectiveness of chemotherapy is greater, the ICER increased to CHF266,267 (USD279,048) per QALY gained. Supplementary Table 13 provides all remaining scenario analysis results (see ESM). Among all scenarios investigated, the ICER of the nivolumab+ipilimumab strategy for patients with the non-epithelioid subtype was the only one below CHF100,000/QALY at current list prices.
Table 2 shows the economically justifiable prices assuming a WTP threshold of CHF100,000/QALY. For nivolumab+ipilimumab to become cost effective for all MPM histologies, a nivolumab+ipilimumab price reduction of 52% (i.e. to 48% of their 2022 list prices) would be required. The combination pemetrexed+carboplatin+bevacizumab would require a bevacizumab price reduction of 72% (to 28%) to become cost effective compared with pemetrexed+carboplatin.
Table 2.
Economically justifiable price at target WTP of CHF100 000/QALY
| Treatment | Discount level of pack prices | Costs (CHF) | QALYs | ΔCosts (CHF) | ΔQALYs | Comment | ICERs (CHF/QALY gained) | Total drug costs (CHF) |
|---|---|---|---|---|---|---|---|---|
| Overall population (carboplatin) | ||||||||
| For Nivo and Ipi | ||||||||
| Pem+Platin | 69,345 | 1.20 | 12,442 | |||||
| Nivo+Ipi | −52% (to 48%) | 125,132 | 1.76 | 55,787 | 0.56 | Cost effective at WTP vs Pem+Platin | 99,128 | 58,707 |
| Pem+Platin+Beva | 163,441 | 1.56 | 38,309 | −0.20 | Absolutely dominated | 92,937 | ||
| For bevacizumab | ||||||||
| Pem+Platin | 76,138 | 1.20 | 19,235 | |||||
| Pem+Platin+Beva | −72% (to 28%) | 112,099 | 1.56 | 35,961 | 0.36 | Cost effective at WTP vs Pem+Platin | 99,928 | 41,595 |
| Epithelioid population (carboplatin) | ||||||||
| For Nivo and Ipi | ||||||||
| Pem+Platin | 68,578 | 1.44 | 10,001 | |||||
| Nivo+Ipi | −73% (to 27%) | 99,606 | 1.75 | 31,028 | 0.32 | Cost effective at WTP | 97,877 | 33,517 |
| Pem+Platin+Beva | 205,983 | 1.95 | 106,377 | 0.20 | 541,636 | 128,999 | ||
| For bevacizumab | ||||||||
| Pem+Platin | 78,693 | 1.44 | 20,116 | |||||
| Pem+Platin+Beva | −72% (to 28%) | 129,529 | 1.95 | 50,837 | 0.51 | 99,017 | 52,546 | |
| Nivo+Ipi | 180,101 | 1.75 | 50,572 | −0.20 | Absolutely dominated | 114,013 | ||
Beva bevacizumab, CHF Swiss Francs, ICER incremental cost-effectiveness ratio, ipi ipilimumab, nivo nivolumab, pem pemetrexed, QALY quality-adjusted life-year, WTP willingness-to-pay
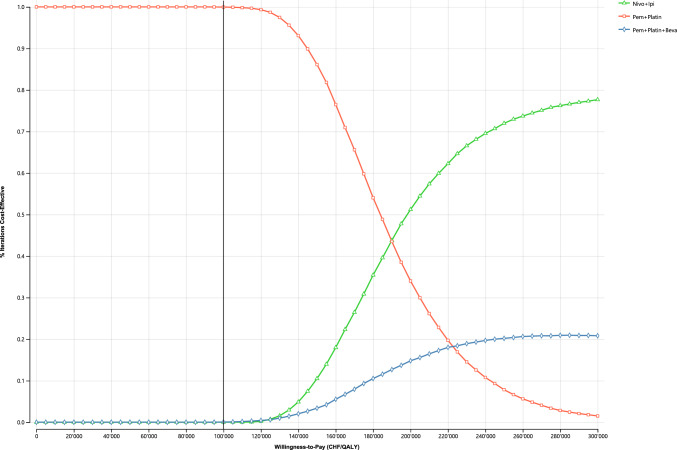
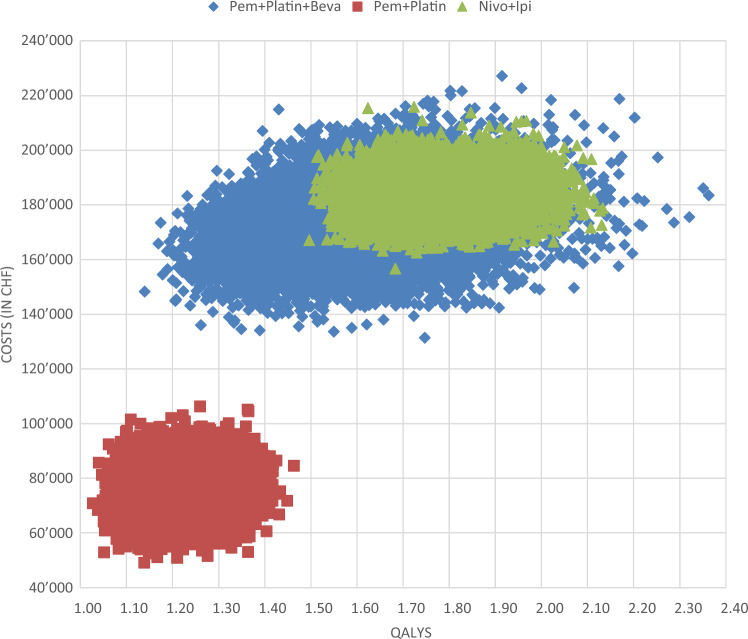
Fig. 3 presents the cost-effectiveness acceptability curve of the PA. It shows that both nivolumab+ipilimumab and chemotherapy+bevacizumab had a 0% probability of being cost effective at a WTP threshold of CHF100,000, whereas chemotherapy was cost effective at 100%. The probability of nivolumab+ipilimumab being cost effective increased to 10.5%, 51.2%, and 72.0% at WTP thresholds of CHF150,000, CHF200,000, and CHF250,000/QALY, respectively. The cost-effectiveness plane in Fig. 4 and Supplementary Fig. 34 (see ESM) show that for chemotherapy+bevacizumab, changes in QALYs were more pronounced (suggesting higher uncertainty) than changes in costs. The ICER cloud for the chemotherapy+bevacizumab strategy largely overlapped the cloud for nivolumab+ipilimumab.
Fig. 3.
Cost-effectiveness acceptability curve. Beva bevacizumab, CHF Swiss Francs, Ipi ipilimumab, Nivo nivolumab, Pem pemetrexed, QALY quality-adjusted life-year
Fig. 4.
Cost-effectiveness plane. Beva bevacizumab, CHF Swiss Francs, Ipi ipilimumab, Nivo nivolumab, Pem pemetrexed, QALY quality-adjusted life-year
With perfect information, we estimated an individual patient EVPI value (opportunity loss) of CHF1.37 for a WTP threshold of CHF100,000/QALY. EVPI locally peaked at approximately CHF11,945 in the graph at the point where the underlying ICER was closest to the WTP (Supplementary Fig. 35, see ESM). The estimated population EVPI over a 5-year time horizon (2022–2026) at a 1.3% annual decline in MPM incidence was CHF1344 (Supplementary Fig. 36, see ESM). This means that at the WTP threshold of CHF100,000/QALY, a new trial would only be beneficial if the cost of the trial is less than CHF1344, which will not be the case. A longer effective lifetime of the treatments (e.g. 10 years instead of 5 years) will most likely increase the benefits, but we do not expect them to reach the costs of a new trial.
Budget Impact Analysis Results
At current 2022 Swiss list prices, the availability of nivolumab+ipilimumab is projected to largely increase the necessary Swiss health budget for this indication due to the increased costs of nivolumab+ipilimumab (Table 3, Supplementary Tables 14 and 15 in ESM). Strictly adhering to the Swissmedic label, by restricting nivolumab+ipilimumab to PD-L1 ≥1% or the non-epithelioid subtype, led to a necessary budget of CHF165.5 million, and CHF127.1 million in the case of no nivolumab+ipilimumab. Following the recommendations of ESMO, treating the largest possible number of MPM patients with ipilimumab+nivolumab is estimated to result in 5-year costs of CHF184.2 million.
Table 3.
Five-year budget impact
| Strategy and treatments | Scenario analysis | Base case | ||
|---|---|---|---|---|
| Excluding FU costs in 2022–2026 of patients who started between 2018–2021 (in CHF) | Including FU costs in 2022–2026 of patients who started between 2018–2021* (in CHF) |
Budget increase | % Budget increase | |
| No nivo+ipi | 110,944,118 | 127,129,866 | Reference | Reference |
| Swissmedic label | 149,294,663 | 165,480,411 | 38,350,545 | 30% |
| Nivo+ipi targeting all patients with unresectable MPM | 167,985,030 | 184,170,779 | 57,040,913 | 45% |
Beva bevacizumab, BIA budget impact analysis, carbo carboplatin, CHF Swiss Francs, FU follow-up, ipi ipilimumab, MPM malignant pleural mesothelioma, nivo nivolumab, pem pemetrexed
*Before 2022, patients had only started on pem+carbo (50% of patients) and pem+carbo+beva (50% of patients) and continued their original treatment during the BIA time horizon
We compared each of the scenarios (2) and (3) to a scenario without nivolumab+ipilimumab (1). This resulted in a necessary 5-year budget increase of CHF38.4 million (30%) applying the Swissmedic label, and of CHF57.0 million (45%) when targeting all patients.
Discussion
We conducted an independent CEA to assess from a Swiss statutory health insurance perspective all first-line therapies recommended for patients with unresectable MPM. We found that substantial discounts for nivolumab+ipilimumab would be necessary to achieve cost effectiveness at the current list prices when assuming a traditional WTP threshold of CHF100,000/QALY (the estimated ICER at the 2022 list prices was CHF192,585/QALY; i.e. USD201,829/QALY). As expected from the clinical subgroup analysis results of the registration trial CM-743, cost effectiveness was more likely for non-epithelioid MPM, supporting the limited label by Swissmedic for this subtype. Based on our model, nivolumab+ipilimumab was regarded as a cost-effective treatment option for the treatment of patients with non-epithelioid MPM (ICER of CHF97,894/QALY, USD102,593/QALY).
Compared with the previous CEAs from a US perspective, our estimated ICERs for Switzerland for nivolumab+ipilimumab versus pemetrexed+carboplatin were lower [19, 20]. The US models were based on less recent CM-743 data, used a more limited selection of grade 3–4 AEs, and importantly used non-small-cell lung cancer (NSCLC) utilities. The US models projected ICERs of USD371,861/QALY [19] (CHF355,053/QALY) and USD475,677/QALY [20] (CHF454,176/QALY), which were above their chosen WTP thresholds of USD207,659/QALY (3 times US GDP per capita in 2021) and USD150,000/QALY. The latter publication even excluded the possibility of nivolumab+ipilimumab becoming cost effective for all histologies and the non-epithelioid subtype.
In a partitioned survival model for a UK NICE submission [1, 2, 14], nivolumab+ipilimumab resulted in incremental costs of GBP50,260 and 0.67 incremental QALYs, yielding an ICER of GBP75,322/QALY, with unknown details of the agreed nivolumab and ipilimumab prices. With discounts that are confidential and not known by us, nivolumab+ipilimumab was regarded as a cost-effective treatment option. Confidential discounts are increasing and may have negative effects on global drug affordability [50]. CADTH reported an ICER of CAD300,921/QALY gained [16] (i.e. CHF219,462/QALY [51]), and estimated that a discount of 72% was required for nivolumab+ipilimumab to be cost effective compared with standard chemotherapy assuming a WTP threshold of CAD50,000/QALY. The model results of both submissions are consistent with our results. For Switzerland, we estimated that a nivolumab+ipilimumab price discount of 52% (i.e. to 48% of its 2022 list prices) for all histologies is necessary to achieve cost effectiveness.
Chemotherapy+bevacizumab was not cost effective in our base case. Two previous CEAs yielded conflicting results. From a US payers’ perspective, the addition of bevacizumab to chemotherapy was cost effective according to commonly used WTP thresholds, with a reported ICER of USD73,081/QALY [21]. In contrast, from a Chinese perspective, pemetrexed+cisplatin+bevacizumab was not cost effective (with an ICER of USD727,203/QALY gained) assuming a WTP threshold of 3*GDP per capita (USD23,970/QALY) [22].
The high costs of nivolumab+ipilimumab, but also of chemotherapy+bevacizumab, are mainly driven by the actual drug prices, and not by toxicity management or palliative care. The high drug costs are also the reason we projected for Switzerland a necessary increase in the 5-year health budget for unresectable MPM of 30% to implement the Swissmedic label, and of 45% to treat all patients with nivolumab+ipilimumab (under the assumption that all other model assumptions hold).
Our CEA has several strengths. To the best of our knowledge, it is the first that analysed three different therapeutic options in one model. We included the most recent update from CM-743, the MAPS study, and applied a life-long time horizon. A further strength of our model is the use of MPM-specific utilities, and that we considered all grade 3–4 AEs occurring in ≥2% in at least one of the treatment arms. Resource use and costs from published and official Swiss sources, coupled with real-world data from a large Swiss hospital, are further strengths. We also tested, and ultimately used when appropriate, spline functions to approximate and extrapolate survival functions. We distinguished between outpatient and inpatient settings. We validated our base-case model by changing Markov model assumptions and developing a partitioned survival model. All three models led to the same overall conclusion for the base case. Our work was funded by the Swiss Institute for Accident Insurance (Suva) and carried out independently from industry.
Potential limitations of our analysis include uncertainties related to model and input parameter values, as no head-to-head clinical trial comparing all three treatment options exists. Since the MAPS trial population differs from the CM-743 population, we had to simulate the chemotherapy+bevacizumab arm by applying its HR to the survival curve of the control arm of CM-743. This worked well for PFS, but introduced a bias to OS, which resulted in an overestimation of treatment benefit for the MAPS chemotherapy+bevacizumab arm during the first 6 months in our model. Nevertheless, our approach led to less bias than merging survival data of different trial populations (data not shown). For the epithelioid and non-epithelioid subtypes, there were no published HRs from MAPS comparing chemotherapy with chemotherapy+bevacizumab. We used the HR of the total population in these cases as well. This procedure had no effect on the comparison of nivolumab+ipilimumab and chemotherapy for the subtypes.
With regard to utilities, there might also be an overestimation of the utility during PFS in the chemotherapy+bevacizumab arm. The fact that bevacizumab maintenance is given until progression led to the on-treatment utility being applied continuously during PFS. However, since our model showed that chemotherapy+bevacizumab was dominated by other treatment strategies, we assumed that chemotherapy+bevacizumab would have been even less cost effective if lower OS or a lower utility were applied.
In the DA and PA, we varied the HRs of the chemotherapy+bevacizumab strategy for OS and PFS separately, but could not do so for the individually estimated OS and PFS curves for the nivolumab+ipilimumab and pemetrexed+platin strategies. To investigate the impact of different OS and PFS, we used the lower and upper bounds of their 95% CIs. In a further scenario analysis, we also modelled the costs of a maximum 24-month nivolumab+ipilimumab treatment duration, whereas OS and PFS from the CH-743 and MAPS trials emerged from shorter treatment durations. Therefore, the results of this scenario should be interpreted with caution.
A further limitation of our model may be related to second- and third-line therapy, as publications from CM-743 and MAPS [12, 13] were not very precise in this regard. Because effectiveness of available further-line treatments remains poor and unclear [1, 2, 52], and because we did not find reliable real-world data for Switzerland, we modelled current clinical practice in Switzerland based on our own clinical experience. With this approach we were also much more in line with the reported FU percentages of the MAPS trial [12]. We assumed the effect of further treatments on the CM-743 survival curves (‘progressed disease part’) as low.
NICE recommended to adjust the survival curves for treatment switching with an appropriate methodology (e.g. inverse probability censoring weights [IPCW] method) [14]. We could not follow their recommendation, since we did not have IPD available, and the switching-adjusted OS curves were not published. Results of the adjusted analysis are redacted in the NICE report, but indicate a reduction in the ICER for nivolumab+ipilimumab versus pemetrexed+platin from GBP75,322 to an unknown value [14].
Conclusion
At current list prices in Switzerland and assuming a WTP threshold of CHF100,000/QALY, nivolumab+ipilimumab is cost effective for the treatment of unresectable MPM for the non-epithelioid subtype but not for all histologies. As suggested by authorities in the UK and Canada, substantial discounts would be necessary to achieve cost effectiveness for all MPM histologies in Switzerland. Chemotherapy+bevacizumab is unlikely to be a cost effective treatment, requiring an even higher discount of its current list price than nivolumab+ipilimumab. Randomized trials testing checkpoint inhibitors in combination with chemotherapy in patients with unresectable MPM are ongoing.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Swiss National Accident Insurance Fund (Suva, Lucerne, Switzerland) for their research grant. We thank the Foundation National Institute for Cancer Epidemiology and Registration (NICER) for information on Swiss MPM incidence and prevalence values, and histologic subtype proportions. We thank Mario Pietrini and Florian Wüthrich (Cantonal Hospital Lucerne, Lucerne, Switzerland) for supporting us with anonymized economic data. We also thank Dr Cédric Panje (Radiooncology, Hirslanden, St. Gallen, Switzerland) for supporting the Suva grant application.
Declarations
Funding
Open access funding provided by University of Basel. This work was funded by the Swiss Institute for Accident Insurance (Suva). Neither the study funder, nor the manufacturer of nivolumab and ipilimumab, were involved in determining the research question, the study design, data collection, analysis, interpretation, decision to publish, or writing of the manuscript.
Conflict of interest/competing interests
Unrelated to the submitted work, MB has received personal fees from Vifor for participation in an advisory board meeting. OG attended advisory boards by Eli Lilly, was consultant for Amgen, and is member of a data safety monitoring board for Boehringer Ingelheim (all honoraria were paid to his institution). AB, AF, EP, and NM have declared no conflict of interest.
Ethics approval
We requested approval from the Ethics Committee Northwest and Central Switzerland for the use of aggregate resource use and cost data of patients treated at the Cantonal Hospital of Lucerne from 2011 to 2022. According to our request (Req-2022-00420), ethical approval was not needed for the use of anonymized and aggregated data.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of Data and Material
The parameter values used in this model are included in the published article (and its supplementary information files) or are available from the corresponding author on reasonable request for non-commercial purposes. Access to underlying data from the National Institute for Cancer Epidemiology and Registration and the Cantonal Hospital of Lucerne is possible via the data owners following their regulations. Where relevant, contact can be established with the corresponding author.
Code availability
The cost-effectiveness model was implemented in TreeAge and is available from the corresponding author (MB) on reasonable request for non-commercial purpose.
Author contributions
MB, AF, and OG conceived and designed the study. MB performed the survival modelling in R, developed and implemented the model in TreeAge, and performed the model validation. AF, OG, and EP provided aggregate resource use and cost data of medical records of patients from the Cantonal Hospital of Lucerne. MB drafted the grant application and the manuscript. AB and NM helped with the resubmission and performed a detailed review of the manuscript. All authors reviewed and commented on the manuscript for important intellectual content and approved the final version.
References
- 1.NICE. Final appraisal document (FAD). Nivolumab with ipilimumab for untreated unresectable malignant pleural mesothelioma. 2022; https://www.nice.org.uk/guidance/ta818/documents/html-content-4. [DOI] [PubMed]
- 2.NICE. Committee papers 2021. Nivolumab with ipilimumab for untreated unresectable malignant pleural mesothelioma [ID1609]. 2021; https://www.nice.org.uk/guidance/ta818/evidence.
- 3.Bibby AC, Tsim S, Kanellakis N, Ball H, Talbot DC, Blyth KG, et al. Malignant pleural mesothelioma: an update on investigation, diagnosis and treatment. Eur Respir Rev. 2016;25(142):472–486. doi: 10.1183/16000617.0063-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima EC, Vellanki PJ, Larkins E, Chatterjee S, Mishra-Kalyani PS, Bi Y, et al. FDA approval summary: nivolumab in combination with ipilimumab for the treatment of unresectable malignant pleural mesothelioma. Clin Cancer Res. 2022;28(3):446–451. doi: 10.1158/1078-0432.CCR-21-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 6.CANCER TODAY International Agency for Research on Cancer. GLOBOCAN 2020 Fact Sheet Mesothelioma. https://gco.iarc.fr/today/fact-sheets-cancers.
- 7.Suva. Unfallstatistik UVG 2020. 2020; https://www.unfallstatistik.ch/d/publik/unfstat/unfstat_d.htm.
- 8.Moore A, Bennett B, Taylor-Stokes G, McDonald L, Daumont MJ. Malignant pleural mesothelioma: treatment patterns and humanistic burden of disease in Europe. BMC Cancer. 2022;22(1):693. doi: 10.1186/s12885-022-09750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Research UK. Mesothelioma statistics. 2020. https://www.cancerresearchuk.org/about-cancer/mesothelioma/survival.
- 10.National Comprehensive Cancer Network©. NCCN Clinical Practice Guidelines in Oncology, Malignant Pleural Mesothelioma. Version 1.2022. 2022; https://www.nccn.org/home.
- 11.Popat S, Baas P, Faivre-Finn C, Girard N, Nicholson A, Nowak A, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2022;33(2):129–142. doi: 10.1016/j.annonc.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. The Lancet. 2016;387(10026):1405–1414. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 13.Peters S, Scherpereel A, Cornelissen R, Oulkhouir Y, Greillier L, Kaplan MA, et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol. 2022;33(5):488–499. doi: 10.1016/j.annonc.2022.01.074. [DOI] [PubMed] [Google Scholar]
- 14.NICE. Committee Papers 2022. Nivolumab with ipilimumab for untreated unresectable malignant pleural mesothelioma [ID1609]. 2022; https://www.nice.org.uk/guidance/ta818/documents/html-content-4.
- 15.Zalcman G, Oulkhouir Y, Cornelissen R, Greillier L, Cid JR, Mazieres J, et al. LBA71 First-line nivolumab (NIVO) plus ipilimumab (IPI) vs chemotherapy (chemo) in patients (pts) with unresectable malignant pleural mesothelioma (uMPM): 4-year update from CheckMate 743. Ann Oncol. 2022;33:S1438–S1439. doi: 10.1016/j.annonc.2022.08.077. [DOI] [PubMed] [Google Scholar]
- 16.CADTH. CADTH Reimbursement Recommendation Nivolumab In Combination With Ipilimumab (Opdivo-Yervoy). 2021; https://www.cadth.ca/sites/default/files/pcodr/Reviews2021/PC0229%20Opdivo-Yervoy%20%20Final%20Rec.pdf.
- 17.IQWiG. Nivolumab (malignes Pleuramesotheliom)—Nutzenbewertung Bericht Nr. 1209. 2021; https://www.iqwig.de/download/a21-89_nivolumab_nutzenbewertung-35a-sgb-v_v1-0.pdf.
- 18.Organisation for Economic Co-operation and Development (OECD). Health spending USD per capita. https://data.oecd.org/healthres/health-spending.htm.
- 19.Ye ZM, Tang ZQ, Xu Z, Zhou Q, Li H. Cost-effectiveness of nivolumab plus ipilimumab as first-line treatment for American patients with unresectable malignant pleural mesothelioma. Front Public Health. 2022;10:947375. doi: 10.3389/fpubh.2022.947375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Cao X, Li N, Zheng B, Liu M, Cai H. Cost-effectiveness analysis of nivolumab plus ipilimumab versus chemotherapy as the first-line treatment for unresectable malignant pleural mesothelioma. Ther Adv Med Oncol. 2022;14:17588359221116604. doi: 10.1177/17588359221116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malacan J, Carlson J. Cost-effectiveness analysis of addition of bevacizumab to a standard chemotherapy doublet (pemetrexed+ cisplatin) in patients with malignant pleural mesothelioma. Value in Health. 2016;19(3):A153. doi: 10.1016/j.jval.2016.03.1610. [DOI] [Google Scholar]
- 22.Zhan M, Zheng H, Xu T, Yang Y, Li Q. Cost-effectiveness analysis of additional bevacizumab to pemetrexed plus cisplatin for malignant pleural mesothelioma based on the MAPS trial. Lung Cancer. 2017;110:1–6. doi: 10.1016/j.lungcan.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Pahuta MA, Werier J, Wai EK, Patchell RA, Coyle D. A technique for approximating transition rates from published survival analyses. Cost Effectiveness Resource Allocation. 2019;17(1):12. doi: 10.1186/s12962-019-0182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.TreeAge LLC. TreeAge User Manual. https://www.treeage.com/support/electronic-help/.
- 25.Santoro A, O'Brien ME, Stahel RA, Nackaerts K, Baas P, Karthaus M, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol. 2008;3(7):756–763. doi: 10.1097/JTO.0b013e31817c73d6. [DOI] [PubMed] [Google Scholar]
- 26.Cinausero M, Rihawi K, Sperandi F, Melotti B, Ardizzoni A. Chemotherapy treatment in malignant pleural mesothelioma: a difficult history. J Thorac Dis. 2018;10(Suppl 2):S304–S310. doi: 10.21037/jtd.2017.10.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meerbeeck JPV, Manegold C, Gaafar R, Klaveren RJV, Marck EAV, Vincent M, et al. A randomized phase III study of cisplatin with or without raltitrexed in patients (pts) with malignant pleural mesothelioma (MPM): an intergroup study of the EORTC Lung Cancer Group and NCIC. J Clin Oncol. 2004;22(14_suppl):7021. doi: 10.1200/jco.2004.22.90140.7021. [DOI] [Google Scholar]
- 28.Exchange Rates UK. Swiss Franc to US Dollar Spot Exchange Rates for 2022. https://www.exchangerates.org.uk/CHF-USD-spot-exchange-rates-history-2022.html.
- 29.Matter-Walstra K, Schwenkglenks M, Aebi S, Dedes K, Diebold J, Pietrini M, et al. A cost-effectiveness analysis of nivolumab versus docetaxel for advanced nonsquamous NSCLC including PD-L1 testing. J Thorac Oncol. 2016;11(11):1846–1855. doi: 10.1016/j.jtho.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Barbier MC, Tomonaga Y, Menges D, Yebyo HG, Haile SR, Puhan MA, et al. Survival modelling and cost-effectiveness analysis of treatments for newly diagnosed metastatic hormone-sensitive prostate cancer. PLoS ONE. 2022;17(11):e0277282. doi: 10.1371/journal.pone.0277282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbier M, Durno N, Bennison C, Örtli M, Knapp C, Schwenkglenks M. Cost-effectiveness and budget impact of venetoclax in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia in Switzerland. Eur J Health Econ. 2022;23:1–10. doi: 10.1007/s10198-021-01398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbier MC, Pardo E, Panje CM, Gautschi O, Lupatsch JE, Research SGfCC. A cost-effectiveness analysis of pembrolizumab with or without chemotherapy for the treatment of patients with metastatic, non-squamous non-small cell lung cancer and high PD-L1 expression in Switzerland. Eur J Health Econ. 2021;22:669–677. doi: 10.1007/s10198-021-01282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhadhuri A, Insinga R, Guggisberg P, Panje C, Schwenkglenks M. Cost effectiveness of pembrolizumab vs chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in Switzerland. Swiss Med Wkly. 2019;149:w20170. doi: 10.4414/smw.2019.20170. [DOI] [PubMed] [Google Scholar]
- 34.Iino H, Hashiguchi M, Hori S. Estimating the range of incremental cost-effectiveness thresholds for healthcare based on willingness to pay and GDP per capita: a systematic review. PLoS ONE. 2022;17(4):e0266934. doi: 10.1371/journal.pone.0266934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swiss Federal Statistical Office. Gross domestic product per capita. 23.11.2022]; https://www.bfs.admin.ch/bfs/en/home/statistics/national-economy/national-accounts/gross-domestic-product.assetdetail.23184194.html.
- 36.Thokala P, Ochalek J, Leech AA, Tong T. Cost-effectiveness thresholds: the past, the present and the future. Pharmacoeconomics. 2018;36(5):509–522. doi: 10.1007/s40273-017-0606-1. [DOI] [PubMed] [Google Scholar]
- 37.DigitizeIt. DigitizeIt software. https://www.digitizeit.xyz/.
- 38.R software. https://www.r-project.org/.
- 39.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Federal Office of Public Health. Swiss Specialty List ("Schweizer Spezialitätenliste"). http://www.spezialitaetenliste.ch/ShowPreparations.aspx?searchType=SUBSTANCE.
- 41.Swiss Federal Statistical Office. Mean body height (in cm). https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit.assetdetail.7586022.html.
- 42.Swiss Federal Statistical Office. Mean body weight (in cm). https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit.assetdetail.7586025.html.
- 43.Online Definitionshandbuch SwissDRG 9.0. 16.11.2022]; https://manual.swissdrg.org/de/11.3/supplements.
- 44.TARMED Online Browser Tarifversion 1.09. 1.2.2021]; https://www.tarmed-browser.ch/de.
- 45.Analysenliste (Version 1.August 2022). Swiss Federal Office of Public Health. 2022; https://www.bag.admin.ch/bag/de/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/Analysenliste.html.
- 46.Swiss Federal Statistical Office. Population size and change in Switzerland in 2021: definitive figures. https://www.bfs.admin.ch/bfs/en/home/statistics/population.gnpdetail.2022-0467.html.
- 47.Swiss Federal Statistical Office. Key population figures, 1950-2021. https://www.bfs.admin.ch/bfs/en/home/statistics/population.assetdetail.23328853.html.
- 48.National Institute for Cancer Epidemiology and Registration (NICER). https://www.nicer.org/en/home.
- 49.Cedrés S, Ponce-Aix S, Zugazagoitia J, Sansano I, Enguita A, Navarro-Mendivil A, et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM) PLoS ONE. 2015;10(3):e0121071. doi: 10.1371/journal.pone.0121071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carl DL, Vokinger KN. Patients’ access to drugs with rebates in Switzerland—empirical analysis and policy implications for drug pricing in Europe. Lancet Regional Health - Europe. 2021;3:100050. doi: 10.1016/j.lanepe.2021.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Exchange Rates UK. Swiss Franc to Canadian Dollar Spot Exchange Rates for 2021. https://www.exchangerates.org.uk/CAD-CHF-spot-exchange-rates-history-2021.html.
- 52.Metaxas Y, Rivalland G, Mauti LA, Klingbiel D, Kao S, Schmid S, et al. Pembrolizumab as palliative immunotherapy in malignant pleural mesothelioma. J Thorac Oncol. 2018;13(11):1784–1791. doi: 10.1016/j.jtho.2018.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.