Abstract
Recent advances in agarose gel electrophoresis protocols established conditions for the high-resolution separation of DNA and RNA using higher voltages combined with short run times. We subsequently developed a protocol for using these conditions to measure the binding affinity of a protein for an RNA ligand on an agarose gel. This native gel mobility shift assay is highly accessible, using common molecular biology reagents found in most laboratories. Here, we describe the protocol for carrying out native agarose gel electrophoresis to characterize the binding affinity of a protein for an RNA ligand. The electrophoresis time is less than 10 min, which minimizes the dissociation of protein and ligand. We have used the p19 siRNA binding protein and its cognate dsRNA ligand to demonstrate strategies for identifying optimal conditions to measure apparent binding constants using this agarose gel shift system.
Keywords: Gel shift, Mobility shift assay, EMSA, RNA binding assay, Native agarose gel
1. Introduction
Native gel electrophoretic mobility shift assays (EMSAs) are widely used to measure the association between biomolecules, such as proteins and nucleic acid oligonucleotides [1]. While this method is inherently non-equilibrium, it requires very little specialized equipment. As a result, an EMSA can be a highly accessible approach for evaluating interactions between biological macromolecules [2]. Conventional EMSA protocols use vertical polyacrylamide gels, which can involve handling of neurotoxic unpolymerized acrylamide solution [3].
Building on previous advances in horizontal agarose gel electrophoresis [4, 5], we have established a method for separating protein-RNA complexes on high-percentage agarose gels in less than 10 min [6]. The short run time is especially useful for EMSAs, as it reduces the amount of time that could allow the bound complex to dissociate. The oligonucleotide ligand is detected using SYBR Gold, a low-toxicity stain that can detect nanogram quantities of nucleic acid. This novel system was used to determine the apparent binding constant for the p19 siRNA-binding protein and its cognate dsRNA ligand. The binding affinity was found to be comparable to the apparent binding constant obtained from a conventional polyacrylamide gel assay performed concurrently [6].
Because this method requires only standard molecular biology reagents and equipment, it is affordable and accessible to a wide range of laboratories. Here, we describe our protocol for rapid native agarose gel electrophoresis to measure quantitatively the association of protein and RNA.
2. Materials
2.1. General Reagents
Double-deionized water for buffers.
Autoclaved water for reactions (see Note 1).
2.2. Gel Electrophoresis
2.3. Binding Reaction Components
Purified protein of interest.
Purified RNA oligonucleotides of interest.
RNA dilution buffer: 5 mM Tris–HCl, pH 8.0 at room temperature.
Binding buffer (see Note 3).
30% glycerol.
2.4. Quantitation
Gel imaging system (Alpha Innotech Imager, BioRad XRS+ ChemiDoc system, or similar).
Imaging software capable of quantitation (ImageQuant, ImageJ, ImageLab, or similar).
Software capable of nonlinear multivariate curve fitting (SigmaPlot, Kaleidagraph, or similar).
3. Methods
3.1. Annealing Complementary Single-Stranded RNA Oligonucleotides
Prepare equimolar mixtures of single-stranded RNA oligonucleotides in the buffered solution of choice. For the reactions described below, each single-stranded RNA was resuspended in nuclease-free water to a stock concentration of ~140 μM. Annealing reactions contained 3.8 μM of each RNA oligonucleotide in RNA dilution buffer.
Gently mix the solution.
Incubate at 90 °C in a dry heating block for 4 min.
Remove tubes to room temperature and allow to equilibrate for 30 min.
Confirm annealing with a 3.5% agarose gel (see Subheadings 3.2 and 3.4).
Store annealed RNA in small aliquots at −20 °C and avoid freeze-thaw cycles. Under these conditions, we found that the annealed dsRNA was stable for several months at −20 °C.
3.2. Preparing a High-Percentage Agarose Gel
Seal the ends of an agarose gel tray with gel-sealing tape, and place it on a level surface in a 60 °C incubator for 2–5 min to prewarm the plastic.
Meanwhile, prepare a solution of 2.5–3.0% agarose dissolved in 0.5× TB buffer (see Notes 4 and 5).
With the gel tray still in the incubator, pour the dissolved agarose solution into the tray. Place the comb and incubate at 60 °C for 2 min (see Note 6).
Remove the gel tray from the incubator, and allow the gel to solidify at room temperature on a level bench for at least 20 min.
3.3. Binding Reaction
Set up the binding reactions in a total volume of 12–14 μL (see Note 7).
Allow reactions to equilibrate under appropriate conditions of time and temperature. Note that several incubation times may need to be evaluated to confirm that the binding reaction has reached equilibrium. For p19, we incubated at room temperature for 1 h.
If glycerol was not included in the binding buffer, add 30% glycerol to each reaction to bring to a final concentration of 5% (v/v) glycerol in preparation for gel loading.
3.4. Running the Gel
Remove the gel tape and comb, and place the gel tray in the gel rig, making sure the gel is completely submerged in fresh 0.5× TB buffer. The level of the buffer should be no more than 4–5 mm above the surface of the gel [4] (see Note 8).
Load ~13 μL of binding reaction + glycerol (see Note 9).
Set the power supply to 20–30 V/cm (for a 14-cm-long gel that translates to 300–420 V). Electrophorese for 6–10 min (see Note 10).
3.5. Imaging the Gel
Staining: place the gel in a dish, and cover it with 2× SYBR Gold, freshly prepared in 0.5× TB buffer, diluted from the 10,000× SYBR Gold stock solution. Shake vigorously for 40 min at room temperature (see Notes 11 and 12).
Destaining: Decant the staining solution, and replace it with enough 0.5× TB buffer to cover the gel. Shake vigorously for 15 min at room temperature (see Note 12).
Image under UV light in an electronic gel imaging system.
3.6. Quantitation and Calculation
Use gel imaging software (ImageQuant, ImageJ, or similar) to quantitate the free and bound DNA bands (see Note 13).
Evaluate the sum of counts (bound + free) across all lanes. Total counts per lane should be approximately the same, because all of the RNA ligand should be detectable in either partition (bound or free).
Calculate fraction of ligand bound = (bound/(bound + free)).
Plot fraction bound as function of total protein concentration.
- Fit the data with the quadratic form of the binding isotherm to solve for (see Note 14):
where is the total protein concentration, is the total ligand concentration, is the fraction of protein and ligand in complex with one another, and is the apparent dissociation constant.(1)
4. Notes
Water should be double-deionized and sterilized and ideally nuclease-free; however, it does not need to be DEPC-treated. For reaction buffers, we use commercial nuclease-free water (e.g., from integrated DNA technologies).
Be sure to use a staining container that is appropriate for the staining agent; for example, SYBR nucleic acid stains should not be used in glass containers (per manufacturer’s recommendation).
The binding buffer contents and conditions should be optimized for the system of interest, including buffering agent, salt concentration, glycerol content, and incubation temperature [6, 7]. For p19 siRNA binding protein, we used the 1× p19 siRNA binding buffer provided by the manufacturer (20 mM Tris–HCl, 100 mM NaCl, 1 mM EDTA, 1 mM TCEP, 0.02% Tween-20, pH 7.0 at 25 °C) and incubated for 1 h at room temperature (~22–25 °C).
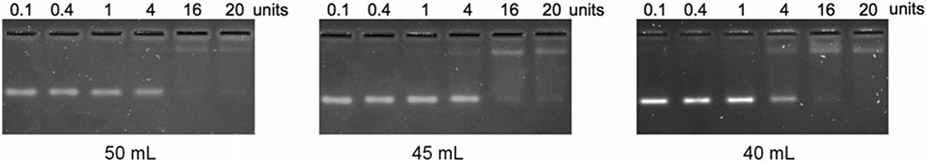
Agarose content should be optimized for the protein/RNA system of interest. We evaluated a range of concentrations from 2.5 to 3.5% agarose. The ~50 mL solution is prepared by microwaving on full power for 45 s, mixing by swirling the flask, and then microwaving on full power in 8 s increments until fully dissolved. We found that 2.5% and 3.0% agarose produced less background fluorescence, which was compatible with SYBR gold staining.
For a gel with dimensions of 11 cm × 14 cm, we prepare gel solutions of 45–55 mL volume. This combination of volume and surface area produces gels that are 6–8 mm thick, which we found to be optimal to reduce background fluorescent signal from the agarose (Fig. 1).
The high agarose content of the gel solution will cause it to solidify quickly if poured at room temperature. By pouring the gel at higher temperatures (60 °C), it prevents distortions in the gel matrix caused by uneven cooling, which would compromise the quality of the electrophoresis.
Ligand concentration should be optimized for the protein and detection systems being used. For high percentage agarose gels that are 6–8 mm thick and stained with SYBR gold, we have found that the minimum amount of dsRNA needed for robust signal over background is ~20 ng.
While 0.5× TB running buffer can be reused for electrophoresis, we found that repeated reuse (more than two electrophoresis runs) resulted in heavily smeared and uninterpretable bands.
To maximize signal, the volume of sample that is loaded onto the gel should be as large as the well can contain. For example, our 10-well gel of dimensions 11 cm × 14 cm × 0.8 cm uses combs that are 1 mm thick, allowing for an approximate well volume of ~ 12–13 μL. If thicker gels or bigger combs are used, more samples can be loaded. Such adjustments may allow for a lower concentration of RNA ligand to be used in the initial binding reaction, since a larger quantity can be loaded into the well.
Gel running time and voltage should be optimized for the system of interest. Higher voltages may allow for faster run times and good band separation but can cause blurry or streaky bands if set too high. We have found that 20–30 V/cm produces the best results in our system (Fig. 2).
For a gel with dimensions of 11 cm × 14 cm × ~0.8 cm, we used 50 mL of staining solution in a container that was ~15% larger than the gel.
Destaining took place in a larger container and used 100–200 mL of destaining solution. SYBR gold has a strong pH dependence, and we found that preparing either the staining or destaining solutions with deionized water resulted in poor signal (Fig. 3). In particular, we observed a significant reduction in band sharpness and intensity when compared to staining and destaining in 0.5× TB. Longer incubations for either staining or destaining (more than 40 min and 15 min, respectively) are not recommended, as they will result in fuzzy and/or undetectable bands due to RNA diffusion within the agarose matrix (Fig. 3).
There are multiple approaches to defining bands for quantitation. Because of the short run times of these experiments, the complex is less likely to dissociate during electrophoresis. The sharp, discrete bands can then be easily defined. However, if the complex dissociates during the run, there may be a smear of SYBR gold signal in the lane (see Fig. 2, left and center panels). Consequently, the entire smear should be included in the definition of “bound” oligonucleotide, using a box that covers the entire lane above the “free” oligonucleotide migration distance.
Because the amount of RNA ligand needed for detection by SYBR gold is likely to be greater than the apparent of the interaction, the binding reaction conditions prevent the common assumption that . As a result, the simplified form of the binding isotherm cannot be used [7]. However, fluorescently or radiolabeled oligonucleotides could be used in place of SYBR gold staining for more sensitive detection of the ligand. By using smaller amounts of ligand, this assumption could then be made, and fitting could be carried out with the simplified form of the binding isotherm [7].
Fig. 1.
Reducing the volume and thickness of the gel improves band signal. 2.5% agarose gel solutions were prepared in volumes of 50, 45, and 40 mL and used to analyze identical binding reactions. All three gels were stained with SYBR Gold and destained in 0.5× TB. The background fluorescence of the SYBR Gold-stained agarose was significantly reduced in the thinner gels. (Data reproduced with permission from Ream et al. [6], Figure 1)
Fig. 2.
Electrophoresis voltage must be optimized for each protein/RNA system. Identical binding reactions of 0, 0.5, 1, 10, and 20 units of p19 protein were separated on identical 2.5% agarose gels at three different voltages: 30, 25, and 20 V/cm, where cm refers to the length of the agarose gel. While higher voltages may allow for reduced run times, we observed that the higher voltages reduced the stability of the protein-RNA complex. The 30 V/cm voltage in particular shows a considerable amount of free RNA at the two highest protein concentrations, while the 20 V/cm separation shows robust RNA-protein complex and very little free RNA. (Data reproduced with permission from Ream et al. [6], Figure 2)
Fig. 3.
Destaining protocol affects the quality of band resolution. Top panels: The appropriate destaining buffer was identified by comparing 15-min destain incubations in either 0.5× TB or ddH2O. The intensity and resolution of bands were superior when gels were destained in 0.5× TB. Bottom panels: Destaining too long will negatively affect band quality. Identical gels were incubated in either 0.5× TB or ddH2O destaining solution for 2 h. Regardless of destain solution, the resulting bands were fuzzy and low-intensity, preventing quantitation or interpretation
Acknowledgments
This work was supported by the National Science Foundation (NSF 1407736) through the Houston Louis-Stokes Alliance for Minority Participation Scholars Program (J.A.R., scholar) and the National Institutes of Health – National Institute for General Medical Sciences (GM099049 to L.K.L. and GM119096 to K.A. L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation. All authors declare no conflict of interest.
References
- 1.Lane D, Prentki P, Chandler M (1992) Use of gel retardation to analyze protein-nucleic acid interactions. Microbiol Rev 56:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helwa R, Hoheisel JD (2010) Analysis of DNA–protein interactions: from nitrocellulose filter binding assays to microarray studies. Anal Bioanal Chem 398:2551–2561. 10.1007/s00216-010-4096-7 [DOI] [PubMed] [Google Scholar]
- 3.Hellman LM, Fried MG (2007) Electrophoretic mobility shift assay (EMSA) for detecting protein–nucleic acid interactions. Nat Protoc 2:1849–1861. 10.1038/nprot.2007.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanderson BA, Araki N, Lilley JL et al. (2014) Modification of gel architecture and TBE/TAE buffer composition to minimize heating during agarose gel electrophoresis. Anal Biochem 454:44–52. 10.1016/j.ab.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez BV, Pescador J, Pollok N et al. (2015) Impact of size, secondary structure, and counterions on the binding of small ribonucleic acids to layered double hydroxide nanoparticles. Biointerphases 10:041007. 10.1116/1.4936393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ream JA, Lewis LK, Lewis KA (2016) Rapid agarose gel electrophoretic mobility shift assay for quantitating protein: RNA interactions. Anal Biochem. 10.1016/j.ab.2016.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altschuler SE, Lewis KA, Wuttke DS (2013) Practical strategies for the evaluation of high-affinity protein/nucleic acid interactions. J Nucleic Acids Investig 4:19–28. 10.4081/jnai.2013.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]