Abstract
The uptake and degradation of nanomolar levels of [methyl-14C]choline in estuarine water samples and in seawater filtrate cultures composed mainly of natural free-living bacteria was studied. Uptake of [14C]choline exhibited Michaelis-Menten kinetics, with Kt + Sn values of 1.7 to 2.9 nM in filtrate cultures and 1.7 to 4.1 nM in estuarine-water samples. Vmax values ranged from 0.5 to 3.3 nM · h−1. The uptake system for choline in natural microbial assemblages therefore displays very high affinity and appears able to scavenge this compound at the concentrations expected in seawater. Uptake of choline was inhibited by some natural structural analogs and p-chloromercuribenzoate, indicating that the transporter may be multifunctional and may involve a thiol binding site. When 11 nM [14C]choline was added to water samples, a significant fraction (>50%) of the methyl carbon was respired to CO2 in incubations lasting 10 to 53 h. Cells taking up [14C]choline produced [14C]glycine betaine ([14C]GBT), and up to 80% of the radioactivity retained by cells was in the form of GBT, a well-known osmolyte. Alteration of the salinity in filtrate cultures affected the relative proportion of [14C]choline degraded or converted to [14C]GBT, without substantially affecting the total metabolism of choline. Increasing the salinity from 14 to 25 or 35 ppt caused more [14C]GBT to be produced from choline but less 14CO2 to be produced than in the controls. Lowering the salinity to 7 ppt decreased [14C]GBT production and increased 14CO2 production slightly. Intracellular accumulations of [14C]GBT in the salt-stressed cultures were osmotically significant (34 mM). Choline may be used as an energy substrate by estuarine bacteria and may also serve as a precursor of the osmoprotectant GBT, particularly as bacteria are mixed into higher-salinity waters.
Choline [(CH3)3N+CH2CH2OH] is a methylated nitrogen compound that is a common constituent of eucaryotic membranes in the form of phosphatidylcholine and therefore should be widespread in the marine environment. The fate of choline in aquatic systems is of interest because it contains nitrogen (C/N = 5) and is a precursor of glycine betaine [(CH3)3N+CH2COOH] (GBT), one of the most potent osmoprotectants known (5, 15, 29). A variety of different bacteria including Escherichia coli, Staphylococcus aureus, Bacillus subtilus, Rhizobium meliloti, Rhodobacter sphaeroides and Vibrio costicola oxidatively convert choline to GBT (1, 3, 4, 11, 19, 27). Choline is oxidized to GBT in a two-step process with betaine aldehyde as an intermediate. In Alcaligenes spp., a soluble choline oxidase can carry out both steps of choline oxidation (16), while in E. coli and other bacteria, a membrane-bound choline dehydrogenase is primarily responsible for oxidation to the aldehyde, which is further oxidized by a soluble betaine aldehyde dehydrogenase (3, 4, 11). The overall reaction requires NAD+ and produces H2O2 in addition to GBT.
A supply of exogenous choline, and its subsequent conversion to GBT, has been shown to confer osmotolerance to bacteria when cells are grown at otherwise inhibitory osmolarities (11, 15). On the other hand, choline has no osmoprotectant effects in mutants which are defective in their ability to convert choline to GBT (26, 27), indicating that conversion to GBT is required for choline to be an osmoprotectant. Furthermore, choline uptake and oxidation activities are under osmotic control in a number of bacteria, with enhanced transport and oxidation at high osmolarities (3, 4, 11).
Choline has recently been measured at nanomolar levels in coastal seawater (22), but its fate in this environment is poorly known. The uptake and degradation of nanomolar levels of [methyl-14C]choline were therefore studied in estuarine water samples and in seawater filtrate cultures composed mainly of natural free-living bacteria. The effects of salinity changes, typical of estuarine environments, on choline uptake and degradation patterns were addressed experimentally.
MATERIALS AND METHODS
Sample collection and incubation.
Estuarine water samples were collected, using an acid-rinsed bucket, from the east end pier on Dauphin Island, Ala. (30°15′N, 88°05′W). This site is located at the mouth of Mobile Bay, a large (1,000-km2), shallow (mean depth, 3 m) subtropical estuary which empties into the northern Gulf of Mexico. Surface water salinities at the sampling site are highly variable over time, due to seasonal rainfall patterns, tidal flushing, and meteorological conditions. The typical salinity range over an annual period is from 1 to 33 ppt. Samples collected for this study had salinities which ranged from 12 to 17 and temperatures which ranged from 15 to 31°C. After collection, the water samples were returned, within minutes, to the laboratory for further processing.
Incubations were carried out with either whole, unfiltered estuarine water or filtrates generated by subjecting water to gravity filtration through 142-mm GF/F filters (nominal retention, >0.7 μm; Whatman). Experiments with whole water were conducted within hours of water collection, while filtrate cultures were incubated in the dark, in 1-liter Teflon bottles, for approximately 24 h prior to initiation of uptake measurements. The GF/F filtrates contained mainly free-living bacteria, since observations by epifluorescence microcroscopy showed that photoautotrophs and microzooplankton were in very low abundance compared with bacteria.
Determination of uptake kinetics.
The dependence of the [14C]choline uptake rate on the concentration of added [14C]choline was examined in both whole water and 24-h-old GF/F filtrate cultures. Aliquots (10 ml) of freshly collected seawater or filtrate cultures were incubated at the in situ temperature in a series of acid-washed glass 125-ml serum bottles. Individual samples received spike additions of [14C]choline to yield a range of added [14C]choline concentrations. The concentrations used varied in each experiment, but the overall range was from 0.2 to 40 nM. The [14C]choline was added as an aqueous stock, and the addition volume never exceeded 100 μl (i.e., <1% of the sample volume). The samples were incubated for 5 or 20 min depending on the experiment. Incubations were kept short to minimize the fraction of added [14C]choline that would be taken up and to minimize potential artifacts such as the induction of enzymatic activity. Incubation was terminated by filtration of the sample onto 0.2-μm-pore-size Supor filters (Gelman) with a vacuum of <10 cm Hg and a multiple-place filtration manifold (Hoefer Scientific). The filters were rinsed three times with 1 ml of 0.2-μm-filtered water of the same salinity as the samples. The amount of 14C activity captured on the Supor filters was assumed to represent the total amount taken up by the cells. This assumption is probably valid because the degradation of the [14C]choline to 14CO2 was found to be <5% of the total uptake in these short incubations (data not shown). Blanks consisting of 0.2-μm-filtered water treated with [14C]choline yielded filter counts (disintegration per minute) near background levels. Kinetic data were analyzed by the method of Wright and Hobbie (28) as described by Kiene et al. (8).
Uptake, retention, and degradation of [14C]choline.
An estuarine water sample (salinity of 24 ppt) was treated with 10.8 nM [14C]choline and incubated in a 1-liter Teflon bottle held in the dark at the in situ temperature (27°C). Incubation was carried out for approximately 53 h. Subsamples (10 ml) were periodically removed for analysis of total 14C-particulates, 14CO2, and [14C]GBT in particulate material. Each fraction was measured in duplicate. Similar experiments were repeated on several occasions with similar results in each case. A representative experiment is presented here.
The total uptake of [14C]choline into particles larger than 0.2 μm was measured by filtering 10-ml water samples (in duplicate) onto 0.2-μm-pore-size Supor filters (Gelman) with a vacuum of <10 cm Hg. The filters were rinsed three times with ∼1 ml of 0.2-μm-filtered water of the same salinity as the sample. These filters were counted directly in 5 ml of Ecolume (ICN Biomedicals) as a measure of total incorporation of radioactivity. Controls consisting of 0.2-μm-filtered water or formalin-treated water gave negligible total uptake (<20 dpm/filter) for the amounts of isotope (<5,000 dpm/ml) used during these experiments.
Production and retention of [14C]GBT in the filterable material were determined by taking subsamples in parallel with those for total uptake, as described above, and placing the filters (in duplicate) into 5 ml of methanol-choroform-water (MCW) (12:5:1) for extraction of [14C]GBT. Approximately 50 nmol of unlabeled GBT was added to each of the extraction vials to aid the recovery of labeled GBT. After >24 h, the filters were removed from the MCW and rinsed gently with methanol into the same vial. The filters were counted separately as a measure of nonextractable material. The sum of [14C]GBT and nonextractable material was typically >80% of the total activity measured on separately collected parallel filters. The MCW extract was evaporated to dryness under a stream of N2 at 45°C and then reconstituted in 0.25 ml of water. The reconstituted extract was filtered through either Z-spin microcentrifuge filters or 0.2-μm-pore-size nylon high-pressure liquid chromatography (HPLC) syringe filters (both from Gelman). The extract was then injected into an HPLC apparatus for separation of GBT and collection of the peak fraction. Separation took place on a Partisil SCX column (4.5 mm [inner diameter] by 250 mm) with 50 mM KH2PO4 containing 2.5% methanol as the eluent. The flow rate was 0.9 ml/min. Because of the addition of unlabeled GBT, the chromatographic peaks were clearly observed with a UV detector (190 nm), and the retention time of GBT was 5.2 min. Fractions corresponding to GBT peaks were collected with a Gilson fraction collector, and each fraction was combined with 5 ml of Ecolume. The radioactivity on the filters and HPLC peak fractions was then determined with a Packard liquid scintillation counter by using the external-standard method and the TSIE quench correction parameter.
At each time point, the amount of 14CO2 produced was determined by pipetting 10 ml of the sample into a 125-ml serum bottle. The sample was then acidified with 0.3 ml of 2 M HCl, and the bottle was quickly capped with a serum stopper fitted with a hanging plastic cup (Kontes). The cup held a wick consisting of a pleated glass fiber filter (Gelman AE) to which 0.3 ml of 1 N NaOH had been added. Acidified samples were set on a rotary shaker (120 rpm) for >4 h, which was sufficient time to allow for >95% of the 14CO2 in the bottle to become trapped in the wick. After the trapping step, the cups with their wicks were placed directly into scintillation vials and counted with Ecolume. Stable counts were obtained after the chemiluminescence had subsided (1 to 3 days).
Effects of salinity on choline uptake and degradation.
Estuarine water with a salinity of 14 ppt was collected and filtered through GF/F filters to yield a filtrate culture composed mainly of free-living bacteria. A filtrate culture was used for this experiment to minimize the possibility of release of either GBT or dimethylsulfoniopropionate (DMSP) from phytoplankton. Release of these compounds might have affected the uptake or metabolism of choline, which, as shown below, was converted to GBT by microorganisms in the samples. A low-salinity (7-ppt) sample was generated by diluting the filtrate 1:1 with 18 MΩ water. Higher-salinity samples were generated by adding NaCl (American Chemical Society reagent grade) to yield 25 and 35 ppt. Salinities were determined with a refractometer. These filtrate cultures were preincubated for 20 h in the dark before [14C]choline was added. Incubation was carried out in 250-ml Teflon bottles. Water from each salinity treatment was spiked with 11 nM [14C]choline, and total uptake into particulates, [14C]GBT in particulates, and 14CO2 production were measured over time. To allow for a greater sampling frequency, only single samples were taken for each analysis. The entire experiment was replicated on a different date and gave similar results (data not shown).
Subsamples from non-14C-treated bottles of each of the salinity treatments were taken for bacterial abundance measurements during the course of the experiment. The bacteria were enumerated by epifluorescence microscopy with 4′,6-diamino-2-phenylindole (DAPI) staining (20). Intracellular concentrations of GBT were calculated by assuming a per-cell biovolume of 0.07 μm3 (12) and by assuming that [14C]GBT was uniformly distributed among all the cells.
Tests of potential inhibitors.
A series of organic compounds were tested for their effects on the short-term uptake of [14C]choline. These included potential competitive inhibitors (GBT, DMSP, carnitine, proline betaine, proline, glycine, ethanolamine, glucose, and unlabeled choline), as well as inhibitors of biochemical energy generation (sodium azide and 2,4-dinitrophenol [DNP]) and thiol-containing enzymes (p-chloromercuribenzoate [p-CMB]). The inhibitor assays were conducted with a 24-h-old filtrate culture generated from Mobile Bay water (salinity, 24 ppt; in situ temperature, 27°C). Aliquots (10 ml) of the filtrate culture were transferred to a series of 50-ml polypropylene centrifuge tubes (Corning). Potential competitive inhibitors were then added to a final concentration of 200 nM (duplicate samples were used for each compound). After thorough mixing (<15 s), each sample received [14C]choline to give a final concentration of 9.4 nM. Incubation proceeded for 20 min and was terminated by filtration through 0.2-μm Supor filters. The total uptake of 14C onto the filters was determined by radioassay. Uptake in the experimental (inhibitor) samples was compared with that in controls receiving only [14C]choline and a volume of water equivalent to the inhibitor additions. The time constraints of filtration prevented more than three compounds from being tested in a single incubation run. Therefore, several runs were carried out over a 4-h period, all with the same filtrate culture. Experimental treatments within each run were compared to controls ([14C]choline only) from the same run, which accounted for changes in gross uptake rates in the controls. These changes amounted to less than 10% of the gross uptake rate. Tests of some of the inhibitors, repeated several hours apart, yielded similar results, indicating that changes in the microbial community in the culture did not affect the treatment comparisons. The endogenous “effective” choline concentration in the water was not known. Therefore, unlabeled choline (200 nM) was added as a positive control inhibitor with which the other inhibitors could be compared.
The effects of the energy production inhibitors sodium azide and DNP, as well as the thiol-binding reagent p-CMB, on [14C]choline uptake were tested similarly, except that these inhibitors were added to a concentration of 100 μM and the samples were preincubated with the inhibitors for ∼1 h before the [14C]choline uptake assay.
Reagents.
[methyl-14C]choline (57 mCi · mmol−1) was obtained from ICN Biomedicals. The primary stock was stored in ethanol at 4°C. Working stocks for addition to samples were prepared by evaporating an aliquot of the ethanolic stock on the day of use and reconstituting in 18 MΩ water. The added volumes of the isotope stock to seawater were always <1% of the sample volume. HPLC analysis of the [14C]choline stock showed that <0.2% of the radioactivity could have been [14C]GBT.
The following reagents were obtained from Sigma: GBT · HCl, carnitine · HCl, choline · HCl, d-glucose, glycine, dimethylglycine, l-carnitine, l-proline, DNP, p-CMB, and sodium azide. DMSP · HCl was obtained from Research Plus Inc. Proline betaine was a gift from David Rhodes.
RESULTS
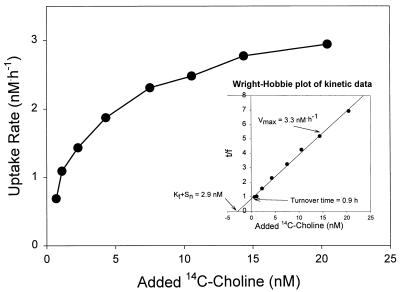
In estuarine waters and GF/F filtrates, the uptake of choline displayed Michaelis-Menten-type saturation kinetics, characteristic of enzyme-mediated transport (Fig. 1). Wright-Hobbie linearization of the kinetic data yielded Kt + Sn values ranging from 1.7 to 4.1 nM, indicative of a high-affinity transport system (Table 1). Vmax values ranged from 0.5 to 3.3 nM · h−1. The turnover times of the endogenous substrate pool (Sn) ranged from 0.9 to 3.2 h, with the shortest turnover times being found in the filtrate cultures.
FIG. 1.
Representative plot of choline uptake kinetics in a 24-h-old filtrate culture (<0.7 μm) generated from Mobile Bay water. The salinity of the sample was 17 ppt, and the culture was incubated at 22°C. The inset shows the uptake rate data linearized by the method of Wright and Hobbie (28), which was used to estimate uptake kinetic parameters.
TABLE 1.
Kinetic parameters for [14C]choline uptakea in estuarine waters and filtrate cultures from Mobile Bay, Ala.
| Sample and date | Salinity (ppt) | Temp (°C) | Kt + Sn (nM) | Vmax (nM · h−1) | Turnover time of Sn (h) |
|---|---|---|---|---|---|
| Whole water | |||||
| 14 Nov 1995 | 12 | 15 | 1.7 | 0.5 | 3.2 |
| 26 June 1996 | 15 | 31 | 4.1 | 3.3 | 1.3 |
| Filtrate culturesb | |||||
| 5 Dec 1996 | 17 | 22 | 2.9 | 3.3 | 0.9 |
| 12 Sept 1997 | 24 | 26 | 1.7 | 1.9 | 0.9 |
Uptake rates were determined by using 5 to 20 min incubations depending on the experiment.
24-h-old filtrate cultures (<0.7 μm) composed mainly of bacteria were used for these experiments.
As expected, addition of a 20-fold excess of unlabeled choline strongly inhibited the uptake of [14C]choline in short-term assays (Table 2). GBT and DMSP proved to be strong inhibitors as well, with each causing the uptake to fall to only 38% of control levels. Moderately inhibitory compounds (resulting in 50 to 82% uptake compared with controls) included carnitine, proline betaine, dimethylglycine, proline, and glycine. Compounds having little effect on choline uptake included ethanolamine (91% of control uptake) and glucose (102% of control uptake). Inhibitors of biochemical energy generation (DNP and azide) were only moderately inhibitory to choline uptake resulting in 71 and 72% of control uptake, respectively, when added at 100 μM 1 h before the choline uptake assays (Table 2). On the other hand, the thiol-binding reagent p-CMB (100 μM) nearly eliminated choline uptake, decreasing it to 4% of that in controls.
TABLE 2.
Effects of potential competitive inhibitors, inhibitors of energy generation, and thiol-containing enzymes on short-term uptake of [14C]choline in an estuarine filtrate culturea
| Inhibitor | Percent uptake in controlb |
|---|---|
| Competitive inhibitorsc | |
| Control (no addition) | 100 ± 4 |
| Glucose | 102 ± 2 |
| Ethanolamine | 91 ± 4 |
| l-Proline | 82 ± 6 |
| Glycine | 79 ± 7 |
| l-Proline | 75 ± 4 |
| Dimethylglycine | 68 ± 3 |
| Proline betaine | 54 ± 4 |
| Carnitine | 50 ± 1 |
| GBT trial 1 | 41 ± 2 |
| DMSP trial 1 | 40 ± 2 |
| DMSP trial 2 | 36 ± 1 |
| GBT trial 2 | 36 ± 1 |
| Choline trial 1 | 14 ± 1 |
| Choline trial 2 | 10 ± 1 |
| Energy uncouplers or thiol-binding reagentsd | |
| DNP | 71 ± 3 |
| Sodium azide | 72 ± 3 |
| p-CMB | 4 ± 3 |
Filtrate culture was generated by filtering water through a GF/F filter by gravity. The culture, containing mainly free-living bacteria, was preincubated for 24 h in the dark prior to choline uptake assays.
Values represent the means of duplicate determinations of total uptake of [14C]choline into filterable material, expressed as a percentage of the controls. The error terms indicate the range about the mean. The total uptake in controls ranged from 640 to 702 dpm · 10 ml of sample−1 depending on the trial. Incubation was carried out for 20 min.
Competitive inhibitors were added at 200 nM immediately before the addition of 9.4 nM [14C]choline.
2,4DNP, azide, and p-CMB were added at 100 μM 1 h before the addition of 9.4 nM [14C]choline.
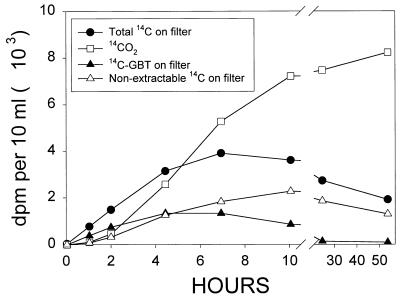
Longer-term uptake and degradation patterns of [14C]choline were investigated with estuarine water samples from Mobile Bay. Following the addition of 10.8 nM [14C]choline, label rapidly accumulated in particles larger than 0.2 μm in diameter, probably bacterial cells (Fig. 2). Maximum accumulation in particulates occurred at around 7 h, after which time the levels of 14C in particulates declined to half the maximum by 53 h. Significant amounts of [14C]GBT accumulated in particulates over the first 4 h of incubation, indicating that [14C]choline was rapidly converted to [14C]GBT. Within the first hour of incubation, >50% of the 14C on the filters was associated with GBT. The concentration of [14C]GBT associated with particulates reached 1.0 nM at 4 to 7 h, which accounted for ∼9% of the added choline. After 7 h, the levels of [14C]GBT associated with particulates declined, and by the end of the incubation, these levels were very low (0.1 nM) but still detectable. Also contributing to the radioactivity on the filters was material which remained on the Supor filters after extraction in MCW and rinsing with methanol (Fig. 2). This is presumably insoluble cell material. The nonextractable material accounted for a small fraction (10%) of the total filterable 14C during the first few hours of incubation but increased to about 68% of the radioactivity on the filters by the end of the incubation.
FIG. 2.
Accumulation of 14C-particulates and 14CO2 plotted against time after the addition of 10.8 nM [14C]choline (∼13,000 dpm/10 ml) to estuarine water from Mobile Bay. Data represent the amount of radioactivity in 10-ml subsamples taken from the water sample at the times indicated. The points represent the mean of duplicate subsamples at each time. Ranges averaged 7.9% of the mean and, for the sake of clarity, are not shown. The salinity of the sample was 24 ppt, and incubation was carried out in the dark at 27°C.
Production of 14CO2 from the methyl carbons of choline was initially slower than the accumulation in particulates but overtook particulate 14C at times longer than 5 h. By 10 h, 50% of the added label was accounted for as 14CO2 and this increased to about 60% at the end of the incubation (53 h). Of the total metabolites recovered (14CO2 + 14C-particulates), 14CO2 accounted for 81% at the end of the incubation.
Effects of salinity on [14C]choline metabolism.
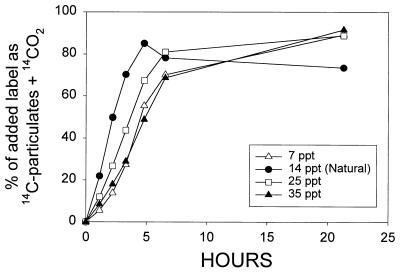
Because choline was converted to GBT, a well-known osmolyte and compatible solute, the effects of salinity on the uptake, degradation, and retention of choline were tested experimentally. The total metabolism of [14C]choline, as judged by the percentage of added label converted to 14CO2 plus 14C-particulates, was fastest in the unaltered sample (14 ppt) (Fig. 3). Samples with altered salinity displayed a lag in choline metabolism, with a shorter lag in the 25-ppt treatment than in the 7- and 35-ppt treatments (Fig. 3). The total amount of choline metabolized was very similar for all treatments after 7 h of incubation. In contrast to the total metabolism, the relative production of 14CO2, 14C-particulates, and [14C]GBT was quite different between the salinity treatments (Fig. 4). Accumulation of labeled particulate material was greater at higher salinities (Fig. 4A) while 14CO2 production from added choline showed the opposite trend, being dramatically lower at the higher salinities (Fig. 4C). [14C]GBT was produced from choline and retained in the cells to a much greater extent in the higher salinity samples (Fig. 4B). In the highest-salinity sample (35 ppt), >80% of the particulate radioactivity was attributable to accumulation of the osmolyte [14C]GBT in the bacterial cells over the entire 22 h incubation. Similar high production of [14C]GBT was observed in the 25-ppt treatment, but the levels declined somewhat by the last time point. The high value for [14C]GBT at about 4 h in the 25-ppt treatment seems to be spurious because the [14C]GBT on the filter should not have been greater than the total activity on the filter.
FIG. 3.
Effects of altered salinity upon the total recovered metabolites (14C-particulates plus 14CO2) obtained from a single addition of 11 nM [14C]choline to estuarine filtrate cultures. The parent water sample was collected from Mobile Bay on 17 April 1997 and had a natural salinity of 14 ppt. Filtration and salinity modifications were performed 20 h before the addition of labeled substrate. The incubation temperature was 25°C. Individual points represent single samples taken at each time point.
FIG. 4.
Effects of altered salinity in estuarine filtrate cultures upon the time courses of [14C]choline uptake into particulates (A), its conversion to [14C]GBT (B), and its degradation to 14CO2 (C). [14C]choline was added to an initial concentration of 11 nM (∼13,000 dpm per 10 ml [the volume filtered]) in each of the salinity treatments. The parent water sample was collected from Mobile Bay on 17 April 1997 and had a natural salinity of 14 ppt. Filtration and salinity modifications were performed 20 h before the addition of labeled substrate. Incubation was carried out in the dark at 25°C. Individual points represent single samples taken at each time point. The datum point for [14C]GBT at 4 h in the 25-ppt treatment seems to be spurious, since it was 50% higher than the total filter activity, of which it should be a subset.
In the low-salinity treatment (7 ppt), roughly equal amounts of 14C were recovered in filterable material and 14CO2, while [14C]GBT production was low. At the natural salinity (14 ppt), labeled particulate material accumulated faster than 14CO2 but 14CO2 eventually reached higher values, similar to what was observed in previous experiments (Fig. 2).
Bacterial abundances in the filtrate cultures used for salinity manipulation experiments increased over time from the start of the choline addition experiment (salinity manipulations had taken place 20 h earlier) (Fig. 5). The ambient-salinity sample (unaltered with respect to the original water salinity) had the largest initial number of cells and the fastest increase with time. The altered-salinity samples had lower initial abundances. The 7-ppt sample was expected to have fewer cells, since this water had been diluted 1:1 with 0.1-mm-filtered pure water. The lower initial abundances in the 25- and 35-ppt salinities must have resulted in slower growth over the 20-h preincubation period than did to the ambient salinity. Thus, salinity stress seemed to lower the net growth of the total bacterial population.
FIG. 5.
Effects of experimental salinity modifications on bacterial abundances (DAPI direct counts) in filtrate cultures. The same cultures were used for [14C]choline uptake and degradation measurements. The parent water sample was collected from Mobile Bay on 17 April 1997 and had a natural salinity of 14 ppt. Filtration and salinity modifications were performed 20 h before the time zero shown. Time zero in this figure corresponds to the time of [14C]choline addition. Incubation was carried out in the dark at 25°C.
The maximum accumulations of particulate [14C]GBT during the salinity modification experiment were used to calculate the intracellular concentration of [14C]GBT by assuming that this [14C]GBT was in bacterial cells (Table 3). An assumed biovolume per cell (0.07 μm3) was used, along with directly determined bacterial abundances (Fig. 5). Intracellular concentrations of [14C]GBT were osmotically significant, ranging from 2.1 to 34 mM. There was a clear trend in the peak intracellular [14C]GBT accumulations, with higher concentrations being found at higher salinities (Table 3).
TABLE 3.
Maximum cellular accumulations of [14C]GBT from supplied [14C]choline in estuarine bacteria grown in filtrate cultures subjected to salinity variationsa
| Salinity (ppt) | Cellular [14C]GBT concn
|
|
|---|---|---|
| 10−18 mol · cell−1 | mM inside cellsb | |
| 7 | 0.2 | 2.1 |
| 14c | 0.6 | 8.2 |
| 25 | 1.4 | 19 |
| 35 | 2.4 | 34 |
[14C]choline was supplied at an initial concentration of 11 nM.
A biovolume of 0.07 μm per cell was assumed. Bacteria were counted by the DAPI staining method.
The natural salinity of the water samples.
DISCUSSION
The results presented here indicate that natural estuarine bacteria are able to rapidly scavenge choline at the low (nanomolar) concentrations expected to be found in estuarine and coastal water (22). After uptake, choline was used by microorganisms as an energy substrate, a source of cell carbon, and a precursor of the osmoprotectant GBT (Fig. 2 and 4). Salt stress was identified as one variable which favored the conversion of choline to GBT and retention of GBT in microorganisms, presumably for its compatible solute functions. The overall uptake and degradation patterns observed for choline were similar to those found when GBT was added directly to the same waters (7). The results of this study and other recent investigations (7, 8) suggest that bacteria in estuarine and coastal shelf waters are well adapted to acquire and use trace levels of naturally occurring osmoprotectant compounds such as choline, GBT, and DMSP.
The choline transport system expressed in the natural microbial populations appears to be somewhat specific for choline-like compounds, because compounds like glucose or ethanolamine, both of which lack onium (N+ or S+) or carboxyl functional groups, did not inhibit uptake (Table 2). On the other hand, inhibitor assays indicated that compounds with close structural similarity to choline, particularly with regard to an onium functional group, might compete with choline for the uptake mechanism. GBT and DMSP, in particular, were strong inhibitors of choline uptake (Table 2), although neither was as effective as unlabeled choline. The latter point suggests that the transport system has a higher affinity for choline than for GBT or DMSP. In contrast to the effects of GBT and DMSP on [14C]choline uptake, choline was a poor inhibitor of [14C]GBT uptake by microorganisms (8, 18). Collectively, these results suggest that GBT and choline are taken up by different transport systems, a conclusion supported by results from studies with bacterial cultures (1, 3). Despite the apparent specificity of the uptake system for choline, the large number of naturally occurring compounds which were somewhat inhibitory to uptake suggests that the choline transporter may be involved in the uptake of a suite of chemically related compounds.
The uptake of choline by natural bacterial populations appears to be energy dependent, since both azide, an inhibitor of respiratory electron transport, and DNP, an uncoupler of ATP synthesis, inhibited choline uptake by about 30% (Table 2). Choline transport into cells is believed to be dependent on the proton motive force (14); therefore, it was not surprising that DNP, which is known to disrupt the proton motive force, was inhibitory. Higher concentrations of the inhibitors would probably have resulted in greater inhibition. Perroud and Le Rudulier (18) observed that GBT uptake in E. coli was inhibited ca. 40% by 500 μM DNP but was inhibited 70% by 1,000 μM DNP. Despite the limited survey of inhibitors used here, it seems clear that choline uptake involved a thiol-binding site, since 100 μM p-CMB nearly completely eliminated choline uptake (Table 2). Tests with a larger suite of energy and transport inhibitors would be required to further elucidate the mechanisms of choline transport operating in the natural populations.
The production of GBT from supplied choline was rapid and represented a substantial fraction of the choline taken up (Fig. 2 and 4). This indicated that estuarine bacteria express a constitutive choline oxidation system, which leads to GBT production. The ability to oxidize choline to GBT is widespread among bacteria in culture (1, 3, 11), and it has often been observed that the choline supply confers protection from osmotic stress. The osmoprotective functions of choline are attributed to the GBT produced rather than to choline itself, because mutants able to take up choline but unable to oxidize it to GBT are not protected from osmotic stress (26, 27). The results of the salinity alteration experiment showed clearly that choline conversion to GBT and the subsequent retention of GBT were under osmotic control. Bacteria subjected to elevated salinities produced more GBT from choline and retained more in the cells (Table 3). The enhanced production and retention of GBT from choline occurred at the expense of its degradation to CO2 (Fig. 4C) and to nonextractable cell material (data not shown). The response observed with the natural populations was similar to that for GBT degradation and retention in cultures of Rhizobium meliloti, which degrade GBT at low osmolality but retain it when the cells become osmotically stressed (2, 14). It should be added that very similar GBT retention and CO2 production patterns, as a function of salinity, were found when [14C]GBT was directly supplied to the same filtrate cultures as used here (7). Taken together, the results discussed above suggest that estuarine bacteria mixed into waters of higher salinity might require osmoprotectants like GBT and that uptake of choline could meet some of the demand for GBT.
The calculated intracellular GBT concentrations resulting from a supply of ∼10 nM choline were osmotically significant, i.e., 34 mM intracellular GBT in the 35-ppt salinity treatment. The 34 mM estimate is conservatively low because it was assumed that the [14C]GBT was evenly distributed among all the cells counted by the DAPI method. It is more likely that only a fraction of the total bacterial population would be involved in the uptake of choline and retention of GBT, although this fraction is currently unknown. If only 10% of the cells in the 35-ppt filtrate culture contained [14C]GBT, the intracellular concentration would be closer to 340 mM, which is at or above the level typically observed in bacterial isolates which have been subjected to salinity stress at near seawater salinity and which have been supplied with exogenous GBT (2, 18). It remains to be demonstrated whether the uptake of osmoprotectants like choline (or GBT) confers survival or growth advantages to estuarine bacteria as salinities increase. GBT and other osmoprotectants can extend the range of salinities at which growth and activity of certain bacteria in culture can take place (6, 13, 21, 25).
Mineralization of the methyl groups of choline (or GBT produced from choline) could take place by methylotrophic (demethylation) metabolism, similar to what has been observed with bacterial isolates (10, 23, 24). Alternatively, choline could be metabolized by a reductive pathway which would lead to the production of trimethylamine as an intermediate. Such a pathway has been observed in anoxic marine sediments (9), but it is not known whether it operates in aerobic seawater. TMA would probably be further metabolized to CO2 by aerobic methylotrophs. In the estuarine water samples used here, total recoveries of 14CO2 plus 14C-particulates from supplied [14C]choline were less than 100% (Fig. 3), indicating the probable production of dissolved 14C-containing compounds which were not degraded on the timescale of the experiments. A similar conclusion was reached when [14C]GBT was supplied to seawater (7). Further research on the production of dissolved intermediates from choline and GBT in seawater is needed to identify these compounds.
The role of choline as a source of carbon and nitrogen for estuarine bacteria needs further investigation. Kortstee (10) found that a large variety of bacteria, including a marine pseudomonad, utilized choline as a source of carbon and nitrogen. The kinetic data collected in this study suggest that the potential for turnover of choline is significant (turnover times of 1.3 to 3.2 h; Vmax values of 0.5 to 3.3 nM · h−1), but true turnover rate estimates for choline will require simultaneous measurements of choline uptake rate constants and dissolved-choline concentrations. Choline concentrations were not measured in the present study, but Kt + Sn estimates place an upper limit on the effective concentration of choline. The Kt + Sn values ranged from 1.7 to 4.1 nM in two whole-water samples (Table 1), values which are in good agreement with the choline concentrations reported by Roulier et al. for coastal waters (22). Use of these upper-limit concentrations and their respective turnover times (Table 1) yields turnover rates, in terms of carbon, of 2.7 to 16 nM C · h−1 (1 mol of choline contains 5 mol of C). These turnover rates can be compared with bacterial carbon demand in the estuarine waters around Dauphin Island, which have recently been estimated to range from 66 to 620 nM C · h−1 (27a). It seems from these estimates that choline could, contribute ∼25%, at most, to the bacterial carbon demand. The actual turnover rates of choline and hence its contribution to bacterial carbon demand are likely to be significantly lower than the upper-limit estimates mentioned above because the Sn must be less than Kt + Sn and other compounds such as GBT or DMSP might contribute to the effective Sn.
Worth mentioning in regard to choline turnover in seawater is the fact that oxidation of choline by choline oxidase can yield H2O2 (16). Roulier et al. (22) have exploited this fact in developing a sensitive enzymatic assay for choline which is based on analysis of H2O2. Hydrogen peroxide is a highly reactive oxidant which is produced in seawater primarily through photochemical processes (30). Choline turnover would be one mechanism for H2O2 production that could be independent of light. Although H2O2 production was not measured here, this phenomenon would be consistent with the dark production of H2O2 reported by Palenik et al. (17) and with the linkage between this process and metabolism of organic nitrogen compounds suggested by those authors. The significance of choline as a source of dark H2O2 production will require further study of choline turnover rates.
In conclusion, low (nanomolar) concentrations of choline were rapidly taken up by estuarine bacteria and a significant fraction of the choline taken up was metabolized to GBT. Mineralization of choline and its conversion to GBT were strongly affected by salinity, and results suggested that bacteria experiencing elevated salinities during estuarine mixing might utilize a greater fraction of choline for GBT synthesis and might then retain the GBT for osmotic purposes. The significance of this phenomenon for bacterial growth dynamics in estuaries remains to be examined.
ACKNOWLEDGMENTS
Funding for this research was provided by the National Science Foundation (grants OCE-92-18511 and OCE-95-30378).
Special thanks are extended to Laura Linn for excellent technical assistance. Joel Walker and Jody Bruton also contributed to some aspects of this work.
Footnotes
This is contribution number 296 of the Dauphin Island Sea Lab.
REFERENCES
- 1.Abee T, Palmen R, Hellingwerf K J, Konings W N. Osmoregulation in Rhodobacter sphaeroides. J Bacteriol. 1990;172:149–154. doi: 10.1128/jb.172.1.149-154.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard T, Pocard J-A, Perroud B, Le Rudulier D. Variations in the response of salt-stressed Rhizobium strains to betaines. Arch Microbiol. 1986;143:359–364. [Google Scholar]
- 3.Boch J, Kempf B, Bremer E. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J Bacteriol. 1994;176:5364–5371. doi: 10.1128/jb.176.17.5364-5371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choquet C G, Ahonkhai I, Klein M, Kushner D J. Formation and role of glycine betaine in the moderate halophile Vibrio costicola. Arch Microbiol. 1991;155:153–158. [Google Scholar]
- 5.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 6.Diaz M R, Visscher P T, Taylor B F. Metabolism of dimethylsulfoniopropionate and glycine betaine by a marine bacterium. FEMS Microbiol Lett. 1992;96:61–66. [Google Scholar]
- 7.Kiene, R. P., and L. P. Hoffmann. Glycine betaine uptake, retention and degradation by microorganisms in seawater. Submitted for publication.
- 8.Kiene, R. P., L. P. Hoffmann, and J. E. Walker. Sea water microorganisms have a high-affinity glycine betaine uptake system which also recognizes dimethylsulfoniopropionate. Aquat. Microbiol. Ecol., in press.
- 9.King G M. Metabolism of trimethylamine, choline, and glycine betaine by sulfate-reducing and methanogenic bacteria in marine sediments. Appl Environ Microbiol. 1984;48:719–725. doi: 10.1128/aem.48.4.719-725.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kortstee G J J. The aerobic decomposition of choline by microorganisms. Arch Mikrobiol. 1970;71:235–244. [PubMed] [Google Scholar]
- 11.Landfald B, Strom A R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986;165:849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Fuhrman J A. Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl Environ Microbiol. 1987;53:1298–1303. doi: 10.1128/aem.53.6.1298-1303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Rudulier D, Bouillard L. Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl Environ Microbiol. 1983;46:152–159. doi: 10.1128/aem.46.1.152-159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Rudulier D, Pocard J A, Boncompagni E, Poggi M C. Osmoregulation in bacteria and transport of onium compounds. In: Kiene R P, Visscher P T, Keller M D, Kirst G O, editors. Biological and environmental chemistry of DMSP and related sulfonium compounds. New York, N.Y: Plenum; 1996. pp. 253–263. [Google Scholar]
- 15.Le Rudulier D, Strom A R, Dandekar A M, L. T. S, Valentine R C. Molecular biology of osmoregulation. Science. 1984;224:1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- 16.Ohta-Fukuyama M, Miyake Y, Emi S, Yamano T. Identification and properties of the prosthetic group of choline oxidase from Alcaligenes sp. J Biochem. 1980;88:197–203. [PubMed] [Google Scholar]
- 17.Palenik B, Zafiriou O C, Morel F M M. Hydrogen peroxide production by a marine phytoplankter. Limnol Oceanogr. 1987;32:1365–1369. [Google Scholar]
- 18.Perroud B, Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985;161:393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pocard J-A, Bernard T, Smith L T, LeRudulier D. Characterization of three choline transport activities in Rhizobium meliloti: modulation by choline and osmotic stress. J Bacteriol. 1989;171:531–537. doi: 10.1128/jb.171.1.531-537.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter K G, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 21.Riou N, Le Rudulier D. Osmoregulation in Azospirillum brasilense: glycine betaine transport enhances growth and nitrogen fixation under salt stress. J Gen Microbiol. 1990;136:1455–1461. doi: 10.1099/00221287-136-8-1455. [DOI] [PubMed] [Google Scholar]
- 22.Roulier M A, Palenik B, Morel F M M. A method for the measurement of choline and hydrogen peroxide in seawater. Mar Chem. 1990;30:409–421. [Google Scholar]
- 23.Shieh H S. Aerobic degradation of choline: some properties of whole cells and cell-free extracts of Achromobacter cholinophagum. Can J Microbiol. 1965;11:375–379. doi: 10.1139/m65-045. [DOI] [PubMed] [Google Scholar]
- 24.Shieh H S. Further studies on the oxidation of betaine by a marine bacterium, Achromobacter cholinophagum. Can J Microbiol. 1966;12:299–302. doi: 10.1139/m66-040. [DOI] [PubMed] [Google Scholar]
- 25.Smith L T, Pocard J, Bernard T, Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988;170:3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strom A R, Falkenberg P, Landfald B. Genetics of osmoregulation in Escherichia coli: uptake and biosynthesis of organic osmolytes. FEMS Microbiol Rev. 1986;39:79–86. [Google Scholar]
- 27.Styrvold O B, Falkenberg P, Landfald B, Eshoo M W, Bjornsen T, Strom A R. Selection, mapping, and characterization of osmoregulatory mutants of Eschericia coli blocked in the choline-glycine betaine pathway. J Bacteriol. 1986;165:856–863. doi: 10.1128/jb.165.3.856-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Walker, J., and R. P. Kiene. Unpublished data.
- 28.Wright R T, Hobbie J E. Use of glucose and acetate by bacteria and algae in aquatic ecosystems. Ecology. 1966;47:447–464. [Google Scholar]
- 29.Yancey P H, Clark M E, Hand S C, Bowlus R D, Somero G N. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 30.Zika R G, Moffett J W, Petasne R G, Cooper W J, Saltzman E S. Spatial and temporal variations of hydrogen peroxide in Gulf of Mexico waters. Geochim Cosmochim Acta. 1985;49:1173–1184. [Google Scholar]