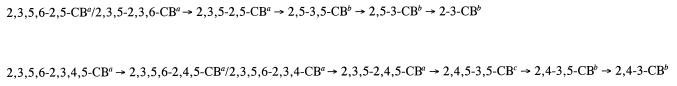Abstract
Reductive dechlorination of Aroclor 1260 was investigated in anaerobic slurries of estuarine sediments from Baltimore Harbor (Baltimore, Md.). The sediment slurries were amended with 800 ppm Aroclor 1260 with and without the addition of 350 μM 2,3,4,5-tetrachlorobiphenyl (2,3,4,5-CB) or 2,3,5,6-tetrachlorobiphenyl (2,3,5,6-CB) and incubated in triplicate at 30°C under methanogenic conditions in an artificial estuarine medium. After 6 months, extensive meta dechlorination and moderate ortho dechlorination of Aroclor 1260 occurred in all incubated cultures except for sterilized controls. Overall, total chlorines per biphenyl decreased by up to 34%. meta chlorines per biphenyl decreased by 65, 55, and 45% and ortho chlorines declined by 18, 12, and 9%, respectively, when 2,3,4,5-CB, 2,3,5,6-CB, or no additional congener was supplied. This is the first confirmed report of microbial ortho dechlorination of a commercial polychlorinated biphenyl mixture. In addition, compared with incubated cultures supplied with Aroclor 1260 alone, the dechlorination of Aroclor 1260 plus 2,3,4,5-CB or 2,3,5,6-CB occurred with shorter lag times (31 to 60 days versus 90 days) and was more extensive, indicating that the addition of a single congener stimulated the dechlorination of Aroclor 1260.
Polychlorinated biphenyls (PCBs) and other anthropogenic pollutants adsorb to sediments due to the hydrophobic nature of the compounds. As sediments settle, adsorbed PCBs accumulate in the lower anoxic layers of the sediment column, where reductive dechlorination of PCBs by anaerobic microorganisms has been demonstrated to occur in the laboratory and in situ (1, 2, 4, 5, 10, 12, 22). The turnover of naturally formed halogenated organics in marine coastal regions suggests that these environments have a significant potential for dechlorination (14, 18). However, few studies have focused on the dechlorination of PCBs in marine and estuarine sediments (2, 11, 20).
Anaerobic PCB dechlorination has the potential to reduce the toxicity of the PCBs (5, 11, 23) and convert highly persistent congeners, frequently the more extensively chlorinated congeners, into forms that are more amenable to aerobic degradation (6, 8, 13, 15, 26). However, only the loss of meta and/or para chlorines has been demonstrated when preexisting or freshly added commercial PCB mixtures (e.g., Aroclors 1242, 1254, and 1260, etc.) have been microbially dechlorinated in sediments from the Hudson River (N.Y.), Silver Lake (Pittsfield, Mass.), Woods Pond (Lenox, Mass.), and Puget Sound (2, 3, 7, 20, 21). ortho dechlorination of an Aroclor has not been demonstrated, and microbial dechlorination of Aroclor 1260, preexisting or freshly added to sediments, has not been very extensive. The addition of single PCB congeners, in a process called priming, stimulated the dechlorination of Aroclor 1260 residue in Woods Pond sediments, but priming did not promote ortho dechlorination of the residual Aroclor 1260 (3, 5, 7, 31).
Baltimore Harbor (BH; Baltimore, Md.) has been heavily impacted by industrial activity over the last 150 years, and PCBs have accumulated in sediments throughout the harbor (27). We recently reported that the single congeners 2,3,5,6-chlorobiphenyl (2,3,5,6-CB), 2,3,5-CB, and 2,3,6-CB were ortho dechlorinated by enrichment cultures that contained sediments collected from the northwest branch of the harbor (9). Here we describe the anaerobic dechlorination of Aroclor 1260 by enrichment cultures prepared with these sediments. The data demonstrate extensive meta dechlorination and moderate ortho dechlorination. Furthermore, we show that the meta and ortho dechlorinations are stimulated by the addition of single PCB congeners.
MATERIALS AND METHODS
Sediment collection and storage.
Collection of estuarine sediments from BH was described previously (9), and the sediment samples were stored anaerobically at room temperature for 14 months in the dark before use in these experiments. No background PCBs were detected in these sediments based on methods described below (detection limit, ∼0.01 μg/g of PCB standard used).
Preparation of slurries and incubation.
Estuarine medium without sulfate (E-Cl) was prepared as described by Berkaw et al. (9). In an anaerobic chamber (Coy Laboratory Products, Ann Arbor, Mich.) containing 95% nitrogen–5% hydrogen, sediment slurries were prepared by mixing 1 volume of wet BH sediment with 4 volumes of E-Cl medium (equivalent to 0.06 g [dry weight] of sediment per ml). Aliquots of the slurries (30 ml) were dispensed into 50-ml serum bottles and allowed to stand for 5 days in the anaerobic chamber.
To prepare sterile controls, slurries were autoclaved twice for 1 h at 121°C on 2 consecutive days. Live cultures and sterile controls prepared in triplicate were amended with 800 ppm Aroclor 1260 (800 μg per g [dry weight] of sediment or 133 μmol per liter of slurry) and either 350 μM (μmol per liter of slurry) 2,3,4,5-CB, 350 μM 2,3,5,6-CB, or no additional congener. All enrichment cultures were incubated at 30°C in the dark. Each month, all enrichments were supplemented with a fatty acid mixture (2.5 mM each acetate, propionate, and butyrate).
Sample preparation, extraction, and analysis.
The dechlorination of 2,3,4,5-CB, 2,3,5,6-CB, and Aroclor 1260 in each culture was analyzed at various time points throughout a 6-month period. Samples were drawn and extracted in ethyl acetate (high-performance liquid chromatography grade; Fisher Scientific, Pittsburgh, Pa.), and the organic fraction was passed over a Florisil-copper column as described previously (9). PCBs were analyzed with a Hewlett-Packard 5890 series II gas chromatograph (GC) equipped with an RTX-1 capillary column (30 m by 0.25 mm [inside diameter] by 0.25 μm; Restek Corp., Bellefonte, Pa.) and a Ni63 electron capture detector as described previously (9).
Congeners 2,3,4,5-CB and 2,3,5,6-CB and their dechlorination products were identified by matching their retention times with those of authentic standards (>90% purity; AccuStandard, New Haven, Conn.) and were quantified by use of a piecewise-fit calibration curve generated from these standards at 9 to 16 calibration levels (9). PCB congeners in Aroclor 1260 and their dechlorination products were identified by matching their GC retention times with a customized PCB standard prepared by supplementing Aroclor 1260 with the dechlorination products observed in Woods Pond (24) or a standard mixture composed of 3-3-CB, 3-4-CB, 3,5-3-CB, 3,5-4-CB, 2,4-3,5-CB, and 2,5-3,5-CB. Congener assignments were made in accordance with those reported by Frame et al. (16). Each congener in the Aroclor mixture was quantified by use of a piecewise-fit calibration curve generated from standards at 4- to 8-point calibration levels. Congener and homolog distributions for each sample were calculated and reported in units of moles percent. Congener distributions for each enrichment culture with Aroclor and 2,3,4,5-CB (or 2,3,5,6-CB) were calculated after subtracting the peaks corresponding to 2,3,4,5-CB (or 2,3,5,6-CB) and their potential dechlorination products. Therefore, values for dechlorination of Aroclor 1260 in those incubations are conservative.
Mass selective analysis was performed with a Hewlett-Packard 6890 series GC equipped with an HP-5MS capillary column (30 m by 0.25 mm [inside diameter] by 0.25 μm; Hewlett-Packard, Atlanta, Ga.) and a Hewlett-Packard 6890 series mass selective detector (MS). Chromatographic conditions were identical to those described previously for the GC-electron capture detector (9). Our analysis found that 2,4-3,5-CB was not resolved with 2,3,6-2,6-CB on a DB-1 column. Thus, we used GC-MS to identify 2,4-3,5-CB (m/z 292) and 2,3,6-2,6-CB (m/z 326) due to different molecular formulas between these two congeners. In addition, analysis of our PCB standard mixtures on an HP-5MS capillary column resulted in the resolution of 2,4-3,5-CB from 2,3,6-2,6-CB. We also found that 2,5-3,5-CB was not resolved from 2,3,4-2-CB, 2,3,6-4-CB, and 2,6-3,4-CB on a DB-1 column as reported previously (16). However, 2,5-3,5-CB was resolved from these congeners by using an HP-5MS column with GC-MS.
RESULTS
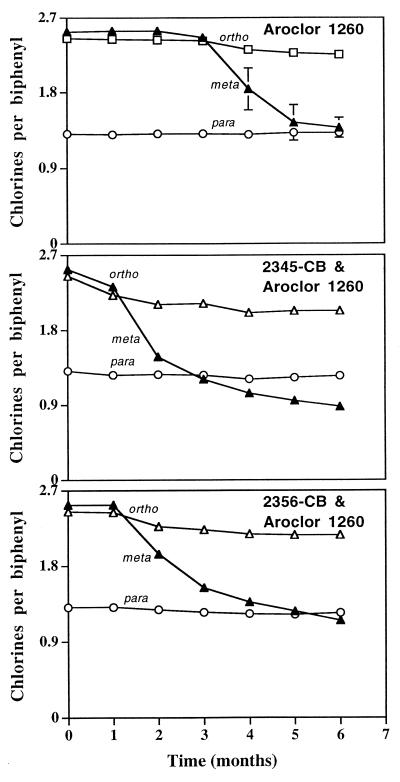
Dechlorination of Aroclor 1260 in BH enrichment cultures was detected within 4 months (Fig. 1). However, the lag time decreased to 31 days in sediment slurries additionally supplied with 2,3,4,5-CB. Congener 2,3,5,6-CB also stimulated the onset of Aroclor dechlorination but not as quickly as 2,3,4,5-CB. In addition, the overall dechlorination of Aroclor 1260 was enhanced more by the presence of 2,3,4,5-CB than by 2,3,5,6-CB (Table 1 and Fig. 1). After 6 months, only a small level of meta dechlorination continued in the congener-supplemented cultures and all ortho dechlorination had ceased. No biphenyl was detected (by GC-MS) in any of the enrichment cultures, and no PCB dechlorination was observed in sterilized slurries (the total chlorine per biphenyl ± standard deviation of triplicate controls was 6.32 ± 0.01).
FIG. 1.
Chlorine distribution of Aroclor 1260 over the incubation time. Averaged data of triplicate samples are presented. Errors bars indicate standard deviations of triplicate samples; if no error bar is evident, the standard deviation is less than 0.09 and is masked by the symbols.
TABLE 1.
Homolog distribution and chlorine distribution of Aroclor 1260 after 181 days of incubation
| Homolog or chlorine | Distributiona
|
|||
|---|---|---|---|---|
| Aroclor 1260 at 0 day | After 181 days
|
|||
| Aroclor 1260 | Aroclor 1260 + 2,3,4,5-CB | Aroclor 1260 + 2,3,5,6-CB | ||
| PCB homologsb | ||||
| Dichlorobiphenyls | 0.10 ± 0.00 | 0.19 ± 0.03 | 4.15 ± 0.56 | 0.98 ± 0.52 |
| Trichlorobiphenyls | 0.71 ± 0.01 | 3.81 ± 0.55 | 14.87 ± 0.42 | 5.71 ± 0.80 |
| Tetrachlorobiphenyls | 1.42 ± 0.03 | 53.36 ± 4.53 | 60.01 ± 1.35 | 61.67 ± 1.30 |
| Pentachlorobiphenyls | 10.81 ± 0.16 | 11.35 ± 0.55 | 10.43 ± 0.52 | 9.84 ± 0.14 |
| Hexachlorobiphenyls | 45.65 ± 0.13 | 12.64 ± 2.66 | 4.60 ± 1.42 | 13.54 ± 1.91 |
| Heptachlorobiphenyls | 35.61 ± 0.18 | 14.33 ± 2.55 | 4.17 ± 1.17 | 5.90 ± 1.22 |
| Octachlorobiphenyls | 5.21 ± 0.09 | 3.84 ± 0.38 | 1.36 ± 0.26 | 1.91 ± 0.31 |
| Nonachlorobiphenyls | 0.49 ± 0.01 | 0.48 ± 0.01 | 0.41 ± 0.03 | 0.45 ± 0.01 |
| Chlorines | ||||
| ortho | 2.49 ± 0.00 | 2.26 ± 0.02 | 2.04 ± 0.01 | 2.19 ± 0.03 |
| meta | 2.55 ± 0.00 | 1.39 ± 0.12 | 0.89 ± 0.07 | 1.15 ± 0.05 |
| para | 1.27 ± 0.00 | 1.28 ± 0.01 | 1.23 ± 0.01 | 1.23 ± 0.01 |
| Total | 6.31 ± 0.01 | 4.93 ± 0.15 | 4.16 ± 0.08 | 4.57 ± 0.07 |
All data are means of triplicate determinations ± standard deviations. Data for PCB homologs are in moles percent, and data for chlorines are per biphenyl.
No mono- or decachlorobiphenyls were detected.
Dechlorination of added congeners 2,3,4,5-CB and 2,3,5,6-CB was detected after 20 and 27 days, respectively, and preceded the dechlorination of Aroclor 1260. After 181 days, 67 mol% of 2,3,4,5-CB and 99 mol% of 2,3,5,6-CB were transformed to the same products reported by Berkaw et al. (9). Monochlorobiphenyls were produced, including 26 and 12 mol% of 3-CB and 4-CB, respectively, in cultures incubated with 2,3,4,5-CB and Aroclor 1260 and 1 and 32 mol% of 2-CB and 3-CB, respectively, in enrichment cultures supplied with Aroclor 1260 plus 2,3,5,6-CB. Previous (9) and subsequent studies of BH sediments incubated with 2,3,5,6-CB alone have not resulted in the production of 2-CB. We cannot exclude the possibility that 2-CB, or any other monochlorobiphenyl produced, came from the transformation of Aroclor 1260. However, since we cannot unequivocally determine the source of these monochlorobiphenyls, they are discounted in our overall assessment of Aroclor dechlorination when the supplemental congeners are added. No monochlorobiphenyls were detected in samples from slurry enrichments supplied with Aroclor 1260 alone.
The homolog distribution data for Aroclor 1260 before and after incubation can be found in Table 1. Overall, hexa- to nonachlorobiphenyls decreased by 65, 75, and 88% in incubated cultures supplied with Aroclor 1260 alone, Aroclor 1260 plus 2,3,5,6-CB, and Aroclor 1260 plus 2,3,4,5-CB, respectively, indicating more extensive dechlorination of Aroclor 1260 in enrichment cultures supplied with 2,3,4,5-CB. Significant decreases were seen in all of the major hexa- and heptachlorobiphenyls, e.g., 2,3,6-2,4,5-CB, 2,4,5-2,4,5-CB, 2,3,4-2,4,5-CB, 2,3,5,6-2,4,5-CB, 2,3,4,5-2,3,6-CB, 2,3,4,5-2,4,5-CB, and 2,3,4,5-2,3,4-CB. Large increases occurred in tri- and tetrachlorobiphenyls such as 2,4-3-CB, 2,4-3,5-CB, 2,4-2,4-CB, 2,4-2,5-CB, and 2,4-2,6-CB. Small changes in the concentration of pentachlorobiphenyls may indicate an intermediary role for these homologs.
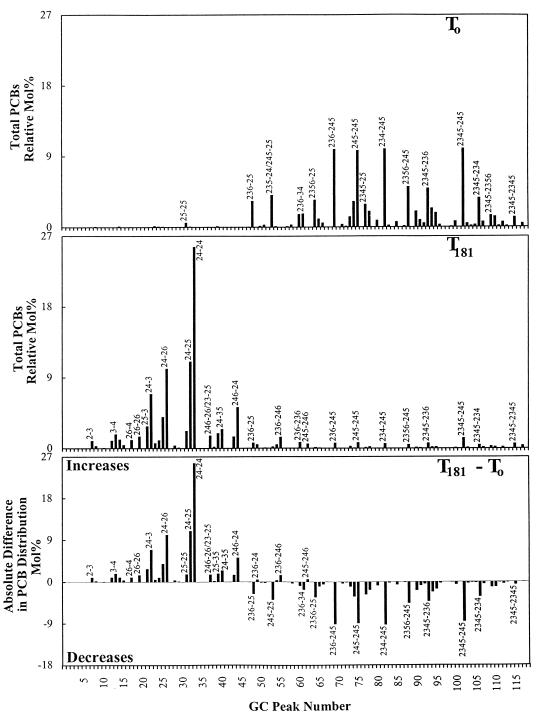
Chlorine distribution of Aroclor 1260 over time indicated that meta dechlorination was predominant but was accompanied by a significant, yet more moderate, level of ortho dechlorination (Fig. 1 and Table 1). After 181 days, 45 to 65% of the meta chlorines and 9 to 18% of the ortho chlorines had been removed depending upon congener supplementation. Only a slight decrease of para chlorines was observed, although significant para dechlorination of 2,3,4,5-CB resulted in cultures supplied with Aroclor 1260 and 2,3,4,5-CB. Comparisons of the congener distributions (± standard deviations) for incubations with Aroclor 1260 alone, Aroclor 1260 plus 2,3,4,5-CB, and Aroclor 1260 plus 2,3,5,6-CB are given in Table 2. Figure 2 presents the data for the 2,3,4,5-CB-supplemented cultures in graphical form, including a difference plot. The data demonstrate that meta dechlorination led to substantial increases in 2,4-2,4-CB, 2,4-2,5-CB, and 2,4-2,6-CB and decreases in 2,4,5-2,5-CB, 2,3,6-2,4,5-CB, 2,4,5-2,4,5-CB, 2,3,4-2,4,5-CB, 2,3,4,5-2,3,4-CB, and 2,3,4,5-2,4,5-CB under all conditions. These changes were similar to results reported previously (5, 21). However, 2,3,5,6-2,4-CB, which increased during meta dechlorination of Aroclor 1260 residue in Woods Pond sediment (5), was not detected after 6 months of incubation.
TABLE 2.
Changes in PCB congeners of Aroclor 1260 after 181 days of incubationa
| DB-1 peak no. | PCB congener(s) | Mol% of total PCBs
|
|||
|---|---|---|---|---|---|
| Aroclor 1260 at 0 day | After 181 days
|
||||
| Aroclor 1260 | Aroclor 1260 + 2,3,4,5-CB | Aroclor 1260 + 2,3,5,6-CB | |||
| 7 | 2-3- | 0.01 ± 0.02 | 0.97 ± 0.69 | ||
| 8 | 2,3-; 2-4- | 0.10 ± 0.00 | 0.18 ± 0.01 | 0.32 ± 0.01 | 0.30 ± 0.02 |
| 10 | 2,6-2- | 0.04 ± 0.01 | 0.08 ± 0.06 | ||
| 12 | 3-3- | 0.99 ± 0.08 | 0.12 ± 0.17 | ||
| 13 | 3,4-; 3-4- | 1.80 ± 0.13 | 0.56 ± 0.37 | ||
| 14 | 2,5-2- | 0.14 ± 0.00 | 0.49 ± 0.05 | 1.13 ± 0.02 | 1.23 ± 0.18 |
| 15 | 2,4-2- | 0.05 ± 0.00 | 0.17 ± 0.02 | 0.44 ± 0.02 | 0.37 ± 0.07 |
| 17 | 2,3-2-; 2,6-4- | 0.06 ± 0.00 | 1.02 ± 0.17 | 1.07 ± 0.19 | 0.50 ± 0.18 |
| 19 | 2,6-2,6- | 1.68 ± 0.31 | 1.51 ± 0.37 | ||
| 21 | 2,5-3- | 0.11 ± 0.02 | 2.83 ± 0.19 | 0.80 ± 0.26 | |
| 22 | 2,4-3- | 0.13 ± 0.06 | 6.96 ± 0.16 | 1.52 ± 0.38 | |
| 23 | 2,5-4- | 0.16 ± 0.00 | 0.29 ± 0.02 | 0.67 ± 0.03 | 0.51 ± 0.08 |
| 24 | 2,4-4- | 0.10 ± 0.00 | 1.13 ± 0.14 | 1.03 ± 0.08 | 0.55 ± 0.11 |
| 25 | 2,5-2,6- | 0.08 ± 0.00 | 3.06 ± 0.21 | 4.01 ± 0.20 | 3.07 ± 0.35 |
| 26 | 2,4-2,6- | 9.13 ± 0.63 | 10.17 ± 0.77 | 4.77 ± 2.15 | |
| 28 | 3,5-3- | 0.36 ± 0.05 | 0.11 ± 0.02 | ||
| 29 | 2,3-2,6- | 0.11 ± 0.00 | 0.11 ± 0.01 | 0.15 ± 0.02 | |
| 30 | 3,5-4- | 0.37 ± 0.09 | —c | 0.06 ± 0.01 | |
| 31 | 2,5-2,5- | 0.58 ± 0.01 | 1.30 ± 0.07 | 2.21 ± 0.06 | 5.08 ± 0.28 |
| 32 | 2,4-2,5- | 0.08 ± 0.00 | 7.92 ± 0.58 | 11.05 ± 0.17 | 14.50 ± 0.16 |
| 33 | 2,4-2,4- | 20.11 ± 1.96 | 25.65 ± 0.85 | 24.51 ± 0.97 | |
| 37 | 2,4,6-2,6-; 2,3-2,5- | 0.04 ± 0.00 | 0.44 ± 0.08 | 1.62 ± 0.17 | 0.77 ± 0.15 |
| 38 | 2,3-2,4- | 0.16 ± 0.01 | 0.19 ± 0.08 | 0.54 ± 0.09 | |
| 39 | 2,5-3,5-b; 2,3,6-4- | 0.14 ± 0.00 | 2.25 ± 0.12 | 1.92 ± 0.08 | 1.86 ± 0.18 |
| 40 | 2,4-3,5- | 6.21 ± 0.98 | 2.45 ± 0.12 | 3.32 ± 0.19 | |
| 43 | 2,4,6-2,5- | 1.05 ± 0.11 | 1.50 ± 0.03 | 1.42 ± 0.05 | |
| 44 | 2,4,6-2,4- | 3.81 ± 0.63 | 5.23 ± 0.46 | 2.60 ± 0.60 | |
| 46 | 2,3,5-2,6- | 0.74 ± 0.09 | 0.01 ± 0.01 | ||
| 47 | 2,5-3,4- | 0.06 ± 0.04 | 0.35 ± 0.08 | 1.54 ± 0.18 | |
| 48 | 2,4-3,4-b; 2,3,6-2,5- | 3.32 ± 0.05 | 0.87 ± 0.20 | 0.70 ± 0.05 | 3.54 ± 0.86 |
| 49 | 2,3,6-2,4- | 1.08 ± 0.02 | 0.49 ± 0.10 | 0.70 ± 0.06 | |
| 50 | 2,3-3,4-; 2,3,4-4- | 0.18 ± 0.00 | |||
| 51 | 2,3,6-2,3-; 2,3,5-2,5 | 0.32 ± 0.00 | 0.11 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 |
| 53 | 2,4,5-2,5-; 2,3,5-2,4- | 4.09 ± 0.17 | 0.94 ± 0.21 | 0.20 ± 0.02 | 0.38 ± 0.03 |
| 54 | 2,4,5-2,4- | 0.13 ± 0.09 | 1.25 ± 0.09 | 0.47 ± 0.03 | 0.74 ± 0.05 |
| 55 | 2,4,6-3,4-; 2,3,6-2,4,6- | 1.07 ± 0.05 | 1.43 ± 0.21 | 1.96 ± 0.13 | |
| 57 | 2,4,5-2,3- | 0.13 ± 0.00 | 0.20 ± 0.02 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| 58 | 2,3,4-2,5- | 0.35 ± 0.01 | 0.03 ± 0.00 | ||
| 60 | 2,3,6-2,3,6- | 1.65 ± 0.01 | 0.52 ± 0.21 | 0.77 ± 0.33 | 2.60 ± 0.49 |
| 61 | 2,3,6-3,4- | 1.72 ± 0.02 | 0.35 ± 0.06 | ||
| 62 | 2,4,5-2,4,6- | 0.33 ± 0.05 | 0.58 ± 0.29 | 1.93 ± 0.43 | |
| 64 | 2,3,5,6-2,5- | 3.47 ± 0.03 | 0.69 ± 0.14 | 0.13 ± 0.03 | 0.23 ± 0.05 |
| 65 | 2,3,5-2,3,6- | 1.07 ± 0.01 | 0.34 ± 0.08 | 0.08 ± 0.02 | 0.13 ± 0.04 |
| 66 | 2,3,4,6-2,5- | 0.57 ± 0.01 | |||
| 69 | 2,4,5-3,4-; 2,3,6-2,4,5- | 9.90 ± 0.04 | 2.23 ± 0.54 | 0.68 ± 0.37 | 5.34 ± 1.46 |
| 71 | 2,3,5,6-2,3- | 0.37 ± 0.00 | 0.05 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| 72 | 2,3,4,6-2,3-; 2,3,5-2,3,5- | 0.14 ± 0.00 | 0.08 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 73 | 2,3,5-2,4,5- | 1.33 ± 0.01 | 0.64 ± 0.10 | 0.27 ± 0.03 | 0.37 ± 0.04 |
| 74 | 2,3,4-3,4-; 2,3,4-2,3,6- | 3.28 ± 0.00 | 0.47 ± 0.10 | 0.08 ± 0.02 | 0.12 ± 0.03 |
| 75 | 2,4,5-2,4,5- | 9.74 ± 0.01 | 2.85 ± 0.59 | 0.73 ± 0.15 | 1.31 ± 0.22 |
| 77 | 2,3,4,5-2,5- | 2.90 ± 0.03 | 0.60 ± 0.11 | 0.07 ± 0.04 | 0.13 ± 0.05 |
| 78 | 2,3,5,6-2,3,6- | 2.00 ± 0.03 | 0.83 ± 0.18 | 0.28 ± 0.04 | 0.40 ± 0.07 |
| 80 | 2,3,4,5-2,4-; 2,3,4-2,3,5-; 2,3,4,6-2,3,6- | 0.87 ± 0.00 | 0.23 ± 0.05 | 0.05 ± 0.01 | 0.09 ± 0.02 |
| 82 | 2,3,4-2,4,5-; 2,3,5,6-3,4-; 2,3,6-3,4,5- | 9.94 ± 0.03 | 2.65 ± 0.51 | 0.61 ± 0.17 | 0.93 ± 0.19 |
| 83 | 2,3,4,6-3,4- | 0.23 ± 0.00 | 0.05 ± 0.01 | ||
| 85 | 2,3,5,6-2,3,5- | 0.69 ± 0.01 | 0.20 ± 0.04 | 0.03 ± 0.01 | 0.05 ± 0.01 |
| 87 | 2,3,4,6-2,3,5- | 0.18 ± 0.01 | 0.08 ± 0.01 | 0.01 ± 0.00 | 0.03 ± 0.01 |
| 88 | 2,3,5,6-2,4,5- | 5.15 ± 0.04 | 2.01 ± 0.36 | 0.50 ± 0.12 | 0.75 ± 0.17 |
| 90 | 2,3,4,6-2,4,5- | 2.00 ± 0.02 | 0.65 ± 0.12 | 0.13 ± 0.03 | 0.22 ± 0.05 |
| 91 | 2,4,5-3,4,5- | 0.93 ± 0.06 | 0.52 ± 0.19 | 0.10 ± 0.03 | 0.14 ± 0.03 |
| 92 | 2,3,4,5,6-2,5- | 0.53 ± 0.00 | 0.18 ± 0.03 | 0.04 ± 0.01 | 0.07 ± 0.01 |
| 93 | 2,3,4,5-2,3,6- | 4.94 ± 0.01 | 1.81 ± 0.33 | 0.68 ± 0.31 | 0.70 ± 0.14 |
| 94 | 2,3,5,6-2,3,4 | 2.39 ± 0.01 | 0.81 ± 0.16 | 0.19 ± 0.05 | 0.29 ± 0.06 |
| 95 | 2,3,4,5-3,4-; 2,3,4,6-2,3,4- | 1.82 ± 0.06 | 0.71 ± 0.12 | 0.20 ± 0.05 | 0.12 ± 0.03 |
| 96 | 2,3,4-3,4,5-; 2,3,5,6-2,3,5,6- | 0.32 ± 0.01 | 0.12 ± 0.02 | 0.04 ± 0.03 | |
| 99 | 2,3,4,6-2,3,5,6- | 0.10 ± 0.01 | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 |
| 100 | 2,3,4,5-2,3,5- | 0.72 ± 0.00 | 0.33 ± 0.05 | 0.10 ± 0.03 | 0.15 ± 0.03 |
| 102 | 2,3,4,5-2,4,5- | 9.98 ± 0.06 | 4.87 ± 0.82 | 1.32 ± 0.32 | 2.03 ± 0.40 |
| 103 | 2,3,5,6-3,4,5- | 0.48 ± 0.01 | 0.35 ± 0.04 | 0.13 ± 0.01 | 0.18 ± 0.05 |
| 104 | 2,3,4,6-3,4,5- | 0.20 ± 0.00 | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.00 |
| 105 | 2,3,4,5,6-2,3,6- | 0.30 ± 0.02 | 0.10 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 |
| 106 | 2,3,4,5-2,3,4- | 3.72 ± 0.02 | 1.55 ± 0.25 | 0.48 ± 0.10 | 0.68 ± 0.14 |
| 107 | 2,3,4,5,6-3,4- | 0.67 ± 0.02 | 0.54 ± 0.09 | 0.18 ± 0.04 | 0.25 ± 0.04 |
| 109 | 2,3,4,5-2,3,5,6- | 1.50 ± 0.01 | 0.92 ± 0.12 | 0.30 ± 0.07 | 0.43 ± 0.08 |
| 110 | 2,3,4,5-2,3,4,6-; 2,3,4,5,6-2,4,5- | 1.35 ± 0.05 | 0.89 ± 0.11 | 0.21 ± 0.06 | 0.31 ± 0.07 |
| 111 | 2,3,4,5-3,4,5- | 0.20 ± 0.00 | 0.13 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 |
| 112 | 2,3,4,5,6-2,3,4- | 0.64 ± 0.00 | 0.50 ± 0.05 | 0.19 ± 0.03 | 0.27 ± 0.03 |
| 113 | 2,3,4,5,6-2,3,5,6- | 0.16 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 115 | 2,3,4,5-2,3,4,5- | 1.26 ± 0.04 | 1.30 ± 0.07 | 0.61 ± 0.09 | 0.81 ± 0.08 |
| 117 | 2,3,4,5,6-2,3,4,5- | 0.48 ± 0.01 | 0.49 ± 0.01 | 0.40 ± 0.02 | 0.44 ± 0.01 |
FIG. 2.
Congener distribution of Aroclor 1260 at time zero (T0) and after 181 days (T181) of incubation in incubated cultures supplied with Aroclor 1260 and 2,3,4,5-CB. Averaged data of triplicate samples are presented.
ortho dechlorination was evident in all enrichment cultures by the appearance of dechlorination products 2,4-3,5-CB, 2,5-3,5-CB, 2,4-3-CB, and 2,5-3-CB, which are not in Aroclor 1260 (16, 17). Both 2,4-3,5-CB and 2,5-3,5-CB were identified by GC-MS analysis (see Materials and Methods). Although 2,4-3,5-CB and 2,5-3,5-CB could be products of meta or para dechlorination rather than ortho dechlorination, the quantity of substrate congener for such reactions is far less than the amounts of 2,4-3,5-CB and 2,5-3,5-CB observed. As reported by Frame et al. (16), congeners in Aroclor 1260 which were transformed to 2,4-3,5-CB or 2,5-3,5-CB exclusively by meta and para dechlorination are 2,3,4-3,4,5-CB (0.02 mol%), 2,4,5-3,4,5-CB (0.21 mol%), and 2,3,4,5-3,4,5-CB (0.08 mol%). Far greater than 1.0 mol% each of 2,4-3,5-CB and 2,5-3,5-CB remained in all of our enrichment cultures after 6 months (Fig. 2). However, even higher levels (3.52 to 8.37 mol% of 2,4-3,5-CB and 1.86 to 4.12 mol% of 2,5-3,5-CB) were present in the slurries at earlier times in the experiment. These levels exceed the combined totals of the substrate congeners by more than an order of magnitude. Therefore, the majority of the observed 2,4-3,5-CB and 2,5-3,5-CB in our enrichment cultures is due to ortho dechlorination of Aroclor 1260. As the levels of 2,4-3,5-CB and 2,5-3,5-CB declined, corresponding increases in 2,4-3-CB and 2,5-3-CB, which are not present in Aroclor 1260, were observed. The presence of both 2,4-3-CB and 2,5-3-CB was further supported by GC-MS analysis showing the presence of a molecular ion of m/z 258 for both of these products. The existence of these trichlorobiphenyls confirms the ortho dechlorination that produced 2,4-3,5-CB and 2,5-3,5-CB.
The formation of the non-ortho-chlorinated biphenyls 3-3-CB, 3-4-CB, and 3,5-3-CB (Table 2 and Fig. 2) was observed in sediment slurries incubated with Aroclor 1260 plus 2,3,4,5-CB or 2,3,5,6-CB. In addition, 3,5-4-CB was produced in cultures incubated with only Aroclor 1260 and was further dechlorinated in the other cultures. Since none of these congeners are present in Aroclor 1260 (16, 17), they must be products of ortho dechlorination because all congeners in virgin Aroclor 1260 contain at least one ortho chlorine. 3-4-CB coelutes with 3,4-CB, which is a potential dechlorination product of 2,3,4,5-CB, but we have not observed the formation of 3,4-CB in incubations with only 2,3,4,5-CB. Therefore, 3-4-CB is most likely the result of Aroclor dechlorination. Due to our discount of monochlorobiphenyl production, we do not know whether the non-ortho congeners were further dechlorinated to monochlorobiphenyls. Conversely, we observed ortho-only-chlorinated congener 2,6-2,6-CB at a low mole percent but no 2,6-2-CB or 2,6-CB/2-2-CB was detected, indicating that dechlorination of 2,6-2,6-CB did not occur in our enrichment cultures.
DISCUSSION
meta dechlorination of Aroclor 1260.
Extensive meta dechlorination of Aroclor 1260 in BH sediment resulted in significant decreases of PCBs with 2,3,4-, 2,4,5-, 2,3,4,5-, and 2,3,4,6-chlorophenyl groups and corresponding increases in 2,4- and 2,4,6-chlorophenyl groups. These products are the same as those found in Aroclor 1260-contaminated freshwater sediments that have been exposed to dechlorination Process N (2, 7, 21, 31). Process N is characterized by an almost exclusive loss of flanked meta chlorines (5, 21). No unflanked meta dechlorination of Aroclor 1260 has been reported. In addition to Process N, we observed unflanked meta dechlorination of PCBs (e.g., 2,4-3,5-CB→2,4-3-CB and 2,5-3,5-CB→2,5-3-CB) with our enrichment cultures. We suspect that this is primarily due to the ortho dechlorination preceding the unflanked meta dechlorination in our enrichments. Quensen et al. (21) reported a 19% decrease in the meta and para chlorines of freshly added Aroclor 1260 with anaerobic microorganisms eluted from Silver Lake after a 19-week incubation. Alder et al. (2) demonstrated a 30% removal of meta and para chlorines from freshly added Aroclor 1260 with PCB-contaminated sediment from Silver Lake after an 11-month incubation. In comparison to the aforementioned investigations, our results exhibited more extensive meta dechlorination (up to 65 mol%) in a relatively shorter period of time. This further demonstrates the potential for reductive dechlorination of haloaromatic compounds in estuarine sediments.
ortho dechlorination of Aroclor 1260.
At least six distinct microbial dechlorination processes can be recognized as occurring in various contaminated sediments on the basis of congener selectivity and the products observed in situ and in laboratory studies (5, 10, 12). In all previous reports, PCBs are dechlorinated only by loss of meta and/or para chlorines. Here we have demonstrated the occurrence of ortho dechlorination of Aroclor 1260 added to BH sediment. The results suggest that such activity could play a role in the bioremediation of Aroclors in marine and estuarine sediments. This is the first confirmed report of ortho dechlorination of PCB mixtures, although ortho dechlorination of single congeners has also been reported (9, 19, 28–30). Maximal chlorine removal appears to require the complementary action of two or more dechlorination processes (5, 21). For example, in Process N (flanked meta dechlorination of Aroclor 1260), elevated amounts of 2,3,5,6-2,4-CB are produced by meta dechlorination of 2,3,5,6-2,4,5-CB and 2,3,5,6-2,3,4-CB, and 2,3,5,6-chlorophenyl substituents are recalcitrant in Aroclor 1260 (5). However, no 2,3,5,6-2,4-CB was observed in our slurry enrichments because the 2,3,5,6-2,4,5-CB and 2,3,5,6-2,3,4-CB were ortho and meta dechlorinated to 2,4-3,5-CB and 2,4-3-CB. Thus, the combination of ortho dechlorination plus flanked and unflanked meta dechlorination resulted in more dechlorination than that produced by the flanked meta dechlorination of Process N.
Specificity of ortho dechlorinating activity.
A modest amount of ortho dechlorination was observed in comparison to the amounts of meta dechlorination in all of our enrichment cultures. We hypothesize that the moderate ortho dechlorination of the Aroclor in our enrichments is dependent on the specificity of ortho dechlorinating microorganisms in BH sediment. Previously, we reported on the ortho dechlorination of a few single PCB congeners (9). Among those congeners, ∼99 mol% of 2,3,5-CB, ∼20 mol% of 2,3,6-CB, and ∼92 mol% of 2,3,5,6-CB were ortho dechlorinated. In that report, no ortho dechlorination was observed in BH sediment incubations supplied with 2-CB, 2,3-CB, 2,4-CB, 2,5-CB, 2,6-CB, 2,4,6-CB, 2,6-2,6-CB, or 2,3,4,5-CB over a 6-month period. However, after incubating the cultures for more than a year, we have now observed the ortho dechlorination of 2,4-CB and 2,4,6-CB to 4-CB and a small amount of 2,6-2,6-CB to 2,6-2-CB in enrichment cultures supplied with these single congeners (data not shown). Others have also reported on the ortho dechlorination of unflanked ortho chlorines after extended incubation (29, 30). These results indicate that although some unflanked ortho dechlorination will occur after extended incubation, the ortho dechlorinators in BH sediment favor removal of flanked ortho chlorines, with the exception of 2,3-CB and 2,3,4,5-CB. In Aroclor 1260 (16), only 12 mol% of the congeners bear 2,3,5- and 2,3,5,6-chlorophenyl groups. Congeners carrying 2,3,6-chlorophenyl groups (14 mol%) and 2,3,4-, 2,4,5-, and 2,3,4,5-chlorophenyl groups (55 mol%) are far more prevalent. Therefore, relatively smaller amounts of PCBs with 2,3,5- and 2,3,5,6-substitutions in Aroclor 1260 may explain why only moderate levels of ortho dechlorination of Aroclor 1260 were observed in our BH sediment enrichment cultures. Based on the results presented here and previously with single congeners (9), we propose pathways for the dechlorination of Aroclor 1260 to the major ortho dechlorination products observed in these experiments (Fig. 3).
FIG. 3.
Proposed pathway of meta and ortho dechlorination of PCB congeners in Aroclor 1260 to produce 2,4-3,5-CB, 2,5-3,5-CB, 2,4-3-CB, 2,5-3-CB, and 2-3-CB. Superscript a designates a decreased congener after incubation; superscript b designates a congener appearing after incubation; superscript c designates a proposed intermediate, which could not be identified due to its coelution with 2,4,5-2,4-CB.
Although we did not perform controlled experiments, we have observed in general that PCB dechlorination is more stable, i.e., more extensive and with shorter lag times, when sediments are stored anaerobically at room temperature (20 to 22°C) than at 4°C. K. R. Sowers has also observed this with the storage of sediments from several sites used for methanogenic enrichments. Room temperature storage is a possible explanation for why ortho dechlorination could be activated even after the sediment had been stored for 14 months. Other laboratory studies have revealed that a prolonged storage time of sediment at 4 to 7°C increased the incubation time required to transform 50% of the substrate tested for chlorophenol dechlorination (32) and changed the PCB dechlorination primed by 4-bromobenzoate (25). However, it is also important to note that room temperature storage of an estuarine or marine sediment does not ensure the development of ortho dechlorination. Using identical storage and enrichment conditions, we have not been able to enrich for ortho dechlorination with three of five Charleston Harbor (Charleston, S.C.) sediments and one sediment from the middle of the Chesapeake Bay near the mouth of the Potomac River. meta or para dechlorination developed with each of these sediments (data not shown). Therefore, something specific to the site, perhaps the microbial population, is more critical for the development of ortho dechlorination than storage temperature.
Effect of added 2,3,4,5-CB and 2,3,5,6-CB on dechlorination of Aroclor 1260.
Microbial PCB dechlorination of Aroclor 1260 residue can be primed by the addition of elevated concentrations (200 to 500 μM) of certain PCB congeners (3, 5, 7, 31). Bedard and colleagues (3, 7) found that they could stimulate Process N and Process P (flanked para dechlorination) of Aroclor 1260 residue in Woods Pond sediment by the addition of 2,3,4,5,6-CB and 2,5-3,4-CB, respectively. The addition of 2,3,4,6-CB also stimulated Process N, Process P, and Process LP (unflanked para dechlorination) of Aroclor 1260 residue in Woods Pond sediment and led to a 34% decrease in meta and para chlorines after 12 months of incubation at 25°C (31). Our results indicate that the addition of single PCB congeners (2,3,4,5-CB and 2,3,5,6-CB) stimulates meta and ortho dechlorination of Aroclor 1260 in these sediments (shorter lag time and more extensive dechlorination), further supporting the hypothesis that anaerobic bacteria derive energy by donating electrons to halogenated biphenyls (10, 12, 22).
In summary, anaerobic microorganisms in BH estuarine sediments reductively dechlorinate Aroclor 1260. The dechlorination of Aroclor 1260 is extensive and results in removal of meta and ortho chlorines. The addition of single PCB congeners stimulates the meta and ortho dechlorination of Aroclor 1260. Reviewed together, these results demonstrate that the biocatalytic capability of anaerobic microorganisms to reductively dechlorinate PCBs is broader than previously realized. Such activity could prove useful in the bioremediation of PCBs and awaits testing with PCB-contaminated (aged) sediments.
ACKNOWLEDGMENTS
We thank Donna L. Bedard and Lynn A. Smullen from General Electric Co. for supplying a customized PCB standard.
The work was supported by the Office of Naval Research, U.S. Department of Defense (grant N00014-96-1-0116 to H.D.M. and grant N00014-96-1-0115 to K.R.S.).
REFERENCES
- 1.Abramowicz D A. Aerobic PCB degradation and anaerobic PCB dechlorination in the environment. Res Microbiol. 1994;145:42–46. doi: 10.1016/0923-2508(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 2.Alder A C, Häggblom M M, Oppenheimer S, Young L Y. Reductive dechlorination of polychlorinated biphenyls in anaerobic sediments. Environ Sci Technol. 1993;27:530–538. [Google Scholar]
- 3.Bedard D L, Bunnell S C, Smullen L A. Stimulation of microbial para-dechlorination of polychlorinated biphenyls that have persisted in Housatonic River sediment for decades. Environ Sci Technol. 1996;30:687–694. [Google Scholar]
- 4.Bedard D L, May R J. Characterization of the polychlorinated biphenyls (PCBs) in the sediment of Woods Pond: evidence for microbial dechlorination of Aroclor 1260 in situ. Environ Sci Technol. 1996;30:237–245. [Google Scholar]
- 5.Bedard D L, Quensen J F., III . Microbial reductive dechlorination of polychlorinated biphenyls. In: Young L Y, Cerniglia C, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss Division, John Wiley & Sons, Inc.; 1995. pp. 127–216. [Google Scholar]
- 6.Bedard D L, Unterman R, Bopp L H, Brennan M J, Haberl M L, Johnson C. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol. 1986;51:761–768. doi: 10.1128/aem.51.4.761-768.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedard D L, Van Dort H M, Bunnell S C, Principe L M, DeWeerd K A, May R J, Smullen L A. Anaerobic dehalogenation and its environmental implications, Abstracts of the 1992 American Society Microbiology Conference. Washington, D.C: Office of Research and Development, U.S. Environmental Protection Agency; 1993. Stimulation of reductive dechlorination of Aroclor 1260 contaminant in anaerobic slurries of Woods Pond sediment; pp. 19–21. [Google Scholar]
- 8.Bedard D L, Wagner R E, Brennan M J, Haberl M L, Brown J F., Jr Extensive degradation of Aroclors and environmentally transformed polychlorinated biphenyls by Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987;53:1094–1102. doi: 10.1128/aem.53.5.1094-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkaw M, Sowers K R, May H D. Anaerobic ortho dechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Appl Environ Microbiol. 1996;62:2534–2539. doi: 10.1128/aem.62.7.2534-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J F, Jr, Bedard D L, Brennan M J, Carnahan J C, Feng H, Wagner R E. Polychlorinated biphenyl dechlorination in aquatic sediments. Science. 1987;236:709–712. doi: 10.1126/science.236.4802.709. [DOI] [PubMed] [Google Scholar]
- 11.Brown J F, Jr, Wagner R E. PCB movement, dechlorination, and detoxication in the Acushnet estuary. Environ Toxicol Chem. 1990;9:1215–1233. [Google Scholar]
- 12.Brown J F, Jr, Wagner R E, Feng H, Bedard D L, Brennan M J, Carnahan J C, May R J. Environmental dechlorination of PCBs. Environ Toxicol Chem. 1987;6:579–593. [Google Scholar]
- 13.Commandeur L C M, May R J, Mokross H, Bedard D L, Reineke W, Govers H A J, Parsons J R. Aerobic degradation of polychlorinated biphenyls by Alcaligenes sp. JB1: metabolites and enzymes. Biodegradation. 1996;7:435–443. doi: 10.1007/BF00115290. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner D J. Marine natural products. Nat Prod Rep. 1995;12:223–269. doi: 10.1039/np9900700269. [DOI] [PubMed] [Google Scholar]
- 15.Focht D D. Microbial degradation of chlorinated biphenyls. In: Bollag J M, Stotzky G, editors. Soil biochemistry. Vol. 8. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 341–400. [Google Scholar]
- 16.Frame G M, Cochran J W, Bøwadt S S. Complete PCB congener distributions for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J High Resolut Chromatogr. 1996;19:657–668. [Google Scholar]
- 17.Frame G M, Wagner R E, Carnahan J C, Brown J F, Jr, May R J, Smullen L A, Bedard D L. Comprehensive quantitative congener-specific analyses of eight Aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere. 1996;33:603–623. [Google Scholar]
- 18.King G M. Dehalogenation in marine sediments containing natural sources of halophenols. Appl Environ Microbiol. 1988;54:3079–3085. doi: 10.1128/aem.54.12.3079-3085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery L, Vogel T M. Dechlorination of 2,3,5,6-tetrachlorobiphenyl by a phototrophic enrichment culture. FEMS Microbiol Lett. 1992;94:247–250. doi: 10.1016/0378-1097(92)90638-5. [DOI] [PubMed] [Google Scholar]
- 20.Øfjord G D, Puhakka J A, Ferguson J F. Reductive dechlorination of Aroclor 1254 by marine sediment cultures. Environ Sci Technol. 1994;28:2286–2294. doi: 10.1021/es00062a012. [DOI] [PubMed] [Google Scholar]
- 21.Quensen J F, III, Boyd S A, Tiedje J M. Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Appl Environ Microbiol. 1990;56:2360–2369. doi: 10.1128/aem.56.8.2360-2369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quensen J F, III, Tiedje J M, Boyd S A. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science. 1988;242:752–754. doi: 10.1126/science.242.4879.752. [DOI] [PubMed] [Google Scholar]
- 23.Quensen J F, III, Tiedje J M, Boyd S A, Enke C, Lopshire R, Giesy J, Mora M, Crawford R, Tillitt D. International Symposium on Soil Decontamination using Biological Processes, Karlsruhe, Germany (December 1992). Frankfurt am Main, Germany: Dechema; 1992. Evaluation of the suitability of reductive dechlorination for the bioremediation of PCB-contaminated soils and sediments; pp. 91–100. [Google Scholar]
- 24.Smullen L A, DeWeerd K A, Bedard D L, Fessler W A, Carnahan J C, Wagner R E. Twelfth Progress Report of Research and Development Program for the Destruction of PCBs. Schenectady, N.Y: General Electric Co. Corporate Research and Development; 1993. Development of a customized congener specific PCB standard for quantification of Woods Pond sediment PCBs; pp. 45–66. [Google Scholar]
- 25.Stokes R W, Deweerd K A, Bedard D L. Thirteenth Progress Report of Research and Development Program for the Destruction of PCBs. Schenectady, N.Y: General Electric Co. Corporate Research and Development; 1994. Variables affecting the microbial dechlorination Aroclor 1260 in Woods Pond sediment slurries stimulated with 4-bromobenzoate; pp. 27–41. [Google Scholar]
- 26.Sylvestre M, Sondossi M. Selection of enhanced polychlorinated biphenyl-degrading bacterial strains for bioremediation: consideration of branching pathways. In: Chaudry G R, editor. Biological degradation and bioremediation of toxic chemicals. Portland, Oreg: Discorides Press; 1994. pp. 47–73. [Google Scholar]
- 27.Technical and Regulatory Services Administration of the Maryland Department of the Environment. Toxics Regional Action Plan for Baltimore Harbor. Baltimore: Technical and Regulatory Services Administration of the Maryland Department of the Environment; 1996. [Google Scholar]
- 28.Van Dort H M, Bedard D L. Reductive ortho and meta dechlorination of a polychlorinated biphenyl congener by anaerobic microorganisms. Appl Environ Microbiol. 1991;57:1576–1578. doi: 10.1128/aem.57.5.1576-1578.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams W A. Microbial reductive dechlorination of trichlorophenyls in anaerobic slurries. Environ Sci Technol. 1994;28:630–635. doi: 10.1021/es00053a015. [DOI] [PubMed] [Google Scholar]
- 30.Wu Q, Bedard D L, Wiegel J. Effect of incubation temperature on the route of microbial reductive dechlorination of 2,3,4,6-tetrachlorobiphenyl in polychlorinated biphenyl (PCB)-contaminated and PCB-free freshwater sediments. Appl Environ Microbiol. 1997;63:2836–2843. doi: 10.1128/aem.63.7.2836-2843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Q, Bedard D L, Wiegel J. Temperature determines the pattern of anaerobic microbial dechlorination of Aroclor 1260 primed by 2,3,4,6-tetrachlorobiphenyl in Woods Pond sediments. Appl Environ Microbiol. 1997;63:4818–4825. doi: 10.1128/aem.63.12.4818-4825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, X., and J. Wiegel. Personal communication.