Abstract
Whether tumor deposits (TDs) should be classified as lymph node metastasis or distant metastasis remains controversial. To address this predicament, we conducted this study to identify the predictive value of TDs on the survival of patients diagnosed with stage III colon cancer (CC). 12,904 eligible patients diagnosed with stage III CC between 2010 and 2015 were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. The best cutoff point of TD quantity was determined based on the difference in survival. Cox proportional hazards model was employed to perform univariate and multivariate analyses. The Kaplan-Meier method and log-rank test were performed to calculate the differences between overall survival (OS). Our results showed that the number of TDs was a significant prognostic factor in patients with stage III CC (P < 0.0001). We added the number of TDs to the pN stage and devised a new pN stage, there were no significant differences in the survival of npN, except npN2a (P > 0.05). Upon re-staging to the same npN stage, the difference in survival between TDs+ and TDs- disappeared (P > 0.05). The median survival times for N2aTDs > 4 and N2bTDs > 4 were 33 and 37 months, respectively, which were significantly shorter than that of N2TDs- (65 months) and represented the worst survival rates among all groups. In conclusion, the number of TDs indicated a poor prognosis for patients with stage III CC. Incorporating TDs into the pN is feasible to predict prognosis.
Keywords: Stage III colon cancer, tumor deposits, lymph node metastasis, new pN staging system, survival
Introduction
The GLOBOCAN 2020 report presents the latest information regarding the global cancer burden. According to the statistics, colorectal cancer (CRC) ranks as the third most common malignancy worldwide among new cancer cases (10.0%). CRC ranks second in mortality among the most frequent malignant neoplasms, after lung cancer (9.4%) [1].
Clinicopathological factors commonly predict the prognosis of patients with CRC. The TNM staging system, proposed by the American Joint Committee on Cancer (AJCC), is the most widely recognized. This system is acknowledged for its sensitivity in identifying patients at risk of recurrence, predicting survival, and guiding clinical decisions. However, it tends to overlook tumor heterogeneity, and differences in the prognosis of patients with similar TNM stages may not be accounted for when relying solely on the staging system. The National Comprehensive Cancer Network (NCCN) identified additional pathological features related to patient survival, including lymphatic vascular infiltration, TDs, and perineural infiltration [2,3]. Among these histopathological features, only TDs are commonly incorporated into TNM staging; their presence is considered a high-risk factor for postoperative recurrence and metastasis and an essential indicator for postoperative adjuvant chemotherapy [4,5].
TDs were first described in the fifth edition of the AJCC TNM staging system. TDs with diameters > 3 mm and without regional lymph node metastasis are classified as pN. In contrast, TDs with diameters < 3 mm were classified as T3. The sixth edition of the TNM defined TDs based on contour. A firm and smooth contour is deemed a positive lymph node, whereas irregular TDs remain categorized as T3. The seventh TNM edition defined TDs as discrete cancerous nodules located around the pericolic or perirectal fat or adjacent mesentery, with no features of lymph node tissue. The seventh and eighth editions included TDs without LNM into the N1c stage of CRC. In cases of LNM, the number of TDs is not calculated into the number of positive lymph nodes. Compared with the seventh edition, the eighth edition only excludes tumor lesions related to “identifiable vascular or neural structures”, classifying them as vascular or perineural invasion. Nevertheless, the guidelines still do not clarify the classification of patients identified simultaneously with LNM and TDs.
Several previous retrospective analyses and meta-analyses indicated a statistically significant difference in the survival of patients confirmed with or without TDs in lymph node-positive [6-8]. Currently, controversies regarding redefining the relationship between LNM and TDs and whether TDs should be regarded as lymph node metastasis or distant metastasis remain unsolved [9-11]. Therefore, it is crucial to clarify the prognostic role of TDs, particularly in evaluating the prognosis of patients with CRC with LNM and TDs. However, previous studies revealing differences between colon and rectal cancer regarding etiology, embryonic origin, and metastasis model. Additionally, variations in surgical methods and systemic treatment, such as neoadjuvant therapy, concurrent chemoradiotherapy, or chemotherapy, exist between patients with stage III colon cancer (CC) and those with stage III rectal cancer. Therefore, separate studies should be conducted to assess the impact of TDs on the prognoses of patients with CC and those with rectal cancer [12-14]. Our study aimed to determine the predictive value of TDs in patients with CC, combine the number of TDs with the pN stage, and explore the possibility of the inclusion of TDs in the pN stage.
Materials and methods
Patients and data sources
We extracted the data of patients with CC from the Surveillance, Epidemiology, and End Results (SEER) database from January 2010 to December 2015 by applying SEER * stat (version 8.3.9.2). The inclusion criteria were as follows: (I) patients aged 18-80 years; (II) patients received resection at the primary site, and the site codes C18.0 and C18.2-18.7 were used; (III) patients were pathologically confirmed to have colon cancer, with histology codes encompassing adenocarcinoma [8140-8144, 8210-8213, 8220-8221, and 8260-8263], mucinous adenocarcinoma [8480-8481], and signet ring cell carcinoma [8490]; (IV) patients were diagnosed at stage III, according to the TNM stage; (V) patients with complete survival information; (VI) patients with follow-up period > 1 month.
This study excluded patients with missing or incomplete clinical basic, clinicopathological, and therapeutic information (see Figure 1 in the supplementary file for details). This study was conducted according to the SEER data use agreement, and patient informed consent was not required given the anonymized, de-identified data in the SEER database.
Figure 1.
The workflow of the patient selection process.
Variables and outcomes
Our primary endpoint was overall survival (OS), defined as the interval from diagnosis to death or the last follow-up. The primary site of the tumor was defined as the right colon (cecum, ascending colon, hepatic flexure of colon, and transverse colon) and the left colon (splenic flexure of the colon, descending colon, and sigmoid colon). Carcinoembryonic antigen (CEA) was considered negative when its levels were within the normal range and positive when its levels were higher than the normal range.
Statistical analysis
All continuous variables were transformed into categorical variables. We used the χ2 test or Fisher exact test to compare categorical variables for demographic and clinicopathological characteristics. Univariate and multivariate analyses were performed using Cox proportional hazards models. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were expressed as numerical values. The Kaplan-Meier method and log-rank test were used to further calculate the difference in OS among groups to determine the independent effect of TD on OS.
All statistical analyses in this study were performed using EmpowerStats 2.0 (R 3.4.3) software and R Statistical Software version 4.1.2 (Vienna, Austria; www.r-project. org). A bilateral p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics of patients with stage III colon cancer
A total of 12,904 patients were included in this study. Patients were categorized into three groups according to the best cutoff point: TDs-, TDs ≤ 4, and TDs > 4 (10884, 1766, and 254, respectively; see Figure 2 in the supplementary file for details).
Figure 2.
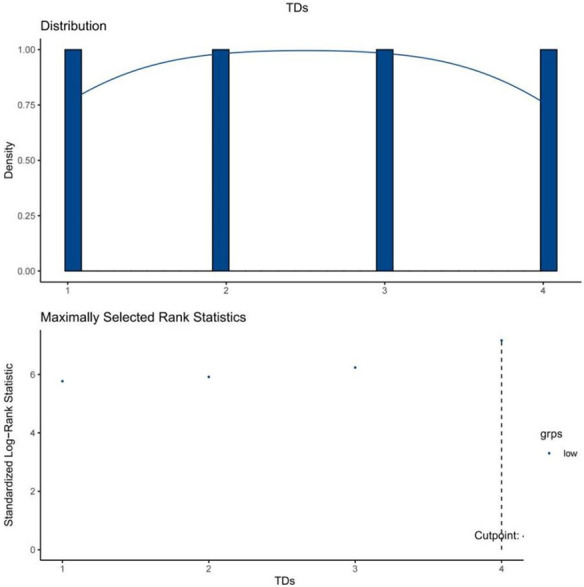
The best cutoff point of tumor deposits quantities.
Table 1 presents the baseline characteristics of patients with stage III CC according to the TDs status. Among the 12904 patients, 2020 (15.65%) were confirmed as TDs+. No significant differences were identified regarding sex, race, age at diagnosis, tumor size, or chemotherapy among the TDs-, TDs ≤ 4, and TDs > 4 groups (P > 0.05).
Table 1.
Clinicopathological features of patients with stage III colon cancer
| Total (N = 12904) | Tumor deposits (TDs) | ||||
|---|---|---|---|---|---|
|
| |||||
| TDs- (n = 10884) | TDs ≤ 4 (n = 1766) | TDs > 4 (n = 254) | P-value | ||
| Sex | 0.328 | ||||
| Male | 6270 (48.59%) | 5311 (48.80%) | 846 (47.90%) | 113 (44.49%) | |
| Female | 6634 (51.41%) | 5573 (51.20%) | 920 (52.10%) | 141 (55.51%) | |
| Age | 0.19 | ||||
| < 65 | 6725 (52.12%) | 5687 (52.25%) | 895 (50.68%) | 143 (56.30%) | |
| ≥ 65 | 6179 (47.88%) | 5197 (47.75%) | 871 (49.32%) | 111 (43.70%) | |
| Race | 0.353 | ||||
| White | 9550 (74.01%) | 8028 (73.76%) | 1328 (75.20%) | 194 (76.38%) | |
| AmericanIndian/Alaska Native | 116 (0.9%) | 97 (0.89%) | 15 (0.85%) | 4 (1.57%) | |
| Black | 1916 (14.85%) | 1620 (14.88%) | 265 (15.01%) | 31 (12.20%) | |
| Asian or Pacific Islanders | 1322 (10.24%) | 1139 (10.46%) | 158 (8.95%) | 25 (9.84%) | |
| Primary site | < 0.001 | ||||
| Right colon | 7472 (57.9%) | 6392 (58.73%) | 965 (54.64%) | 115 (45.28%) | |
| Left colon | 5432 (42.1%) | 4492 (41.27%) | 801 (45.36%) | 139 (54.72%) | |
| Pathology | 0.002 | ||||
| Adenocarcinoma | 11551 (89.51%) | 9745 (89.54%) | 1595 (90.32%) | 211 (83.07%) | |
| Mucinous adenocarcinoma | 1186 (9.19%) | 1006 (9.24%) | 145 (8.21%) | 35 (13.78%) | |
| Signet ring cell carcinoma | 167 (1.29%) | 133 (1.22%) | 26 (1.47%) | 8 (3.15%) | |
| Degree of differentiation | < 0.001 | ||||
| Well | 642 (4.98%) | 566 (5.20%) | 68 (3.85%) | 8 (3.15%) | |
| Moderately | 9217 (71.43%) | 7778 (71.46%) | 1285 (72.76%) | 154 (60.63%) | |
| Poorly | 2534 (19.64%) | 2120 (19.48%) | 334 (18.91%) | 80 (31.50%) | |
| Undifferentiated | 511 (3.96%) | 420 (3.86%) | 79 (4.47%) | 12 (4.72%) | |
| pT stage | < 0.001 | ||||
| T1 | 495 (3.84%) | 473 (4.35%) | 20 (1.13%) | 2 (0.79%) | |
| T2 | 1229 (9.52%) | 1116 (10.25%) | 108 (6.12%) | 5 (1.97%) | |
| T3 | 8450 (65.48%) | 7181 (65.98%) | 1133 (64.16%) | 136 (53.54%) | |
| T4 | 2730 (21.16%) | 2114 (19.42%) | 505 (28.60%) | 111 (43.70%) | |
| pN stage | < 0.001 | ||||
| N1a | 4198 (32.53%) | 3842 (35.30%) | 335 (18.97%) | 21 (8.27%) | |
| N1b | 4095 (31.73%) | 3625 (33.31%) | 422 (23.90%) | 48 (18.90%) | |
| N1c | 474 (3.67%) | 0 (0.00%) | 447 (25.31%) | 27 (10.63%) | |
| N2a | 2416 (18.72%) | 2048 (18.82%) | 309 (17.50%) | 59 (23.23%) | |
| N2b | 1721 (13.34%) | 1369 (12.58%) | 253 (14.33%) | 99 (38.98%) | |
| Tumor size(cm) | 0.187 | ||||
| < 5 | 7264 (56.29%) | 6153 (56.53%) | 981 (55.55%) | 130 (51.18%) | |
| ≥ 5 | 5640 (43.71%) | 4731 (43.47%) | 785 (44.45%) | 124 (48.82%) | |
| CEA | < 0.001 | ||||
| Negative | 7637 (59.18%) | 6526 (59.96%) | 984 (55.72%) | 127 (50.00%) | |
| Positive | 5267 (40.82%) | 4358 (40.04%) | 782 (44.28%) | 127 (50.00%) | |
| Nerve invasion | < 0.001 | ||||
| No | 10821 (83.86%) | 9323 (85.66%) | 1343 (76.05%) | 155 (61.02%) | |
| Yes | 2083 (16.14%) | 1561 (14.34%) | 423 (23.95%) | 99 (38.98%) | |
| Chemotherapy | 0.644 | ||||
| No/Unknown | 2969 (23.01%) | 2488 (22.86%) | 420 (23.78%) | 61 (24.02%) | |
| Yes | 9935 (76.99%) | 8396 (77.14%) | 1346 (76.22%) | 193 (75.98%) | |
Regarding the degree of differentiation, moderately differentiated tumors constituted the highest proportion, followed by poorly differentiated tumors. Within the moderately differentiated category, TDs > 4 accounted for 60.63%, lower than TDs- (71.46%) and TDs ≤ 4 (72.76%). Nevertheless, TDs > 4 accounted for 31.50% in the poorly differentiated category, which was significantly higher than the TDs- (19.48%) and TDs ≤ 4 (18.91%).
Regarding the T stage, the proportion of patients with TDs in stages ≥ T3 was higher than those without TDs (TDs- vs. TDs ≤ 4 vs. TDs > 4: 85.4% vs. 92.76% vs. 97.24%, P < 0.001). Regarding the N stage, the N1 stage (N1a, N1b, N1c) constituted the primary phase in TDs- and TDs ≤ 4, accounting for 64.26% and 68.17%, respectively. Conversely, the main phase shifted to the N2 stage (N2a, N2b) in cases of TDs > 4, accounting for 62.31% (P < 0.001). As the number of TDs increased, the scale of CEA positivity and nerve invasion gradually increased (CEA positivity: 40.04% vs. 44.28% vs. 50.00%; nerve invasion: 14.34% vs. 23.95% vs. 38.98%).
The median follow-up time in this study was 53.0 months (range 1.0-107.0 months). Overall, 4376 (33.91%, n = 12904) patients died by the final follow-up.
Identification of independent prognostic factors for overall survival in patients with stage III colon cancer
Univariate and multivariate analyses were performed to evaluate the predictive differences in OS among patients with stage III CC (Table 2). In addition to the number of TDs, other significant prognostic factors included sex, age, race, primary tumor site, pathological type, histological grade, T stage, N stage, CEA levels, nerve invasion, and chemotherapy.
Table 2.
Univariate and multivariate Cox regression analysis of overall survival rate of patients with stage III colon cancer
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| H.R. | 95% CI | P-value | H.R. | 95% CI | P-value | |
| Sex | ||||||
| Male | 1 | |||||
| Female | 1.16 | 1.09, 1.23 | < 0.0001 | 1.32 | 1.22, 1.43 | < 0.0001 |
| Age | ||||||
| < 65 | 1 | |||||
| ≥ 65 | 1.84 | 1.73, 1.95 | < 0.0001 | 1.90 | 1.75, 2.06 | < 0.0001 |
| Race | ||||||
| White | 1 | |||||
| American Indian/Alaska Native | 0.93 | 0.67, 1.29 | 0.6695 | 0.97 | 0.63, 1.50 | 0.9034 |
| Black | 1.26 | 1.16, 1.36 | < 0.0001 | 1.44 | 1.29, 1.61 | < 0.0001 |
| Asian or Pacific Islanders | 0.88 | 0.79, 0.97 | 0.0137 | 0.84 | 0.74, 0.97 | 0.0141 |
| Primary site | ||||||
| Right colon | 1 | |||||
| Left colon | 0.72 | 0.68, 0.77 | < 0.0001 | 0.79 | 0.73, 0.86 | < 0.0001 |
| Pathology | ||||||
| Adenocarcinoma | 1 | |||||
| Mucinous adenocarcinoma | 1.21 | 1.09, 1.33 | 0.0002 | 1.08 | 0.94, 1.23 | 0.2915 |
| Signet ring cell carcinoma | 3.12 | 2.58, 3.76 | < 0.0001 | 2.46 | 1.72, 3.52 | < 0.0001 |
| Degree of differentiation | ||||||
| Well | 1 | |||||
| Moderately | 1.15 | 0.99, 1.34 | 0.072 | 1.07 | 0.89, 1.30 | 0.4765 |
| Poorly | 1.69 | 1.45, 1.99 | < 0.0001 | 1.24 | 1.01, 1.53 | 0.0383 |
| Undifferentiated | 2.06 | 1.70, 2.50 | < 0.0001 | 1.39 | 1.07, 1.82 | 0.0152 |
| pT stage | ||||||
| T1 | 1 | |||||
| T2 | 1.26 | 0.98, 1.62 | 0.0762 | 1.15 | 0.86, 1.53 | 0.355 |
| T3 | 2.17 | 1.74, 2.71 | < 0.0001 | 1.82 | 1.40, 2.35 | < 0.0001 |
| T4 | 4.06 | 3.24, 5.09 | < 0.0001 | 3.40 | 2.60, 4.44 | < 0.0001 |
| pN stage | ||||||
| N1a | 1 | |||||
| N1b | 1.31 | 1.21, 1.42 | < 0.0001 | 1.36 | 1.23, 1.51 | < 0.0001 |
| N1c | 1.41 | 1.19, 1.67 | < 0.0001 | 0.94 | 0.74, 1.21 | 0.6483 |
| N2a | 1.66 | 1.52, 1.81 | < 0.0001 | 1.71 | 1.52, 1.92 | < 0.0001 |
| N2b | 2.54 | 2.32, 2.78 | < 0.0001 | 2.62 | 2.30, 2.98 | < 0.0001 |
| Tumor size (cm) | ||||||
| < 5 | 1 | |||||
| ≥ 5 | 1.25 | 1.18, 1.33 | < 0.0001 | 0.94 | 0.86, 1.02 | 0.1221 |
| CEA | ||||||
| Negative | 1 | |||||
| Positive | 1.64 | 1.55, 1.74 | < 0.0001 | 1.53 | 1.41, 1.66 | < 0.0001 |
| Nerve invasion | ||||||
| no | 1 | |||||
| yes | 1.53 | 1.42, 1.65 | < 0.0001 | 1.27 | 1.14, 1.41 | < 0.0001 |
| Tumor deposits | ||||||
| No | 1 | |||||
| ≤ 4 | 1.37 | 1.26, 1.48 | < 0.0001 | 1.24 | 1.10, 1.41 | 0.0007 |
| > 4 | 2.67 | 2.28, 3.14 | < 0.0001 | 2.48 | 1.87, 3.29 | < 0.0001 |
| Chemotherapy | ||||||
| No/Unknown | 1 | |||||
| Yes | 0.45 | 0.42, 0.47 | < 0.0001 | 0.40 | 0.37, 0.44 | < 0.0001 |
Differences in overall survival in patients with stage III colon cancer with different lymph node stages and tumor deposits
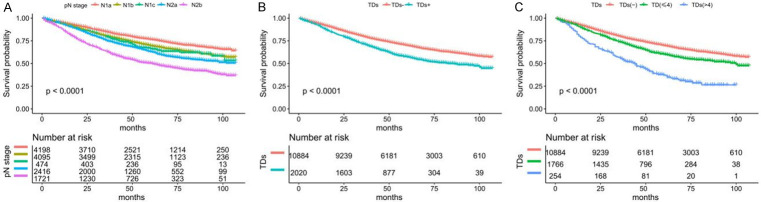
When ignoring the number of TDs, the 5-year overall survival rate of the N1a stage was 76.48%, constituting the longest survival time. Subsequently, the Kaplan-Meier curves reveal no survival difference between N1b and N1c (69.57% vs. 66.28%), indicating that the survival of patients with TDs and stage II CC is similar to that of patients with stage III CC without LNM. Finally, the 5-year overall survival of the N2a and N2b stages were 63.03% and 50.25% (P < 0.0001) (Figure 3A).
Figure 3.
Survival prognosis of lymph node stage and tumor deposits in patients with stage III colon cancer. A. The overall survival (OS) of patients with different pN stages. B. The OS of patients with TDs and without TDs. C. The OS of patients with TDs-, TDs ≤ 4, and TDs > 4.
Regarding the TDs status, the survival prognosis of the TDs+ was worse than that of the TDs- (P < 0.0001) (Figure 3B). We performed further categorization based on the best cutoff point of TDs, and the OS of TDs- was the best, followed by TDs ≤ 4, and TDs > 4 was the worst (P < 0.0001) (Figure 3C). The Kaplan-Meier curve showed that the OS of patients with stage III CC was worse with increased TDs.
Survival analysis of varying numbers of tumor deposits in patients with stage III colon cancer
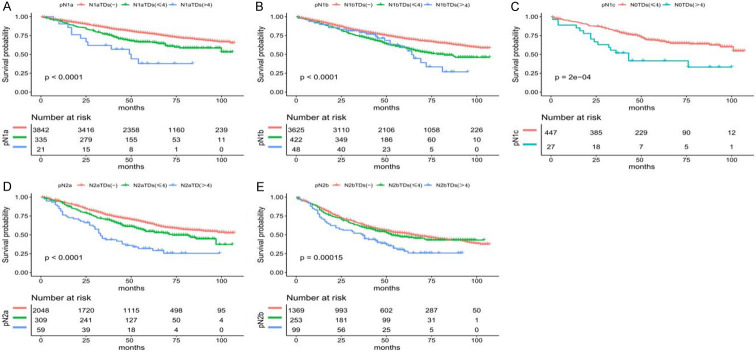
In stage N0, the survival prognosis of TDs ≤ 4 (N0TDs ≤ 4) was better than that of TDs > 4 (N0TDs > 4) (P < 0.05) (Figure 4C). Among patients with existing LNM (N1a, N1b, N2a, N2b), those with TDs- (N1-2TDs-) had the longest survival, followed by those with TDs ≤ 4 (N1-2TDs ≤ 4), whereas patients with TDs > 4 (N1-2TDs > 4) had the shortest survival (P < 0.05) (Figure 4A, 4B, 4D, 4E).
Figure 4.
Kaplan-Meier curve shows the difference in survival of tumor deposit groups under the same lymph node stage in patients with stage III colon cancer. A. The overall survival (OS) of patients with different numbers of TDs in the pN1a stage. B. The OS of patients in the pN1b stage. C. The OS of patients in the pN1c stage. D. The OS of in the pN2a stage. E. The OS of patients in the pN2b stage.
The Kaplan-Meier curve indicated that the OS of patients with stage III CC decreases gradually with an increasing number of LNMs and TDs, suggesting significant differences in the survival prognosis among different TDs quantities in the same pN stage (Table 3).
Table 3.
1-, 3-, and 5-year overall survival rate and median survival time after reorganization according to tumor deposits and lymph node stage
| III stage | 1-year survival (%) | 3-year survival (%) | 5-year survival (%) | mOS (months) |
|---|---|---|---|---|
| pN0 | ||||
| N0TDs ≤ 4 | 93.46 | 80.93 | 67.77 | NA (101, NA) |
| N0TDs > 4 | 88.89 | 51.85 | 41.48 | 43 (26, NA) |
| pN1a | ||||
| N1aTDs- | 94.80 | 85.46 | 77.56 | NA |
| N1aTDs ≤ 4 | 92.47 | 76.83 | 66.39 | NA (100, NA) |
| N1aTDs > 4 | 90.48 | 61.90 | 37.83 | 50 (25, NA) |
| pN1b | ||||
| N1bTDs- | 93.83 | 80.87 | 70.89 | NA |
| N1bTDs ≤ 4 | 91.93 | 73.55 | 58.90 | 82 (66, NA) |
| N1bTDs > 4 | 91.67 | 79.05 | 59.08 | 64 (52, NA) |
| pN2a | ||||
| N2aTDs- | 93.63 | 76.48 | 65.17 | NA (102, NA) |
| N2aTDs ≤ 4 | 91.87 | 70.09 | 54.42 | 73 (58, NA) |
| N2aTDs > 4 | 76.27 | 43.49 | 31.81 | 33 (29, 51) |
| pN2b | ||||
| N2bTDs- | 86.88 | 64.25 | 52.24 | 65 (58, 73) |
| N2bTDs ≤ 4 | 86.12 | 60.59 | 47.30 | 53 (44, NA) |
| N2bTDs > 4 | 77.62 | 51.07 | 28.16 | 37 (24, 47) |
Restaging according to different lymph node stages and groups of tumor deposits to develop a new pN stage
The 1-year, 3-year, and 5-year survival rates for patients with stage III CC when integrating the pN stages with the TDs groups are shown in Table 3. After interpreting the aforementioned survival, we performed restaging when similar survival prognoses were observed (Table 4).
Table 4.
Re-staging consists of tumor deposits and lymph node staging
| The new pathology lymph node staging (npN) | |
|---|---|
| N1a | N1aTDs- |
| N1b | N0TDs ≤ 4, N1aTDs ≤ 4, N1bTDs- |
| N1c | N0TDs > 4 |
| N2a | N1bTDs ≤ 4, N2aTDs- |
| N2b | N2aTDs ≤ 4, N2bTDs- |
| N2c | N1aTDs > 4, N1bTDs > 4, N2bTDs ≤ 4 |
| clinical metastasis | N2aTDs > 4, N2bTDs > 4 |
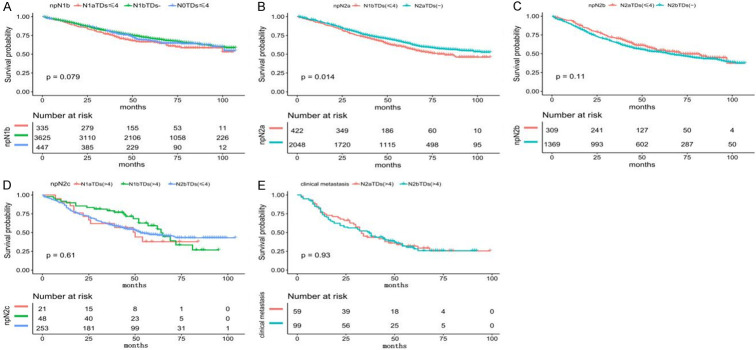
The 1-year, 3-year, and 5-year OS rates in N1aTDs- were 94.80%, 85.46%, and 77.56%, respectively, representing the best survival prognoses among all groups. The Kaplan-Meier curve demonstrated that N0TDs ≤ 4, N1aTDs ≤ 4, and N1bTDs- had similar OS (P = 0.079) (Figure 5A). The 1-year, 3-year, and 5-year OS rates in N0TDs > 4 were 88.89%, 51.85%, and 41.48%, respectively. The 1-year, 3-year, and 5-year OS rates in N1bTDs ≤ 4 were 91.93%, 73.55%, and 58.90%, slightly lower than those of N2aTDs- (93.63%, 76.48%, and 65.17%). The Kaplan-Meier survival curve indicated a statistically significant survival difference (P = 0.014) (Figure 5B). No significant difference was observed between N2aTDs ≤ 4 and N2bTDs- (P = 0.11) (Figure 5C) or among N1aTDs > 4, N1bTDs > 4, and N2bTDs ≤ 4 (P = 0.61) (Figure 5D). The median overall survival rates (mOS) of N2aTDs > 4 and N2bTDs > 4 were 33 and 37 months, respectively, constituting the shortest rates among all groups (P = 0.93) (Figure 5E).
Figure 5.
Kaplan-Meier survival curve showing the survival after regrouping. A. The overall survival (OS) in the N0-1aTDs ≤ 4 and N1bTDs-. B. The OS in the N1bTDs ≤ 4 and N2aTDs-. C. The OS in the N2aTDs ≤ 4 and N2bTDs-. D. The OS in the N1a-bTDs > 4 and N2bTDs ≤ 4. E. The OS in the N2TDs > 4.
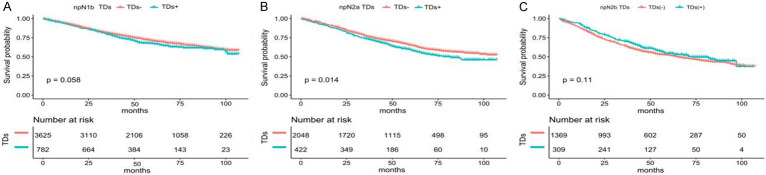
After the re-staging, we compared the TDs- and TDs+ in the new pathology lymph node stage. We found no significant difference in survival between the TDs- and the TDs+ in npN1b and npN2b (P = 0.058 and P = 0.11) (Figure 6A, 6C). The survival of TDs+ was slightly lower than that of TDs- in the npN2a stage (P = 0.014) (Figure 6B).
Figure 6.
Difference in survival of tumor deposits after re-staging. A. The overall survival (OS) of patients with TDs and without TDs in the npN1b stage. B. The OS of patients with TDs and without TDs in the npN2a stage. C. The OS of patients with TDs and without TDs in the npN2b stage.
Discussion
In this study, we investigated the predictive value of TDs and their relationship with LNM in stage III CC using the SEER database. Our findings demonstrated that TDs independently influenced the survival of patients with stage III CC, based on rigorous univariate and multivariate Cox regression analysis. Notably, N1c showed slightly worse survival than N1a and overlaps with N1b, supporting the classification of patients with stage II CC without LNM but with TDs+ as stage III, as advocated by Belt, Al Sahaf et al. [15-18]. Their conclusions also highlight the presence of TDs as a predictor of poorer survival.
Our study determined the optimal cutoff point for TDs and found that the number of TDs independently predicted prognosis in patients with stage III CC. By combining the pN stage and TDs, we observed that increasing TDs at the same pN stage corresponded to a worse prognosis in stage III CC. These findings are consistent with those of Bai et al. [19], who identified a cutoff point of 1 and 5 for TDs. Patients with TDs ≥ 5 had the worst survival, followed by those in the TDs 2-4 groups, whereas patients with TDs = 1 demonstrated a better prognosis.
A previous study [20] determined that the presence of TDs was an independent adverse prognostic factor in patients with CRC. However, their findings showed that the number of TDs had no association with OS. Another study [21] analyzed 146 patients with simultaneous CRC and liver metastases who underwent R0 resection. This study found a significant correlation between TDs and shorter disease-free survival (DFS). However, the patients in the aforementioned study were in the TNM stage IV or had liver metastasis, which could mask the impact of TDs owing to adverse factors associated with distant metastasis, rendering the number of TDs irrelevant to survival prognosis.
Post-hoc analysis of two phase 3 clinical trials [22,23] revealed a linear and negative correlation between the number of TDs and DFS and OS. Upon combining the TDs and LNM, some patients were restaged from the pN1 stage to the pN2 stage. No differences in DFS were observed between patients re-staged to npN2 and those originally staged to pN2. Song and Pei et al’s. [24,25] conclusion aligned with this post-hoc analysis, suggesting that integrating TDs into LNM to form the npN stage has a higher prediction ability than the pN stage. Importantly, Song’s research found no significant prognosis difference between TDs+ and TDs- in the same npN stage. The findings of Li [26] and Ueno [27] also support that incorporating the number of TDs into the number of LNM can enhance prognosis accuracy. However, Liu [20] and the results of our study indicate that the number of TDs and the number of LNMs do not have a one-to-one correspondence. Consequently, our study first grouped TDs based on the impact on survival in patients with stage III CC before combining them with pN staging. We proposed an adjustment of pN staging following the seventh edition of the TNM guidelines. After regrouping TDs and LNM, all the npN stages did not significantly differ in OS, except for npN2a. Survival of TDs- was equal to that of TDs+ in the same npN stage. Therefore, we believe it is feasible to include TDs in the LNM approach.
Tong [28] analyzed 1541 patients with stage I-IV CRC using subgroup analysis and found that the 5-year OS of T3N2bM0TD+ was significantly lower than that of T3N2bM0TD- (7.8% vs. 46.9%; P = 0.012), which was similar to that of stage IV (7.8% vs. 9.9%; P = 0.730). Furthermore, the 5-year OS of T4N2bM0 was 0%, with or without TDs. Lino-Silva [10] found that the average OS of patients complicated with TDs+ in stage I-III was 69.3 months, which was different from that of stage III (110.5 months), stage II (135.7 months), and stage I (114.9 months) and similar to that of stage IV (64.6 months), and recommended to treat these patients as clinical IV-TD+.
Chemotherapy combined with targeted therapy has significantly improved the survival of patients with advanced CRC, and the mOS increased from 13 to nearly 30 months. Among patients with advanced CRC where surgery is feasible, those treated with surgery and systemic treatment can achieve a 5-year OS of 25-55%, with a mOS of 31-64.7 months [29-34]. Importantly, the OS of N2aTDs > 4 and N2bTDs > 4 was significantly lower than that of N2TDs-; the 5-year OS was 31.81% and 28.16%, with mOS of 33 and 37 months, respectively. Compared with simply adding the number of TDs to LNM in other research, our study initially grouped the TDs before combining them with the pN stage. This approach revealed that patients with N2TDs > 4 had a worse prognosis than those with distant metastasis. Therefore, we propose the classification of clinical metastasis when N2TDs > 4, and more active treatments are recommended to improve the survival of patients at this stage.
Our study had notable strengths. First, we used SEER data, which offers a large sample of evidence-based medicine, reduces potential biases arising from limited sample sizes, and enhances clinical value. Second, our study improves the accuracy of prognostic evaluation in patients with stage III colon cancer by incorporating the number of TDs into LNM. Notably, our findings demonstrated that patients with N2TDs > 4 exhibited the worst prognosis, suggesting its potential classification as clinically distant metastasis.
However, our study also had limitations. First, this was a retrospective study, and inherent selection biases may exist in the inclusion process, exclusion criteria, and variable screening. Second, the SEER database lacks key factors affecting survival outcomes, such as surgical approach, treatment regimens, and genetic mutations. Finally, the small sample size of patients in the TDs > 4 groups (n = 254) may introduce bias in the analysis of survival prognosis. Future studies should incorporate multicenter data to further validate our conclusions.
In summary, the number of TDs had predictive value for the prognosis of patients with stage III CC, and the presence and number of TDs should be considered to guide treatment decisions. Finally, it is feasible to include TDs in the LNM. We propose the classification of clinical metastasis when the staging diagnosis is N2TDs > 4.
Acknowledgements
This work was supported by the Natural Science Foundation of China (Nos. 81302067 and 81502360); The Natural Science Foundation of Fujian Province (Nos. 2016J01576 and 2020J011147); The Science and Technology Innovation Joint Foundation of Fujian Province (No. 2017Y9125).
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:329–359. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 3.Li X, An B, Zhao Q, Qi J, Wang W, Zhang D, Li Z, Qin C. Impact of tumor deposits on the prognosis and chemotherapy efficacy in stage III colorectal cancer patients with different lymph node status: a retrospective cohort study in China. Int J Surg. 2018;56:188–194. doi: 10.1016/j.ijsu.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Yabata E, Udagawa M, Okamoto H. Effect of tumor deposits on overall survival in colorectal cancer patients with regional lymph node metastases. J Rural Med. 2014;9:20–26. doi: 10.2185/jrm.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouquot M, Creavin B, Goasguen N, Chafai N, Tiret E, Andre T, Flejou JF, Parc Y, Lefevre JH, Svrcek M. Prognostic value and characteristics of N1c colorectal cancer. Colorectal Dis. 2018;20:O248–O255. doi: 10.1111/codi.14289. [DOI] [PubMed] [Google Scholar]
- 6.Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot T, Ueno H, Quirke P. Tumor deposits in colorectal cancer: improving the value of modern staging-a systematic review and meta-analysis. J. Clin. Oncol. 2017;35:1119–1127. doi: 10.1200/JCO.2016.68.9091. [DOI] [PubMed] [Google Scholar]
- 7.Jin M, Roth R, Rock JB, Washington MK, Lehman A, Frankel WL. The impact of tumor deposits on colonic adenocarcinoma AJCC TNM staging and outcome. Am J Surg Pathol. 2015;39:109–115. doi: 10.1097/PAS.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YI, Cho H, Kim CW, Park Y, Kim J, Ro JS, Lee JL, Yoon YS, Park IJ, Lim SB, Yu CS, Kim JC. Prognostic impact of extranodal extension in rectal cancer patients undergoing radical resection after preoperative chemoradiotherapy. Clin Colorectal Cancer. 2021;20:e35–e42. doi: 10.1016/j.clcc.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Puppa G, Ueno H, Kayahara M, Capelli P, Canzonieri V, Colombari R, Maisonneuve P, Pelosi G. Tumor deposits are encountered in advanced colorectal cancer and other adenocarcinomas: an expanded classification with implications for colorectal cancer staging system including a unifying concept of in-transit metastases. Mod Pathol. 2009;22:410–415. doi: 10.1038/modpathol.2008.198. [DOI] [PubMed] [Google Scholar]
- 10.Lino-Silva LS, Anchondo-Nunez P, Chit-Huerta A, Aguilar-Romero E, Morales-Soto J, Salazar-Garcia JA, Guzman-Lopez CJ, Maldonado-Martinez HA, Meneses-Garcia A, Salcedo-Hernandez RA. Stage I-III colon cancer patients with tumor deposits behave similarly to stage IV patients. Cross-section analysis of 392 patients. J Surg Oncol. 2019;120:300–307. doi: 10.1002/jso.25482. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Xing S, Li J, Yang S, Hu J, Liu H, Du F, Yin J, Liu S, Li C, Yuan J, Lv B. Novel lymph node ratio predicts prognosis of colorectal cancer patients after radical surgery when tumor deposits are counted as positive lymph nodes: a retrospective multicenter study. Oncotarget. 2016;7:73865–73875. doi: 10.18632/oncotarget.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, Colditz GA. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamas K, Walenkamp AM, de Vries EG, van Vugt MA, Beets-Tan RG, van Etten B, de Groot DJ, Hospers GA. Rectal and colon cancer: not just a different anatomic site. Cancer Treat Rev. 2015;41:671–679. doi: 10.1016/j.ctrv.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61:794–797. doi: 10.1136/gutjnl-2012-302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belt EJ, van Stijn MF, Bril H, de Lange-de Klerk ES, Meijer GA, Meijer S, Stockmann HB. Lymph node negative colorectal cancers with isolated tumor deposits should be classified and treated as stage III. Ann Surg Oncol. 2010;17:3203–3211. doi: 10.1245/s10434-010-1152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pricolo VE, Steingrimsson J, McDuffie TJ, McHale JM, McMillen B, Shparber M. Tumor deposits in stage III colon cancer: correlation with other histopathologic variables, prognostic value, and risk stratification-time to consider “N2c”. Am J Clin Oncol. 2020;43:133–138. doi: 10.1097/COC.0000000000000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al Sahaf O, Myers E, Jawad M, Browne TJ, Winter DC, Redmond HP. The prognostic significance of extramural deposits and extracapsular lymph node invasion in colon cancer. Dis Colon Rectum. 2011;54:982–988. doi: 10.1097/DCR.0b013e31821c4944. [DOI] [PubMed] [Google Scholar]
- 18.Ueno H, Mochizuki H, Shirouzu K, Kusumi T, Yamada K, Ikegami M, Kawachi H, Kameoka S, Ohkura Y, Masaki T, Kushima R, Takahashi K, Ajioka Y, Hase K, Ochiai A, Wada R, Iwaya K, Nakamura T, Sugihara K. Actual status of distribution and prognostic impact of extramural discontinuous cancer spread in colorectal cancer. J. Clin. Oncol. 2011;29:2550–2556. doi: 10.1200/JCO.2010.33.7725. [DOI] [PubMed] [Google Scholar]
- 19.Bai R, Tan Y, Li D, Yang M, Yu L, Yuan Y, Fang X. Development and validation of a novel prognostic nomogram including tumor deposits could better predict survival for colorectal cancer: a population-based study. Ann Transl Med. 2021;9:620. doi: 10.21037/atm-20-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Zhao J, Li C, Wu Y, Song W, Guo T, Chen S, Cai S, Huang D, Xu Y. The unique prognostic characteristics of tumor deposits in colorectal cancer patients. Ann Transl Med. 2019;7:769. doi: 10.21037/atm.2019.11.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Q, Wei Y, Ren L, Zhong Y, Qin C, Zheng P, Xu P, Zhu D, Ji M, Xu J. Tumor deposit is a poor prognostic indicator in patients who underwent simultaneous resection for synchronous colorectal liver metastases. Onco Targets Ther. 2015;8:233–240. doi: 10.2147/OTT.S71414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen R, Shi Q, Meyers J, Jin Z, Svrcek M, Fuchs C, Couture F, Kuebler P, Ciombor KK, Bendell J, De Jesus-Acosta A, Kumar P, Lewis D, Tan B, Bertagnolli MM, Philip P, Blanke C, O’Reilly EM, Shields A, Meyerhardt JA. Combining tumor deposits with the number of lymph node metastases to improve the prognostic accuracy in stage III colon cancer: a post hoc analysis of the CALGB/SWOG 80702 phase III study (Alliance)☆. Ann Oncol. 2021;32:1267–1275. doi: 10.1016/j.annonc.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delattre JF, Cohen R, Henriques J, Falcoz A, Emile JF, Fratte S, Chibaudel B, Dauba J, Dupuis O, Becouarn Y, Bibeau F, Taieb J, Louvet C, Vernerey D, Andre T, Svrcek M. Prognostic value of tumor deposits for disease-free survival in patients with stage III colon cancer: a post hoc analysis of the IDEA France phase III trial (PRODIGE-GERCOR) J. Clin. Oncol. 2020;38:1702–1710. doi: 10.1200/JCO.19.01960. [DOI] [PubMed] [Google Scholar]
- 24.Song YX, Gao P, Wang ZN, Liang JW, Sun Z, Wang MX, Dong YL, Wang XF, Xu HM. Can the tumor deposits be counted as metastatic lymph nodes in the UICC TNM staging system for colorectal cancer? PLoS One. 2012;7:e34087. doi: 10.1371/journal.pone.0034087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei JP, Zhang CD, Liang Y, Zhang C, Wu KZ, Li YZ, Zhao ZM, Dai DQ. A modified pathological N stage including status of tumor deposits in colorectal cancer with nodal metastasis. Front Oncol. 2020;10:548692. doi: 10.3389/fonc.2020.548692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Yang S, Hu J, Liu H, Du F, Yin J, Liu S, Li C, Xing S, Yuan J, Lv B, Fan J, Leng S, Zhang X, Wang B. Tumor deposits counted as positive lymph nodes in TNM staging for advanced colorectal cancer: a retrospective multicenter study. Oncotarget. 2016;7:18269–18279. doi: 10.18632/oncotarget.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno H, Mochizuki H, Shirouzu K, Kusumi T, Yamada K, Ikegami M, Kawachi H, Kameoka S, Ohkura Y, Masaki T, Kushima R, Takahashi K, Ajioka Y, Hase K, Ochiai A, Wada R, Iwaya K, Nakamura T, Sugihara K Study Group for Tumor Deposits without Lymph Node Structure in Colorectal Cancer projected by the Japanese Society for Cancer of the Colon and Rectum. Multicenter study for optimal categorization of extramural tumor deposits for colorectal cancer staging. Ann Surg. 2012;255:739–746. doi: 10.1097/SLA.0b013e31824b4839. [DOI] [PubMed] [Google Scholar]
- 28.Tong LL, Gao P, Wang ZN, Song YX, Xu YY, Sun Z, Xing CZ, Xu HM. Is the seventh edition of the UICC/AJCC TNM staging system reasonable for patients with tumor deposits in colorectal cancer? Ann Surg. 2012;255:208–213. doi: 10.1097/SLA.0b013e31821ad8a2. [DOI] [PubMed] [Google Scholar]
- 29.Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O’Neil BH, Atkins JN, Berry S, Polite BN, O’Reilly EM, Goldberg RM, Hochster HS, Schilsky RL, Bertagnolli MM, El-Khoueiry AB, Watson P, Benson AB 3rd, Mulkerin DL, Mayer RJ, Blanke C. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317:2392–2401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Kaiser F, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Moehler M, Scheithauer W, Held S, Miller-Phillips L, Modest DP, Jung A, Kirchner T, Stintzing S. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br J Cancer. 2020;124:587–594. doi: 10.1038/s41416-020-01140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vigano L, Russolillo N, Ferrero A, Langella S, Sperti E, Capussotti L. Evolution of long-term outcome of liver resection for colorectal metastases: analysis of actual 5-year survival rates over two decades. Ann Surg Oncol. 2012;19:2035–2044. doi: 10.1245/s10434-011-2186-1. [DOI] [PubMed] [Google Scholar]
- 32.Muratore A, Ribero D, Zimmitti G, Mellano A, Langella S, Capussotti L. Resection margin and recurrence-free survival after liver resection of colorectal metastases. Ann Surg Oncol. 2010;17:1324–1329. doi: 10.1245/s10434-009-0770-4. [DOI] [PubMed] [Google Scholar]
- 33.Lemke J, Cammerer G, Ganser J, Scheele J, Xu P, Sander S, Henne-Bruns D, Kornmann M. Survival and prognostic factors of colorectal liver metastases after surgical and nonsurgical treatment. Clin Colorectal Cancer. 2016;15:e183–e192. doi: 10.1016/j.clcc.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Moris D, Ronnekleiv-Kelly S, Rahnemai-Azar AA, Felekouras E, Dillhoff M, Schmidt C, Pawlik TM. Parenchymal-sparing versus anatomic liver resection for colorectal liver metastases: a systematic review. J Gastrointest Surg. 2017;21:1076–1085. doi: 10.1007/s11605-017-3397-y. [DOI] [PubMed] [Google Scholar]