Abstract
MR elastography (MRE), first described in 1995 and FDA-cleared in 2009, has emerged as an important tool for non-invasively detecting and staging liver fibrosis in patients with known or suspected chronic liver disease. This review focuses on a series of practical questions about the clinical use of MRE. Most head-to-head comparison studies with other laboratory and imaging-based tests have concluded that MRE has the highest diagnostic performance among tests for staging liver fibrosis. Limitations in the accuracy of biopsy as a standard of truth in staging liver fibrosis are increasingly being recognized. MRE-based measurements show promise as quantitative surrogates of disease severity and predictors of important clinical outcomes. The appropriate role of MRE in management of patients with chronic liver disease is being actively incorporated into recognized clinical guidelines. Growing evidence shows that MRI measurement of elevated liver fat is the most important single biomarker for detecting non-alcoholic steatohepatitis (NASH), while MRE-based liver stiffness is the most important single biomarker for detecting at-risk NASH (i.e., NASH with stage ≥F2 fibrosis). Advances in MRE technology are offering higher precision and new biomarkers, which have potential to allow independent assessment of inflammation and other histologic processes in addition to fibrosis.
The centuries-old physical examination technique of palpation owes its diagnostic value to the fact that many disease conditions such as inflammation, fibrosis, and neoplasia cause marked localized changes in tissue stiffness. Although medical imaging technologies have been available for decades for characterizing tissue properties such as CT attenuation, ultrasound (US) echogenicity, MRI relaxation times, and water proton diffusion, the ability to quantitatively image the mechanical properties of tissue is a more recent development.
Over the last 15 years, MRI-based and US-based quantitative elastography techniques have become available. These techniques measure shear wave propagation in tissue and process the resulting data to estimate mechanical stiffness.
This review focuses on practical questions of current interest regarding the performance and clinical role of MR elastography (MRE). First described in 1995, MRE technology was cleared as a system upgrade by the U.S FDA in 2009 and is now deployed on MRI systems worldwide. Currently, the main clinical indication for MRE is detecting and staging liver fibrosis [1–3]. The presence and severity of liver fibrosis are the most important histologic findings for predicting progression and mortality in chronic liver disease [4]. Liver fibrosis may not cause changes detectable with conventional anatomic imaging until very late-stage disease develops [5]. Liver tissue stiffness is known to increase progressively with liver fibrosis severity [1–3]. MRE-based measurement of liver stiffness has emerged as a reliable and less expensive alternative to liver biopsy for detecting and staging disease.
What Does MRE Measure?
Best practices for conducting MRE examinations have been documented in the literature and in a consensus document by the Quantitative Imaging Biomarkers Alliance (QIBA) of the Radiological Society of North America [3,5]. These references provide detailed information on patient preparation and setup, assessment of examination quality, analysis, and troubleshooting. During examinations, a drum-like vibration source is placed on the right anterior chest wall to generate mechanical waves in the liver during imaging (Fig. 1). These propagating shear waves are imaged using a special MRI sequence. The scanner automatically processes the wave images to generate cross-sectional images that quantitatively depict tissue stiffness. All commercially available versions of MRE use a standardized algorithm for calculating tissue stiffness, expressed as the magnitude of the complex shear modulus (in kilopascals).
Figure 1.
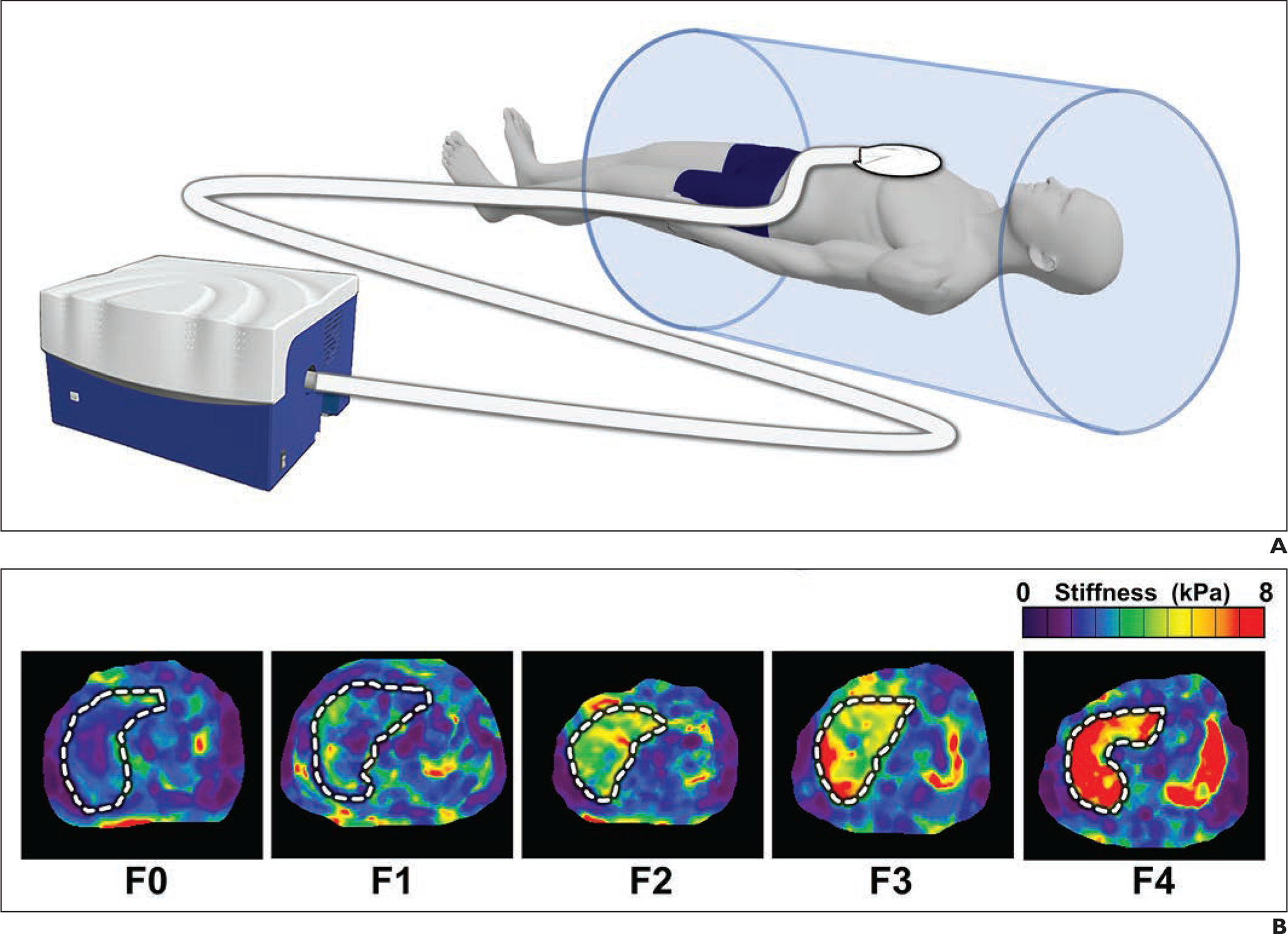
(A) Schematic illustration of driver system for MR elastography. (B) Representative MR elastograms of separate patients with no liver fibrosis (F0), mild fibrosis (F1), significant fibrosis (F2), advanced fibrosis (F3), and cirrhosis (F4).
What is the Reference Standard for Diagnosing Liver Fibrosis?
The standard of truth for staging liver fibrosis is liver biopsy and histologic analysis by an expert pathologist. Liver biopsy is an invasive procedure with risk of complications and is not suited for repeated testing [6]. Moreover, histopathologic assessment of liver fibrosis is subjective, with several different and in some cases, etiology-specific, systems. Fibrosis severity is usually reported according to limited number of stages rather than on a continuous quantitative scale [7]. Commonly, severity is assigned to one of five stages: no fibrosis (F0), mild fibrosis (F1), significant fibrosis (F2), advanced fibrosis (F3), or cirrhosis (F4). Studies have shown substantial inter-observer variability in liver fibrosis staging, with expert pathologists assigning different stages for the same specimen in nearly 50% of cases (Fig. 2) [8–10]. Furthermore, fibrosis severity is often regionally heterogeneous within the liver, causing sampling error when evaluating fibrosis using a single representative needle biopsy specimen. When two needle biopsy specimens are obtained from different regions of the liver, the histologic fibrosis stage may differ in approximately one-third of specimen pairs [11].
Figure 2.
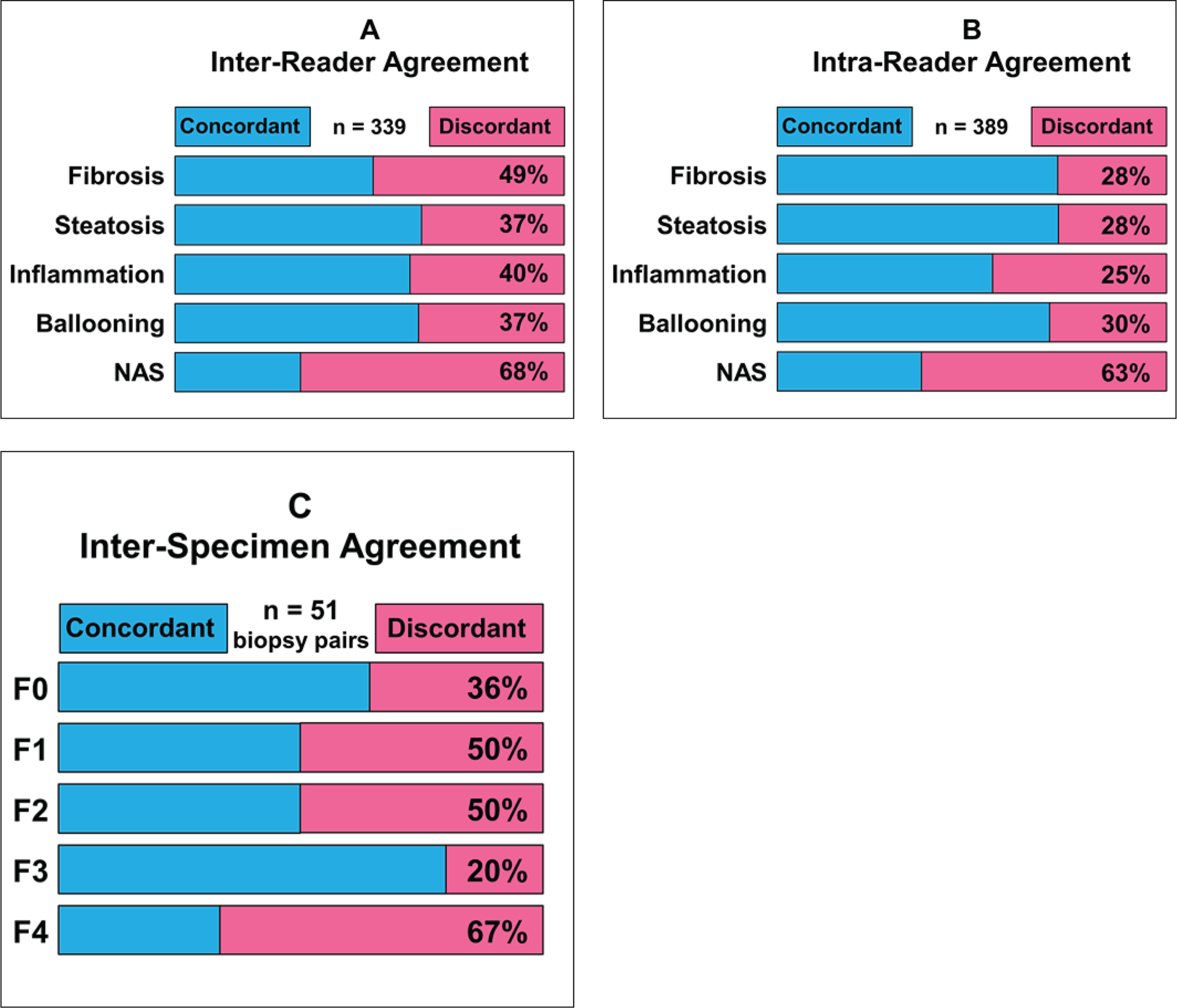
Metrics showing limitations in diagnostic performance of biopsy for evaluation of liver fibrosis, as reported in literature.
A-In study in which 339 liver biopsy specimens were each evaluated independently by two expert histopathologists, reported stage of fibrosis in specimens was discordant in nearly half of specimens. Substantial discordance was also present for grading of steatosis, inflammation, ballooning, and assignment of nonalcoholic fatty liver disease activity score (NAS) [9}.
B-In same study, 389 liver biopsy specimens were each evaluated at two separate times by same expert histopathologist. Reported severity of fibrosis and other histopathologic findings for same specimens were discordant in 25–63% of cases [9].
C-In study that obtained two percutaneous liver biopsy specimens from each of 51 patients, stage of fibrosis in pairs of specimens evaluated by expert histopathologist was discordant in 20–67% of cases [12].
Limitations of liver biopsy in terms of accuracy and precision pose a challenge in assessing the diagnostic performance of non-invasive tests for detecting and staging liver fibrosis. Nevertheless, most existing published evidence on the performance of non-invasive tests for liver fibrosis has used biopsy as the reference standard.
What is the Diagnostic Performance of MRE for Diagnosing Liver Fibrosis?
Given the importance of detecting and staging liver fibrosis in managing chronic liver disease, many noninvasive tests have been evaluated for this task, including blood-based tests and clinical indices [e.g., AST-to-platelet ratio index (APRI), FIB-4 index (computed using patient age, AST, ALT, and platelet count)], and proprietary indices [12]. Imaging methods that have been tested include US- and MRI-based elastography, MRI-based measurement of water diffusion (ADC), MRI relaxation times (T1, T1ρ), CT and MRI liver texture analysis, and various anatomic features such as hepatic surface nodularity [1,13–21].
The diagnostic performance of quantitative biomarkers for ruling-in or ruling-out a given condition is often evaluated by ROC analysis [22], whereby AUC measures the test performance. AUC of 0.5 indicates that a test is no better than a coin flip in making a diagnosis, whereas AUC of 1.0 corresponds to a test with perfect performance. ROC analysis also provides a method for identifying optimal threshold values for ruling in or ruling out a condition using a biomarker.
Over the last decade, multiple studies have used ROC analysis to evaluate the performance of candidate biomarkers for staging liver fibrosis. Individual cohort studies and meta-analyses have validated MRE-based liver stiffness as a biomarker for liver fibrosis, with head-to-head comparisons showing MRE generally outperforming ultrasound-based elastography [vibration-controlled transient elastography (VCTE), ultrasound shear wave elastography (SWE), and 2D SWE], other quantitative MRI biomarkers [e.g., T1 relaxation time, DWI, intravoxel incoherent motion analysis and dynamic contrast enhanced (DCE) MRI], and serum-based (e.g., FIB-4 index) [1,14,17,23–28].
Given the extensive evidence supporting the diagnostic performance of MRE in assessing liver fibrosis, it is increasingly used as an alternative to biopsy as a reference standard for evaluating the performance of other non-invasive tests [29,30]
While studies have generally been consistent in demonstrating high diagnostic performance in fibrosis staging with MRE (AUC of approximately 0.9 for advanced fibrosis), optimum thresholds for each stage have varied widely across studies (Table 1). For example, among twelve studies, the reported optimum stiffness threshold for diagnosing advanced fibrosis (≥F3) ranged from 2.9 kPa to 4.8 kPa.
Table 1.
Reported thresholds (in kilopascals) for staging of liver fibrosis with MR elastography.
| Population and Study | N | Fibrosis Stage | |||
|---|---|---|---|---|---|
| ≥F1 | ≥F2 | ≥F3 | F4 | ||
| Chronic liver disease and healthy volunteers | |||||
| Choi et al, 2013. Invest Radiol. 48: 607–613. | 168 | 2.9 | 3.0 | 3.4 | 4.00 |
| Ichikawa et al, 2015. J Magn Reson Imaging. 42: 204–210. | 129 | 2.60 | 2.80 | 3.60 | 4.00 |
| Yoshimitsu et al, 2016. Eur Radiol. 26: 656–663. | 70 | 3.13 | 3.85 | 4.28 | 5.38 |
| Morisaka et al, 2018. J Magn Reson Imaging. 47: 12681275. | 80 | 2.32 | 2.61 | 3.02 | 4.23 |
| Chronic hepatitis B or C | |||||
| Venkatesh et al, 2014. Eur Radiol. 24(1): 70–78. | 63 | 2.74 | 3.2 | 3.7 | 4.33 |
| Chang et al, 2016. Radiology. 280: 88–97. | 352 | 2.56 | 2.57 | 2.92 | 3.67 |
| Shi et al, 2016. Am J Gastroenterol. 111: 823–833. | 179 | 3.08 | 3.13 | 3.74 | 4.18 |
| Loomba et al, 2014. Hepatology. 60: 1920–1928. | 117 | 3.02 | 3.58 | 3.64 | 4.67 |
| Fatty liver disease | |||||
| Chen et al, 2017. Radiology. 283: 418–428. | 111 | 2.60 | 3.50 | 3.60 | 4.52 |
| Imajo et al, 2016. Gastroenterology. 150: 626–637. | 142 | 2.50 | 3.40 | 4.80 | 6.70 |
| Park et al, 2017. Gastroenterology. 152: 598–607. | 104 | 2.65 | 2.86 | 2.99 | 3.35 |
| Imajo et al, 2022. Clin Gastroent and Hepatol. 20: 908–917. | 231 | 2.92 | 3.19 | 3.90 | 4.62 |
The primary driver of this variability is spectrum bias [31]. Using ROC analysis, the stiffness threshold that optimally categorizes a given fibrosis stage in a test cohort is strongly affected by the distribution of fibrosis stages in the cohort [15]. These effects can be mitigated by studying larger cohorts and by ensuring that the distribution of fibrosis stages in the cohort used to develop the optimum thresholds is as close as possible to the distribution in the population in which MRE will be used.
In many studies, the AUC of MRE for staging ≥F2, ≥F3, and F4 fibrosis is at the upper limit of what can be demonstrated when using biopsy as standard of truth. In practical terms, the uncertainty resulting from observer variation and sampling effects when using biopsy as standard of truth imposes a ceiling on the diagnostic performance that can be demonstrated with a comparative test, no matter how accurate. For instance, it is estimated that even with generous assumptions about the accuracy of biopsy for staging significant fibrosis, the maximum AUC that might be demonstrated for a perfect non-invasive test, using biopsy as standard of truth, could be as low as 0.90 [7,32].
Support is growing for using MRE-based liver stiffness measurement as a primary surrogate of disease severity, rather than simply assigning a histologic stage [21]. This approach leverages the inherently quantitative continuous scale of the biomarker rather than collapsing it into a limited set of predicted histologic stages. The approach is especially attractive when longitudinal MRE data are available, allowing recognition of changes in disease severity that may not be detectable with biopsy-based staging. Multiple studies have shown that MRE-based liver stiffness measurements are help predict future outcomes, including development of cirrhosis and hepatic decompensation, as well as non-hepatic events such as cardiovascular disease and heart failure-related hospitalization and death [33–37]. According to a 2021 study, a patient with known cirrhosis secondary to fatty liver disease and liver stiffness of 5.0 kPa has an approximately 20% risk of decompensation or death within 3 years [38]. If a subsequent MRE examination shows that the liver stiffness has risen to 8 kPa, the risk of decompensation or death within 3 years would double to approximately 40% (Fig. 3). Yet, biopsies at both times would simply indicate stage F4 fibrosis.
Figure 3.
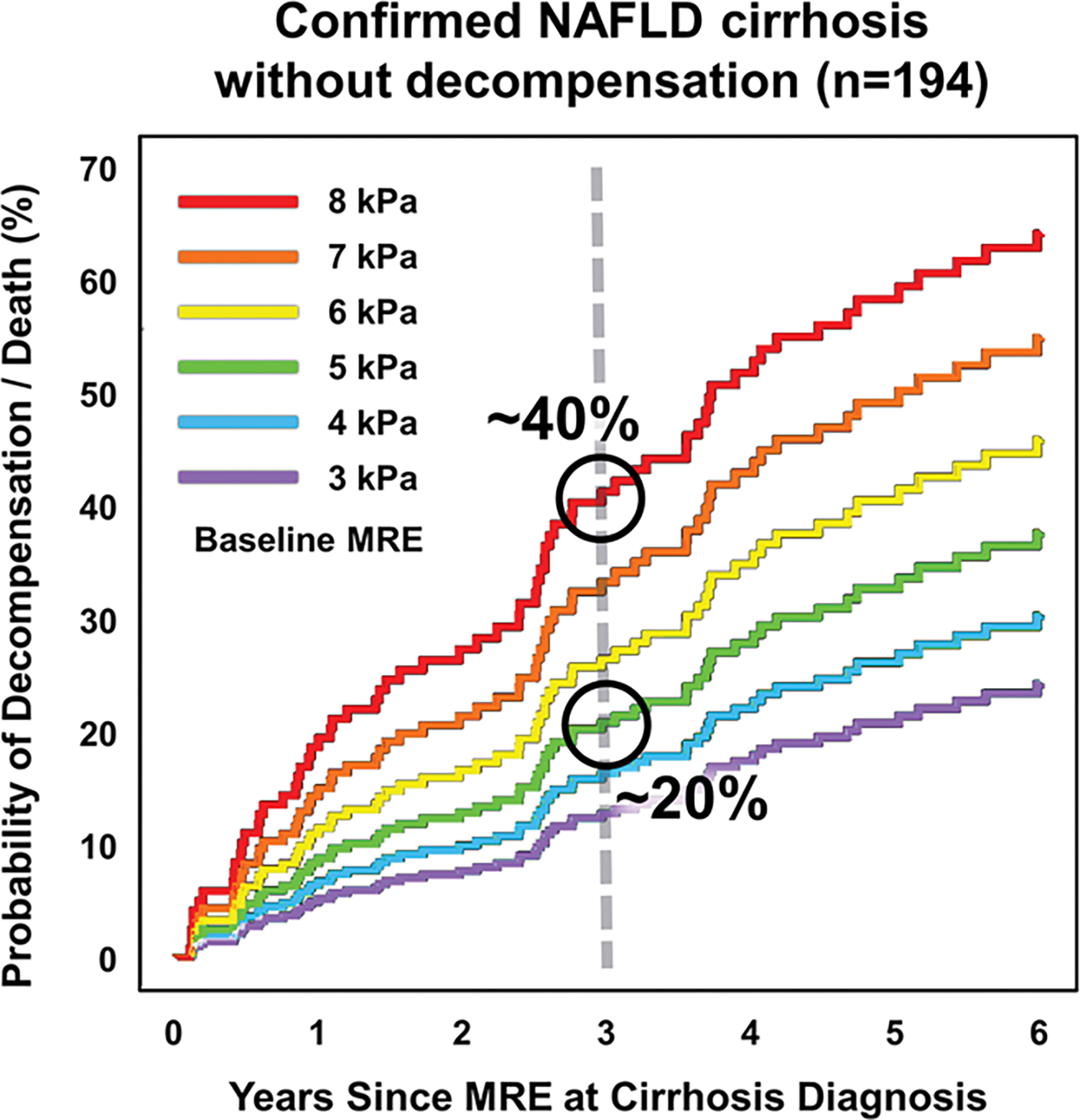
Graph showing probability of decompensation or death over time in years in patients with histologically confirmed nonalcoholic fatty liver disease and baseline non-decompensated cirrhosis, stratified by baseline liver stiffness. Baseline liver stiffness is strongly linked to probability of subsequent decompensation or death. For example, patient with cirrhosis and baseline liver stiffness value of 5 kPa has approximately 20% probability of decompensation or death after 3 years, whereas patient with baseline stiffness of 8 kPa has approximately twice risk of decompensation or death in same interval. NAFLD = nonalcoholic fatty liver disease; MRE = MR elastography. (Adapted from Clinical Gastroenterology and Hepatology, 19, Gidener T, Ahmed OT, Larson JJ, et al. Liver stiffness by magnetic resonance elastography predicts future cirrhosis, decompensation, and death in NAFLD, 1915–1924, Copyright 2021, with permission from Elsevier, https://www.sciencedirect.com/journal/clinical-gastroenterology-and-hepatology)
Should Different Stiffness Thresholds be Applied When Using MRE to Stage Fibrosis With Different Etiologies?
Progressive liver fibrosis is a wound-healing response to chronic inflammation and hepatocyte injury caused by various conditions, including viral hepatitis, metabolic fatty liver disease, toxic agents, and cholestatic diseases. Some etiologies are associated with distinct spatial distributions of fibrosis at histology (e.g., periportal, septal, or diffuse). The structural arrangement of fibrosis associated with a particular etiology may have a different impact on liver stiffness compared with another etiology with the same histologic stage of fibrosis. Furthermore, inflammation alone can increase liver stiffness [39]; the intensity of inflammation varies with etiology which may also suggest the need for etiology-specific thresholds for staging liver fibrosis with MRE.
Some ultrasound elastography publications have suggested the use of etiology-specific liver stiffness thresholds for staging fibrosis [40–41]. However, as previously noted, reported stiffness thresholds in etiology-specific cohorts have varied over a wide range, comparable to variations between etiologies. There is currently a paucity of published evidence that would support etiology-specific thresholds for MRE-based liver fibrosis staging.
What Factors Cause Technical Failures in MRE?
MRE examinations may fail if the amplitude of shear waves generated in the liver is too low, or if the waves cannot be adequately visualized due to motion artifacts or other technical problems. Studies have typically found little or no impact of obesity and high BMI on the technical success rate of MRE [42]. In patients with hemochromatosis or other causes of severe iron overload, the signal intensity of the liver may be so low that shear waves cannot be imaged reliably, causing MRE examinations to fail [3].
The first commercial implementations of MRE used a gradient-recalled echo (GRE) sequence. This technique was found to work well on 1.5-T systems. However, magnetic susceptibility effects that account for the short T2* of normal liver are more severe at higher field strength, and early experience with 3-T systems demonstrated that liver signal was too low for reliable stiffness estimation in 5–10% of examinations using a GRE MRE sequence [42]. The introduction of MRE sequences based on spin-echo echo planar imaging (SE-EPI) addressed this problem. Recent studies and meta-analyses have shown that, with use of appropriate sequences, the technical failure rate of clinical MRE is 2% or less, comparable to other MRI techniques [43,44]. Also, use of appropriate MRE sequences can provide successful liver stiffness measurements in patients with moderate iron overload [45].
Other factors that can cause technical failure in MRE examinations include patient motion, poor breath-holding, and procedural failures (e.g., improper positioning of shear wave driver on the body wall). As with other MRI techniques, advances in acquisition speed and patient ergonomics will likely further increase the technical reliability and efficiency of the examination.
What is the Precision of Liver Stiffness Measurement With MRE?
In clinical practice and pharmaceutical trails, MRE can be repeated at intervals of time to monitor disease progression or response to therapeutic measures. In this context, it is important to be aware of the precision or consistency of repeated liver stiffness measurements. Multiple studies have evaluated the repeatability of MRE-based liver stiffness measurements performed on different days using the same platform and technique. Based on evidence from ten studies[46,47], the QIBA Profile for Magnetic Resonance Elastography of the Liver concluded that the repeatability coefficient of MRE, if performed in a standardized fashion, is 19% [5]. According to the profile, therefore, “a measured change in hepatic stiffness of 19% or larger indicates that a true change in stiffness has occurred with 95% confidence” [5].
A portion of observed variability in liver stiffness is due to a known effect on liver tissue stiffness caused by variations in portal blood flow, hydration, and mechanical deformation of the liver by surrounding structures [48–52].
Due to a lack of standardization in acquisition and processing techniques across vendor platforms, quantitative MRI biomarkers are typically not directly reproducible if obtained on systems from different manufacturers. However, for MRE, all FDA-cleared implementations, currently available from five different MRI manufacturers, use equivalent acquisition techniques, as well as identical driver systems and processing methods [53]. Studies of cross-platform reproducibility have provided preliminary evidence of the validity of comparisons of MRE-based liver stiffness obtained on different MRI systems [54].
Can MRE-Based Stiffness Measurements be Combined With Other Noninvasive Biomarkers to Further Improve Diagnostic Performance?
Clinical recommendations for managing liver disease are often presented in flow charts with a sequential series of decisions based on clinical information and non-invasive test results. For instance, the 2023 AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease initially uses the FIB-4 test to screen for presence of advanced fibrosis [55]. If the FIB-4 value is greater than 2.67 or if ALT or AST is persistently elevated, then the patient may proceed to further evaluation with MRE, to provide a more reliable assessment (including high PPV) of fibrosis.
Can MRE-Based Stiffness Measurements be Used to Diagnose NASH?
Nonalcoholic fatty liver disease (NAFLD) now affects approximately one-quarter of the global population [56] and approximately one third of the adult U.S. population [57]. Approximately 25% of patients with NAFLD will develop some degree of nonalcoholic steatohepatitis (NASH), characterized by inflammation and hepatocellular injury in addition to steatosis. It is estimated that 10–15% of patients with NAFLD will develop progressive liver fibrosis leading to end-stage liver disease (cirrhosis) and an annual risk of approximately 4% of developing hepatocellular carcinoma [58]. Accordingly, the hepatology community has identified a strong clinical need for reliable non-invasive methods to diagnose NASH and, critically, to identify patients with NASH who are progressing to clinically significant fibrosis (F2 or greater) [55,59].
Consensus is emerging that MRI-assessed proton-density fat fraction (PDFF) quantifies the extent of steatosis with high accuracy [60], and that MRE-assessed liver stiffness is the most reliable method for detecting and staging liver fibrosis in patients with NAFLD [1,25,61].
Additionally, in patients with NAFLD, unlike ultrasound-based methods, steatosis severity has no significant influence on MRE-based liver stiffness measurement [62]. In head-to-head comparisons with other quantitative MRI biomarkers (e.g., T1), PDFF has generally showed the most power in diagnosing NASH in multiparametric models [63]. On the other hand, MRE has demonstrated the highest performance of any single biomarker in distinguishing at-risk NASH (i.e., NASH with clinically significant fibrosis ≥F2) in multiparametric models [63].
Other strategies have evaluated combinations of imaging- and laboratory-based biomarkers, with the goal of increasing diagnostic performance. These include the MAST index (computed from MRE, PDFF, and AST measurements), and the MEFIB index (computed from MRE and FIB-4). Studies have reported higher performance in diagnosing NASH and at-risk NASH (i.e., NASH with significant fibrosis), and in predicting clinical outcomes, using these indices rather than using single biomarkers alone [24,64,65].
Can Further Technical Advances Augment the Diagnostic Performance of MRE?
The technical approach for liver MRE used in current FDA-cleared commercial versions of the technology was guided by a goal of minimizing the engineering challenge of implementation on many different MRI platforms. The driver system was designed to create a predictable pattern of shear waves in the liver, with the main direction of propagation in the transverse plane, allowing reasonably valid wave speed to be obtained from individual transverse phase images. In addition, valid results may be obtained with a single direction of motion sensitization (perpendicular to the transverse imaging plane). This approach, which enables easy acquisition of valid MRE data for one or more individual transverse images through the liver in a single breath hold, is known as 2D MRE [53].
Although clinical experience and published evidence have validated the streamlined 2D MRE technique, a more rigorous approach would avoid the approximation that all shear waves are propagating parallel to an imaging plane and instead acquire and analyze the pattern of propagating shear waves throughout a 3D volume. In addition, if cyclic tissue in each voxel can be recorded in all three directions instead of only in the z direction, then more advanced algorithms can be used to calculate tissue stiffness. This approach is known as 3D vector MRE [66,67]. Although 3D MRE requires acquisition of more than ten times the amount of data compared with 2D MRE, advanced techniques now allow the approach to be accomplished in just 3 to 6 breath-holds [68].
The 3D vector MRE technique provides several advantages in liver disease assessment. The volume of liver imaged is much larger than with 2D MRE [53]. Most importantly, by analyzing a much more complete data set, the tissue stiffness calculation is, in principle, more accurate and precise [69]. A preliminary study suggested that the repeatability coefficient for 3D MRE of the liver is approximately 11% (vs 19% for 2D MRE), allowing detection of smaller changes in liver stiffness in longitudinal studies [70].
In addition, the more complete data obtained with 3D MRE allows calculation of additional biomarkers, including the two individual components of the complex shear modulus, with the real part known as the storage modulus and the imaginary part known as the loss modulus [69]. The biomarker known as stiffness in FDA-cleared commercial versions of MRE is the magnitude of the vector formed by the real and imaginary components of the complex shear modulus [71]. The storage modulus measures the elastic (spring-like) properties of tissue, whereas the loss modulus reflects viscous (energy dissipating) mechanical properties [86–88]. Other biomarkers that can be extracted include shear wave attenuation, volumetric strain, and local shear strain [72–74]. Volumetric strain reflects tissue compressibility and has promise as a biomarker of interstitial tissue pressure and microvascular turgidity [96, 98]. Current research indicates that these biomarkers have potential applications for characterizing inflammation, estimating tissue interstitial pressure in chronic liver disease, and characterizing microvascular invasion in hepatocellular carcinoma [67–75].
A potential source of variability in 2D and 3D MRE is the subjectivity involved when an analyst defines ROIs to perform a measurement. Automated tools for MRE analysis are being introduced. Studies show that these tools can provide measurements that are equivalent to those obtained by a highly experienced analyst [61,76].
The standard shear wave frequency used in liver MRE is 60 Hz. This frequency represents an optimization between increased elastographic resolution obtained with higher frequencies and better penetration of shear waves obtained with lower frequencies. The measured shear stiffness of tissue typically varies with frequency. This characteristic (called dispersion), which can be studied with multifrequency MRE acquisition, is another biomarker that has shown potential for advanced characterization of liver pathology with MRE [67,75,77].
What Conditions Other Than Fibrosis can Affect Liver Stiffness and Potentially the Accuracy of Fibrosis Staging With MRE?
Unlike biomarkers such as PDFF (a direct measurement of liver fat content via chemical-shift MRI), MRE-based liver stiffness is not a direct measure of liver fibrosis but rather a surrogate biomarker that correlates strongly with severity. Any other conditions that can affect liver stiffness might impact the reliability of this biomarker as a fibrosis surrogate.
Steatosis has no Significant Effect on Liver Stiffness
Accumulation of excess lipid in hepatocytes is the hallmark of fatty liver disease and the most common abnormality affecting the liver [55]. Adipose tissue is one of the softest tissues in the body, and initially, there was some concern in the early evaluation of MRE that hepatic steatosis might have a softening effect that could mask the effect of co-existing fibrosis. However, published data establish that mild, moderate, and severe hepatic steatosis have no significant effect on MRE-assessed liver stiffness [62]. It is notable that there is little published information on the effect of steatosis on the subjective histologic staging of liver fibrosis.
Inflammation can Increase Liver Stiffness
Inflammation is a central factor in development of liver injury, and chronic inflammation can lead to progressive liver fibrosis. Fulminant acute hepatitis due to infection or toxic agents is known to markedly elevate liver stiffness in the absence of fibrosis [78]. Therefore, MRE interpretation must take into account the clinical setting.
Chronic inflammation also elevates liver stiffness measurement to a variable but usually small extent [48]. The stiffness variability introduced by chronic inflammation may impact the reliability of fibrosis staging at the F0-F1 end of the fibrosis spectrum, where stiffness differences between stages are smallest [79].
Growing evidence indicates that the elevation of liver stiffness with inflammation is due to increased vascular permeability that results in increased interstitial free fluid [67,80,81]. Research using 3D vector MRE demonstrates a characteristic increase in the imaginary component of the complex shear modulus, known as the loss modulus. Given that loss modulus reflects viscous fluid-like mechanical properties, MRE may depict an aspect of inflammation that cannot be evaluated with conventional histopathology [67] [Fig. 4]. Moreover, advanced multiparametric 3D vector MRE may distinguish inflammation from fibrosis and thereby more precisely assess treatment effects [67,82].
Figure 4.
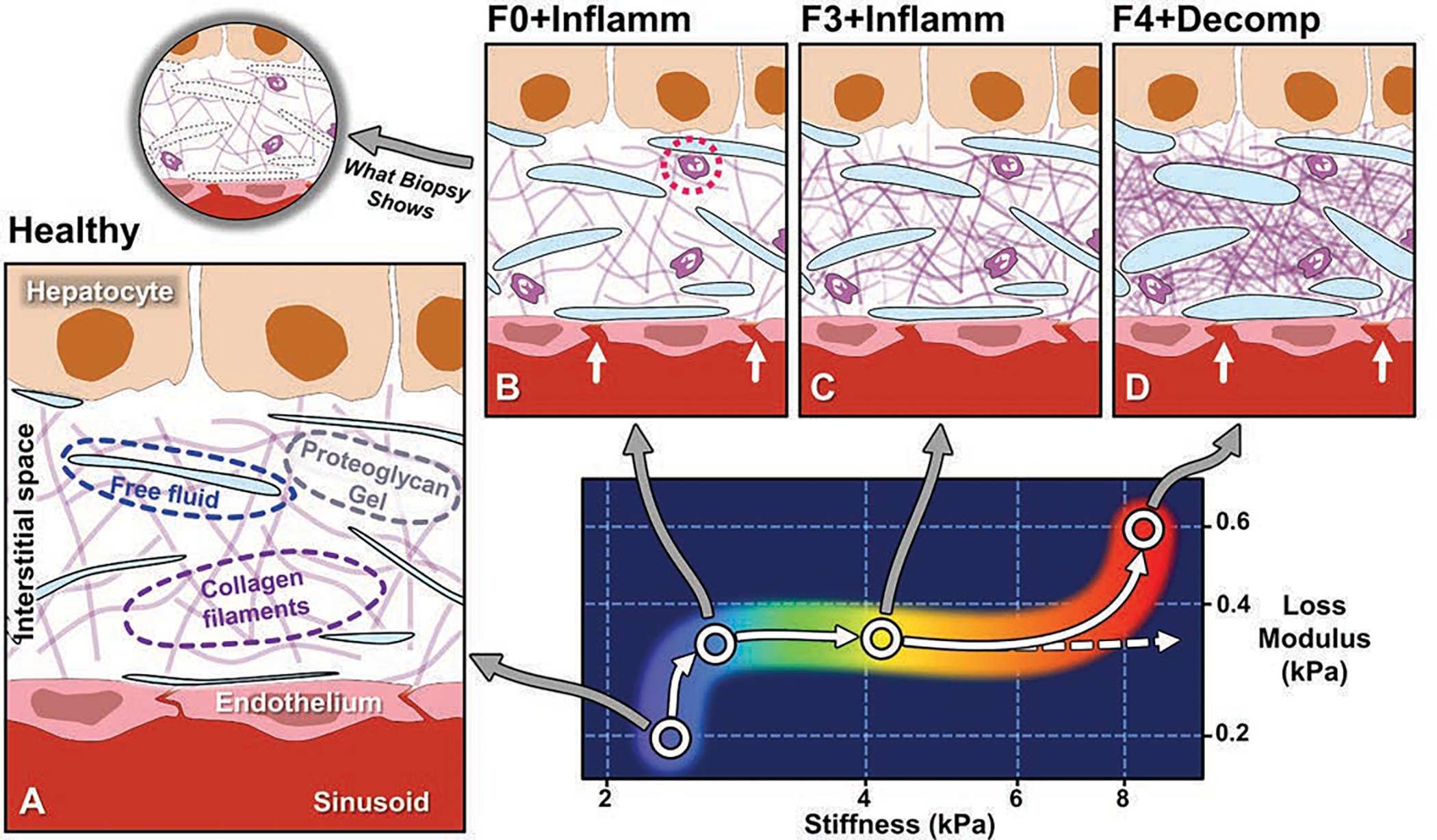
Conceptual illustration of emerging understanding of correlations between MRE-based quantitative biomarkers and spectrum of histopathology in chronic liver disease [67,79,81]. Panel A depicts hepatic interstitial space in healthy state. Interstitium is interposed between hepatic sinusoids and hepatocytes and consists of proteoglycan gel matrix with collagen filaments and other macromolecules. Normal interstitium also contains tiny clefts of free fluid, representing transudate from sinusoids, which is drained from interstitial space by lymphatics. These clefts of fluid are lost in standard process of biopsy tissue fixation and sectioning and therefore not generally visible at histologic review. Panel B shows inflammation, which is key mechanism in chronic liver disease and is detected at histopathology by presence of leukocytes in interstitium (dotted circle). Inflammation is also characterized by increased vascular permeability (arrows), resulting in increased interstitial fluid. Loss modulus is MRE-measured biomarker that is sensitive to fluid phase in tissue and rises with onset of inflammation. Increased loss modulus associated with inflammation also causes small increase in liver stiffness, independent of effect of fibrosis. In panel C, with activation of stellate cells, increased amounts of collagen accumulate in interstitial space. MRE-assessed liver stiffness largely reflects elastic properties of solid phase of tissue, and increases rapidly with fibrosis. Panel D depicts end-stage liver disease, in which accumulation of collagen leads to ever-increasing stiffness values (dashed arrow). Studies suggest that hepatic decompensation is marked by further elevation of loss modulus, with incrementally increased vascular permeability (arrows) and increased interstitial free fluid, potentially overloading lymphatic drainage of liver and resulting in ascites. MRE = MR elastography.
Portal Hypertension and Hepatic Venous Congestion can Increase Liver Stiffness
MRE examinations of patients with advanced liver fibrosis often incidentally demonstrate elevated spleen stiffness (Fig. 1) [83]. This phenomenon reflects elevated portal pressure, which is common in advanced liver disease [84].
Studies have demonstrated that liver stiffness is also increased by elevated portal pressure [84,85]. In patients with chronic liver disease, MRE-assessed liver stiffness may rise acutely by as much as 40% immediately after food intake [50,51,86]. This effect is attributed to impaired autoregulation of hepatic sinusoidal resistance, which in healthy individuals keeps portal pressure relatively constant even when splanchnic flow doubles in the postprandial state [49,50]. The standard requirement of 4 hours of fasting before an MRE examination helps to avoid fibrosis overstaging in these circumstances. A stiffness index adjustment, calculated as the ratio of liver to spleen stiffness, has been proposed to account for the effects of portal hypertension on liver stiffness measurements and for other related purposes [85,87].
Conditions associated with hepatic venous congestion, including Budd-Chiari syndrome, cardiac failure, and prior Fontan surgery, are known to increase liver stiffness, independent of presence of fibrosis [88,9–90]. Nonetheless, these conditions are also potent causes of progressive liver fibrosis. Congestion-induced liver fibrosis occurs in nearly all patients with Fontan circulation, and accurate staging is important for management. Yet, presence of hepatic venous congestion in these patients can lead to overestimation of fibrosis severity if standard stiffness thresholds are employed [89–91]. Splenic stiffness measurements have been used to address this challenge [91].
As with causes of inflammation, preliminary evidence indicates that advanced 3D MRE may help differentiate these conditions that cause elevated liver stiffness. Specifically, volumetric strain, a biomarker that decreases with increasing tissue turgidity, is being investigated for its potential to distinguish the effects of vascular congestion from fibrosis [92,93].
Cholestasis can Increase Liver Stiffness
Disruption and dysregulation of bile flow resulting in cholestasis is associated with increased liver stiffness, hepatic inflammation, and consequent cellular injury [94]. In this setting, clinical information greatly aids MRE interpretation. Cholestasis is typically associated with elevated levels of serum bilirubin, alkaline phosphatase, and gamma-glutamyl transferase (serum biomarkers of bile duct obstruction). Cholestatic liver disease can also cause ductal dilatation, which can be seen on MRI or other imaging modalities. A multiparametric approach, considering all available imaging and biochemical data, as well as the patient’s medical history and clinical examination, are critical for appropriate interpretation.
Ischemia May Decrease Liver Stiffness.
Few conditions other than ischemia are known to decrease tissue stiffness from normal values. A component of the mechanical stiffness of most living soft tissues reflects the presence of vascular perfusion. Studies of extracted healthy tissue specimens usually show lower stiffness in comparison with the same healthy perfused tissue in vivo [95]. This effect is prominent in the kidneys, where reduced renal blood flow may cause a marked decrease in parenchymal stiffness even when substantial renal fibrosis is present [96,97]. Although not well studied, ischemia or necrosis of the liver is likely to present with decreased liver stiffness in the affected regions, possibly offsetting the increased stiffness that might be present due to fibrosis. However, this situation is unlikely to result in misdiagnosis via MRE when accounting for clinical information.
What Factors Have Influenced Adoption of MRE in Clinical Practice?
Adoption of new technologies in clinical practice is influenced by many variables, including availability, standardization, diagnostic performance, insurance coverage, cost, and ultimately, recognition as a best practice in applicable clinical guidelines.
When MRE was first introduced as a commercially available FDA-cleared option for MRI systems in 2009, it was initially only accessible at a handful of academic institutions. However, availability has steadily grown. Currently, MRE is available from most major MRI manufacturers, and the technology is now deployed on more than 2100 MRI systems worldwide [53].
A factor that has aided MRE adoption is that all regulatory-approved versions of the technology use equivalent acquisition sequences, shear wave driver systems, processing algorithms, image scaling, and display color tables. This standardization has enabled use of standardized stiffness thresholds for staging liver fibrosis for examinations obtained on different vendor platforms [54,98].
In the United States, a prerequisite for insurance coverage of a medical procedure is that the procedure has achieved sufficient availability and documented efficacy to be recognized by the American Medical Association (AMA) though assignment of a CPT code. In 2018, the American College of Radiology presented evidence of the wide availability and the high diagnostic performance of MRE to the AMA, which granted the procedure a full Category-I CPT code (code 76391), to be used either in combination with an abdominal MRI examination or as a standalone test. In 2019, CMS assigned a relative value to the service, which determines the reimbursable cost. Because the acquisition time for MRE is very short (as little as 15–20 seconds), the assigned value equates to a government insurance reimbursement of approximately $240 per procedure. Although MRI is generally considered to be a high-cost modality, the rapidity of MRE and resulting modest cost has aided adoption. A recent study employing Markov modeling and updated CMS reimbursement data showed that MRE is more cost-effective than VCTE as a frontline modality for staging advanced fibrosis [98].
As consensus has emerged over the last decade on the performance of MRE for non-invasively detecting and staging liver fibrosis, its role as a key tool has increasingly been recognized in professional clinical guidelines for liver disease management, including those developed by the American College of Radiology, the American Gastroenterologic Association, and the American Association for the Study of Liver Disease [53,99,100].
Future Directions
Although the literature has already addressed many important questions about the diagnostic performance of MRE in assessing liver disease, further prospective testing and tuning of quantitative thresholds in larger patient cohorts will continue to define the clinical role of MRE in specific populations. Additional longitudinal studies are needed to understand the clinical implications of quantitative changes in liver stiffness over time. Ongoing studies will assess the potential to use novel biomarkers provided by 3D vector MRE to differentiate fibrosis from processes such as inflammation and hepatic venous congestion.
Highlights.
MRE has high diagnostic performance for staging liver fibrosis, but interpretation must include consideration of co-existing conditions such as inflammation and hepatic venous congestion.
MRE-based liver stiffness has emerged as the most effective single non-invasive biomarker for detecting at-risk NASH (i.e., NASH with stage ≥F2 fibrosis).
Advances in MRE technology are providing new biomarkers that have potential to allow independent assessment of inflammation and other histologic processes, in addition to fibrosis.
Funding:
NIH grants R37 EB001981, R01 EB17197.
Footnotes
Disclosures: MY, RLE, and the Mayo Clinic have intellectual property rights and a financial interest in magnetic resonance elastography technology.
Publisher's Disclaimer: The publication of this Accepted Manuscript is provided to give early visibility to the contents of the article, which will undergo additional copy-editing, typesetting, and review before it is published in its final form. During the production process, errors may be discovered that could affect the content of the Accepted Manuscript. All legal disclaimers that apply to the journal pertain. The reader is cautioned to consult the definitive version of record before relying on the contents of this document.
References
- 1.Imajo K, Honda Y, Kobayashi T, et al. Direct comparison of US and MR elastography for staging liver fibrosis in patients with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology 2022; 20:908–917 [DOI] [PubMed] [Google Scholar]
- 2.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 2017; 66:1486–1501 [DOI] [PubMed] [Google Scholar]
- 3.Pepin KM, Welle CL, Guglielmo FF, Dillman JR, Venkatesh SK. Magnetic resonance elastography of the liver: everything you need to know to get started. Abdom Radiol (NY). 2022; 47:94–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng CH, Lim WH, Hui Lim GE, et al. Mortality Outcomes by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clinical Gastroenterology and Hepatology 2023; 21:931–939 e9355. [DOI] [PMC free article] [PubMed] [Google Scholar]; Budai BK, Toth A, Borsos P, et al. Three-dimensional CT texture analysis of anatomic liver segments can differentiate between low-grade and high-grade fibrosis. BMC Medical Imaging 2020; 20:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RSNA-QIBA. QIBA Profile: MR Elastography of the Liver. In. https://qibawiki.rsna.org/index.php/Profiles: RSNA Quantitative Imaging Biomarkers Alliance, 2022 [Google Scholar]
- 6.Zhou F, Stueck A, McLeod M. Liver biopsy complication rates in patients with non-alcoholic fatty liver disease. Canadian Liver Journal 2022; 5:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. Journal of Hepatology 2009; 50:1–3 [DOI] [PubMed] [Google Scholar]
- 8.Davison BA, Harrison SA, Cotter G, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. Journal of Hepatology 2020; 73:1322–1332 [DOI] [PubMed] [Google Scholar]
- 9.Brunt EM. Liver biopsy reliability in clinical trials: Thoughts from a liver pathologist. Journal of Hepatology 2020; 73:1310–1312 [DOI] [PubMed] [Google Scholar]
- 10.Kim HP, Idowu MO, Mospan AR, et al. Liver biopsy in the real world-reporting, expert concordance and correlation with a pragmatic clinical diagnosis. Alimentary Pharmacology and Therapeutics 2021; 54:1472–1480 [DOI] [PubMed] [Google Scholar]
- 11.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128:1898–1906 [DOI] [PubMed] [Google Scholar]
- 12.Vali Y, Lee J, Boursier J, et al. Liver Investigation: Testing Marker Utility in Steatohepatitis (LITMUS) consortium investigators. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol Hepatol 2023; Mar 20:S2468–1253(23)00017–1. doi: 10.1016/S2468-1253(23)00017-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Hennedige TP, Wang G, Leung FP, et al. Magnetic resonance elastography and diffusion weighted imaging in the evaluation of hepatic fibrosis in chronic hepatitis B Gut and Liver 2017; 11:401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman DH, Ayoola A, Nickel D, Han F, Chandarana H. T1 mapping, T2 mapping and MR elastography of the liver for detection and staging of liver fibrosis. Abdominal Radiology 2020; 45:692–700 [DOI] [PubMed] [Google Scholar]
- 15.Besa C, Wagner M, Lo G, et al. Detection of liver fibrosis using qualitative and quantitative MR elastography compared to liver surface nodularity measurement, gadoxetic acid uptake, and serum markers. Journal of Magnetic Resonance Imaging 2018; 47:1552–1561 [DOI] [PubMed] [Google Scholar]
- 16.Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound elastography and MR elastography for assessing liver fibrosis: part 2, diagnostic performance, cofounders, and future directions. AJR 2015; 205:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs. transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clinical Gastroenterology and Hepatology 2019; 17:630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvaraj EA, Mozes FE, Jayaswal ANA, et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: a systematic review and meta-analysis. Journal of Hepatology 2021; 75:770–785 [DOI] [PubMed] [Google Scholar]
- 19.Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clinical Gastroenterology and Hepatology 2015; 13:440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Yin M. Liver Fibrosis: Counterpoint- MR elastography is the noninvasive imaging modality of choice for detecting and staging liver fibrosis. AJR 2022; 219:384–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesh SK, Torbenson MS. Liver fibrosis quantification. Abdominal Radiology 2022; 47:1032–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Erkel AR, Pattynama PM. Receiver operating characteristic (ROC) analysis: basic principles and applications in radiology. European Journal of Radiology 1998; 27:88–94 [DOI] [PubMed] [Google Scholar]
- 23.Lefebvre T, Wartelle-Bladou C, Wong P, et al. Prospective comparison of transient, point shear wave, and magnetic resonance elastography for staging liver fibrosis. European Radiology 2019; 29:6477–6488 [DOI] [PubMed] [Google Scholar]
- 24.Jung J, Loomba RR, Imajo K, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut 2021; 70:1946–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang YN, Fowler KJ, Boehringer AS, et al. Comparative diagnostic performance of ultrasound shear wave elastography and magnetic resonance elastography for classifying fibrosis stage in adults with biopsy-proven nonalcoholic fatty liver disease. European Radiology 2022; 32:2457–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Yin M, Talwalkar JA, et al. Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology 2017; 283:418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 2016; 150:626–637 [DOI] [PubMed] [Google Scholar]
- 28.Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 2017; 152:598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nogami A, Iwaki M, Kobayashi T, et al. Real-world assessment of SmartExam, a novel FibroScan computation method: a retrospective single-center cohort study. Journal of Gastroenterology and Hepatology 2023; 38(2):321–329 [DOI] [PubMed] [Google Scholar]
- 30.Torres L, Schuch A, Longo L, et al. New FIB-4 and NFS cutoffs to guide sequential non-invasive assessment of liver fibrosis by magnetic resonance elastography in NAFLD. Annals of Hepatology 2023; 28:100774. [DOI] [PubMed] [Google Scholar]
- 31.Goehring C, Perrier A, Morabia A. Spectrum bias: a quantitative and graphical analysis of the variability of medical diagnostic test performance. Statistics in Medicine 2004; 23:125–135 [DOI] [PubMed] [Google Scholar]
- 32.Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. Journal of Hepatology 2009; 50:36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gidener T, Yin M, Dierkhising RA, Allen AM, Ehman RL, Venkatesh SK. Magnetic resonance elastography for prediction of long-term progression and outcome in chronic liver disease: a retrospective study. Hepatology 2022; 75:379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tada A, Nagai T, Kato Y, et al. Liver stiffness assessed by magnetic resonance elastography predicts clinical outcomes in patients with heart failure and without chronic liver disease. European Radiology 2023; 33(3):2062–2074 [DOI] [PubMed] [Google Scholar]
- 35.Han MAT, Vipani A, Noureddin N, et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: a multicenter study. Liver International 2020; 40:2242–2251 [DOI] [PubMed] [Google Scholar]
- 36.Higuchi M, Tamaki N, Kurosaki M, et al. Longitudinal association of magnetic resonance elastography-associated liver stiffness with complications and mortality. Alimentary Pharmacology and Therapeutics 2022; 55:292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JG, Jun J, Verma KK, et al. Liver stiffness by magnetic resonance elastography is associated with increased risk of cardiovascular disease in patients with non-alcoholic fatty liver disease. Alimentary Pharmacology and Therapeutics 2021; 53:1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gidener T, Ahmed OT, Larson JJ, et al. Liver stiffness by magnetic resonance elastography predicts future cirrhosis, decompensation, and death in NAFLD. Clinical Gastroenterology and Hepatology 2021; 19:1915–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Guo Q, Xia F, et al. MR elastography for the assessment of hepatic fibrosis in patients with chronic Hepatitis B infection: does histologic necroinflammation influence the measurement of hepatic stiffness? Radiology 2014; 273:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C. Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. Ultraschall in der Medizin - European Journal of Ultrasound 2016; 37:1–5 [DOI] [PubMed] [Google Scholar]
- 41.Cristoferi L, Nardi A, Carbone M. Transient elastography in chronic liver disease: Beware of the cut-offs! Journal of Hepatology 2021; 75:1245–1246 [DOI] [PubMed] [Google Scholar]
- 42.Yin M, Glaser KJ, Talwalkar JA, Chen J, Manduca A, Ehman RL. Hepatic MR elastography: clinical performance in a series of 1377 consecutive patients. Radiology 2016; 278:114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gill HE, Lisanti CJ, Schwope RB, Kim J, Katz M, Harrison S. Technical success rate of MR elastography in a population without known liver disease. Abdominal Radiology 2021; 46:590–596 [DOI] [PubMed] [Google Scholar]
- 44.Kim DW, Kim SY, Yoon HM, et al. Comparison of technical failure of MR elastography for measuring liver stiffness between gradient-recalled echo and spin-echo echo-planar imaging: A systematic review and meta-analysis. J Magn Reson Imaging 2020; 51(4):1086–1102. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Glaser KJ, Zhang T, et al. Assessment of advanced hepatic MR elastography methods for susceptibility artifact suppression in clinical patients. Journal of Magnetic Resonance Imaging 2018; 47:976–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serai SD, Obuchowski NA, Venkatesh SK, et al. Repeatability of MR elastography of liver: a meta-analysis. Radiology 2017; 285:92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gidener T, Dierkhising R, Mara KC, et al. Change in serial liver stiffness measurement by magnetic resonance elastography and outcomes in non-alcoholic fatty liver disease. Hepatology 2023. Jan 1;77(1):268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dittmann F, Tzschatzsch H, Hirsch S, et al. Tomoelastography of the abdomen: tissue mechanical properties of the liver, spleen, kidney, and pancreas from single MR elastography scans at different hydration states. Magnetic Resonance in Medicine 2017; 78:976–983 [DOI] [PubMed] [Google Scholar]
- 49.Obrzut M, Atamaniuk V, Chen J, et al. Postprandial hepatic stiffness changes on a magnetic resonance elastography in healthy volunteers. Scientific Reports 2021; 11:19786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin M, Talwalkar JA, Glaser KJ, et al. Dynamic postprandial hepatic stiffness augmentation assessed with MR elastography in patients with chronic liver disease. AJR 2011; 197:64–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hines CDG, Lindstrom MJ, Varma AK, Reeder SB. Effects of postprandial state and mesenteric blood flow on the repeatability of MR elastography in asymptomatic subjects. Journal of Magnetic Resonance Imaging 2011; 33:239–244 [DOI] [PubMed] [Google Scholar]
- 52.Ren H, Yang D, Xu H, et al. Effect of breath holding at the end of the inspiration and expiration phases on liver stiffness measured by 2D-MR elastography. Abdominal Radiology 2021; 46(6):2516–2526 [DOI] [PubMed] [Google Scholar]
- 53.Ehman RL. Magnetic resonance elastography: from invention to standard of care. Abdominal Radiology 2022; 47:3028–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trout AT, Serai S, Mahley AD, et al. Liver Stiffness Measurements with MR Elastography: Agreement and Repeatability across Imaging Systems, Field Strengths, and Pulse Sequences. Radiology 2016; 281(3):793–804. [DOI] [PubMed] [Google Scholar]
- 55.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023; 77(5):1797–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84 [DOI] [PubMed] [Google Scholar]
- 57.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40:1387–1395 [DOI] [PubMed] [Google Scholar]
- 58.Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-analysis, and Meta-regression. Clinical Gastroenterology and Hepatology 2022; 20:283–292 e210 [DOI] [PubMed] [Google Scholar]
- 59.Kang KA, Jun DW, Kim MS, Kwon HJ, Nguyen MH. Prevalence of significant hepatic fibrosis using magnetic resonance elastography in a health check-up clinic population. Alimentary Pharmacology and Therapeutics 2020; 51:388–396 [DOI] [PubMed] [Google Scholar]
- 60.Gu J, Liu S, Du S, et al. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. European Radiology 2019; 29:3564–3573 [DOI] [PubMed] [Google Scholar]
- 61.Tang A, Dzyubak B, Yin M, et al. MR elastography in nonalcoholic fatty liver disease: inter-center and inter-analysis-method measurement reproducibility and accuracy at 3T. European Radiology 2022; 32:2937–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J, Allen AM, Therneau TM, et al. Liver stiffness measurement by magnetic resonance elastography is not affected by hepatic steatosis. European Radiology 2022; 32:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, Lu X, Zhu Z, et al. Head-to-head comparison of MR Elastography-based liver stiffness, fat fraction, and T1 relaxation time in identifying at-risk NASH. Hepatology 2023; Apr 24. doi: 10.1097/HEP.0000000000000417. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noureddin M, Truong E, Gornbein JA, et al. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. Journal of Hepatology 2022; 76:781–787 [DOI] [PubMed] [Google Scholar]
- 65.Kim BK, Tamaki N, Imajo K, et al. Head-to-head comparison between MEFIB, MAST, and FAST for detecting stage 2 fibrosis or higher among patients with NAFLD. Journal of Hepatology 2022; 77:1482–1490 [DOI] [PubMed] [Google Scholar]
- 66.Arunachalam SP, Rossman PJ, Arani A, et al. Quantitative 3D magnetic resonance elastography: comparison with dynamic mechanical analysis. Magnetic Resonance in Medicine 2017; 77:1184–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Allen AM, Shah VH, Manduca A, Ehman RL, Yin M. Longitudinal changes in MR elastography-based biomarkers in obese patients treated with bariatric surgery. Clinical Gastroenterology and Hepatology 2023; 21(1):220–222.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li M, Yang H, Liu Y, et al. Comparison of the diagnostic performance of 2D and 3D MR elastography in staging liver fibrosis. European Radiology 2021; 31:9468–9478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manduca A, Bayly PJ, Ehman RL, et al. MR elastography: principles, guidelines, and terminology. Magnetic Resonance in Medicine 2021; 85:2377–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen J, Chen J, Heilman JA, et al. Abdominal MR elastography with multiple driver arrays: performance and repeatability. Abdominal Radiology 2023; Mar 16. doi: 10.1007/s00261-023-03866-5. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reeder SB. Emergence of 3D MR Elastography-based Quantitative Markers for Diffuse Liver Disease. Radiology 2021; 301:163–165 [DOI] [PubMed] [Google Scholar]
- 72.Domire ZJ, McCullough MB, Chen Q, An KN. Wave attenuation as a measure of muscle quality as measured by magnetic resonance elastography: initial results. Journal of Biomechanics 2009; 42:537–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirsch S, Guo J, Reiter R, et al. Towards compression-sensitive magnetic resonance elastography of the liver: sensitivity of harmonic volumetric strain to portal hypertension. Journal of Magnetic Resonance Imaging 2014; 39:298–306 [DOI] [PubMed] [Google Scholar]
- 74.Yin Z, Lu X, Cohen Cohen S, et al. A new method for quantification and 3d visualization of brain tumor adhesion using slip interface imaging in patients with meningiomas. European Radiology 2021; 31:5554–5564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M, Yin Z, Guo N, et al. MR elastography-based shear strain mapping for assessment of microvascular invasion in hepatocellular carcinoma. European Radiology 2022; 32:5024–5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dzyubak B, Li J, Chen J, et al. Automated analysis of multiparametric magnetic resonance imaging/ magnetic resonance elastography exams for prediction of nonalcoholic steatohepatitis. Journal of Magnetic Resonance Imaging 2021; 54:121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allen AM, Shah VH, Therneau TM, et al. The role of three-dimensional magnetic resonance elastography in the diagnosis of nonalcoholic steatohepatitis in obese patients undergoing bariatric surgery. Hepatology 2020; 71:510–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Venkatesh SK, Wells ML, Miller FH, et al. Magnetic resonance elastography: beyond liver fibrosis- a case-based pictorial review. Abdominal Radiology 2018; 43:1590–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi Y, Qi YF, Lan GY, et al. Three-dimensional MR Elastography Depicts Liver Inflammation, Fibrosis, and Portal Hypertension in Chronic Hepatitis B or C. Radiology 2021; 301:154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stewart RH. A Modern View of the Interstitial Space in Health and Disease. Frontiers in Veterinary Science 2020; 7:609583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yin M, Glaser KJ, Manduca A, et al. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology 2017; 284:694–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komiyama Y, Motosugi U, Maekawa S, et al. Early diagnosis of hepatic inflammation in Japanese nonalcoholic fatty liver disease patients using 3D MR elastography. Hepatology Research 2023; 53(3):208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Talwalkar JA, Yin M, Venkatesh SK, et al. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR 2009; 193:122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reiberger T The value of liver and spleen stiffness for evaluation of portal hypertension in compensated cirrhosis. Hepatology Communications 2022; 6:950–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navin PJ, Gidener T, Allen AM, et al. The role of magnetic resonance elastography in the diagnosis of noncirrhotic portal hypertension. Clinical Gastroenterology and Hepatology 2020; 18:3051–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS ONE 2014; 9:e97355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singh R, Wilson MP, Katlariwala P, Murad MH, McInnes MDF, Low G. Accuracy of liver and spleen stiffness on magnetic resonance elastography for detecting portal hypertension: a systematic review and meta-analysis. European Journal of Gastroenterology and Hepatology 2021; 32:237–245 [DOI] [PubMed] [Google Scholar]
- 88.Xu P, Lyu L, Lu X, Hu C, Xu K. Evaluating the short-term clinical efficacy of magnetic resonance elastography in patients with Budd-Chiari syndrome. Academic Radiology 2021; 28:S179–S183 [DOI] [PubMed] [Google Scholar]
- 89.Cho Y, Kabata D, Ehara E, et al. Assessing liver stiffness with conventional cut-off values overestimates liver fibrosis staging in patients who received the Fontan procedure. Hepatology Research 2021; 51:593–602 [DOI] [PubMed] [Google Scholar]
- 90.Serai SD, Tsitsiou Y, Wilkins BJ, et al. MR elastography-based staging of liver fibrosis in Fontan procedure associated liver disease is confounded by effects of venous congestion. Clinical Radiology 2022; 77:e776–e782 [DOI] [PubMed] [Google Scholar]
- 91.Kennedy P, Stocker D, Carbonell G, et al. MR elastography outperforms shear wave elastography for the diagnosis of clinically significant portal hypertension. European Radiology 2022; 32:8339–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ronot M, Lambert S, Elkrief L, et al. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. European Radiology 2014; 24:1394–1402 [DOI] [PubMed] [Google Scholar]
- 93.Guo J, Buning C, Schott E, et al. In vivo abdominal magnetic resonance elastography for the assessment of portal hypertension before and after transjugular intrahepatic portosystemic shunt implantation. Investigative Radiology 2015; 50:347–351 [DOI] [PubMed] [Google Scholar]
- 94.Guo H, Liao M, Jin J, et al. How intrahepatic cholestasis affects liver stiffness in patients with chronic hepatitis B: a study of 1197 patients with liver biopsy. European Radiology 2020; 30:1096–1104 [DOI] [PubMed] [Google Scholar]
- 95.Liu D, Li GY, Su C, et al. Effect of ligation on the viscoelastic properties of liver tissues. Journal of Biomechanics 2018; 76:235–240 [DOI] [PubMed] [Google Scholar]
- 96.Warner L, Yin M, Glaser KJ, et al. Noninvasive in vivo assessment of renal tissue elasticity during graded renal ischemia using MR elastography. Investigative Radiology 2011; 46:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Korsmo MJ, Ebrahimi B, Eirin A, et al. Magnetic resonance elastography noninvasively detects in vivo renal medullary fibrosis secondary to swine renal artery stenosis. Investigative Radiology 2013; 48:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sangha K, Chang ST, Cheung R, Deshpande VS. Cost-effectiveness of MRE versus VCTE in staging fibrosis for nonalcoholic fatty liver disease (NAFLD) patients with advanced fibrosis. Hepatology 2023; 77(5):1702–1711 [DOI] [PubMed] [Google Scholar]
- 99.Long MT, Noureddin M, Lim JK. AGA Clinical Practice Update: Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Lean Individuals: Expert Review. Gastroenterology 2022; 163:764–774 e761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Expert Panel on Gastrointestinal I, Bashir MR, Horowitz JM, et al. ACR Appropriateness Criteria(R) Chronic Liver Disease. Journal of American College of Radiology 2020; 17:S70–S80 [DOI] [PubMed] [Google Scholar]


