Abstract
Background
Rapid deployment aortic valve replacement (RD-AVR) has been recently introduced with encouraging results. Outcomes of isolated RD-AVR include good hemodynamic profile, facilitation of minimally invasive techniques, and reduction of surgical times. However, role of this prosthesis in concomitant surgery is not well known.
Methods
In 2016, we formed a registry to monitor the introduction of this prosthesis, RApid Deployment Aortic Replacement (RADAR). We aim to report mid-term outcomes focusing on patients who had RD-AVR combined with other surgical procedures.
Results
Between July 2012 and February 2021, 370 patients were included in this registry (mean age, 75.8±8.0 years; 64.32% male; mean EuroSCORE II, 3.5±2.8). Of these, 128 (34.59%) had concomitant procedures including myocardial revascularization surgery in 69 patients (53.91%), surgery on the ascending aorta in 34 (26.56%), and procedures on other valves in 10 patients (7.81%). There were no significant differences between the isolated AVR and concomitant AVR groups in postoperative complications, in-hospital mortality (4.72% vs. 3.32%, P=0.524), or hemodynamic behavior of these prostheses. Three-year survival was 83.73% and 89.89% in the isolated and concomitant AVR group respectively. There was no difference in survival between the two groups (log-rank test, P=0.4124).
Conclusions
Our results support the safety and efficacy of the Edwards INTUITY valve system even in complex aortic valve disease with additional cardiac procedures. RD-AVR could become a useful tool for concomitant surgeries where surgical times are expected to be prolonged.
Keywords: Rapid deployment (RD), aortic valve replacement (AVR), combined surgery
Highlight box.
Key findings
• There was no difference in complications or hemodynamics between the isolated and combined cardiac surgery groups with INTUITY valve.
What is known and what is new?
• Rapid deployment (RD) valves reduce cross clamp times and myocardial ischemia in isolated aortic valve replacement (AVR).
• We analyze the RApid Deployment Aortic Replacement registry to show if outcomes of RD-AVR procedures are impacted by concomitant procedures.
What is the implication, and what should change now?
• Concomitant procedures do not impact the outcomes of RD-AVR procedures. It may safely improve outcomes and could have an important impact.
Introduction
Rapid deployment valves (RDVs) are relatively new in clinical practice for aortic valve replacement (AVR) with encouraging results (1,2). Different studies have reported advantages of this new technology compared to conventional bioprosthesis, such as improved hemodynamic profile, significant decrease in surgical times, and facilitation of minimally invasive approaches (1-6). Conversely, these rapid deployment (RD) aortic valves present an increase in postoperative permanent pacemaker implantation (PPI) rate compared with conventional bioprosthesis (7,8).
Due to the increasing age of the population in Europe, the use of biological prostheses in aortic position has increased in recent decades (9). In addition, many older patients require additional cardiac surgical procedures, besides AVR (9). However, the role of RDV with concomitant procedures is not well studied. The objective of this study is to analyze the results of patients included in the RApid Deployment Aortic Replacement (RADAR) registry that received the Edwards INTUITY valve system (Edwards Lifesciences, Irvine, CA, USA) in combination with other cardiac surgical procedures. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-191/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The master Ethics Committee at the main center was the Official IRB of Galicia (Spain). The protocol was approved by all local institutional Ethics Committees on 1/16/2020 (Study No. Xunta de Galicia/Conselleria de Sanidade: 2016/018). Written informed consent was obtained from patients or patients’ authorized representatives prior to study inclusion.
Study valve implantation
The Edwards INTUITY valve system is a stented bioprosthesis based on the Edwards Perimount valve design (Edwards Lifesciences). The main feature is the sub-annular balloon expandable stainless stent inflow frame and the cloth skirt that stabilizes the valve in the left ventricle outflow tract. The implant procedure of this prosthesis has been previously described (1-6). After a hockey stick aortotomy towards the non-coronary sinus, the native aortic valve is resected with the aortic annulus decalcified in a standard fashion. Three sutures are implanted at the nadir of the aortic sinuses and the valve is guided down until placed in a supra-annular position. The infra-annular frame is expanded with a balloon at a pressure between 3 and 5 atmospheres, depending on the size of the prosthesis for 10 seconds, and finally the three guide sutures are tied down.
Registry design
In 2016, the RADAR registry began to collect the experience with the Edwards INTUITY valve system in 8 Spanish centers. The study protocol of the RADAR registry was previously published (10). This registry is a real-world multicenter, observational, prospective, single-arm, non-randomized study; its main objective is to obtain information about this relatively novel type of prosthesis and to facilitate its use in current clinical practice.
The aim of the present study is to analyze the results of patients included in the RADAR registry for having received the Edwards INTUITY RD aortic prosthesis with other concomitant cardiac surgical procedures.
Statistical analysis
A descriptive analysis of all the variables included in the study was undertaken. A normality assessment of the quantitative variables was performed with the Shapiro-Wilk test. The quantitative variables were expressed as median (interquartile range) or mean ± standard deviation (SD) as appropriate. The qualitative variables were expressed as n (%). The comparison of means was made by Student’s t-test or Mann-Whitney U test, as appropriate. The difference between group variables was analyzed using the Student’s t-test for independent data. The association of qualitative variables was estimated using either the chi-square statistic or Fisher’s test. A value of P<0.05 was considered statistically significant. Overall survival analysis was performed using Kaplan-Meier analysis with in- or out-of-hospital mortality established as a terminal event. Survival was compared using the log-rank test. The lost values were treated statistically as unknown values. StataCorp 2015 software package was used (Stata Statistical Software: Release 14, College Station, TX, USA; StataCorp LP for statistical analysis).
Results
Between July 2012 and February 2021, 370 patients were included in the RADAR registry [mean age 75.8±8.0 years; 64.32% male; mean EuroSCORE II, 3.5±2.8; 92.70% were New York Heart Association (NYHA) functional grade II or III]. Of these, 128 (34.59%) had other cardiac surgery associated with AVR (combined surgery) (Figure 1). This population constitutes the objective of this study (Table 1).
Figure 1.
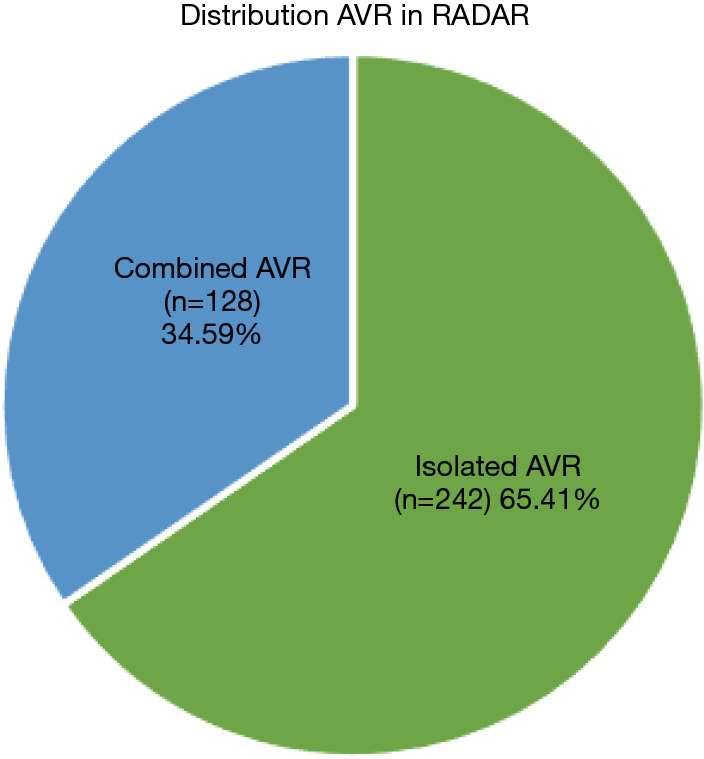
Patient distribution in the RADAR registry. AVR, aortic valve replacement; RADAR, RApid Deployment Aortic Replacement.
Table 1. Demographics and characteristics.
| Parameters | Isolated AVR (n=242) | Combined AVR (n=128) |
|---|---|---|
| Age (years) | 76.13±0.39 | 75.28±0.52 |
| Gender (male) | 148 (61.16) | 90 (70.21) |
| Weight (kg) | 76.36±0.85 | 76.30±1.05 |
| Height (cm) | 161.94±0.57 | 163.48±0.84 |
| EuroSCORE II | 2.59±0.16 | 5.36±0.36 |
| Smoker | 67 (27.69) | 31 (24.41) |
| AHT | 169 (70.12) | 104 (81.89) |
| DM | 91 (37.60) | 47 (36.72) |
| Dyslipidemia | 144 (59.75) | 85 (66.41) |
| Previous CVA | 12 (4.96) | 6 (4.69) |
| Arteriopathy | 18 (7.44) | 11 (8.59) |
| COPD | 34 (14.05) | 16 (12.50) |
| PHT | 14 (5.79) | 11 (8.59) |
| Previous cardiac insufficiency | 35 (14.46) | 70 (54.69) |
| NYHA functional grade | ||
| I | 5 (2.10) | 4 (3.20) |
| II | 115 (48.32) | 60 (48.00) |
| III | 111 (46.64) | 57 (45.60) |
| IV | 5 (2.10) | 3 (2.40) |
| Creatinine (mg/dL) | 1.09±0.05 | 1.13±0.05 |
Data were expressed as mean ± SD or n (%). AVR, aortic valve replacement; AHT, arterial hypertension; DM, diabetes mellitus; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease; PHT, pulmonary hypertension; NYHA, New York Heart Association; SD, standard deviation.
The most common concomitant procedures were coronary artery bypass grafting (CABG) surgery in 69 patients (53.91% of the concomitant group and 18.65% of the total registry), ascending aorta surgery in 34 (26.56% and 9.19%, respectively), followed at a distance by procedures on other valves in 10 patients (1 case associating CABG corresponding to the 0.27% of the total RADAR registry and 0.78% of the concomitant group and 9 cases with exclusively multi-valve surgery corresponding to 2.43% and 7.03%, respectively). Two cases of atrial fibrillation surgery (1.56% and 0.54% respectively) and 3 left atrial appendage (LAA) ligation (2.34% and 0.81% respectively). In the combined procedures on other valves, 8 mitral prostheses were implanted (2 mechanical prostheses and 6 biological prostheses), 2 mitral rings, and 1 tricuspid ring (Table 2).
Table 2. Concomitant procedures.
| AVR procedure distribution | N | Total RADAR registry (n=370), % | Combined AVR group (n=128), % |
|---|---|---|---|
| Isolated AVR | 242 | 65.41 | NA |
| AVR & ascending aortic surgery | 34 | 9.19 | 26.56 |
| AVR & maze | 2 | 0.54 | 1.56 |
| AVR & LAA ligation | 3 | 0.81 | 2.34 |
| AVR & CABG | 69 | 18.65 | 53.91 |
| AVR & another valve procedure | 9 | 2.43 | 7.03 |
| AVR & CABG & ascending aortic surgery | 3 | 0.81 | 2.34 |
| AVR & CABG & another valvular procedure | 1 | 0.27 | 0.78 |
| AVR & other procedures | 7 | 1.89 | 5.47 |
AVR, aortic valve replacement; RADAR, RApid Deployment Aortic Replacement; LAA, left atrial appendage; CABG, coronary artery bypass grafting.
The number of grafts in the cases of concomitant CABG: 26 patients (38%) received one graft, another 26 (38%) received two grafts, 13 patients (19%) received three grafts, and 4 patients (6%) received four grafts. Considering the sizes of the implanted prostheses in the combined surgery group (n=139), the most frequently implanted sizes were 23 mm in 45 patients (35%), 25 mm in 38 patients (30%), 21 mm in 30 patients (24%), 27 mm in 8 patients (6%), and 19 mm in 6 patients (5%). In isolated AVR, the mean times of myocardial ischemia and cardiopulmonary bypass (CPB) were 45±18 and 63±25 min, respectively. For concomitant surgery, mean times of myocardial ischemia and CPB rose to 87±28 and 118±41 min, respectively. The stay in the intensive care unit (ICU) was shorter for the isolated valve surgery population (isolated vs. concomitant: 3.32±4.70 vs. 4.24±7.09 days, P=0.203), while the hospital stay was slightly shorter in patients with concomitant procedure (concomitant vs. isolated: 6.00±4.75 vs. 7.00±5.00 days, P=0.265) but without statistical significance in both cases. Regarding perioperative complications, no significant differences were found between the two groups for in-hospital mortality (4.72% vs. 3.32%, P=0.524) (Table 3).
Table 3. Perioperative complications.
| Complication | Isolated AVR (n=242) | Combined AVR (n=128) | Total (n=370) | P value |
|---|---|---|---|---|
| Bleeding reintervention | 12 (4.96) | 11 (8.66) | 23 (6.23) | 0.162 |
| Atrial fibrillation | 59 (24.38) | 37 (29.13) | 96 (26.02) | 0.482 |
| Postoperative PPI | 19 (7.85) | 10 (7.87) | 29 (7.86) | 0.769 |
| CVA | 6 (2.48) | 2 (1.57) | 8 (2.21) | 0.571 |
| Perioperative AMI | 2 (0.83) | 1 (0.79) | 3 (0.81) | 0.968 |
| AKI requiring hemofiltration | 10 (4.15) | 10 (7.94) | 20 (5.45) | 0.129 |
| Intrahospitalary death | 9 (3.32) | 7 (4.72) | 16 (3.70) | 0.524 |
Data were expressed as n (%). AVR, aortic valve replacement; PPI, permanent pacemaker implantation; CVA, cerebrovascular accident; AMI, acute myocardial infarction; AKI, acute kidney injury.
The incidence of postoperative atrial fibrillation was 26.02%, and the early rate (in-hospital) PPI was 7.86%. When analyzing the hemodynamic behavior of the RD prosthesis in the aortic position, no significant differences were observed between both populations for the peak gradient, mean gradient, or effective valve area (Table 4).
Table 4. Hemodynamic results of isolated AVR vs. AVR with concomitant procedure.
| Hemodynamic behavior in follow up | Isolated AVR | Combined AVR | Test Wilcoxon P value | |||
|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | |||
| Peak to peak gradient (mmHg) | 142 | 17.70 (8.62) | 54 | 15.77 (9.76) | 0.163 | |
| Mean gradient (mmHg) | 135 | 9.33 (5.23) | 47 | 10.03 (4.76) | 0.389 | |
| Effective valvular area (cm2) | 57 | 1.76 (0.45) | 10 | 1.84 (0.51) | 0.608 | |
AVR, aortic valve replacement; SD, standard deviation.
During the in-hospital stay, no cases of prosthetic endocarditis, thrombosis, early structural failure, or hemolysis were reported. During follow-up, 27 patients (7.6%) died. All-cause mortality of 11%. The mean follow-up was 1.48 years, with 564.36 patient-years of time at risk. The 3-year survival of the entire population was 85.31% (Figure 2). No difference in survival between the two groups at 3 years was found (log-rank test, P=0.4124) (Figure 3). There were no cases of reoperation during follow-up due to significant periprosthetic leak, structural valve deterioration, or endocarditis. We did not find any case of endocarditis, valve thrombosis, prosthesis displacement or migration, or hemolysis during follow-up.
Figure 2.
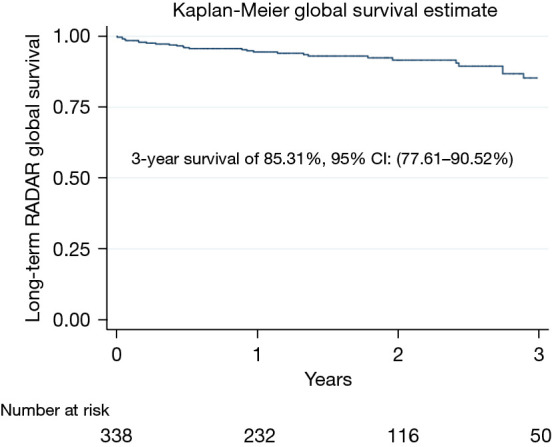
Global survival. RADAR, RApid Deployment Aortic Replacement; CI, confidence interval.
Figure 3.
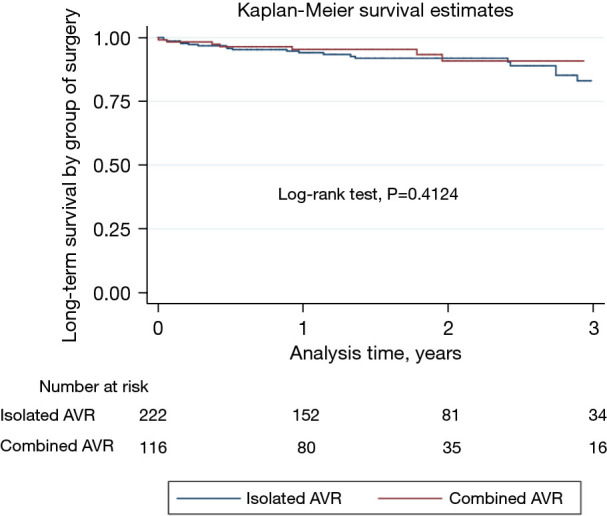
Isolated AVR vs. AVR with concomitant procedure. AVR, aortic valve replacement.
Discussion
Our results show that the implantation of an Edwards INTUITY valve system in the context of AVR with concomitant procedures is a feasible, reproducible, and safe procedure with good clinical and hemodynamic results observed. Due to both the progressive aging of the population in the Western world and technological advances in cardiac surgery and anesthesia, patients who benefit from cardiac surgery are getting older, more fragile with greater comorbidities, which is why they face increasingly complex interventions (9). Previous studies have linked the longer duration of myocardial ischemia and CPB times with higher perioperative mortality and morbidity; paradoxically, this does not happen in our series (11,12).
RD aortic prostheses first appeared in the 1960s (13) and re-emerged in the second decade of the 21st century within the group of biological prostheses (1-6). These prostheses make it possible to shorten surgical times, reduce myocardial ischemia and CPB times, and favors less invasive approaches (1-6). Previous studies with this technology have focused mainly on the isolated AVR procedure (1-6,14). In 2016, the RADAR registry began with the aim of bringing together the experience with the Edwards INTUITY RD aortic prosthesis in 8 Spanish centers (10). Around 35% of the cases included in this registry had concomitant surgery added to AVR. This percentage is similar to that of other previous series of RD and sutureless aortic prostheses (15,16). In our series, CABG was also the most frequently associated procedure (53.91%), followed by surgery on the ascending aorta (26.56%), and surgery on other heart valves, mainly the mitral valve (7.81%).
Mitro-aortic surgery using an Edwards INTUITY RD prosthesis poses a certain challenge since the presence of the infra-annular stent, by which the prosthesis is attached to the left ventricular outflow tract, could deform the outflow tract and interfere with the previously implanted mitral prosthesis. The length of the infra-annular stent varies between 6 and 8 mm depending on the size of the Edwards INTUITY prosthesis (1,8,9), so we do not recommend implanting the Edwards INTUITY prosthesis if the aortic-mitral distance does not measure 8 mm. If 8 mm distance is maintained, no interference with the mitral prosthesis has been reported (17,18). Although, no problems were seen in our series for combined aortic and mitral valve replacements. Regarding the number of grafts in the cases of concomitant coronary surgery, it should be noted that 75% of the patients received one or two grafts and the remaining 25% received three or more grafts.
Considering the size of the implanted RD prosthesis, it should be noted that 30% of the patients analyzed received a small prosthesis (19–21 mm) and the remaining 70% received 23 mm or larger valve similar to the previous series (19). As expected, the myocardial ischemia and CPB times were higher in the combined surgery group. When analyzing the perioperative complications of both groups, we did not find significant differences in any variable. The incidence of postoperative atrial fibrillation was 26%, similar to that reported by other groups (14,15). The early rate of PPI was 7.8%, which is lower than that reported by other groups with this type of prosthesis (7,20). Unlike other studies, we found no differences in the incidence of postoperative pacemakers between isolated AVR with RD prosthesis and cases of concomitant or combined surgery (6). We have previously reported that low preoperative weight (as a surrogate for small aortic valve annulus) and preoperative arrhythmias were related to the need for postoperative PPI. Moreover, we recommend not to oversize the valve and carefully consider the implantation of this technology in patients with pre-existing arrhythmia to minimize the risk for postoperative PPI (21).
Hospital mortality was 3.7%, which is slightly higher than that calculated by preoperative EuroSCORE II (3.5%) but lower than the published registry of the Spanish Society of Cardiovascular and Endovascular Surgery (SECCE) in 2019 than was 5.75% for this type of intervention (22). The stay both in the ICU and in the hospital for both the groups was short, especially in the case of the concomitant surgery group given their complexity. Shorter surgical times for AVR surgery have been related to shorter intensive care and intubation times, outcomes, and in-hospital stay; thus, utilization of the Edwards INTUITY valve system could be of benefit in terms of recovery after cardiac surgery for this challenging subset of patients (23). Regarding hemodynamic profile, the mean trans-prosthetic gradients at discharge were low in both groups, and the mean valve effective area upon leaving the hospital was 1.8 cm2. Our results align with other publications for this RD aortic valve and confirm the good hemodynamics of the Edwards INTUITY valve system (1,14,15,19,24). Survival of the entire series at 3 years was 85.31%, and we found no difference in terms of survival between the two groups. These values are in line with previous studies for both combined surgery with sutureless prostheses (16) and surgery with the Edwards INTUITY system (14,25). This could especially be interesting for patients undergoing AVR with concomitant procedures because of their increased perioperative surgical risk.
Limitations
There are some limitations to this study in relation to its retrospective nature, the number of patients per center was not homogeneous. First, follow-up was not available for all patients due to the continuous actualization of the data and its corresponding center. Echocardiographic examinations were performed by different teams and technicians, and adverse events were not reviewed by an external committee. Second, there is no control group with conventional biological prostheses and concomitant surgery, but we believe that this study is a good reflection of the daily clinical practice.
Conclusions
In a “real-world” setting, data from the RADAR registry show 237 excellent outcomes of both isolated- AVR and AVR with concomitant procedures using an RDV. Our results support the safety of the Edwards INTUITY valve system even for complex aortic valve disease with additional cardiac procedures. RD-AVR could become a useful tool for combined and complex surgeries where surgical times are expected to be prolonged.
Supplementary
The article’s supplementary files as
Acknowledgments
The present manuscript underwent oral and in-person presentation at the 35th EACTS Annual Meeting in Barcelona on October 14th, 2021.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The master Ethics Committee at the main center was the Official IRB of Galicia (Spain). The protocol was approved by all local institutional Ethics Committees on 1/16/2020 (Study No. Xunta de Galicia/Conselleria de Sanidade: 2016/018). Written informed consent was obtained from patients or patients’ authorized representatives prior to study inclusion.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-191/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-191/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-191/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-191/coif). All authors report that this registry was supported by a research grant provided by Edwards Lifesciences. The authors have no other conflicts of interest to declare.
References
- 1.Kocher AA, Laufer G, Haverich A, et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110-5; discussion 115-6. 10.1016/j.jtcvs.2012.07.108 [DOI] [PubMed] [Google Scholar]
- 2.Haverich A, Wahlers TC, Borger MA, et al. Three-year hemodynamic performance, left ventricular mass regression, and prosthetic-patient mismatch after rapid deployment aortic valve replacement in 287 patients. J Thorac Cardiovasc Surg 2014;148:2854-60. 10.1016/j.jtcvs.2014.07.049 [DOI] [PubMed] [Google Scholar]
- 3.Borger MA, Dohmen PM, Knosalla C, et al. Haemodynamic benefits of rapid deployment aortic valve replacement via a minimally invasive approach: 1-year results of a prospective multicentre randomized controlled trial. Eur J Cardiothorac Surg 2016;50:713-20. 10.1093/ejcts/ezw042 [DOI] [PubMed] [Google Scholar]
- 4.Wahlers TC, Haverich A, Borger MA, et al. Early outcomes after isolated aortic valve replacement with rapid deployment aortic valve. J Thorac Cardiovasc Surg 2016;151:1639-47. 10.1016/j.jtcvs.2015.12.058 [DOI] [PubMed] [Google Scholar]
- 5.Andreas M, Wallner S, Habertheuer A, et al. Conventional versus rapid-deployment aortic valve replacement: a single-centre comparison between the Edwards Magna valve and its rapid-deployment successor. Interact Cardiovasc Thorac Surg 2016;22:799-805. 10.1093/icvts/ivw052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahmanian PB, Kaya S, Eghbalzadeh K, et al. Rapid Deployment Aortic Valve Replacement: Excellent Results and Increased Effective Orifice Areas. Ann Thorac Surg 2018;105:24-30. 10.1016/j.athoracsur.2017.07.047 [DOI] [PubMed] [Google Scholar]
- 7.Barnhart GR, Accola KD, Grossi EA, et al. TRANSFORM (Multicenter Experience With Rapid Deployment Edwards INTUITY Valve System for Aortic Valve Replacement) US clinical trial: Performance of a rapid deployment aortic valve. J Thorac Cardiovasc Surg 2017;153:241-251.e2. 10.1016/j.jtcvs.2016.09.062 [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Barbeito M, Arribas JM, Vazquez A, et al. Risk Factors for Postoperative Pacemaker Implantation After Rapid Deployment Aortic Valve Replacement: Results from the RADAR Registry. Adv Ther 2021;38:1832-42. 10.1007/s12325-021-01622-z [DOI] [PubMed] [Google Scholar]
- 9.Beckmann A, Meyer R, Lewandowski J, et al. German Heart Surgery Report 2020: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 2021;69:294-307. 10.1055/s-0041-1730374 [DOI] [PubMed] [Google Scholar]
- 10.Bautista-Hernandez V, Cal-Purriños N, Arribas-Leal JM, et al. Rapid Deployment Aortic Replacement (RADAR) Registry in Spain: a protocol. BMJ Open 2017;7:e011437. 10.1136/bmjopen-2016-011437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814-22. 10.1053/j.jvca.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 12.Al-Sarraf N, Thalib L, Hughes A, et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg 2011;9:104-9. 10.1016/j.ijsu.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 13.Magovern GJ, Cromie HW. Sutureless prosthetic heart valves. J Thorac Cardiovasc Surg 1963;46:726-36. [PubMed] [Google Scholar]
- 14.Andreas M, Coti I, Rosenhek R, et al. Intermediate-term outcome of 500 consecutive rapid-deployment surgical aortic valve procedures†. Eur J Cardiothorac Surg 2019;55:527-33. 10.1093/ejcts/ezy273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Onofrio A, Tessari C, Filippini C, et al. Early and Mid-Term Results of Rapid Deployment Valves: The Intuity Italian Registry (INTU-ITA). Ann Thorac Surg 2018;106:1742-9. 10.1016/j.athoracsur.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 16.Shrestha M, Folliguet TA, Pfeiffer S, et al. Aortic valve replacement and concomitant procedures with the Perceval valve: results of European trials. Ann Thorac Surg 2014;98:1294-300. 10.1016/j.athoracsur.2014.05.033 [DOI] [PubMed] [Google Scholar]
- 17.Schlömicher M, Bechtel M, Taghiyev Z, et al. The Use of Rapid Deployment Valves in Combined Aortic and Mitral Valve Surgery: One-Year Clinical and Echocardiographic Outcomes. Innovations (Phila) 2017;12:201-6. 10.1097/IMI.0000000000000373 [DOI] [PubMed] [Google Scholar]
- 18.Schlömicher M, Bechtel M, Taghiyev Z, et al. Intermediate Outcomes after Rapid Deployment Aortic Valve Replacement in Multiple Valve Surgery. Thorac Cardiovasc Surg 2020;68:595-601. 10.1055/s-0039-1685178 [DOI] [PubMed] [Google Scholar]
- 19.Laufer G, Haverich A, Andreas M, et al. Long-term outcomes of a rapid deployment aortic valve: data up to 5 years. Eur J Cardiothorac Surg 2017;52:281-7. 10.1093/ejcts/ezx103 [DOI] [PubMed] [Google Scholar]
- 20.Deutsch O, Deisenhofer I, Koch-Buettner K, et al. Need for permanent pacemaker implantation following implantation of the rapid deployment valve in combined procedures: a single centre cohort study. J Thorac Dis 2021;13:2128-36. 10.21037/jtd-20-3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Barbeito M, Arribas JM, Vazquez A, et al. Risk Factors for Postoperative Pacemaker Implantation After Rapid Deployment Aortic Valve Replacement: Results from the RADAR Registry. Adv Ther 2021;38:1832-42. 10.1007/s12325-021-01622-z [DOI] [PubMed] [Google Scholar]
- 22.Cuerpo Caballero G, López Menéndez J, Polo López L, et al. Cirugía cardiovascular en España en el año 2019. Registro de intervenciones de la Sociedad Española de Cirugía Cardiovascular y Endovascular. Cir Cardiov 2021;28:162-76. [Google Scholar]
- 23.Santarpino G, Pfeiffer S, Concistré G, et al. The Perceval S aortic valve has the potential of shortening surgical time: does it also result in improved outcome? Ann Thorac Surg 2013;96:77-81; discussion 81-2. 10.1016/j.athoracsur.2013.03.083 [DOI] [PubMed] [Google Scholar]
- 24.Iacovelli F, Desario P, Cafaro A, et al. The hemodynamic performance of balloon-expandable aortic bioprostheses in the elderly: a comparison between rapid deployment and transcatheter implantation. Hellenic J Cardiol 2022;68:9-16. 10.1016/j.hjc.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 25.Arribas JM, Rivera-Caravaca JM, Moreno JA, et al. Experiencia de 7 años con la protesis aortica de rápido despliegue Edwards Intuity. Cir Cardiov 2021;28:77-83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


