Abstract
Increased particulate matter (PM) exposure is positively associated with increased incidence and mortality of many human malignancies. However, evidence of urologic cancer is limited. We aimed to evaluate the association between PM10 exposure and the relative risk of urologic cancer. This nationwide longitudinal cohort study included 231,997 participants who underwent a baseline health examination in 2008 from the National Health Information Database of Korea. The primary endpoint was newly diagnosed urologic cancer according to PM10 exposure. Of the total 231,99 participants, 50,677 developed urologic cancer during a median follow-up of 6.7 years. After controlling for confounding factors, participants in the high PM10 exposure group had a higher risk of kidney cancer (hazard ratio [HR] 1.111, 95% confidence interval [CI] 1.068-1.157) and prostate cancer (HR 1.083, 95% CI 1.058-1.109) than those in the low PM10 exposure group. However, in urothelial cell carcinoma, there was no significant increase in the HRs in the high PM10 exposure group. For kidney cancer, participants with the following characteristics were more susceptible: age < 65 years, female sex, decreased regular physical activity, current smoking, no diabetes, no hypertension, normal body mass index, and desirable total cholesterol level. For prostate cancer, participants with the following characteristics were more susceptible: decreased regular physical activity, current smoking, and no hypertension. High PM10 exposure is associated with an increased risk of overall urologic cancers, especially kidney and prostate cancer.
Keywords: Urologic cancer, particulate matter exposure, kidney cancer, prostate cancer, urothelial cell carcinoma
Introduction
Air pollution has been associated with detrimental effects on human health including increased risk of premature mortality, cardiovascular and respiratory diseases, adverse birth outcomes, and several cancers [1,2]. The World Health Organization has reported that ambient air pollution and household air pollution are associated with 7 million premature deaths annually, and has declared air pollution as the “silent killer” [3]. Air pollution is a complex mixture of pollutants including sulfur oxides, nitrogen oxides, ambient ozone, and particulate matter (PM). PM, a mixture of toxic and carcinogenic substances, is classified by the aerodynamic diameter of particles: PM10 is < 10 μm in diameter; PM2.5 is < 2.5 μm; PM0.1 particles are < 0.1 μm. PM can easily penetrate the respiratory system and enter the blood, causing extensive inflammatory reactions, endothelial cell damage, increased oxidative stress, and ultimately carcinogenesis [4-6].
Ambient PM has been designated as a Group 1 human carcinogen by the International Agency for Research on Cancer (IARC) in 2013 [7]. In a meta-analysis originated by the IARC, the relative risk for lung cancer associated with PM2.5 and PM10 was 1.09 (95% confidential interval [CI]: 1.04, 1.14), and 1.08 (95% CI: 1.00, 1.17), respectively [8]. In addition, a growing body of evidence has demonstrated that increased PM exposure is positively associated with the increased incidence and mortality of many human malignancies including breast cancer [9,10], colorectal cancer [11], thyroid cancer [12], liver cancer [13], and childhood cancer [14]. Although there is sufficient evidence regarding the association between PM exposure and human malignancies, evidence for urologic cancer is limited [15]. Thus, in this nationwide longitudinal cohort study, we aimed to evaluate the association between PM10 exposure and the relative risks of urologic cancers such as kidney cancer, prostate cancer, and urothelial cell carcinoma.
Methods
Data source
We used a customized database (DB) from the National Health Information (NHI) DB of the National Health Insurance Service (NHIS), which covers nearly the entire South Korean population. The NHIS maintains a health examination database for all insured persons in South Korea consisting of anthropometric measurements, laboratory tests, and self-report questionnaires. The NHI DB includes qualification information, insurance rates, health check-up results, treatment details, long-term care insurance data for the elderly, treatment status, cancer, and rare disease registration information. The NHIS has provided data including a sample cohort DB, customized DB, health disease index for academic research in the health and medical sector.
Study population
This study received exemption status from the Institutional Review Board at Seoul St. Mary’s Hospital and Dankook University. Participants who underwent a baseline health examination in 2008 were included in the NHI DB (N=10,581,265). We excluded participants with any cancer within the first year from the index data (N=20,961), those with no data regarding air pollution between 2005 and 2007 (N=2,010,736), those who did not receive more than four consecutive health examinations from 2010 to 2018 (N=6,226,437), and those with missing data on health examination (N=2,058,371). A total of 231,997 participants were included in the final analysis (Figure 1). The primary endpoint was newly diagnosed urologic cancer (N=50,677). Figure 2 is a diagram to help understand the participant timeline.
Figure 1.
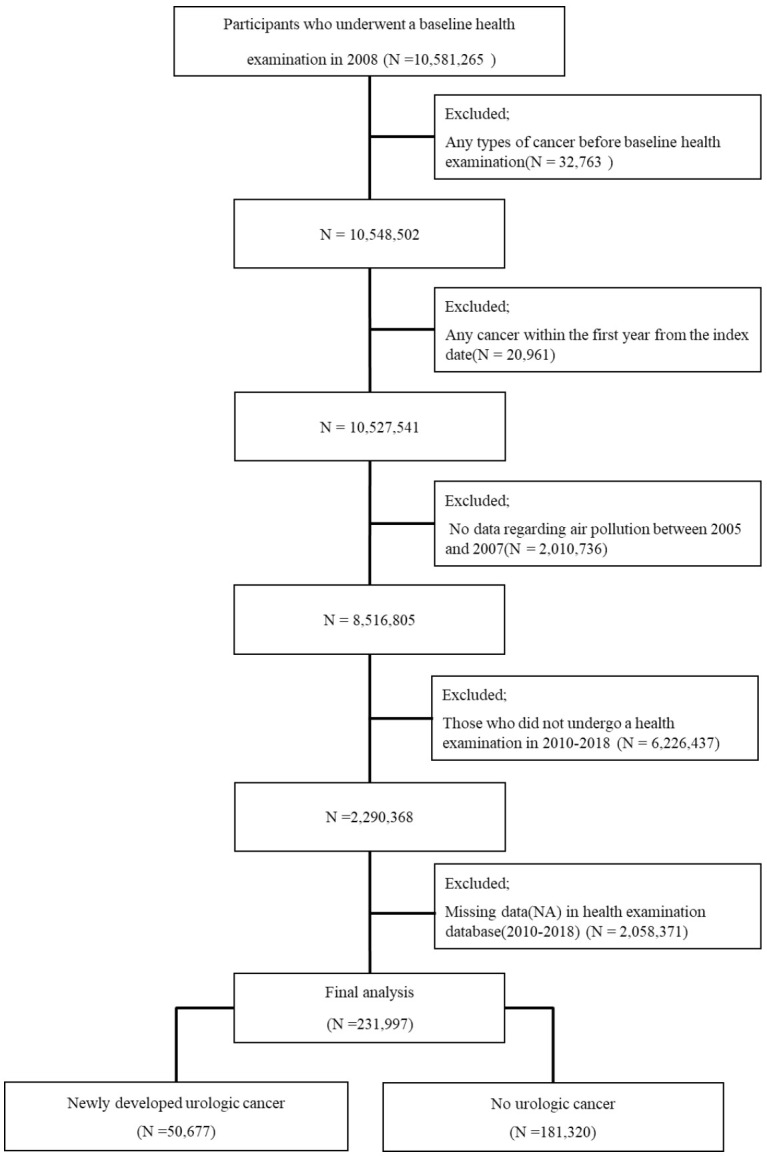
Flowchart of participant inclusion.
Figure 2.
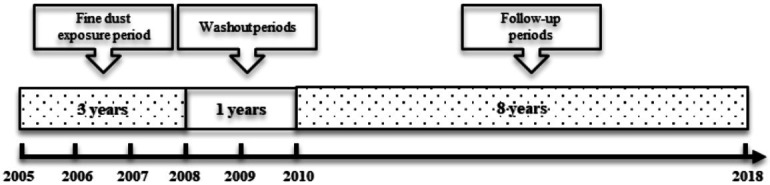
Diagram for a timeline of participants.
Definition of urologic cancer
We focused on newly diagnosed urologic cancers, such as kidney cancer, prostate cancer, and urothelial cell carcinoma. Kidney cancer was defined as C64x according to ICD-10 codes. Prostate cancer was defined as C61 and urothelial cell carcinoma was defined as C65x, C66x, and C67 according to ICD-10 codes. Among the newly diagnosed urologic cancer cases (N=50,677), 9,736 were kidney cancer (19.2%), 28,440 were prostate cancers (56.1%), and 12,501 were urothelial cell carcinoma (24.6%).
Estimation of individual particulate matter exposure
We used air pollution data from AirKorea provided by the Korean Environment Corporation which collects local pollution information through the National Ambient Air Quality Monitoring Information System and provides it through the AirKorea website [16]. In South Korea, PM10 standard was newly established and has been applied since 1995. In 2008, 426 measuring stations were installed nationwide (as of the end of December 2008) [17].
Covariate assessment
Regular physical activity was divided into three groups: no exercise, 1-4 times per week, and ≥ 5 days per week. Alcohol consumption was divided into three groups: non-drinkers, drinkers (2-8 times per month), and heavy drinkers (≥ 4 times per week). There were three groups of smokers: non-smokers, ex-smokers, and smokers. Diabetes mellitus (DM) and hypertension were classified according to the presence or absence of disease using the ICD-10 codes. There were three groups of body mass index (BMI): below 25 (underweight and normal), 25-29.9 (overweight), and over 30 kg/m2 (obesity) [18]. There were three groups of fasting blood glucose levels: normal, pre-diabetic, and diabetic [19]. There were four groups of total cholesterol: desirable level (< 200 mg/dL), borderline high level (200-239 mg/dL), and high level (≥ 240 mg/dL) [20].
Statistical analysis
Statistical analyses were performed using R version 4.0.3 (2020-10-10) and SPSS Statistics version 25.0 (SPSS Inc.). R was used for data preprocessing and visualization of the PM10 data distribution. The baseline demographic data of the participants were compared using the independent t-test for continuous variables and the chi-squared test for categorical variables. The incidence rates of urologic cancer were calculated per 1,000 person-years. The association between the incidence of urologic cancer and PM10 exposure was assessed using multivariate Cox proportional hazards regression analysis. All statistical tests were two-tailed, and the significance level was set at P-values < 0.05.
Results
Baseline statistics of air pollutant
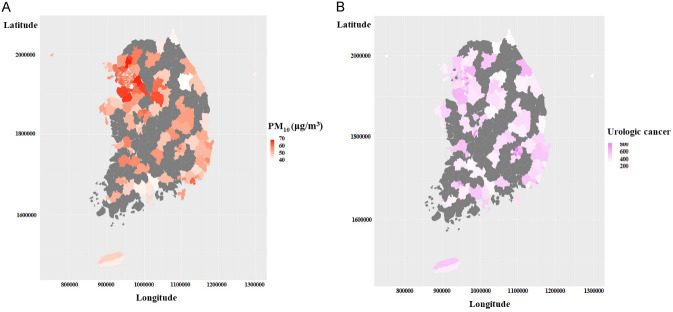
We used 3-year average PM10 concentration data measured at 398 stations from 2005 to 2007 (based on 2008). To categorize the participants according to PM10 concentration, we explored the PM10 concentration data. The mean and median PM10 concentration were 56.24 and 56 μg/m3, respectively. The skewness and kurtosis were 0.133 and 0.139, respectively (Figure 3). There are four criteria for PM10 concentrations in South Korea: good (0-30 μg/m3), moderate (31-80 μg/m3), unhealthy (81-150 μg/m3), and very unhealthy (> 150 μg/m3) [21]. Due to the nature of the data, it was not possible to classify the participants according to the Korean criteria for PM10 concentration. Considering the characteristics of the data regarding PM10 concentration, the participants were divided into two groups according to the median concentration of PM10 (56 μg/m3). The location-specific distribution of urologic cancer incidence and PM10 concentration in South Korea showed similar patterns as shown in Figure 4.
Figure 3.
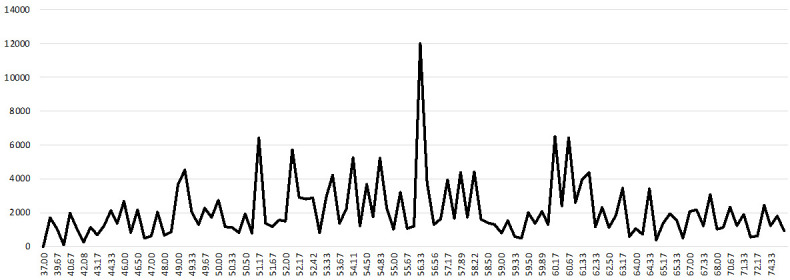
Distribution of PM10 concentration. X-axis: PM10 concentration (μg/m3), Y-axis: frequency.
Figure 4.
Distribution of (A) PM10 concentration and (B) incidence of urologic cancer in South Korea.
Baseline characteristics of the participants
Of the total 231,99 participants, 21.8% developed urologic cancer during the median follow-up of 6.7 years (Table 1). Of the total 50,677 urologic cancer patients, 56.1% had prostate cancer, 24.7% had urothelial cell carcinoma, and 19.2% had kidney cancer. The age ratio of those aged ≥ 65 years was 49.5%, similar to that of those aged < 60 years. The proportion of female was 77.3%. Fifty percent of participants did not exercise regularly, 56.8% of the total did not drink alcohol. 60.7% of the total were non-smokers. Only 28.5% had hypertension and 12.4% had DM.
Table 1.
Demographics characteristics
| Variables | N | % | |
|---|---|---|---|
| Class | No urologic cancer | 181,320 | 78.2 |
| Newly developed urologic cancer | 50,677 | 21.8 | |
| PM10 exposure | Low PM10 group (Under 56 μg/m3) | 123,081 | 53.1 |
| High PM10 group (Over 56 μg/m3) | 108,916 | 46.9 | |
| Age | < 65 years | 117,210 | 50.5 |
| ≥ 65 years | 114,787 | 49.5 | |
| Gender | Male | 179,255 | 77.3 |
| Female | 52,742 | 22.7 | |
| Regular physical activity | No exercise | 116,041 | 50.0 |
| 1-4 times per week | 82,687 | 35.6 | |
| 5 or more times per week | 33,269 | 14.3 | |
| Alcohol consumption | No Alcohol | 131,840 | 56.8 |
| 2-8 times per month | 66,146 | 28.5 | |
| 4 or more times per week | 34,011 | 14.7 | |
| Smoking | Non-smoker | 140,864 | 60.7 |
| Ex-smoker | 30,289 | 13.1 | |
| Smoker | 60,844 | 26.2 | |
| Hypertension | No | 165,945 | 71.5 |
| Yes | 66,052 | 28.5 | |
| Diabetes mellitus | No | 203,175 | 87.6 |
| Yes | 28,822 | 12.4 | |
| Death | No | 193,334 | 83.3 |
| Yes | 38,663 | 16.7 | |
| BMI (kg/m2) | Less than 25 | 154,449 | 66.6 |
| 25-29.9 | 70,804 | 30.5 | |
| Over 30 | 6,744 | 2.9 | |
| Fasting blood glucose | Normal | 128,388 | 55.3 |
| Pre-diabetic | 71,372 | 30.8 | |
| Diabetic | 32,237 | 13.9 | |
| sBP | Less than 120: Healthy | 82,933 | 35.7 |
| 120-129: Elevated | 57,366 | 24.7 | |
| 130=139: Stage1 hypertension | 48,592 | 20.9 | |
| 140 or higher: Stage2 hypertension | 43,106 | 18.6 | |
| dBP | Less than 80: Normal | 156,814 | 67.6 |
| 80-89: Prehypertension | 55,550 | 23.9 | |
| 90 or higher: hypertension | 19,633 | 8.5 | |
| Total cholesterol | Less than 200: Desirable level | 143,028 | 61.7 |
| 200-239: Borderline High Level | 66,143 | 28.5 | |
| 240 and above: High level | 22,826 | 9.8 | |
| Total | 231,997 | 100 | |
BMI, body mass index; sBP, systolic blood pressure; dBP, diastolic blood pressure.
We divided the extracted data into two groups based on PM10 concentration (Table 2). According to the PM10 concentration, there were significant differences in almost all baseline characteristics. Regardless of the occurrence of urological cancer, the proportion of participants older than 65 years and males was significantly higher in the high PM10 group. The prevalence of comorbidities such as DM and hypertension was also higher in the high PM10 group.
Table 2.
Demographics according to the PM10 exposure
| Variables | No urologic cancer | Newly developed urologic cancer | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Low PM10 | High PM10 | p-value | Low PM10 | High PM10 | p-value | ||
| No. of patients | 97,330 (100%) | 83,990 (100%) | 181,320 | 25,751 (100%) | 24,926 (100%) | 50,677 | |
| Age | < 65 years | 41,406 (42.5) | 38,708 (46.1) | 0.000 | 18,576 (72.1) | 18,520 (74.3) | 0.000 |
| ≥ 65 years | 55,924 (57.5) | 45,282 (53.9) | 7,175 (27.9) | 6,406 (25.7) | |||
| Gender | Male | 71,711 (73.7) | 62,462 (74.4) | 0.000 | 22,835 (88.7) | 22,247 (89.3) | 0.039 |
| Female | 25,619 (26.3) | 21,528 (25.6) | 2,916 (11.3) | 2,679 (10.7) | |||
| Regular physical activity | No | 52,107 (53.5) | 43,077 (51.3) | 0.000 | 10,626 (41.3) | 10,231 (41.0) | 0.000 |
| Yes: 1-4 times per week | 31,087 (31.9) | 28,596 (34) | 11,471 (44.5) | 11,533 (46.3) | |||
| Yes: 5 or more times per week | 14,136 (14.5) | 12,317 (14.7) | 3,654 (14.2) | 3,162 (12.7) | |||
| Smoking | Non-smoker | 61,017 (62.7) | 51,203 (61) | 0.000 | 14,822 (57.6) | 13,822 (55.5) | 0.000 |
| Ex-smoker | 11,345 (11.7) | 10,649 (12.7) | 4,075 (15.8) | 4,220 (16.9) | |||
| Smoker | 24,968 (25.7) | 22,138 (26.4) | 6,854 (26.6) | 6,884 (27.6) | |||
| Alcohol consumption | No | 59,228 (60.9) | 49,182 (58.6) | 0.000 | 12,149 (47.2) | 11,281 (45.3) | 0.000 |
| Yes: 2-8 times per month | 23,737 (24.4) | 22,545 (26.8) | 9,806 (38.1) | 10,058 (40.4) | |||
| Yes: 4 or more times per week | 14,365 (14.8) | 12,263 (14.6) | 3,796 (14.7) | 3,587 (14.4) | |||
| Diabetes mellitus | No | 84,048 (86.4) | 72,219 (86) | 0.023 | 23,829 (92.5) | 23,079 (92.6) | 0.000 |
| Yes | 13,282 (13.6) | 11,771 (14) | 1,922 (7.5) | 1,847 (7.4) | |||
| Hypertension | No | 68,976 (70.9) | 58,093 (69.2) | 0.000 | 19,775 (76.8) | 19,101 (76.6) | 0.817 |
| Yes | 28,354 (29.1) | 25,897 (30.8) | 5,976 (23.2) | 5,825 (23.4) | |||
| BMI | 25 | 82,896 (85.2) | 71,553 (85.2) | 0.000 | 16,272 (63.2) | 15,394 (61.8) | 0.001 |
| 25-29.9 | 36,796 (37.8) | 34,008 (40.5) | 8,823 (34.3) | 8,808 (35.3) | |||
| Over 30 | 3,389 (3.5) | 3,355 (4) | 656 (2.5) | 724 (2.9) | |||
| sBP | Less than 120: Healthy | 34,474 (35.4) | 28,686 (34.2) | 0.000 | 10,113 (39.3) | 9,660 (38.8) | 0.000 |
| 120-129: Elevated | 23,916 (24.6) | 20,272 (24.1) | 6,882 (26.7) | 6,296 (25.3) | |||
| 130=139: Stage1 hypertension | 20,293 (20.8) | 18,002 (21.4) | 5,136 (19.9) | 5,161 (20.7) | |||
| 140 or higher: Stage2 hypertension | 18,647 (19.2) | 17,030 (20.3) | 3,620 (14.1) | 3,809 (15.3) | |||
| dBP | Less than 80: Normal | 66,567 (68.4) | 56,260 (67) | 0.000 | 17,545 (68.1) | 16,442 (66) | 0.000 |
| 80-89: Prehypertension | 22,686 (23.3) | 20,230 (24.1) | 6,298 (24.5) | 6,336 (25.4) | |||
| 90 or higher: hypertension | 8,077 (8.3) | 7,500 (8.9) | 1,908 (7.4) | 2,148 (8.6) | |||
| Fasting blood sugar | Normal | 52,389 (53.8) | 45,635 (54.3) | 0.078 | 15,367 (59.7) | 14,997 (60.2) | 0.093 |
| Pre diabetic | 30,211 (31) | 25,702 (30.6) | 7,846 (30.5) | 7,613 (30.5) | |||
| Diabetic (125 or higher) | 14,730 (15.1) | 12653 (15.1) | 2,538 (9.9) | 2,316 (9.3) | |||
| Total Cholesterol (mg/dL) | Less than 200: Desirable level | 61,110 (62.8) | 51715 (61.6) | 0.000 | 15,462 (60) | 14,741 (59.1) | 0.093 |
| 200-239: Borderline High Level | 26,690 (27.4) | 23856 (28.4) | 7,818 (30.4) | 7,779 (31.2) | |||
| 240 and above: High level | 9,530 (9.8) | 8419 (10) | 2,471 (9.6) | 2,406 (9.7) | |||
BMI, body mass index; sBP, systolic blood pressure; dBP, diastolic blood pressure.
Risk of urologic cancer according to the PM10 exposure
Table 3 presents the HRs (95% CIs) for urologic cancer according to PM10 concentrations. High PM10 exposure was associated with an increased overall risk of urologic cancer. It was observed that the HRs for urologic cancer in the high PM10 exposure group increased significantly after adjusting for confounding variables (P for trend < 0.001): over 56 μg/m3 (Model 1: 1.068, 1.050-1.087; Model 2: 1.604, 1.046-1.083; Model 3: 1.604, 1.046-1.083).
Table 3.
Hazard ratios of urologic cancer according to the PM10 exposure
| Cancer | PM10 concentration (unit: μg/m3) | Event | Person-years | Incidence ratea | HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Model 1b | Model 2c | Model 3d | |||||
| Urologic cancer (N=50,677, 100%) | < 56 | 25,751 | 799,101 | 32.225 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| ≥ 56 | 24,926 | 706,259 | 35.293 | 1.068 (1.050, 1.087) | 1.064 (1.046, 1.083) | 1.064 (1.046, 1.083) | |
| P for trend | < 0.001 | < 0.001 | < 0.001 | ||||
| Kidney cancer (N=9,736, 19.2%) | < 56 | 4,817 | 660,560 | 7.292 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| ≥ 56 | 4,919 | 573,552 | 8.576 | 1.122 (1.064, 1.115) | 1.113 (1.070, 1.158) | 1.111 (1.068, 1.157) | |
| P for trend | < 0.001 | < 0.001 | < 0.001 | ||||
| Prostate cancer (N=28,440, 56.1%) | < 56 | 14,379 | 560,576 | 25.650 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| ≥ 56 | 14,061 | 496,225 | 28.336 | 1.089 (1.064, 1.115) | 1.083 (1.058, 1.108) | 1.083 (1.058, 1.109) | |
| P for trend | < 0.001 | < 0.001 | < 0.001 | ||||
| Urothelial cancer (N=12,501, 24.7%) | < 56 | 6,555 | 671,750 | 9.758 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| ≥ 56 | 5,946 | 579,292 | 10.264 | 1.032 (0.996, 1.069) | 1.027 (0.992, 1.064) | 1.029 (0.993, 1.066) | |
| P for trend | n.s | n.s | n.s | ||||
Incidence per 1,000 person-years.
Model 1 was adjusted for age and gender.
Model 2 was adjusted for age, gender, regular activity smoking status, and alcohol consumption.
Model 3 was adjusted for age, gender, regular activity, smoking status, alcohol consumption, diabetes mellitus, and hypertension.
HR, hazard ratio; CI, confidence interval.
We found similar results for kidney and prostate cancers (P for trend < 0.001). For kidney cancer, participants in the high PM10 exposure group had a higher risk than those in the low PM10 exposure group: over 56 μg/m3 (Model 1: 1.122, 1,064-1.115; Model 2: 1.113, 1.070-1.158; Model 3: 1.111, 1.068-1.157). For prostate cancer, participants in the high PM10 exposure group had a higher risk than those in the low PM10 exposure group: over 56 μg/m3 (Model 1: 1.089, 1.064-1.115; Model 2: 1.083, 1.058-1.108; Model 3: 1.083, 1.058-1.109). However, in urothelial cell carcinoma, there was no significant increase in the HRs in the high PM10 exposure group.
Risk of urologic cancer in clinically relevant subgroup
We conducted subgroup analysis based on several clinically relevant variables (Table 4). We observed the same trend of increasing risk of kidney and prostate cancer in participants in the high PM10 group compared with those in the low PM10 group. For kidney cancer, participants with the following characteristics were more susceptible to these associations: age < 65 years, female sex, decreased regular physical activity, current smoking, no DM, no hypertension, normal BMI, and desirable total cholesterol level. For prostate cancer, participants with the following characteristics were more susceptible to these associations: decreased regular physical activity, current smoking, and no hypertension.
Table 4.
Hazard ratios (95% CI) of urologic cancer according to the PM10 exposure in clinically relevant subgroups
| Variables | Kidney cancer | Prostate cancer | ||
|---|---|---|---|---|
|
|
|
|||
| Low PM10 | High PM10 | Low PM10 | High PM10 | |
|
|
|
|||
| < 56 μg/m3 | ≥ 56 μg/m3 | < 56 μg/m3 | ≥ 56 μg/m3 | |
| Age | ||||
| < 65 years | 1 (ref.) | 1.135 (1.088, 1.185)*** | 1 (ref.) | 1.077 (1.048, 1.108)*** |
| ≥ 65 years | 1 (ref.) | 1.051 (0.943, 1.173) | 1 (ref.) | 1.119 (1.072, 1.168)*** |
| Gender | ||||
| Male | 1 (ref.) | 1.176 (1.122, 1.233)*** | 1 (ref.) | 1.118 (1.093, 1.145)*** |
| Female | 1 (ref.) | 1.214 (1.128, 1.306)*** | ||
| Regular physical activity | ||||
| No | 1 (ref.) | 1.300 (1.224, 1.381)*** | 1 (ref.) | 1.146 (1.104, 1.189)*** |
| Yes: 1-4 times per week | 1 (ref.) | 1.083 (1.021, 1.148)** | 1 (ref.) | 1.104 (1.067, 1.142)*** |
| Yes: 5 or more times per week | 1 (ref.) | 1.084 (0.958, 1.226) | 1 (ref.) | 1.021 (0.960, 1.085) |
| Alcohol consumption | ||||
| No | 1 (ref.) | 1.183 (1.117, 1.252)*** | 1 (ref.) | 1.113 (1.075, 1.153)*** |
| Yes: 2-8 times per month | 1 (ref.) | 1.148 (1.079, 1.221)*** | 1 (ref.) | 1.114 (1.074, 1.156)*** |
| Yes: 4 or more times per week | 1 (ref.) | 1.182 (1.049, 1.331)** | 1 (ref.) | 1.085 (1.023, 1.151)** |
| Smoking | ||||
| Non-smoker | 1 (ref.) | 1.177 (1.118, 1.240)*** | 1 (ref.) | 1.094 (1.061, 1.129)*** |
| Ex-smoker | 1 (ref.) | 1.116 (0.996, 1.250)* | 1 (ref.) | 1.129 (1.070, 1.191)*** |
| Smoker | 1 (ref.) | 1.238 (1.149, 1.333)*** | 1 (ref.) | 1.164 (1.111, 1.220)*** |
| Diabetes mellitus | ||||
| No | 1 (ref.) | 1.196 (1.147, 1.246)*** | 1 (ref.) | 1.117 (1.090, 1.144)*** |
| Yes | 1 (ref.) | 1.097 (0.939, 1.282) | 1 (ref.) | 1.147 (1.052, 1.251)** |
| Hypertension | ||||
| No | 1 (ref.) | 1.227 (1.174, 1.284)*** | 1 (ref.) | 1.138 (1.108, 1.169)*** |
| Yes | 1 (ref.) | 1.087 (0.998, 1.185)* | 1 (ref.) | 1.075 (1.025, 1.127)** |
| BMI | ||||
| 25 | 1 (ref.) | 1.207 (1.144, 1.273)*** | 1 (ref.) | 1.127 (1.095, 1.161)*** |
| 25-29.9 | 1 (ref.) | 1.142 (1.072, 1.217)*** | 1 (ref.) | 1.086 (1.044, 1.130)*** |
| Over 30 | 1 (ref.) | 1.130 (0.948, 1.347) | 1 (ref.) | 1.192 (1.006, 1.411)** |
| Fasting blood sugar | ||||
| Normal | 1 (ref.) | 1.204 (1.145, 1.266)*** | 1 (ref.) | 1.113 (1.080, 1.147) |
| Pre diabetic | 1 (ref.) | 1.197 (1.111, 1.289)*** | 1 (ref.) | 1.131 (1.085, 1.179) |
| Diabetic (125 or higher) | 1 (ref.) | 1.026 (0.898, 1.173) | 1 (ref.) | 1.100 (1.020, 1.187) |
| Total cholesterol | ||||
| Less than 200: Desirable level | 1 (ref.) | 1.244 (1.181, 1.311)*** | 1 (ref.) | 1.119 (1.086, 1.153)*** |
| 200-239: Borderline High Level | 1 (ref.) | 1.119 (1.042, 1.201)** | 1 (ref.) | 1.108 (1.063, 1.156)*** |
| 240 and above: High level | 1 (ref.) | 1.061 (0.940, 1.198) | 1 (ref.) | 1.104 (1.021, 1.194)** |
HR, hazard ratio; CI, confidence interval. HRs (95% CIs) were obtained using multivariable Cox proportional hazard regression analysis after adjusting age, gender, regular physical activity, alcohol consumption, smoking, diabetes mellitus, hypertension, BMI, fasting blood sugar, and total cholesterol.
P < 0.05;
P < 0.01;
P < 0.001.
Discussion
Considering the rapidly urbanizing world, it is becoming increasingly important to evaluate air pollution as a determinant of cancer, especially with respect to the reduction of some traditional risk factors, such as smoking, workplace carcinogens, and solid fuel, which may be improved over time with various smoking control activities, workplace safety measures, and socioeconomic development. In this respect, there were several important observations in our study regarding the association of urologic cancer and PM10 exposure. We found that high PM10 exposure was associated with an increased risk of overall urologic cancer, especially kidney and prostate cancers. We also found that the increased risk of kidney and prostate cancer related to high PM10 exposure was significantly augmented among participants with current smoking, female sex (only in kidney cancer), and no features of metabolic disorders, and decreased among participants with regular physical activity five or more times per week.
Currently available studies on the association between the risk of urologic cancer and air pollution have reported conflicting results. Fourteen cohorts from the European Study of Cohorts for Air Pollution Effects study demonstrated higher HRs for kidney parenchyma cancer in association with higher PM2.5 concentrations (HRs 1.57, 95% CI 0.81-3.01, per 5 μg/m3 PM2.5), although not statistically significant [22]. In a population-based study in Shanghai, China, waste gas emissions were positively associated with multiple cancers, incidence including kidney, bladder, and prostate cancer [23]. In a recent Canadian case-control study, positive associations were found between exposure to PM2.5 and NO2 over the previous 20 years and prostate cancer [24]. However, a Danish cohort study that investigated the association between NO2 and 20 selected cancers reported an association between NO2 exposure and increased risk of cervical and brain cancer but not with bladder, kidney, and prostate cancer [25]. Thus, a recent systematic review of 20 studies concluded that most previous studies reported a positive association between air pollution and bladder or kidney cancer risk, but only a few reached statistical significance; also most studies inadequately addressed confounding factors, and cohort studies had insufficient numbers of participants and follow-up duration [15]. Our study is meaningful in that it presents positive results using a large number of participants, after controlling for sufficient confounding factors.
One of the interesting details of our study is that the impact of PM10 exposure on subsequent kidney and prostate cancer risks was more prominent in participants with specific characteristics. First, the association between PM10 exposure and risk of kidney cancer was more prominent in females than males. It is not clear whether females are more susceptible to cancers associated with air pollution. Similar to our findings, Li et al. reported that females were more susceptible to the association between the risk of esophageal cancer and air pollution than males [26]. Although it is not a cancer research, Liu et al. also reported that susceptibility to cardiovascular mortality from air pollution was higher among females than males [27]. Further researches on the difference in urologic cancer susceptibility from air pollution in males and females is needed.
Second, the association between PM10 exposure and the risk of kidney and prostate cancers was more prominent in participants without features of metabolic disorders. The rationale for these results is unclear. However, it is necessary to consider that metabolic disorders such as DM, hypertension, and dyslipidemia are important risk factors for urologic cancer. A recent meta-analysis of 151 cohort studies comprising 32 million participants showed that DM was associated with an increased risk of kidney (pooled relative risk [RR] 1.32, 95% CI 1.21-1.44) and bladder (pooled RR 1.19, 95% CI 1.09-1.29) cancer [28]. Another meta-analysis of 24 studies including a total of 132,589 participants reported an association between metabolic syndrome and prostate cancer incidence (odds ratio [OR] 1.17, 95% CI 1.00-1.36) and between metabolic syndrome and high-grade prostate cancer (OR 1.89, 95% CI 1.50-2.38) [29]. Considering the well-known mechanisms linking DM, hypertension, and dyslipidemia to cancer [30-32], it can be assumed that the risk of urologic cancer did not increase as similar mechanisms of these chronic diseases and PM10 are complementary.
Finally, the association between PM10 concentration and the risks of both kidney and prostate cancers differed according to the category of regular physical activity. In participants with no regular physical activity or regular physical activity 1-4 times per week, high PM10 exposure was significantly associated with increased risks of kidney and prostate cancer. In contrast, in participants who engaged in regular physical activity five or more times per week, high PM10 exposure was not associated with an increased risk of kidney and prostate cancer. Although regular physical activity is known to reduce the incidence and mortality from cardiovascular diseases, type 2 DM, and several cancers [33,34], there might be some concerns regarding exposure to air pollution during physical activity. However, numerous epidemiological and modeling studies have suggested that the long-term benefits of physical activity in urban areas outweigh the risks of exposure to air pollution [35]. The results of our study might provide additional evidence that regular physical activity offsets the risks of kidney and prostate cancer associated with PM10 exposure.
This study had some limitations. First, we excluded participants who did not undergo more than four consecutive health examinations from 2010 to 2018 to avoid the bias of cancer not being diagnosed, even though it has actually occurred. However, the risk of another selection bias may have occurred because a significant number of participants who did not receive more than four consecutive health examinations were excluded. Second, we estimated the level of individual exposure to PM10 by using fixed outdoor measurement stations near their residential regions. This approach may ignore the possible differences between individuals within the same residential region with regard to occupational exposure, daily activity area, indoor or outdoor exposure time, and the use of air purifiers. However, previous studies have shown that personal PM concentrations correlate reasonably well with PM concentrations measured at fixed outdoor measuring stations [36]. Finally, in 2008, the level of air pollution in South Korea was less severe than it is now, and the distribution or number of measurements of air pollutants was not as large as it is now. Therefore, this should be considered when interpreting our results. However, the major strength of our study was its large sample size, which allowed us to comprehensively control for important confounding factors.
Conclusions
High PM10 exposure is associated with an increased risk of overall urologic cancer, especially kidney and prostate cancers. Additionally, the increased risk of kidney and prostate cancer related to high PM10 exposure is significantly increased by current smoking, female sex (only in kidney cancer), and no features of metabolic disorders, and is decreased with regular physical activity five or more times a week. Although further studies are needed, physicians should consider more attentive lifestyle interventions, including air quality improvement and encouraging regular physical activity, to decrease the risk of urological cancers.
Acknowledgements
This study was performed using the database from the National Health Insurance System (NHIS-2020-1-432), and the results do not necessarily represent the opinion of the National Health Insurance Corporation. We used the public dataset from the National Health Insurance System, which is not individually identifiable after approval by the Institutional Review Board of Seoul St. Mary’s Hospital (IRB-KC19ZNSI0771) and Dankook University (IRB-DKU2022-06-002). The data used in this study are available only on the servers of the National Health Insurance system. Therefore, the corresponding author cannot provide these data to other researchers independently. However, PM10 data from 2008 can be provided.
This study was supported by a grant from the Korean Urological Association (2019-KUA-002) and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2022R1G1A1011635).
Disclosure of conflict of interest
Mi Jung Rho and Jihwan Park are married couples and are part of Dankook University, co-participating in the project. None of the contributing authors has any conflicts of interest, including specific financial interests, relationships, or affiliations relevant to the subject matter or materials discussed in the manuscript.
References
- 1.Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, Perez-Padilla R, Rice MB, Riojas-Rodriguez H, Sood A, Thurston GD, To T, Vanker A, Wuebbles DJ. Air pollution and noncommunicable diseases: a review by the forum of international respiratory societies’ environmental committee, part 1: the damaging effects of air pollution. Chest. 2019;155:409–416. doi: 10.1016/j.chest.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, Perez-Padilla R, Rice MB, Riojas-Rodriguez H, Sood A, Thurston GD, To T, Vanker A, Wuebbles DJ. Air pollution and noncommunicable diseases: a review by the forum of international respiratory societies’ environmental committee, part 2: air pollution and organ systems. Chest. 2019;155:417–426. doi: 10.1016/j.chest.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Air pollution. Accessed May 12, 2022. https://www.who.int/health-topics/air-pollution.
- 4.Achilleos S, Kioumourtzoglou MA, Wu CD, Schwartz JD, Koutrakis P, Papatheodorou SI. Acute effects of fine particulate matter constituents on mortality: a systematic review and meta-regression analysis. Environ Int. 2017;109:89–100. doi: 10.1016/j.envint.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim KH, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int. 2015;74:136–143. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Morakinyo OM, Mokgobu MI, Mukhola MS, Hunter RP. Health outcomes of exposure to biological and chemical components of inhalable and respirable particulate matter. Int J Environ Res Public Health. 2016;13:592. doi: 10.3390/ijerph13060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scientific I. Air pollution and cancer. Lyon: IARC Scientific; 2013. [Google Scholar]
- 8.Hamra GB, Guha N, Cohen A, Laden F, Raaschou-Nielsen O, Samet JM, Vineis P, Forastiere F, Saldiva P, Yorifuji T, Loomis D. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect. 2014;122:906–911. doi: 10.1289/ehp/1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White AJ, Keller JP, Zhao S, Carroll R, Kaufman JD, Sandler DP. Air pollution, clustering of particulate matter components, and breast cancer in the sister study: a U.S.-wide cohort. Environ Health Perspect. 2019;127:107002. doi: 10.1289/EHP5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Q, Wang X, Gao Y, Zhou J, Huang C, Zhang Z, Chu H. Relationship between particulate matter exposure and female breast cancer incidence and mortality: a systematic review and meta-analysis. Int Arch Occup Environ Health. 2021;94:191–201. doi: 10.1007/s00420-020-01573-y. [DOI] [PubMed] [Google Scholar]
- 11.Chu H, Xin J, Yuan Q, Wu Y, Du M, Zheng R, Liu H, Wu S, Zhang Z, Wang M. A prospective study of the associations among fine particulate matter, genetic variants, and the risk of colorectal cancer. Environ Int. 2021;147:106309. doi: 10.1016/j.envint.2020.106309. [DOI] [PubMed] [Google Scholar]
- 12.Karzai S, Zhang Z, Sutton W, Prescott J, Segev DL, McAdams-DeMarco M, Biswal SS, Ramanathan M Jr, Mathur A. Ambient particulate matter air pollution is associated with increased risk of papillary thyroid cancer. Surgery. 2022;171:212–219. doi: 10.1016/j.surg.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng H, Eckel SP, Liu L, Lurmann FW, Cockburn MG, Gilliland FD. Particulate matter air pollution and liver cancer survival. Int J Cancer. 2017;141:744–749. doi: 10.1002/ijc.30779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JM, Lee TH, Kim S, Song M, Bae S. Association between long-term exposure to particulate matter and childhood cancer: a retrospective cohort study. Environ Res. 2022;205:112418. doi: 10.1016/j.envres.2021.112418. [DOI] [PubMed] [Google Scholar]
- 15.Zare Sakhvidi MJ, Lequy E, Goldberg M, Jacquemin B. Air pollution exposure and bladder, kidney and urinary tract cancer risk: a systematic review. Environ Pollut. 2020;267:115328. doi: 10.1016/j.envpol.2020.115328. [DOI] [PubMed] [Google Scholar]
- 16.AirKorea. Annual report of air quality in Korea. 2008. Accessed March 26, 2021. https://www.airkorea.or.kr/web/detailViewDown?pMENU_NO=125.
- 17.AirKorea. Measuring stations in Korea. 2008. Accessed March 26, 2021. https://www.airkorea.or.kr/web/stationInfo?pMENU_NO=93.
- 18.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Accessed April 1, 2021. https://www.cdc.gov/diabetes/data/statistics-report/index.html.
- 20.Jeong SM, Choi S, Kim K, Kim SM, Lee G, Park SY, Kim YY, Son JS, Yun JM, Park SM. Effect of change in total cholesterol levels on cardiovascular disease among young adults. J Am Heart Assoc. 2018;7:e008819. doi: 10.1161/JAHA.118.008819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AirKorea. Introduction to the CAI. Accessed April 1, 2021. https://www.airkorea.or.kr/eng/khaiInfo?pMENU_NO=166.
- 22.Raaschou-Nielsen O, Pedersen M, Stafoggia M, Weinmayr G, Andersen ZJ, Galassi C, Sommar J, Forsberg B, Olsson D, Oftedal B, Krog NH, Aasvang GM, Pyko A, Pershagen G, Korek M, De Faire U, Pedersen NL, Ostenson CG, Fratiglioni L, Sorensen M, Eriksen KT, Tjonneland A, Peeters PH, Bueno-de-Mesquita HB, Plusquin M, Key TJ, Jaensch A, Nagel G, Foger B, Wang M, Tsai MY, Grioni S, Marcon A, Krogh V, Ricceri F, Sacerdote C, Migliore E, Tamayo I, Amiano P, Dorronsoro M, Sokhi R, Kooter I, de Hoogh K, Beelen R, Eeftens M, Vermeulen R, Vineis P, Brunekreef B, Hoek G. Outdoor air pollution and risk for kidney parenchyma cancer in 14 European cohorts. Int J Cancer. 2017;140:1528–1537. doi: 10.1002/ijc.30587. [DOI] [PubMed] [Google Scholar]
- 23.Cong X. Air pollution from industrial waste gas emissions is associated with cancer incidences in Shanghai, China. Environ Sci Pollut Res Int. 2018;25:13067–13078. doi: 10.1007/s11356-018-1538-9. [DOI] [PubMed] [Google Scholar]
- 24.Youogo LMK, Parent ME, Hystad P, Villeneuve PJ. Ambient air pollution and prostate cancer risk in a population-based Canadian case-control study. Environ Epidemiol. 2022;6:e219. doi: 10.1097/EE9.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raaschou-Nielsen O, Andersen ZJ, Hvidberg M, Jensen SS, Ketzel M, Sorensen M, Hansen J, Loft S, Overvad K, Tjonneland A. Air pollution from traffic and cancer incidence: a Danish cohort study. Environ Health. 2011;10:67. doi: 10.1186/1476-069X-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Jing J, Guo W, Guo X, Hu W, Qi X, Wei WQ, Zhuang G. The associations of air pollution and socioeconomic factors with esophageal cancer in China based on a spatiotemporal analysis. Environ Res. 2021;196:110415. doi: 10.1016/j.envres.2020.110415. [DOI] [PubMed] [Google Scholar]
- 27.Liu M, Xue X, Zhou B, Zhang Y, Sun B, Chen J, Li X. Population susceptibility differences and effects of air pollution on cardiovascular mortality: epidemiological evidence from a time-series study. Environ Sci Pollut Res Int. 2019;26:15943–15952. doi: 10.1007/s11356-019-04960-2. [DOI] [PubMed] [Google Scholar]
- 28.Ling S, Brown K, Miksza JK, Howells L, Morrison A, Issa E, Yates T, Khunti K, Davies MJ, Zaccardi F. Association of type 2 diabetes with cancer: a meta-analysis with bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people. Diabetes Care. 2020;43:2313–2322. doi: 10.2337/dc20-0204. [DOI] [PubMed] [Google Scholar]
- 29.Gacci M, Russo GI, De Nunzio C, Sebastianelli A, Salvi M, Vignozzi L, Tubaro A, Morgia G, Serni S. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis. 2017;20:146–155. doi: 10.1038/pcan.2017.1. [DOI] [PubMed] [Google Scholar]
- 30.Labochka D, Moszczuk B, Kukwa W, Szczylik C, Czarnecka AM. Mechanisms through which diabetes mellitus influences renal cell carcinoma development and treatment: a review of the literature. Int J Mol Med. 2016;38:1887–1894. doi: 10.3892/ijmm.2016.2776. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto K, Morishita R, Moriguchi A, Tomita N, Yo Y, Nishii T, Nakamura T, Higaki J, Ogihara T. Prevention of renal damage by angiotensin II blockade, accompanied by increased renal hepatocyte growth factor in experimental hypertensive rats. Hypertension. 1999;34:279–284. doi: 10.1161/01.hyp.34.2.279. [DOI] [PubMed] [Google Scholar]
- 32.Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Ann N Y Acad Sci. 2012;1271:82–87. doi: 10.1111/j.1749-6632.2012.06737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnohr P, Lange P, Scharling H, Jensen JS. Long-term physical activity in leisure time and mortality from coronary heart disease, stroke, respiratory diseases, and cancer. The Copenhagen city heart study. Eur J Cardiovasc Prev Rehabil. 2006;13:173–179. doi: 10.1097/01.hjr.0000198923.80555.b7. [DOI] [PubMed] [Google Scholar]
- 34.Johnsen NF, Ekblond A, Thomsen BL, Overvad K, Tjonneland A. Leisure time physical activity and mortality. Epidemiology. 2013;24:717–725. doi: 10.1097/EDE.0b013e31829e3dda. [DOI] [PubMed] [Google Scholar]
- 35.Tainio M, Jovanovic Andersen Z, Nieuwenhuijsen MJ, Hu L, de Nazelle A, An R, Garcia LMT, Goenka S, Zapata-Diomedi B, Bull F, Sa TH. Air pollution, physical activity and health: a mapping review of the evidence. Environ Int. 2021;147:105954. doi: 10.1016/j.envint.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssen NA, Hoek G, Brunekreef B, Harssema H, Mensink I, Zuidhof A. Personal sampling of particles in adults: relation among personal, indoor, and outdoor air concentrations. Am J Epidemiol. 1998;147:537–547. doi: 10.1093/oxfordjournals.aje.a009485. [DOI] [PubMed] [Google Scholar]