Abstract
BACKGROUND:
Little is known about the adherence rate to Allergen Immunotherapy (AIT) labeling guidelines.
OBJECTIVE:
Assess adherence to labeling guidelines of AIT Practice Parameter 2011 at University of Michigan Health Service (UMHS).
METHODS:
AIT vials of 320 patients receiving their care at UMHS were reviewed. Data collected looked at patient identifiers (PI), concentrations in volume/volume (v/v) format, color-coding, allergen content, expiration date and instructions about AIT dosing and systemic reaction treatment. Data was analyzed using chi square/fisher exact tests and logistic regression.
RESULTS:
Of 238 non-University formulated labels, 65% had 2 PI, 62% had a v/v concentration, 41% had color coding, 71% had the content listed and 100% had a recorded expiration date. Only 21% had all 5 recommended components. All 82 University vials had 5 components. Labels with 2 PI were more likely to have a v/v concentration with its corresponding color coding (OR 3.84, p<0.001, CI 1.9–7.7). Labels specifying the extract’s content were more likely to be color coded or to have a v/v concentration listed (OR 6.3, p<0.001, CI 3.4–11.8). For all AIT vials, complete labels were significantly more likely to have clear buildup schedule (OR 9.6, p<0.001, CI=4.2–23.2), dosing adjustment after a missed dose (OR 8.2, p<0.001, CI 3.4–19.8) or after a reaction (OR 13.7, p<0.001, CI 7.8–2.1) and clear systemic reaction treatment instructions (OR 9.7, p<0.001, CI 7.8–24.1).
CONCLUSION:
Less than 25% of the non-University prescribers adhered with AIT Practice Parameters 5 years post-publication. Recording 2 PI, v/v concentration or color-coding increased the likelihood of having a complete label. Complete label contents were associated with clear instructions about AIT dosing and reaction treatment/dose adjustments.
Keywords: allergen immunotherapy, practice parameters, practice guidelines, immunotherapy labeling, allergen extracts, student health services, adherence, health services research
Introduction:
Allergen immunotherapy (AIT) was first used successfully in 1911 by Leonard Noon, who noticed an association between allergic symptoms and the grass pollination season in England1. Recent advances in the clinical application of AIT, as recommended by recent practice parameters, have aimed to significantly improve AIT quality and its clinical outcomes2–5. AIT clinical outcomes require the provision of high quality and safe AIT extract preparation and labeling. There has been an iterative process in terms of expanding labeling guidelines during the past 11 years and now 3 sets of published AIT guidelines exist2–5, most recently the 3rd AIT practice parameter update published in 20115. Additional up-to-date references are available for the AIT prescriber and the preparers of AIT extracts in addition to the practice parameters, including allergen extract manufacturer preparation manuals8–11, Allergen Immunotherapy Extract Preparation Manual6, and the United Sates Pharmacopeia’s General Chapter 797 (USP 797)7.
Guidelines recommend that all extract vials label should contain two or more patient identifiers (PI)2–5, expiration date or beyond use date2–5, abbreviations or names of allergen content or a link to a document that lists specific allergen content2–5. Labels should also include the concentration of each vial listed in a volume to volume (v/v) format with 1:1 indicating the maintenance concentrate. During the build-up phase of immunotherapy, four (and in some instances five) dilutions of the patient’s maintenance concentrate are needed. Guidelines recommend using a color-coding system with a red cap indicating the 1:1 v/v concentrate, yellow for the 1:10 v/v, blue for the 1:100 v/v, green for 1: 1,000 v/v and silver for the 1:10,000 v/v dilution (if a fifth dilution is necessary)2–5. It is also permissible, per the practice parameter recommendations, to use an alphanumeric system beginning at “1” or “A” for the maintenance vials or to list the concentrations of allergens in each vial as alternative labeling methods3–5. However, these may be a source of confusion for healthcare personnel administering allergy injections, especially if one chose to label in a reverse order with “5” being the concentrate.
The effects of labeling variation, or errors resulting from such labeling variation, have not undergone robust prior study. Differences in labeling of AIT extracts can potentially lead to confusion, dosing errors, and increased systemic reactions in non-Allergy healthcare settings (such as a primary care office or college health clinic) who administer but do not prescribe AIT. Such settings handle AIT written by numerous, differing providers (from both Allergists and Otolaryngologists) which may not be similar in style and have to handle high degrees of labeling disparities that may potentially exist. We thus conducted a study at the University of Michigan (U of M) University Health Services Center (UHS) to determine the rate of adherence of non-U of M referring prescribers of patients receiving AIT at UHS to the AIT practice parameters guidelines. UHS is a health clinic servicing the U of M undergraduate and graduate campuses, provides the opportunity for patients to continue to receive AIT injections from multiple independent providers using their originally prescribed and mixed serum extract.
Methods:
AIT extract vials labels of all 320 patients receiving their care at UHS during the fall of 2013 were reviewed. UHS is a health care clinic located on the central campus of the U of M. It is accredited by the Accreditation Association for Ambulatory Health Care, Inc. (AAAHC), a nationally recognized organization. With approximately 80,000 total visits per year and approximately 5000 AIT injection visits per year, UHS is a highly utilized campus resource. UHS services are available to any enrolled undergraduate/graduate student, faculty/staff and retirees, alumni, and spouses/domestic partners/dependents of these individuals. All AIT extract vials of patients receiving their injections at UHS are stored in alphabetical order in one refrigerator. Labels of all 320 available AIT extract vials in the storage refrigerator were checked for the different elements recommended by the third update AIT practice parameter including:
Two PI, including patient’s full name, date of birth, medical record number2–5
Color or an alphanumeric-coding system which indicates a dilution from the corresponding maintenance concentrate vial3–5
Corresponding medical records for each AIT extract vial were screened for presence of a clear buildup schedule stipulated by the prescriber, as well as for the presence of instructions/guidance of a clear definition of the signs of a reaction, treatment instructions of a local or systemic reaction, clear instructions about AIT dosing adjustment after a reaction, and clear instructions on dosing adjustments after a missed scheduled dose. All data were recorded into a MS Excel (Redmond, WA) spreadsheet for data keeping. Finally we performed a web-based search of each provider listed for the external prescribers, using the American Board of Medical Specialties (www.abms.org, for Allergy/Immunology and for Otolaryngology) and both the American Academy of Allergy, Asthma, and Immunology (www.aaaai.org) and the American College of Allergy, Asthma and Immunology (www.acaai.org) websites to determine the number of years that each prescribing provider has been in practice. AIT written by U of M Allergy/Immunology faculty were not included for final analysis, per pre-specified intent, because all U of M Allergy/Immunology prescribed labels are adherent with the 5 components of the labeling guidelines described above.
The specific outcomes of this study were to determine the extent to which external providers to UHS complied with labeling guidelines and assess factors associated with labeling adherence. Data were uploaded from the excel spreadsheet into Stata 13 SE (College Station, TX), and analyzed for descriptive attributes using frequency analysis, as well analyzed for inferential attributes using chi square, fisher exact tests, and bivariate logistic regression where appropriate. A predictive model of adherence was formulated using linear regression and analysis of marginal means. This study was determined to be exempt from ongoing review by the University of Michigan Medical School IRB (IRBMED) under a waiver for quality assurance/quality improvement research.
Results:
A total of 320 AIT extract vials labels were reviewed, 238 of which were from patients from independent prescribers and 82 belonged to patients from U of M affiliated prescribing physicians with University-formulated IT extracts. These 82 patients were restricted from analysis, as all vials were adherent to all aspects of the latest practice parameter labeling guidelines.5 The characteristics of the prescribing physicians are summarized in Table I.
Table 1.
Characteristics of the AIT prescribing physicians
| Characteristics of the non-University prescribing physicians (n= 114) | |
|---|---|
| Specialty of prescribers | Allergy n= 107 |
| Otolaryngology n= 7 | |
| Location of practice | Michigan n= 55 |
| Out-of-state n=59 | |
| Allergy/Immunology board certified | Certified n= 102 |
| Non-certified n= 12 | |
| Number of years in practice | ≤10 years n= 20 |
| >10 years n= 94 | |
Of 238 non-U of M formulated AIT extract vials, 65.5% (n=156) had 2 PI, of which 91.7% (n=143) included the patient’s name and date of birth and 8.3% (n=13) included the patient’s name and medical record number. Other identifiers used included the prescribing physician’s name (n=86), the clinic name (n=21), barcode (n=1), lot number (n=1), and the patient’s picture (n=3). For extract concentration labeling, 62% (n=148) of the vials had a v/v concentration; 41% (n=98) were color-coded, and 2% (n=4) were alphanumerically coded. In terms of extract content labeling, 71% (n=169) of the labels had allergen content listed, of which 64% (n=108) had the content fully written and 36% (n=61) abbreviated (e.g., T for Trees, G for grass, etc.). Finally, all the labels analyzed had a recorded expiration date. Only 20.6% of the 238 non-U of M formulated AIT extract vials had a complete label, which per the 2011 practice parameters indicated the following elements were necessary: two PI, concentration of the vial in v/v format, allergen content and expiration (or beyond use date), and either color or alphanumerically coding as per guidelines recommendations (Figure 1).5
Figure 1. Number of Recommended Label Components.
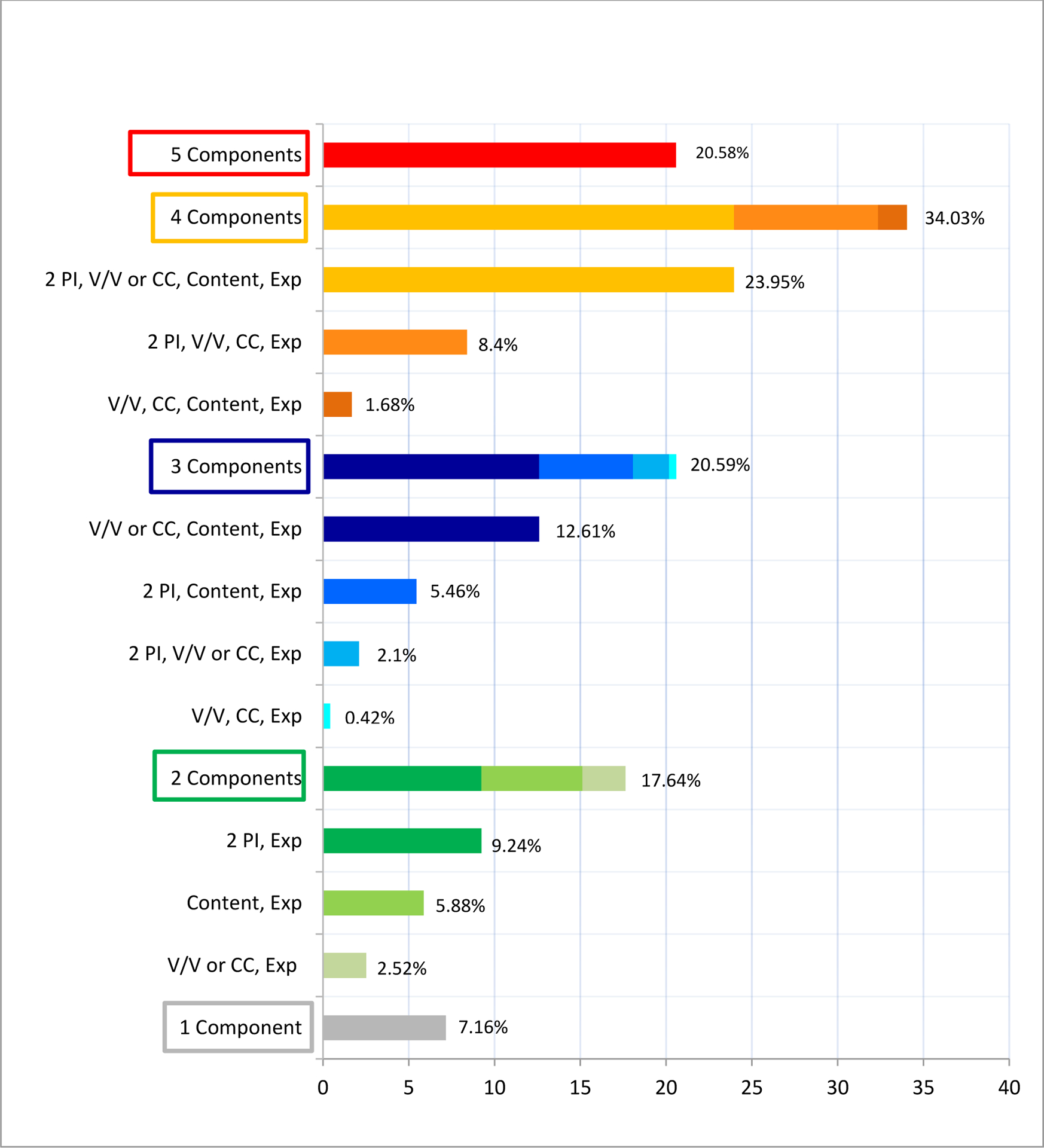
PI, Patient identifiers; V/V, concentration in volume to volume format; CC, color coding; Exp, expiration date.
Labels with 2 or more PI had 3.8 times higher odds to have both a v/v concentration with its corresponding color-coding (OR 3.8; p<0.001; CI 1.9–7.7). Labels specifying the extract’s content had higher odds to have a v/v concentration listed (OR 3.8; p<0.001; CI 1.8–7.9) and had 6 times higher odds to be either color coded or to have a v/v concentration listed (OR 6.3; p<0.001; CI 3.4–11.8) AIT extract vials that were color coded and had a complete label had higher odds of having clear buildup schedule (OR 12.6, P<0.001, CI 4.4–35.9) provided in the corresponding patient chart, and were more likely to have clear instructions about dosing adjustment after a missed AIT injection (OR 8.2; p<0.001; CI 3.2–21.5) and after a systemic reaction (OR 12.7; p<0.001; CI 7.2–22.4). These vials were also 10 times higher odds to have clear instructions about how to treat a systemic reaction (OR 9.7; p<0.001; CI 2.9–32.6); (Table II).
Table 2.
Trends of AIT Extract Vials Labeling, Dosing and Treatment Instructions
| Trend | OR | P | 95% Cl |
|---|---|---|---|
| If 2 patient identifiers present: | |||
| v/v listed | 2.2 | 0.005 | 2.3–3.8 |
| Color coding present | 2.8 | 0.001 | 1.6–5.1 |
| Has both v/v & color coding | 3.84 | <0.001 | 1.9–7.7 |
| If content present: | |||
| v/v listed | 3.8 | <0.001 | 1.8–7.9 |
| Color coding present | 11.2 | 0.019 | 1.5–84.5 |
| Has either v/v or color coding | 6.3 | <0.001 | 3.4–11.8 |
| If complete label present, clear instructions about the following also present: | |||
| Clear dosing buildup schedule | 12.6 | <0.001 | 4.4–35.9 |
| Adjustment for missed dose | 8.2 | <0.001 | 3.2–21.5 |
| Instructions for treatment of a reaction | 9.7 | <0.001 | 2.9–32.6 |
| Dosing adjustment after a reaction | 12.7 | <0.001 | 7.2–22.4 |
v/v, volume to volume
An additional, pre-specified analysis was targeted towards exploring the relationship between labeling content adherence and years in practice. Time in practice has been previously identified as a factor that is influential of adherence and adherence with guidelines18–21. When we adjusted for AIT extract vials labels having both 2 PI and allergen content listed, the predicted probability of having both a v/v concentration on the label and the AIT vial being color coded decreased by 4% for every year in practice of the respective prescribing physician. (Figure 2)
Figure 2. Predicted probability of color coding & V/V concentration labeling compliance.
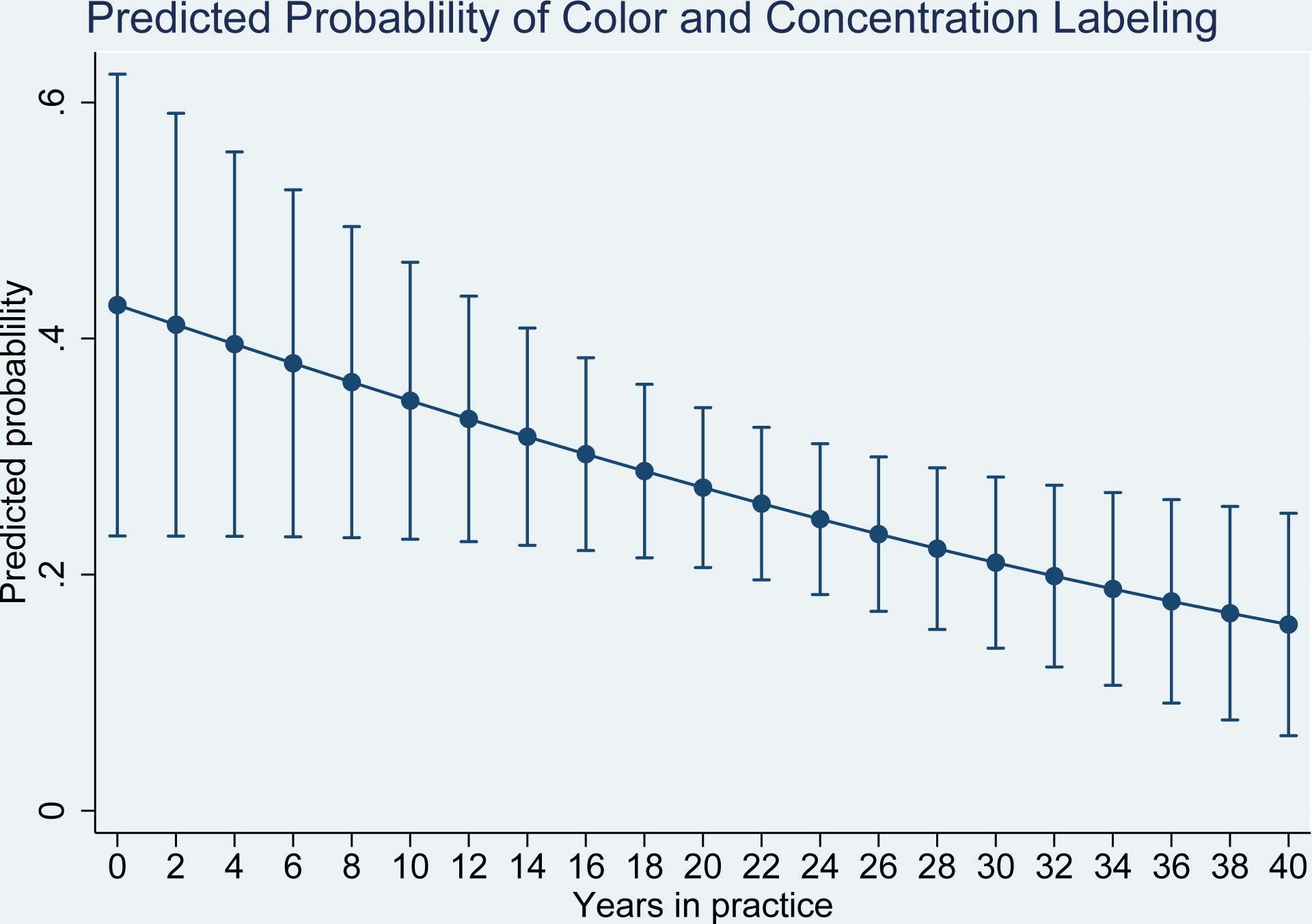
Relationship between compliance with allergen extract labels containing both color coding and v/v concentration information as a function of number of years of practice. Error bars represent 95% confidence intervals.
Discussion:
Allergen immunotherapy (AIT) has been a staple and distinguishing feature of clinical allergy practice for over a century1. The first clinical practice guidelines for AIT were only recently published in 1994, but have been updated 3 times in the past 20 years, most recently in 20115. Within this time frame there have been comprehensive recommendations to the practice of AIT that include use of standardized dosing regimens for selected antigens, more efficient mixing processes that minimize the effects of protease on extract stability, better classification and monitoring of systemic reactions, use of concentrate for maintenance extracts, and comprehensive, standardized labeling guidelines to enhance communication and improve safety. These guideline changes have been positioned to enhance AIT clinical outcomes, and there remains a continued push to further standardize the AIT process in order to improve patient safety and improve communication between healthcare providers.
While much of the focus of AIT practice improvement is globally targeted at the reduction and prevention of systemic reactions to immunotherapy11–13, there are many potentially overlooked areas of importance, such as patient outcomes related to the safety of extract mixed by an allergist but administered at a non-allergist’s office because of patient-level convenience and logistical factors. This is a common occurrence that arises secondary to access to care issues or for convenience to the patient, and results in allergy extracts that are prepared by specialists are subsequently administered in the offices of primary care physicians or specialists or other health service facilities. These practices may not know one another, and the practice administering the AIT likely does not have familiarity with inter-office notation or convention “style” that may be regularly used for AIT in the specialist office. Such jargon or inside familiarity may not translate to the external practice. These aspects may be neglected when AIT is distributed to that outside practice for administration. For Universities and other institutes of higher learning, it is a relatively common occurrence to accommodate students from outside the area, to allow students to continue already established therapy. Differences in labeling of AIT extracts can potentially lead to increased confusion and possible errors for healthcare personnel administering the AIT, especially in the setting of a campus health service that may not also have a specialty allergy clinic or experienced AIT nursing to administer injections. However, to our knowledge, there is no literature describing labeling adherence or the ramifications that could occur as a result.
This study was exploratory, to investigate the extent to which labeling variation may occur. Our site was ideal for this since we have a robust UHS allergy service for AIT, a large number of prescribers external to the University over which to explore prescribing variation, and have a “gold standard” control population of n=82 internally written AIT prescriptions that were 100% adherent to the latest practice parameter recommendations for comparison of effect. Overall there is limited data regarding allergist adherence with any of the updated practice parameter guidelines in the past 11 years. However, there is no previous data, in particular, with respect to vial label guideline adherence and the proxy experience in administering AIT written by an outside office.
In the UHS population of AIT written by external providers, we found that less than 25 % of AIT prescribers were adherent to AIT practice parameter guidelines as reflected by the high number of incomplete labels reviewed, compared to 100% of AIT prescriptions written by U of M Division of Allergy and Clinical Immunology faculty. These issues raise concern for patient safety, considering we show a strong association between having labels with 2 or more PI and both v/v concentration and color coding (what we declared a “completely adherent” label) and adherence to other safety measures, such has having instructions in how to modify the dosage schedule if the patient does not adhere to the recommended buildup schedule, or how to both modify a dose and if a reaction occurs as well as how to treat a reaction.
The adherence issue is important because these patients are being provided AIT in a venue that may not be staffed by an allergist and is not a formal allergy office, and therefore guidance on how to manage a local or systemic reaction in particular is essential to avoid potentially catastrophic patient-care outcomes. Perhaps more concerning are the data suggesting a trend in the odds of adherence with what is considered to be a “complete label” decreased among this prescribing population in a linear fashion by 4% for each year in practice. This suggests a fundamental problem in implementation of updated allergy practice parameters for AIT, considering that it has been 11 years since the 2003 recommendations for color coding (summary statement 90 in the 2011 practice parameters), and 7 years since the 2007 recommendation for v/v and 2 PI on the label (summary statement 87 in the 2011 practice parameters). Additionally, this suggests potential problems with adherence to the Joint Commission National Patient Safety Goals that reinforce identification of patients using two identifiers.22 An alternative explanation may be that established practices continue to use labeling system which predates the 2003 and 2007 practice parameters, and are still in the process of transition toward the uniform recommended labeling system. Though the practice parameters suggests that transition toward the recommended uniform labeling system should be done gradually to reduce potential errors,5 our data raise concern that such a transition may not even be occurring, given 6–7 years later most practices were not in compliance.
There are several limitations to this study. The study was exploratory for descriptive differences in adherence to recommendations for labeling, and did not investigate if such lack of adherence, as described, actually led to a higher rate of adverse events. This is a subject presently being studied by our group. Although our patient population transferred their care to UHS from diverse geographical location, it is hard to generalize our 21% adherence rate to providers in other states without further studies. As well, most of our providers have been in practice for >20 years, which may create a bias towards older practice styles, and there are no data we are aware of, to date, that demonstrate any risk from lack of adherence. We have not assessed provider experience or personal views on the parameter updates, and have not assessed the effect of provider sub-specialty fellowship training on adaption and implementation of new information. The standard for determining the clarity of AIT dosing scheduling instructions, reactions definition and treatment instructions was done by our group of academic allergists, and could be considered biased and/or excessive compared to what may be the “community” standard for Allergists or Otolaryngologists who prescribe AIT (who would not necessarily be inclined to follow AAAAI/ACAAI guidelines). Motivations for adherence or lack thereof, and providers’ willingness to make a requested change to labeling were also not considered and are being investigated in an ongoing study. Lastly, UHS does not have a policy to which it holds outside AIT prescribers in terms of what labeling components are expected for extracts administered remotely in the UHS office.
This is, to our knowledge, the first study that explores adherence to AIT extract labeling guidelines, and is the first description of remote AIT prescribing behavior in a University Health Services setting. We note poor adherence with labeling guidelines among the outside prescribers of AIT administered at UHS, and it is worrisome that such variation demonstrated an inversely proportional, linear relationship to time in practice, a specific finding requiring further follow-up study. Such variation highlights the significant potential for adverse events that could result from AIT not labeled to the most current practice parameter recommendations. These data suggest there is considerable room for improvement in adherence to these guidelines, all of which have been recommended for several years. We are conducting ongoing study to evaluate both provider motivations to comply with guidelines, and the effects of labeling variation or poor adherence on dosing errors and adverse events. Adherence with AIT clinical practice is important to patient safety which is especially important in the practice of administering AIT in settings which are not allergy offices—such sites generally lack robust experience in providing AIT compared to an Allergy practice, and likely lack direct access to board-certified allergists when issues arise. Therefore, maximizing labeling adherence is even more critical for such settings, given the potential for error that can be made based on any existing variation.
What is known about this topic:
There are exceptionally limited data assessing prescribers’ adherence to AIT labeling guidelines.
What does this study add to our knowledge:
This study highlights poor adherence to AIT extract vials labeling guidelines and notes this as a potential function of the number of years in practice.
How does this study impact current management guidelines:
This study highlights the potential for adverse events that could result from non-adherence to AIT extract vial labeling guidelines, and raises questions about reasons of non-adherence and ways to best implement practice guidelines.
Disclosures:
Dr. Greenhawt receives support from NIH grant #2KL2TR000434, has received support from NIH grant #UL1RR024986, and receives support from the University of Michigan Food Allergy Center. He is an associate editor for Annals of Allergy, Asthma, and Immunology and receives honoraria for that. He has no other conflicts relevant to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noon BC Prophylactic inoculation against hay fever. The Lancet 1911;177:1572–1573. [Google Scholar]
- 2.American Academy of Allergy and Immunology. Guidelines to minimize the risk from systemic reactions caused by immunotherapy with allergenic extracts. J Allergy Clin Immunol 1994;93:811–2. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter. Ann Allergy Asthma Immunol 2003; 90(suppl 1):1–40. [PubMed] [Google Scholar]
- 4.Cox L, Li J, Lockey R, Nelson H. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol 2007;120(suppl):S25–85, IV. [DOI] [PubMed] [Google Scholar]
- 5.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1–55. Erratum in: J Allergy Clin Immunol 2011 ;127: 840. [DOI] [PubMed] [Google Scholar]
- 6.Nelson MR, Cox L . Allergen immunotherapy extract preparation guidelines, in Lee, T, editor. Practice Management Resource Guide (2012. edition). http://www.aaaai.org/practice-esources/tools-andtechnology/practice-management-resources/practice-managementresource-guide.aspx. Accessed May 10, 2014.
- 7.US Pharmacopeial Convention, Inc. United States Pharmacopeia 27, Supplement 1. Chapter <797> “Pharmaceutical Compounding-Sterile Preparations”. Revision Bulletin. Rockville, MD: US Pharmacopeial Convention, Inc.; 2008:16. [Google Scholar]
- 8.Cox L, Esch RE,Corbett M, Hankin C, Nelson M, Plunkett G. Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes. Ann Allergy Asthma Immunol 2011. ; 107: 289–99. [DOI] [PubMed] [Google Scholar]
- 9.Nelson M, Petersen M, Wolverton W, Mikita C. Allergen Immunotherapy Extract Treatment Set Preparation : Making a Safer and Higher Quality Product for Patients. Curr Allergy Asthma Rep 2013; 13: 399–405 [DOI] [PubMed] [Google Scholar]
- 10.Nelson HS. Preparing and mixing allergen vaccines for subcutaneous immunotherapy. Clin Allergy Immunol 2008;21:303–20 [PubMed] [Google Scholar]
- 11.Esch RE, Plunkett GA. Immunotherapy preparation guidelines, rules, and regulation. Curr Allergy Asthma Rep 2013; 13:406–13 [DOI] [PubMed] [Google Scholar]
- 12.Bernstein DI, Wanner M, Borish L, Liss GM, and Immunotherapy Committee, American Academy of Allergy, Asthma and Allergy. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J Allergy Clin Immunol 2004;113: 1129–1136 [DOI] [PubMed] [Google Scholar]
- 13.Aaronson DW, Gandhi TK. Incorrect allergy injections: allergists’ experiences and recommendations for prevention. J Allergy Clin Immunol 2004; 113: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 14.Borchers AT, Ken CL, Greshwin ME. Fatalities following allergen immunotherapy. Clin Rev Allergy Imunnol 2004; 2 7: 147–158 [DOI] [PubMed] [Google Scholar]
- 15.Charron M, Kramer J, Crocetti S. Allergy immunotherapy in the primary care setting. JNurs Care Qual 2006; 21:187–193 [DOI] [PubMed] [Google Scholar]
- 16.Lay PC. Injectable immunotherapy: recommendations for safe allergen vial preparation in the office setting. Curr Opin Otolaryngol Head Neck Surg 2009;17: 223–5 [DOI] [PubMed] [Google Scholar]
- 17.Lin SY, Houser SM, Gross G, Aaronson D. Impact of newly revised sterile medication compounding guidelines USP {797} on allergy vial preparation. Otolaryngol Head Neck Surg 2008; 139: 5–6. [DOI] [PubMed] [Google Scholar]
- 18.Greenhawt M, Wang J. Physician compliance with updated practice parameters for influenza vaccination in individuals with egg allergies. J Allergy Clin Immunol-In Practice 2013;1:602–7 [DOI] [PubMed] [Google Scholar]
- 19.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abbound PA, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–65. [DOI] [PubMed] [Google Scholar]
- 20.Marceau L, McKinlay J, Shackelton R, Link C. The relative contribution of patient, provider and organizational influences to the appropriate diagnosis and management of diabetes mellitus. J Eval Clin Pract 2011;17:1122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conway PH, Edwards S, Stucky ER, Chiang VW, Ottolini MC, Landrigan CP. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics 2006;118:441–7. [DOI] [PubMed] [Google Scholar]
- 22.The Joint Commission Electronic Accreditation & Certification Manual. Two Patient Identifiers-NPSG-Goal 1. Publication date December 9, 2008. Accessed at http://www.jointcommission.org/standards_information/jcfaqdetails.aspx7StandardsFaqId=145&ProgramId=47. Accessed June 26, 2014. [Google Scholar]


