Abstract
Introduction
Humans use discriminative touch to perceive texture through dynamic interactions with surfaces, activating low‐threshold mechanoreceptors in the skin. It was largely assumed that texture was processed in primary somatosensory regions in the brain; however, imaging studies indicate heterogeneous patterns of brain activity associated with texture processing.
Methods
To address this, we conducted a coordinate‐based activation likelihood estimation meta‐analysis of 13 functional magnetic resonance imaging studies (comprising 15 experiments contributing 228 participants and 275 foci) selected by a systematic review.
Results
Concordant activations for texture perception occurred in the left primary somatosensory and motor regions, with bilateral activations in the secondary somatosensory, posterior insula, and premotor and supplementary motor cortices. We also evaluated differences between studies that compared touch processing to non‐haptic control (e.g., rest or visual control) or those that used haptic control (e.g., shape or orientation perception) to specifically investigate texture encoding. Studies employing a haptic control revealed concordance for texture processing only in the left secondary somatosensory cortex. Contrast analyses demonstrated greater concordance of activations in the left primary somatosensory regions and inferior parietal cortex for studies with a non‐haptic control, compared to experiments accounting for other haptic aspects.
Conclusion
These findings suggest that texture processing may recruit higher order integrative structures, and the secondary somatosensory cortex may play a key role in encoding textural properties. The present study provides unique insight into the neural correlates of texture‐related processing by assessing the influence of non‐textural haptic elements and identifies opportunities for a future research design to understand the neural processing of texture.
Keywords: activation likelihood estimation meta‐analysis, discriminative touch, functional magnetic resonance imaging, systematic review
Our review provides unique insight into the understanding of texture perception in the brain by investigating texture processing compared to non‐haptic and haptic baselines. Activation likelihood estimation demonstrated concordant activation for texture processing in primary and high‐order sensorimotor regions. Further, contrast analysis suggested texture processing preferentially recruits high‐order integrative structures.

1. INTRODUCTION
Humans typically explore and gather haptic information using discriminative touch through the glabrous skin on their hands and digits (Gibson, 1962; Lederman & Klatzky, 1993; Wagner & Gibson, 2016). Previous research investigating texture perception and brain activation commonly focus on one textural feature, with roughness the most studied (Hollins et al., 2000). This meta‐analysis aimed to collate research articles using functional magnetic resonance imaging (fMRI) methods to identify regions of the brain associated with texture perception of various stimuli during discriminative touch. For the purpose of this review, texture perception was defined as the activation of low‐threshold mechanoreceptors (LTMRs) and the dorsal column‐medial lemniscus (DCML) pathway (Gardner & Johnson, 2012a). Importantly, this excludes thermal perception via thermoreceptors and the spinothalamic tract, which has previously been included as a dimension of texture (Okamoto et al., 2013).
The glabrous skin of hands contains LTMRs, which transduce incoming tactile information from surface texture (Gomez‐Ramirez et al., 2016; Harvey et al., 2013; Johnson et al., 2000; McGlone & Reilly, 2010). Tactile information from LTMRs travels through the DCML pathway to the brain, and thalamocortical afferents convey signals to the primary (SI) and secondary somatosensory cortices (SII; Klingner et al., 2016; Raju & Tadi, 2021)). In humans, texture processing elicits bilateral activation in the SI and SII (Genna et al., 2018; Simões‐Franklin et al., 2011). Lesions in the macaque SI and SII lead to impairment of texture perception (Garcha & Ettlinger, 1980; Randolph & Semmes, 1974). Moreover, the SII is hypothesized to be responsible for roughness discrimination (Kitada et al., 2005; Sathian et al., 2011; Servos et al., 2001; Stilla & Sathian, 2008).
The activation of LTMRs requires voluntary movement or dynamic passive touch and contact pressure. The MI and non‐primary motor regions, split into the supplementary motor area (SMA) and premotor cortex, are responsible for planning and initiating limb movements (Rizzolatti & Kalaska, 2012; Rizzolatti & Luppino, 2001). Therefore, in contrast to employing voluntary movement (i.e., active touch), the MI and premotor areas would be activated due to motor preparation and execution.
Somatosensory information is also processed in the insula, with the posterior region of the insular cortex found to be functionally connected to sensorimotor areas including the SI, SII, primary motor cortex (MI), and SMA (Deen et al., 2011; Taylor et al., 2009). The insula is conceptualized as an integration hub as it is connected to many brain regions and is associated not only with sensory inputs (Craig et al., 2000; Jensen et al., 2016; Segerdahl et al., 2015) but also with affective processing (Björnsdotter et al., 2009, 2014; Morrison, 2016; Olausson et al., 2016) and higher level cognition such as decision‐making (Gogolla, 2017; Uddin et al., 2017). Therefore, texture perception in the brain likely involves the insula (Kitada et al., 2005; Stilla & Sathian, 2008).
The posterior parietal cortex (PPC) is linked to multisensory integration, combining inputs from several brain areas, including somatosensory, auditory, visual, motor, cingulate, and prefrontal cortices (Buneo & Andersen, 2006; Whitlock, 2017). Increased activation in the PPC has been demonstrated during grasping, reaching, and interacting with objects (Vingerhoets, 2014). In humans, the PPC can be divided into three regions: the superior parietal lobule (SPL), the inferior parietal lobule (IPL), and the intraparietal sulcus (IPS; Gardner & Johnson, 2012b). The SPL integrates proprioceptive information to guide motor actions (Hadjidimitrakis et al., 2019; Johns, 2014; Kalaska, 1996), while the IPL interprets sensory information to aid in the preparation of motor acts such as hand grasping movement for object manipulation (Bodegård et al., 2001; Vingerhoets, 2014). Similarly, the IPS plays a role in the multisensory processing of vision and touch (Amedi et al., 2005; Cusack, 2005; Grefkes et al., 2002; Peltier et al., 2007; Saito et al., 2003; Tal & Amedi, 2009), contributing to a range of cognitive functions including spatial imagery (Cohen et al., 1996) and attention (Macaluso et al., 2002). Lesions within these areas impact sensorimotor integration and somatosensory processing (Freund, 2001; Murray & Mishkin, 1984); lesions to the SPL and surrounding IPS can impair the sensorimotor integration of proprioceptive signals and cause optic ataxia (Andersen et al., 2014; Perenin & Vighetto, 1988), while lesions to the IPL can cause tactile agnosia (Reed & Caselli, 1994; Reed et al., 1996). Therefore, it is likely that texture discrimination through active touch activates the PPC to aid sensorimotor integration and somatosensory processing.
In the present study, a coordinate‐based meta‐analysis was performed with an activation likelihood estimation (ALE) of published fMRI findings relating to the neural correlates of texture perception (Eickhoff et al., 2009, 2012). First, we aimed to identify key brain regions involved in texture perception at hand and/or digit skin sites using concordance analysis to identify regions of the brain with the highest activation likelihood. Second, we attempted to identify key brain regions involved in texture‐specific perception when controlling for other haptic elements involved in discriminative touch (e.g., location, orientation, and shape). For this purpose, we performed conjunction and contrast analyses to compare fMRI studies which contrasted texture perception with a resting or non‐haptic control with those which used a haptic baseline to control for these non‐texture aspects of discriminative touch.
We hypothesized that areas consistently reported in tactile perception studies would result in activation, which are bilateral SI, SII, and insular cortices. Further, we expected that areas associated with voluntary movement and motor planning would show activation, including bilateral MI, SMA, premotor cortex, and PPC. When controlling for the influence of haptic processing, we anticipated an increased likelihood of activation in medial brain regions associated with higher order processing or texture‐specific processing such as the SII and insular cortex.
2. METHOD
This systematic review is reported following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines (Moher et al., 2009). The review protocol was registered on Open Science Framework (http://osf.io/kz7mg/?view_only=f47532ac93e64598b56c5c488e651845) on November 3, 2020.
2.1. Data search and extraction
Three electronic databases were examined during February 2023 (PubMed, PsycINFO, and Web of Science) using the Medical Subject Headings search terms (magnetic resonance imaging OR fMRI) AND (functional OR brain activation OR neural activity OR BOLD) AND (texture OR rough* OR smooth* OR soft*) AND (touch OR tactile OR haptic OR somatosensory). No date limit was set for the searches. A citation search was conducted of the five most recent research papers accepted for analysis.
2.2. Article selection and extraction of data
Article selection consisted of two stages and was conducted by the same two authors (Jessica Henderson and Tyler Mari). First, the title and abstract for all unique search results were assessed separately by the two authors, and studies identified as relevant were retrieved for full‐text review. During the second stage, full‐text articles, retrieved from stage one, were reviewed independently for inclusion, and disagreements were resolved via discussion or presented to a third arbiter (Nicholas Fallon). One author (Jessica Henderson) extracted the relevant coordinate data, which were cross‐checked and confirmed by a second author (Tyler Mari). Studies that reported coordinates in Talairach space were converted into Montreal Neurologic Institute (MNI) using the GingerALE software for analysis and reporting (Eickhoff et al., 2009, 2012; Turkeltaub et al., 2012). Studies that employed a region‐of‐interest (ROI) analysis to investigate the contrast of interest were included in the cohort when whole‐brain statistical data were available from online repositories, such as NeuroVault (Gorgolewski et al., 2015). In such instances, the unthresholded t‐maps resulting from the fMRI analysis were manually thresholded at uncorrected voxelwise throughout the whole brain with a cluster‐level correction to give whole‐brain results.
2.3. Eligibility criteria
The criteria for inclusion were as follows: (i) fMRI studies; (ii) original English language articles; (iii) published in a peer‐reviewed journal; (iv) healthy human participants aged 18+; (v) using a paradigm where the hand and/or fingers are either passively or actively stimulated by textured stimuli, that is, three‐dimensional (3D)‐printed texture, natural texture, or man‐made textures; (vi) coordinates were reported in the paper or supplementary material in either MNI (Evans et al., 1994) or Talairach space (Talairach & Tournoux, 1988); (vii) studies which analyzed either of the two contrasts of interest: (1) texture perception through hand and/or finger stimulation compared to non‐haptic control conditions, such as rest, visual control (e.g., visual instructions or rating scales with the absence of textured stimuli) or motor control (e.g., hand motion with the absence of textured stimuli), and (2) texture perception through hand and/or fingers stimulation compared to haptic control conditions, which included shape, location, and orientation tasks. See Figure 1 for a flowchart showing the study selection steps.
FIGURE 1.

Flow chart depicting the screening process. ALE, activation likelihood estimation.
ALE meta‐analyses were performed in BrainMap GingerALE v3.0.2 (Eickhoff et al., 2009, 2012; Turkeltaub et al., 2012). The ALE method computes an ALE value for each voxel in the brain and performs tests to determine the null distribution of the ALE statistic at each voxel, with increased ALE values suggestive of more studies reporting activated peaks in specific loci or at neighbouring voxels using a Gaussian distribution. Next, p‐values computed from the previous step are used to calculate a thresholded ALE map, and thereafter cluster analysis is performed on the thresholded map.
For the primary analyses, the comparison of texture perception > control, texture perception > non‐haptic control, and texture perception > haptic control was evaluated with permutation analyses performed with 5000 permutations. First, a cluster‐forming threshold of uncorrected was applied (Eickhoff et al., 2012), followed by cluster‐level family‐wise error correction as recommended (Eickhoff et al., 2016). For the secondary analyses, the thresholded ALE images from the primary analysis were compared using conjunction and contrast analyses; this was executed by permutation analysis with 10,000 permutations and a cluster‐level false discovery rate threshold of , with a minimum cluster size of 200 mm3 as recommended (Eickhoff et al., 2016), and in line with previous research (Morrison, 2016).
3. RESULTS
A total of 870 articles were identified from searches (PubMed, 244; PsycInfo, 362; Web of Science, 264; Figure 1). Of these, 485 articles were removed due to duplication from repeated searches. An additional 337 articles were removed during the titles and abstracts review stage. Studies excluded at this stage included those where it was clear and obvious that no suitable population was reported (106), not an experimental report published in a peer‐reviewed journal (16), did not use fMRI methods (80), not using suitable textured stimuli (130), and not addressing one of the outcomes outlined (5). Following full‐text review, a further 35 articles were removed including those that used an unsuitable contrast (121), did not utilize appropriate textured stimuli (13), did not conduct an fMRI contrast study (5), were not an experimental report journal article (3), only reported ROI analysis and whole brain data were not available (1; see Section 2.2), or which did not report findings in English (1). This resulted in a final cohort of 13 studies for the analyses of texture perception (Table 1), with the age range of participants recruited being 18–47 years. The citation search did not lead to the inclusion of any additional studies. Studies contributing to this ALE stimulated the right hand, with (Kitada et al., 2006) stimulating both hands and combining results. Therefore, concordant activation in the left and right hemispheres correspond to contralateral and ipsilateral activation, respectively.
TABLE 1.
Studies included in activation likelihood estimation (ALE) meta‐analysis.
| Author | Year | Title | N | Mean age (SD) | Description of tactile stimuli | Stimulation site | Type of stimulation | Task used in contrast | Included experiments |
|---|---|---|---|---|---|---|---|---|---|
| Gurtubay‐Antolin et al. | 2018 | Neural evidence of hierarchical cognitive control during haptic processing: An fMRI study | 17 | 23.4 (1.5) | Six real 3D objects and six textures | Right palm/hand | Active | Congruency | Haptic texture > Haptic shape |
| Kim et al. | 2015 | Decoding accuracy in supplementary motor cortex correlates with perceptual sensitivity to tactile roughness | 13 | 25.3 (3.8) | Five grades of aluminum oxide sandpaper | Right index fingertip | Active | Perception | Haptic texture (3 μm) > rest |
| Haptic texture (5 μm) > rest | |||||||||
| Haptic texture (9 μm) > rest | |||||||||
| Haptic texture (12 μm) > rest | |||||||||
| Haptic texture (40 μm) > rest | |||||||||
| Kitada et al. | 2006 | Multisensory activation of the intraparietal area when classifying grating orientation: A functional magnetic resonance imaging study | 16 | 22–47 a (range) | Nine rectangular gratings with three degrees of roughness | Right and left middle finger | Passive | Estimation | Roughness task > button press |
| Roughness task > tactile orientation b | |||||||||
| Kitada et al. | 2005 | Tactile estimation of the roughness of gratings yields a graded response in the human brain: An fMRI study | 14 | 23–26 a (range) | Linear gratings with three ridge heights | Right middle fingertip | Passive | Perception | No estimation > Rest |
| Mueller et al. | 2019 | Neural correlates of top‐down modulation of haptic shape versus roughness perception | 21 | 25.33 (3.44) | 3D‐printed cuboids with five levels of shape and roughness | Right thumb and index finger | Active | Comparison | Roughness > Rest |
| Podrebarac et al. | 2014 | Are visual texture‐selective areas recruited during haptic texture discrimination? | 13 | 27 a | Two 3D shapes with two indented texture patterns | Right hand | Active | Comparison | Haptic texture > haptic shape |
| Sathian et al. | 2011 | Dual pathways for haptic and visual perception of spatial and texture information | 18 | 20.8 a | Textiles attached to a piece of cardboard | Right hand | Active | Comparison | Haptic texture > haptic location |
| Simões‐Franklin et al. | 2011 | Active and passive touch differentially activate somatosensory cortex in texture perception | 16 | 23.6 a | Three grades of aluminum oxide sandpaper | Right middle finger | Active and passive | Estimation | Active and passive > control |
| Stilla and Sathian | 2008 | Selective visual‐haptic processing of shape and texture | 6 | 22 a | 3D meaningless objects. Textiles attached to a piece of cardboard | Right hand | Active | Comparison | Haptic texture > haptic shape |
| Tang et al. | 2021 | Brain activation related to the tactile perception of touching ridged texture using fingers | 10 | 22 (2.3) | Ridged textures with different edge shapes | Right index finger | Passive | Perception | Sharp shape > rest |
| Rounded shape > rest | |||||||||
| Flat shape > rest | |||||||||
| Wang et al. | 2016 | Brain discriminative cognition on the perception of touching different fabric using fingers actively | 8 | 28.6 a | Silk and linen swatches | Right thumb and index finger | Active | Estimation | Linen > rest |
| Silk > rest | |||||||||
| Yang et al. | 2021 | Different activation signatures in the primary sensorimotor and higher‐level regions for haptic three‐dimensional curved surface exploration | 20 | 22 (0.63) | 3D‐printed surfaces with four levels of raised dot patterns and four types of curvature, plus one flat surface | Right index and middle finger | Active | Estimation | Roughness estimation > hand motion and visual control |
| (Roughness estimation—hand motion and visual control) > (curve estimation—hand motion and visual control) b | |||||||||
| Yang et al. | 2017 | Brain networks involved in tactile speed classification of moving dot patterns: The effects of speed and dot periodicity | 20 | 21.9 (2.6) | Two surfaces with identical or pseudo‐randomly distributed dot patterns | Right middle fingertip | Passive | Estimation | Speed classification periodic > visual motor control periodic |
| Speed classification non‐periodic > visual motor control non‐periodic |
Abbreviation: 3D, three‐dimensional.
Did not report standard deviation; Estimation = Participants estimated textural properties, for example, roughness; Comparison = Presented participants with two stimuli in succession, and participants had to indicate if the stimuli were identical or different; Congruency = Judge whether a touched stimulus corresponded to an expected stimulus which had been presented before stimulus exploration; Perception = Exploration of the textured stimuli with a rest period between trials.
Not combined as a single experiment because they contribute to different analyses (i.e., texture perception > non‐haptic control or texture perception > haptic control).
3.1. Primary analyses
3.1.1. Texture perception > control
Texture perception > control contrast ALE meta‐analysis pooled data from 13 studies which contributed 15 experiments, with a total of 228 participants and 275 reported foci. The results revealed seven significant clusters (Table 2). One cluster originated from the right hemisphere and spanned from the superior temporal gyrus to the postcentral gyrus, corresponding to both the right posterior insula (PI) and SII. One cluster was identified in the left hemisphere from the supramarginal gyrus (SMG) in the IPL to the SI. Further clusters were identified in the bilateral SMA with one peak in the right SMA, two clusters encompassed the bilateral PI, and two clusters encompassed the bilateral ventral premotor cortex (PMv). Significant ALE cluster locations are illustrated in Figure 2.
TABLE 2.
Locations of significant clusters from the activation likelihood estimation (ALE) map of texture perception > control.
| Cluster # | Label | Volume (mm3) | BA | x | y | z | #Experiments | ALE |
|---|---|---|---|---|---|---|---|---|
| 1 | Insula R | 1752 | 13 | 58 | −20 | 20 | 7 | 0.021 |
| Postcentral gyrus R | 3 | 64 | −16 | 22 | 0.021 | |||
| 2 | Postcentral gyrus L | 1600 | 2 | −54 | −20 | 48 | 8 | 0.023 |
| 3 | Precentral gyrus L | 1424 | 6 | −48 | 6 | 24 | 7 | 0.019 |
| Precentral gyrus L | 6 | −58 | 8 | 28 | 0.014 | |||
| Precentral gyrus L | 6 | −58 | 2 | 32 | 0.012 | |||
| 4 | Insula L | 1224 | 13 | −36 | −6 | 10 | 6 | 0.022 |
| Insula L | 13 | −42 | −4 | 2 | 0.014 | |||
| 5 | Inferior frontal gyrus R | 984 | 9 | 50 | 8 | 24 | 4 | 0.025 |
| 6 | Insula R | 816 | 13 | 40 | −8 | 8 | 4 | 0.020 |
| 7 | Superior frontal gyrus R | 816 | 6 | 4 | 16 | 48 | 4 | 0.018 |
Abbreviations: BA, Brodmann area; L, left hemisphere; R, right hemisphere.
FIGURE 2.
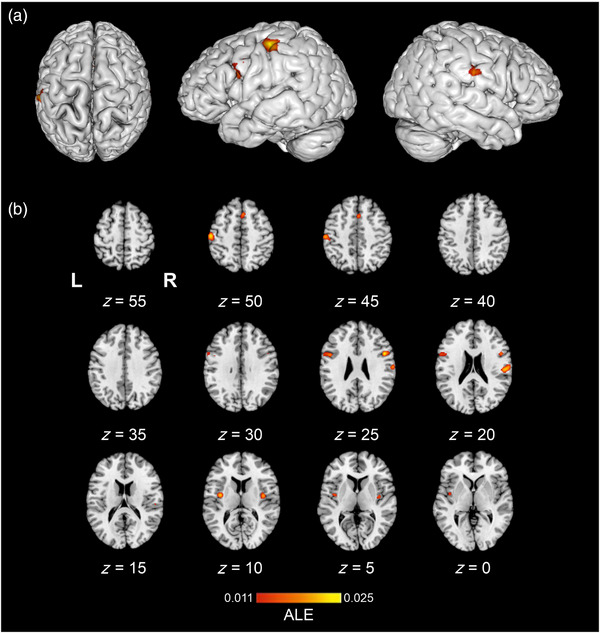
The location of significant activation likelihood estimation (ALE) clusters from the meta‐analysis of concordant activations for texture perception > control. Results are displayed overlaid onto a standardized Montreal Neurologic Institute (MNI) template anatomical brain in (a). Three‐dimensional (3D) surface projection from superior, left, and right views, respectively. (b) As a montage of coronal slices throughout the whole brain, L and R denote the left and right hemispheres, respectively. ALE scores are indicated by the color bar.
3.1.2. Texture perception > non‐haptic control
The texture perception > non‐haptic control (e.g., rest or visual control) contrast ALE meta‐analysis pooled data from nine studies which contributed to nine experiments, with a total of 138 participants and 240 reported foci. The results revealed six significant clusters (Table 3); one cluster spanned from the right superior temporal gyrus to the postcentral gyrus, consistent with both PI and SII. Cluster two, in the left hemisphere, spanned from the SMG in the IPL to the SI. Cluster six corresponded to the left SI and MI. Further clusters encompassed the bilateral SMA and the bilateral PMv. Significant ALE cluster locations are illustrated in Figure 3.
TABLE 3.
Locations of significant clusters from the activation likelihood estimation (ALE) map of texture perception > non‐haptic control.
| Cluster # | Label | Volume (mm3) | BA | x | y | z | #Experiments | ALE |
|---|---|---|---|---|---|---|---|---|
| 1 | Insula R | 1864 | 13 | 58 | −20 | 20 | 7 | 0.021 |
| Postcentral gyrus R | 3 | 64 | −16 | 22 | 0.021 | |||
| 2 | Postcentral gyrus L | 1832 | 2 | −54 | −20 | 48 | 8 | 0.023 |
| 3 | Inferior frontal gyrus L | 1336 | 9 | −58 | 6 | 22 | 5 | 0.016 |
| Precentral gyrus L | 6 | −58 | 8 | 28 | 0.014 | |||
| Precentral gyrus L | 6 | −58 | 6 | 14 | 0.013 | |||
| Precentral gyrus L | 6 | −58 | 2 | 32 | 0.012 | |||
| 4 | Inferior frontal gyrus R | 1120 | 9 | 50 | 8 | 24 | 4 | 0.025 |
| 5 | Superior frontal gyrus R | 944 | 6 | 4 | 16 | 48 | 4 | 0.018 |
| 6 | Postcentral gyrus L | 752 | 3 | −44 | −12 | 58 | 0.016 | |
| Precentral gyrus L | 4 | −38 | −20 | 52 | 4 | 0.013 |
Abbreviations: BA, Brodmann area; L, left hemisphere; R, right hemisphere.
FIGURE 3.

The location of significant activation likelihood estimation (ALE) clusters from the meta‐analysis of concordant activations for texture perception > haptic control. Results are displayed overlaid onto a standardized Montreal Neurologic Institute (MNI) template anatomical brain as a montage of coronal slices throughout the whole brain, L and R denote the left and right hemispheres, respectively. ALE scores are indicated by the color bar.
3.1.3. Texture perception > haptic control
The texture > haptic control contrast ALE meta‐analysis pooled data from a total of six studies which contributed to six experiments, with 90 participants and 35 reported foci. Findings demonstrated one significant left hemisphere cluster located in the SII (Table 4). Figure 4 illustrates the location of significant ALE clusters from the meta‐analysis of texture perception when controlling for other haptic processes.
TABLE 4.
Locations of significant clusters from the activation likelihood estimation (ALE) map of texture perception > haptic control.
| Cluster # | Label | Volume (mm3) | BA | x | y | z | #Experiments | ALE |
|---|---|---|---|---|---|---|---|---|
| 1 | Postcentral gyrus L | 608 | 43 | −54 | −14 | 22 | 2 | 0.010 |
| Postcentral gyrus L | 43 | −50 | −10 | 16 | 0.009 |
Abbreviations: BA, Brodmann area; L, left hemisphere.
FIGURE 4.
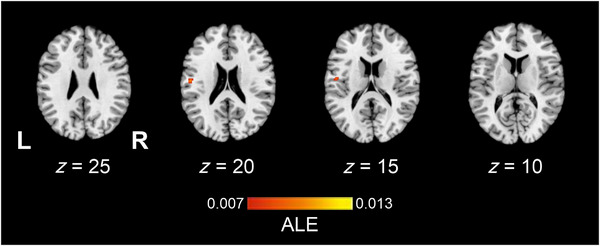
The location of significant clusters from contrast analysis of activation likelihood estimation (ALE) maps for a greater likelihood of texture perception > non‐haptic control relative to texture perception > haptic control. All clusters are overlaid onto a standardized Montreal Neurologic Institute (MNI) template anatomical brain in (a). Three‐dimensional (3D) surface projection from superior, left, and right views, respectively. (b) As a montage of coronal slices throughout the whole brain, L and R denote the left and right hemispheres, respectively. Relative Z scores are indicated by the color bar.
3.2. Secondary analyses
3.2.1. Conjunction analysis
The conjunction analysis of ALE maps representing texture perception relative to non‐haptic control and texture‐specific perception (relative to haptic control) pooled data from a total of 23 experiments, with a total of 245 participants and 273 reported foci. There were no findings of overlap of activation likelihood coordinates across both contrast types.
3.2.2. Contrast analyses
Contrast analysis comparing the ALE maps of concordant activations for each process pointed to a significantly greater likelihood of activation during general texture perception (texture perception > non‐haptic control) relative to texture‐specific perception (texture perception > haptic control) in three clusters (Figure 5, Table 5). Two left clusters included the SI and the SMG in the IPL, as well as the SI, MI, and premotor areas. The third cluster was located in the right IPL and corresponded to the SMG. The reverse contrast did not reveal any clusters indicative of increased activation likelihood estimates during the texture‐specific perception relative to general texture perception studies.
FIGURE 5.

The location of significant clusters from contrast analysis of activation likelihood estimation (ALE) maps for a greater likelihood of texture perception > non‐haptic control relative to texture perception > haptic control. All clusters are overlaid onto a standardized Montreal Neurologic Institute (MNI) template anatomical brain in (a). Three‐dimensional (3D) surface projection from superior, left, and right views, respectively. (b) As a montage of coronal slices throughout the whole brain, L and R denote the left and right hemispheres, respectively. Relative Z scores are indicated by the color bar.
TABLE 5.
Locations of significant clusters from contrast analysis of non‐haptic–haptic control.
| Cluster # | Label | Volume (mm3) | BA | x | y | z | #Experiments | Extrema (Z) |
|---|---|---|---|---|---|---|---|---|
| 1 | Inferior parietal lobule L | 1832 | 40 | −53.3 | −22.3 | 48.2 | 8 | 3.156 |
| 2 | Postcentral gyrus L | 3 | −41.2 | −12.3 | 55.2 | 4 | 3.719 | |
| Postcentral gyrus L | 640 | 3 | −40.9 | −18.7 | 54.5 | 3.156 | ||
| 3 | Inferior parietal lobule R | 232 | 40 | 55.4 | −24.6 | 19.8 | 1 | 3.036 |
Abbreviations: BA, Brodmann area; L, left hemisphere; R, right hemisphere.
3.3. Sensitivity analysis
To assess the stability of results, 13 leave‐one‐out analyses (also known as jack‐knife analyses) were conducted, whereby the primary analysis of texture perception > control was rerun, each time excluding a different single study (Supporting Information 1; Radua & Mataix‐Cols, 2009; Radua et al., 2012). The sensitivity analysis confirmed the stability of the right SII cluster and left SI/IPL across all 13 studies. Leaving out Yang et al. (2017) resulted in the left PMv and left PI clusters no longer reaching significance. The SMA cluster was not identified when leaving out Mueller et al. (2019), Simões‐Franklin et al. (2011), Yang et al. (2017), and Kitada et al. (2006). In addition, leaving out Kitada et al. (2006) and Mueller et al. (2019) resulted in the loss of the right PMv cluster. The right PI cluster was not identified as significant when leaving out Kitada et al. (2006), Mueller et al. (2019), Sathian et al. (2011), and Stilla and Sathian (2008). Further, an additional cluster in the right PI/SII was identified when removing Simões‐Franklin et al. (2011), Mueller et al. (2019), Wang et al. (2016), Yang et al. (2021), and Kim et al. (2015). Removing Kitada et al. (2005) and Yang et al. (2021) led to the identification of a further cluster in the left dorsolateral prefrontal cortex (DLPFC). Finally, a cluster from the left SI to the left MI was uncovered when removing Kitada et al. (2005), Sathian et al. (2011), Simões‐Franklin et al. (2011), and Yang et al. (2017, 2021).
4. DISCUSSION
Findings from the primary ALE meta‐analysis of texture perception relative to control revealed nine significant clusters with activation in the left SI and SMG, bilateral PMv and SMA, and the right SII and PI, consistent with our hypotheses that texture perception would activate the sensorimotor regions that are well associated with tactile perception and movement planning and execution. The presence of haptic control conditions during texture perception identified activation in the left SII, in line with our hypothesis that texture processing activates brain regions associated with higher order processing. Further, contrast analyses revealed sensorimotor (SI, MI, and SMG) activations as more predominant in the non‐haptic control contrast than when controlling for other haptic processing, suggesting that texture‐specific processing may require the activation of higher order cortical regions.
Left SII was the only region selectively activated when contrasting texture processing with a haptic control, indicating its role in texture‐specific processing. The human parietal operculum comprises four distinct cytoarchitectonic areas (OP1−4; Eickhoff et al., 2006, 2007), with concordant activation in the left SII cluster corresponding to OP4. The SII has previously been implicated in higher order processes, including attention (Chen et al., 2008; Hämäläinen et al., 2000), learning (Mishkin, 1979; Ridley & Ettlinger, 1976), and roughness discrimination (Kitada et al., 2005; Sathian et al., 2011; Servos et al., 2001; Stilla & Sathian, 2008). Additionally, concordant activation was found in OP4 during a detect‐discriminate touch meta‐analysis (Morrison, 2016), and this region, along with OP1 and OP3, has been proposed as texture selective (Sathian et al., 2011; Stilla & Sathian, 2008). Research with non‐human primates shows that lesions to the SII result in deficits in texture and shape discrimination (Garcha & Ettlinger, 1980; Murray & Mishkin, 1984), while humans with lesions in the area suffer from tactile apraxia (Binkofski et al., 2001). (Jiang et al., 1997; Pruett et al., 2000; Sinclair & Burton, 1993). Furthermore, low‐ and high‐frequency vibrotactile cues elicit differential patterns of brain activation, with increased SII activation during high‐frequency vibrotactile stimulation (Chung et al., 2013; Francis et al., 2000; Han et al., 2013; Harrington & Hunter Downs, 2001; Ryun, Kim, Lee, et al., 2017). Concordant activation in the SII when contrasting with haptic control was observed only in response to textiles, which are more likely to induce high‐frequency vibrations due to their finer grained surface texture. Taken together the results of this meta‐analysis, it is likely that the SII plays a significant role in the higher order encoding of textural properties, which may be correlated with high‐frequency vibrotactile information.
Conjunction and contrast analyses were computed to examine similarities and differences between the processing of generic discriminative touch, by comparing texture processing to non‐haptic control conditions, relative to brain regions that are more selectively activated during texture processing/evaluations, which were determined by comparing texture processing to haptic control conditions. Concordant activation in the left SI (Brodmann's area; BA 3) and bilateral SMG was more likely to be activated during texture perception compared to non‐haptic control contrast, relative to touch minus haptic control contrast tasks that accounted for other aspects of discrimination (e.g., shape). Interestingly, the conjunction analysis did not identify any overlap of findings across studies with these differing approaches. Demonstrating that concordant activation of the SII cluster in the texture relative to haptic control ALE analysis may be specific to texture processing. These findings indicate that broad aspects of tactile information are processed in the sensorimotor areas, hence the dominance in contrasts which do not correct for haptic processing in the baseline. However, the absence of deeper SII or PI clusters indicates that important aspects of texture‐specific processing may occur in higher order medial regions such as the SII and insular cortex (Eck et al., 2016; Jiang et al., 1997; Roland et al., 1998), which may require careful consideration of experimental design, and particularly baseline, to investigate. This aligns with the presence of an SII cluster in the texture relative to haptic control ALE analysis.
Bilateral PI was found to be active when investigating texture processing compared to control (i.e., non‐haptic and haptic control conditions combined). The insular cortex has been linked with somatosensation (Kurth et al., 2010; Preusser et al., 2015) and has been associated with the intensity processing of thermosensory (Craig et al., 2000) and noxious stimuli (Frot et al., 2007; Iannetti et al., 2005). Roughness‐related activation has been reported in the parietal operculum and insula (Kitada et al., 2005). Therefore, the insula may play a role in the processing or evaluation of texture intensity. For the present meta‐analysis, 10 studies were included where participants were asked to complete an estimation or comparison task, evaluating textural features with a response (Gurtubay‐Antolin et al., 2018; Kitada et al., 2006; Mueller et al., 2019; Podrebarac et al., 2014; Sathian et al., 2011; Simões‐Franklin et al., 2011; Stilla & Sathian, 2008; Wang et al., 2016; Yang et al., 2017, 2021). The insula is anatomically interconnected to the prefrontal cortex (Burton & Sinclair, 2000; Preusser et al., 2015); as such, the insula is conceptualized as an integrative region associated with both sensory input (Craig et al., 2000; Jensen et al., 2016; Segerdahl et al., 2015) and cognitive processes, such as decision‐making (Gogolla, 2017; Uddin et al., 2017). Therefore, insula activation may reflect the integration of sensory input, which may be crucial for higher order cognitive decisions based on sensory features.
SI and SMG were found to be active when comparing texture processing to both control and non‐haptic control conditions. The SI processes tactile information and encompasses BA 3a, 3b, 1, and 2 (Case et al., 2016; Chapman, 1994; Lieber & Bensmaia, 2019, 2020; Lin et al., 1996; Purves et al., 2001). Concordant activation peaked in BA 2 of the left SI, which is associated with the processing of tactile and proprioceptive input (Hyvärinen & Poranen, 1978; Padberg et al., 2019; Pons et al., 1985). Furthermore, the tactile stimulation of the fingers is consistently associated with activation of BA 2 (Hlushchuk & Hari, 2006; Holmes et al., 2019; Janko et al., 2022; Puckett et al., 2020), suggesting concordant activation in the SI corresponds to the finger area. Active touch engages sensorimotor circuits in the PPC, including the SMG located in the IPL, and activation in these regions has been associated with sensorimotor integration (Arikan et al., 2021; Batista et al., 1999; Battaglia‐Mayer et al., 2000; Buneo et al., 2002; Buneo & Andersen, 2006; Ferraina et al., 1997; Hyvärinen, 1982; Lamp et al., 2019; Mountcastle et al., 1975; Naito et al., 2005; Snyder et al., 1997).
The MI demonstrated concordant activation only when compared to the non‐haptic control condition. Concordant activation of the MI, associated with the execution of voluntary movement (Kalaska & Rizzolatti, 2012), was observed to be predominantly engaged within the hand knob area (Bonzano et al., 2022; Hardwick et al., 2013; Puce et al., 1995; Yousry et al., 1997). An exploratory analysis of experiments employing active touch found concordant activation in premotor and motor areas, while passive touch did not (Supporting Information 2). However, two studies contributing to the concordant activation identified in the MI cluster employed dynamic passive touch (Kitada et al., 2005; Tang et al., 2021); therefore, concordance of MI activation may also be due to force exerted by the finger during passive paradigms rather than solely due to active touch (Dettmers et al., 1995).
Concordant activation was identified in both the bilateral PMv and SMA across two ALE analyses, one that considered all studies irrespective of the control condition and the other that only considered baselines that did not account for other haptic elements (i.e., non‐haptic control). The PMv is associated with both tactile processing (Avanzini et al., 2016; Ryun et al., 2023) and movement‐related functions, including grasping and object manipulation (Davare et al., 2006, 2008, 2009; Fogassi et al., 2001; Reader & Holmes, 2018; Vingerhoets et al., 2013). In non‐human primates, the PMv has been linked to the evaluation of sensory information for guiding motor action (Romo et al., 2004). Five studies that contributed to the bilateral PMv clusters involved active touch (Mueller et al., 2019; Sathian et al., 2011; Simões‐Franklin et al., 2011; Wang et al., 2016; Yang et al., 2021). Accordingly, SMA neurons discharge before and during coordinated voluntary movement (Tanji, 2001; Tanji & Shima, 1996), such as button pressing. Therefore, PMv and SMA activation may reflect the evaluation of sensory information to inform response behavior during experimental paradigms.
The current meta‐analysis is impacted by the limited number of studies, partially due to the absence of a standardized paradigm, resulting in the rejection of 130 studies due to stimuli/paradigm discrepancies. Therefore, establishing a standardized texture perception paradigm would benefit the field. Further, a vast range of tactile stimuli are used in texture perception paradigms, including gratings (Kitada et al., 2005, 2006), 3D‐printed textures (Mueller et al., 2019; Yang et al., 2021), dot patterns (Yang et al., 2017, 2021), and textiles (Gurtubay‐Antolin et al., 2018; Wang et al., 2016), each varying considerably in their tactile properties. Textiles are often finer grained and therefore are more likely to rely on vibrational cues generated through movement, while coarse textures such as gratings depend on distinct spatial patterns (Moungou et al., 2016; Weber et al., 2013). Consequently, findings are difficult to collate to investigate texture‐specific processing. In the future, a standardized battery of textural stimuli would aid researchers to align and compare findings across studies, laboratories, and geographical regions. Furthermore, concordant activation identified in the SI, MI, PMv, IPL, SII, and insula parallels human electrophysiological mapping studies (Ryun et al., 2023; Ryun, Kim, Jeon, et al., 2017; Ryun, Kim, Lee, et al., 2017), providing further evidence that these areas contribute to tactile perception. While a larger sample size of 17–20 studies is recommended to detect small effects (Eickhoff et al., 2016), the alignment of these findings suggests that the current findings are robust.
Studies identified by systematic review are limited by modest participant numbers. The recommended sample size for investigating sensorimotor effects with 3T scanners is a minimum of 20 participants, and preferably 27 participants (Thirion et al., 2007). The average number of participants recruited in the studies contributing to this meta‐analysis was 15 ± 4.63 (M ± SD) with only three studies (Mueller et al., 2019; Yang et al., 2017, 2021) recruiting 20 participants or more. Therefore, contributing foci are potentially underpowered. However, a leave‐one‐out analysis was conducted to assess the sensitivity of results (Supporting Information 2; Acar et al., 2018), which demonstrated that clusters in the right SII and left SI/IPL were stable across all 13 studies. During leave‐one‐out analyses, additional clusters were identified in the left SII and left DLPFC and SI/MI, which may indicate that bilateral SII and higher order prefrontal regions are important for texture processing. However, the identification of concordant activation in these areas may be dependent on task design and/or stimuli utilized, hence the sensitivity to leave‐one‐out procedures. Thus, the importance of a standardized procedure in the field of texture processing is highlighted.
To conclude, findings revealed expected concordance in sensorimotor areas including higher order structures associated with top‐down mechanisms. Analysis of studies that included a haptic baseline to control for non‐textual processing revealed concordance solely in the left SII. Furthermore, the contrast analysis demonstrated that lateral SI and IPL are significantly more predominant when utilizing a resting baseline than in studies where textural aspects of discriminative touch are accounted for in the baseline. These findings point toward the preferential processing of texture in higher order structures, particularly the SII. Further research should carefully consider research design, and particularly the use of appropriate baseline contrasts to uncover the role of higher order structures in texture processing. Overall, the present study has furthered our understanding of texture perception, specifically when accounting for the influence of other haptic processes that offer unique insight into the neural correlates of texture‐related processing.
AUTHOR CONTRIBUTIONS
Jessica Henderson: Conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft, writing—review and editing, visualization, project administration. Tyler Mari: Investigation, writing—review and editing. Danielle Hewitt: Writing—review and editing. Alice Newton-Fenner: Writing—review and editing. Timo Giesbrecht: Conceptualization, funding acquisition, supervision. Alan Marshall: Conceptualization, supervision, writing—review and editing. Andrej Stancák: Conceptualization, supervision, writing—review and editing. Nicholas Fallon: Conceptualization, methodology, writing—review and editing, supervision, funding acquisition.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.3264.
Supporting information
Supplementary table S1. Locations of significant clusters when leaving out Gurtubay‐Antolin et al. (2018).
Supplementary table S2. Locations of significant clusters when leaving out Kim et al. (2015).
Supplementary table S3. Locations of significant clusters when leaving out Kitada et al. (2005).
Supplementary table S4. Locations of significant clusters when leaving out Kitada et al. (2006).
Supplementary table S5. Locations of significant clusters when leaving out Mueller et al. (2019).
Supplementary table S6. Locations of significant clusters when leaving out Podrebarac et al. (2014).
Supplementary table S7. Locations of significant clusters when leaving out Sathian et al. (2011).
Supplementary table S8. Locations of significant clusters when leaving out Simões‐Franklin et al. (2011).
Supplementary table S9. Locations of significant clusters when leaving out Stilla and Sathian (2008).
Supplementary table S10. Locations of significant clusters when leaving out Tang et al. (2021).
Supplementary table S11. Locations of significant clusters when leaving out Wang et al. (2016).
Supplementary table S12. Locations of significant clusters when leaving out Yang et al. (2017).
Supplementary table S13. Locations of significant clusters when leaving out Yang et al. (2021).
Supplementary table S14. Locations of significant clusters for both active and passive stimulation, with the exception of Simões‐Franklin et al. (2011).
Supplementary table S15.
Supplementary table S16.
Henderson, J. , Mari, T. , Hewitt, D. , Newton‐Fenner, A. , Giesbrecht, T. , Marshall, A. , Stancak, A. , & Fallon, N. (2023). The neural correlates of texture perception: A systematic review and activation likelihood estimation meta‐analysis of functional magnetic resonance imaging studies. Brain and Behavior, 13, e3264. 10.1002/brb3.3264
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are openly available on Open Science Framework (Henderson et al., 2020).
REFERENCES
- Acar, F. , Seurinck, R. , Eickhoff, S. B. , & Moerkerke, B. (2018). Assessing robustness against potential publication bias in Activation Likelihood Estimation (ALE) meta‐analyses for fMRI. PLoS ONE, 13(11), e0208177. 10.1371/JOURNAL.PONE.0208177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedi, A. , Von Kriegstein, K. , Van Atteveldt, N. M. , Beauchamp, M. S. , & Naumer, M. J. (2005). Functional imaging of human crossmodal identification and object recognition. Experimental Brain Research, 166(3–4), 559–571. 10.1007/S00221-005-2396-5 [DOI] [PubMed] [Google Scholar]
- Andersen, R. A. , Andersen, K. N. , Hwang, E. J. , & Hauschild, M. (2014). Optic ataxia: From Balint's syndrome to the parietal reach region. Neuron, 81(5), 967. 10.1016/J.NEURON.2014.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikan, B. E. , Voudouris, D. , Voudouri‐Gertz, H. , Sommer, J. , & Fiehler, K. (2021). Reach‐relevant somatosensory signals modulate activity in the tactile suppression network. NeuroImage, 236, 118000. 10.1016/J.NEUROIMAGE.2021.118000 [DOI] [PubMed] [Google Scholar]
- Avanzini, P. , Abdollahi, R. O. , Sartori, I. , Caruana, F. , Pelliccia, V. , Casaceli, G. , Mai, R. , Lo Russo, G. , Rizzolatti, G. , & Orban, G. A. (2016). Four‐dimensional maps of the human somatosensory system. Proceedings of the National Academy of Sciences of the United States of America, 113(13), E1936–E1943. 10.1073/PNAS.1601889113/SUPPL_FILE/PNAS.1601889113.SM04.GIF [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista, A. P. , Buneo, C. A. , Snyder, L. H. , & Andersen, R. A. (1999). Reach plans in eye‐centered coordinates. Science, 285(5425), 257–260. 10.1126/SCIENCE.285.5425.257 [DOI] [PubMed] [Google Scholar]
- Battaglia‐Mayer, A. , Ferraina, S. , Mitsuda, T. , Marconi, B. , Genovesio, A. , Onorati, P. , Lacquaniti, F. , & Caminiti, R. (2000). Early coding of reaching in the parietooccipital cortex. Journal of Neurophysiology, 83(4), 2374–2391. 10.1152/jn.2000.83.4.2374 [DOI] [PubMed] [Google Scholar]
- Binkofski, F. , Kunesch, E. , Classen, J. , Seitz, R. J. , & Freund, H. J. (2001). Tactile apraxiaUnimodal apractic disorder of tactile object exploration associated with parietal lobe lesions. Brain, 124(1), 132–144. 10.1093/BRAIN/124.1.132 [DOI] [PubMed] [Google Scholar]
- Björnsdotter, M. , Gordon, I. , Pelphrey, K. A. , Olausson, H. , & Kaiser, M. D. (2014). Development of brain mechanisms for processing affective touch. Frontiers in Behavioral Neuroscience, 8, 24. 10.3389/fnbeh.2014.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsdotter, M. , Löken, L. , Olausson, H. , Vallbo, Å. , & Wessberg, J. (2009). Somatotopic organization of gentle touch processing in the posterior insular cortex. Journal of Neuroscience, 29(29), 9314–9320. 10.1523/JNEUROSCI.0400-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodegård, A. , Geyer, S. , Grefkes, C. , Zilles, K. , & Roland, P. E. (2001). Hierarchical processing of tactile shape in the human brain. Neuron, 31(2), 317–328. 10.1016/S0896-6273(01)00362-2 [DOI] [PubMed] [Google Scholar]
- Bonzano, L. , Bortoletto, M. , Zazio, A. , Iester, C. , Stango, A. , Gasparotti, R. , Miniussi, C. , & Bove, M. (2022). The hand motor hotspot for seed‐based functional connectivity of hand motor networks at rest. Frontiers in Neuroscience, 16, 896746. 10.3389/FNINS.2022.896746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo, C. A. , & Andersen, R. A. (2006). The posterior parietal cortex: Sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia, 44(13), 2594–2606. [DOI] [PubMed] [Google Scholar]
- Buneo, C. A. , Jarvis, M. R. , Batista, A. P. , & Andersen, R. A. (2002). Direct visuomotor transformations for reaching. Nature, 416(6881), 632–636. 10.1038/416632A [DOI] [PubMed] [Google Scholar]
- Burton, H. , & Sinclair, R. J. (2000). Attending to and remembering tactile stimuli: A review of brain imaging data and single‐neuron responses. Journal of Clinical Neurophysiology, 17(6), 575–591. 10.1097/00004691-200011000-00004 [DOI] [PubMed] [Google Scholar]
- Case, L. K. , Laubacher, C. M. , Olausson, H. , Wang, B. , Spagnolo, P. A. , & Bushnell, M. C. (2016). Encoding of touch intensity but not pleasantness in human primary somatosensory cortex. Journal of Neuroscience, 36(21), 5850–5860. 10.1523/JNEUROSCI.1130-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, C. E. (1994). Active versus passive touch: Factors influencing the transmission of somatosensory signals to primary somatosensory cortex. Canadian Journal of Physiology and Pharmacology, 72(5), 558–570. 10.1139/y94-080 [DOI] [PubMed] [Google Scholar]
- Chen, T. L. , Babiloni, C. , Ferretti, A. , Perrucci, M. G. , Romani, G. L. , Rossini, P. M. , Tartaro, A. , & del Gratta, C. (2008). Human secondary somatosensory cortex is involved in the processing of somatosensory rare stimuli: An fMRI study. NeuroImage, 40(4), 1765–1771. 10.1016/J.NEUROIMAGE.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Chung, Y. G. , Kim, J. , Han, S. W. , Kim, H. S. , Choi, M. H. , Chung, S. C. , Park, J. Y. , & Kim, S. P. (2013). Frequency‐dependent patterns of somatosensory cortical responses to vibrotactile stimulation in humans: A fMRI study. Brain Research, 1504, 47–57. 10.1016/j.brainres.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Cohen, M. S. , Kosslyn, S. M. , Breiter, H. C. , Digirolamo, G. J. , Thompson, W. L. , Anderson, A. K. , Bookheimer, S. Y. , Rosen, B. R. , & Belliveau, J. W. (1996). Changes in cortical activity during mental rotation A mapping study using functional MRI. Brain, 119(1), 89–100. 10.1093/BRAIN/119.1.89 [DOI] [PubMed] [Google Scholar]
- Craig, A. D. , Chen, K. , Bandy, D. , & Reiman, E. M. (2000). Thermosensory activation of insular cortex. Nature Neuroscience, 3(2), 184–190. 10.1038/72131 [DOI] [PubMed] [Google Scholar]
- Cusack, R. (2005). The intraparietal sulcus and perceptual organization. Journal of Cognitive Neuroscience, 17(4), 641–651. 10.1162/0898929053467541 [DOI] [PubMed] [Google Scholar]
- Davare, M. , Andres, M. , Cosnard, G. , Thonnard, J. L. , & Olivier, E. (2006). Dissociating the role of ventral and dorsal premotor cortex in precision grasping. Journal of Neuroscience, 26(8), 2260–2268. 10.1523/JNEUROSCI.3386-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare, M. , Lemon, R. , & Olivier, E. (2008). Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. The Journal of Physiology, 586(11), 2735–2742. 10.1113/JPHYSIOL.2008.152603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare, M. , Montague, K. , Olivier, E. , Rothwell, J. C. , & Lemon, R. N. (2009). Ventral premotor to primary motor cortical interactions during object‐driven grasp in humans. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 45(9), 1050–1057. 10.1016/J.CORTEX.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen, B. , Pitskel, N. B. , & Pelphrey, K. A. (2011). Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex, 21(7), 1498–1506. 10.1093/CERCOR/BHQ186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmers, C. , Fink, G. R. , Lemon, R. N. , Stephan, K. M. , Passingham, R. E. , Silbersweig, D. , Holmes, A. , Ridding, M. C. , Brooks, D. J. , & Frackowiak, R. S. (1995). Relation between cerebral activity and force in the motor areas of the human brain. Journal of Neurophysiology, 74(2), 802–815. 10.1152/jn.1995.74.2.802 [DOI] [PubMed] [Google Scholar]
- Eck, J. , Kaas, A. L. , Mulders, J. L. , Hausfeld, L. , Kourtzi, Z. , & Goebel, R. (2016). The effect of task instruction on haptic texture processing: The neural underpinning of roughness and spatial density perception. Cerebral Cortex, 26(1), 384–401. 10.1093/CERCOR/BHU294 [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Amunts, K. , Mohlberg, H. , & Zilles, K. (2006). The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cerebral Cortex, 16(2), 268–279. 10.1093/CERCOR/BHI106 [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Bzdok, D. , Laird, A. R. , Kurth, F. , & Fox, P. T. (2012). Activation likelihood estimation meta‐analysis revisited. NeuroImage, 59(3), 2349–2361. 10.1016/J.NEUROIMAGE.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Grefkes, C. , Zilles, K. , & Fink, G. R. (2007). The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cerebral Cortex, 17(8), 1800–1811. 10.1093/CERCOR/BHL090 [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. 10.1002/HBM.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Nichols, T. E. , Laird, A. R. , Hoffstaedter, F. , Amunts, K. , Fox, P. T. , Bzdok, D. , & Eickhoff, C. R. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage, 137, 70. 10.1016/J.NEUROIMAGE.2016.04.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, A. C. , Collins, D. L. , Mills, S. R. , Brown, E. D. , Kelly, R. L. , & Peters, T. M. (1994). 3D statistical neuroanatomical models from 305 MRI volumes. IEEE Nuclear Science Symposium & Medical Imaging Conference, 3, 1813–1817. 10.1109/nssmic.1993.373602 [DOI] [Google Scholar]
- Ferraina, S. , Garasto, M. R. , Battaglia‐Mayer, A. , Ferraresi, P. , Johnson, P. B. , Lacquaniti, F. , & Carniniti, R. (1997). Visual control of hand‐reaching movement: Activity in parietal area 7m. European Journal of Neuroscience, 9(5), 1090–1095. 10.1111/j.1460-9568.1997.tb01460.x [DOI] [PubMed] [Google Scholar]
- Fogassi, L. , Gallese, V. , Buccino, G. , Craighero, L. , Fadiga, L. , & Rizzolatti, G. (2001). Cortical mechanism for the visual guidance of hand grasping movements in the monkey: A reversible inactivation study. Brain, 124, 571–586. [DOI] [PubMed] [Google Scholar]
- Francis, S. T. , Kelly, E. F. , Bowtell, R. , Dunseath, W. J. R. , Folger, S. E. , & McGlone, F. (2000). fMRI of the responses to vibratory stimulation of digit tips. NeuroImage, 11(3), 188–202. 10.1006/nimg.2000.0541 [DOI] [PubMed] [Google Scholar]
- Freund, H. J. (2001). The parietal lobe as a sensorimotor interface: A perspective from clinical and neuroimaging data. NeuroImage, 14(1), S142–S146. 10.1006/NIMG.2001.0863 [DOI] [PubMed] [Google Scholar]
- Frot, M. , Magnin, M. , Mauguière, F. , & Garcia‐Larrea, L. (2007). Human sii and posterior insula differently encode thermal laser stimuli. Cerebral Cortex, 17(3), 610–620. 10.1093/CERCORBHK007 [DOI] [PubMed] [Google Scholar]
- Garcha, H. S. , & Ettlinger, G. (1980). Tactile discrimination learning in the monkey: The effects of unilateral or bilateral removals of the second somatosensory cortex (area SII). Cortex, 16(3), 397–412. 10.1016/S0010-9452(80)80041-4 [DOI] [PubMed] [Google Scholar]
- Gardner, E. P. , & Johnson, K. O. (2012a). The somatosensory system: receptors and central pathways. In Schwartz J., Jessell T., Siegelbaum S., & Hudspeth A. J. (Eds.), Principles of neural science (5th ed., pp. 475–497). McGraw‐Hill Publishing. https://ebookcentral.proquest.com/lib/liverpool/detail.action?docID=4959346 [Google Scholar]
- Gardner, E. P. , & Johnson, K. O. (2012b). Touch. In Kandel E. R., Schwartz J. H., Jessell T. M., Siegelbaum S. A., & Hudspeth A. J. (Eds.), Principles of neural science ( 5th ed., pp. 498–527). McGraw‐Hill Publishing. [Google Scholar]
- Genna, C. , Oddo, C. , Fanciullacci, C. , Micera, S. , Artoni, F. , Chisari, C. , Micera, S. , & Artoni, F. (2018). Bilateral cortical representation of tactile roughness. Brain Research, 1699, 79–88. 10.1016/j.brainres.2018.06.014 [DOI] [PubMed] [Google Scholar]
- Gibson, J. J. (1962). Observations on active touch. Psychological Review, 69(6), 477–491. 10.1037/h0046962 [DOI] [PubMed] [Google Scholar]
- Gogolla, N. (2017). The insular cortex. Current Biology, 27(12), R580–R586. 10.1016/J.CUB.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Gomez‐Ramirez, M. , Hysaj, K. , & Niebur, E. (2016). Neural mechanisms of selective attention in the somatosensory system. Journal of Neurophysiology, 116(3), 1218–1231. 10.1152/jn.00637.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski, K. J. , Varoquaux, G. , Rivera, G. , Schwarz, Y. , Ghosh, S. S. , Maumet, C. , Sochat, V. V. , Nichols, T. E. , Poldrack, R. A. , Poline, J. B. , Yarkoni, T. , & Margulies, D. S. (2015). NeuroVault.org: A web‐based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics, 9(APR), 8. 10.3389/FNINF.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes, C. , Weiss, P. H. , Zilles, K. , & Fink, G. R. (2002). Crossmodal processing of object features in human anterior intraparietal cortex: An fMRI study implies equivalencies between humans and monkeys. Neuron, 35(1), 173–184. 10.1016/S0896-6273(02)00741-9 [DOI] [PubMed] [Google Scholar]
- Gurtubay‐Antolin, A. , León‐Cabrera, P. , & Rodríguez‐Fornells, A. (2018). Neural evidence of hierarchical cognitive control during haptic processing: An fMRI study. eNeuro, 5(6), ENEURO. 10.1523/ENEURO.0295-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjidimitrakis, K. , Bakola, S. , Wong, Y. T. , & Hagan, M. A. (2019). Mixed spatial and movement representations in the primate posterior parietal cortex. Frontiers in Neural Circuits, 13, 426944. 10.3389/FNCIR.2019.00015/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen, H. , Hiltunen, J. , & Titievskaja, I. (2000). fMRI activations of SI and SII cortices during tactile stimulation depend on attention. Neuroreport, 11(8), 1673–1676. 10.1097/00001756-200006050-00016 [DOI] [PubMed] [Google Scholar]
- Han, S. W. , Chung, Y. G. , Kim, H. S. , Chung, S. C. , Park, J. Y. , & Kim, S. P. (2013). Evaluation of somatosensory cortical differences between flutter and vibration tactile stimuli. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Osaka, Japan. 10.1109/EMBC.2013.6610522 [DOI] [PubMed]
- Hardwick, R. M. , Rottschy, C. , Miall, R. C. , & Eickhoff, S. B. (2013). A quantitative meta‐analysis and review of motor learning in the human brain. NeuroImage, 67, 283–297. 10.1016/J.NEUROIMAGE.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington, G. S. , & Hunter Downs, J. (2001). fMRI mapping of the somatosensory cortex with vibratory stimuli: Is there a dependency on stimulus frequency? Brain Research, 897(1–2), 188–192. 10.1016/S0006-8993(01)02139-4 [DOI] [PubMed] [Google Scholar]
- Harvey, M. A. , Saal, H. P. , Dammann, J. F. , & Bensmaia, S. J. (2013). Multiplexing stimulus information through rate and temporal codes in primate somatosensory cortex. PLoS Biology, 11(5), e1001558. 10.1371/journal.pbio.1001558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, J. , Mari, T. , Giesbrecht, T. , Marshall, A. , Stancak, A. , & Fallon, N. (2020). A systematic review and meta‐analysis of fMRI studies investigating texture perception during active and passive stimulation. Open Science Framework. 10.17605/OSF.IO/KZ7MG [DOI] [Google Scholar]
- Hlushchuk, Y. , & Hari, R. (2006). Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. The Journal of Neuroscience, 26(21), 5819. 10.1523/JNEUROSCI.5536-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollins, M. , Bensmaïa, S. , Karlof, K. , & Young, F. (2000). Individual differences in perceptual space for tactile textures: Evidence from multidimensional scaling. Perception & Psychophysics, 62(8), 1534–1544. 10.3758/BF03212154 [DOI] [PubMed] [Google Scholar]
- Holmes, N. P. , Tamè, L. , Beeching, P. , Medford, M. , Rakova, M. , Stuart, A. , & Zeni, S. (2019). Sensory processing: Locating primary somatosensory cortex in human brain stimulation studies: Experimental evidence. Journal of Neurophysiology, 121(1), 336. 10.1152/JN.00641.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen, J. (1982). Posterior parietal lobe of the primate brain. Physrev, 62(3), 1060–1129. 10.1152/PHYSREV.1982.62.3.1060 [DOI] [PubMed] [Google Scholar]
- Hyvärinen, J. , & Poranen, A. (1978). Receptive field integration and submodality convergence in the hand area of the post‐central gyrus of the alert monkey. The Journal of Physiology, 283(1), 539–556. 10.1113/JPHYSIOL.1978.SP012518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti, G. D. , Zambreanu, L. , Cruccu, G. , & Tracey, I. (2005). Operculoinsular cortex encodes pain intensity at the earliest stages of cortical processing as indicated by amplitude of laser‐evoked potentials in humans. Neuroscience, 131(1), 199–208. 10.1016/J.NEUROSCIENCE.2004.10.035 [DOI] [PubMed] [Google Scholar]
- Janko, D. , Thoenes, K. , Park, D. , Willoughby, W. R. , Horton, M. , & Bolding, M. (2022). Somatotopic mapping of the fingers in the somatosensory cortex using functional magnetic resonance imaging: A review of literature. Frontiers in Neuroanatomy, 16, 866848. 10.3389/FNANA.2022.866848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, K. B. , Regenbogen, C. , Ohse, M. C. , Frasnelli, J. , Freiherr, J. , & Lundström, J. N. (2016). Brain activations during pain: A neuroimaging meta‐analysis of patients with pain and healthy controls. Pain, 157(6), 1279–1286. 10.1097/J.PAIN.0000000000000517 [DOI] [PubMed] [Google Scholar]
- Jiang, W. , Tremblay, F. , & Chapman, C. E. (1997). Neuronal encoding of texture changes in the primary and the secondary somatosensory cortical areas of monkeys during passive texture discrimination. Journal of Neurophysiology, 77(3), 1656–1662. 10.1152/JN.1997.77.3.1656 [DOI] [PubMed] [Google Scholar]
- Johns, P. (2014). Functional neuroanatomy. In P. Johns (Ed.), Clinical neuroscience (pp. 27–47). Churchill Livingstone. [Google Scholar]
- Johnson, K. O. , Yoshioka, T. , & Vega Bermudez, F. (2000). Tactile functions of mechanoreceptive afferents innervating the hand. Journal of Clinical Neurophysiology, 17(6), 539–558. 10.1097/00004691-200011000-00002 [DOI] [PubMed] [Google Scholar]
- Kalaska, J. F. (1996). Parietal cortex area 5 and visuomotor behavior. Canadian Journal of Physiology and Pharmacology, 74(4), 483–498. 10.1139/Y96-040/ASSET/Y96-040.FP.PNG_V03 [DOI] [PubMed] [Google Scholar]
- Kalaska, J. F. , & Rizzolatti, G. (2012). Voluntary movement: The primary motor cortex. In Kandel E. R., Schwartz J. H., Jessell T. M., Siegelbaum S. A., & Hudspeth A. J. (Eds.), Principles of neural science ( 5th ed., pp. 835–863). McGraw‐Hill Publishing. [Google Scholar]
- Kitada, R. , Hashimoto, T. , Kochiyama, T. , Kito, T. , Okada, T. , Matsumura, M. , Lederman, S. J. , & Sadato, N. (2005). Tactile estimation of the roughness of gratings yields a graded response in the human brain: An fMRI study. NeuroImage, 25(1), 90–100. 10.1016/j.neuroimage.2004.11.026 [DOI] [PubMed] [Google Scholar]
- Kitada, R. , Kito, T. , Saito, D. N. , Kochiyama, T. , Matsumura, M. , Sadato, N. , & Lederman, S. J. (2006). Multisensory activation of the intraparietal area when classifying grating orientation: A functional magnetic resonance imaging study. Journal of Neuroscience, 26(28), 7491–7501. 10.1523/JNEUROSCI.0822-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Chung, Y. G. , Park, J.‐Y. , Chung, S.‐C. , Wallraven, C. , Bülthoff, H. H. , & Kim, S.‐P. (2015). Decoding accuracy in supplementary motor cortex correlates with perceptual sensitivity to tactile roughness. PloS One, 10(6), e0129777. 10.1371/journal.pone.0129777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingner, C. M. , Brodoehl, S. , Huonker, R. , & Witte, O. W. (2016). The processing of somatosensory information shifts from an early parallel into a serial processing mode: A combined fMRI/MEG study. Frontiers in Systems Neuroscience, 10(DEC), 103. 10.3389/FNSYS.2016.00103/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth, F. , Zilles, K. , Fox, P. T. , Laird, A. R. , & Eickhoff, S. B. (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta‐analysis. Brain Structure & Function, 214(5–6), 519. 10.1007/S00429-010-0255-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamp, G. , Goodin, P. , Palmer, S. , Low, E. , Barutchu, A. , & Carey, L. M. (2019). Activation of bilateral secondary somatosensory cortex with right hand touch stimulation: A meta‐analysis of functional neuroimaging studies. Frontiers in Neurology, 10(JAN), 424645. 10.3389/FNEUR.2018.01129/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman, S. J. , & Klatzky, R. L. (1993). Extracting object properties through haptic exploration. Acta Psychologica, 84, 29–40. 10.1016/0001-6918(93)90070-8 [DOI] [PubMed] [Google Scholar]
- Lieber, J. D. , & Bensmaia, S. J. (2019). High‐dimensional representation of texture in somatosensory cortex of primates. Proceedings of the National Academy of Sciences of the United States of America, 116(8), 3268–3277. 10.1073/PNAS.1818501116/SUPPL_FILE/PNAS.1818501116.SAPP.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber, J. D. , & Bensmaia, S. J. (2020). Emergence of an invariant representation of texture in primate somatosensory cortex. Cerebral Cortex, 30(5), 3228–3239. 10.1093/cercor/bhz305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, W. , Kuppusamy, K. , Haacke, E. M. , & Burton, H. (1996). Functional MRI in human somatosensory cortex activated by touching textured surfaces. Journal of Magnetic Resonance Imaging, 6(4), 565–572. 10.1002/jmri.1880060402 [DOI] [PubMed] [Google Scholar]
- Macaluso, E. , Frith, C. D. , & Driver, J. (2002). Directing attention to locations and to sensory modalities: Multiple levels of selective processing revealed with PET. Cerebral Cortex, 12(4), 357–368. 10.1093/CERCOR/12.4.357 [DOI] [PubMed] [Google Scholar]
- McGlone, F. , & Reilly, D. (2010). The cutaneous sensory system. Neuroscience and Biobehavioral Reviews, 34(2), 148–159. 10.1016/j.neubiorev.2009.08.004 [DOI] [PubMed] [Google Scholar]
- Mishkin, M. (1979). Analogous neural models for tactual and visual learning. Neuropsychologia, 17(2), 139–151. 10.1016/0028-3932(79)90005-8 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ, 339(7716), 332–336. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, I. (2016). ALE meta‐analysis reveals dissociable networks for affective and discriminative aspects of touch. Human Brain Mapping, 37(4), 1308–1320. 10.1002/hbm.23103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moungou, A. , Thonnard, J. L. , & Mouraux, A. (2016). EEG frequency tagging to explore the cortical activity related to the tactile exploration of natural textures. Scientific Reports, 6(January), 1–10. 10.1038/srep20738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle, V. B. , Lynch, J. C. , Georgopoulos, A. , Sakata, H. , & Acuna, C. (1975). Posterior parietal association cortex of the monkey: Command functions for operations within extrapersonal space. Journal of Neurophysiology, 38(4), 871–908. 10.1152/jn.1975.38.4.871 [DOI] [PubMed] [Google Scholar]
- Mueller, S. , de Haas, B. , Metzger, A. , Drewing, K. , Fiehler, K. , Haas, B. , Metzger, A. , Drewing, K. , & Fiehler, K. (2019). Neural correlates of top‐down modulation of haptic shape versus roughness perception. Human Brain Mapping, 40(18), 5172–5184. 10.1002/hbm.24764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, E. A. , & Mishkin, M. (1984). Relative contributions of SII and area 5 to tactile discrimination in monkeys. Behavioural Brain Research, 11(1), 67–83. 10.1016/0166-4328(84)90009-3 [DOI] [PubMed] [Google Scholar]
- Naito, E. , Roland, P. E. , Grefkes, C. , Choi, H. J. , Eickhoff, S. , Geyer, S. , Zilles, K. , & Ehrsson, H. H. (2005). Dominance of the right hemisphere and role of area 2 in human kinesthesia. Journal of Neurophysiology, 93(2), 1020–1034. 10.1152/JN.00637.2004/ASSET/IMAGES/LARGE/Z9K0020543650005.JPEG [DOI] [PubMed] [Google Scholar]
- Okamoto, S. , Nagano, H. , & Yamada, Y. (2013). Psychophysical dimensions of tactile perception of textures. IEEE Transactions on Haptics, 6(1), 81–93. 10.1109/TOH.2012.32 [DOI] [PubMed] [Google Scholar]
- Olausson, H. , Wessberg, J. , Morrison, I. , & McGlone, F. (2016). Affective touch and the neurophysiology of CT afferents. Springer. 10.1007/978-1-4939-6418-5 [DOI] [Google Scholar]
- Padberg, J. , Cooke, D. F. , Cerkevich, C. M. , Kaas, J. H. , & Krubitzer, L. (2019). Cortical connections of area 2 and posterior parietal area 5 in macaque monkeys. Journal of Comparative Neurology, 527(3), 718–737. 10.1002/CNE.24453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier, S. , Stilla, R. , Mariola, E. , LaConte, S. , Hu, X. , & Sathian, K. (2007). Activity and effective connectivity of parietal and occipital cortical regions during haptic shape perception. Neuropsychologia, 45(3), 476–483. 10.1016/J.NEUROPSYCHOLOGIA.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Perenin, M. T. , & Vighetto, A. (1988). Optic ataxia: A specific disruption in visuomotor mechanismsi. Different aspects of the deficit in reaching for objects. Brain, 111(3), 643–674. 10.1093/BRAIN/111.3.643 [DOI] [PubMed] [Google Scholar]
- Podrebarac, S. K. , Goodale, M. A. , & Snow, J. C. (2014). Are visual texture‐selective areas recruited during haptic texture discrimination? NeuroImage, 94, 129–137. 10.1016/j.neuroimage.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Pons, T. P. , Garraghty, P. E. , Cusick, C. G. , & Kaas, J. H. (1985). The somatotopic organization of area 2 in macaque monkeys. Journal of Comparative Neurology, 241(4), 445–466. 10.1002/CNE.902410405 [DOI] [PubMed] [Google Scholar]
- Preusser, S. , Thiel, S. D. , Rook, C. , Roggenhofer, E. , Kosatschek, A. , Draganski, B. , Blankenburg, F. , Driver, J. , Villringer, A. , & Pleger, B. (2015). The perception of touch and the ventral somatosensory pathway. Brain, 138(3), 540. 10.1093/BRAIN/AWU370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett, J. R. , Sinclair, R. J. , & Burton, H. (2000). Response patterns in second somatosensory cortex (SII) of awake monkeys to passively applied tactile gratings. Journal of Neurophysiology, 84(2), 780–797. 10.1152/JN.2000.84.2.780/ASSET/IMAGES/LARGE/9K0801162014.JPEG [DOI] [PubMed] [Google Scholar]
- Puce, A. , Constable, R. T. , Luby, M. L. , McCarthy, G. , Nobre, A. C. , Spencer, D. D. , Gore, J. C. , & Allison, T. (1995). Functional magnetic resonance imaging of sensory and motor cortex: Comparison with electrophysiological localization. Journal of Neurosurgery, 83(2), 262–270. 10.3171/JNS.1995.83.2.0262 [DOI] [PubMed] [Google Scholar]
- Puckett, A. M. , Bollmann, S. , Junday, K. , Barth, M. , & Cunnington, R. (2020). Bayesian population receptive field modeling in human somatosensory cortex. NeuroImage, 208, 116465. 10.1016/J.NEUROIMAGE.2019.116465 [DOI] [PubMed] [Google Scholar]
- Purves, D. , Augustine, G. J. , Fitzpatrick, D. , Katz, L. C. , LaMantia, A.‐S. , McNamara, J. O. , & Williams, S. M. (2001). The somatic sensory cortex . https://www.ncbi.nlm.nih.gov/books/NBK11153/
- Radua, J. , & Mataix‐Cols, D. (2009). Voxel‐wise meta‐analysis of grey matter changes in obsessive–compulsive disorder. British Journal of Psychiatry, 195(5), 393–402. 10.1192/bjp.bp.108.055046 [DOI] [PubMed] [Google Scholar]
- Radua, J. , Mataix‐Cols, D. , Phillips, M. L. , El‐Hage, W. , Kronhaus, D. M. , Cardoner, N. , & Surguladze, S. (2012). A new meta‐analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry, 27(8), 605–611. 10.1016/j.eurpsy.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Raju, H. , & Tadi, P. (2021). Neuroanatomy, somatosensory cortex. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK555915/ [PubMed] [Google Scholar]
- Randolph, M. , & Semmes, J. (1974). Behavioral consequences of selective subtotal ablations in the postcentral gyrus of Macaca mulatta . Brain Research, 70(1), 55–70. 10.1016/0006-8993(74)90211-x [DOI] [PubMed] [Google Scholar]
- Reader, A. T. , & Holmes, N. P. (2018). The left ventral premotor cortex is involved in hand shaping for intransitive gestures: Evidence from a two‐person imitation experiment. Royal Society Open Science, 5(10). 181356. 10.1098/RSOS.181356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, C. L. , & Caselli, R. J. (1994). The nature of tactile agnosia: A case study. Neuropsychologia, 32(5), 527–539. 10.1016/0028-3932(94)90142-2 [DOI] [PubMed] [Google Scholar]
- Reed, C. L. , Caselli, R. J. , & Farah, M. J. (1996). Tactile agnosia. Underlying impairment and implications for normal tactile object recognition. Brain, 119, 875–888. [DOI] [PubMed] [Google Scholar]
- Ridley, R. M. , & Ettlinger, G. (1976). Impaired tactile learning and retention after removals of the second somatic sensory projection cortex (SII) in the monkey. Brain Research, 109(3), 656–660. 10.1016/0006-8993(76)90048-2 [DOI] [PubMed] [Google Scholar]
- Rizzolatti, G. , & Kalaska, J. F. (2012). Voluntary movement: The parietal and premotor cortex. In Schwartz J., Jessell T., Siegelbaum S., & Hudspeth A. J. (Eds.), Principles of neural science (5th ed., pp. 865–892). McGraw‐Hill Publishing. [Google Scholar]
- Rizzolatti, G. , & Luppino, G. (2001). The cortical motor system. Neuron, 31(6), 889–901. 10.1016/S0896-6273(01)00423-8 [DOI] [PubMed] [Google Scholar]
- Roland, P. E. , O'Sullivan, B. , & Kawashima, R. (1998). Shape and roughness activate different somatosensory areas in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 95(6), 3295–3300. 10.1073/PNAS.95.6.3295/ASSET/887A97C5-67C3-42E1-B544-CDB3A4E81D0E/ASSETS/GRAPHIC/PQ0383981001.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo, R. , Hernández, A. , & Zainos, A. (2004). Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron, 41(1), 165–173. 10.1016/S0896-6273(03)00817-1 [DOI] [PubMed] [Google Scholar]
- Ryun, S. , Kim, J. S. , Jeon, E. , & Chung, C. K. (2017). Movement‐related sensorimotor high‐gamma activity mainly represents somatosensory feedback. Frontiers in Neuroscience, 11(JUL), 276548. 10.3389/FNINS.2017.00408/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryun, S. , Kim, J. S. , Lee, H. , & Chung, C. K. (2017). Tactile frequency‐specific high‐gamma activities in human primary and secondary somatosensory cortices. Scientific Reports, 7(1), 15442. 10.1038/s41598-017-15767-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryun, S. , Kim, M. , Kim, J. S. , & Chung, C. K. (2023). Cortical maps of somatosensory perception in human. NeuroImage, 276, 120197. 10.1016/J.NEUROIMAGE.2023.120197 [DOI] [PubMed] [Google Scholar]
- Saito, D. N. , Okada, T. , Morita, Y. , Yonekura, Y. , & Sadato, N. (2003). Tactile‐visual cross‐modal shape matching: A functional MRI study. Cognitive Brain Research, 17(1), 14–25. 10.1016/S0926-6410(03)00076-4 [DOI] [PubMed] [Google Scholar]
- Sathian, K. , Lacey, S. , Stilla, R. , Gibson, G. O. , Deshpande, G. , Hu, X. , LaConte, S. , & Glielmi, C. (2011). Dual pathways for haptic and visual perception of spatial and texture information. NeuroImage, 57(2), 462–475. 10.1016/J.NEUROIMAGE.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerdahl, A. R. , Mezue, M. , Okell, T. W. , Farrar, J. T. , & Tracey, I. (2015). The dorsal posterior insula subserves a fundamental role in human pain. Nature Neuroscience, 18(4), 499–500. 10.1038/NN.3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servos, P. , Lederman, S. , Wilson, D. , & Gati, J. (2001). fMRI‐derived cortical maps for haptic shape, texture, and hardness. Cognitive Brain Research, 12(2), 307–313. 10.1016/s0926-6410(01)00041-6 [DOI] [PubMed] [Google Scholar]
- Simões‐Franklin, C. , Whitaker, T. A. , & Newell, F. N. (2011). Active and passive touch differentially activate somatosensory cortex in texture perception. Human Brain Mapping, 32(7), 1067–1080. 10.1002/hbm.21091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, R. J. , & Burton, H. (1993). Neuronal activity in the second somatosensory cortex of monkeys (Macaca mulatta) during active touch of gratings. Journal of Neurophysiology, 70(1), 331–350. 10.1152/jn.1993.70.1.331 [DOI] [PubMed] [Google Scholar]
- Snyder, L. H. , Batista, A. P. , & Andersen, R. A. (1997). Coding of intention in the posterior parietal cortex. Nature, 386(6621), 167–170. 10.1038/386167A0 [DOI] [PubMed] [Google Scholar]
- Stilla, R. , & Sathian, K. (2008). Selective visuo‐haptic processing of shape and texture. Human Brain Mapping, 29(10), 1123–1138. 10.1002/hbm.20456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji, J. , & Shima, K. (1996). Supplementary motor cortex in organization of movement. European Neurology, 36(Suppl.1), 13–19. 10.1159/000118878 [DOI] [PubMed] [Google Scholar]
- Tal, N. , & Amedi, A. (2009). Multisensory visual–tactile object related network in humans: Insights gained using a novel crossmodal adaptation approach. Experimental Brain Research, 198(2–3), 165. 10.1007/S00221-009-1949-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach, J. , & Tournoux, T. (1988). Co‐planar stereotaxic atlas of the human brain. Thieme Medical Publishers. [Google Scholar]
- Tang, W. , Shu, Y. , Bai, S. , Peng, Y. , Yang, L. , & Liu, R. (2021). Brain activation related to the tactile perception of touching ridged texture using fingers. Skin Research and Technology, 28, 254–264. 10.1111/srt.13122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji, J. (2001). Sequential organization of multiple movements involvement of cortical motor areas. Annual Review of Neuroscience, 24(1), 631–651. 10.1146/annurev.neuro.24.1.631 [DOI] [PubMed] [Google Scholar]
- Taylor, K. S. , Seminowicz, D. A. , & Davis, K. D. (2009). Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping, 30(9), 2731. 10.1002/HBM.20705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirion, B. , Pinel, P. , Mériaux, S. , Roche, A. , Dehaene, S. , & Poline, J. B. (2007). Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. NeuroImage, 35(1), 105–120. 10.1016/J.NEUROIMAGE.2006.11.054 [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eickhoff, S. B. , Laird, A. R. , Fox, M. , Wiener, M. , & Fox, P. (2012). Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Human Brain Mapping, 33(1), 1–13. 10.1002/HBM.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, L. Q. , Nomi, J. S. , Hébert‐Seropian, B. , Ghaziri, J. , & Boucher, O. (2017). Structure and function of the human insula. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 34(4), 300. 10.1097/WNP.0000000000000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets, G. (2014). Contribution of the posterior parietal cortex in reaching, grasping, and using objects and tools. Frontiers in Psychology, 5(MAR), 151. 10.3389/FPSYG.2014.00151/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets, G. , Nys, J. , Honoré, P. , Vandekerckhove, E. , & Vandemaele, P. (2013). Human left ventral premotor cortex mediates matching of hand posture to object use. PLoS One, 8(7), e70480. 10.1371/JOURNAL.PONE.0070480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, A. , & Gibson, J. J. (2016). Pre‐Gibsonian observations on active touch. History of Psychology, 19(2), 93–104. 10.1037/hop0000028 [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Yu, W. , Chen, K. , & Zhang, Z. (2016). Brain discriminative cognition on the perception of touching different fabric using fingers actively. Skin Research and Technology, 22(1), 63–68. 10.1111/srt.12229 [DOI] [PubMed] [Google Scholar]
- Weber, A. I. , Saal, H. P. , Lieber, J. D. , Cheng, J. W. J.‐W. , Manfredi, L. R. , Dammann, J. F. , & Bensmaia, S. J. (2013). Spatial and temporal codes mediate the tactile perception of natural textures. Proceedings of the National Academy of Sciences, 110(42), 17107–17112. 10.1073/pnas.1305509110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock, J. R. (2017). Posterior parietal cortex. Current Biology, 27(14), R691–R695. 10.1016/J.CUB.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Yang, J. , Kitada, R. , Kochiyama, T. , Yu, Y. , Makita, K. , Araki, Y. , Wu, J. , & Sadato, N. (2017). Brain networks involved in tactile speed classification of moving dot patterns: The effects of speed and dot periodicity. Scientific Reports, 7(1), 40931. 10.1038/srep40931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Molfese, P. J. , Yu, Y. , Handwerker, D. A. , Chen, G. , Taylor, P. A. , Ejima, Y. , Wu, J. , & Bandettini, P. A. (2021). Different activation signatures in the primary sensorimotor and higher‐level regions for haptic three‐dimensional curved surface exploration. NeuroImage, 231, 117754. 10.1016/j.neuroimage.2021.117754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry, T. A. , Schmid, U. D. , Alkadhi, H. , Schmidt, D. , Peraud, A. , Buettner, A. , & Winkler, P. (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain: A Journal of Neurology, 120(1), 141–157. 10.1093/BRAIN/120.1.141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table S1. Locations of significant clusters when leaving out Gurtubay‐Antolin et al. (2018).
Supplementary table S2. Locations of significant clusters when leaving out Kim et al. (2015).
Supplementary table S3. Locations of significant clusters when leaving out Kitada et al. (2005).
Supplementary table S4. Locations of significant clusters when leaving out Kitada et al. (2006).
Supplementary table S5. Locations of significant clusters when leaving out Mueller et al. (2019).
Supplementary table S6. Locations of significant clusters when leaving out Podrebarac et al. (2014).
Supplementary table S7. Locations of significant clusters when leaving out Sathian et al. (2011).
Supplementary table S8. Locations of significant clusters when leaving out Simões‐Franklin et al. (2011).
Supplementary table S9. Locations of significant clusters when leaving out Stilla and Sathian (2008).
Supplementary table S10. Locations of significant clusters when leaving out Tang et al. (2021).
Supplementary table S11. Locations of significant clusters when leaving out Wang et al. (2016).
Supplementary table S12. Locations of significant clusters when leaving out Yang et al. (2017).
Supplementary table S13. Locations of significant clusters when leaving out Yang et al. (2021).
Supplementary table S14. Locations of significant clusters for both active and passive stimulation, with the exception of Simões‐Franklin et al. (2011).
Supplementary table S15.
Supplementary table S16.
Data Availability Statement
Data that support the findings of this study are openly available on Open Science Framework (Henderson et al., 2020).


