Abstract
Background
The biologic disease‐modifying anti‐rheumatic drugs (DMARDs) are very effective in treating rheumatoid arthritis (RA), however there is a lack of head‐to‐head comparison studies.
Objectives
To compare the efficacy and safety of abatacept, adalimumab, anakinra, etanercept, infliximab, and rituximab in patients with RA.
Methods
This ‘Overview of Reviews’ was done by including all Cochrane Reviews on Biologics for RA available in The Cochrane Library. We included only data on standard dosing regimens for these biologic DMARDs from placebo‐controlled trials. The primary efficacy and safety outcomes were ACR50 and withdrawals due to adverse events. We calculated Odds Ratios (OR) for efficacy and safety outcomes and combined estimates of events across the placebo groups as the expected Control Event Rate (CER). Indirect comparisons of biologics were performed for efficacy and safety using a hierarchical generalized linear mixed model (GLMM) incorporating the most important study‐level characteristic (i.e. type of biologic) as a fixed factor and study and study*drug interaction as random factors.
Main results
From the six available Cochrane reviews, we obtained data from seven studies on abatacept, eight on adalimumab, five on anakinra, four on etanercept, four on infliximab, and three on rituximab.
The indirect comparison estimates showed similar efficacy for the primary efficacy outcome for all biologics with three exceptions. Anakinra was less efficacious than etanercept with a ratio of ORs (95% CI; P value) of 0.34 (0.14, 0.81; P=0.015); and likewise adalimumab was more efficacious than anakinra, 2.20 (1.01, 4.75; P=0.046).
In terms of safety, adalimumab was more likely to lead to withdrawals compared to etanercept, with a ratio of ORs of 1.89 (1.18 to 3.04; P = 0.009); anakinra more likely than etanercept, 2.05 (1.27 to 3.29; P = 0.003); and likewise etanercept less likely than infliximab, 0.37 (0.19 to 0.70; P = 0.002).
Authors' conclusions
Based upon indirect comparisons, anakinra seemed less efficacious than etanercept and adalimumab. Etanercept seemed to cause fewer withdrawals due to adverse events than adalimumab, anakinra and infliximab. Significant heterogeneity in characteristics of trial populations imply that these finding must be interpreted with caution. These findings can inform physicians and patients regarding their choice of biologic for treatment of RA.
Keywords: Humans; Abatacept; Adalimumab; Antibodies, Monoclonal; Antibodies, Monoclonal/adverse effects; Antibodies, Monoclonal/therapeutic use; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Murine‐Derived; Antirheumatic Agents; Antirheumatic Agents/adverse effects; Antirheumatic Agents/therapeutic use; Arthritis, Rheumatoid; Arthritis, Rheumatoid/drug therapy; Biological Products; Biological Products/adverse effects; Biological Products/therapeutic use; Etanercept; Immunoconjugates; Immunoconjugates/adverse effects; Immunoconjugates/therapeutic use; Immunoglobulin G; Immunoglobulin G/adverse effects; Immunoglobulin G/therapeutic use; Infliximab; Interleukin 1 Receptor Antagonist Protein; Interleukin 1 Receptor Antagonist Protein/adverse effects; Interleukin 1 Receptor Antagonist Protein/therapeutic use; Patient Compliance; Receptors, Tumor Necrosis Factor; Receptors, Tumor Necrosis Factor/therapeutic use; Review Literature as Topic; Rituximab
Plain language summary
Biologics for rheumatoid arthritis: an overview of Cochrane reviews
This summary of a Cochrane review presents what we know from research about the effect of biologics on Rheumatoid Arthritis (RA).
The review shows that in people with RA;
‐ abatacept, adalimumab, etanercept, infliximab, and rituximab probably improve signs of rheumatoid arthritis such as the number of tender or swollen joints and other outcomes such as pain and disability.
‐ anakinra probably improves signs of rheumatoid arthritis such as the number of tender or swollen joints and other outcomes such as pain and disability (but not as well as the others).
We do not have precise information about possible side effects and complications. This is particularly true for rare but serious side effects. Possible side effects may include a serious infection or upper respiratory infection. Rare complications may include certain types of cancer.
What is Rheumatoid arthritis (RA) and what are biologics?
When you have rheumatoid arthritis, your immune system, which normally fights infection, attacks the lining of your joints making them inflamed. This inflammation causes your joints to be hot, swollen, stiff, and painful. The small joints of your hands and feet are usually affected first. If the inflammation goes on without treatment, it can lead to damaged joints. Once the joint is damaged it cannot be repaired, so treating rheumatoid arthritis early is important.
Biologics are a group of medications that suppress the immune system and reduce the inflammation in the joints. Even though suppressing the immune system can make it slightly harder to fight off infections, it also helps to stabilize an overactive immune system. By reducing the inflammation, the aim is to help prevent damage to the joints.
Best estimate of what happens to people with rheumatoid arthritis who take biologics:
ACR 50 (number of tender or swollen joints and other doctor or patient assessed aspects of rheumatoid arthritis)
Among people who took abatacept, 44 people out of 100 experienced improvement in the signs of their rheumatoid arthritis compared to 21 people out of 100 who took a placebo (23% absolute improvement).
Among people who took adalimumab 49 people out of 100 experienced improvement in the signs of their rheumatoid arthritis compared to 21 people out of 100 who took a placebo (28% absolute improvement).
Among people who took anakinra 30 people out of 100 experienced improvement in the signs of their rheumatoid arthritis compared to 21 people out of 100 who took a placebo (9% absolute improvement).
Among people who took etanercept 57 people out of 100 experienced improvement in the signs of their rheumatoid arthritis compared to 21 people out of 100 who took a placebo (36% absolute improvement).
Among people who took infliximab 43 people out of 100 experienced improvement in the signs of their rheumatoid arthritis compared to 21 people out of 100 who took a placebo (22% improvement).
Among people who took rituximab 52 people out of 100 experienced improvement in the signs of their rheumatoid arthritis compared to 21 people out of 100 who took a placebo (31% improvement).
Side effects
Among people who took adalimumab 8 people out of 100 dropped out of the study because of the side effects compared to 5 people out of 100 who took a placebo (3% absolute difference).
Among people who took anakinra 9 people out of 100 dropped out of the study because of the side effects compared to 5 people out of 100 who took a placebo (4% absolute difference).
Among people who took infliximab 11 people out of 100 dropped out of the study because of the side effects compared to 5 people out of 100 who took a placebo (6% absolute difference).
There may be little or no difference in people who dropped out because of side effects with abatacept, etanercept, and rituximab compared to people who took a placebo (fake pill).
Background
Description of the condition
Rheumatoid arthritis (RA) is a systemic inflammatory disease characterized by inflammation of the synovial lining of the joints, tendons and periarticular structures (Lee 2001). RA affects 0.5% to 1.0% of the population in Western countries (Kvien 2004). Untreated, RA leads to joint destruction, functional limitation and severe disability (Odegard 2005; Yelin 2007) and has a significant impact on health‐related quality of life (HRQoL) (Kvien 2005; Lubeck 2004).
Treatment options for rheumatoid arthritis include non‐steroidal anti‐inflammatory drugs (NSAIDs), glucocorticoids, traditional DMARDs (disease‐modifying anti‐rheumatic drugs, e.g. methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, cyclosporine) and biologic DMARDs. The use of DMARDs leads to an improvement in pain and functioning for patients with RA as well as more long‐term outcomes such as reduced radiographic progression (Finckh 2006; Pincus 2002) and disability (Cash 1994; Strand 2008).
Description of the interventions
The introduction of biologic DMARDs has revolutionized the management of RA. Biologic DMARDs, although not achieving remission often, provide clinically important improvement in pain and function in patients not responding to traditional DMARDs such as methotrexate. Biologic DMARDs appear to have fewer side‐effects and have much greater success in slowing structural joint destruction than methotrexate. Biologics are much more costly than traditional DMARDs.
Biologic DMARDs are commonly used for patients with suboptimal response or intolerance to traditional DMARDs such as methotrexate (MTX). Many DMARDs are used in combination with MTX in patients with a suboptimal response to MTX. The biologic DMARDs include three tumor necrosis factor inhibitors (Scott 2006): infliximab (REMICADE, approved 1998 in the U.S.) (FDA 1999), etanercept (ENBREL, approved 1998) (FDA 1998), and adalimumab (HUMIRA, approved, 2002) (FDA 2002); anti‐CD28 therapy ‐ abatacept (ORENCIA, approved 2005) (FDA 2005; FDA 2008a); anti‐IL1 therapy ‐ anakinra (KINERET, approved 2001) (FDA 2001); and anti‐B‐cell therapy ‐ rituximab (RITUXAN/MABTHERA, approved 1997 for lymphoma and 2006 for RA) (Drugs 2006). These biologic DMARDs have been approved for use in RA patients internationally, although the indications for use differ slightly between countries.
How the intervention might work
The mechanism of action of the biologic DMARDs is summarized in the individual Cochrane systematic reviews and is not repeated here for brevity. The systemic and joint inflammation in RA is mediated by activation of T‐cells (Cope 2008), B‐cells, macrophages (Szekanecz 2007), and other immune cells (Woolley 2003). These interactions lead to expression of chemokines, metalloproteinases and inflammatory cytokines such as tumor necrosis factor‐alpha (TNF‐alpha) and various interleukins (IL) (Brennan 2008; Choy 2001). Interaction of lymphocytes and inflammatory cytokines with host cells such as fibroblasts, osteoclasts and chondrocytes leads to bone and cartilage destruction, a hallmark of RA (Brennan 2008; Connell 2006). It is possible that, due to different contributions of these cytokines and processes to the disease expression, the use of therapy targeting one cytokine may be more efficacious or safer than therapy targeting other mechanisms. As briefly described above the mechanism of action differs between the biologics (TNF‐alpha versus Interleukin‐1 versus B‐cells versus T‐cell co‐stimulatory molecule).
Furst 2008 summarized the specifics regarding the use of each biologic DMARD in their consensus statement as follows:
"1. Anti‐TNFs (adalimumab (Ada), etanercept (Eta), infliximab (Inf)) are used in conjunction with another DMARD, usually methotrexate (MTX), for the treatment of RA. These drugs are also effective for the treatment of RA in MTX‐naive patients and have been used successfully with other DMARDs such as sulfasalazine and leflunomide.
2. Anakinra is recommended for the treatment of active RA after an adequate trial of another conventional DMARD, for example, MTX. It may be used alone or with MTX.
3. Abatacept is recommended for treatment of active RA, alone or with background DMARDs, in patients with an inadequate response to MTX or another effective DMARD.
4. Rituximab is approved in the USA for the treatment of moderate‐to‐severe RA in patients who have had an inadequate response to at least one TNF blocking agent or have at least moderate disease activity despite MTX therapy. It may be used alone or in combination with MTX. It may also be used when TNF inhibitors are not suitable."
Why it is important to do this overview
As shown in the six Cochrane systematic review published in The Cochrane Library, these six biologic DMARDs all provide clinically important improvement in pain and disability in treating RA, compared to placebo. The existing six Cochrane systematic reviews, however, only reviewed each agent on its own. Patients, clinicians and policy‐makers need to know if there are any important differences between them in terms of efficacy and safety. Ideally this requires head‐to‐head comparison studies. To our knowledge only one study to date had two biologic arms (abatacept and infliximab) but this study was only powered for comparisons to placebo, not the two biologics to each other (Schiff 2008). In the absence of superiority studies, indirect comparisons provide the best evidence for demonstrating any differences between the available biologics (Kristensen 2007). When randomized trials fail to make head to head comparisons, a common comparator can be used to make an indirect comparison (Song 2003).
This is an overview of several Cochrane Systematic reviews. It differs in methodology from Cochrane Systematic reviews, such that it is not intended to examine only one intervention for RA (Becker 2008). It aims to systematically review the existing updated Cochrane systematic reviews of Biologic DMARDs for RA.
Objectives
To determine the comparative efficacy and safety of currently available biologic DMARDs in adults with rheumatoid arthritis.
Methods
Criteria for considering reviews for inclusion
Completed/updated/available Cochrane systematic reviews of biologic DMARDs for RA.
Search methods for identification of reviews
We searched the Cochrane Database of Systematic Reviews using the search term “Rheumatoid” in the title. We scrutinized these titles for systematic reviews of biologics in rheumatoid arthritis.
Types of studies
Cochrane systematic reviews of randomized controlled trials (RCTs) of biologic DMARDs including but not limited to abatacept, adalimumab, anakinra, etanercept, infliximab, and rituximab in patients with RA. A review was included if it contained at least one RCT, had clinically relevant outcomes, and included clear inclusion and exclusion criteria for studies.
We only included studies using the standard dosing regimens of these biologic DMARDs. Specifically, the studies with the following doses: abatacept every 4 weeks intravenously at 500 mg dose in patients < 60 kg, 750 mg in patients 60 kg to 100 kg and 1000 mg in patients > 100 kg, after the initial dosing regimen of baseline, 2 and 4‐week infusions; adalimumab 40 mg subcutaneous every 2 weeks; anakinra 100 mg subcutaneous every day; etanercept 25 mg subcutaneous twice weekly; infliximab: 3 mg/kg intravenous every 8 weeks after initial dosing at 0, 2 and 6 weeks; rituximab, two 1000 mg IV doses 2 weeks apart.
Types of participants
Adults 18 years or older, with RA meeting the 1987 American College of Rheumatology Classification criteria for RA (Arnett 1988).
Types of interventions
Biologic DMARDs (including abatacept, adalimumab, anakinra, etanercept, infliximab, rituximab and other biologic DMARDs) alone used in standard, approved‐doses or in combination with other biologic/traditional DMARD compared to placebo alone or to placebo plus biologic/traditional DMARD.
Types of outcome measure
Primary/major outcomes
Binary: ACR50 defined as 50% improvement in both tender and swollen joint counts and 50% improvement in three of the five following five variables: patient global assessment, physician global assessments, pain scores, Health Assessment Questionnaire (HAQ) score, and acute phase reactants (Erythrocyte Sedimentation Rate (ESR) or C‐Reactive Protein (CRP) (Chung 2006; Felson 1995). ACR50 was chosen as clinical and statistical evidence shows that this is the preferred endpoint for contemporary RA clinical trials.
Withdrawal due to adverse events was used as a proxy measure of safety.
Minor outcomes
ACR20 and ACR70 defined as 20% and 70% improvement in variables defined above under major outcome (Felson 1995).
Withdrawal for any reason.
Continuous outcomes: changes in either Disease Activity Score (DAS), a composite index of tender and swollen joint counts, patient global assessment and ESR (van der Heijde 1993) or DAS28 score (Prevoo 1995).
Achieving a "good state": (a) good European League Against Rheumatism (EULAR) response ‐ defined by a decrease in the DAS or DAS 28 of ≥ 1.2 from baseline with a final DAS < 2.4 (or DAS 28 < 3.2) (Fransen 2005; van Gestel 1996); (b) low disease activity defined by DAS < 2.4 or DAS28 ≤ 3.2 (Fransen 2005; van Gestel 1996); (c) remission defined as DAS < 1.6 or DAS28 < 2.6 (Fransen 2005; Prevoo 1996).
Quality of Life, measured by Short‐Form‐36 *(SF‐36) (i.e. continuous data, 8 domains; and two summary score, physical and mental component summary) and function measured by HAQ score or modified HAQ calculated as score changes (Fries 1980; Pincus 1983) and the proportion achieving minimally clinically important difference on HAQ ≤ 0.22 (Wells 1993).
Radiographic progression, as measured by Larsen/Sharp/modified Sharp scores (Larsen 1977; Sharp 1971; van der Heijde 1989).
Number and type of adverse effects (AEs).
Withdrawal due to lack of efficacy.
Death
We recognize that randomized controlled trials included in this overview are limited in their ability to assess safety. We therefore also searched the U.S. Food and Drug Administration (FDA) web site for labels and warnings. We also searched other similar regulatory agencies' web sites from Canada (Health Canada) and Europe (European Medicines Agency, EMEA) to summarize warnings related to each of the biologic DMARDs.
Data collection and analysis
Selection of reviews
We included all completed/updated/available Cochrane systematic reviews of biologic DMARDs for RA, if they had been completed and submitted for review and/or updated by 30 May 2009. Other systematic reviews were not included as it was thought this would be duplicative. If a review was incomplete and/or not updated recently, we contacted the authors of the review and requested data and/or an update to the review.
Two authors (JS and RC) reviewed the results of the search (titles and abstracts), and obtained the full text of reviews identified as relevant for review.
Data extraction and management
Two authors (JS and GW) independently extracted data from the reviews using a predefined data extraction form created as a Microsoft Excel® spreadsheet. A third author (ML) double‐checked the data entry. Disagreements were resolved by discussion. We obtained additional information from the original RCT reports where necessary.
Assessment of methodological quality of included reviews
Two authors (JS and GW) independently evaluated the methodological quality of the included studies for each included review.
Quality of included reviews
Two authors (JS and GW) independently assessed the methodological quality of the included reviews using the 'assessment of multiple systematic reviews' (AMSTAR) instrument (Shea 2007). The AMSTAR instrument uses the following assessment criteria:
1. Was an a priori design provided?
2. Was there duplicate study selection and data extraction?
3. Was a comprehensive literature search performed?
4. Was the status of publication (i.e. grey literature) used as an inclusion criterion?
5. Was a list of studies (included and excluded) provided?
6. Were the characteristics of the included studies provided?
7. Was the scientific quality of the included studies assessed and documented?
8. Was the scientific quality of the included studies used appropriately in formulating conclusions?
9. Were the methods used to combine the findings of studies appropriate?
10. Was the likelihood of publication bias assessed?
11. Was the conflict of interest stated?
Quality of evidence in included reviews
Two authors (JS and ETG) independently assessed the overall quality of the evidence for each study/outcome using the GRADE approach (Atkins 2004). The GRADE approach specifies four levels of quality:
High quality for randomized trials; or double‐upgraded observational studies.
Moderate quality for downgraded randomized trials; or upgraded observational studies.
Low quality for double‐downgraded randomized trials; or observational studies and
Very low quality for triple‐downgraded randomized trials; or downgraded observational studies; or case series/case reports.
Authors could downgrade randomized trial evidence by one or two levels depending on the presence of five factors:
Serious (‐1) or very serious (‐2) limitation to study quality
Important inconsistency (‐1)
Some (‐1) or major (‐2) uncertainty about directness
Imprecise or sparse data (‐1)
High probability of reporting bias (‐1).
Data synthesis
Statistical Analyses
For dichotomous outcomes (i.e. the number of patients achieving more than 50% symptomatic improvement (ACR50), ACR20, ACR70, and those who withdrew due to adverse events and overall withdrawals), we performed the meta‐analysis by combining trials of various drugs versus placebo to obtain mutually independent estimates using odds ratios (OR) as effect measures. For data on withdrawals due to adverse events we anticipated that events might be rather rare (Bradburn 2007; Sweeting 2004). In order to handle these expected sparse data, an empirical Bayes (treatment arm‐based) (Salanti 2008) approach was applied.
When two drugs are compared with a common standard, the difference between these two drugs with respect to the common standard forms the basis of indirect comparisons. In our case, most biologics were used in conjunction with other baseline disease‐modifying antirheumatic drugs (most commonly methotrexate, but others in some cases, which leads to clinical heterogeneity). Heterogeneity is a common issue encountered while performing meta‐analyses (Thompson 1999; Higgins 2002). They were compared to placebo plus the same baseline therapy. Indirect comparisons can be analyzed by various methods according to the different networks applied, including the star, ladder, closed and partially closed‐loop designs (Wells 2009). We used the star design and included one active and one placebo group from each available trial, independent of concomitant medication use. Individual trial data were extracted from the available Cochrane reviews. We did an arm‐based, random‐effects model within an empirical Bayes framework using generalized linear mixed models (GLMM; i.e. a mixed effects logistic regression) (Platt 1999). We modelled the binary outcomes in every treatment group of every study, and specified the relations among the odds ratios (ORs) across studies making different comparisons. The GLMM models fit by the PROC GLIMMIX in SAS (SAS® 9.1.3, SAS Institute Inc, Cary, NC, USA) extend the general linear model by incorporating correlations among the responses. The class statement informs the procedure to treat the variables drug, study, and the stratifying subgroups as classification variables. The model statement specifies the response variable as a sample proportion using an events/observations syntax; the procedure defaults to the binomial distribution. The denominator degrees of freedom for the tests of fixed effects resulting from the model were based on a general Satterthwaite approximation (ddfm=SATTERTH). A ‘random statement’ specifies that the linear predictor contains an intercept term that randomly varies at the level of the ‘Study’ as well as ‘Study by Drug’ interaction. The indirect comparison (D) of each biologic to each other was done on the log‐scale, thus D = log(A) – log(B) = log(A/B) results in a modified Ratio of Odds Ratios (ROR) when back‐transformed: ROR = exp(log[A/B]). The corresponding 95% Confidence Intervals were based on ±1.96×SE(log[A/B]). We present the inconsistency index (Platt 1999) for each of the drugs compared with placebo (ranging from 0% to 100%, higher values indicate more heterogeneity). I2 is a statistic for quantifying inconsistency of the results in the individual reviews (Higgins 2003) and combines the χ2 statistic and the number of studies contributing to each summary estimate in the figure. We evaluated heterogeneity for the indirect comparison analyses using τ2, which examines heterogeneity because of study and study × drug interaction (smaller values indicate a better model). There is no specific range for this measure.
On the basis of the comparison of the individual odds ratio (OR) values to the overall event rate in the placebo groups as a proxy for baseline risk, we estimated the number needed to treat for benefit and harm, with 95% confidence intervals (CIs). This method enables direct translation into clinical practice (Osiri 2003), using Visual Rx with the overall (pooled) number of responders within the available studies as proxy for the expected rate of responders in a given RA population (Cates 2009). We considered p values less than 0.05 and 95% CIs that did not include 1 to be statistically significant. In all the forest plots presenting effect measure data per drug, the average (random‐effects model) applied as default option (Dersimonian 2007) for illustrative purposes, and we used I2 values to evaluate inconsistencies across drugs interpretable as differences not related to random variations.
Sub‐group analyses/planned comparisons
We compared the six biologic DMARDs with regard to efficacy and safety as the main analysis. In addition, we performed the following analyses for the main efficacy outcome, ACR50:
Concomitant methotrexate vs. no methotrexate
RA disease duration ‐ categorized as early RA defined as duration of less than 2 years (Boers 2001) vs. established RA, duration 2 to10 years vs. late RA defined as > 10 years (Barlow 1999)
Anti‐TNF biologic DMARDs vs. other biologic DMARDs
Use in patients who have traditional DMARD‐failure (most commonly Methotrexate) vs. biologic DMARD‐failure vs. none
Single biologic DMARD agent vs. combination biologic therapy
Treatment duration with biologic DMARD: Short (<= 6 months), intermediate duration (> 6 to 12 months) or long‐duration (> 1 year)
Prior failure of TNF‐biologic versus non‐failure
Results
Description of included reviews
Figure 1 shows the details of reviews that were considered and met the criteria for inclusion in this overview. Of the 54 reviews identified, seven reviews were of potential interest. One review (certolizumab) was at the protocol stage and did not have any data available for analysis. Thus, six Cochrane reviews were included in this overview ‐ abatacept (Maxwell 2008), adalimumab (Navarro‐Sarabia 2005), anakinra (Mertens 2008), etanercept (Lethaby 2003), infliximab (Blumenauer 2003) and rituximab (Lopez‐Olivo 2008) (listed alphabetically here and throughout the overview and analysis).
1.
Study Selection Flow Chart
Methodological quality of included reviews
Quality of included reviews
A priori, the research question and inclusion criteria were provided in the published protocols of all six reviews: abatacept (Maxwell 2008), adalimumab (Navarro‐Sarabia 2005; update in press), anakinra (Mertens 2008), etanercept (Lethaby 2003; update in press), infliximab (Blumenauer 2003) and rituximab (Lopez‐Olivo 2008; full review in press).
Two authors (JS and GW) independently selected studies and extracted data from each of the six reviews: abatacept (Maxwell 2008), adalimumab (Navarro‐Sarabia 2005), anakinra (Mertens 2008), etanercept (Lethaby 2003), infliximab (Blumenauer 2003) and rituximab (Lopez‐Olivo 2008).
We conducted a comprehensive literature search in all six reviews (Blumenauer 2003; Lethaby 2003; Lopez‐Olivo 2008; Maxwell 2008; Mertens 2008; Navarro‐Sarabia 2005) without any language restriction. We also searched grey literature.
All six reviews provided a list of included and excluded studies as well as the characteristics of the included studies (Blumenauer 2003; Lethaby 2003; Lopez‐Olivo 2008; Maxwell 2008; Mertens 2008; Navarro‐Sarabia 2005).
All six reviews assessed and documented the scientific quality of included studies (Blumenauer 2003; Lethaby 2003; Lopez‐Olivo 2008; Maxwell 2008; Mertens 2008; Navarro‐Sarabia 2005).
All six reviews considered the results of the methodological quality assessment in the analysis. The conclusions were available for four out of six reviews (66.7%), abatacept (Maxwell 2008), adalimumab (Navarro‐Sarabia 2005), anakinra (Mertens 2008), and etanercept (Lethaby 2003) . Two reviews [infliximab (Blumenauer 2003) and rituximab (Lopez‐Olivo 2008)] were still in progress and had no conclusions yet.
All six reviews used appropriate statistical methods to pool results (Blumenauer 2003; Lethaby 2003; Lopez‐Olivo 2008; Maxwell 2008; Mertens 2008; Navarro‐Sarabia 2005).
Three out of six (50%) reviews assessed publication bias: abatacept, anakinra, etanercept. Publication bias was not applicable in the infliximab review (16.7%) because it only included three studies. It was not possible to determine if publication bias had been assessed in two (33.3%) of the six reviews: one review update (adalimumab, Navarro‐Sarabia 2005) and one new review (rituximab, Lopez‐Olivo 2008).
All six reviews addressed conflicts of interest (Blumenauer 2003; Lethaby 2003; Lopez‐Olivo 2008; Maxwell 2008; Mertens 2008; Navarro‐Sarabia 2005).
The inclusion criteria for studies included in each of the systematic reviews were similar as follows:
(1) Etanercept: "All randomized controlled (RCTs) or controlled clinical trials (CCTs) comparing etanercept to placebo, etanercept to methotrexate, or etanercept plus methotrexate to methotrexate alone that were at least six months long were eligible for inclusion."
(2) Infliximab: "All randomized controlled trials comparing infliximab 1, 3, 5 or 10 mg/kg with methotrexate (MTX) to MTX alone, or without MTX to placebo, with a minimum duration of 6 months and at least 2 infusions were eligible."
(3) Adalimumab: "All randomised controlled trials (RCTs) or controlled clinical trials (CCTs) comparing adalimumab alone or in combination with DMARDs to placebo or other DMARDs."
(4) Anakinra: "All randomised controlled trials (RCTs) comparing anakinra alone or in combination with DMARDs or biologics to placebo or other DMARDs or biologics in patients with rheumatoid arthritis will be considered."
(5) Abatacept: "RCTs comparing abatacept alone or in combination with DMARDs to placebo or other DMARDs. There will be no restrictions with regard to dosage or duration of intervention."
(6) Rituximab: "Treatment with rituximab in combination with any DMARD or rituximab alone versus placebo or other DMARDs or biologic will be eligible for inclusion. Doses of rituximab eligible for inclusion include 300 mg/m2, 350 mg/m2, 500 mg/m2 and 600 mg/m2."
The participants included in the reviews were similar:
1) Etanercept: "Patients 16 years of age or older meeting the ACR 1987 revised criteria (Arnett 1988) for RA. Patients had to have evidence of active disease as demonstrated by at least two of: 1. Tender joint count; 2. Swollen joint count; 3. Duration of early morning stiffness > 30 minutes; 4. Acute phase reactants such as Westergren erythrocyte sedimentation rate (ESR) or C reactive protein (CRP)."
2) Infliximab: "Patients at least 16 years of age meeting the ACR 1987 revised criteria (Arnett 1988) for RA. These patients must have evidence of active disease as demonstrated by at least 2 of: 1. Tender joint count 2. Swollen joint count; 3. Duration of early morning stiffness > 30 minutes.; 4. Acute phase reactants such as Westergren erythrocyte sedimentation rate (ESR) or C‐ reactive protein (CRP)."
3) Adalimumab: "Patients with confirmed RA according to the American College of Rheumatology 1987 revised criteria (Arnett 1988), who had active disease as defined in every study. Patients who have failed methotrexate or other DMARDs therapy, and, also, DMARDs naive patients."
4) Anakinra: "Adults aged 18 years and above meeting the ACR 1987 revised criteria for rheumatoid arthritis (Arnett 1988)."
5) Abatacept: "Patients at least 16 years of age meeting the ACR 1987 revised criteria for rheumatoid arthritis (Arnett 1988)."
6) Rituximab: "Patients at least 16 years of age meeting the American College of Rheumatology 1987 revised criteria (Arnett 1988) for rheumatoid arthritis and active disease as described by authors in relation to the outcome measures."
The outcomes in the reviews were similar. The efficacy outcomes in all reviews (Appendix 4) included the American College of Rheumatology (ACR) improvement criteria along with the core set of disease activity variables and/or Disease Activity Score (DAS). Many studies also included Health Assessment Questionnaire (HAQ) or modified HAQ. Quality of life (QoL) was assessed by Short‐form 36 in many studies. Radiographic progression was frequently assessed by Sharp, modified Sharp or Larsen scores. The safety outcomes (Appendix 5) included adverse events, serious adverse events including infections and malignancy, withdrawals (total), withdrawals and mortality. Withdrawals due to adverse events were reported in all but one systematic review (Mertens 2008). Withdrawals due to inefficacy were reported in three of the six systematic reviews (Blumenauer 2003; Lethaby 2003; Lopez‐Olivo 2008).
Quality of evidence in included reviews
A list of all studies included for the review is presented in Table 1. The main outcomes are reported in Appendix 2. The following section describes GRADE ratings of the included studies (also presented in the Summary of Findings tables ‐ Table 2, Table 3, and Table 4) followed by the AMSTAR rating.
1. Characteristics of the included study populations of the included reviews, including important potentially confounding covariates.
|
Study Name and year |
Study code |
Trial duration |
Disease duration in years |
Concomitant use of MTX | RA duration | Biologic is anti‐TNF | Prior drugs failed | Prior failure of TNF biologic | Combination biologic therapy | Duration of randomized trial | Comparison | Biologic naive |
| Abatacept | ||||||||||||
| Moreland 2002 |
A0001 | 3 months | 3.30 | no | ES | no | both | yes | yes | short | PL | no |
| Genovese 2005 |
A0002 | 6 months | 11.90 | no | LA | no | biologic | yes | yes | short | DMARD | no |
| Schiff 2008 |
A0003 | 6 months | 8.10 | yes | ES | no | dmard | no | yes | short | MTX | yes |
| Kremer 2003 |
A0004 | 6 months | 9.20 | yes | ES | no | dmard | no | yes | short | MTX | yes |
| Kremer 2006 |
A0005 | 12 months | 8.60 | yes | ES | no | dmard | no | yes | intermediate | MTX | yes |
| Weinblatt 2007 |
A0006 | 12 months | 12.90 | no | LA | no | biologic | yes | no | intermediate | ETN | no |
| ASSURE 2006 |
A0007 | 12 months | 9.70 | yes | ES | no | both | yes | yes | intermediate | DMARD_BIO | no |
| Adalimumab | ||||||||||||
| Bejarano 2008 |
A0008 | 13 months | 0.90 | yes | EA | yes | dmard | no | yes | long | MTX | yes |
| Breedveld 2006 |
A0009 | 24 months | 0.70 | yes | EA | yes | dmard | no | yes | long | MTX | yes |
| Furst 2003 |
A0010 | 6 months | 10.40 | no | LA | yes | dmard | no | yes | short | DMARD | yes |
| Keystone 2004 |
A0011 | 12 months | 11.00 | yes | LA | yes | dmard | no | yes | intermediate | MTX | yes |
| Kim 2007 |
A0012 | 6 months | 6.80 | yes | ES | yes | dmard | no | yes | short | MTX | yes |
| Miyasaka 2008 |
A0013 | 6 months | 9.80 | no | ES | yes | dmard | no | yes | short | PL | yes |
| Van De Putte 2004 |
A0014 | 6 months | 11.10 | no | LA | yes | dmard | no | yes | short | PL | yes |
| Weinblatt 2003 |
A0015 | 6 months | 11.70 | yes | LA | yes | dmard | no | yes | short | MTX | yes |
| Anakinra | ||||||||||||
| Bresnihan 1998 |
A0016 | 6 months | 4.10 | no | ES | no | dmard | no | yes | short | PL | yes |
| Fleischiman 2003 |
A0017 | 6 months | 10.30 | no | LA | no | dmard | no | yes | short | DMARD | yes |
| Cohen 2002 |
A0018 | 6 months | 7.80 | yes | ES | no | dmard | no | yes | short | MTX | yes |
| Cohen 2004 |
A0019 | 6 months | 10.50 | yes | LA | no | dmard | no | yes | short | MTX | yes |
| Genovese 2004 |
A0020 | 6 months | 10.20 | yes | LA | no | dmard | no | no | short | ETN_MTX | yes |
| Etanercept | ||||||||||||
| Moreland 1999 |
A0021 | 6 months | 11.50 | no | LA | yes | dmard | no | yes | short | PL | yes |
| Weinblatt 1999 |
A0022 | 6 months | 13.00 | yes | LA | yes | dmard | no | yes | short | MTX | yes |
| COMET 2008 |
A0023 | 12 months | 0.90 | yes | EA | yes | none | no | yes | intermediate | MTX | yes |
| TEMPO 2004 |
A0024 | 12 months | 6.80 | yes | ES | yes | dmard | no | yes | intermediate | MTX | yes |
| Infliximab | ||||||||||||
| Maini 1998 |
A0025 | 6 months | 9.90 | yes | ES | yes | dmard | no | yes | short | MTX | yes |
| ASPIRE 2004 |
A0026 | 12 months | 0.80 | yes | EA | yes | dmard | no | yes | intermediate | MTX | yes |
| ATTRACT 2000 |
A0027 | 12 months | 10.50 | yes | LA | yes | dmard | no | yes | intermediate | MTX | yes |
| Quinn 2005 |
A0028 | 12 months | 0.70 | yes | EA | yes | none | no | yes | intermediate | MTX | yes |
| Rituximab | ||||||||||||
| Edwards 2004 |
A0029 | 6 months | 11.50 | yes | LA | no | dmard | no | yes | short | MTX | yes |
| DANCER 2006 |
A0030 | 6 months | 10.10 | yes | LA | no | both | yes | yes | short | MTX | no |
| REFLEX 2006 |
A0031 | 6 months | 11.90 | yes | LA | no | biologic | yes | yes | short | MTX | no |
MTX = methotrexate; ES = Established; PL = placebo; LA = late; DMARD = disease‐modifying antirheumatic drug; ETN = etanercept; BIO = biologic
2. Summary of Findings Table.
| Biologics for rheumatoid arthritis: combined 3‐, 6‐ and 12‐month outcome data, adjusted for Control Event Rate (CER) in the placebo group | |||||||
| Outcome | Intervention and Comparison intervention |
Illustrative comparative risks (95%) CI) |
Relative effect (95% CI) |
Number of participants (studies) |
Quality of the evidence (GRADE) |
NNT (95% CI) |
|
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| ACR20 by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
382 per 1000 | 270 per 1000 | OR 3.03 (2.02 to 4.55) | 1712 (6 studies) | ⊕⊕⊕⊝ moderate1 | 4 (3 to 6) |
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
382 per 1000 | 275 per 1000 | OR 3.09 (2.18 to 4.39) | 2269 (8 studies) | ⊕⊕⊕⊝ moderate2 | 4 (3 to 6) |
| Anakinra | Anakinra +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
382 per 1000 | 112 per 1000 | OR 1.58 (0.97 to 2.56) | 1164 (4 studies) | ⊕⊕⊕⊝ moderate3 | Not statistically significant |
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
382 per 1000 | 352 per 1000 | OR 4.47 (2.70 to 7.38) | 1205 (4 studies) | ⊕⊕⊕⊝ moderate4 | 3 (3 to 5) |
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
382 per 1000 | 200 per 1000 | OR 2.26 (1.21 to 4.21) | 819 (3 studies) | ⊕⊕⊕⊕ high |
5 (3 to 22) |
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
382 per 1000 | 307 per 1000 | OR 3.59 (2.02 to 6.37) | 823 (3 studies) | ⊕⊕⊕⊝ moderate5 | 4 (3 to 6) |
| ACR50 by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
207 per 1000 | 437 per 1000 (319–565) | OR 2.98 (1.79 to 4.97) | 1712 (6 studies) |
⊕⊕⊕⊝ moderate1 | 5 (3 to 10) |
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
207 per 1000 | 491 per 1000 (385–598) | OR 3.70 (2.40 to 5.70) | 2269 (8 studies) |
⊕⊕⊕⊝ moderate2 | 4 (3 to 6) |
| Anakinra | Anakinra +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
207 per 1000 | 304 per 1000 (178–472) | OR 1.68 (0.83 to 3.41) | 815 (3 studies) |
⊕⊕⊕⊝ moderate3 | Not statistically significant |
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
207 per 1000 | 565 per 1000 (414–704) | OR 4.97 (2.70 to 9.13) | 1205 (4 studies) |
⊕⊕⊕⊝ moderate4 | 3 (3 to 5) |
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
207 per 1000 | 433 per 1000 (263–619) | OR 2.92 (1.37 to 6.24) | 819 (3 studies) |
⊕⊕⊕⊕ high |
5 (3 to 18) |
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
207 per 1000 | 518 per 1000 (346–1000) | OR 4.10 (2.02 to 8.33) | 823 (3 studies) |
⊕⊕⊕⊝ moderate5 | 4 (3 to 8) |
| ACR70 by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
106 per 1000 | 360 per 1000 |
OR 4.00 (2.21 to 7.21) |
1712 (6 studies) | ⊕⊕⊕⊝ moderate1 | 5 (3 to 10) |
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
106 per 1000 | 425 per 1000 |
OR 3.98 (2.48 to 6.4) |
2269 (8 studies) | ⊕⊕⊕⊝ moderate2 | 5 (4 to 9) |
| Anakinra | Anakinra +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
106 per 1000 | 130 per 1000 |
OR 1.63 (0.72 to 3.65) |
815 (3 studies) | ⊕⊕⊕⊝ moderate3 | Not statistically significant |
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
106 per 1000 | 320 per 1000 |
OR 4.05 (2.07 to 7.93) |
1205 (4 studies) | ⊕⊕⊕⊝ moderate4 | 5 (3 to 11) |
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
106 per 1000 | 264 per 1000 | OR 3.23 (1.42 to 7.37) | 819 (3 studies) | ⊕⊕⊕⊕ high |
6 (3 to 27) |
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
106 per 1000 | 475 per 1000 |
OR 5.30 (2.35 to 11.92) |
823 (3 studies) | ⊕⊕⊕⊝ moderate5 | 4 (3 to 9) |
| Safety (withdrawals due to AE) by Drug*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
54 per 1000 | 66 per 1000 |
OR 1.24 (0.88 to 1.76) |
1441 (6 studies) |
⊕⊕⊕⊝ moderate1 | Not statistically significant |
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
54 per 1000 | 81 per 1000 |
OR 1.54 (1.12 to 2.12) |
2944 (8 studies) |
⊕⊕⊝⊝ low2,6 | 39 (19 to 162) |
| Anakinra | Anakinra +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
54 per 1000 | 87 per 1000 | OR 1.67 (1.22 to 2.29) | 2619 (5 studies) | ⊕⊕⊕⊝ moderate3 | 31 (17 to 92) |
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
54 per 1000 | 45 per 1000 |
OR 0.82 (0.56 to 1.19) |
1248 (4 studies) |
⊕⊕⊕⊝ moderate4 | Not statistically significant |
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
54 per 1000 | 112 per 1000 |
OR 2.21 (1.28 to 3.82) |
835 (3 studies) |
⊕⊕⊕⊕ high |
18 (8 to 72) |
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
54 per 1000 | 71 per 1000 |
OR 1.34 (0.65 to 2.76) |
938 (3 studies) |
⊕⊕⊕⊝ moderate5 | Not statistically significant |
| Total withdrawals by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
272 per 1000 | 183 per 1000 |
OR 0.52 (0.35 to 0.75) |
3169 (7 studies) |
⊕⊕⊕⊝ moderate1 | 10 (7 to 21) |
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
272 per 1000 | 223 per 1000 |
OR 0.64 (0.45 to 0.92) |
2860 (7 studies) |
⊕⊕⊝⊝ low2,6 | 13 (8 to 47) |
| Anakinra | Anakinra +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
272 per 1000 | 286 per 1000 |
OR 1.12 (0.69 to 1.80) |
2118 (4 studies) |
⊕⊕⊕⊝ moderate3 | Not statistically significant |
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
272 per 1000 | 163 per 1000 |
OR 0.37 (0.23 to 0.62) |
1248 (4 studies) |
⊕⊕⊕⊝ moderate4 | 7 (5 to 11) |
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
272 per 1000 | 219 per 1000 |
OR 0.60 (0.32 to 1.13) |
835 (3 studies) |
⊕⊕⊕⊕ high |
Not statistically significant |
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
272 per 1000 | 127 per 1000 |
OR 0.29 (0.16 to 0.54) |
938 (3 studies) |
⊕⊕⊕⊝ moderate5 | 6 (5 to 10) |
The assumed risk is based on the empirical control event rate (CER) across all drugs and all studies for outcome data at 3, 6 and 12 months combined.
The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
NNT = number needed to treat
DMARD = disease‐modifying anti‐rheumatic drugs
AE = adverse events
OR = odds ratio
1 Kremer 2006: intention to treat analysis not performed ‐ 9 patients in abatacept group and 5 in control group not included in analysis. 2 Randomization and blinding were not described and also allocation concealment was not clear in 7 studies: Breedveld 2007; Furst 2003; Keystone 2004; Kim 2007; Miyasaka 2008; van de Putte 2004; Weinblatt 2003. 3 Randomization not described in all four studies; intention to treat analysis not performed in three studies (Bresnihan 1998; Cohen 2004; Genovese 2004); blinding not described and > 20% dropout in Cohen 2002; allocation concealment not described in Genovese 2004. 4 Randomization not described in TEMPO 2004; allocation concealment and blinding not described in COMET 2008. 5 Randomization and allocation concealment not described in all three studies; blinding not clear in Emery (DANCER) 2006; Attrition not clear in Cohen (REFLEX) 2006 study. 6 Analysis includes non‐standard doses.
3. Summary of Findings Table.
| Biologics for rheumatoid arthritis: 6‐month outcome data provided in original reviews (not involving indirect comparisons) | |||||||
| Outcome | Intervention and Comparison intervention |
Illustrative comparative risks (95%) CI) |
Relative effect (95% CI) |
Number of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| ACR50 at 6 months by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
137 per 1000 | 338 per 1000 (274 to 421) |
RR 2.47 (2 to 3.07) |
1648 (5 studies) | ⊕⊕⊕⊝ moderate1 | |
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
109 per 1000 | 380 per 1000 (258 to 559) |
RR 3.49 (2.37 to 5.13) |
1195 (4 studies) | ⊕⊕⊕⊝ moderate2a |
|
| Adalimumab vs placebo | 73 per 1000 |
232 per 1000 (142 to 382) |
RR 3.18 (1.94 to 5.23) | 506 (3 studies) | ⊕⊕⊕⊝ moderate2b |
||
| Anakinra | Anakinra + DMARD vs placebo + DMARD |
74 per 1000 | 186 per 1000 (115 to 298) | RR 2.51 (1.56 to 4.03) | 654 (2 studies) | ⊕⊕⊕⊝ moderate3 |
|
| Anakinra + Biologic vs placebo + Biologic |
412 per 1000 | 310 per 1000 |
RR 0.75 (0.49 to 1.14) |
161 (1 study) | ⊕⊕⊕⊝ moderate4 |
||
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
45 per 1000 | 400 per 1000 (162 to 985) | RR 8.89 (3.61 to 21.89) | 247 (2 studies) | ⊕⊕⊕⊝ moderate5 |
|
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
85 per 1000 | 316 per 1000 (218 to 457) |
RR 3.72 (2.57 to 5.38) |
823 (3 studies) | ⊕⊕⊕⊝ moderate6 |
|
| DAS low disease activity at 6 months by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
75 per 1000 |
252 per 1000 (171 to 372) |
RR 3.36 (2.28 to 4.96) |
1027 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| Physical function ‐ HAQ at 6 months by DRUG*studies | |||||||
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
The mean change in the control groups was ‐0.24 points | The mean change in the intervention groups was 0.32 lower (0.4 to 0.24 lower) |
PDC*** 182% improvement |
663 (3 studies) | ⊕⊕⊕⊝ moderate7a | |
| Adalimumab vs placebo | The mean change in the control groups was ‐0.07 points | The mean change in the intervention groups was 0.31 lower (0.42 to 0.19 lower) |
PDC*** 25% improvement |
401 (2 studies) | ⊕⊕⊕⊝ moderate7b | ||
| Anakinra | Anakinra + DMARD vs placebo + DMARD |
The mean change in the control groups was ‐0.18 points | The mean change in the intervention groups was 0.19 lower (0.3 to 0.09 lower) |
PDC*** 61% improvement |
951 (2 studies) | ⊕⊕⊝⊝ low8,9 | |
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
The mean change in the control group was 1.1 points | The mean change in the intervention group was 0.3 lower (0.65 lower to 0.05 higher) |
PDC*** 75% improvement |
89 (1 study) | ⊕⊕⊕⊕ high |
|
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
The mean change in the control groups was 1.8 points | The mean change
in the intervention groups was 0.3 lower (0.37 to 0.22 lower) |
PDC*** 182% improvement |
823 (3 studies) | ⊕⊕⊕⊝ moderate6 |
|
| Radiographic score (total) at 6 months by DRUG*studies | |||||||
| Anakinra (Larsen score) |
Anakinra + DMARD vs placebo + DMARD |
The mean change in the control group was 6.4 | The mean change
in the intervention group was 2.5 lower (5.56 lower to 0.56 higher) |
PDC** 39.1% less progression |
172 (1 study) | ⊕⊕⊕⊝ moderate10 |
|
| AE (total) at 6 months by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
770 per 1000 |
816 per 1000 (747 to 885) |
RR 1.06 (0.97 to 1.15) |
657 (2 studies) | ⊕⊕⊕⊝ moderate11 | |
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
893 per 1000 |
920 per 1000 (893 to 947) |
RR 1.03 (1 to 1.06)12 |
1987 (6 studies) | ⊕⊕⊝⊝ low12a,13 | |
| Adalimumab vs placebo | 779 per 1000 |
974 per 1000 (748 to 1000) |
RR 1.25 (0.96 to 1.61) |
854 (3 studies) |
⊕⊕⊝⊝ low12b,13 | ||
| Anakinra | Anakinra + DMARD vs placebo + DMARD |
879 per 1000 | 923 per 1000 (826 to 1000) |
RR 1.05 (0.94 to 1.17) |
1894 (2 studies) | ⊕⊕⊕⊝ moderate3 | |
| Anakinra + Biologic vs placebo + Biologic |
973 per 1000 |
1000 per 1000 (924 to 1000) |
RR 1.04 (0.95 to 1.14) | 155 (1 study) | ⊕⊕⊕⊝ moderate4 | ||
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
804 per 1000 | 860 per 1000 (732 to 1000) |
RR 1.07 (0.91 to 1.26) |
938 (3 studies) | ⊕⊕⊝⊝ low6,11 | |
| SAE (total) at 6 months for rheumatoid arthritis | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
97 per 1000 |
78 per 1000 (48 to 127) |
RR 0.8 (0.49 to 1.31) | 703 (2 studies) | ⊕⊕⊕⊕ high |
|
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
90 per 1000 | 84 per 1000 (67 to 131) | RR 0.93 (0.60 to 1.45) | 843 (4 studies) | ⊕⊕⊝⊝ low13,14 |
|
| Adalimumab vs placebo | 105 per 1000 |
111 per 1000 (69 to 179) |
RR 1.06 (0.66 to 1.7) |
1111
(4 studies) |
⊕⊕⊝⊝ low12b,13 |
||
| Anakinra | Anakinra + DMARD vs placebo + DMARD |
56 per 1000 |
86 per 1000 (38 to 195) |
RR 1.53 (0.67 to 3.48) |
1900 (2 studies) | ⊕⊕⊕⊝ moderate3 | |
| Anakinra + Biologic vs placebo + Biologic |
25 per 1000 |
148 per 1000 (34 to 641) |
RR 5.93 (1.37 to 25.64) |
161 (1 study) | ⊕⊕⊝⊝ low4,5 | ||
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
70 per 1000 |
71 per 1000 (45 to 113) |
RR 1.01 (0.64 to 1.61) |
938 (3 studies) | ⊕⊕⊕⊝ moderate6 |
|
| Total withdrawals at 6 months‐ by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
235 per 1000 |
148 per 1000 (94 to 237) |
RR 0.63 (0.4 to 1.01) |
891 (3 studies) | ⊕⊕⊕⊕ high |
|
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
261 per 1000 |
204 per 1000 (172 to 240) |
RR 0.78 (0.66 to 0.92) |
1964 (7 studies) | ⊕⊕⊝⊝ low13,15 |
|
| Adalimumab vs placebo | 345 per 1000 |
190 per 1000 (117 to 310) |
RR 0.55 (0.34 to 0.9) |
1180 (3 studies) | ⊕⊕⊝⊝ low13,16 |
||
| Anakinra | Anakinra + DMARD vs placebo + DMARD |
222 per 1000 | 231 per 1000 (191 to 282) | RR 1.04 (0.86 to 1.27) | 1957 (3 studies) | ⊕⊕⊕⊝ moderate3,10 | |
| Anakinra + Biologic vs placebo + Biologic |
62 per 1000 | 184 per 1000 (70 to 482) | RR 2.96 (1.13 to 7.77) | 162 (1 study) | ⊕⊕⊕⊝ moderate4 |
||
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
545 per 1000 |
185 per 1000 (120 to 272) |
RR 0.34 (0.22 to 0.5) |
247 (2 studies) | ⊕⊕⊕⊕ high |
|
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
379 per 1000 |
148 per 1000 (117 to 186) |
RR 0.39 (0.31 to 0.49) |
938 (3 studies) | ⊕⊕⊕⊝ moderate6 |
|
The assumed risk is based on the empirical control event rate (CER) across all studies with 6 month outcome data, provided in the original reviews.
The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
DMARD = disease‐modifying anti‐rheumatic drugs
AE = adverse events
SAE = serious adverse events
DAS = disease activity score
HAQ = Health Assessment Questionnaire
RR = risk ratio
***PDC = percent difference in improvement in physical function between the intervention and control groups relative to improvement in control group.
**PDC = percent difference in radiographic progression between the intervention and control group relative to progression in control group.
1 Kremer 2006: intention to treat analysis not performed ‐ 9 patients in abatacept group and 5 in control group not included in analysis. 2a Randomization and blinding were not described and also allocation concealment was not clear in four studies: Furst 2003; Keystone 2004; Kim 2007; Weinblatt 2003. 2b Randomization and blinding were not described and also allocation concealment was not clear in three studies: Weinblatt 2003; Miyasaka 2008; van de Putte 2004. 3 Randomization not described in both studies (Cohen 2002; Cohen 2004) ; intention to treat analysis not performed in Cohen 2004 study; blinding not described and > 20% dropout in Cohen 2002 study. 4 Genovese 2004: allocation concealment not described and ITT analysis not performed. 5 Wide confidence interval. 6 Randomization and allocation concealment not described in all three studies; blinding not clear in Emery (DANCER) 2006; Attrition not clear in Cohen (REFLEX) 2006 study. 7a Randomization and blinding were not described and also allocation concealment was not clear in all three studies: Keystone 2004; Kim 2007; Weinblatt 2003. 7b Randomization and blinding were not described and also allocation concealment was not clear in both studies: Miyasaka 2008; van de Putte 2004. 8 Randomization not described and intention to treat analysis not performed in both studies (Bresnihan 1998; Cohen 2004); 9 Bresnihan 1998: non‐standard dose included. 10 Bresnihan 1998: randomization not described and intention to treat analysis not performed. 11 Unexplained heterogeneity. 12a Randomization and blinding were not described and also allocation concealment was not clear in five studies: Breedveld 2007; Furst 2003; Keystone 2004; Kim 2007; Rau 2004. 12b Randomization and blinding were not described and also allocation concealment was not clear in three studies: Furst 2003; Miyasaka 2008; van de Putte 2004. 13 Analysis includes non‐standard doses. 14 Randomization and blinding were not described and also allocation concealment was not clear in three studies: Furst 2003; Kim 2007; Rau 2004. 15 Randomization and blinding were not described and also allocation concealment was not clear in four studies: Breedveld 2007; Furst 2003; Keystone 2004; Kim 2007.
4. Summary of Findings Table.
| Biologics for rheumatoid arthritis: 12‐month outcome data provided in original reviews (not involving indirect comparisons) | |||||||
| Outcome | Intervention and Comparison intervention |
Illustrative comparative risks (95%) CI) |
Relative effect (95% CI) |
Number of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| ACR50 at 12 months by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
179 per 1000 | 396 per 1000 (310 to 505) |
RR 2.21 (1.73 to 2.82) |
993 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
321 per 1000 | 603 per 1000 (258 to 559) |
RR 1.88 (1 to 3.55) |
1080 (3 studies) | ⊕⊕⊝⊝ low2,3 | |
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
461 per 1000 | 701 per 1000 (627 to 784) | RR 1.52 (1.36 to 1.7) | 958 (2 studies) | ⊕⊕⊕⊝ moderate4 |
|
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
266 per 1000 | 404 per 1000 (333 to 492) | RR 1.52 (1.25 to 1.85) | 819 (3 studies) | ⊕⊕⊕⊕ high |
|
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
115 per 1000 | 470 per 1000 (241 to 917) |
RR 3.72 (2.57 to 5.38) |
353 (3 studies) | ⊕⊕⊕⊝ moderate5 |
|
| DAS low disease activity at 12 months by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
98 per 1000 |
424 per 1000 (278 to 646) |
RR 4.33 (2.84 to 6.59) |
638 (1 study) | ⊕⊕⊕⊝ moderate1 | |
| Physical function ‐ HAQ at 12 months by DRUG*studies | |||||||
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
The mean change in the control groups was ‐0.80 points | The mean change in the intervention groups was 0.32 lower (0.39 to 0.25 lower) |
PDC*** 81% improvement |
1080 (3 studies) | ⊕⊕⊕⊝ moderate2 | |
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
The mean change in the control group was 0.9 points | The mean change in the intervention group was 0.25 lower (0.65 lower to 0.05 higher) |
PDC *** 25% improvement |
956 (2 studies) | ⊕⊕⊕⊝ moderate6 | |
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
The mean change in the control groups was 0.68 points | The mean change in the intervention groups was 0.13 higher (0.05 to 0.22 lower) |
PDC *** 62% improvement |
835 (3 studies) | ⊕⊕⊕⊕ high |
|
| Radiographic score (total) at 12 months by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
The mean change in the control group was 0.27 | The mean change in the intervention group was 0.27 lower (0.42 lower to 0.12 higher) |
PDC ** 100% less progression |
586 (1 study) | ⊕⊕⊕⊝ moderate1 |
|
| Adalimumab (modified Sharp score) |
Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
The mean change in the control group was 2.7 points | The mean change in the intervention groups was 2.60 lower (3.83 to 1.37 lower) |
PDC ** 96% less progression |
880 (2 studies) | ⊕⊕⊕⊝ moderate2 | |
| Etanercept (Sharp score) |
Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
The mean change in the control group was 2.4 points | The mean change in the intervention group was 2.21 lower (2.99 lower to 1.43 higher) |
PDC ** 92% less progression |
894 (2 studies) | ⊕⊕⊕⊝ moderate4 |
|
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
The mean change in the control groups was 3.7 points | The mean change in the intervention groups was 3.69 lower (0.05 to 0.22 lower) |
PDC ** 99.7% less progression |
776 (2 studies) | ⊕⊕⊕⊕ high |
|
| AE (total) at 12 months by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
859 per 1000 |
893 per 1000 (868 to 928) |
RR 1.04 (1.01 to 1.08) |
2214 (3 studies) | ⊕⊕⊕⊝ moderate7 | |
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
893 per 1000 |
920 per 1000 (893 to 947) |
RR 1.03 (1 to 1.06) |
1987 (6 studies) | ⊕⊕⊝⊝ low8,9 | |
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
133 per 1000 | 137 per 1000 (97 to 196) |
RR 1.03 (0.73 to 1.47) |
835 (2 studies) | ⊕⊕⊕⊕ high |
|
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
850per 1000 | 1000 per 1000 (289 to 1000) |
RR 1.24 (0.34 to 4.43) |
80 (1 study) | ⊕⊕⊕⊝ moderate10 | |
| SAE (total) at 12 months for rheumatoid arthritis | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
123 per 1000 |
138 per 1000 (109 to 171) |
RR 1.12 (0.89 to 1.39) | 2448 (4 studies) | ⊕⊕⊕⊝ moderate11 | |
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
90 per 1000 | 84 per 1000 (67 to 131) | RR 0.93 (0.60 to 1.45) | 843 (4 studies) | ⊕⊕⊝⊝ low9,12 |
|
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
133 per 1000 |
137 per 1000 (97 to 196) |
RR 1.03 (0.73 to 1.47) |
835 (2 studies) | ⊕⊕⊕⊕ high |
|
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
100 per 1000 |
100 per 1000 (23 to 431) |
RR 1.00 (0.23 to 4.31) |
80 (1 study) | ⊕⊕⊕⊝ moderate10 |
|
| Total withdrawals from therapy at 12 months by DRUG*studies | |||||||
| Abatacept | Abatacept + DMARD/Biologic vs placebo + DMARD/Biologic |
241 per 1000 |
147 per 1000 (123 to 174) |
RR 0.61 (0.51 to 0.72) |
2448 (4 studies) | ⊕⊕⊕⊝ moderate11 |
|
| Adalimumab | Adalimumab +/‐ DMARD/Biologic vs placebo +/‐ DMARD/Biologic |
261 per 1000 |
204 per 1000 (172 to 240) |
RR 0.78 (0.66 to 0.92) |
1964 (5 studies) | ⊕⊕⊝⊝ low9,13 |
|
| Etanercept | Etanercept +/‐ DMARD vs placebo +/‐ DMARD |
545 per 1000 |
185 per 1000 (120 to 272) |
RR 0.34 (0.22 to 0.5) |
247 (2 studies) | ⊕⊕⊕⊝ moderate4 |
|
| Infliximab | Infliximab + DMARD vs placebo + DMARD |
274 per 1000 | 197 per 1000 (153 to 252) |
RR 0.72 (0.56 to 0.92) |
835 (3 studies) | ⊕⊕⊕⊕ high |
|
| Rituximab | Rituximab + DMARD vs placebo + DMARD |
379 per 1000 |
148 per 1000 (117 to 186) |
RR 0.39 (0.31 to 0.49) |
938 (3 studies) | ⊕⊕⊕⊝ moderate10 |
|
The assumed risk is based on the empirical control event rate (CER) across all studies with 12 month outcome data, provided in the original reviews.
The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
DMARD = disease‐modifying anti‐rheumatic drugs
AE = adverse events
SAE = serious adverse events
DAS = disease activity score
RR = risk ratio
***PDC = percent difference in improvement in physical function between the intervention and control groups relative to improvement in control group.
**PDC = percent difference in radiographic progression between the intervention and control group relative to progression in control group.
1 Kremer 2006: intention to treat analysis not performed ‐ 9 patients in abatacept group and 5 in control group not included in analysis. 2 Randomization and blinding were not described and also allocation concealment was not clear in two studies: Breedveld 2006; Keystone 2004. 3 Unexplained substantial heterogeneity. 4 Randomization not described in TEMPO 2004; allocation concealment and blinding not described in COMET 2008 5 Randomization and allocation concealment not described in all three studies; blinding not clear in Emery (DANCER) 2006; Attrition not clear in Cohen (REFLEX) 2006 study. 6 TEMPO 2004: randomization not described. 7 Intention to treat analysis not performed in two studies: Kremer 2006; Weinblatt 2006. Risk of attrition bias (< 80% completion rate in treatment groups at 12 months) in Weinblatt 2007. 8 Randomization and blinding were not described and also allocation concealment was not clear in five studies: Breedveld 2007; Furst 2003; Keystone 2004; Kim 2007; Rau 2004. 9 Analysis includes non‐standard doses. 10 Edwards 2004: randomization and allocation concealment not described. 11 Intention to treat analysis not performed in two studies: Kremer 2006; Weinblatt 2006. Risk of attrition bias (< 80% completion rate in treatment groups at 12 months) in Kremer 2003 and Weinblatt 2007. 12 Randomization and blinding were not described and also allocation concealment was not clear in three studies: Furst 2003; Kim 2007; Rau 2004. 13 Randomization and blinding were not described and also allocation concealment was not clear in four studies: Breedveld 2006; Furst 2003; Keystone 2004; Kim 2007.
Abatacept for RA
Seven studies were included in this review (Maxwell 2008). Intention to treat analysis was not performed in two studies. There was risk of attrition bias with < 80 % completion rate in the treatment groups at 12 months in two studies. Radiographic data were not obtained for 90% of the study population. Physical function was measured as a categorical outcome of HAQ by a decrease in the minimal clinically important change. The quality of the evidence was moderate because of these limitations in the study design.
Adalimumab for RA
Eight studies were included from this review (Navarro‐Sarabia 2005). There were limitations in the study design of six studies ‐ the method of randomization was not described, allocation concealment was not reported, and blinding was not described. There was unexplained substantial heterogeneity or inconsistency of results. Reported data were sparse. The quality of the evidence was moderate for efficacy outcomes. The quality for safety outcomes was downgraded to low because the data reported included both standard and non‐standard doses.
Anakinra for RA
Five studies were included from this review (Mertens 2008) with limitations in study design including methods of randomization not described in all five, allocation concealment was not reported in one study, and blinding was not described in one study. Intention‐to‐treat analysis was not performed in four studies. There was > 20% attrition in two studies. Data on all withdrawals from therapy were not reported. This resulted in a downgrading of the GRADE quality of evidence to moderate.
Etanercept for RA
Four studies were included from this review (Lethaby 2003) and four had limitations in study design including one or more of the following: method of randomization was not described, allocation concealment was not reported, and blinding was not described. There was unexplained substantial heterogeneity in the results. There was imprecision of results due to wide confidence interval and sparse data. The quality of the evidence was moderate.
Infliximab for RA
Only four studies were included from this review (Blumenauer 2003) and intention‐to‐treat analysis was not performed in one. Data were missing for important outcomes such as total adverse events and infections as well as physical function (HAQ). The quality of the evidence was high as a result of high quality studies.
Rituximab for RA
Only three studies were included (Lopez‐Olivo 2008). The method of randomization and allocation concealment was not described in all three studies. Blinding was not described in two and there was risk of attrition bias in one study. There was unexplained substantial heterogeneity in some results. Radiographic scores were not reported. The evidence for rituximab was moderate.
AMSTAR rating for the reviews
Most reviews scored very high on the AMSTAR criteria. These are summarized in Appendix 3.
Effect of interventions
All data were extracted from the updated Cochrane systematic reviews addressing the six medications listed above. Review of the data revealed that both primary/major outcomes including ACR50 and number of withdrawals due to adverse events were uniformly reported in these systematic reviews.
Several other outcomes as pre‐specified in "Types of Outcome Measures" such as ACR20, DAS/DAS28, Disease state (good EULAR response, low disease activity or remission), HRQoL, HAQ, radiographic progression, number of adverse events, number of serious adverse events, specific adverse events (infections, malignancy) and death were only reported in few, but not all systematic reviews (Appendix 4; Appendix 5). Analyses and comparisons for these outcomes were done where possible. When analysis was impossible due to lack of data for comparisons, data were simply described in the text or depicted in tables. In contrast, two outcomes, ACR70, ACR20 and all withdrawals (any cause) were reported in all reviews (or obtained from authors), with at least one study presenting these outcomes. These were analyzed for each drug separately for the main comparison between the six biologics.
For the main efficacy outcome, we performed all seven comparisons in the order of pre‐specified analyses as listed in the "Sub‐group analyses/planned comparisons" above. Safety as assessed by withdrawals due to adverse events was compared between six biologics. Additional outcomes available from all studies, including ACR70 and all withdrawals were also compared between the six biologics. For data that could not be analyzed, a summary following these outcomes is provided in the text and/or in the tables.
Upon review of individual systematic reviews, it was not possible to abstract all study characteristics. GW and JS therefore abstracted study characteristics independently from the methods section of each published study.
Main analysis: comparison of the six biologic DMARDs with regard to efficacy and safety
Primary/major efficacy outcome: ACR50
Of the 31 studies included in the Cochrane systematic reviews, 27 studies reported ACR50. Of these, 20 studies had concomitant DMARD therapy (most often methotrexate) and 7 studies had no concomitant DMARD therapy.
The use of biologic DMARD therapy was associated with a significantly higher likelihood of achieving an ACR50 response, compared to placebo with an OR of 3.35 (2.62 to 4.29) (Figure 2) although based on results with a substantial degree of heterogeneity, with I2 of 69% (Figure 3).
2.
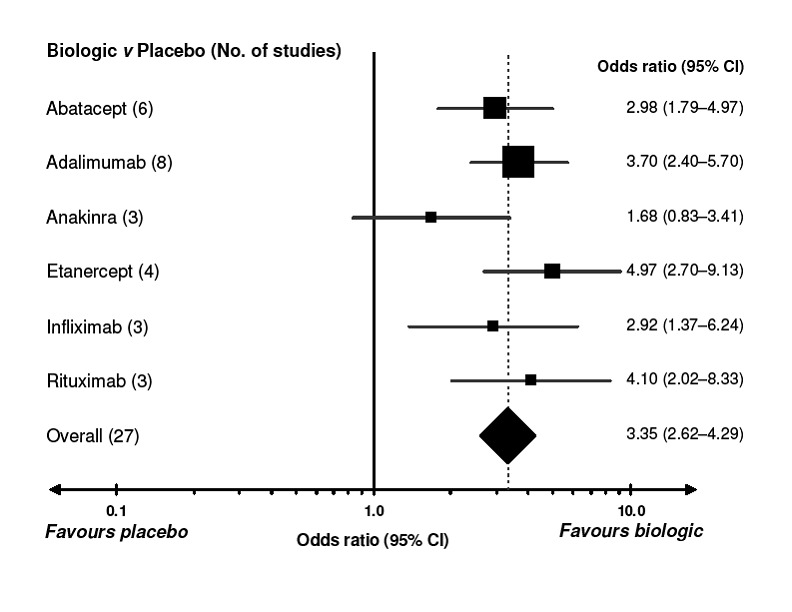
Comparison of each biologic to placebo for benefit (defined as a 50% improvement in patient‐ and physician‐reported criteria of the American College of Rheumatology [ACR50]). A value greater than 1.0 indicates a benefit from the biologic. CI = confidence interval. For details of studies included for each biologic, refer to Appendix. I2 values for the studies are presented in Figure 7. Every square represents the individual study’s effect measure with 95% CI indicated by horizontal lines. Square sizes are proportional to the precision of the estimate.
3.
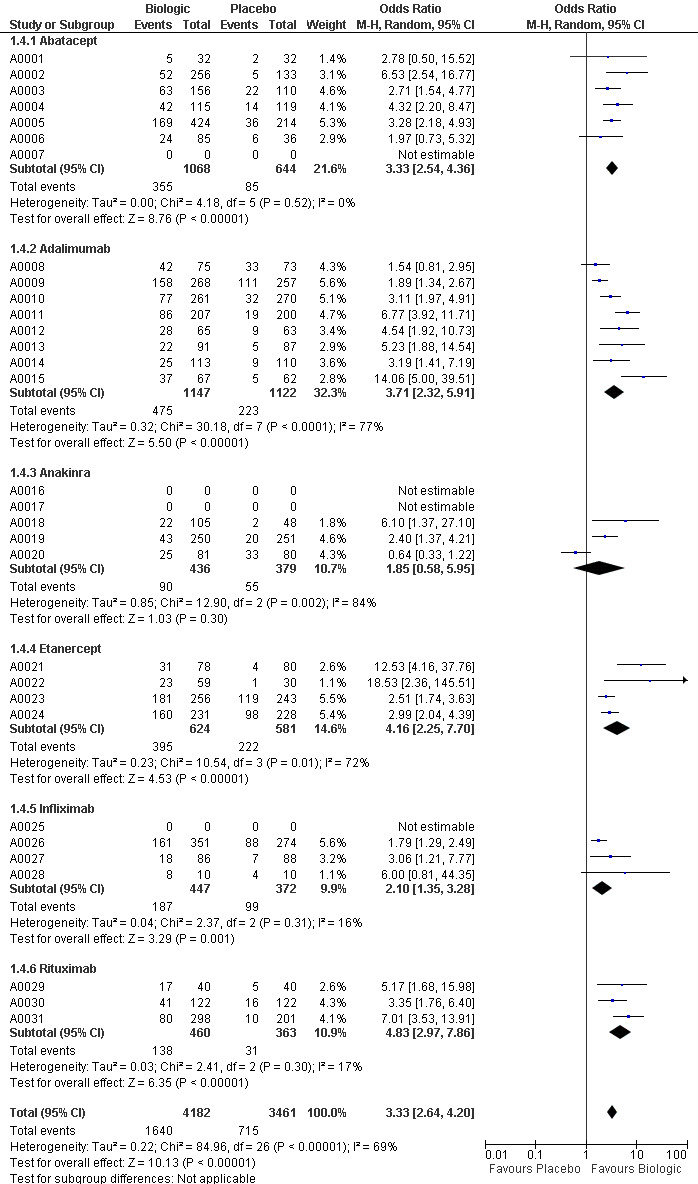
Forest plots for ACR50 (grouped by drug)
Compared to patients receiving placebo, patients receiving each biologic, except anakinra, were significantly more likely to achieve an ACR50 with OR ranging from 2.92 to 4.97 times (Figure 2). In this combined model, Anakinra was not statistically different from placebo with OR of 1.68 (95%CI: 0.83 to 3.41) (Figure 2).
The indirect comparisons for ACR50 are presented in Figure 4. Comparing the six biologics to each other revealed no significant differences between biologics for patients achieving ACR50 with two exceptions: anakinra was less efficacious than etanercept, with a ratio of OR of 0.34 (0.14 to 0.81) and adalimumab was more efficacious than anakinra, ratio of OR = 2.20 (95%CI: 1.01 to 4.75) (Figure 4).
4.
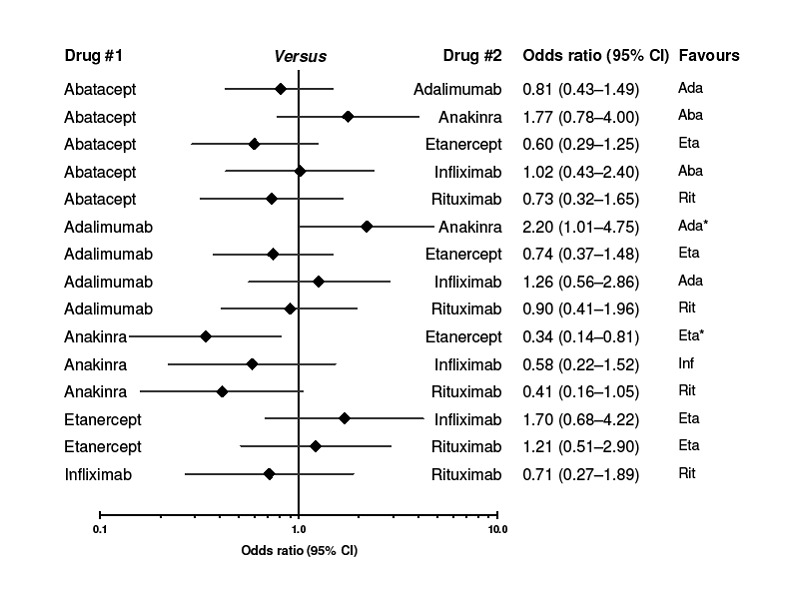
Indirect comparison of each biologic to each other for benefit (ACR50). CI = confidence interval. I2 values for the studies are presented in Figure 7.
Primary/major safety outcome‐ withdrawals due to adverse events
Of the 31 studies included in the Cochrane systematic reviews, 29 studies reported withdrawals due to adverse events. Of these, 21 studies had concomitant DMARD therapy (most often methotrexate) and 8 studies had no concomitant DMARD therapy.
Compared to placebo, patients receiving adalimumab, anakinra and infliximab were at significantly higher risk of withdrawals due to adverse events with ORs ranging from1.54 to 2.21 (Figure 5).
5.
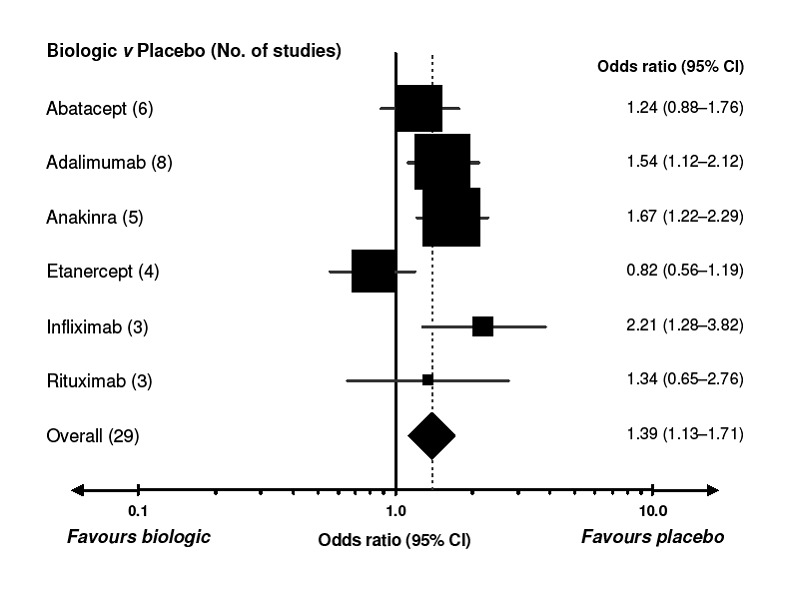
Comparison of each biologic to placebo for safety (determined by number of withdrawals because of adverse events). A value less than 1.0 indicates a benefit from the biologic. CI = confidence interval. For details of the studies included for each biologic, refer to Appendix. I2 values for the studies are presented in Figure 9. Every square represents the individual study’s effect measure with 95% CI indicated by horizontal lines. Square sizes are proportional to the precision of the estimate.
Patients receiving abatacept, etanercept and rituximab did not differ significantly from placebo with regard to safety ‐ with ORs ranging from 0.82 to 1.34 (Figure 5).
The indirect comparisons for withdrawals due to adverse events showed three significant differences between drugs, favoring etanercept (Figure 6). Adalimumab was more likely to lead to withdrawals compared to etanercept with a ratio of OR (95% CI) of 1.89 (1.18 to 3.04); anakinra more likely than etanercept, 2.05 (1.27 to 3.29); and etanercept less likely than infliximab, 0.37 (0.19 to 0.70).
6.
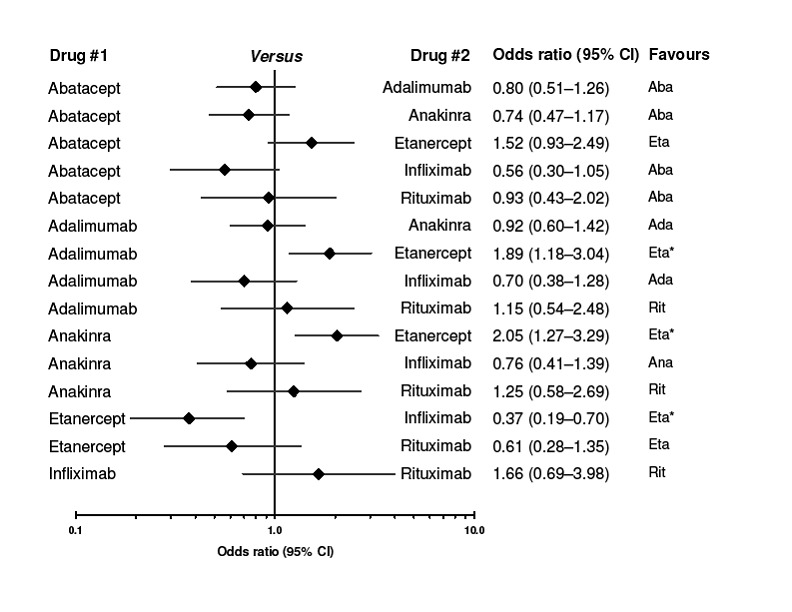
Indirect comparison of biologics to each other for safety (determined by number of withdrawals because of adverse events). CI = confidence interval. I2 values for the studies are presented in Figure 9.
Number needed to treat to benefit (NNTB) and number needed to treat to harm (NNTH)
As the NNTB and NNTH are considered helpful for clinicians, translating the results into an absolute value, we used the relative measures (Figure 2 and Figure 5) to assess these. The analyses were based on the empirical control event rate (CER) across all drugs, all studies. Thus, the expected CER for patients responding (ACR50) to placebo therapy was set to 20.7%, whereas the CER for patients withdrawing from therapy was expected to be 5.4% (Table 2).
Based on these NNTB values were as follows: 5 (95% confidence interval [CI] 3 to 10) for abatacept, 4 (95% CI 3 to 6) patients for adalimumab, 3 (95% CI 3 to 5) patients for etanercept, 5 (95% CI 3 to18) for infliximab and 4 (95% CI 3 to 8) patients for rituximab. For anakinra the number needed to treat for a benefit was not significant.
The NNTH (withdrawal due to adverse event) and 95% confidence interval (CI) compared to placebo were as follows: 39 (95% CI 19 to 162) for adalimumab; 31 (95% CI 17 to 92) for anakinra; and 18 (95% CI 8 to 72) for infliximab. The NNTH for abatacept, etanercept and rituximab were not significant.
Stratified analyses for ACR50
1. Concomitant methotrexate vs. no methotrexate
Twenty studies included concomitant methotrexate and seven studies did not. The use of biologic DMARDs was associated with a significantly higher likelihood of achieving ACR50 compared to the placebo group in both groups of patients, those receiving were concomitant methotrexate, OR = 3.16 (95% CI 2.40 to 4.16) and those not receiving concomitant methotrexate, OR = 4.18 (95% CI 2.48 to 7.06) (Table 5).
5. Stratified meta‐analyses for benefit and safety for biologics used in the treatment of rheumatoid arthritis.
| Benefit (ACR 50)* | |
Safety† | |||||||
| Group | No. of trials | OR (95% CI) |
Tau2 (study)‡ |
Tau2 (study x drug)‡ |
No. of trials | OR (95% CI) |
Tau2 (study)‡ |
Tau2 (study x drug)‡ |
|
| Concomitant use of methotrexate | 0.40 | 0.14 | 0.33 | 0.04 | |||||
| Yes | 20 | 3.16 (2.40 ‐ 4.16) | 21 | 1.30 (1.02 ‐ 1.65) | |||||
| No | 7 | 4.18 (2.48 ‐ 7.06) | 8 | 1.70 (1.12 ‐ 2.57) | |||||
| Rheumatoid arthritis duration | 0.23 | 0.12 | 0.34 | 0.04 | |||||
| Early | 5 | 2.05 (1.24 ‐ 3.38) | 5 | 1.45 (0.92 ‐ 2.28) | |||||
| Established | 8 | 3.47 (2.26 ‐ 5.33) | 9 | 1.25 (0.87 ‐ 1.78) | |||||
| Late | 14 | 4.02 (2.89 ‐ 5.59) | 15 | 1.52 (1.09 ‐ 2.11) | |||||
| Biologic is TNF‐inhibitor | 0.45 | 0.14 | 0.27 | 0.05 | |||||
| Yes | 15 | 3.57 (2.57 ‐ 4.97) | 15 | 1.27 (0.94 ‐ 1.69) | |||||
| No | 12 | 3.10 (2.12 ‐ 4.53) | 14 | 1.55 (1.14 ‐ 2.11) | |||||
| Prior drugs failed | 0.33 | 0.15 | 0.32 | 0.04 | |||||
| Biologic | 5 | 4.09 (2.17 ‐ 7.69) | 5 | 1.74 (1.02 ‐ 2.96) | |||||
| DMARD | 20 | 3.27 (2.46 ‐ 4.35) | 22 | 1.41 (1.11 ‐ 1.79) | |||||
| None | 2 | 3.00 (1.11 ‐ 8.13) | 2 | 0.85 (0.41 ‐ 1.76) | |||||
| Combination biologic therapy | 0.57 | 0.09 | N.E | N.E | |||||
| Yes | 2 | 1.00 (0.45 ‐ 2.23) | 2 | N.E | |||||
| No | 25 | 3.60 (2.89 ‐ 4.49) | 27 | N.E | |||||
| Duration of randomized trial | 0.29 | 0.13 | 0.28 | 0.04 | |||||
| Short | 17 | 4.03 (2.93 ‐ 5.54) | 18 | 1.46 (1.07 ‐ 1.99) | |||||
| Intermediate | 8 | 2.92 (1.91 ‐ 4.46) | 9 | 1.31 (0.94 ‐ 1.82) | |||||
| Long | 2 | 1.73 (0.78 ‐ 3.82) | 2 | 1.47 (0.71 ‐ 3.03) | |||||
| Prior failure of TNF biologic | 0.45 | 0.14 | 0.29 | 0.05 | |||||
| Yes | 5 | 4.11 (2.21 ‐ 7.63) | 5 | 1.76 (1.01 ‐ 3.06) | |||||
| No | 22 | 3.24 (2.48 ‐ 4.22) | 24 | 1.34 (1.06 ‐ 1.69) | |||||
DMARD = disease‐modifying antirheumatic drug, NE = not estimable, OR = odds ratio, RA = rheumatoid arthritis, TNF = tumor necrosis factor.
*Defined as 50% improvement in American College of Rheumatology symptomatic criteria (ACR50).
†As measured by number of withdrawals related to adverse events.
‡Tau2 (τ2) is the measure of heterogeneity between various drugs. Tau‐squared is presented as that which is due to study and due to
study × drug interaction. The overall τ2 is the sum of the τ2 due to study and that due to study×drug interaction. For example, the overall τ2 for ACR50 for the use of methotrexate background therapy is 0.40 + 0.14 = 0.54.
2. RA disease duration ‐ categorized as early RA vs. established RA vs. late RA
Five studies assessed early RA, eight assessed established RA and 14 assessed late RA.
Table 5 shows these results. Overall, in patients with early RA, ACR50 for biologic DMARD did significantly differ from placebo; OR = 2.05 (95% CI 1.24 to 3.38). For established RA and late RA, use of biologics was associated with a significantly higher chance of achieving ACR50 compared to placebo, with OR 3.47 and 4.02, respectively (Table 5).
3. Anti‐TNF biologic DMARDs vs. other biologic DMARDs
Fifteen studies included anti‐TNF biologic DMARDs (adalimumab, etanercept and infliximab) and 12 studies included other biologic DMARDs (abatacept, anakinra, rituximab). Both anti‐TNF DMARDs (OR 3.57) and other biologic DMARDs (OR 3.10) were associated with a significantly greater likelihood of achieving ACR50 than placebo (Table 5).
4. Use in patients who had failed traditional DMARDs vs. biologic DMARDs (or both) vs. none
Twenty studies included patients who had had traditional DMARD failure (most commonly methotrexate failure), five studies included patients who were biologic DMARD failures and two studies included treatment‐naive patients. Patients who had failed biologic DMARDs were more likely to achieve ACR50 when they were treated with biologic DMARDs compared to placebo (OR 4.09; 95% CI 2.17 to 7.69). Those who had failed traditional DMARDs and DMARD‐naive patients were more likely to achieve ACR50 when they were treated with biologic DMARDs compared to placebo (OR 3.27 and 3.00 respectively) (Table 5).
5. Single biologic DMARD agent vs. combination biologic therapy
Twenty‐five studies included use of a single biologic DMARD and two studies used combination biologic DMARDs. Use of single biologic DMARD was significantly superior to placebo in achieving ACR50 (Table 5). Use of combination biologic was not associated with significantly different results with regard to ACR50 when compared to placebo.
6. Treatment duration with biologic DMARD: short (6 months or less), intermediate duration (> 6 to 12 months) or long‐duration (> 1 year)
Seventeen studies had a short duration, 8 had an intermediate‐duration and 2 had long duration. Biologic DMARDs were significantly superior to placebo in achieving ACR50 in both short‐term and intermediate‐term RCTs, but not in long‐term studies (Table 5).
7. Prior failure of TNF‐biologic
Twenty‐two studies had patients without prior failure of TNF biologics and 5 with prior TNF biologic‐failure. Biologic DMARDs were superior to placebo in achieving ACR50 in both groups (Table 5).
Stratified analyses for Withdrawals due to Adverse Events
1. Concomitant methotrexate vs. no methotrexate
Twenty one studies included concomitant methotrexate and eight studies did not. Biologic DMARDs were significantly more likely to lead to withdrawals due to adverse events compared to placebo in both groups, 1.30 (95% CI 1.02 to 1.65) in those with concomitant methotrexate and 1.70 (95% CI 1.12 to 2.57) in those not receiving concomitant methotrexate (Table 5).
2. RA disease duration ‐ categorized as early RA vs. established RA vs. late RA
Five studies assessed early RA, nine assessed established RA and 15 assessed late RA. Table 3 shows these results. Biologic DMARDs were associated with a significantly higher risk of withdrawals due to adverse events compared to placebo in patients with late RA, OR 1.52 (1.09 to 2.11), but not in patients with early or established RA (Table 5).
3. Anti‐TNF biologic DMARDs vs. other biologic DMARDs
Fifteen studies included anti‐TNF biologic DMARDs (adalimumab, etanercept and infliximab) and 14 studies included other biologic DMARDs (abatacept, anakinra, rituximab). Non anti‐TNF biologics were more likely to lead to withdrawals due to adverse events compared to placebo, OR, 1.55 (1.14 to 2.11). Anti‐TNF biologics were not associated with more withdrawals due to adverse events compared to placebo, OR 1.27 (0.94 to 1.69) (Table 5).
4. Use in patients who had failed traditional DMARDs vs. biologic DMARDs (or both) vs. none
Twenty‐two studies included patients who have had traditional DMARD failure (most commonly methotrexate failure), five studies included patients who were biologic DMARD failures and two studies included treatment‐naive patients. Biologic DMARDs were significantly more likely to lead to withdrawals due to adverse events compared to placebo in both traditional DMARD‐ and biologic‐failure, OR, 1.41 (1.11 to 1.79) and 1.74 (1.02 to 2.96). Biologic use in DMARD‐naive patients was not associated with any higher risk of withdrawals due to adverse events compared to placebo, OR, 0.85 (0.41 to 1.76) (Table 5).
5. Single biologic DMARD agent vs. combination biologic therapy
Twenty‐seven studies included use of a single biologic DMARD and two studies used combination biologic DMARDs. Estimates could not be obtained for withdrawals since the model failed to converge (Table 5).
6. Treatment duration with biologic DMARD: short (6 months or less), intermediate duration (> 6 to 12 months) or long‐duration (> 1 year)
Eighteen studies had a short duration, 9 had an intermediate‐duration and 2 had long duration. Biologic DMARDs led to more withdrawals due to adverse events compared to placebo in short‐term trials, but not in intermediate‐ or long‐term trials (Table 5).
7. Prior failure of TNF‐biologic
Twenty‐four studies had patients without prior failure of TNF biologics and 5 with prior TNF biologic‐failure. Biologic DMARDs were associated with more withdrawals due to adverse events compared to placebo in both groups (Table 5).
Other outcomes
ACR70, ACR20 and overall withdrawals rates were available for all biologic DMARDs and are presented by each drug in Table 2. For both ACR70 and ACR20, all biologics except anakinra were associated with significantly better rates than placebo. Similarly, all withdrawals were significantly lower than placebo for abatacept, adalimumab, etanercept, infliximab and rituximab (Table 2). Anakinra and infliximab were similar to placebo with regards to total withdrawal rates.
The details of ACR70 by each drug are provided in the summary of findings table (Table 2).Compared to placebo, patients receiving each biologic except anakinra were significantly more likely than placebo to achieve ACR70. The OR of ACR70 with each biologic DMARDs were as follows: abatacept, 4.00 (95% CI 2.21 to 7.21); adalimumab, 3.98 (95% CI 2.48 to 6.40); anakinra, 1.63 (95% CI 0.72 to 3.65); etanercept, 4.05 (95% CI 2.07 to 7.93); infliximab, 3.23 (95% CI 1.42 to 7.37); and rituximab, 5.30 (95% CI 2.35 to 11.92).
The risk of overall withdrawals from biologic DMARDs was significantly lower compared to control/placebo at 0.57 OR (95% CI 0.45 to 0.71). Patients were significantly less likely to withdraw from therapy with abatacept, adalimumab, etanercept or rituximab as compared to control/placebo. For anakinra or infliximab, the total withdrawals were not significantly lower than control/placebo (Figure 7).
7.
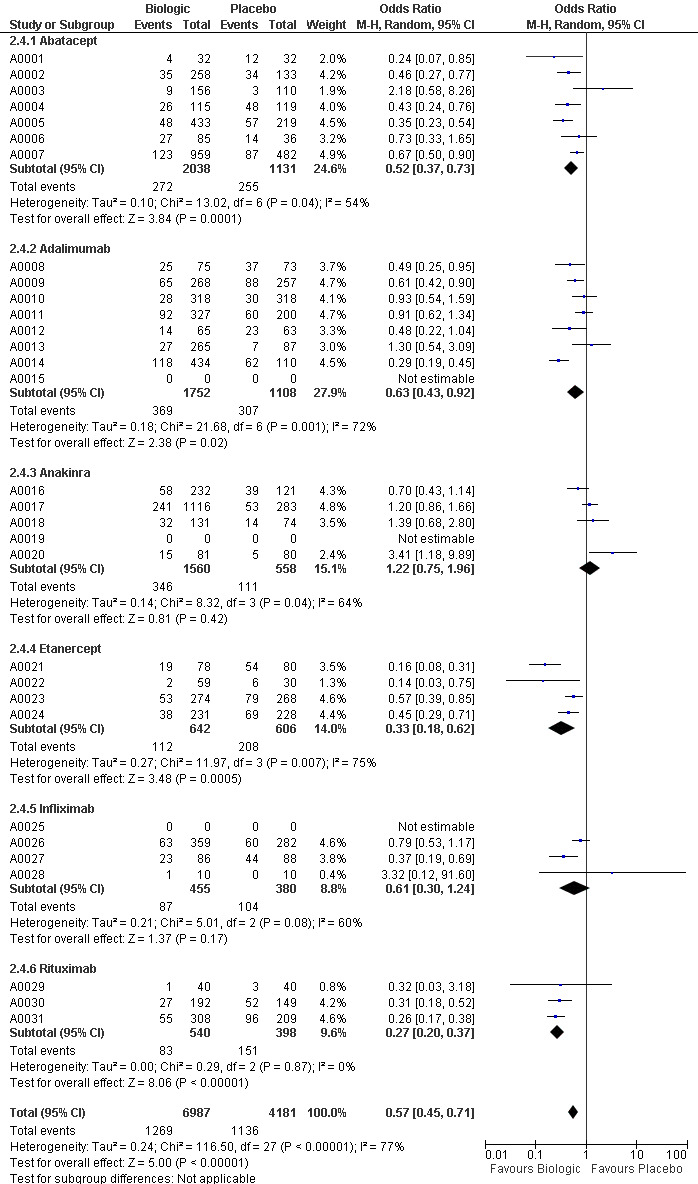
Forest plots for All withdrawals (grouped by drug)
HAQ scores as continuous outcomes were available for all reviews except abatacept for which categorical outcome of HAQ decrease by minimal clinically important change was provided. Radiographic progression/scores were reported by even fewer studies. Similarly, other outcomes as pre‐specified in our protocol were not available from most reviews to perform indirect comparisons. However, these are important outcomes for both patients and physicians, and are therefore provided in the Summary of Findings tables (Table 3; Table 4).
The forest plots for ACR20, ACR50, ACR70, withdrawals due to adverse events and overall withdrawals are provided in Figure 8, Figure 3, Figure 9, Figure 10 and Figure 7 respectively.
8.
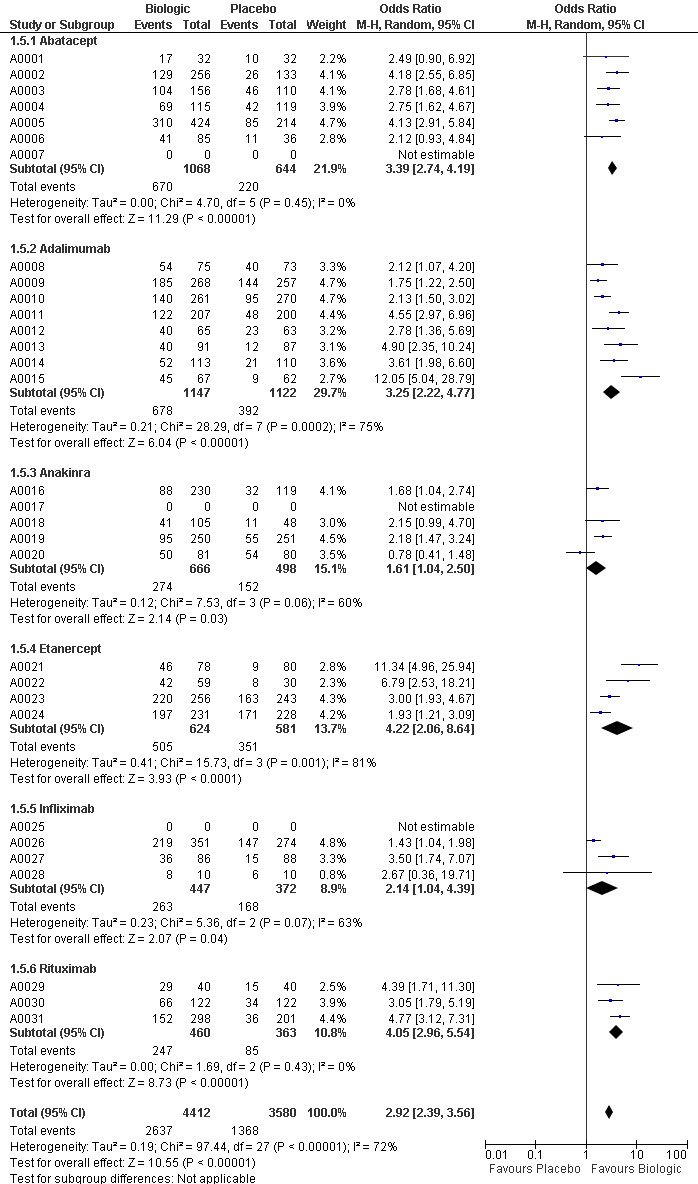
Forest plots for ACR20 (grouped by drug)
9.
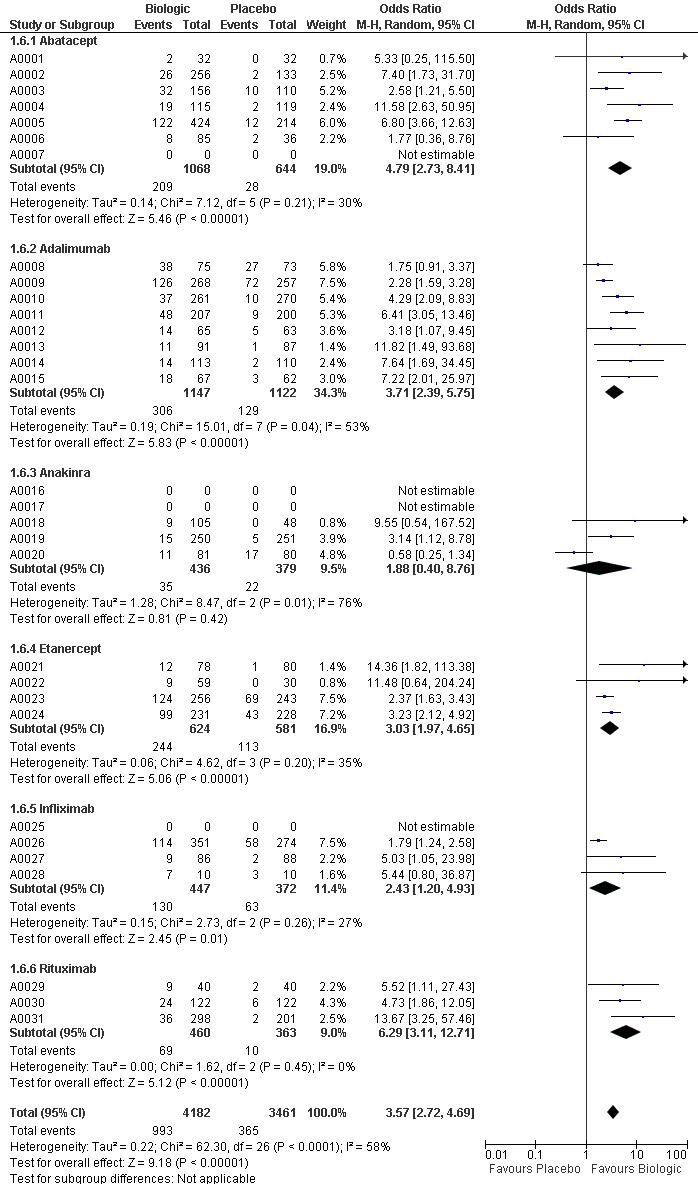
Forest plots for ACR70 (grouped by drug)
10.
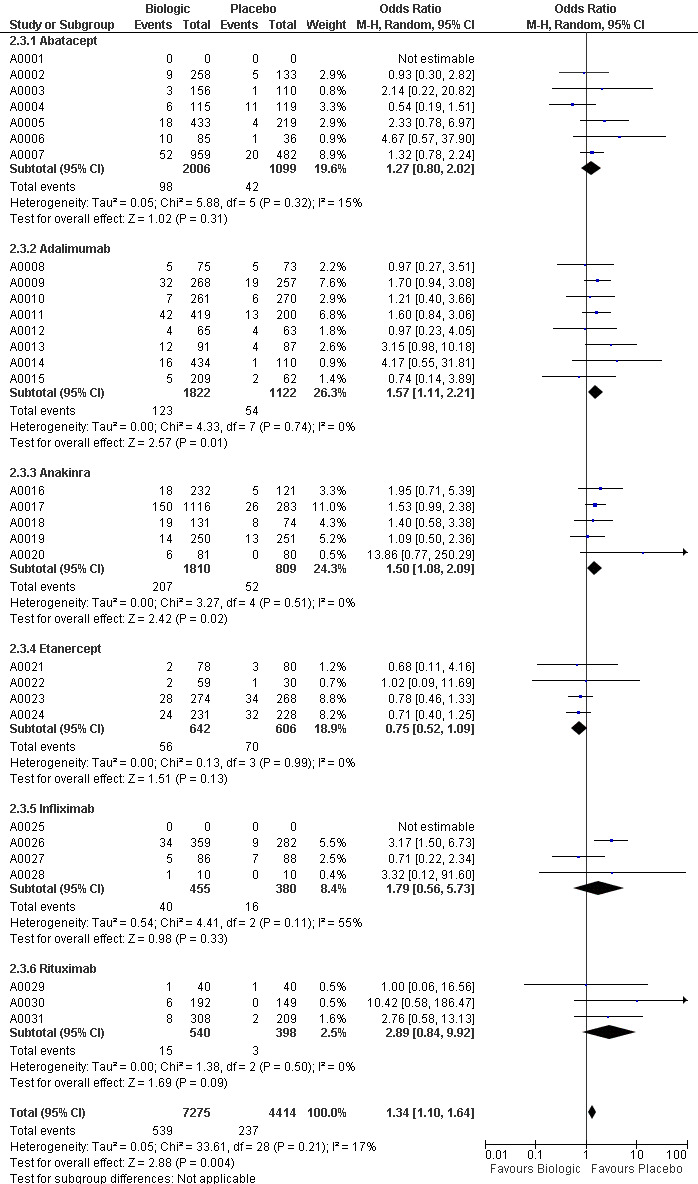
Forest plots for Withdrawals due to adverse events (grouped by drug)
Summary of safety warnings from regulatory agencies
Evidence from RCTs is limited in informing patients and physicians regarding uncommon or rare adverse events. In the section below, we summarize with warnings from the FDA, EMEA and Health Canada, the regulatory agencies in the USA, Europe and Canada, respectively.
Abatacept
No recent warnings have been issued with regard to abatacept. On the product label of abatacept, the FDA warns against known safety implications reporting, "In controlled clinical trials, patients receiving concomitant abatacept and TNF antagonist therapy experienced more infections (63%) and serious infections (4.4%) compared to patients treated with only TNF antagonists (43% and 0.8%, respectively). Concurrent administration of a TNF antagonist with abatacept has been associated with an increased risk of serious infections and no significant additional efficacy over use of the TNF antagonists alone" (FDA 2007). Furthermore, the FDA reports that, "rare occurrences of anaphylaxis or anaphylactoid reactions have been observed in two of 2,688 patients treated with abatacept in clinical trials" (FDA 2007). Trials have also shown that, "COPD patients treated with abatacept developed adverse events more frequently than those treated with placebo, including COPD exacerbations, cough, rhonchi, and dyspnea" (FDA 2007).
The effects of abatacept on pregnant women, pediatric patients, and the development of malignancies is "not yet fully understood" (FDA 2007). The European Medicines Agency (EMEA) reports the adverse reactions in patients treated with abatacept, ranking the occurrences of such reactions as very common (≤ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); and very rare (< 1/10,000). EMEA 2009a reports increase in blood pressure, abnormal liver function test (transaminases increased) and headaches are very common adverse reactions. Dizziness, cough, rash including dermatitis, diarrhea, nausea, dyspepsia, abdominal pain, lower respiratory tract infection (including bronchitis), urinary tract infection, herpes simplex, upper respiratory tract infection, hypertension, flushing, fatigue and asthenia are common (EMEA 2009a). Overall, "the most commonly reported adverse events (occurring in 10% or more of patients) were headaches, upper respiratory tract infection, nasopharyngitis, and nausea. The adverse events most commonly resulting in clinical intervention were due to infection" (FDA 2007).
Adalimumab
The updated 2008 FDA label for adalimumab reports "Serious infections, sepsis, tuberculosis and cases of opportunistic infections, including fatalities, have been reported with the use of TNF blocking agents including Humira® [adalimumab]" (FDA 2008). "Other serious infections seen in clinical trials include pneumonia, pyelonephritis, septic arthritis and septicaemia. Hospitalization or fatal outcomes associated with infections have been reported" (EMEA 2009b). Furthermore, hepatitis B reactivation has been shown to be associated with adalimumab treatment (Health Canada 2006). The FDA reports, "As observed with other TNF blocking agents, tuberculosis associated with the administration of Humira® in clinical trials has been reported" (FDA 2008).
In rare instances, adalimumab has been associated with, "new onset or exacerbation of clinical symptoms and/or radiographic evidence of demyelinating disease including multiple sclerosis" (EMEA 2009b). Furthermore, "In the controlled portions of clinical trials of some TNF‐blocking agents, including Humira, more cases of malignancies have been observed among patients receiving those TNF blockers compared to control patients" (FDA 2008).
"Some of these hepatosplenic T‐cell lymphomas have occurred in young adult patients on concomitant treatment with azathioprine or 6‐mercaptopurine used for Crohn’s disease". Thus, the risk of the development of hepatosplenic T‐cell lymphoma cannot be excluded for patients treated with adalimumab (EMEA 2009b). Though the causal relationship of hematological reactions and the use of adalimumab remain unclear as of 2008, the FDA label states, "Rare reports of pancytopenia including aplastic anemia have been reported with TNF blocking agents". Furthermore, the FDA reports "Treatment with Humira® [adalimumab] may result in the formation of autoantibodies and, rarely, in the development of a lupus‐like syndrome" (FDA 2008).
Anakinra
Anakinra leads to an increased rate of infections (2%) versus placebo (less than 1%). Following the EMEA standard of classification of frequency of the occurrence of "undesirable effects" mentioned above, neutropenia and serious infection requiring hospitalization were common (between 1/10 and 1/100) and headaches and injection site reactions were very common occurring in 1/10 or more patients treated with anakinra (EMEA 2004). "A… clinical trial sponsored by Amgen Inc. showed a higher incidence of serious infection and of neutropenia in anakinra and etanercept combination group than patients receiving Enbrel [etanercept] alone and higher than observed in previous trials where Kineret [anakinra] was used alone (EMEA 2003), therefore, the use of etanercept and anakinra is not recommended as it leads to safety complications)". Furthermore, the FDA reports in its most recent report on anakinra that "Hypersensitivity reactions associated with Kineret [anakinra] administration are rare" (FDA 2001). Moreover, the FDA reports the effects of anakinra on the hematologic conditions of patients stating that, "In placebo‐controlled studies with Kineret® [anakinra], treatment was associated with small reductions in the mean values for total white blood count, platelets, and absolute neutrophil count (ANC), and a small increase in the mean eosinophil differential percentage" (FDA 2001). With regard to the development of malignancies for patients treated with anakinra, trials show that, "among 5300 RA patients treated with Kineret [anakinra] clinical trials for a mean of 15 months (approximately 6400 patient years of treatment), lymphomas were observed for a rate of 0.12 cases per 100 patient years. This is 3.6 fold higher than the rate of lymphomas expected in the general population, based on the National Cancer Institutes Surveillance Epidemiology and End Results (SEER) database" (FDA 2001).
Etanercept
In the post‐marketing reports of etanercept, "Infections, including serious infections leading to hospitalization or death, have been observed in patients treated with Enbrel® [etanercept]" (FDA 2008b). Furthermore, "Data from clinical trials and preclinical studies suggest that the risk of reactivation of latent tuberculosis infection is lower with Enbrel® than with TNF‐blocking monoclonal antibodies. Nonetheless, post‐marketing cases of tuberculosis reactivation have been reported for TNF blockers, including Enbrel® [etanercept]. Patients receiving Enbrel® should be monitored closely for signs and symptoms of active tuberculosis. The possibility of tuberculosis should be considered, especially in patients who have travelled to countries with a high prevalence of tuberculosis or had close contact with a person with active tuberculosis. All patients treated with Enbrel® should have a thorough history taken prior to initiating therapy" (FDA 2008b). This finding is also stated in an important health warning issued by Health Canada in 2006 (Health Canada 2006).
Furthemore, etanercept has been associated with the risk of histoplasmosis and other invasive fungal infections. Health Canada 2009 states, "...although no histoplasmosis infections were reported among 17,696 patients from the United States and Canada who were treated with Enbrel®, in 38 clinical trials and four cohort studies involving all authorized indications, post marketing cases of serious and sometimes fatal fungal infections, including histoplasmosis, have been reported with TNF blockers, including Enbrel®." The FDA also outlines the risk of nervous system complications stating, "nervous system complications such as multiple sclerosis, seizures, or inflammation of the nerves of the eyes have occurred in rare cases" (FDA 2008b).
Infections, including serious infections leading to hospitalization or death, have been observed in patients treated with Enbrel® [etanercept] (FDA 2008b). The FDA reports on the risk of malignancies for patients on etanercept treatment, stating "Patients have been observed in clinical trials with Enbrel® for over five years. Among 4462 rheumatoid arthritis patients treated with Enbrel® in clinical trials for a mean of 27 months (approximately 10000 patient‐years of therapy), lymphomas were observed for a rate of 0.09 cases per 100 patient‐years. This is 3‐fold higher than the rate of lymphomas expected in the general population based on the Surveillance, Epidemiology, and End Results Database. Rare reports of pancytopenia including aplastic anemia, some with a fatal outcome, have been reported in patients treated with Enbrel®" (FDA 2008b). The FDA also reports, "Treatment with Enbrel® may result in the formation of autoantibodies and, rarely, in the development of a lupus‐like syndrome or autoimmune hepatitis which may resolve following withdrawal of Enbrel®" (FDA 2008b).
The use of etanercept has also been associated with the relapse of hepatitis B (Health Canada 2006).
Infliximab
In its recent revised report on infliximab, the EMEA reports on the risk of infusion reactions and hypersensitivity, stating, "An infusion‐related reaction was defined in clinical studies as any adverse event occurring during an infusion or within 1 to 2 hours after an infusion. In clinical studies, approximately 20% of infliximab‐treated patients compared with approximately 10% of placebo‐treated patients experienced an infusion‐related effect. Approximately 3% of patients discontinued treatment due to infusions reactions" (EMEA 2009a). Infliximab is also associated with the relapse of hepatitis B as reported by Health Canada in 2006 (Health Canada 2006). "Opportunistic infections have been reported in patients treated with infliximab, suggesting that host defence against infection is compromised. It should be noted that suppression of TNF‐alpha may also mask symptoms of infection such as fever." There is also a possible association between infliximab and heptosplenix T‐Cell lymphoma in pediatric and young adult patients with Crohn’s disease (Health Canada 2006b).
"In a study designed to evaluate Remicade® [infliximab] in congestive heart failure (CHF), 150 patients with moderate to severe (NYHA class II‐IV) CHF were treated with three infusions of Remicade 5mg/ kg, or placebo over six weeks. Higher incidences of mortality and hospitalization for worsening heart failure were seen in those patients treated with Remicade®, especially have treated with the higher dose of 10mg/kg. At present 7 out of 101 patients treated with Remicade® have died compared to no deaths among 49 patients on placebo" (EMEA 2001). In a May 2009 revision of the Remicade label, the FDA warns, "Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving TNF‐ blocking agents. Among opportunistic infections, tuberculosis, histoplasmosis, aspergillosis, candidiasis, coccidioidomycosis, listeriosis, and pneumocystosis were the most commonly reported. Patients have frequently presented with disseminated rather than localized disease, and are often taking concomitant immunosuppressants such as methotrexate or corticosteroids with Remicade®" (FDA 2009). In an investigation of neurological events, EMEA reports "Infliximab and other agents that inhibit TNF‐alpha have been associated in rare cases with optic neuritis, seizure and new onset or exacerbation of clinical symptoms and/or radiographic evidence of central nervous system demyelinating disorders, including multiple sclerosis, and peripheral demyelinating disorders, including Guillain‐Barré syndrome" (EMEA 2009a).
The increased risk of developing lymphoma is also reported. "In the controlled portions of clinical trials of all the TNF‐blocking agents, more cases of lymphoma have been observed among patients receiving a TNF blocker compared with control patients… In the combined clinical trial population for rheumatoid arthritis, Crohn's disease, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and plaque psoriasis, lymphomas were observed for a rate of 0.10 cases per 100 patient‐years of follow‐up, which is approximately 4‐fold higher than expected in the general population. Patients with Crohn's disease, rheumatoid arthritis or plaque psoriasis, particularly patients with highly active disease and/or chronic exposure to immunosuppressant therapies, may be at a higher risk (up to several fold) than the general population for the development of lymphoma, even in the absence of TNF‐blocking therapy" (EMEA 2009a).
TNF‐blockers as a group
In 2008, the FDA issued a safety alert regarding anti‐TNF biologic DMARDs, which stated that the risk of pulmonary and disseminated histoplasmosis, coccidioidomycosis, blastomycosis and other opportunistic infections were not consistently recognized in patients taking tumor necrosis factor‐alpha blockers (TNF blockers including etanercept, adalimumab, infliximab or certolizumab), which resulted in the delay of proper antifungal treatment and at times led to death (FDA 2008a). The FDA reviewed 240 reports of histoplasmosis, an infection caused by the fungus Histoplasma capsulatum, in patients being treated with Enbrel, Humira, or Remicade. The majority of the reports involved people in the Ohio River and Mississippi River valleys (the fungus is commonly found in those areas). In at least 21 of the reports, histoplasmosis was initially not recognized by healthcare professionals, and antifungal treatment was delayed. Twelve of those patients died. The FDA recommended that for patients at risk of histoplasmosis and other invasive fungal infections, clinicians should consider empiric antifungal treatment until the pathogen(s) are identified.
Rituximab
While no reviews exist for rituximab in the EMEA website and Health Canada’s reviews are outdated, the FDA provides its most recent label for Rituxan® [rituximab] from 2008. In this label, the possible safety complications of Rituxan® use included "tumor lysis syndrome which necessitates clinicians to administer prophylaxis and monitor patients renal function, hepatitis B reactivation with fulminant hepatitis, which can sometimes [be] fatal and the risk of progressive multifocal leukoencephalopathy" (Drugs 2006).
FDA and Genentech informed healthcare professionals of important emerging safety information about Rituxan®. "Two patients died after being treated with Rituxan® for systemic lupus erythematosus (SLE). Rituxan® is approved for the above indication and is prescribed off‐label for other serious diseases and conditions such as SLE. The cause of death was progressive multifocal leukoencephalopathy, a viral infection of the brain (that is caused by reactivated JC virus which is present in about 80% of adults" (FDA 2006b). Further risks include "cardiac arrhythmias and angina" which can be life threatening, and "bowel obstruction and perforation" (FDA 2008b).
Health Canada 2006 also provide warnings of bowel obstruction and perforation, "Reports of abdominal pain, bowel obstruction, and perforation, in some cases leading to death, have been observed in patients receiving Rituxan®. The majority of reports, including all deaths, have occurred in patients receiving Rituxan in combination with chemotherapy for NHL [(non‐Hodgkin’s Lymphoma)] indication. A causal relationship has not been established".
Discussion
Summary of main results
This is the first overview of Cochrane systematic reviews of biologic DMARDs for RA. We included updated reviews of six biologic DMARDs, including abatacept, adalimumab, anakinra, etanercept, infliximab and rituximab in recommended approved doses only.
Five of the six biologics were statistically significantly better than placebo in achieving ACR50, the main efficacy variable, as opposed to anakinra which was no different than placebo. The likelihood of achieving ACR50 varied with different biologic DMARDs. On the nominal level, all biologics had similar efficacy for ACR50 in indirect comparisons; it was evident that anakinra was half as efficacious as adalimumab, etanercept, and rituximab. This is an important observation, in the absence of direct comparisons of these biologic DMARDs in RCTs. While we noted that different types of patient populations were treated with different biologic DMARDs, with some biologics being used more in patients with longer disease duration and more DMARD failures than others, we are also aware of the limitations of such analyses, even when they were pre‐specified. Most RCTs reported mean duration of RA (used for defining early, established and late RA), which may lead to ecological fallacy. Additionally, while these definitions of RA duration may not be universally accepted, these were perhaps the only clinically acceptable definitions available to us from the published literature available in existing Cochrane reviews and other published literature.
The main safety outcome was withdrawals due to adverse events. Withdrawals due to adverse events were significantly higher in patients allocated to treatment than in those allocated to placebo for three biologic DMARDs, adalimumab, anakinra and infliximab. Abatacept, etanercept and rituximab did not differ significantly from placebo in withdrawals due to adverse events. Indirect comparisons for withdrawals due to adverse events showed that adalimumab, anakinra and infliximab each were significantly more likely to lead to withdrawals compared to etanercept. This seems to be driven primarily by low withdrawal rates in the etanercept group due to better tolerability rather than higher rates in the other biologics. In clinical practice, These comparisons may have implications for adherence to treatment with biologic DMARDs in clinical practice.
The results from the other two efficacy outcomes, ACR20 and ACR70 mirrored those for ACR50. This was very reassuring, considering the heterogeneity of studies. Similarly, results for all withdrawals were similar to withdrawals due to adverse events. Patients were significantly less likely to withdraw for any reason from four of the six biologic DMARDs, namely, abatacept, adalimumab, etanercept and rituximab, compared to placebo. Withdrawal from anakinra and infliximab were not different compared to placebo. This provides patients and physicians with an estimate of overall drug continuation. In clinical practice, use of these drugs is meant for much longer duration than the duration of randomized trials, most limited to 6 to12 months. Additionally, these medications may be used in patients who may have higher comorbidity than those enrolled in the RCTs. Therefore, these numbers are at best rough guides for short‐ to intermediate‐term continuation rates of use of these biologic DMARDs.
Our overview provides means for indirect comparisons of the biologics in the absence of head‐to‐head studies. Due to several limitations outlined in the section below, these findings must be interpreted with caution.
Stratified analyses showed lack of superiority of biologics as a group compared to placebo in subgroups of patients with DMARD‐naive disease (2 studies), in long trial duration of one or more years (2 studies) and use of multiple biologics for treatment (2 studies). Lack of significance in all three instances seems to be related to small sample size with type II error, rather than a lack of efficacy in these groups. These analyses also showed lack of efficacy of biologics compared to placebo in early RA (5 studies), whereas biologics were more effective than placebo in established and late RA. In most studies, placebo groups also received background methotrexate similar to the biologics groups, which is a very effective DMARD for many patients. It is possible that in early disease what matters the most is the initiation of DMARD, not necessarily the type of DMARD or whether it is a single versus combination DMARD therapy. It is also possible that a small sample size (5 studies) led to lack of significance.
Overall completeness and applicability of evidence
We had prespecified ACR50 and withdrawals due to adverse events as as the primary efficacy and safety outcome, which were reported by almost all of the studies included in each Cochrane systematic review. The review includes completed and recently updated Cochrane reviews of six biologics, therefore the evidence report is up to date and current. In the absence of direct comparisons, this study provides indirect comparisons of six biologics.
Quality of the evidence
Six Cochrane systematic reviews (Blumenauer 2003; Lethaby 2003; Lopez‐Olivo 2008; Maxwell 2008; Mertens 2008; Navarro‐Sarabia 2005) with 31 studies were included in this overview of reviews (Appendix 1).
The quality of evidence reflects the extent to which we are confident that an estimate of the effect is correct. It was assessed for each important outcome in this review. The Cochrane Collaboration recommends the use of GRADE in assessing quality of evidence (Higgins 2008). For most systematic reviews method of randomization and allocation concealment were not described, although some other deficits were noted in other studies.
The AMSTAR rating was good for all reviews with minor exceptions.
Outcome measures for this overview of reviews were selected following the recommendations of the Outcome Measures in Rheumatolgy (OMERACT) group. Data on several important outcomes such as HAQ and radiographic scores were not reported in all the included reviews. Some measurements varied across the reviews and meta‐analysis was not possible. For example, radiographic scores were not reported in the rituximab review. It was impossible to pool the results for physical function as HAQ scores were reported as a categorical outcome in the abatacept review and as a continuous outcome in the other reviews. To combine results, it is important that they are similar.
Complete risk of bias assessment was performed in two included reviews. Risk of bias assessment had to be performed on the other four reviews to complete the assessment of quality of evidence in this overview of reviews. Some study characteristics were not provided in the included reviews. These were obtained from the original studies. The methods used in some included studies were not clearly described and could be a source of potential bias.
Potential biases in the overview process
Our review has several limitations. Lack of reporting of many important outcomes from RCTs (HAQ, SF‐36 scores, radiographic scores etc.) limited our ability to analyze them and compare them between biologic DMARDs. Since our data extraction was limited to Cochrane reviews and not the original studies, it is possible that we may not have captured all minor outcomes. However, primary/major safety and efficacy outcomes were available for all included reviews and for most studies within the reviews.
To our knowledge there are no head‐to‐head comparisons of efficacy and safety of various biologic DMARDs in patients with RA. The Schiff study (Schiff 2008) had three arms (abatacept, infliximab and placebo) but was not powered enough to find changes between abatacept and infliximab. With the introduction of multiple DMARDs whose efficacies have yet to be compared to one another, it is unclear which biologic DMARD is more effective, safer, and best tailored to different subgroups of patients suffering from RA. In the absence of such direct comparisons, indirect comparisons such as these can provide useful information. Indirect comparisons have several limitations. RCTs differ in patient population characteristics, most prominently in mean RA disease duration, prior failed therapy, concomitant use of DMARDs and trial duration. Even though several RCTs have been done for most biologics, we were limited in that many biologics only had three to five studies. This makes many of our analyses prone to type II error, i.e., missing a significant result, when difference exists between biologic DMARDs. Therefore, these results must be interpreted with caution.
Additionally, some studies presented data on safety for all doses of the biologic, not just the recommended dose and in some cases presented data for the entire study duration, including the open‐label phase. This limited our ability to get the data for the approved dose only during the randomized phase. Several studies allowed continuation of DMARDs such as methotrexate in a proportion of the patients, but not all patients ‐ 58% of patients in an anakinra study (Fleischman 2003) and 78% in an abatacept study continued on methotrexate (Genovese 2005). These studies make the placebo group heterogeneous compared to other studies, where patients are usually randomized to placebo + methotrexate or placebo alone. Some Cochrane reviews did not present key information that was included in the randomized trials. This included exclusion of ACR20 in the rituximab review (Lopez‐Olivo 2008; full review in press) and withdrawals due to adverse events in an anakinra review (Mertens 2008). We requested and obtained this information from the authors of these reviews and included it in this systematic review. Several key study characteristics were impossible to extract from each Cochrane systematic review, it was therefore neccesary to abstract this information from individual studies.
One must be careful in interpreting the odds ratios that may look slightly different from each other numerically, but not statistically. It is important to consider the 95% confidence intervals while interpreting these numbers.
JS and GW abstracted all data independently for this review. Most review data had previously also been abstracted independently by two review authors. This we believe minimizes errors in data abstractions, and biases due to this error. The quality of RCTs was reasonably good, although some RCTs did not report certain quality characteristics.
Agreements and disagreements with other studies or reviews
Our results agree with several similar analyses in the past. However, since our review has several more studies than included in the previous reviews/overviews, some estimates differ, as expected. Additionally, our review includes six biologics used in standard approved doses, while most previous reviews have included anti‐TNF biologic DMARDs with few exceptions, and many used RCT data from non‐standardized doses.
In a meta‐analysis of RCTs of anti‐TNF biologic DMARDs for RA (Alonso‐Ruiz 2008), compared to control treatment, the relative risk of achieving ACR50 was 2.6 for adalimumab, 2.1 for infliximab and 2.6 for etanercept. The relative risk of withdrawals due to adverse events was 1.42 (1.01 to 1.99) for adalimumab, 0.70 (0.53 to 0.92) for etanercept and 2.04 (1.34 to 3.12) for infliximab compared to control treatment. The pattern of these relative risks are very similar to those reported in our study.
In a systematic review of four biologics for RA, TNF‐inhibitors were similar to each other in efficacy, but seemed to be better than anakinra (Gartlehner 2006). There were no differences between three anti‐TNF biologic DMARDs (adalimumab, etanercept and infliximab) and anakinra for achieving ACR50. Our study confirms this finding and extends it to comparisons of six biologics, including abatacept and rituximab.
Kristensen 2007 examined patients with established RA and based on ACR50 following one year of therapy, estimated the NNTB to be 8 (5 to 38) patients for infliximab, 4 (3 to 6) for etanercept and 4 (3 to 6) for adalimumab. Our NNT estimates for achieving ACR50 were very similar to this previous study: 5 for infliximab, 3 for etanercept and 4 for adalimumab. In comparison, we report the NNTB for rituximab was 4 and for abatacept, 5 patients. We have also provided NNTH for these biologic DMARDs.
Lee 2008 indirectly compared TNF‐inhibitors (etanercept, infliximab and adalimumab) to each other for RCTs of 50 weeks or longer in RA . Three studies, Lipsky 2000 (infliximab); Klareskog 2004 (etanercept); and Keystone 2004 (adalimumab), qualified and were included. The RR (95% CI, P value) of achieving ACR 50 were as follows: etanercept versus infliximab, 0.59 (0.27 to 1.29, P = 0.19); etanercept versus adalimumab, 0.37 (0.22 to 0.60, P < 0.0001); and infliximab versus adalimumab, 0.62 (0.25 to 1.49, P = 0.28). However, the authors failed to reduce the between study variation, and did not interpret their data in absolute terms. Withdrawals due to adverse events among the three TNF inhibitors were as follows: etanercept versus infliximab, 1.01 (0.30 to 3.42, P = 0.98); etanercept versus adalimumab, 0.38 (0.17 to 0.86, P = 0.02); and infliximab versus adalimumab, 0.37 (0.11 to 1.36, P = 0.14). Our estimates differ from this study such that ACR50 were not significantly different among the three TNF‐inhibitors. In our study, withdrawals due to adverse events were significantly lower in etanercept compared to adalimumab and infliximab. This is likely due to inclusion of more RCTs and RCTs of all length in our study (including those RCTs 50‐weeks or more from Lee 2008).
Nixon 2007 performed indirect comparisons of TNF‐inhibitors and anakinra for RA including RCTs with both standard approved doses as well as RCTs with non‐standardized doses (Nixon 2007). Similar to our study, they found that ACR50 was similar between the various TNF‐blockers, with odds ratios (95% CI) as follows: infliximab/etanercept, 0.98 (0.45 to 1.93); adalimumab/etanercept, 0.94 (0.51 to 1.62); and adalimumab/infliximab, 0.96 (0.48 to 1.90).
Donahue 2008 performed a qualitative synthesis (and where meta‐analyses provided quantitative analysis) of RCTs, observational studies and meta‐analyses of traditional and biologic DMARDs for RA (Donahue 2008). They reported that anakinra led to lower ACR50 compared to anti‐TNF drugs and similar ACR50 among the anti‐TNF drugs. These findings are in agreement with our quantitative approach.
Previous studies have analyzed the risk of malignancy and serious adverse events from RCTs of infliximab and adalimumab (Bongartz 2006) and etanercept (Bongartz 2009). Odds Ratio of malignancy was 3.3 (1.2 to 9.1) for all doses of infliximab and adalimumab and 2.0 (1.3 to 3.1) for serious infections, when compared to control treatment. The risk of malignancy was 1.87 (0.75 to 5.59) in a patient level meta‐analysis of etanercept versus control treatment (Bongartz 2009). Due to a pre‐specified requirement that outcomes to be compared across six biologics should have been presented in each review, these outcomes could not be compared between biologics in our study, since they were not presented for all six biologics.
Authors' conclusions
Implications for practice.
In the absence of direct comparisons of biologic DMARDs in patients with RA, practitioners are faced with a dilemma when choosing biologic DMARDs, for patients who have failed traditional DMARDs. This review provides summarization of data regarding these six biologics for which Cochrane reviews have been completed and updated. Anakinra was less efficacious than the other five biologics and etanercept led to lower withdrawal rates due to adverse events compared to adalimumab, anakinra and infliximab.
Implications for research.
We believe that RCTs of direct head‐to‐head comparisons of biologic agents in patients with RA are needed. These RCTs should examine the relative efficacy and safety of biologics for various stages of the disease (early, established and late RA), various levels of functional limitation (mild, moderate and severe limitation) and the nature of prior treatment (traditional DMARD‐naive, traditional DMARD‐failure, biologic‐failure, multiple biologic failure).
What's new
| Date | Event | Description |
|---|---|---|
| 15 January 2013 | Amended | Minor edits |
| 1 March 2010 | Amended | Odds ratios have been used in the network meta‐analyses. See 'Published notes' for details. |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 25 February 2010 | Amended | CMSG ID: C187‐R |
Notes
This review was co‐published in the Canadian Medical Association Journal (CMAJ 2009 DOI:10.1503/cmaj.091391) and because of separate review processes, both versions were substantively different ‐ risk ratios were used in the network meta‐analyses in the Cochrane version and odds ratios in the CMAJ version. Since the indirect comparisons approach used requires odds ratios, we have now harmonized the network meta‐analyses using odds ratios in the Cochrane version as well.
Acknowledgements
We thank the authors of the individual Cochrane systematic reviews of biologic DMARDs, who provided us with the review data and/or the updates. We thank Shahrzad Noorbaloochi for helping gather and summarize warnings from the websites of regulatory agencies.
Appendices
Appendix 1. List of studies included for analyses in this overview
| Study Name | Year | Study code | Reference | Trial Duration in months | RA disease duration in years | Intervention | Comparator |
| ABATACEPT | |||||||
| Moreland | 2002 | A0001 | Moreland LW, Alten R, Van den Bosch F, Appelboom T, Leon M, Emery P, et al. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose‐finding, double‐blind, placebo‐controlled clinical trial evaluating CTLA‐4Ig and LEA29Y eighty‐five days after the first infusion. Arthritis and Rheumatism. 2002 Jun;46(6):1470‐9. | 3 | 3.3 | ABA | PL |
| Genovese | 2005 | A0002 | Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. New England Journal of Medicine. 2005 Sep 15;353(11):1114‐23 | 6 | 11.9 | ABA + DMARD | DMARD + PL |
| Schiff | 2008 | A0003 | Schiff MH, Pritchard C, Huffstutter JE, Rodriguez‐Valverde V, Durez P, Zhou X, et al. The 6‐month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti‐TNF therapy or were directly switched to abatacept: the ARRIVE trial. Annals of the Rheumatic Diseases. 2008 Dec 15. | 6 | 8.1 | ABA + MTX | MTX + PL |
| Kremer | 2003 | A0004 | Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. New England Journal of Medicine. 2003 Nov 13;349(20):1907‐15. | 6 | 9.2 | ABA + MTX | MTX + PL |
| Kremer | 2006 | A0005 | Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud‐Mendoza C, et al. Effects of abatacept in patients with methotrexate‐resistant active rheumatoid arthritis: a randomized trial. Annals of Internal Medicine. 2006 Jun 20;144(12):865‐76. | 12 | 8.6 | ABA + MTX | MTX + PL |
| Weinblatt | 2007 | A0006 | Weinblatt M, Schiff M, Goldman A, Kremer J, Luggen M, Li T, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Annals of the Rheumatic Diseases. 2007 Feb;66(2):228‐34. | 12 | 12.9 | ABA + ETN | ETN + PL |
| Weinblatt (ASSURE) | 2006 | A0007 | Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease‐modifying antirheumatic drugs: A one‐year randomized, placebo‐controlled study. Arthritis Rheum. 2006 Sep;54(9):2807‐16.. | 12 | 9.7 | ABA + DMARD(biologic + non‐biologic) | DMARD(biologic + non‐biologic) + PL |
| ADALIMUMAB | |||||||
| Bejarano | 2008 | A0008 | Bejarano V, Quinn M, Conaghan PG, Reece R, Keenan AM, Walker D, et al. Effect of the early use of the anti‐tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthritis and Rheumatism. 2008 Oct 15;59(10):1467‐74. | 13 | 0.9 | ADA + MTX | MTX + PL |
| Breedveld | 2006 | A0009 | Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: A multicenter, randomized, double‐blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis and Rheumatism. 2006 Jan;54(1):26‐37. | 24 | 0.7 | ADA + MTX | MTX + PL |
| Furst | 2003 | A0010 | Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor‐alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). The Journal of Rheumatology. 2003 Dec;30(12):2563‐71. | 6 | 10.4 | ADA + DMARD | DMARD + PL |
| Keystone | 2004 | A0011 | Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti‐tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo‐controlled, 52‐week trial. Arthritis and Rheumatism. 2004 May;50(5):1400‐11. | 12 | 11.0 | ADA + MTX | MTX + PL |
| Kim | 2007 | A0012 | Kim H, Lee S, Song Y, Yoo D, Koh EM, Yoo B, et al. A randomized, double‐blind, placebo‐controlled, phase III study of the human anti‐tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexate. APLAR Journal of Rheumatology. 2007;10:9‐16. | 6 | 6.8 | ADA + MTX | MTX + PL |
| Miyasaka | 2008 | A0013 | Miyasaka N. Clinical investigation in highly disease‐affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Modern Rheumatology. 2008;18(3):252‐62. | 6 | 9.8 | ADA | PL |
| Van de Putte | 2004 | A0014 | van de Putte LB, Atkins C, Malaise M, Sany J, Russell AS, van Riel PL, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Annals of the Rheumatic Diseases. 2004 May;63(5):508‐16. | 6 | 11.1 | ADA | PL |
| Weinblatt | 2003 | A0015 | Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis and Rheumatism. 2003 Jan;48(1):35‐45. | 6 | 11.7 | ADA + MTX | MTX + PL |
| ANAKINRA | |||||||
| Bresnihan | 1998 | A0016 | Bresnihan B, Alvaro‐Gracia JM, Cobby M, Doherty M, Domljan Z, Emery P, et al. Treatment of rheumatoid arthritis with recombinant human interleukin‐1 receptor antagonist. Arthritis and Rheumatism. 1998 Dec;41(12):2196‐204. | 6 | 4.1 | ANA | PL |
| Fleischiman | 2003 | A0017 | Fleischmann RM, Schechtman J, Bennett R, Handel ML, Burmester GR, Tesser J, et al. Anakinra, a recombinant human interleukin‐1 receptor antagonist (r‐metHuIL‐1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo‐controlled trial. Arthritis and Rheumatism. 2003 Apr;48(4):927‐34. | 6 | 10.3 | ANA + DMARD | PL + DMARD |
| Cohen | 2002 | A0018 | Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin‐1 receptor antagonist, in combination with methotrexate: results of a twenty‐four‐week, multicenter, randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism. 2002 Mar;46(3):614‐24. | 6 | 7.8 | ANA + MTX | MTX + PL |
| Cohen | 2004 | A0019 | Cohen SB, Moreland LW, Cush JJ, Greenwald MW, Block S, Shergy WJ, et al. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Annals of the Rheumatic Diseases. 2004 Sep;63(9):1062‐8. | 6 | 10.5 | ANA + MTX | MTX + PL |
| Genovese | 2004 | A0020 | Genovese MC, Cohen S, Moreland L, Lium D, Robbins S, Newmark R, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis and Rheumatism. 2004 May;50(5):1412‐9. | 6 | 10.2 | ANA + ETN +MTX | ETN + MTX + PL |
| ETANERCEPT | |||||||
| Moreland | 1999 | A0021 | Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Annals of Internal Medicine. 1999 Mar 16;130(6):478‐86. | 6 | 11.5 | ETA | PL |
| Weinblatt | 1999 | A0022 | Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. New England Journal of Medicine. 1999 Jan 28;340(4):253‐9. | 6 | 13.0 | ETA + MTX | MTX + PL |
| Emery (COMET) | 2008 | A0023 | Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double‐blind randomised controlled trial. Lancet. 2004 Feb 28;363(9410):675‐81. | 12 | 0.9 | ETA + MTX | MTX + PL |
| Klareskog (TEMPO) | 2004 | A0024 | Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double‐blind, parallel treatment trial. Lancet. 2008 Aug 2;372(9636):375‐82. | 12 | 6.8 | ETA + MTX | MTX + PL |
| INFLIXIMAB | |||||||
| Maini | 1998 | A0025 | Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor alpha monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis and Rheumatism. 1998 Sep;41(9):1552‐63. | 6 | 9.9 | INF + MTX | MTX + PL |
| St. Clair (ASPIRE) | 2004 | A0026 | St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis and Rheumatism. 2004 Nov;50(11):3432‐43. | 12 | 0.8 | INF + MTX | MTX + PL |
| Lipsky (ATTRACT) | 2000 | A0027 | Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. New England Journal of Medicine. 2000 Nov 30;343(22):1594‐602. | 12 | 10.5 | INF + MTX | MTX + PL |
| Quinn | 2005 | A0028 | Quinn MA, Conaghan PG, O'Connor PJ, Karim Z, Greenstein A, Brown A, et al. Very early treatment with infliximab in addition to methotrexate in early, poor‐prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve‐month randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism. 2005 Jan;52(1):27‐35. | 12 | 0.7 | INF + MTX | MTX + PL |
| RITUXIMAB | |||||||
| Edwards | 2004 | A0029 | Edwards JC, Szczepanski L, Szechinski J, Filipowicz‐Sosnowska A, Emery P, Close DR, et al. Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. New England Journal of Medicine. 2004 Jun 17;350(25):2572‐81. | 6 | 11.5 | RIT + MTX | MTX + PL |
| Emery (DANCER) | 2006 | A0030 | Emery P, Fleischmann R, Filipowicz‐Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double‐blind, placebo‐controlled, dose‐ranging trial. Arthritis and Rheumatism. 2006 May;54(5):1390‐400. | 6 | 10.1 | RIT + MTX | MTX + PL |
| Cohen (REFLEX) | 2006 | A0031 | Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy: Results of a multicenter, randomized, double‐blind, placebo‐controlled, phase III trial evaluating primary efficacy and safety at twenty‐four weeks. Arthritis and Rheumatism. 2006 Sep;54(9):2793‐806. | 6 | 11.9 | RIT + MTX | MTX + PL |
Appendix 2. Characteristics of included Cochrane systematic reviews
| Review title | Date assessed as up to date |
RA Disease Duration (number of studies) |
Comparison interventions [number of studies] |
Outcomes for which data were reported | Review limitations |
| Abatacept for rheumatoid arthritis | 1 April 2009 | Established RA (4) Late RA (3) |
Placebo [1] MTX (+ Placebo) [3] DMARD (+ placebo) [2] Etanercept (+ placebo) [1] |
ACR20 ACR50 ACR70 DAS HAQ SF‐36 Radiographic scores AE Serious AE Infections Malignancies Withdrawals Death |
There was moderate quality of evidence for most outcomes because of limitations in study design (ITT analysis not performed, less than 80% risk of attrition bias). Few included studies and all data not available. |
| Adalimumab for rheumatoid arthritis | 9 February 2009 | Early RA (2) Established RA (3) Late RA (3) |
Placebo [2] MTX (+ placebo) [5] DMARD (+ placebo) [1] |
ACR20 ACR50 ACR70 Good EULAR response DAS HAQ Radiographic scores AE Serious AE Infections Malignancies Withdrawals |
Limitations in study design include method of randomization not clearly described. Few included studies and sparse data with many different doses used resulting in heterogeneity. |
| Anakinra for rheumatoid arthritis | 5 February 2008 | Established RA (3) Late RA (2) |
Placebo [3] MTX (+ Placebo) [2] Etanercept & MTX (+ Placebo) [1] |
ACR20 ACR50 ACR70 HAQ Radiographic scores AE Serious AE Infections Withdrawals Death |
Limitations in study design include method of randomization not clearly described and ITT analysis not performed. Few included studies and sparse data with different doses. One included study used a different comparison resulting in heterogeneity. |
| Etanercept for rheumatoid arthritis | 25 June 2008 | Established RA (2) Late RA (3) |
Placebo [1] MTX (+ placebo) [4] |
ACR20 ACR50 ACR70 DAS HAQ SF‐36 Radiographic scores Infections Malignancies Withdrawals Death |
Limitations in study design include method of randomization not described, method of concealment not reported, blinding not done. Few included studies and all data not available. |
| Infliximab for rheumatoid arthritis | 17 March 2009 | Early RA (1) Established RA (3) |
MTX (+ placebo) [4] | ACR20 ACR50 ACR70 DAS HAQ SF‐36 Radiographic scores Serious AE Infections Malignancies Withdrawals Death |
Limitations in study design not yet fully assessed. Very few included studies with different doses and not all data available. |
| Rituximab for rheumatoid arthritis | 17 March 2009 | Late RA (3) | MTX (+ placebo) [3] | ACR50 ACR70 Good EULAR response DAS HAQ SF‐36 Radiographic scores AE Serious AE Infections Withdrawals Death |
Limitations in study design include method of randomization not described, method of concealment not reported, blinding not described. Few included studies and all data not available. |
Appendix 3. AMSTAR ratings for each Cochrane systematic review
| AMSTAR criteria | Abatacept | Adalimumab | Anakinra | Etanercept | Infliximab | Rituximab |
| A priori design | yes | yes | yes | yes | yes | yes |
| Duplicate extraction | yes | yes | yes | yes | yes | yes |
| Literature search Comprehensive | yes | yes | yes | yes | yes | yes |
| Status of publication used as criteria | yes | yes | yes | yes | yes | yes |
| Excluded/Included list provided | yes | yes | yes | yes | yes | yes |
| Study Characteristics Provided | yes | yes | yes | yes | yes | yes |
| Quality assessed/presented | yes | yes | yes | yes | yes | yes |
| Quality impacted conclusions | yes | yes | yes | yes | can't answer | can't answer |
| Heterogeneity tested before combining | yes | yes | yes | yes | yes | yes |
| Publication bias assessed | yes | can't answer | yes | yes | Not possible (2 studies) | can't answer |
| Conflict stated | yes | yes | yes | yes | yes | yes |
Appendix 4. Efficacy outcomes reported in each Cochrane systematic review
| Cochrane Library | ACR‐20 | ACR‐50 | ACR‐70 |
Good EULAR response, Low Ds Activity, Remission |
HAQ Ch>=0.22 HAQ =0 |
SF36 PCS & MCS Ch>=5 | Radiographic Progression (continuous or categorical) | HAQ Scores continuous | SF‐36 PCS or MCS continuous | DAS or DAS28 continuous |
| ABATACEPT | ||||||||||
| Moreland (2002) | Y | Y | Y | |||||||
| Genovese (2005) | Y | Y | Y | Y | Y | Y | Y | |||
| Schiff (2008) | Y | Y | Y | Y | Y | Y | ||||
| Kremer (2003) | Y | Y | Y | Y | Y | |||||
| Kremer (2006) | Y | Y | Y | Y | Y | Y | ||||
| Weinblatt (2007) | Y | Y | Y | |||||||
| Weinblatt (ASSURE) (2006) | ||||||||||
| ADALIMUMAB | ||||||||||
| Bejarano (2008) | Y | Y | Y | Y | Y | |||||
| Breedveld (2006) | Y | Y | Y | Y | Y | Y | Y | |||
| Furst | Y | Y | Y | |||||||
| Keystone (2004) | Y | Y | Y | Y | Y | |||||
| Kim (2007) | Y | Y | Y | Y | ||||||
| Miyasaka (2008) | Y | Y | Y | Y | ||||||
| Van de Putte (2004) | Y | Y | Y | Y | Y | Y | ||||
| Weinblatt (2003) | Y | Y | Y | Y | ||||||
| ANAKINRA* | ||||||||||
| Bresnihan (1998) | Y | Y | Y | |||||||
| Fleischiman (2003) | ||||||||||
| Cohen (2002) | Y | Y | Y | |||||||
| Cohen (2004) | Y | Y | Y | Y | ||||||
| Genovese (2004) | Y | Y | Y | |||||||
| ETANERCEPT | ||||||||||
| Moreland (1999) | Y | Y | Y | Y | ||||||
| Weinblatt (1999) | Y | Y | Y | Y | ||||||
| Emery (COMET) (2008) | Y | Y | Y | Y | Y | Y | ||||
| Klareskog (TEMPO) (2004) | Y | Y | Y | Y | Y | Y | ||||
| INFLIXIMAB | ||||||||||
| Maini (1998) | ||||||||||
| ASPIRE (2004) | Y | Y | Y | Y | Y | Y | Y | |||
| Lipsky (ATTRACT) (2000) | Y | Y | Y | Y | Y | Y | ||||
| Quinn (2005) | Y | Y | Y | Y | Y | |||||
| RITUXIMAB** | ||||||||||
| Edwards (2004) | Y | Y | Y | Y | Y | Y | ||||
| Emery (DANCER) (2006) | Y | Y | Y | Y | Y | Y | ||||
| Cohen (REFLEX) (2006) | Y | Y | Y | Y | Y | |||||
*Anakinra review did not present withdrawals due to adverse events, which were obtained from the authors (reported in the studies)
**Rituximab review did not present ACR20, which were obtained from the authors (reported in the studies)
Appendix 5. Safety outcomes reported in each Cochrane systematic review
| Cochrane Library |
Total AE |
Total SE |
Infections, Serious & Lung Infections, Tuberculosis |
Cancer | Withdrawals (all) | Withdrawals (SE) |
Withdrawals (Inefficacy) |
Death |
| ABATACEPT | ||||||||
| Moreland (2002) | Y | |||||||
| Genovese (2005) | Y | Y | Y | Y | Y | Y | ||
| Schiff (2008) | Y | Y | Y | Y | Y | Y | Y | |
| Kremer (2003) | Y | Y | Y | Y | Y | |||
| Kremer (2006) | Y | Y | Y | Y | Y | Y | Y | |
| Weinblatt (2007) | Y | Y | Y | Y | Y | Y | Y | |
| Weinblatt (ASSURE) (2006) | Y | Y | Y | Y | Y | Y | Y | |
| ADALIMUMAB | ||||||||
| Bejarano (2008) | Y | Y | Y | Y | Y | |||
| Breedveld (2006) | Y | Y | Y | Y | Y | |||
| Furst | Y | Y | Y | Y | Y | |||
| Keystone (2004) | Y | Y | Y | Y | ||||
| Kim (2007) | Y | Y | Y | Y | Y | Y | ||
| Miyasaka (2008) | Y | Y | Y | Y | Y | Y | ||
| Van de Putte (2004) | Y | Y | Y | Y | Y | |||
| Weinblatt (2003) | Y | |||||||
| ANAKINRA* | ||||||||
| Bresnihan (1998) | Y | |||||||
| Fleischiman (2003) | Y | Y | Y | Y | Y | |||
| Cohen (2002) | Y | |||||||
| Cohen (2004) | Y | Y | Y | |||||
| Genovese (2004) | Y | Y | Y | Y | ||||
| ETANERCEPT | ||||||||
| Moreland (1999) | Y | Y | Y | |||||
| Weinblatt (1999) | Y | Y | Y | Y | Y | |||
| Emery (COMET) (2008) | Y | Y | Y | Y | Y | |||
| Klareskog (TEMPO) (2004) | Y | Y | Y | Y | Y | Y | ||
| INFLIXIMAB | ||||||||
| Maini (1998) | Y | Y | Y | |||||
| ASPIRE (2004) | Y | Y | Y | Y | Y | Y | Y | |
| Lipsky (ATTRACT) (2000) | Y | Y | Y | Y | Y | Y | ||
| Quinn (2005) | Y | Y | Y | |||||
| RITUXIMAB** | ||||||||
| Edwards (2004) | Y | Y | Y | Y | Y | Y | Y | |
| Emery (DANCER) (2006) | Y | Y | Y | Y | Y | Y | Y | |
| Cohen (REFLEX) (2006) | Y | Y | Y | Y | Y | Y | ||
| *Anakinra review did not present withdrawals due to adverse events, which were obtained from the authors (reported in the studies) **Rituximab review did not present ACR20, which were obtained from the authors (reported in the studies) | ||||||||
Contributions of authors
JS, RC, MC, RB, ML, GW, PT‐ study concept
JS, RC, GW ‐ protocol development
JS, RC, MC, RB, ML, PT, GW, ETG ‐ protocol editing
JS, RC, ML, GW, ETG ‐ data extraction
JS, RC, GW ‐ data analysis
JS, RC, MC, RB, ML, GW, PT, ETG ‐ writing and editing results and conclusions
Sources of support
Internal sources
-
The Oak Foundation, Switzerland.
Provide support for The Parker Institute: Musculoskeletal Statistics Unit.
External sources
-
Jasvinder Singh, USA.
NIH CTSA Award 1 KL2 RR024151‐01 (Mayo Clinic Center for Clinical and Translational Research)
Declarations of interest
JS ‐ speaker honoraria from Abbott; research grants from AMGEN, Allergan, Takeda, Savient; consultant fee from Savient, URL pharma
RC ‐ research grant and consultant fee from Bristol‐Myers Squibb and Abbott
GW ‐ research grant and consultant fee from Bristol‐Myers Squibb
MS ‐ speaker honoraria from Bristol‐Myers Squibb and Roche and consultant fee from Amgen
RB ‐ none
ML ‐ none
ETG ‐ none
PT ‐ grants/honoraria from Bristol Myers, Chiltern International, and UCB
Edited (no change to conclusions)
References
References to included reviews
Blumenauer 2003
- Blumenauer BBTB, Judd M, Wells GA, Burls A, Cranney A, Hochberg MC, et al. Infliximab for the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003785] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lethaby 2003
- Lethaby A, Lopez‐Olivo MA, Blumenauer BBTB, Cranney A, Burls A, Coyle D, et al. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004525] [DOI] [PubMed] [Google Scholar]
Lopez‐Olivo 2008
- Lopez‐Olivo MA, Amezaga M, McGahan L, Suarez‐Almazor ME. Rituximab for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007356] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maxwell 2008
- Maxwell L, Singh JA. Abatacept for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007277] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mertens 2008
- Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005121.pub2] [DOI] [PubMed] [Google Scholar]
Navarro‐Sarabia 2005
- Navarro‐Sarabia F, Ariza‐Ariza R, Hernandez‐Cruz B, Villanueva I. Adalimumab for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005113.pub2] [DOI] [PubMed] [Google Scholar]
Additional references
Alonso‐Ruiz 2008
- Alonso‐Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, Calabozo M, Quintana A. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and meta analysis of efficacy and safety. BMC Musculoskeletal Disorders 2008;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnett 1988
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 1988;31(3):315‐24. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barlow 1999
- Barlow JH, Cullen LA, Rowe IF. Comparison of knowledge and psychological well‐being between patients with a short disease duration (< or = 1 year) and patients with more established rheumatoid arthritis (> or = 10 years duration). Patient Education and Counseling 1999;38:195‐203. [DOI] [PubMed] [Google Scholar]
Becker 2008
- Becker LA, Oxman AD. Chapter 22: Overviews of Reviews. In: Higgins JP, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochranehandbook.org.
Boers 2001
- Boers M. Treatment of early disease. Rheumatic Diseases Clinics of North America 2001;27:405‐14, x. [DOI] [PubMed] [Google Scholar]
Bongartz 2006
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti‐TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta‐analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295(19):2275‐85. [DOI] [PubMed] [Google Scholar]
Bongartz 2009
- Bongartz T, Warren FC, Mines D, Matteson EL, Abrams KR, Sutton AJ. Etanercept therapy in rheumatoid arthritis and the risk of malignancies: a systematic review and individual patient data meta‐analysis of randomised controlled trials. Annals of the Rheumatic Diseases 2009;68(7):1177‐83. [DOI] [PubMed] [Google Scholar]
Bradburn 2007
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: A comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26:53‐77. [DOI] [PubMed] [Google Scholar]
Brennan 2008
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. Journal of Clinical Investigation 2008;118(11):3537‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cash 1994
- Cash JM, Klippel JH. Second‐line drug therapy for rheumatoid arthritis. New England Journal of Medicine 1994;330(19):1368‐75. [DOI] [PubMed] [Google Scholar]
Cates 2009 [Computer program]
- Dr Chris Cates' EBM web site. Available from http://www.Nntonline.Net/visualrx/. Visual Rx NNT Calculator version 3. Dr Chris Cates' EBM web site. Available from http://www.Nntonline.Net/visualrx/, Accessed 13 February 2009.
Choy 2001
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. New England Journal of Medicine 2001;344(12):907‐16. [DOI] [PubMed] [Google Scholar]
Chung 2006
- Chung CP, Thompson JL, Koch GG, Amara I, Strand V, Pincus T. Are American College of Rheumatology 50% response criteria superior to 20% criteria in distinguishing active aggressive treatment in rheumatoid arthritis clinical trials reported since 1997? A meta‐analysis of discriminant capacities. Annals of the Rheumatic Diseases 2006;65(12):1602‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Connell 2006
- Connell L, McInnes IB. New cytokine targets in inflammatory rheumatic diseases. Best Practice and Research Clinical Rheumatology 2006;20(5):865‐78. [DOI] [PubMed] [Google Scholar]
Cope 2008
- Cope AP. T cells in rheumatoid arthritis. Arthritis Research and Therapy 2008;10 Suppl 1:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dersimonian 2007
- DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemporary Clinical Trials 2007;28(2):105‐14. [DOI] [PubMed] [Google Scholar]
Donahue 2008
- Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas BL, et al. Systematic review: comparative effectiveness and harms of disease‐modifying medications for rheumatoid arthritis. Annals of Internal Medicine 2008;148(2):124‐34. [DOI] [PubMed] [Google Scholar]
Drugs 2006
- Drug Information Online. Drugs.com. FDA Approves First Rheumatoid Arthritis Indication for Rituxan. http://www.drugs.com/news/fda‐approves‐first‐rheumatoid‐arthritis‐indication‐rituxan‐1745.html 03/01/2006.
EMEA 2001
- EMEA. EMEA Public Statement on Infliximab (Remicade): Increased incidence of mortality and hospitalization for worsening Congestive Heart Failure. http://www.emea.europa.eu/pdfs/human/press/pus/325701en.pdf.
EMEA 2003
- EMEA. EMEA public statement: Increased risk of serious infection and neutropenia in patients treated concurrently with Kineret (anakinra) and Enbrel (etanercept). http://www.emea.europa.eu/humandocs/PDFs/EPAR/kineret/3163102en.pdf.
EMEA 2004
- European Medicines Agency. EPARs for authorized medicinal products for human use: Anakinra: European Public Assessment Report. http://www.emea.europa.eu/humandocs/Humans/EPAR/kineret/kineret.htm Revision 11, published 27/01/09.
EMEA 2009a
- EMEA. Remicade (inflixmab). http://www.emea.europa.eu/humandocs/PDFs/EPAR/Remicade/H‐240‐PI‐en.pdf.
EMEA 2009b
- European Medicines Agency. EPARs for authorized medicinal products for human use: Humira: European Public Assessment Report. http://www.emea.europa.eu/humandocs/Humans/EPAR/humira/humira.htm Revision 15, Published 11/05/09.
FDA 1998
- Food, Drug Administration. Etanercept Product Approval Information‐ Licensing Action 12/2/1998. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm080536.htm 1998.
FDA 1999
- Food, Drug Administration. Remicade® (infliximab) for IV Injection. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm107722.pdf 1999.
FDA 2001
- Food, Drug Administration. Kineret® (anakinra) Physician packet insert: Kineret® (anakinra) prescribing information. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm080650.htm 2001.
FDA 2002
- Food, Drug Administration. Package Insert. HUMIRA® (adalimumab). Abbott Laboratories. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm080610.htm 2002.
FDA 2005
- Food, Drug Administration. Patient Information Sheet. Abatacept (marketed as Orencia®). http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/FastTrackApprovalReports/ucm082380.htm 2005.
FDA 2006b
- Food, Drug Administration. Rituxan® (rituximab). http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150747.htm.
FDA 2007
- Food, Drug Administration. Drug details: Orencia. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
FDA 2008
- Food, Drug Administration. Humira (adalimumab): Highlights of prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125057s114lbl.pdf.
FDA 2008a
- Food, Drug Administration. FDA: Manufacturers of TNF‐blocker drugs must highlight risk of fungal infections. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116942.htm.
FDA 2008b
- Food, Drug Administration. Medication Guide: Enbrel® (etanercept). http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/103795s5359lbl.pdf.
FDA 2009
- Food, Drug Administration. Remicade® (infliximab) for IV Injection. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/103772s5234lbl.pdf.
Felson 1995
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1995;38(6):727‐35. [DOI] [PubMed] [Google Scholar]
Finckh 2006
- Finckh A, Liang MH, Herckenrode CM, Pablo P. Long‐term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta‐analysis. Arthritis and Rheumatism 2006;55(6):86‐72. [DOI] [PubMed] [Google Scholar]
Fleischman 2003
- Fleischmann RM, Schechtman J, Bennett R, Handel ML, Burmester GR, Tesser J, et al. Anakinra, a recombinant human interleukin‐1 receptor antagonist (r‐metHuIL‐1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo‐controlled trial. Arthritis and Rheumatism 2003;48(4):927‐34. [DOI] [PubMed] [Google Scholar]
Fransen 2005
- Fransen J, Riel PL. The Disease Activity Score and the EULAR response criteria. Clinical and Experimental Rheumatology 2005;23(5 Suppl 39):S93‐9. [PubMed] [Google Scholar]
Fries 1980
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis and Rheumatism 1980;23(2):137‐45. [DOI] [PubMed] [Google Scholar]
Furst 2008
- Furst DE, Keystone EC, Kirkham B, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2008. Annals of the Rheumatic Diseases 2008;67 Suppl 3(Suppl 3):iii2‐25. [DOI] [PubMed] [Google Scholar]
Gartlehner 2006
- Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. The Journal of Rheumatology 2006;33(12):2398‐408. [PubMed] [Google Scholar]
Genovese 2005
- Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. New England Journal of Medicine 2005;353(11):1114‐23. [DOI] [PubMed] [Google Scholar]
Health Canada 2006
- Health Canada. Important Safety Information on anti‐TNFα Therapy: Enbrel (etanercept), Humira (adalimumab), and Remicade (infliximab) ‐ For Health Professionals. http://www.hc‐sc.gc.ca/dhp‐mps/medeff/advisories‐avis/prof/_2006/anti‐tnf_therap_hpc‐cps‐eng.php.
Health Canada 2006b
- Health Canada. Possible association of Remicade (infliximab) with hepatosplenic T‐cell lymphoma in pediatric and young adult patients with Crohn's disease ‐ For Health Professionals. http://www.hc‐sc.gc.ca/dhp‐mps/medeff/advisories‐avis/prof/_2006/remicade_3_hpc‐cps‐eng.php.
Health Canada 2009
- Health Canada. Association of Enbrel (etanercept) with histoplasmosis and other invasive fungal infections ‐ for health professionals. http://www.hc‐sc.gc.ca/dhp‐mps/medeff/advisories‐avis/prof/_2009/enbrel_hpc‐cps‐eng.php.
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Keystone 2004
- Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti‐tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo‐controlled, 52‐week trial. Arthritis Rheum 2004;50(5):1400‐11. [DOI] [PubMed] [Google Scholar]
Klareskog 2004
- Klareskog L, Heijde D, Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double‐blind randomised controlled trial. Lancet 2004;363(9410):675‐81. [DOI] [PubMed] [Google Scholar]
Kristensen 2007
- Kristensen LE, Christensen R, Bliddal H, Geborek P, Danneskiold‐Samsoe B, Saxne T. The number needed to treat for adalimumab, etanercept, and infliximab based on ACR50 response in three randomized controlled trials on established rheumatoid arthritis: A systematic literature review. Scandinavian Journal of Rheumatology 2007;36:411‐7. [DOI] [PubMed] [Google Scholar]
Kvien 2004
- Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics 2004;22(2 Suppl 1):1‐12. [DOI] [PubMed] [Google Scholar]
Kvien 2005
- Kvien TK, Uhlig T. Quality of life in rheumatoid arthritis. Scandinavian Journal of Rheumatology 2005;34(5):333‐41. [DOI] [PubMed] [Google Scholar]
Larsen 1977
- Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiologica: Diagnosis (Stockholm) 1977;4:481‐91. [DOI] [PubMed] [Google Scholar]
Lee 2001
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001;358(9285):903‐11. [DOI] [PubMed] [Google Scholar]
Lee 2008
- Lee YH, Woo JH, Rho YH, Choi SJ, Ji JD, Song GG. Meta‐analysis of the combination of TNF inhibitors plus MTX compared to MTX monotherapy, and the adjusted indirect comparison of TNF inhibitors in patients suffering from active rheumatoid arthritis. Rheumatology International 2008;28(6):553‐9. [DOI] [PubMed] [Google Scholar]
Lipsky 2000
- Lipsky PE, Heijde DM, Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 2000;343(22):1594‐602. [DOI] [PubMed] [Google Scholar]
Lubeck 2004
- Lubeck DP. Patient‐reported outcomes and their role in the assessment of rheumatoid arthritis. Pharmacoeconomics 2004;22(2 Suppl 1):27‐38. [DOI] [PubMed] [Google Scholar]
Nixon 2007
- Nixon RM, Bansback N, Brennan A. Using mixed treatment comparisons and meta‐regression to perform indirect comparisons to estimate the efficacy of biologic treatments in rheumatoid arthritis. Statistics in Medicine 2007;26(6):1237‐54. [DOI] [PubMed] [Google Scholar]
Odegard 2005
- Odegard S, Finset A, Kvien TK, Mowinckel P, Uhlig T. Work disability in rheumatoid arthritis is predicted by physical and psychological health status: a 7‐year study from the Oslo RA register. Scandinavian Journal of Rheumatology 2005;34(6):441‐7. [DOI] [PubMed] [Google Scholar]
Osiri 2003
- Osiri M, Suarez‐Almazor ME, Wells GA, Robinson V, Tugwell P. Number needed to treat (NNT): Implication in rheumatology clinical practice. Annals of the Rheumatic Diseases 2003;62:316‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pincus 1983
- Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis and Rheumatism 1983;26(11):1346‐53. [DOI] [PubMed] [Google Scholar]
Pincus 2002
- Pincus T, Ferraccioli G, Sokka T, Larsen A, Rau R, Kushner I, et al. Evidence from clinical trials and long‐term observational studies that disease‐modifying anti‐rheumatic drugs slow radiographic progression in rheumatoid arthritis: updating a 1983 review. Rheumatology (Oxford) 2002;41(12):1346‐56. [DOI] [PubMed] [Google Scholar]
Platt 1999
- Platt RW, Leroux BG, Breslow N. Generalized linear mixed models for meta‐analysis. Statistics in Medicine 1999;18(6):643‐54. [DOI] [PubMed] [Google Scholar]
Prevoo 1995
- Prevoo ML, 't Hof MA, Kuper HH, Leeuwen MA, Putte LB, Riel PL. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis and Rheumatism 1995;38(1):44‐8. [DOI] [PubMed] [Google Scholar]
Prevoo 1996
- Prevoo ML, Gestel AM, van THMA, Rijswijk MH, Putte LB, Riel PL. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. British Journal of Rheumatology 1996;35(11):1101‐5. [DOI] [PubMed] [Google Scholar]
Salanti 2008
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17(3):279‐301. [DOI] [PubMed] [Google Scholar]
Schiff 2008
- Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi‐centre, randomised, double‐blind, placebo‐controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Annals of the Rheumatic Diseases 2008;8:1096‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2006
- Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. New England Journal of Medicine 2006;355(7):704‐12. [DOI] [PubMed] [Google Scholar]
Sharp 1971
- Sharp JT, Lidsky MD, Collins LC, Moreland J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis and Rheumatism 1971;14(6):706‐20. [DOI] [PubMed] [Google Scholar]
Shea 2007
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2003
- Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: Empirical evidence from published meta‐analyses. BMJ 2003;326:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strand 2008
- Strand V, Singh JA. Improved health‐related quality of life with effective disease‐modifying antirheumatic drugs: evidence from randomized controlled trials. American Journal of Managed Care 2008;14(4)(4):234‐54. [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23:1351‐75. [DOI] [PubMed] [Google Scholar]
Szekanecz 2007
- Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Current Opinion in Rheumatology 2007;19(3):289‐95. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [DOI] [PubMed] [Google Scholar]
van der Heijde 1989
- Heijde DM, Riel PL, Nuver‐Zwart IH, Gribnau FW, vad de Putte LB. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet 1989;1(8646):1036‐8. [DOI] [PubMed] [Google Scholar]
van der Heijde 1993
- Heijde DM, 't Hof M, Riel PL, Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. Journal of Rheumatology 1993;20(3):579‐81. [PubMed] [Google Scholar]
van Gestel 1996
- Gestel AM, Prevoo ML, 't Hof MA, Rijswijk MH, Putte LB, Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis and Rheumatism 1996;39:34‐40. [DOI] [PubMed] [Google Scholar]
Wells 1993
- Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient's perspective. The Journal of Rheumatology 1993;20(3):557‐60. [PubMed] [Google Scholar]
Wells 2009
- Wells GA, Sultan SA, Chen L, Khan M, Coyle D. Indirect evidence: indirect treatment comparisons in meta‐analysis. Canadian Agency for Drugs and Technologies in Health. Ottawa 2009.
Woolley 2003
- Woolley DE. The mast cell in inflammatory arthritis. New England Journal of Medicine 2003;348(17):1709‐11. [DOI] [PubMed] [Google Scholar]
Yelin 2007
- Yelin E. Work disability in rheumatic diseases. Current Opinion in Rheumatology 2007;19(2):91‐6. [DOI] [PubMed] [Google Scholar]


