Abstract
Objective:
The goal of this study was to look at quinolone-resistant (QR) Escherichia coli (E. coli) from retail beef and poultry meat in Egypt by looking at the QR mechanisms in the resistant strains.
Materials and Methods:
In total, 120 samples of raw poultry meat (n = 60) and beef meat (n = 60) were purchased from Mansoura retail stores between January and March 2021, and evaluated microbiologically for E. coli. Then, an antimicrobial sensitivity test was applied to all isolates. The prevalence of QR E. coli with concern for the QR determinants, including quinolone resistance-determining regions (QRDRs) mutations, the plasmid-mediated quinolone resistance gene (PMQR), and the efflux pump activity were determined.
Results:
The total prevalence of E. coli was 34.2% (41/120). Noticeably, the prevalence of E. coli in poultry meat (40%, 24/60) was higher than that of beef (28%, 17/60). All strains were assessed for their antimicrobial susceptibility using the disc diffusion technique; the highest rate of resistance (100%) was displayed to clindamycin and cefuroxime, followed by ampicillin (97.6%), doxycycline (92.7%), amoxicillin-clavulanate (92.7%), nalidixic acid (NA) (80.5%), sulfamethoxazole/trimethoprim (70.7%), chloramphenicol (63.4%), gentamicin, and azithromycin (58.5% each). Multiple antimicrobial resistance (strains resistant to three or more antimicrobial classes) was displayed by 97.6% of E. coli isolates. Regarding QR, 37 isolates could resist at least one of the examined quinolones. Regarding PMQR genes, qnrS was determined in 70% (7/10) of QR E. coli, while qnrA, qnrB, and qnrD were not identified. While the mutations determined regions of QR in the resistant E. coli isolates, S83L was the most prevalent in gyrase subunit A either alone or combined with D87N and D87Y, and three isolates of QR E. coli isolates revealed a topoisomerase IV subunit mutation harboring S80I. 20% of the isolates displayed efflux activity, as NA showed a considerable difference between its zones of inhibition.
Conclusion:
The high prevalence of antimicrobial-resistant E. coli, with concern for QR strains harboring different resistance mechanisms in poultry meat and beef, threatens the public’s health. Thus, standard manufacturing procedures and adequate hygiene conditions must be followed in all phases of meat preparation, production, and consumption, and public knowledge should be improved.
Keywords: Escherichia coli, retail poultry meat, beef, quinolone-resistant determinants, PMQR
Introduction
Antibiotics are routinely used in intensive farming systems, resulting in gene reservoirs for antimicrobial resistance that could transfer to other hosts or the environment. As a matter of truth, antibiotics are routinely used in an unsustainable way as precautionary preventative procedures, for mass therapy without a precise diagnosis, or for infections that can be avoided [1]. Antimicrobial resistance develops in pathogenic and commensal microorganisms due to their use in food-producing animals [2]. Horizontal gene transfer can transfer resistance genes carried on mobile genetic materials to the human flora during the human body’s transit or colonization [3,4]. As a result, the human flora, containing microorganisms that are possibly hazardous to humans, such as nosocomial pathogens, may become resistant [5]. Furthermore, given the tremendous selection pressure of continual antimicrobial medication use, new [multidrug-resistant (MDR)] variants may colonize animals. Prospective variants could be highly virulent and better suited to humans, creating a public health risk [6].
The gastrointestinal system, in particular, is a hotspot for horizontal gene transfer between species and within species. Escherichia coli and Enterococcus spp., for example, are well-known inhabitants of the mammalian gastrointestinal tract and have been demonstrated to be effective suppliers and recipients of antibiotic resistance determinants [7]. As a result, these resistance genes constitute an indirect threat.
Resistance determinants are usually present on portable genetic materials, as well as the high bacterial load in the intestine is advantageous for genetic translocation; hence, food is likely to have a role in commensal bacteria resistance transmission. Transmission can happen through the exposure or ingestion of tainted foods, which can expose the public and food handlers. Furthermore, food handlers may act as reservoirs, resulting in widespread foodborne outbreaks. The extent to which individuals are subjected to foods infected with antimicrobial-resistant bacteria is determined by various factors that might increase or decrease the bacterial load, as well as hygiene precautions implemented during food processing, shipping, and preparation. Antimicrobial-resistant bacteria can be transmitted through surfaces, hands, equipment, and so on, from one food to another [8].
The fluoroquinolone antimicrobial agent class has gained widespread acceptability among patients in hospitals and those residing in the community, and its use seems to be on the rise [9]. Because they directly block DNA synthesis, fluoroquinolones (and prior quinolones) are unique among antibacterial drugs in clinical usage. The medicine appears to inhibit DNA gyrase and topoisomerase IV by binding with combinations containing DNA and one of the two specific enzymes. During the topoisomerization phase, fluoroquinolones appear to trap the enzyme on DNA, producing a physical barrier to replication fork [10], RNA polymerases in addition to helicase movement in the transcription process [11].
Resistance to quinolones is caused by three mechanisms: drug-targeting mutations, drug-accumulation mutations, and plasmids that shield cells from the toxic impacts of quinolones [12]. fluoroquinolones´ resistance in Gram-negative bacteria is linked to a decrease in porins and drug accumulation. However, measures of diffusion rates imply that porin reductions alone are not enough for resistance to show [13].
Bacteria, which resist different antimicrobials and pass naturally from vertebrates into people, also can pose human illness and are a direct threat to human health. In Egypt, there is limited information on quinolone-resistant (QR) E. coli recovered from various food samples. Because of their dissemination as opportunistic pathogens, the constant rise in the presence rate of QR E. coli isolates is especially concerning and demonstrates the importance of expanding our research of their origins, reservoirs, and transmission routes. Thus, this study aimed to investigate the prevalence of QR E. coli in retail beef and poultry meat in retail stores located in Mansoura, Egypt. Moreover, for determination of QR determinants in the resistant E. coli isolates.
Materials and Methods
Samples collection
In total, 120 samples of meat consisting of retail poultry meat (n = 60) and fresh raw beef (n = 60) were collected from retail stores located in Mansoura, Egypt between February and April 2021. These samples were gathered and labeled in tightly closed plastic bags and transferred in an ice box under aseptic conditions to the Bacteriology, Mycology, and Immunology Department Laboratory, Faculty of Veterinary Medicine at Mansoura University, Mansoura to further bacteriological investigations.
Isolation and identification
About 25 gm of every sample were suspended in 225 ml of Tryptone Soya Broth (TSB; Oxoid, UK) and incubated for 18–24 h at 37°C. A loopful from each pre-enriched broth was streaked on Eosin methylene blue (EMB) medium (Oxoid) and then incubated for 24 h at 37°C. Presumed colonies (green with metallic shin) were picked up and purified on Tryptone Soya Agar (TSA; Oxoid, UK) and biochemically characterized according to MacWilliams [14]. Subsequently, retrieved E. coli isolates were confirmed by PCR targeting 16S rRNA.
Molecular identification of E. coli isolates
Genomic DNA was extracted following Ramadan et al. [15]. In brief, three to five E. coli colonies were inoculated into TSB and then incubated for 18 h at 37°C. One milliliter of that overnight bacterial culture was separated using centrifugation for 2 min at 10,000×g. The sediment was cleared with DNA/RNA-free water, homogenized, and then boiled for 15 min at 95°C. The supernatants from boiled lysates were employed as DNA templates. The recovered DNA templates were adjusted to a concentration of 100 ng/l using a Nanodrop (Nanodrop 1000, Thermo Scientific, UK). PCR directed at the region of 16S ribosomal RNA was done to confirm E. coli, and a uniplex PCR assay was conducted using the following primer: F: GACCTCGGTTTAGTTCACAGA and R: CACACGCTGACGCTGACCA using the cyclic condition illustrated in Table 1 as described previously by the referred author [16].
Table 1. List of oligonucleotide primers used in this study.
| Gene | Primer name | Primer sequence (5¢→3¢) | (bp) | Reference |
|---|---|---|---|---|
| 16S rRNA | 16S-F | F: GACCTCGGTTTAGTTCACAGA | 585 | [16] |
| 16S-R | R: CACACGCTGACGCTGACCA | |||
| DNA gyrA | gyrA-F | F: TACACCGGTCAACATTGAGG | 648 | [20] |
| gyrA-R | R: TTAATGATTGCCGCCGTCGG | |||
| Topoisomerase IV subunit C | Par C-F | F: AAACCTGTTCAGCGCCGCATT | 395 | [22] |
| Par C-R | R: GTGGTGCCGTTAAGCAAA | |||
| PMQR | qnrS-F | F: ACGACATTCGTCAACTGCAA | 417 | [25] |
| qnrS-R | R: TAAATTGGCACCCTGTAGGC | |||
| QnrA-F | F: TCAGCAAGAGGATTTCTCA | 627 | [23] | |
| QnrA_R | R: GGCAGCACTATTACTCCCA | |||
| QnrB-F | F: CGACCTGAGCGGCACTGAAT | 515 | [24] | |
| QnrB-R | R: TGAGCAACGATGCCTGGTAG | |||
| QnrD-F | F:CGAGATCAATTTACGGGGAATA | 582 | [26] | |
| QnrD-R | R: AACAAGCTGAAGCGCCTG |
Antimicrobial susceptibility test
To determine antibiotic resistance phenotypic profiles, the Clinical and Laboratory Standards Institute’s (CLSI) instructions for the diffusion technique were followed [17]. Antimicrobial susceptibility tests were conducted using such antimicrobial discs (Bioanalyze/Turkey): amoxicillin-clavulanate (AMC, 30 µg), cefuroxime (CXM; 30 µg), ampicillin (AM; 10 µg), chloramphenicol (C; 30 µg), gentamicin (CN; 10 µg), doxycycline (DO; 30 µg), clindamycin (DA; 2 µg), azithromycin (AZM; 15 µg), sulfamethoxazole/trimethoprim (SXT; 25 µg), nalidixic acid (NA; 30 µg), norfloxacin (NOR; 10 µg), ciprofloxacin (CIP; 5 µg), and levofloxacin (LEV; 5 µg). Escherichia coli ATCC 25922 was used in the study as a quality assurance. MDR E. coli isolates exhibit resistance to over three distinct antibiotic classes [18]. In addition, the MAR index was calculated using the approach given by Osundiya et al. [19], which involves the antibiotics number that an isolate showed resistance (a) divided by the total antibiotics number utilized in this research (b). The following is the calculation formula: MAR index = a/b.
Detection for the determining regions of the QR
QR was assessed through DNA gyrase subunit A (gyrA) as well as topoisomerase IV subunit C (parC) mutation analysis of the quinolones’ resistance determining regions (QRDR). The gyrA and parC genes in the areas that determine QR were amplified using PCR. For the gyrA gene, the forward primer’s sequence: 5¢-TAC ACC GGT CAA CAT TGA GG-3¢ and the reverse primer: 5¢-TTA ATG ATT GCC GCC GTC GG-3¢ were used. The amplification was performed in a 96-well Applied Biosystem, 2720 thermal cycler as follows: (i) a first denaturation phase lasting 4 min at 94°C, then 30 cycles of denaturation lasting 1 min at 92°C, annealing of 1 min at 64°C, and extension for 2 min at 74°C; then finally, (ii) a last extension process lasting 10 min at 74°C [20]. For parC, a PCR protocol for amplification of parC following Weigel et al. [21] was conducted using the following primers pair [22]: 5¢-AAA CCT GTT CAG CGC CGC ATT-3¢ and 5¢- GTG GTG CCG TTA AGC AAA-3¢ with an initial denaturation of 94°C lasting for 4 min, then 30 cycles of, denaturation lasting 1 min, annealing lasting 30 sec at 55°C, extension lasting 45 sec at 72°C, and lastly a final cycle running 10 min at 72°C. Amplification results were seen using an electrophorized agarose gel stained with ethidium bromide to check the gene fragment’s sizes. The purified products of PCR from both genes were subsequently subjected to sequencing.
Analysis of QRDRs
QIA quick PCR product extraction kit from Qiagen Inc. in Valencia, California, was utilized for the refinement of the PCR products. Cycle sequencing kit with Bigdye Terminator V3.1, cat-number 4336817, from the Perkin-Elmer in Foster city, California, and Applied Biosystems 3130 genetic analyzer (HITACHI, Japan) were used to do gene sequencing and analysis. In addition, kit number CS-901 of 100 reactions was utilized to purify the sequence reaction, as well as Applied Biosystems 3130 automated DNA sequencer (ABI, 3,130, United States of America). Through the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST), the Basic Local Alignment Search Tool was applied to verify the nucleotide sequences with those in the GenBank database.
Detection for plasmid-mediated quinolone resistance (PMQR) gene
Escherichia coli isolates were submitted to PCR for determination of PMQR, including qnrA [23], qnrB [24], qnrS [25], and qnrD [26] using specific primers (Metabion international/AG/Germany) shown in Table 1, and a thermal cycler condition mentioned by the referred authors was used to amplify the specific fragments. PCR products were separated by electrophoresis on a gel of agarose 1% having 0.5 mg/l of ethidium bromide and then photographed under UV light with a Gel Documentation System (Cleaver Scientific Ltd, UK).
Assessment of antibiotic sensitivity test in the availability of the efflux pump inhibitor
The antibiotic sensitivity testing of QR isolates was done in the existence and absence of the inhibitor of the efflux pump, epinephrine [27]. The broth dilution technique was employed to identify the minimal inhibitory dose of epinephrine for the isolates under research, following the CLSI guidelines [28]. At 37°C, two tubes of Muller Hinton (MH) broth (Oxoid) and MH broth containing 150 g/ml of epinephrine (MISR CO.-EGYPT) were inoculated with each strain for 24 h. On MH agar plates, a loopful of each tube was spread, antibiotic discs were inserted, and the plates were incubated at 37°C overnight. A ruler was used to measure the inhibition zones encircling the antibiotic discs. The distinction in the inhibition zones was evaluated in the existence and absence of epinephrine [29].
Results
Prevalence of E. coli isolates
In this work, 120 samples were examined for the existence of E. coli using the conventional cultural techniques stated to evaluate the prevalence of E. coli from poultry meat (n = 60) and beef meat (n = 60). Forty-one isolates (34.2%) were biochemically revealed to be E. coli from poultry meat (24/60; 40%) and beef meat samples (17/60; 28.33%). All 41 biochemically identified isolates were then confirmed as E. coli by PCR (Figure 1).
Figure 1. Escherichia coli identification targeting 16S rRNA (585 bp). Lane L: 100:3,000 bp DNA size marker, Lane 1:24, 26, 28: positive samples. Lane 25, 27: negative samples.

Antibiotic susceptibility testing
The tested isolates exhibited a remarkable resistance to DA and CXM (41/41, 100%), followed by AM (40/41, 97.6%), DO (38/41, 92.7%), AMC (38/41, 92.7%), SXT (29/41, 70.7%), and C (26/41, 63.4%), CN and AZM (24/41, 58.5% each). Regarding resistance to quinolones, 80.5% (33/41) of E. coli isolates had resistance to NA, followed by CIP (20/41, 48.78%), LEV (18/41, 43.9%), and NOR (16/41, 39%) (Table 2). Escherichia coli isolates exhibiting resistance to three or more different antimicrobial classes were termed MAR. In accordance with the prior terminology, MAR was detected in 97.6% (40/41) of the tested isolates (Table 3).
Table 2. Antimicrobial susceptibility testing results.
| Antibiotics | Family | Disc code | CPD | Chicken meat isolates (n = 24) | Beef meat isolates (n = 17) | Total (n = 41) | |||
|---|---|---|---|---|---|---|---|---|---|
| Resistant | Sensitive | Resistant | Sensitive | Resistant | Sensitive | ||||
| AM | β-lactam | AM | 10 | 24 (100%) | 0 (0.00%) | 16 (94%) | 1 (6%) | 40 (97.6%) | 1 (2.4%) |
| AMC | Β-lactams | AMC | 30 | 24 (100%) | 0 (0.00%) | 14 (82.4%) | 3 (17.6%) | 38 (92.7%) | 3 (7.3%) |
| CN | Aminoglycoside | CN | 10 | 18 (75%) | 6 (25%) | 6 (35.3%) | 11 (65.7%) | 24 (58.5%) | 17 (41.5%) |
| DO | Tetracycline | DO | 30 | 23 (95.8%) | 1(4.2%) | 15 (88.2%) | 2 (11.8%) | 38 (92.7%) | 3 (7.3%) |
| AZM | Macrolide | AZM | 15 | 16 (66.7) | 8 (33.3%) | 8 (47%) | 9 (53%) | 24 (58.5%) | 17 (41.5%) |
| C | Phenicols | C | 30 | 22 (91.7%) | 2 (8.3%) | 4 (23.5%) | 13 (76.5%) | 26 (63.4%) | 15 (36.6%) |
| DA | Lincosamide | DA | 2 | 24 (100%) | 0 (0.00%) | 17 (100%) | 0 (0.00%) | 41 (100%) | 0 (0.00) |
| SXT | Sulphonamide | SXT | 25 | 23 (95.8%) | 1 (4.2%) | 6 (35.3%) | 11 (65.7%) | 29 (70.7%) | 12 (29.3%) |
| CXM | Cephalosporin | CXM | 30 | 24 (100%) | 0 (0.00%) | 17 (100%) | 0 (0.00%) | 41 (100%) | 0 (0.00%) |
| NA | Quinolones | NA | 30 | 23 (95.8%) | 1 (4.2%) | 10 (58.8%) | 7 (41.2%) | 33(80.5%) | 8 (19.5%) |
| NOR | Fluoroquinolone | NOR | 10 | 12 (50%) | 12 (50%) | 4 (23.5%) | 13 (76.5%) | 16 (39%) | 25 (61%) |
| CIP | Fluoroquinolone | CIP | 5 | 13 (54.2%) | 11 (45.8%) | 7 (41%) | 10 (59%) | 20 (48.78%) | 21 (51.22%) |
| LEV | Fluoroquinolone | LEV | 5 | 10 (41.7%) | 14 (58.3%) | 8 (47%) | 9 (53%) | 18 (43.9%) | 23 (56.1%) |
Table 3. Pattern of antimicrobial susceptibility testing.
| Pattern | Resistance pattern | Isolates no.(%) N = 41 | MAR index |
|---|---|---|---|
| 1 | AMC, AM, DO, AZM, C, SXT, CXM, CN, DA, NA | 4(10%) | 0.77 |
| 2 | AMC, AM, DO, AZM, C, SXT, CXM, CN, DA, NA, NOR | 1(2.4%) | 0.85 |
| 3 | AMC, AM, DO, AZM, C, SXT, CXM, CN, DA, NA, NOR, LEV | 1(2.4%) | 0.92 |
| 4 | AMC, AM, DO, AZM, C, SXT, CXM, CN, DA, NA, CIP, LEV | 1(2.4%) | 0.92 |
| 5 | AMC, AM, DO, AZM, C, SXT, CXM, CN, DA, NA, NOR, CIP, LEV | 6(15%) | 1 |
| 6 | AMC, AM, DO, C, SXT, CXM, CN, DA, NA | 2(5%) | 0.69 |
| 7 | AMC, AM, DO, C, SXT, CXM, CN, DA, NA, CIP | 1(2.4%) | 0.77 |
| 8 | AMC, AM, DO, C, SXT, CXM, CN, DA, NA, NOR, CIP, LEV | 1(2.4%) | 0.92 |
| 9 | AMC, AM, DO, C, SXT, CXM, AZM, DA, NA, NOR, CIP, LEV | 2(5%) | 0.92 |
| 10 | AMC, AM, DO, C, CXM, AZM, CN, DA, NA | 1 (2.4%) | 0.69 |
| 11 | AMC, AM, DO, C, CXM, AZM, CN, DA, NA, CIP | 1(2.4%) | 0.77 |
| 12 | AMC, AM, DO, SXT, CXM, AZM, DA, NA | 1(2.4%) | 0.6 |
| 13 | AMC, AM, DO, SXT, CXM, AZM, DA, NA, NOR, CIP | 1(2.4%) | 0.77 |
| 14 | AMC, AM, DO, SXT, CXM, C, DA, NA | 1(2.4%) | 0.6 |
| 15 | AMC, AM, DO, SXT, CXM, C, DA, NA,NOR, CIP | 1(2.4%) | 0.77 |
| 16 | AMC, AM, SXT, CXM, C, AZM, DA, NA,NOR,CIP,LEV | 1(2.4%) | 0.85 |
| 17 | AMC, AM, DO, CXM, AZM, CN, DA, LEV | 1(2.4%) | 0.6 |
| 18 | AMC, AM, DO, CXM, AZM, CN, DA,NOR,CIP, LEV | 1(2.4%) | 0.77 |
| 19 | AMC, AM, DO, CXM, SXT, CN, DA, NA | 1 (2.4%) | 0.6 |
| 20 | AMC, AM, DO, CXM, AZM,C, DA, NA, CIP | 1(2.4%) | 0.69 |
| 21 | AMC, AM, DO, CXM, SXT, DA, NA | 1(2.4%) | 0.54 |
| 22 | AMC, AM, DO, CXM, SXT, DA, NA, CIP | 1(2.4%) | 0.6 |
| 23 | AMC, AM, DO, CXM, SXT, DA, NA, NOR, LEV | 1(2.4%) | 0.69 |
| 24 | AMC, AM, DO, CXM,CN, DA, NA, LEV | 1(2.4%) | 0.6 |
| 25 | AMC, AM, DO, CXM,CN, DA, CIP, LEV | 1(2.4%) | 0.6 |
| 26 | AM, DO, CXM, DA, NA | 1(2.4%) | 0.38 |
| 27 | AM, AMC, DO, CXM, DA, CIP, LEV | 1(2.4%) | 0.54 |
| 28 | AMC, AM, C, SXT, CXM, DA. | 1 (2.4%) | 0.46 |
| 29 | AMC, DO, AZM, CXM, DA | 1 (2.4%) | 0.38 |
| 30 | AM, DO, CXM, DA | 1 (2.4%) | 0.31 |
| 31 | CXM, DA | 1 (2.4%) | 0.15 |
Detection of QRDRs
Ten QR isolates (isolates displayed resistance to all quinolone antimicrobials used) were investigated for mutations in gyrA of QRDR (Figure 2) and parC (Figure 3) by PCR and then DNA sequencing. The results of sequencing revealed that 5 out of the 10 isolates held S83L mutation in gyrA; among them, 2 isolates harbored D87N and D87Y alterations at the same gene of gyrA at 83 and 87 residues (S83L/D87N/D87Y). The obtained sequences for mutations in DNA gyrA were submitted to GenBank under the accession numbers OM105873, OM105874, OM105875, OM105876, and OM105877. For parC, one typed mutation at the parC gene coding Ser80I was found in three isolates, and they were uploaded in the GenBank under the following accession numbers: OM105878, OM105880, and OM105882.
Figure 2. Amplification of gyrA at 648-bp in the QRDRs in QR E. coli strains. Lane L: 100:3,000-bp DNA size marker, Lane 1:10 QR E. coli strains.

Figure 3. Amplification of parC at (395-bp) in the QRDRs. Lane L: 100:3,000-bp DNA size marker, Lane 1:10 QR E. coli strains.

Determination of PMQR genes
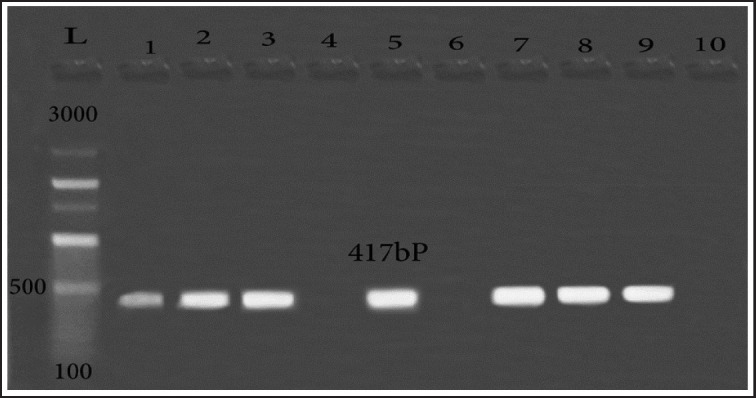
The PMQR was evaluated to explore further resistance’s mechanisms in QR E. coli isolates. The qnrs gene encoding QR was observed in 7 isolates out of the 10 resistant isolates (Figure 4). While, qnrA, qnrB, and qnrD were not determined in the examined isolates.
Figure 4. Amplification of PMQR (qnrS, 417-bp) in QR E. coli strains. Lane L: 100:3,000-bp DNA size marker, Lane 1,2,3,5,7,8,9: positive samples. Lane 4,6,10: negative samples.
The inhibitor of the efflux pump (epinephrine) influence on the antibiograms
Epinephrine, as an inhibitor of the efflux pump, was employed to evaluate the action of efflux for 10 QR isolates. The minimal inhibitory concentration (MIC) of epinephrine was found to be 500 µg/ml. Lower doses for the inhibitor of the efflux pump (150 µg/ml) than inhibitory ones were used. All E. coli strains that appeared to a specific antibiotic resistance were checked. Sensitivity tests for antibiotics were run with and without epinephrine, measuring the distinction in the inhibition zones (Abp—Ab 0) with availability or lack of epinephrine. The considerable discrepancy was 2 mm at least. (2 of 10 samples) for NA showed considerable distinctions in the zones of inhibition suggesting that it was possibly effluxed out of the cells. While 98% of the tested isolates did not show the difference in the inhibition zone (with and without the addition of epinephrine) with NA. The disparity in the inhibition zones of NOR, CIP, and LEV for all the tested isolates was determined to be insignificant, indicating that no role to the efflux pump in the resistance of these isolates.
Discussion
The spreading of Qs resistance in E. coli has considerably increased recently as a result of MDR phenotypes commonly emerge in this organism [30,31]. Accordingly, the already high health risk posed by these strains as food-chain intermediaries for antibiotic-resistance genes is greatly increased. In this study, a total of 120 poultry meat and beef samples were investigated for E. coli contamination. Escherichia coli prevalence in the examined samples was 34.2% (41/120). Noticeably, the contamination rate (40%, 24/60) of poultry meat was higher than that of beef samples (28.3%, 17/60). In agreement with our findings, Moawad et al. [32] and Belotindos et al. [33] have recorded a high rate of contamination of poultry meat compared with beef. While, Adzitey et al. [34] recorded an overall prevalence rate of 55% of E. coli from both poultry meat and beef, while, beef samples were reported to be more contaminated (80%) than poultry samples (20%).
Antibiotics are frequently administered to chickens during the raising process, both for disease prevention and treatment and for body development [35]. Penicillins, sulfonamides, tetracyclines, and quinolones are the most often utilized antibiotic classes in bred poultry [36]. Consequently, in this research, the greatest resistance frequency was detected against DA and CXM with a percentage of 100% followed by AM (97.6%), and then DO and AMC with a percentage of 92.7%, and then NA, SXT, and C with percentages of 80.5%, 70.7%, and 63.4%, respectively. In addition, 58.5% of the tested strains were resistant to CN and AZM. These findings are concerning and directly threaten human health. Antimicrobial resistance has the potential to minimize the effectiveness of first-trial zoonosis treatment and constrict postdiagnosis therapeutic options. Resistant (foodborne) bacteria strains are more likely than susceptible types to produce invasive sickness, greater mortality, and hospitalization [37].
Fluoroquinolones are synthesized drugs with a broad range of action that are frequently applied for treating bacterial infections [38]. As a result of their widespread use, fluoroquinolones resistance has emerged, raising the possibility of treatment failure [39]. In this study, 90.2% (37/41) of QR E. coli isolated were detected from both sources. Twenty-three E. coli isolates out of 24 isolates from poultry samples were resistant to quinolones (95.8%), while, 82% (14/17 isolates) of beef isolates showed QR which goes in line with Caruso et al. [1] and Belotindos et al. [33]. The highest resistance against the quinolone group was recorded against NA (80.5%) followed by CIP (48.78%), LEV (43.9%), and then NOR (39%).
Alterations in the genes encoding the relevant enzymes are known to be the most frequent mechanism of Qs resistance. Genetic analysis of selected E. coli isolates relied on the gyrA and parC genes’ sequences revealed that 5 strains carried a point mutation at gyrA with a serine to leucine change at position 83, one of the widely recorded resistance granting mutations [40]. In addition, other gyrA mutations were detected at position 87 with an aspartate to asparagine substitution and an aspartate to tyrosine substitution. Single-or double-point alterations at gyrA were recorded in this work. Regarding parC of QRDR, a serine into isoleucine substitution at position 80 was detected in 3 isolates. The combination between both single and double gyrA mutations with parC mutations has been recorded. Similarly, prior researches report that the most frequent forms of known amino acid change in E. coli were in gyrA (S83 L and D87 N) as well as parC (S80I) [40–42].
The majority of the genes that cause antibacterial resistance are found on plasmids and other portable genetic components, which can and frequently disseminate to bacteria of various genera and species. [43,44]. In this research, most of the Qs-resistant isolates (7/10) were PMQR-positive (qnrS). Similarly, previous reports worldwide have reported a high prevalence of PMQR-positive among Q-resistant E. coli [45–48] which highlights their significant contribution to acquiring Qs resistance. On the other hand, a lower prevalence of PMQRs was mentioned among QR E. coli in Egypt (26.6%) [49], Taiwan (14.9%) [50], and Germany (3.7%) [51]. According to Woyda et al. [52], chicken production isolates were more likely than human clinical isolates to carry acquired AMR genes (65.7%), and they also carried an average of more acquired AMR genes per isolate. Furthermore, Juraschek et al. [51] verified that the existence of qnr alone can result in phenotypic (fluoro) QR without the need for PMQR or point mutations in the relevant chromosomal area.
Efflux pumps, also known as MDR pumps, have been identified as a major determinant of antibiotic concentration within a bacterial cell. As a result, inhibiting efflux pumps can be used to increase the concentration of antibiotics inside a pathogenic cell, thereby increasing the efficacy of these drugs [53]. In the present study, epinephrine was used as an EPI, by investigating its effect in certain concentrations (150 mg/ml) on the inhibition zone values of selected antibiotics. 20% of the isolates revealed the efflux action indicating that this drug was probably effluxed from the cells, while, 80% of the tested isolates did not show that action with NA. NOR, CIP, and LEV showed no differences in their inhibition zones for all tested isolates, indicating that no role to the efflux pump action in the resistance. Similarly, a lower prevalence of efflux pump effect was recorded by Hooper and Jacoby [54] (12.1%), Hamed et al. [55] (18.3%), and Vieira et al. [56] among quinolone resistant (QR) isolates suggested a higher contribution of other resistance mechanisms. Porin reductions and decreased bacterial drug accumulation are linked to fluoroquinolone resistance in Gram-negative bacteria, although assessments of diffusion levels indicate that reductions of porin alone, typically are not enough to cause resistance [13,57,58].
In this study, 97.6% of the tested isolates expressed MDR phenotypes with a MAR index of more than 0.2. The highest resistance rate was recorded in 32% of these tested isolates that resisted 9 various antibiotic classes and 20% of E. coli isolates resisted 8 different antibiotic classes. While, 21%, 12%, and 10% of tested isolates displayed resistance to 7, 6, and 5 different antibiotic classes, respectively. The lowest percentage of isolates (2.5%) resist to two and four different antibiotic classes. These findings agree with those of recent investigations [33, 58–60] demonstrating greater incidence of MDR between Q-resistant E. coli. The high percentages of resistant strains isolated in this study may pose a direct risk to consumers by colonizing their intestinal tract until conditions are favorable for extraintestinal infection, or indirectly by transferring resistance genes to human commensal flora [61]. The MAR index is a reliable, valid, and affordable method for tracing the origins of antibiotic-resistant organisms [62]. A MAR of more than 0.2 indicates a great threat of contamination origin in areas where antimicrobials are routinely utilized [63]. In this investigation, the highest MDR was presented in E. coli with a MAR value of 1.00, in 17% of the tested isolates which goes in line with Ayandele et al. [64] who recorded a high MDR with a MAR index of 1.00 in 34% of E. coli isolates and Jaja et al. [65] who reported MAR indexes ranged from 0.2 to 0.5. Antimicrobial drugs used in veterinary medicine must be used responsibly and prudently to preserve both animal and human health [66]. To address the antimicrobial resistance crisis, various degrees of safety precautions must be considered such as "tertiary prevention" (improving the immune systems ability of animals to react to diseases) [67] and vaccines based on broadly preserved antigens [68,69].
Conclusion
In conclusion, personal protective precautions, such as the wearing of safety clothes such as gloves and masks, as well as the usage of basic health measures such as hand washing and showering, should be promoted to avoid the dissemination of resistant microbes from food of animal origins to humans. Furthermore, slaughterhouses in addition to food handling practices must be considered in the effort to reduce foodborne transmission, in accordance with the concept of “farm-to-fork”.
Acknowledgments
The authors appreciated the Department of Bacteriology, Mycology, and Immunology staff at the Faculty of Veterinary Medicine at Mansoura University for their support during this study.
List of Abbreviations
AM, Ampicillin; AMC, Amoxicillin-clavulanate; AZM, Azithromycin; C, Chloramphenicol; CIP, Ciprofloxacin; CLSI, Clinical and Laboratory Standards Institute’s; CN, Gentamicin; CXM, Cefuroxime; DA, Clindamycin; DO, Doxycycline;gyrA, DNA gyrase subunit; EPI, efflux pump inhibitor; LEV, Levofloxacin; MAR, multiple antibiotic resistance; MDR, Multidrug-resistant; MH, Muller Hinton; NA, Nalidixic acid; NOR, Norfloxacin; parC, Topoisomerase IV subunit; PCR, polymerase chain reaction; PMQR, Plasmid-mediated quinolone resistance gene; QIA, Qiagen; QR, Quinolone resistant; QRDRs, Quinolone resistance-determining regions; TSA, Tryptone Soya Agar; TSB, Tryptone Soya Broth; SXT, Sulfamethoxazole/trimethoprim.
Availability of data and materials
The corresponding author will supply the datasets produced and/or analyzed in the present research upon reasonable request. Sequence data for gyrA and parC genes available in the GenBank repository under the dissertation title: Studies on the mechanisms of quinolones resistance in E. coli isolated from retail poultry and meat.
Conflict of interests
According to the research authors, there is not any conflict of interest.
Authors’ contributions
Conceptualization, AA; methodology, DI; validation, AA; investigation, AA, DI, writing—original draft preparation, DI; writing—review and editing, AA; supervision, AA, GY.
References
- [1].Caruso G, Giammanco A, Cardamone C, Oliveri G, Mascarella C, Capra G, et al. Extra-intestinal fluoroquinolone-resistant Escherichia coli strains isolated from meat. BioMed Res Int. 2018;2018:1–7. doi: 10.1155/2018/8714975. https://doi.org/10.1155/2018/8714975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Varga C, Rajić A, McFall ME, Reid-Smith RJ, Deckert AE, Checkley SL, et al. Associations between reported on-farm antimicrobial use practices and observed antimicrobial resistance in generic fecal Escherichia coli isolated from Alberta finishing swine farms. Prev Vet Med. 2009;88(3):185–92. doi: 10.1016/j.prevetmed.2008.10.002. https://doi.org/10.1016/j.prevetmed.2008.10.002. [DOI] [PubMed] [Google Scholar]
- [3].Andreoletti O, Budka H, Buncic S, Colin P, Collins JD, Koeijer AD, et al. Scientific opinion of the panel on biological hazards on a request from the European food safety authority on foodborne antimicrobial resistance as a biological hazard. Eur Food Saf Authority J. 2008;765:1–87. https://doi.org/10.2903/j.efsa.2008.765. [Google Scholar]
- [4].Hunter PR, Wilkinson DC, Catling LA, Barker GC. Meta-analysis of experimental data concerning antimicrobial resistance gene transfer rates during conjugation. Appl Environ Microbiol. 2008;74(19):6085–90. doi: 10.1128/AEM.01036-08. https://doi.org/10.1128/AEM.01036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis. 2004;39(2):219–26. doi: 10.1086/422002. https://doi.org/10.1086/422002. [DOI] [PubMed] [Google Scholar]
- [6].Geenen PL, Koene MG, Blaak H, Havelaar AH, Van de Giessen AW. National Institute of Public Health and the Environment (RIVM); Bilthoven, Utrecht: 2010. Description of the micrological hazards. Risk profile on antimicrobial resistance transmissible from food animals to humans; pp. 15–21. [Google Scholar]
- [7].Barlow M. What antimicrobial resistance has taught us about horizontal gene transfer. In: Gogarten MB, Gogarten JP, Olendzenski LC, editors. Horizontal gene transfer: genomes in flux. Humana Press; Totowa, NJ: 2009. pp. 397–411. https://doi.org/10.1007/978-1-60327-853-9_23. [DOI] [PubMed] [Google Scholar]
- [8].EFSA Panel on Biological Hazards (BIOHAZ) Koutsoumanis K, Allende A, Álvarez‐Ordóñez A, Bolton D, Bover‐Cid S, et al. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA J. 2021;19(6):e06651. doi: 10.2903/j.efsa.2021.6651. https://doi.org/10.2903/j.efsa.2021.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Apley MD. Antimicrobial chemotherapy and antimicrobial resistance. Vet Microbiol. 2022;69:771–802. https://doi.org/10.1002/9781119650836.ch69. [Google Scholar]
- [10].Wentzell LM, Maxwell A. The complex of DNA gyrase and quinolone drugs on DNA forms a barrier to the T7 DNA polymerase replication complex. J Mol Biol. 2000;304(5):779–91. doi: 10.1006/jmbi.2000.4266. https://doi.org/10.1006/jmbi.2000.4266. [DOI] [PubMed] [Google Scholar]
- [11].Bush NG, Diez-Santos I, Abbott LR, Maxwell A. Quinolones: mechanism, lethality and their contributions to antibiotic resistance. Molecules. 2020;25(23):5662. doi: 10.3390/molecules25235662. https://doi.org/10.3390/molecules25235662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hooper DC. Mechanisms of quinolone resistance. Quinolone Antimicrob Agents. 2003;12:41–67. https://doi.org/10.1128/9781555817817.ch3. [Google Scholar]
- [13].Correia S, Poeta P, Hébraud M, Capelo JL, Igrejas G. Mechanisms of quinolone action and resistance: where do we stand? J Med Microbiol. 2017;66(5):551–9. doi: 10.1099/jmm.0.000475. https://doi.org/10.1099/jmm.0.000475. [DOI] [PubMed] [Google Scholar]
- [14].MacWilliams MP. American Society for Microbiology; Washington, DC: 2012. Indole test protocol. [Google Scholar]
- [15].Ramadan H, Awad A, Ateya A. Detection of phenotypes, virulence genes and phylotypes of avian pathogenic and human diarrheagenic Escherichia coli in Egypt. J Infect Dev Count. 2016;10(06):584–91. doi: 10.3855/jidc.7762. https://doi.org/10.3855/jidc.7762. [DOI] [PubMed] [Google Scholar]
- [16].Amit-Romach E, Sklan D, Uni Z. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult Sci. 2004;83(7):1093–8. doi: 10.1093/ps/83.7.1093. https://doi.org/10.1093/ps/83.7.1093. [DOI] [PubMed] [Google Scholar]
- [17].CLSI. CLSI Supplements M; Wayne, PA: 2017. CLSI performance standards for antimicrobial susceptibility testing; p. 100. [Google Scholar]
- [18].Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. doi: 10.1111/j.1469-0691.2011.03570.x. https://doi.org/10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- [19].Osundiya OO, Oladele RO, Oduyebo OO. Multiple antibiotic resistance (MAR) indices of Pseudomonas and Klebsiella species isolates in Lagos University Teaching Hospital. Afr J Clin Exp Microbiol 2013 Aug. 5;14(3):164–8. https://doi.org/10.4314/ajcem.v14i3.8. [Google Scholar]
- [20].Dutta S, Kawamura Y, Ezaki T, Nair GB, Iida KI, Yoshida SI. Alteration in the GyrA subunit of DNA gyrase and the parC subunit of topoisomerase IV in quinolone-resistant Shigella dysenteriae serotype 1 clinical isolates from Kolkata, India. Antimicrob Agents Chemother. 2005;49(4):1660. doi: 10.1128/AAC.49.4.1660-1661.2005. https://doi.org/10.1128/AAC.49.4.1660-1661.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Weigel LM, Steward CD, Tenover FC. gyrA mutations associated with fluoroquinolone resistance in eight species of Enterobacteriaceae. Antimicrob Agents Chemother. 1998;42(10):2661–7. doi: 10.1128/aac.42.10.2661. https://doi.org/10.1128/AAC.42.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fendukly F, Karlsson I, Hanson HS, Kronvall G, Dornbusch K. Patterns of mutations in target genes in septicemia isolates of Escherichia coli and Klebsiella pneumoniae with resistance or reduced susceptibility to ciprofloxacin. Apmis. 2003;111(9):857–66. doi: 10.1034/j.1600-0463.2003.1110904.x. https://doi.org/10.1034/j.1600-0463.2003.1110904.x. [DOI] [PubMed] [Google Scholar]
- [23].Cambau E, Lascols C, Sougakoff W, Bebear C, Bonnet R, Cavallo JD, et al. Occurrence of qnrA-positive clinical isolates in French teaching hospitals during 2002–2005. Clin Microbiol Infect. 2006;12(10):1013–20. doi: 10.1111/j.1469-0691.2006.01529.x. https://doi.org/10.1111/j.1469-0691.2006.01529.x. [DOI] [PubMed] [Google Scholar]
- [24].Jiang Y, Zhou Z, Qian Y, Wei Z, Yu Y, Hu S, et al. Plasmid-mediated quinolone resistance determinants qnr and aac(6’)-Ib-cr in extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in China. J Antimicrob Chemother. 2008;61(5):1003–6. doi: 10.1093/jac/dkn063. https://doi.org/10.1093/jac/dkn063. [DOI] [PubMed] [Google Scholar]
- [25].Yue L, Jiang HX, Liao XP, Liu JH, Li SJ, Chen XY, et al. Prevalence of plasmid-mediated quinolone resistance qnr genes in poultry and swine clinical isolates of Escherichia coli. Vet Microbiol. 2008;132(3–4):414–20. doi: 10.1016/j.vetmic.2008.05.009. https://doi.org/10.1016/j.vetmic.2008.05.009. [DOI] [PubMed] [Google Scholar]
- [26].Cavaco LM, Hasman H, Xia S, Aarestrup FM. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob Agents Chemother. 2009;53(2):603–8. doi: 10.1128/AAC.00997-08. https://doi.org/10.1128/AAC.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Piddock LJ, Garvey MI, Rahman MM, Gibbons S. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in Gram-negative bacteria. J Antimicrob Chemother. 2010;65(6):1215–23. doi: 10.1093/jac/dkq079. https://doi.org/10.1093/jac/dkq079. [DOI] [PubMed] [Google Scholar]
- [28].CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-10th edition, CLSI document M07-A10. Wayne PA: 2015. [Google Scholar]
- [29].Anbazhagan PV, Thavitiki PR, Varra M, Annamalai L, Putturu R, Lakkineni VR, et al. Evaluation of efflux pump activity of multidrug-resistant Salmonella typhimurium isolated from poultry wet markets in India. Infect Drug Resist. 2019;12:1081–8. doi: 10.2147/IDR.S185081. https://doi.org/10.2147/IDR.S185081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Seo KW, Lee YJ. Prevalence and characterization of β-lactamases genes and class 1 integrons in multidrug-resistant Escherichia coli isolates from chicken meat in Korea. Microbial Drug Resistance. 2018;24(10):1599–606. doi: 10.1089/mdr.2018.0019. https://doi.org/10.1089/mdr.2018.0019. [DOI] [PubMed] [Google Scholar]
- [31].Seo KW, Lee YJ. Detection of plasmid-mediated quinolone resistance genes in β-lactamase-producing Escherichia coli isolates from layer hens. Poult Sci. 2019;98(3):1480–7. doi: 10.3382/ps/pey545. https://doi.org/10.3382/ps/pey545. [DOI] [PubMed] [Google Scholar]
- [32].Moawad AA, Hotzel H, Awad O, Tomaso H, Neubauer H, Hafez HM, et al. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Path. 2017;9:1–3. doi: 10.1186/s13099-017-0206-9. https://doi.org/10.1186/s13099-017-0206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Belotindos L, Villanueva M, Miguel Jr J, Bwalya P, Harada T, Kawahara R, et al. Prevalence and characterization of quinolone-resistance determinants in Escherichia coli isolated from food-producing animals and animal-derived food in the Philippines. Antibiotics. 2021;10(4):413. doi: 10.3390/antibiotics10040413. https://doi.org/10.3390/antibiotics10040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Adzitey F, Huda N, Shariff AH. Phenotypic antimicrobial susceptibility of Escherichia coli from raw meats, ready-to-eat meats, and their related samples in one health context. Microorganisms. 2021;9(2):326. doi: 10.3390/microorganisms9020326. https://doi.org/10.3390/microorganisms9020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Haque MH, Sarker S, Islam MS, Islam MA, Karim MR, Kayesh ME, et al. Sustainable antibiotic-free broiler meat production: current trends, challenges, and possibilities in a developing country perspective. Biology. 2020;9(11):411. doi: 10.3390/biology9110411. https://doi.org/10.3390/biology9110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jacoby GA, Strahilevitz J, Hooper DC. Plasmid‐mediated quinolone resistance. Biol Impact Biotechnol Discov. 2015;25:475–503. https://doi.org/10.1128/microbiolspec.PLAS-0006-2013. [Google Scholar]
- [37].Angulo FJ, Mølbak K. Human health consequences of antimicrobial drug—resistant salmonella and other foodborne pathogens. Clin Infect Dis. 2005;41(11):1613–20. doi: 10.1086/497599. https://doi.org/10.1086/497599. [DOI] [PubMed] [Google Scholar]
- [38].Animal and Plant Quarantine Agency (QIA) Antimicrobial use and monitoring in animals and animal products. QIA; Gimcheon, South Korea: 2017. [Google Scholar]
- [39].Li Y, Chen L, Wu X, Huo S. Molecular characterization of multidrug-resistant avian pathogenicEscherichia coli isolated from septicemic broilers. Poult Sci. 2015;94(4):601–11. doi: 10.3382/ps/pev008. https://doi.org/10.3382/ps/pev008. [DOI] [PubMed] [Google Scholar]
- [40].Awad A, Arafat N, Elhadidy M. Genetic elements associated with antimicrobial resistance among avian pathogenic Escherichia coli. Ann Clin Microbiol Antimicrob. 2016;15(1):1–8. doi: 10.1186/s12941-016-0174-9. https://doi.org/10.1186/s12941-016-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Temmerman R, Garmyn A, Antonissen G, Vanantwerpen G, Vanrobaeys M, Haesebrouck F, et al. Evaluation of fluoroquinolone resistance in clinical avian pathogenic Escherichia coli isolates from Flanders (Belgium) Antibiotics. 2020;9(11):800. doi: 10.3390/antibiotics9110800. https://doi.org/10.3390/antibiotics9110800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baez M, Espinosa I, Collaud A, Miranda I, Montano DD, Feria AL, et al. Genetic features of extended-spectrum β-lactamase-producing Escherichia coli from poultry in Mayabeque Province, Cuba. Antibiotics. 2021;10(2):107. doi: 10.3390/antibiotics10020107. https://doi.org/10.3390/antibiotics10020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Son SH, Seo KW, Kim YB, Jeon HY, Noh EB, Lee YJ. Molecular characterization of multidrug-resistant Escherichia coli isolates from edible offal in Korea. J Food Protect. 2019;82(7):1183–90. doi: 10.4315/0362-028X.JFP-18-458. https://doi.org/10.4315/0362-028X.JFP-18-458. [DOI] [PubMed] [Google Scholar]
- [44].Uddin TM, Chakraborty AJ, Khusro A, Zidan BR, Mitra S, Emran TB, et al. Antibiotic resistance in microbes: history, mechanisms, therapeutic strategies and future prospects. J Infect Public Health. 2021;14(12):1750–66. doi: 10.1016/j.jiph.2021.10.020. https://doi.org/10.1016/j.jiph.2021.10.020. [DOI] [PubMed] [Google Scholar]
- [45].Piekarska K, Wołkowicz T, Zacharczuk K, Rzeczkowska M, Chróst A, Bareja E, et al. Co-existence of plasmid-mediated quinolone resistance determinants and mutations in gyrA and parC among fluoroquinolone-resistant clinical Enterobacteriaceae isolated in a tertiary hospital in Warsaw, Poland. Int J Antimicrob Agents. 2015;45(3):238–43. doi: 10.1016/j.ijantimicag.2014.09.019. https://doi.org/10.1016/j.ijantimicag.2014.09.019. [DOI] [PubMed] [Google Scholar]
- [46].Ferjani S, Saidani M, Amine FS, Boutiba-Ben Boubaker I. Prevalence and characterization of plasmid-mediated quinolone resistance genes in extended-spectrum β-lactamase-producing Enterobacteriaceae in a Tunisian hospital. Microb Drug Resist. 2015;21(2):158–66. doi: 10.1089/mdr.2014.0053. https://doi.org/10.1089/mdr.2014.0053. [DOI] [PubMed] [Google Scholar]
- [47].Ayobola ED, Oscar WO, Ejovwokoghene EF. Plasmid-mediated quinolone resistance genes transfer among enteric bacteria isolated from human and animal sources. AIMS Microbiol. 2021;7(2):200–15. doi: 10.3934/microbiol.2021013. https://doi.org/10.3934/microbiol.2021013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Haeili M, Salehzeinali H, Mirzaei S, Pishnian Z, Ahmadi A. Molecular characterization of quinolone resistance and antimicrobial resistance profiles of Klebsiella pneumoniae and Escherichia coli isolated from human and broiler chickens. Int J Environ Health Res. 2022;32(6):1382–92. doi: 10.1080/09603123.2021.1885632. https://doi.org/10.1080/09603123.2021.1885632. [DOI] [PubMed] [Google Scholar]
- [49].Hassan WM, Hashim A, Domany RA. Plasmid mediated quinolone resistance determinants qnr, aac (6¢)-Ib-cr, and qep in ESBL-producing Escherichia coli clinical isolates from Egypt. Indian J Med Microbiol. 2012;30(4):442–7. doi: 10.4103/0255-0857.103766. https://doi.org/10.4103/0255-0857.103766. [DOI] [PubMed] [Google Scholar]
- [50].Kao CY, Wu HM, Lin WH, Tseng CC, Yan JJ, Wang MC, et al. Plasmid-mediated quinolone resistance determinants in quinolone-resistant Escherichia coli isolated from patients with bacteremia in a university hospital in Taiwan, 2001–2015. Sci Rep. 2016;6(1):32281. doi: 10.1038/srep32281. https://doi.org/10.1038/srep32281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Juraschek K, Deneke C, Schmoger S, Grobbel M, Malorny B, Käsbohrer A, et al. Phenotypic and genotypic properties of fluoroquinolone-resistant, qnr-carrying Escherichia coli isolated from the German food chain in 2017. Microorganisms. 2021;9(6):1308. doi: 10.3390/microorganisms9061308. https://doi.org/10.3390/microorganisms9061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Woyda R, Oladeinde A, Abdo Z. Chicken production and human clinical Escherichia coli isolates differ in their carriage of antimicrobial resistance and virulence factors. Appl Environ Microbiol. 2023;89;(2):e01167–22. doi: 10.1128/aem.01167-22. https://doi.org/10.1128/aem.01167-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].K Bhardwaj A, Mohanty P. Bacterial efflux pumps involved in multidrug resistance and their inhibitors: rejuvinating the antimicrobial chemotherapy. Recent Patents Anti-Infect Drug Discov. 2012;7(1):73–89. doi: 10.2174/157489112799829710. https://doi.org/10.2174/157489112799829710. [DOI] [PubMed] [Google Scholar]
- [54].Hooper DC, Jacoby GA. Topoisomerase inhibitors: fluoroquinolone mechanisms of action and resistance. Cold Spring Harbor Perspect Med. 2016;6(9):a025320. doi: 10.1101/cshperspect.a025320. https://doi.org/10.1101/cshperspect.a025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hamed SM, Elkhatib WF, El-Mahallawy HA, Helmy MM, Ashour MS, Aboshanab K. Multiple mechanisms contributing to ciprofloxacin resistance among Gram negative bacteria causing infections to cancer patients. Sci Rep. 2018;8(1):1–0. doi: 10.1038/s41598-018-30756-4. https://doi.org/10.1038/s41598-018-30756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vieira DC, Lima WG, de Paiva MC. Plasmid-mediated quinolone resistance (PMQR) among Enterobacteriales in Latin America: a systematic review. Mol Biol Rep. 2020;47(2):1471–83. doi: 10.1007/s11033-019-05220-9. https://doi.org/10.1007/s11033-019-05220-9. [DOI] [PubMed] [Google Scholar]
- [57].Wong MH, Chan EW, Chen S. Evolution and dissemination of OqxAB-like efflux pumps, an emerging quinolone resistance determinant among members of Enterobacteriaceae. Antimicrob Agents Chemother. 2015;59(6):3290–7. doi: 10.1128/AAC.00310-15. https://doi.org/10.1128/AAC.00310-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bhargavi D, Sahu R, Nishanth MA, Doijad SP, Niveditha P, Kumar OR, et al. Genetic diversity and risk factor analysis of drug-resistant Escherichia coli recovered from broiler chicken farms. Comp Immunol Microbiol Infect Dis. 2023;93:101929. doi: 10.1016/j.cimid.2022.101929. https://doi.org/10.1016/j.cimid.2022.101929. [DOI] [PubMed] [Google Scholar]
- [59].Eltai NO, Yassine HM, El-Obeid T, Al-Hadidi SH, Al Thani AA, Alali WQ. Prevalence of antibiotic-resistant Escherichia coli isolates from local and imported retail chicken carcasses. J Food Protect. 2020;83(12):2200–8. doi: 10.4315/JFP-20-113. https://doi.org/10.4315/JFP-20-113. [DOI] [PubMed] [Google Scholar]
- [60].Shawa M, Furuta Y, Paudel A, Kabunda OB, Mulenga E, Mubanga M, et al. Clonal relationship between multidrug-resistant Escherichia coli ST69 from poultry and humans in Lusaka, Zambia. FEMS Microbiol Lett. 2021;368(21–24):fnac004. doi: 10.1093/femsle/fnac004. https://doi.org/10.1093/femsle/fnac004. [DOI] [PubMed] [Google Scholar]
- [61].Jakobsen L, Spangholm DJ, Pedersen K, Jensen LB, Emborg HD, Agersø Y, et al. Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. Int J Food Microbiol. 2010;142(1–2):264–72. doi: 10.1016/j.ijfoodmicro.2010.06.025. https://doi.org/10.1016/j.ijfoodmicro.2010.06.025. [DOI] [PubMed] [Google Scholar]
- [62].Sandhu R, Dahiya S, Sayal P. Evaluation of multiple antibiotic resistance (MAR) index and doxycycline susceptibility of Acinetobacter species among inpatients. Indian J Microbiol Res. 2016;3(3):299. https://doi.org/10.5958/2394-5478.2016.00064.9. [Google Scholar]
- [63].Davis R, Brown PD. Multiple antibiotic resistance index, fitness and virulence potential in respiratory Pseudomonas aeruginosa from Jamaica. J Med Microbiol. 2016;65(4):261–71. doi: 10.1099/jmm.0.000229. https://doi.org/10.1099/jmm.0.000229. [DOI] [PubMed] [Google Scholar]
- [64].Ayandele AA, Oladipo EK, Oyebisi O, Kaka MO. Prevalence of multi-antibiotic resistant Escherichia coli and Klebsiella species obtained from a tertiary medical institution in Oyo State, Nigeria. Qatar Med J. 2020;2020(1):9. doi: 10.5339/qmj.2020.9. https://doi.org/10.5339/qmj.2020.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jaja IF, Oguttu J, Jaja CJ, Green E. Prevalence and distribution of antimicrobial resistance determinants of Escherichia coli isolates obtained from meat in South Africa. PloS One. 2020;15(5):e0216914. doi: 10.1371/journal.pone.0216914. https://doi.org/10.1371/journal.pone.0216914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Anthony F, Acar J, Franklin A, Gupta R, Nicholls T, Tamura Y, et al. Antimicrobial resistance: responsible and prudent use of antimicrobial agents in veterinary medicine. Rev Sci Tech-OIE. 2001;20(3):829–48. doi: 10.20506/rst.20.3.1318. https://doi.org/10.20506/rst.20.3.1318. [DOI] [PubMed] [Google Scholar]
- [67].Maldonado NC, de Ruiz CS, Otero MC, Sesma F, Nader-Macías ME. Lactic acid bacteria isolated from young calves—characterization and potential as probiotics. Res Vet Sci. 2012;92(2):342–9. doi: 10.1016/j.rvsc.2011.03.017. https://doi.org/10.1016/j.rvsc.2011.03.017. [DOI] [PubMed] [Google Scholar]
- [68].Wieser A, Romann E, Magistro G, Hoffmann C, Nörenberg D, Weinert K, et al. A multiepitope subunit vaccine conveys protection against extraintestinal pathogenic Escherichia coli in mice. Infect Immun. 2010;78(8):3432–42. doi: 10.1128/IAI.00174-10. https://doi.org/10.1128/IAI.00174-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dobrindt U, Hacker J. Targeting virulence traits: potential strategies to combat extraintestinal pathogenic E. coli infections. Curr Opin Microbiol. 2008;11(5):409–13. doi: 10.1016/j.mib.2008.09.005. https://doi.org/10.1016/j.mib.2008.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author will supply the datasets produced and/or analyzed in the present research upon reasonable request. Sequence data for gyrA and parC genes available in the GenBank repository under the dissertation title: Studies on the mechanisms of quinolones resistance in E. coli isolated from retail poultry and meat.


