Abstract
Birds in seasonal habitats rely on intricate strategies for optimal timing of migrations. This is governed by environmental cues, including photoperiod. Genetic factors affecting intrinsic timekeeping mechanisms, such as circadian clock genes, have been explored, yielding inconsistent findings with potential lineage-dependency. To clarify this evidence, a systematic review and phylogenetic reanalysis was done. This descriptor outlines the methodology for sourcing, screening, and processing relevant literature and data. PRISMA guidelines were followed, ultimately including 66 studies, with 34 focusing on candidate genes at the genotype-phenotype interface. Studies were clustered using bibliographic coupling and citation network analysis, alongside scientometric analyses by publication year and location. Data was retrieved for allele data from databases, article supplements, and direct author communications. The dataset, version 1.0.2, encompasses data from 52 species, with 46 species for the Clock gene and 43 for the Adcyap1 gene. This dataset, featuring data from over 8000 birds, constitutes the most extensive cross-species collection for these candidate genes, used in studies investigating gene polymorphisms and seasonal bird migration.
Subject terms: Behavioural genetics, Animal migration, Animal migration
Background & Summary
Birds occupy nearly every habitat and ecoregion on Earth, however, many of these habitats experience large seasonal shifts in key ecological attributes such as length of day1, temperature2, rainfall3,4, and associated food and nesting material availability5. This has necessitated the adaptive evolution of complex strategies to maximise survival through seasonal migrations between breeding and wintering ranges. Migrations are carefully timed events, scheduled in such a manner that birds can optimise hours of daylight6, nighttime visibility7,8, and time spent at stop-over sites9 along their migration route to ensure timely arrivals for optimal habitat use. While most of the ecological attributes play some role in the timing of migration, one of the best studied attributes that serve as a trigger to initiate migration is the length of day or photoperiod. The photoperiod is primarily responsible for daily oscillations within the regulatory feedback loops of the circadian clock, which differentially expresses genes during light or dark phases to maintain sleep-wake cycles in most organisms10.
One conundrum regarding migration in birds is how differential migration patterns are established and maintained within singular species, even in the absence of extrinsic environmental triggers. For example, several species within the order Coraciiformes have distinct populations that are either year-round residents, with minimal altitudinal movement, or long-distance migrants. This includes such species as the Lilac-breasted roller11 (Coracias caudatus) and Woodland kingfisher12 (Halcyon senegalensis), both having subspecies that are delineated by differential migration, as well as the European bee-eater13 (Merops apiaster), which is considered monotypic but has a distinct resident population in Southern Africa. Understanding how differential migration is established and maintained between such species is key to assessing connectivity14, speciation at a subspecies level15, and potential population fitness16. This is particularly pertinent with regards to the plasticity or ability to switch between behaviours17,18 should environmental conditions change considerably due to climate change19–21 or anthropogenic activity22–25.
Several studies have explored the possible genetic components that affect intrinsic time keeping mechanisms and migration. Although variable methods have been used, including genomic26, epigenetic27, and transcriptomic approaches28, most studies sought to identify genes or gene regions that show variation in either the sequence itself or the gene expression that can be correlated to divergent migratory behaviour. The key, however, is identifying variation that is linked to processes that interface with annual life events. Thus, variation that is either connected to the endocrine or metabolic changes29, in preparation for migration and breeding, or intrinsic time-keeping mechanisms, such as the rhythmic expression of circadian genes; particularly those that interface with environmental changes that my serve as cues such as photoperiod, temperature, lunar cycles, and food availability30. This is needed to exclude variants that co-vary with migration phenotypes but are not actively involved in shaping them. It is therefore no surprise that many candidate gene studies have explored variation within the network of genes of the circadian clock. Several associated candidate genes have been suggested, with length polymorphisms within short repeats of the Clock and Adcyap1 genes being the focus of many studies31–33.
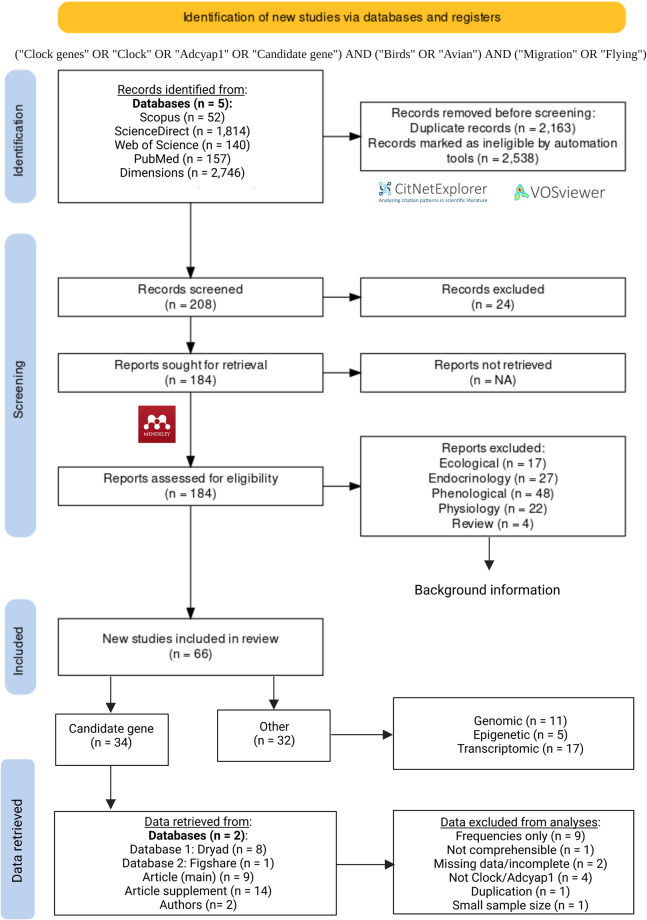
To clarify the role of these genes in migratory phenotypes, a systematic review (Fig. 1) was conducted to identify, synthesise, and provide a reappraisal of the available evidence34. Structured searches of the literature with an optimised Boolean search string were done in five scientific databases. Search results were exported in formats compatible with citation network analysis software35. After duplicate entries were removed, citation network analyses were used for the automated screening of database results to identify the central literature on the topic. Publications identified from the citation network analyses were subjected to manual screening of the title, abstract, and key words to assess the potential eligibility for inclusion in the review. The final list of most eligible publications was sought for full text retrieval. A total of 66 studies were included in the final review of which 34 were candidate gene studies and 32 were other, migration-related, studies. These included latitude/longitude/spatial analyses, timing of migration, and timing of egg laying/breeding. Most of the studies using a candidate gene approach were used for data retrieval. For these studies, datasets were retrieved as either diploid allele data of individuals or allele frequencies. Data sources included the main text of articles, supplementary materials, databases such as Dryad (https://datadryad.org/) or Figshare (https://figshare.com/), data extraction, or data received directly from authors. Unpublished data for an additional 12 species were also included. The dataset included individual level allele data from 52 species of which data was available for 46 species for the Clock gene and 43 species for the Adcyap1 gene. This dataset represents the largest collection of cross species allele data for two candidate genes used to test a putative association between clock gene polymorphisms and divergent migration in birds, which enables the testing for patterns of inheritance, evolutionary selection, relation to divergence times, and associations across a globally distributed dataset.
Fig. 1.
PRISMA statement for the systematic approach used to identify studies that measured clock gene polymorphisms in relation to annual synchronicity of live events such as breeding and migration in birds. Further details are also provided for the retrieval of allele data for individual studies from various sources as well as reasons for exclusion of studies. (image edited in BioRender.com).
This data descriptor summarises both the methodology used to screen the literature as well as to compile the data concisely and presents the resulting data used in prior analyses in an easy-to-understand format. At present, none of the scientific databases that collect genetic variation data is suitable for the deposit of this specific type of data. The barcode of life data system (BOLD, https://boldsystems.org/), which does accept length polymorphism data from microsatellite markers, currently only accepts data for markers used in barcoding or population assignment experiments and does not specifically store data for markers used in behavioural or phenotype associated studies. The European variant archive (EVA, https://www.ebi.ac.uk/eva/), which also accepts variant data that includes length polymorphisms, currently only accepts data for species with reference genomes, which is still unavailable for most avian species. To overcome this, we have endeavoured to create a central compilation of the available data in two standard formats which is archived in parallel to this data descriptor; with an additional online version on GitHub36 (https://github.com/LSLeClercq/AvianClocksData) that will be maintained and updated over time as more data is made available. This may greatly facilitate the reuse of the data where it may be applicable to other forms of analyses within migration genetics and beyond.
Methods
Literature search and automated screening
Literature was searched using systematic review methods, in line with PRISMA Ecology and Evolution guidelines37, to identify and synthesize relevant sources. The overall approach is depicted in the PRISMA statement38 in Fig. 1 that was supplemented with further information on the data retrieval and screening process. Literature was searched between January and September of 2022 on five databases: Scopus (N = 52, www.scopus.com), ScienceDirect (N = 1814, www.sciencedirect.com), Web of Science (N = 140, https://clarivate.com/), PubMed (N = 157, https://pubmed.ncbi.nlm.nih.gov/), and Dimensions (N = 2746, www.dimensions.ai). Databases were searched using an optimized Boolean search string derived from the PICO terms for the aim and objectives of the review. The final search string was as follows: (“Birds” OR “Avian”) AND (“Clock genes” OR “Clock” OR “Adcyap1” OR “Candidate gene”) AND (“Migration” OR “Flying”). As needed, this was complemented by ancillary ‘free term’ searches based on citations in articles or to include other relevant aspects such as “Breeding”, “Moult”, “Genomics”, “Transcriptomics” or “Photoperiod”. For the Scopus and Dimensions database searches, the results were exported in the comma separated value (CSV) format, while the results from the ScienceDirect, Web of Science, and PubMed database search were exported in the research information systems (RIS) format.
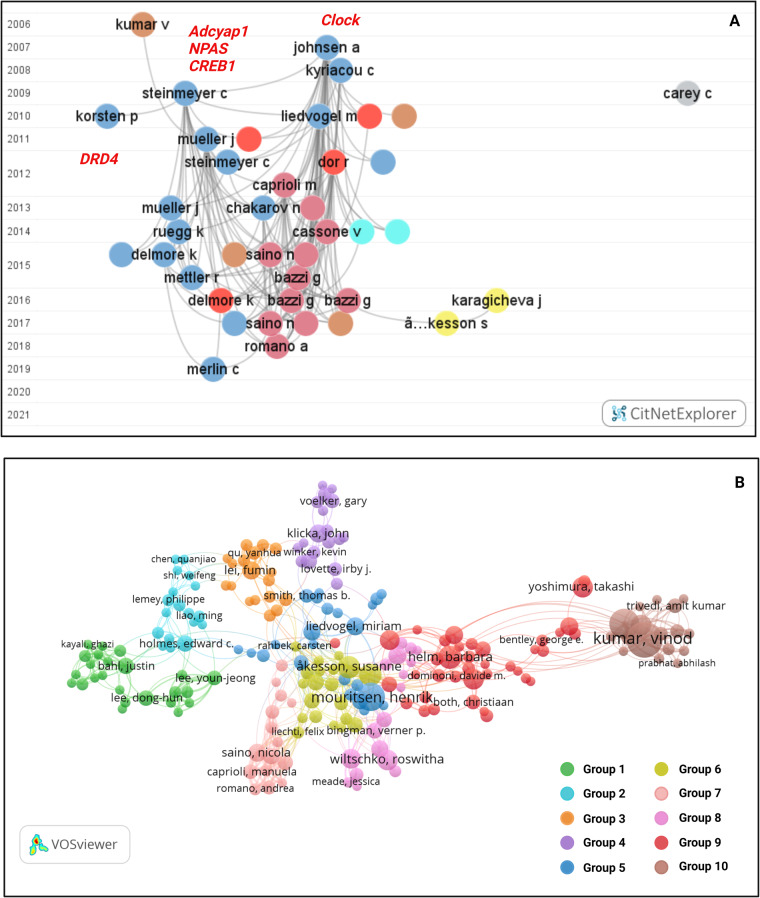
Automated screening for inclusion was done through citation network analyses. For the Scopus database, the results were merged and reformatted with the R package ‘Scopus2CitNet 0.1.0.0’ (https://github.com/MichaelBoireau/Scopus2CitNet) in RStudio 1.4.110639, running R 4.0.540. The results were subsequently visualized by year in CitNetExplorer 1.0.0., keeping only those papers that overlapped in terms of references cited and the largest connected set (Fig. 2a). The results from the search on the Dimensions and ScienceDirect databases were visualized in VOSviewer 1.6.1635 by group as well as by year, keeping only those papers that are connected by citations and reference lists (Fig. 2b). The size of bubbles corresponds to citations and the number of cross-links between studies.
Fig. 2.
Visualised citation network for studies identified in literature searches. (A) Citation network of the Scopus and PubMed database in CitNetExplorer. Publications are organized by year (2006–2021) with the name and first initial of the first author indicating individual studies. The relationship between studies by virtue of co-citations in the reference lists are indicated by grey lines. Subgroup analyses identified several key groups, indicated by the colour code from VOSviewer. Key candidate genes are indicated in red italics and show studies that assayed polymorphisms in the Clock, Adcyap1, CREB1, NPAS, and DRD4 genes. (B) Citation network for studies identified in literature searches of the Dimensions and ScienceDirect database in VOSviewer. First authors are labelled by surname and first name. Automated group analyses identified ten clusters of related studies of which the studies identified from Scopus formed part of five groups, indicated as groups 2, 5, 6, 7, 9, and 10. This network shows the larger field of migration studies including non-candidate gene studies such as transcriptomic studies (group 10). (image edited in BioRender.com).
Manual title-abstract screening and full text retrieval
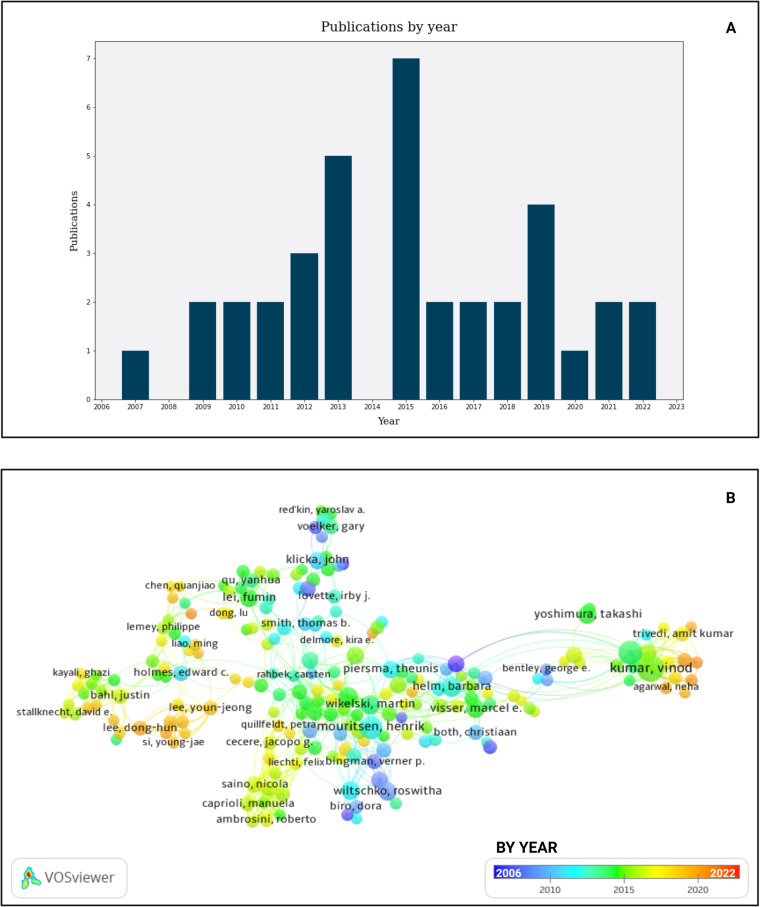
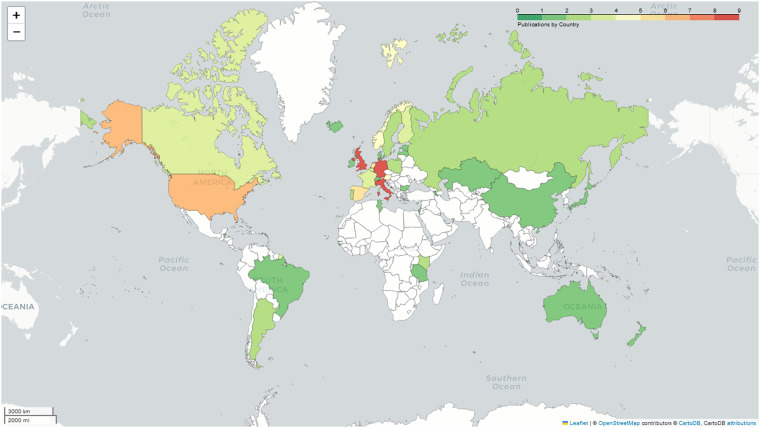
Sources identified from the citation networks were imported (citation and abstract) into Mendeley citation manager (www.mendeley.com) for further screening. Several types of studies relating to migration genetics were included in preliminary screening such as candidate gene studies, genomic studies, transcriptomic studies, and epigenetic studies. Studies with a focus on endocrine systems, physiology, or telomeres were excluded. Studies on migration phenology, without an evident genetic link, were also excluded. The inclusion criteria of candidate gene studies were confined to studies that primarily measure Clock or Adcyap1 gene polymorphisms (as well as other candidate genes studied in parallel e.g., NPAS, CREB1, and DRD4: indicated on Fig. 234) within bird populations to compare putative variation to the annual synchronicity in life events and differential migration. These included latitude/longitude/spatial analyses, timing of migration, migratory restlessness, timing of egg laying/breeding, clutch size, moult, urbanisation, and exploratory behaviour. The final set of studies that passed preliminary screening were sought during full text retrieval and added to the imported reference if it wasn’t already included. A total of 66 studies were included in the final review of which 34 were candidate gene studies and 32 were other, migration related, studies using genetic methods. Some basic scientometric assessments of the final set of studies, including the plotting of publications per year (Fig. 3) as well as the geographic distribution (Fig. 4) of studies, was conducted using ABCal version 1.0.241 (https://github.com/LSLeClercq/ABCal).
Fig. 3.
Plots indicating the distribution for publications by year. (A) Histogram for publications by year indicating the first publications starting in 2007 up to more recent publications in 2022, with the largest number of publications between 2013–2015 and in 2019. (B) Density gradient display of studies in VOSviewer based on year of publication, indicated most studies were published between 2006 (blue) and 2022 (red) with a high number of publications emanating from 2013–2016 (green to orange). (image edited in BioRender.com).
Fig. 4.
Geographic distribution of candidate gene studies included in the final review dataset (N = 34) based on sampling locations. Related migration studies (N = 32), such as transcriptomic or epigenetic studies, were excluded. The density gradient plots the number of studies per country ranging from one study (green) to more than eight studies (red); countries in white are data deficient. The overall plot indicates that most studies emanated from sampling locations in Europe and North America, with only a small number of studies including sampling from parts of Africa and South America.
Published datasets
A total of 34 studies were identified that used a candidate gene approach for which data retrieval was done. Data was retrieved from either the main text, supplementary material of the article, online data repositories such as Dryad42–49 and Figshare50, or additional data received directly from authors. Data types varied from allele frequencies to individual level diploid allele data. Allele data for the Barn swallow51 was retrieved from the text while data for the Yellow-legged gull52 was extracted from images using WebPlotDigitizer version 4.653. Allele data was generally derived from a single source with the exception of the European pied flycatcher44,49 and Willow warbler54–56. The species, data sources, and data types are summarized in Table 1 along with the sampling location and sample sizes. Frequency data was available for most published studies, with the exception of the bluebird species18, and those species for which allele data was unavailable are summarised in Table 2. This includes species for which only frequency data was reported, species for which a non-clock gene approach was used, and studies for which only data summaries without frequencies were reported.
Table 1.
List of species for which published allele data was collected and/or included in the review and data article.
| Common name | Latin binomial | Study | Data | Type | Location | N |
|---|---|---|---|---|---|---|
| Barn swallow* | Hirundo rustica | 51,62,63 | 51 | CA | Switzerland, Italy | 64 |
| Bar-tailed godwit | Limosa lapponica baueri | 64 | 64 | CA | New Zealand | 135 |
| Blackpoll warbler* | Setophaga striata | 65 | 43 | CA, AA | USA | 72 |
| Blue tit* | Cyanistes caeruleus | 31,32,66,67 | 31,42,45 | CA | Europe | 950 |
| Collared flycatcher* | Ficedula albicollis | 68 | 47 | CA, AA | Czechia | 406 |
| Collared plover | Charadrius collaris | 69 | 69 | AA | Brazil | 14 |
| Common buzzard | Buteo buteo | 70 | 48 | AA | Germany | 978 |
| Common nightingale* | Luscinia megarhynchos | 54,71 | 44, Authors | CA, AA | Italy | 150 |
| Common redstart* | Phoenicurus phoenicurus | 54 | Authors | CA, AA | Italy | 43 |
| Common whitethroat* | Sylvia communis | 54 | Authors | CA, AA | Italy | 25 |
| Dark-eyed junco* | Junco hyemalis | 72 | 72 | CA, AA | USA | 36 |
| Eastern subalpine warbler* | Curruca cantillans | 54 | Authors | CA, AA | Italy | 31 |
| Eurasian blackbird* | Turdus merula | 22 | 22 | CA, AA | Europe, Tunisia | 792 |
| Eurasian blackcap* | Sylvia atricapilla | 73,74 | 50,73 | AA | Europe | 936 |
| Eurasian golden oriole* | Oriolus oriolus | 54 | Authors | CA, AA | Italy | 30 |
| Eurasian hoopoe* | Upupa epops | 54 | Authors | CA, AA | Italy | 25 |
| Eurasian reed warbler* | Acrocephalus scirpaceus | 54 | Authors | CA, AA | Italy | 24 |
| Eurasian wryneck* | Jynx torquilla | 54 | Authors | CA, AA | Italy | 30 |
| European bee-eater* | Merops apiaster | 54 | Authors | CA, AA | Italy | 35 |
| European nightjar* | Caprimulgus europaeus | 54 | Authors | CA, AA | Italy | 39 |
| European pied flycatcher* | Ficedula hypoleuca | 71,75,76 | 44,49, Authors | CA, AA | Italy | 226 |
| European turtle dove* | Streptopelia turtur | 54 | Authors | CA, AA | Italy | 29 |
| Garden warbler* | Sylvia borin | 54 | Authors | CA, AA | Italy | 31 |
| Great reed warbler* | Acrocephalus arundinaceus | 54 | Authors | CA, AA | Italy | 20 |
| Icterine warbler* | Hippolais icterina | 54 | Authors | CA, AA | Italy | 29 |
| Northern wheatear* | Oenanthe oenanthe | 54 | Authors | CA, AA | Italy | 30 |
| Painted bunting* | Passerina ciris | 77 | 77 | CA, AA | USA | 60 |
| Sedge warbler* | Acrocephalus schoenobaenus | 54 | Authors | CA, AA | Italy | 30 |
| Semipalmated plover | Charadrius semipalmatus | 69 | 69 | AA | Brazil | 13 |
| Semipalmated sandpiper | Calidris pusilla | 69 | 69 | AA | Brazil | 14 |
| Spotted flycatcher* | Muscicapa striata | 54 | Authors | CA, AA | Italy | 29 |
| Spotted sandpiper | Actitis macularius | 69 | 69 | AA | Brazil | 12 |
| Tree pipit* | Anthus trivialis | 54,71 | 44, Authors | CA, AA | Italy | 153 |
| Tree swallow* | Tachycineta bicolor | 16,78 | 46 | CA, AA | Canada | 921 |
| Whinchat* | Saxicola rubetra | 54,71 | 44, Authors | CA, AA | Italy | 208 |
| Willow warbler* | Phylloscopus trochilus | 54–56 | Authors | CA, AA | Italy | 495 |
| Wilson’s warbler* | Cardellina pusilla | 76 | Authors | CA, AA | USA | 102 |
| Wood warbler* | Phylloscopus sibilatrix | 54 | Authors | CA, AA | Italy | 30 |
| Woodchat shrike* | Lanius senator | 54 | Authors | CA, AA | Italy | 20 |
| Yellow-legged gull | Larus michahellis | 52 | 52 | CA, AA | Italy | 64 |
Species indicated with an asterisk (*) were included in the allele dataset for population genetics analyses34. The primary study, specific data source, location of the study sites and the sample size (N) is given. CA: Clock gene alleles, AA: Adcyap1 gene alleles.
Table 2.
List of species for which other published data was collected and/or included in the review and data article.
| Common name | Latin binomial | Study | Data | Type | Location | N |
|---|---|---|---|---|---|---|
| African stonechat | Saxicola torquatus | 79 | 79 | CF | Kenya, Tanzania | 172 |
| Asian short-toed lark | Alaudala cheleensis | 80 | 80 | CF | China | 257 |
| Black swan | Cygnus atratus | 81 | 81 | Non-CA | Australia | 100 |
| Bluethroat | Luscinia svecica | 31 | 31 | CF | Europe | 369 |
| Blue-winged warbler | Vermivora cyanoptera | 82 | 82 | Non-CA | USA | 24 |
| Canary Island stonechat | Saxicola dacotiae | 79 | 79 | CF | Canary Islands | 61 |
| Chilean swallow | Tachycineta meyeni | 16 | 16 | CF | Argentina | 88 |
| Common buzzard | Buteo buteo | 70 | 48 | CF | Germany | 978 |
| Eurasian blackcap | Sylvia atricapilla | 73,74 | 50,73 | CF | Europe | 936 |
| European roller | Coracias garrulus | 14 | 14 | Non-CA | Europe | 32 |
| European stonechat | Saxicola rubicola | 79 | 79 | CF | Europe | 382 |
| Golden winged warbler | Vermivora chrysoptera | 82 | 82 | Non-CA | USA | 42 |
| Great tit | Parus major | 83–85 | 83 | CF | UK | 225 |
| Mangrove swallow | Tachycineta albilinea | 16 | 16 | CF | Belize | 163 |
| Mountain bluebird | Sialia currucoides | 18 | 18 | NA | Canada | 11 |
| Northern goshawk | Accipiter gentilis | 70 | 48 | CF | Germany | 15 |
| Red kite | Milvus milvus | 70 | 48 | CF, AF | Germany | 20 |
| Seychelles warbler | Acrocephalus sechellensis | 86 | 86 | Non-CA | Seychelles | 57 |
| Siberian stonechat | Saxicola maurus | 79 | 79 | CF | Kazakhstan, Japan | 101 |
| Song sparrow | Melospiza melodia | 87 | 87 | Non-CA | Canada | 78 |
| Violet-green swallow | Tachycineta thalassina | 16 | 16 | CF | USA | 48 |
| Western bluebird | Sialia mexicana | 18 | 18 | NA | Canada | 127 |
| White-rumped swallow | Tachycineta leucorrhoa | 16 | 16 | CF | Argentina | 169 |
| Yellow-eyed junco | Junco phaeonotus | 72 | 72 | CF, AF | USA | 178 |
The primary study, specific data source, location of the study sites and the sample size (N) is given. CF: Clock gene frequencies, AF: Adcyap1 gene frequencies, Non-CA: Non clock gene study, NA: Not Available.
Unpublished datasets
This study included unpublished data for twelve species in total, summarised in Table 3. The six North American species were sampled at Long Point Old Cut, Ontario, Canada, and included the American redstart (N = 26), Common yellowthroat (N = 31), Hermit thrush (N = 30), Magnolia warbler (N = 33), Swainson’s thrush (N = 29), and White-throated sparrow (N = 32). The six European species included the Common chiffchaff (N = 55) and five species of shearwaters: Barolo shearwater (N = 15), Boyd’s shearwater (N = 25), Great shearwater (N = 25), Manx shearwater (N = 23), and Yelkouan shearwater (N = 15). The Common chiffchaff was sampled from several locations in Sweden (N = 30, subspecies abietinus) and Kazakhstan (N = 25, spp. tristis). Blood samples were taken from the brachial vein and stored in SET buffer at –80 °C. Shearwaters were sampled from several locations in Europe including France and Portugal while several species were sampled from islands such as Iceland, Cape Verde, and territories of the United Kingdom such as Gough Island. A 1 ml blood sample was taken from the tarsal or the brachial vein during geolocator retrieval. Samples were collected in 1.5 ml plastic tubes containing 70% ethanol and stored at –20 °C until further analysis.
Table 3.
List of species for which unpublished data was collected and/or included in the review and data article.
| Common name | Latin binomial | Study | Data | Type | Location | N |
|---|---|---|---|---|---|---|
| American redstart* | Setophaga ruticilla | 34 | Authors | CA, AA | Canada | 26 |
| Barolo shearwater | Puffinus baroli | 88 | Authors | CA | Portugal | 15 |
| Boyd’s shearwater | Puffinus boydi | 88 | Authors | CA | Cape Verde | 25 |
| Common chiffchaff* | Phylloscopus collybita | 34 | Authors | CA | Sweden, Kazakhstan | 55 |
| Common yellowthroat* | Geothlypis trichas | 34 | Authors | CA, AA | Canada | 31 |
| Great shearwater | Ardenna gravis | 88 | Authors | CA | UK | 25 |
| Hermit thrush* | Catharus guttatus | 34 | Authors | CA, AA | Canada | 30 |
| Magnolia warbler* | Setophaga magnolia | 34 | Authors | CA, AA | Canada | 33 |
| Manx shearwater | Puffinus puffinus | 88 | Authors | CA | Iceland | 23 |
| Swainson’s thrush* | Catharus ustulatus | 34 | Authors | CA, AA | Canada | 29 |
| White-throated sparrow* | Zonotrichia albicollis | 34 | Authors | CA, AA | Canada | 32 |
| Yelkouan shearwater | Puffinus yelkouan | 88 | Authors | CA | France | 15 |
Species indicated with an asterisk (*) were included in the allele dataset for population genetics analyses34. The primary study, specific data source, location of the study sites and the sample size (N) is given. CA: Clock gene alleles, AA: Adcyap1 gene alleles.
Samples were genotyped using established methods54. Briefly, samples of North American species were preserved in a buffer at room temperature until extraction with the ArchivePure DNA purification kit (5 PRIME, Hilden, Germany). Then, polymorphism at Clock and Adcyap1 3′-UTR was determined as before54, with PCR products labelled with HEX (Clock), 6-FAM (Clock and Adcyap1) or TAMRA (Adcyap1) dyes. For the Common chiffchaff, genomic DNA was extracted using a standard ammonium acetate protocol. All 55 samples were successfully genotyped and analysed for length polymorphism in the poly-Q repeat of the Clock gene following previously published protocols31. For Shearwater samples, total genomic DNA was extracted from blood samples using the Speedtools® Tissue DNA Extraction kit (Biotools, Madrid, Spain) following the manufacturer’s instructions. Genotyping was subsequently performed with methods adapted from the literature31. Briefly, PCR products were generated with shearwater specific primers for the Clock gene labelled with 6-FAM or HEX, followed by fragment analysis as in54 to determine the size of the poly-Q repeat.
Data Records
The data collated during the systematic review and meta-analysis were made available to via the Zenodo repository at the time of publication. Additional inclusion and exclusion criteria were applied and a final set of 40 species (indicated by asterisk in Tables 1, 3) were included in the comparative analyses using mantel and phylogenetic generalised least squares methods to test for an association between migratory phenotypes and candidate gene genotypes34,57. This data are available on Zenodo57, and includes a workbook with the allele data as well as a results workbook with various population genetics measures including allele frequencies, Homozygosity (Ho), Heterozygosity (He), Hardy-Weinberg equilibrium58,59, and Ewens-Watterson60 results. The complete dataset was reformatted for distribution with this data descriptor and is available from two sources, from the Figshare61 depository, as submitted with this article, and from a maintained repository with version histories on GitHub36.
Data (version 1.0.2) are available as a spreadsheet workbook, labelled “Avian Clock Gene Dataset” with multiple sheets. The first sheet of the workbook, labelled “Index”, contains the table of contents which has several columns (Table 4) that list species by common names, indicates data availability for Clock and Adcyap1, and total sample size (N). Furthermore, the taxonomic classifications including genus, species, family, superfamily, parvorder, and order are also given. The species codes are hyperlinked to the allele data for individual species, contained in separate sheets within the same workbook. Individual sheets for species contain several columns including the species name, sample ID, and diploid alleles for Clock and/or Adcyap1 genes. Alleles are expressed as the number of polyglutamine repeats (QN) for Clock while the Adcyap1 alleles represent the amplified fragment length in base pairs (bp). The sum and average of alleles is also provided, and missing data is labelled as NA. For the purpose of individual species analyses, the species sheets from the workbook are also provided as individual comma separated value (CSV) files. The same data is also available on GitHub with the workbooks available in the root directory while the individual CSV files are available in a subfolder with the title “CSV”. The repository also contains a “README” file which provides some basic background and details on the data. Both the workbook as well as CSV files can be read by Microsoft® Office (https://www.office.com/) as well as StarOffice™ (https://www.staroffice.com/), OpenOffice™ (https://www.openoffice.org/), and LibreOffice™ (https://www.libreoffice.org/).
Table 4.
Description of field names and data for workbook and CSV files.
| Field name | Data |
|---|---|
| General (Index): | |
| Species | Common name in English for species |
| Clock | Logical binary for data availability of Clock gene e.g., “Yes” or “No” |
| Adcyap1 | Logical binary for data availability of Adcyap1 gene e.g., “Yes” or “No” |
| Code | Abbreviation used for species tabs |
| Sample (N) | Size (N) of the total individuals for which data are available |
| Taxonomy (Index): | |
| Genus | Latin name for genus e.g., “Hirundo” |
| Species | Latin name for species e.g., “rustica” |
| Family | Latin name for family e.g., “Hirundinidae” |
| Superfamily | Latin name for superfamily e.g., “Locustelloidea” |
| Parvorder | Latin name for parvorder e.g., “Sylviida” |
| Order | Latin name for order e.g., “Passeriformes” |
| Species sheet: | |
| Species | Common name in English for species |
| Sample ID | Sample ID used in raw data for individuals |
| Clock 1 | 1st diploid allele for Clock gene (individual) as QN |
| Clock 2 | 2nd diploid allele for Clock gene (individual) as QN |
| Sum | Sum of two alleles for Clock gene as QN |
| Mean | Mean value of diploid alleles for Clock gene as QN |
| Adcyap 1 | 1st diploid allele for Adcyap1 gene (individual) in base pairs (bp) |
| Adcyap 2 | 2nd diploid allele for Adcyap1 gene (individual) in base pairs (bp) |
| Sum | Sum of two alleles for Adcyap1 gene in base pairs (bp) |
| Mean | Mean value of diploid alleles for Adcyap1 gene in base pairs (bp) |
Technical Validation
Allele data comprises the heterozygous or homozygous diploid allele for one or both studied clock genes as well as the sum and average of allele sizes. The data for Clock was normalized according to the poly-glutamine repeat size (QN) by subtracting the conserved non-repeat size (LC) in base pairs from the total fragment size (LT) and dividing by codon size, following Eq. 1.
| 1 |
Data for Adcyap1 was generated using the same published primers and was kept as the total fragment size.
Acknowledgements
Open access provided by the library of University of the Free State (Grant number: 13560591). The authors would like to thank Chris Guglielmo, Yolanda Morbey, and Ivan Maggini for support with field data collection at Long Point Bird Observatory (Canada), Staffan Bensch for field data collection in both Sweden and Kazakhstan, and Andrea Galimberti, Cristina Possenti, Fernando Spina and the late Nicola Saino for support with genetic analyses and field data collection in Ventotene Island (Italy). Images were created/edited in BioRender.com. This work is based on the research supported wholly/in part by the National Research Foundation of South Africa (Grant Number: 112062). The study received partial financial support from the PRIN 2017 funding scheme (grant number 20178T2PSW to Diego Rubolini), from the ICREA Academia award (to Jacob González-Solís), and from the Ministerio de Ciencia e Innovación, Agencia Estatal de Investigación, España (PID2021-125792NB-I00 to Marta Riutort).
Author contributions
Le Clercq, L.S.: Conceptualisation, Methodology, Data Curation, Formal Analysis, Writing – Original Draft, Writing – Review & Editing, Funding acquisition. Bazzi, G.: Resources, Data Curation. Ferrer Obiol, J.: Resources, Data Curation, Writing - Review & Editing. Cecere, J.G.: Resources, Data Curation, Funding acquisition. Gianfranceschi, L.: Resources, Data Curation. Grobler, J.P.: Writing – Review & Editing, Resources, Supervision. Kotzé, A.: Writing – Review & Editing, Resources, Supervision. Riutort León, M.: Resources, Data Curation. González-Solís, J.: Resources, Data Curation. Rubolini, D.: Resources, Data Curation, Writing - Review & Editing, Funding acquisition. Liedvogel, M.: Data Curation, Writing - Review & Editing. Dalton, D.L.: Conceptualisation, Writing – Review & Editing, Resources, Supervision.
Code availability
The custom R code used to convert data retrieved from Scopus to the appropriate format for visualisation in CitNetExplorer is available from GitHub (https://github.com/MichaelBoireau/Scopus2CitNet). The custom PYTHON script used for plotting the scientometric aspects of the included literature is also available from GitHub (https://github.com/LSLeClercq/ABCal).
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/13/2024
A Correction to this paper has been published: 10.1038/s41597-024-03352-7
References
- 1.Leclerc B, et al. Photoperiodic modulation of clock gene expression in the avian premammillary nucleus. J. Neuroendocrinol. 2010;22:119–128. doi: 10.1111/j.1365-2826.2009.01942.x. [DOI] [PubMed] [Google Scholar]
- 2.Pancerasa M, Ambrosini R, Saino N, Casagrandi R. Barn swallows long-distance migration occurs between significantly temperature-correlated areas. Sci. Rep. 2018;8:12359. doi: 10.1038/s41598-018-30849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saino N, et al. Temperature and rainfall anomalies in Africa predict timing of spring migration in trans-Saharan migratory birds. Clim. Res. 2007;35:123–134. doi: 10.3354/cr00719. [DOI] [Google Scholar]
- 4.Studds CE, Marra PP. Linking fluctuations in rainfall to nonbreeding season performance in a long-distance migratory bird, Setophaga ruticilla. Clim. Res. 2007;35:115–122. doi: 10.3354/cr00718. [DOI] [Google Scholar]
- 5.Hau M, Gwinner E. Food as a circadian zeitgeber for house sparrows: The effect of different food access durations. J. Biol. Rhythms. 1996;11:196–207. doi: 10.1177/074873049601100302. [DOI] [PubMed] [Google Scholar]
- 6.Sockman KW, Hurlbert AH. How the effects of latitude on daylight availability may have influenced the evolution of migration and photoperiodism. Funct. Ecol. 2020;34:1752–1766. doi: 10.1111/1365-2435.13578. [DOI] [Google Scholar]
- 7.Norevik G, Åkesson S, Andersson A, Bäckman J, Hedenström A. The lunar cycle drives migration of a nocturnal bird. PLoS Biol. 2019;17:e3000456. doi: 10.1371/journal.pbio.3000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyle P, Nur N, Henderson RP, DeSante DF. The Effects of Weather and Lunar Cycle on Nocturnal Migration of Landbirds at Southeast Farallon Island, California. Condor. 1993;95:343. doi: 10.2307/1369357. [DOI] [Google Scholar]
- 9.Roques S, et al. When to depart from a stopover site? Time since arrival matters more than current weather conditions. Ornithology. 2022;139:1–13. doi: 10.1093/ornithology/ukab057. [DOI] [Google Scholar]
- 10.Aguilar-Roblero, R., Díaz-Muñoz, M. & Fanjul-Moles, M. L. Mechanisms of circadian systems in animals and their clinical relevance. Mechanisms of Circadian Systems in Animals and Their Clinical Relevance. 10.1007/978-3-319-08945-4 (Springer International Publishing, 2015).
- 11.Fry, H., Kirwan, G. M. & Boesman, P. F. D. Lilac-breasted Roller (Coracias caudatus). in Birds of the World (eds. del Hoyo, J., Elliott, A., Sargatal, J., Christie, D. & de Juana, E.) 10.2173/bow.librol2.01.1 (Cornell Lab of Ornithology, 2021).
- 12.Woodall, P. F. Woodland Kingfisher (Halcyon senegalensis), version 1.0. in Birds of the World (eds. et al). 10.2173/BOW.WOOKIN1.01 (Cornell Lab of Ornithology, 2020).
- 13.Fry, H. & Boesman, P. F. D. European Bee-eater (Merops apiaster), version 1.0. in Birds of the World (eds. del Hoyo, J., Elliott, A., Sargatal, J., Christie, D. A. & de Juana, E.). 10.2173/BOW.EUBEAT1.01 (Cornell Lab of Ornithology, 2020).
- 14.Finch T, et al. A pan-European, multipopulation assessment of migratory connectivity in a near-threatened migrant bird. Divers. Distrib. 2015;21:1051–1062. doi: 10.1111/ddi.12345. [DOI] [Google Scholar]
- 15.Mwale M, et al. Genetic and morphological variation of Woodland Kingfisher Halcyon senegalensis reveals cryptic mitochondrial lineages and patterns of mitochondrial–nuclear discordance. Ostrich. 2022;93:192–207. doi: 10.2989/00306525.2022.2066215. [DOI] [Google Scholar]
- 16.Dor R, et al. Clock gene variation in tachycineta swallows. Ecol. Evol. 2012;2:95–105. doi: 10.1002/ece3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegemann A, Fudickar AM, Nilsson JÅ. A physiological perspective on the ecology and evolution of partial migration. J. Ornithol. 2019 1603. 2019;160:893–905. [Google Scholar]
- 18.Sauve D, Dale CA, Tigano A, Ratcliffe LM, Friesen VL. Do candidate genes for migration and behavior explain migratory variation in bluebirds (Sialia spp.)? Wilson J. Ornithol. 2021;132:820–829. doi: 10.1676/19-00120. [DOI] [Google Scholar]
- 19.Carey C. The impacts of climate change on the annual cycles of birds. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:3321–3330. doi: 10.1098/rstb.2009.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saino N, et al. Climate change effects on migration phenology may mismatch brood parasitic cuckoos and their hosts. Biol. Lett. 2009;5:539–541. doi: 10.1098/rsbl.2009.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beresford AE, et al. Phenology and climate change in Africa and the decline of Afro-Palearctic migratory bird populations. Remote Sens. Ecol. Conserv. 2019;5(1):55–69. doi: 10.1002/rse2.89. [DOI] [Google Scholar]
- 22.Mueller JC, Partecke J, Hatchwell BJ, Gaston KJ, Evans KL. Candidate gene polymorphisms for behavioural adaptations during urbanization in blackbirds. Mol. Ecol. 2013;22:3629–3637. doi: 10.1111/mec.12288. [DOI] [PubMed] [Google Scholar]
- 23.Güneralp B, Lwasa S, Masundire H, Parnell S, Seto KC. Urbanization in Africa: Challenges and opportunities for conservation. Environ. Res. Lett. 2018;13:015002. doi: 10.1088/1748-9326/aa94fe. [DOI] [Google Scholar]
- 24.Navarro, L. M. & Pereira, H. M. Rewilding abandoned landscapes in Europe. in Rewilding European Landscapes 3–23. 10.1007/978-3-319-12039-3_1 (2015).
- 25.Hoogendoorn G, Meintjes D, Kelso C, Fitchett J. Tourism as an incentive for rewilding: the conversion from cattle to game farms in Limpopo province, South Africa. J. Ecotourism. 2019;18:309–315. doi: 10.1080/14724049.2018.1502297. [DOI] [Google Scholar]
- 26.Delmore KE, et al. Genomic analysis of a migratory divide reveals candidate genes for migration and implicates selective sweeps in generating islands of differentiation. Mol. Ecol. 2015;24:1873–1888. doi: 10.1111/mec.13150. [DOI] [PubMed] [Google Scholar]
- 27.Saino N, et al. Inter-generational resemblance of methylation levels at circadian genes and associations with phenology in the barn swallow. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-42798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frias-Soler RC, Pildaín LV, Pârâu LG, Wink M, Bairlein F. Transcriptome signatures in the brain of a migratory songbird. Comp. Biochem. Physiol. - Part D Genomics Proteomics. 2020;34:100681. doi: 10.1016/j.cbd.2020.100681. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Tripathi V, Kumar V. Control and adaptability of seasonal changes in behavior and physiology of latitudinal avian migrants: Insights from laboratory studies in Palearctic-Indian migratory buntings. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022;337:902–918. doi: 10.1002/jez.2631. [DOI] [PubMed] [Google Scholar]
- 30.Åkesson S, et al. Timing avian long-distance migration: from internal clock mechanisms to global flights. Philos. Trans. R. Soc. B Biol. Sci. 2017;372:20160252. doi: 10.1098/rstb.2016.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnsen A, et al. Avian Clock gene polymorphism: Evidence for a latitudinal cline in allele frequencies. Mol. Ecol. 2007;16:4867–4880. doi: 10.1111/j.1365-294X.2007.03552.x. [DOI] [PubMed] [Google Scholar]
- 32.Steinmeyer C, Mueller JC, Kempenaers B. Search for informative polymorphisms in candidate genes: Clock genes and circadian behaviour in blue tits. Genetica. 2009;136:109–117. doi: 10.1007/s10709-008-9318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinmeyer C, Kempenaers B, Mueller JC. Testing for associations between candidate genes for circadian rhythms and individual variation in sleep behaviour in blue tits. Genetica. 2012;140:219–228. doi: 10.1007/s10709-012-9673-6. [DOI] [PubMed] [Google Scholar]
- 34.Le Clercq L. S., et al. Time trees and clock genes: a systematic review and comparative analysis of contemporary avian migration genetics. Biol. Rev. 2023;98:1051–1080. doi: 10.1111/brv.12943. [DOI] [PubMed] [Google Scholar]
- 35.van Eck NJ, Waltman L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics. 2017;111:1053–1070. doi: 10.1007/s11192-017-2300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Clercq, L. S. AvianClocksData: Dataset of Clock and Adcyap1 alleles for Birds. Version 1.0.2. https://github.com/LSLeClercq/AvianClocksData (2023).
- 37.O’Dea RE, et al. Preferred reporting items for systematic reviews and meta-analyses in ecology and evolutionary biology: a PRISMA extension. Biol. Rev. 2021;96:1695–1722. doi: 10.1111/brv.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022;18:e1230. doi: 10.1002/cl2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.RStudio Team. RStudio: Integrated Development Environment for R. Version 4.0.5. http://www.rstudio.com/ (2021).
- 40.R Core Team. R: A Language and Environment for Statisitical Computing. Version 1.4.1106. https://www.r-project.org/ (2020).
- 41.Le Clercq, L. S. ABCal: Author Bias Computation and Scientometric Plotting. Version 1.0.2. https://github.com/LSLeClercq/ABCal (2023).
- 42.Liedvogel M, Cornwallis CK, Sheldon BC. 2012. Data from: Integrating candidate gene and quantitative genetic approaches to understand variation in timing of breeding in wild tit populations. Dryad Dataset. [DOI] [PubMed]
- 43.Ralston J, 2019. Data from: Length polymorphisms at two candidate genes explain variation of migratory behaviors in blackpoll warblers (Setophaga striata) Dryad Dataset. [DOI] [PMC free article] [PubMed]
- 44.Saino N, 2015. Data from: Polymorphism at the Clock gene predicts phenology of long-distance migration in birds. Dryad Dataset. [DOI] [PubMed]
- 45.Liedvogel M, Szulkin M, Knowles S, Wood M, Sheldon B. 2010. Data from: Phenotypic correlates of Clock gene variation in a wild blue tit population: evidence for a role in seasonal timing of reproduction. Dryad Dataset. [DOI] [PubMed]
- 46.Bourret A, Garant D. 2015. Data from: Candidate gene-environment interactions and their relationships with timing of breeding in a wild bird population. Dryad Dataset. [DOI] [PMC free article] [PubMed]
- 47.Krist M, Munclinger P, Briedis M, Adamík P. 2021. Data from: The genetic regulation of avian migration timing: combining candidate genes and quantitative genetic approaches in a long-distance migrant. Dryad Dataset. [DOI] [PubMed]
- 48.Chakarov N, Jonker RM, Boerner M, Hoffman JI, Krüger O. 2013. Data from: Variation at phenological candidate genes correlates with timing of dispersal and plumage morph in a sedentary bird of prey. Dryad Dataset. [DOI] [PubMed]
- 49.Kuhn K, 2014. Data from: Differentiation in neutral genes and a candidate gene in the pied flycatcher: using biological archives to track global climate change. Dryad Dataset. [DOI] [PMC free article] [PubMed]
- 50.Mettler R, Segelbacher G, Martin Schaefer H. 2016. Fileset: PONE-D-15-12289. Figshare Dataset. [DOI]
- 51.Bazzi G, et al. Clock gene polymorphism and scheduling of migration: A geolocator study of the barn swallow Hirundo rustica. Sci. Rep. 2015;5:12443. doi: 10.1038/srep12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romano A, et al. Circadian genes polymorphism and breeding phenology in a resident bird, the yellow‐legged gull. J. Zool. 2018;304:117–123. doi: 10.1111/jzo.12501. [DOI] [Google Scholar]
- 53.Rohatgi, A. WebPlotDigitizer: Version 4.6. https://automeris.io/WebPlotDigitizer (2022).
- 54.Bazzi G, et al. Clock gene polymorphism, migratory behaviour and geographic distribution: a comparative study of trans-Saharan migratory birds. Mol. Ecol. 2016;25:6077–6091. doi: 10.1111/mec.13913. [DOI] [PubMed] [Google Scholar]
- 55.Bazzi G, et al. Candidate genes have sex-specific effects on timing of spring migration and moult speed in a long-distance migratory bird. Curr. Zool. 2017;63:479–486. doi: 10.1093/cz/zow103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sokolovskis K, et al. Phenotypic and genetic characterization of the East Siberian Willow Warbler (Phylloscopus trochilus yakutensis Ticehurst, 1935) in relation to the European subspecies. J. Ornithol. 2019;160:721–731. doi: 10.1007/s10336-019-01653-y. [DOI] [Google Scholar]
- 57.Le Clercq LS, 2022. Time trees and Clock genes: a Systematic Review and Comparative Analysis of Contemporary Avian Migration Genetics (Dataset) Zenodo. [DOI] [PubMed]
- 58.Hardy GH. Mendelian proportions in a mixed population. Science. 1908;28:49–50. doi: 10.1126/science.28.706.49. [DOI] [PubMed] [Google Scholar]
- 59.Weinberg, W. On the demonstration of heredity in man. Pap. Hum. Genet. (1963).
- 60.Slatkin M. An exact test for neutrality based on the Ewens sampling distribution. Genet. Res. 1994;64:71–74. doi: 10.1017/S0016672300032560. [DOI] [PubMed] [Google Scholar]
- 61.Le Clercq LS, 2023. Birds of a feather flock together—A compilation of data for two candidate genes for migration genetics. Figshare Dataset. [DOI]
- 62.Dor R, et al. Low variation in the polymorphic Clock gene poly-Q region despite population genetic structure across Barn Swallow (Hirundo rustica) populations. PLoS One. 2011;6:e0028843. doi: 10.1371/journal.pone.0028843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caprioli M, et al. Clock gene variation is associated with breeding phenology and maybe under directional selection in the migratory barn swallow. PLoS One. 2012;7:e0035140. doi: 10.1371/annotation/b738de1b-6b12-4f1b-9736-7d7e0be5c0da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parody-Merino ÁM, Battley PF, Conklin JR, Fidler AE. No evidence for an association between Clock gene allelic variation and migration timing in a long-distance migratory shorebird (Limosa lapponica baueri) Oecologia. 2019;191:843–859. doi: 10.1007/s00442-019-04524-8. [DOI] [PubMed] [Google Scholar]
- 65.Ralston J, et al. Length polymorphisms at two candidate genes explain variation of migratory behaviors in blackpoll warblers (Setophaga striata) Ecol. Evol. 2019;9:8840–8855. doi: 10.1002/ece3.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liedvogel M, Szulkin M, Knowles SCL, Wood MJ, Sheldon BC. Phenotypic correlates of clock gene variation in a wild blue tit population: Evidence for a role in seasonal timing of reproduction. Mol. Ecol. 2009;18:2444–2456. doi: 10.1111/j.1365-294X.2009.04204.x. [DOI] [PubMed] [Google Scholar]
- 67.Liedvogel M, Cornwallis CK, Sheldon BC. Integrating candidate gene and quantitative genetic approaches to understand variation in timing of breeding in wild tit populations. J. Evol. Biol. 2012;25:813–823. doi: 10.1111/j.1420-9101.2012.02480.x. [DOI] [PubMed] [Google Scholar]
- 68.Krist M, Munclinger P, Briedis M, Adamík P. The genetic regulation of avian migration timing: combining candidate genes and quantitative genetic approaches in a long-distance migrant. Oecologia. 2021;196:373–387. doi: 10.1007/s00442-021-04930-x. [DOI] [PubMed] [Google Scholar]
- 69.de Almeida Miranda D, et al. Shorebirds’ Longer Migratory Distances Are Associated With Larger ADCYAP1 Microsatellites and Greater Morphological Complexity of Hippocampal Astrocytes. Front. Psychol. 2022;12:6679. doi: 10.3389/fpsyg.2021.784372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chakarov N, Jonker RM, Boerner M, Hoffman JI, Krüger O. Variation at phenological candidate genes correlates with timing of dispersal and plumage morph in a sedentary bird of prey. Mol. Ecol. 2013;22:5430–5440. doi: 10.1111/mec.12493. [DOI] [PubMed] [Google Scholar]
- 71.Saino N, et al. Polymorphism at the Clock gene predicts phenology of long-distance migration in birds. Mol. Ecol. 2015;24:1758–1773. doi: 10.1111/mec.13159. [DOI] [PubMed] [Google Scholar]
- 72.Peterson MP, et al. Variation in candidate genes CLOCK and ADCYAP1 does not consistently predict differences in migratory behavior in the songbird genus Junco. F1000Research. 2013;2:115. doi: 10.12688/f1000research.2-115.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mueller JC, Pulido F, Kempenaers B. Identification of a gene associated with avian migratory behaviour. Proc. R. Soc. B Biol. Sci. 2011;278:2848–2856. doi: 10.1098/rspb.2010.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mettler R, Segelbacher G, Schaefer HM. Interactions between a Candidate Gene for Migration (ADCYAP1), Morphology and Sex Predict Spring Arrival in Blackcap Populations. PLoS One. 2015;10:e0144587. doi: 10.1371/journal.pone.0144587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuhn K, et al. Differentiation in neutral genes and a candidate gene in the pied flycatcher: Using biological archives to track global climate change. Ecol. Evol. 2013;3:4799–4814. doi: 10.1002/ece3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bazzi G, et al. Adcyap1 polymorphism covaries with breeding latitude in a Nearctic migratory songbird, the Wilson’s warbler (Cardellina pusilla) Ecol. Evol. 2016;6:3226–3239. doi: 10.1002/ece3.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Contina A, Bridge ES, Ross JD, Shipley JR, Kelly JF. Examination of Clock and Adcyap1 gene variation in a neotropical migratory passerine. PLoS One. 2018;13:e0190859. doi: 10.1371/journal.pone.0190859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bourret A, Garant D. Candidate gene-environment interactions and their relationships with timing of breeding in a wild bird population. Ecol. Evol. 2015;5:3628–3641. doi: 10.1002/ece3.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Justen H, et al. Population-specific association of Clock gene polymorphism with annual cycle timing in stonechats. Sci. Rep. 2022;12:1–13. doi: 10.1038/s41598-022-11158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang S, Xu X, Wang W, Yang W, Liang W. Clock gene is associated with individual variation in the activation of reproductive endocrine and behavior of Asian short toed lark. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-15064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Dongen WFD, Robinson RW, Weston MA, Mulder RA, Guay PJ. Variation at the DRD4 locus is associated with wariness and local site selection in urban black swans. BMC Evol. Biol. 2015;15:253. doi: 10.1186/s12862-015-0533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toews DPL, Taylor SA, Streby HM, Kramer GR, Lovette IJ. Selection on VPS13A linked to migration in a songbird. Proc. Natl. Acad. Sci. USA. 2019;116:18272–18274. doi: 10.1073/pnas.1909186116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liedvogel M, Sheldon BC. Low variability and absence of phenotypic correlates of Clock gene variation in a great tit Parus major population. J. Avian Biol. 2010;41:543–550. doi: 10.1111/j.1600-048X.2010.05055.x. [DOI] [Google Scholar]
- 84.Korsten P, et al. Association between DRD4 gene polymorphism and personality variation in great tits: A test across four wild populations. Mol. Ecol. 2010;19:832–843. doi: 10.1111/j.1365-294X.2009.04518.x. [DOI] [PubMed] [Google Scholar]
- 85.Mueller JC, et al. Haplotype structure, adaptive history and associations with exploratory behaviour of the DRD4 gene region in four great tit (Parus major) populations. Mol. Ecol. 2013;22:2797–2809. doi: 10.1111/mec.12282. [DOI] [PubMed] [Google Scholar]
- 86.Edwards HA, Hajduk GK, Durieux G, Burke T, Dugdale HL. No association between personality and candidate gene polymorphisms in a wild bird population. PLoS One. 2015;10:e0138439. doi: 10.1371/journal.pone.0138439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Posliff, C. D. Correlations in movement behaviour over large and small geographic scales in song sparrows (Melospiza melodia). (Western University of Ontario, 2020).
- 88.Ferrer Obiol, J. New insights into the genetic base of bird migration: a population genetics study based on candidate genes. (University of Barcelona, 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Liedvogel M, Cornwallis CK, Sheldon BC. 2012. Data from: Integrating candidate gene and quantitative genetic approaches to understand variation in timing of breeding in wild tit populations. Dryad Dataset. [DOI] [PubMed]
- Ralston J, 2019. Data from: Length polymorphisms at two candidate genes explain variation of migratory behaviors in blackpoll warblers (Setophaga striata) Dryad Dataset. [DOI] [PMC free article] [PubMed]
- Saino N, 2015. Data from: Polymorphism at the Clock gene predicts phenology of long-distance migration in birds. Dryad Dataset. [DOI] [PubMed]
- Liedvogel M, Szulkin M, Knowles S, Wood M, Sheldon B. 2010. Data from: Phenotypic correlates of Clock gene variation in a wild blue tit population: evidence for a role in seasonal timing of reproduction. Dryad Dataset. [DOI] [PubMed]
- Bourret A, Garant D. 2015. Data from: Candidate gene-environment interactions and their relationships with timing of breeding in a wild bird population. Dryad Dataset. [DOI] [PMC free article] [PubMed]
- Krist M, Munclinger P, Briedis M, Adamík P. 2021. Data from: The genetic regulation of avian migration timing: combining candidate genes and quantitative genetic approaches in a long-distance migrant. Dryad Dataset. [DOI] [PubMed]
- Chakarov N, Jonker RM, Boerner M, Hoffman JI, Krüger O. 2013. Data from: Variation at phenological candidate genes correlates with timing of dispersal and plumage morph in a sedentary bird of prey. Dryad Dataset. [DOI] [PubMed]
- Kuhn K, 2014. Data from: Differentiation in neutral genes and a candidate gene in the pied flycatcher: using biological archives to track global climate change. Dryad Dataset. [DOI] [PMC free article] [PubMed]
- Mettler R, Segelbacher G, Martin Schaefer H. 2016. Fileset: PONE-D-15-12289. Figshare Dataset. [DOI]
- Le Clercq LS, 2022. Time trees and Clock genes: a Systematic Review and Comparative Analysis of Contemporary Avian Migration Genetics (Dataset) Zenodo. [DOI] [PubMed]
- Le Clercq LS, 2023. Birds of a feather flock together—A compilation of data for two candidate genes for migration genetics. Figshare Dataset. [DOI]
Data Availability Statement
The custom R code used to convert data retrieved from Scopus to the appropriate format for visualisation in CitNetExplorer is available from GitHub (https://github.com/MichaelBoireau/Scopus2CitNet). The custom PYTHON script used for plotting the scientometric aspects of the included literature is also available from GitHub (https://github.com/LSLeClercq/ABCal).