Abstract
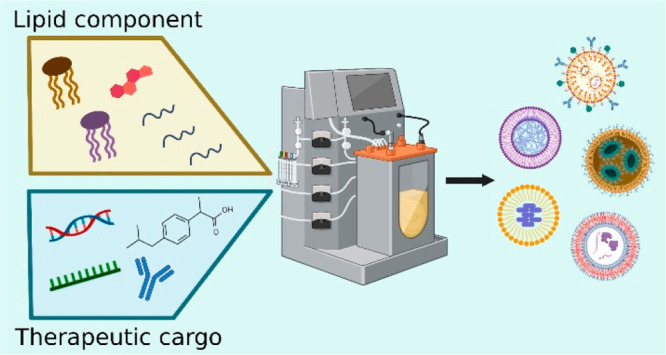
Over the past decade, the therapeutic potential of nanomaterials as novel drug delivery systems complementing conventional pharmacology has been widely acknowledged. Among these nanomaterials, lipid-based nanoparticles (LNPs) have shown remarkable pharmacological performance and promising therapeutic outcomes, thus gaining substantial interest in preclinical and clinical research. In this review, we introduce the main types of LNPs used in drug formulations such as liposomes, nanoemulsions, solid lipid nanoparticles, nanostructured lipid carriers, and lipid polymer hybrid nanoparticles, focusing on their main physicochemical properties and therapeutic potential. We discuss computational studies and modeling techniques to enhance the understanding of how LNPs interact with therapeutic cargo and to predict the potential effectiveness of such interactions in therapeutic applications. We also analyze the benefits and drawbacks of various LNP production techniques such as nanoprecipitation, emulsification, evaporation, thin film hydration, microfluidic-based methods, and an impingement jet mixer. Additionally, we discuss the major challenges associated with industrial development, including stability and sterilization, storage, regulatory compliance, reproducibility, and quality control. Overcoming these challenges and facilitating regulatory compliance represent the key steps toward LNP’s successful commercialization and translation into clinical settings.
Keywords: Manufacturing, Lipid-based nanoparticles, Delivery systems, Lipid-based nanoparticle synthesis, Industrial challenges
1. Introduction
Lipid-based nanoparticles (LNPs) are a highly adaptable class of nanocarriers that have gained widespread usage in medical research and pharmacology.1 They can encapsulate various therapeutic agents including small molecules, nucleic acids and monoclonal antibodies for a diverse range of applications.2,3 LNPs offer several benefits, such as safeguarding drugs from in vivo degradation, boosting their solubility and efficacy, enabling targeted drug delivery to the disease site, regulating drug release, and altering drug biodistribution.4 These engineered nanocarriers hold the potential to overcome significant limitations of traditional therapeutic products such as inadequate efficacy, susceptibility to enzymatic degradation, low bioavailability, and off-target side effects.1,5
The potential for LNP-based pharmaceuticals has been increasingly recognized in recent years, with significant growth in both research and industrial sectors.6 The LNP-based gene therapy market’s value reached about $3.5 billion in 2021, with a projected compound annual growth rate of around 18.5% from 2021 to 2026.7 The current market value of liposomal therapeutics used for cancer treatment (e.g., Doxil, DaunoXome, Myocet, DepoCyt, Marqibo, Onivyde) was approximately $3.72 billion in 2021 and is anticipated to reach almost $7 billion by 2027.8 In addition to cancer treatment, LNP-based therapies have gained FDA approval for treating other diseases, including COVID-19 vaccine (Spikevax , Comirnaty ) and Amyloidosis (Onpattro ), by delivering mRNA and siRNA, respectively.9−11 Nevertheless, to be clinically relevant, LNP-based therapies must be produced through techniques that ensure stability during storage, compatibility with sterilization, quality control, and regulatory compliance.4 These factors are crucial to the successful development and translation of LNP-based therapeutics for clinical applications.
In addition to LNPs, metal–organic frameworks (MOFs) have emerged as a promising class of materials for drug delivery applications. MOFs possess unique porous properties that enable them to store and release molecules effectively, making them attractive candidates for drug delivery.12 Zinc-based MOFs and chitosan/graphene oxide bionanocomposite beads efficiently loaded the antibiotic drug metronidazole, showing excellent releasing power.13 Lipid-coated MOFs efficiently store dye molecules within the porous scaffold with the lipid bilayer preventing premature release. The lipid coating significantly improved nanoparticle stability, promising an innovative cancer therapy.14
Our review offers a comprehensive analysis of diverse LNP formulations, emphasizing their distinctive structural and physicochemical characteristics. We also briefly discuss computational modeling techniques such as molecular dynamics (MD) simulations, quantum mechanical calculations, and molecular docking studies in predicting the structural properties and dynamic behavior of LNPs.15,16 Furthermore, we provide a thorough overview of the LNP manufacturing process, including both laboratory- and industrial-scale production. We also identify the crucial obstacles associated with the industrialization of LNP production that must be addressed to further the clinical implementation of LNPs in drug and gene delivery applications.
2. General Characteristics of LNPs
2.1. Main Components of LNPs
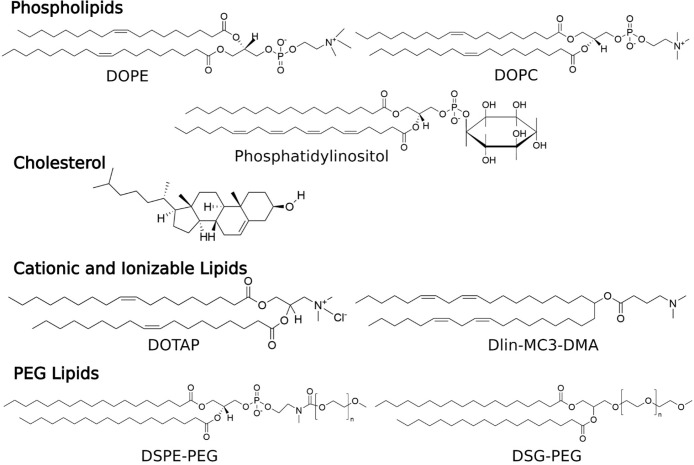
LNPs typically consist of four main lipid components: phospholipids and cholesterol, which are necessary for particle formation and stability; cationic or ionizable lipids, which enable binding with negatively charged nucleic acids, thereby increasing drug loading; and PEGylated lipids, which contribute to enhanced particle stability and circulation time within the biological system17 (Figure 1). Due to their biomimetic architecture and the similarity of their lipid components to those of cell membranes, LNPs can easily cross the cell membrane to deliver nanoparticle contents to the desired intracellular sites. After crossing the cell membrane, LNPs can take advantage of the low pH environment within the target cells, promoting endosomal escape and releasing the therapeutic cargo into the cytoplasm.2
Figure 1.
Chemical structures of various components used in LNP formulations. These include phospholipids (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), and phosphatidylinositol), cholesterol, cationic and ionizable lipids (1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), (1,2-dilinoleyloxy-3-dimethylaminopropane) (Dlin-MC3-DMA)), and PEG lipids (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)](DSPE-PEG), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[succinyl(polyethylene glycol)] (DSG-PEG)).
2.2. Characteristics of LNPs
Understanding key factors related to LNP characteristics is essential before delving into LNP categories and synthesis methods. These factors include LNP size, surface charge, morphology, stability, loading efficacy, and entrapment efficiency.
Nanoparticle size plays a vital role in LNP properties, influencing stability, biodistribution, cellular uptake, and overall therapeutic efficacy.18 Smaller LNPs (size <100 nm) tend to exhibit enhanced cellular uptake and prolonged circulation time, while larger LNPs (size >100 nm) may offer higher drug loading capacity but reduced cellular uptake.19 Dynamic Light Scattering (DLS) is the most preferred method for characterizing LNP sizes due to its effectiveness and convenience.20,21
Surface charge of LNPs, a critical parameter that indicates LNP stability in suspension, is commonly characterized through zeta potential measurements.22 A highly positive or negative Zeta potential indicates a strong surface charge, which results in electrostatic repulsion between particles and enhancement of their stability in the dispersion. On the other hand, a Zeta potential close to zero suggests a low surface charge and a higher tendency for particle aggregation or coalescence, potentially leading to the destabilization of the LNP suspension.23
Surface morphology assessment of LNPs can be performed using transmission electron microscopy (TEM), scanning electron microscopy (SEM), and atomic force microscopy (AFM). These methods provide valuable insights into the physical structure and characteristics of the outer surface of the LNP.24 A well-defined and smooth surface morphology is highly advantageous for LNPs in biological environments as it contributes to enhanced stability and mitigates the risk of opsonization, where serum proteins bind to the surface, triggering immune system clearance. This smooth surface minimizes unwanted interactions with biological components, promoting favorable LNP biodistribution and ensuring optimal therapeutic efficacy.25 On the other hand, LNPs with rough or irregular surface morphology may exhibit increased interactions with biological entities, potentially influencing LNP biodistribution patterns and impacting overall therapeutic effectiveness.25
LNP surface modification by PEGylation or targeting ligands can further influence the surface morphology of LNPs. For instance, these modifications can improve the stealth properties of LNPs, reducing recognition by the immune system and therefore extending drug circulation time for better drug delivery efficiency.26 In short, accurate size, surface charge, and morphology determination of LNPs are crucial for optimizing LNP production and maintaining consistency in their properties. This precision plays a vital role in achieving a reliable and effective drug delivery using LNPs.
LNP stability, loading efficacy, and entrapment efficiency are critical factors in the development and optimization of LNP-based drug delivery systems. LNP stability refers to the capability of LNPs to preserve their physical and chemical properties over time under various conditions. These properties include shape, size, and lipid component integrity during long-term storage or exposure to different biological environments like blood, plasma, or varying pH conditions.27 LNP drug loading capacity quantifies the total amount of drugs that can be loaded into the delivery system, while entrapment efficiency measures the effectiveness of the formulation process in retaining drug within the LNP.18
2.3. Computational Modeling of LNPs: Insights into Structure, Behavior, and Interactions
Computational modeling of LNPs utilizes computer-based methods such as MD simulations, quantum mechanical calculations, and molecular docking studies to analyze and predict the behavior, structure, and interactions of LNPs at the molecular level.28,29 Among these, MD simulations have emerged as the primary method for studying LNPs. This technique was initially introduced by Chaban and Khandelia to characterize the molecular structure of lipid droplets, which consisted of varying fractions of cholesteryl oleate and triolein.30 Using coarse-grained MD simulations, this group investigated the structure and transport properties of lipid droplets. Their findings revealed that cholesterol is uniformly distributed within the lipid droplet, and the extent of phospholipid coverage does not impact this distribution.31
Computational modeling is also a powerful tool for investigating the interaction between LNPs and different drug formulations.32 Metwally and colleagues employed MD simulations to predict curcumin loading efficiency in solid LNPs.33 The authors also used docking calculations to determine the binding energies between curcumin and solid LNPs. By establishing a correlation between the docking binding energy of the drug and LNPs and the entrapment efficiencies in LNPs, this method offers valuable insights into drug loading capacity. Altogether, combination of bioinformatics and computational modeling could assist in selecting the best drug/carrier combinations for further investigation.33
In summary, computational tools can be used to optimize LNP formulations, screen potential drug candidates, and predict their behavior in biological environments, complementing experimental investigations in LNP research.32,34 The following section will provide further insights into the application of computational modeling to study different types of LNPs.
3. Main Types of LNPs
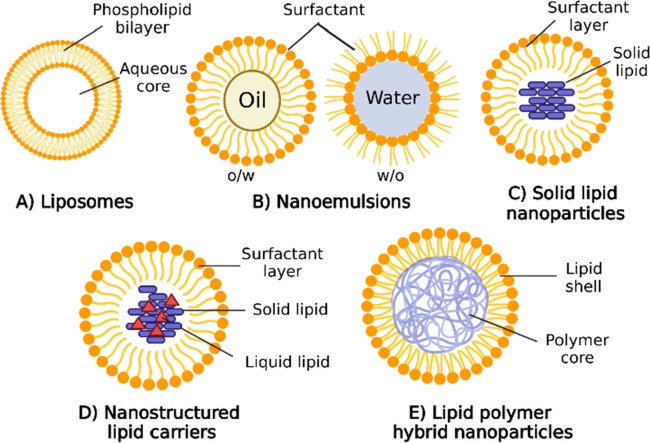
LNPs can be categorized into five subgroups: liposomes, lipid nanoemulsions, solid lipid nanoparticles, nanostructured lipid carriers, and lipid–polymer hybrid nanoparticles (Figure 2). This section offers an outline of the structural elements, physicochemical characteristics, computational modeling studies, and clinical applications of these LNP subgroups.
Figure 2.
Schematic illustration depicts the structure of various LNP formulations used in drug delivery. (A) Liposomes are spherical vesicles with phospholipid bilayers, encapsulating hydrophilic drugs in their aqueous core and incorporating hydrophobic drugs within the lipid bilayers. (B) Nanoemulsions comprise oil droplets dispersed in an aqueous phase stabilized by surfactants, accommodating lipophilic drugs in the oil phase while preventing droplet aggregation. (C) Solid lipid nanoparticles (SLNs) consist of solid lipid matrices entrapping hydrophobic drugs, forming nanoscale particles with a lipid core. (D) Nanostructured lipid carriers (NLCs) are similar to SLNs but contain a combination of solid and liquid lipids, resulting in a more stable matrix and improved drug loading capacity. (E) Lipid polymer hybrid nanoparticles combine lipid-based and polymer-based components, offering the benefits of both systems. These nanoparticles can effectively encapsulate various types of drugs and exhibit enhanced stability and controlled release properties. Each type of LNP structure provides unique advantages and can be tailored for targeted drug delivery, enabling the encapsulation of a diverse range of therapeutic agents. Figure created with BioRender.com.
3.1. Liposomes
Liposomes, first discovered in 1965 by Bangham and colleagues, are self-assembling nanosized lipid vesicles comprising one or more concentric phospholipid bilayers that enclose discrete aqueous spaces.35 Liposomes were quickly recognized for their potential as drug delivery systems due to their ability to carry a diverse range of therapeutic drugs, with hydrophilic drugs contained within their aqueous core and hydrophobic drugs integrated into the lipid bilayer.36,37 Additionally, liposomes can also carry other macromolecules such as different types of imaging agents, nucleic acids, and proteins, thus making them an extremely versatile drug delivery platform.4 Liposomes can be synthesized into unilamellar or multilamellar vesicles, with sizes varying from 20 to 1000 nm, depending on the specific formulations and synthesis procedures.2 Particle size is an important parameter for the pharmaceutical applications of liposomes. Small unilamellar liposomes (≤100 nm) exhibit higher encapsulation efficiency, improved drug half-life, and the ability to evade the immune system upon administration.38
MD simulations have been instrumental in understanding liposome characteristics, including vesicle formation and conformational stability.39−41 Furthermore, these simulations have been utilized to enhance liposome thermal stability by formulating them with different phospholipid components.40
In the context of PEGylation, MD simulations have shed light on its impact on the drug-loading efficiency in lipid membranes. Dzieciuch et al. conducted a study using MD simulations to investigate the interaction between a hydrophobic molecule, p-THPP, and lipid bilayers. Their findings revealed that in PEGylated membranes, p-THPP wraps around the PEG corona, resulting in increased exposure to the solvent when compared to zwitterionic membranes.42 PEGylation enhances hydrophobicity and protects drug molecules like hematoporphyrin under physiological conditions.42 However, MD simulations revealed that PEGylation may not always improve the targeting efficiency. For example, a research group studied an activated endothelium targeting peptide’s effectiveness in directing liposomes to vascular endothelium.43 Upon peptide anchorage to a PEGylated liposome, MD simulations did not show evidence of improved targeting capability, as the ligand was embedded within the PEG layer, reducing its exposure to the solvent.
Additionally, computational prodrug design methodology has been applied to explore various active pharmaceutical ingredients for optimizing drug encapsulation and release from liposomes.30 By leveraging computational simulations, this approach showcases the promise of guiding the design and advancement of liposome-based drug delivery systems, thereby providing improved therapeutic efficacy and controlled drug release.
Liposomes are widely recognized as a versatile drug delivery system with advantages such as rapid absorption, improved drug bioavailability, reduced toxicity, and protection against hydrolysis and oxidation.4 However, their clinical applications face challenges due to short half-lives, low biostability, and the risk of drug leakage.44 To address these limitations, strategies have been developed, including targeted liposomes with surface-attached ligands and “stealth” liposomes enclosed with biocompatible polymers like PEG to evade the immune response.45−47 For example, a preclinical study demonstrated successful delivery of therapeutic agents for glioblastoma across the blood-brain barrier using ApoE-functionalized liposomal nanoplatform based on artesunate-phosphatidylcholine (ARTPC) encapsulated with Temozolomide.48
Thanks to the rapid development of liposome formulations in both the research community and the industry area, there has been a wide range of liposome drugs approved and applied in medical practice. For example, Doxil is the first FDA-approved liposomal drug containing doxorubicin (DOX) which is used to treat solid tumors.49,50 Following the successful application of Doxil, various liposome-formulated drugs have been approved for disease treatments2 (Table 1).
Table 1. LNPs in the Commercial Market and Clinical Trials.
| LNP subgroup | active substance | disease/applications | products | ref |
|---|---|---|---|---|
| liposomes | doxorubicin/daunorubicin | cancer | Doxil, Myocet, Vixeos, DaunoXome, Transdrug | (85−89) |
| other anticancer agents | cancer | Mepact, Depocyt, Marqibo, Onivyde | (90−93) | |
| paclitaxel | cancer | Abraxane | (94−96) | |
| amphotericin B | visceral leishmaniasis | Albecet, Ambisome, Amphotec | (97−99) | |
| vaccine | HAV viral vaccine | Epaxal, Inflexal | (100, 101) | |
| verteporfin | age-related macular degeneration | Visudyne | (102) | |
| nanoemulsions | etomidate, profol | anesthetics | Etomidat-Lipuro, Diprivan | (103, 104) |
| heparinoid | superficial thrombophlebitis | Nanoemulsions carrying heparinoid for topical delivery | (105) | |
| ibuprofen | pain relief | Topical delivery of ibuprofen-nanoemulsions | (106) | |
| cyclosporin A | immunosuppressants | Sandimmune and Sandimmun Neoral | (107) | |
| ritonavir | antiviral HIV-1 medicine in children | Norvir | (108) | |
| saquinavir | antiviral HIV-1 medicine in adult | Fortovase | (108) | |
| solid lipid nanoparticles | mitoxantrone | hepatocarcinoma | Mitoxantrone-loaded polybutylcyanacrylate nanoparticles (DHAD-PBCA-NPs) (phase II clinical trial) | (109) |
| doxorubicin | hepatocarcinoma | Doxorubicin Transdrug (DT) (Phase III clinical trial) | (110) | |
| oxiconazole | tinea fungal infection | Oxiconazole nitrate solid lipid nanoparticles loaded gel (Phase I clinical trial) | (111) | |
| halobetasol propionate | inflammation | Pluronic gel Halobetasol propionate-loaded lipid nanoparticles | (112) | |
| Duobril (Phase IV clinical trial) | (113) | |||
| siRNA targeting transthyretin gene | amyloidosis | Onpattro (Patisiran) | (10) | |
| nanostructured lipid carriers | acitretin | psoriasis | Acitretin Precirol ATO 5/oleic acid/Tween 80 (Randomized Controlled Trial) | (114) |
| all-trans retinoic acids | keratinization disorders | Oleic acid/Cetyl palmitate/Cineole/Limonene/Transcutol/Butylated hydroxytoluene/Tween 20/Tween 80 | (115) | |
| self-amplifying RNA | COVID-19 | THEMBA II T-CELL Vaccine (phase I/II clinical trial) | (116, 117) | |
| mRNA-1273 | COVID-19 | Spikevax | (118) | |
| BNT162b2 mRNA | COVID-19 | Comirnaty | (119) | |
| lipid polymer hybrid nanoparticle | docetaxel | pancreatic cancer | Docetaxel polymeric nanoparticle (phase I clinical trial) | (120, 121) |
| docetaxel | lung cancer with KRAS mutation | BIND-014 (Docetaxel Nanoparticles for Injectable Suspension) (phase II clinical trial) | (122) | |
| docetaxel | prostate cancer | BIND-014 (Docetaxel Nanoparticles for Injectable Suspension) (phase II clinical trial) | (123) |
3.2. Nanoemulsions
Nanoemulsions are another type of LNP that consist of spherical biphasic liquid droplets ranging in size from 50 to 500 nm.51,52 They are composed of an internally dispersed oil phase covered by an external continuous phase. Nanoemulsions can be prepared as oil-in-water (o/w) or water-in-oil (w/o) droplets to carry either hydrophobic or hydrophilic active compounds, respectively.53 To stabilize these small droplets, different types of emulsifiers, such as surfactants, phospholipids, proteins, polysaccharides or polymers such as poly(vinyl alcohol), can be added during the synthesis procedure.54,55 These surfactants, whether ionic or nonionic, prevent droplet aggregation through electrostatic repulsion or steric hindrance, hydration, and thermal fluctuation interactions.55,56
Nanoemulsions have a colloidal structure that enables solubilization and encapsulation of hydrophobic drugs, reducing adverse effects.57 They are mainly used for topical drug delivery (Table 1) but have potential for other modes of administration like intravenous, ocular, intranasal, and oral delivery.58−61 Nanoemulsions find applications in the food industry as flavoring, coloring, nutraceutical, or preservative agents.53 They offer advantages as drug delivery systems, skincare materials, or food additives due to their cost-effectiveness, improved drug bioavailability, physical stability, and nontoxic nature.60 However, they may be thermodynamically unstable, requiring surfactants, and can be influenced by environmental factors like pH and temperature, making them less suitable for clinical applications compared to other types of lipid nanoparticles.61
MD simulations are commonly used to explore the dynamic behavior, interactions, and stability of nanoemulsions at molecular levels under different conditions. For instance, Pirhadi and Amani used the MD simulations to study a siRNA-loaded nanoemulsion with benzakonium chloride as a surfactant, cyclohexane as the oil phase, and ethanol as the cosurfactant.62 MD simulations revealed oil molecules in the hydrophobic core, increasing the nanoemulsion size, while benzalkonium chloride’s polar terminal groups were mainly on the surface. In another study, MD simulations were used to predict the interactions between polymeric surfactant and seven different hydrophobic drugs within nanoemulsions for improved drug solubilization and stability.63 Besides MD simulations, mesoscale simulations, like dissipative particle dynamics, extend the scope to study larger scales and timeframes, providing a comprehensive understanding of nanoemulsion systems’ collective behavior and stability.64 In summary, these simulations aid in developing efficient and stable nanoemulsion formulations for drug delivery, cosmetics, and food technology.65
3.3. Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) were initially formulated as small spherical particles with a solid lipid core at room temperature, and subsequent advancements have led to the development of flat ellipsoidal or disc-like shapes, exhibiting sizes between 50 and 100 nm.18,66,67 Within SLNs, the active substance can be added to the lipid core or lipid shell or dispersed within the whole lipid matrix. The solid lipid core of SLNs is a new method to improve particle stability compared to liposomes.68 The incorporation of cationic lipids into SLN shell formulations can enhance cellular internalization of the particles, potentially improving tumor targeting, blood-brain barrier penetration, and gene transfection efficiency.69 Additionally, the outer shell of SLNs can be modified with macromolecules such as oligosaccharides, proteins, specific ligands, and antibodies to enhance their specificity at desired therapeutic sites.70−73
SLNs carrying several therapeutic agents including antioxidants, anticancer agents, nucleic acids, antibiotics, cytokines, and other hydrophobic drugs have currently been examined in clinical trials69 (Table 1). Additional research is needed to comprehensively investigate the therapeutic capabilities of SLNs as drug carriers.
Using computational modeling, specifically MD simulations, offers valuable insights into the physicochemical properties and behavior of SLNs at the molecular level.74 These simulations explore the dynamic behavior and interactions of lipid molecules within SLNs, shedding light on their stability, structural changes, and drug encapsulation efficiency under various conditions.75 MD simulations were employed to investigate the structure and conformational differences of tripalmitin SLNs and the morphology of SLN.76 Additionally, docking simulations were utilized to predict the loading of therapeutic agents into the SLN core. These computational modeling methods offer advantages in optimizing SLN formulations for specific applications by predicting drug release profiles, biodistribution, and cellular uptake.
From the pharmaceutical aspect, SLNs have both advantages and disadvantages compared to other types of drug carriers. SLNs offer several technical advantages such as drug protection from chemical and enzymatic degradation, improved physical stability, ease of scale-up production, omission of the need for organic solvents, ease of sterilization process, and ability to codelivery two active agents.68,77−79 They also have great potential for various routes of administration.80 This material also has reputation for being biodegradable and biocompatible.81
However, SLNs are facing the main technological issues of limited drug loading efficiency, short shelf life due to cold storage requirements, poor long-term drug retention, low drug loading capacity, polydispersity, and high operative temperature.69,82,83 Indeed, specific values for these parameters can vary significantly depending on various factors, including formulation composition, lipid type, encapsulated drug, storage conditions, and intended application. For instance, the entrapment efficiency of Pomegranate extract within SLN has been reported within only 25.15–63.5%.84 Therefore, researchers and manufacturers must carefully optimize these parameters to address the challenges and ensure the stability and efficacy of SLNs for specific applications.
3.4. Nanostructured Lipid Carriers
As mentioned earlier, solid lipid nanoparticles (SLNs) have a key weakness in limited drug loading capacity.124 To address this challenge, researchers have introduced the second generation of SLNs, known as nanostructured lipid carriers (NLCs), which incorporate both solid and liquid lipids in their cores, resulting in a more disordered lipid structure. This structural characteristic allows for higher drug loading capacity and improved drug release kinetics compared to SLNs.125,126 The ratio between solid lipid (e.g., cetyl palmitate), liquid oil (e.g., caprylic triglyceride), and surfactant (e.g., polysorbate 80) plays a critical role in determining the entrapment efficiency and stability of therapeutic agents in vivo.127 NLCs can be modified with ligands or polymers for targeted delivery of hydrophilic and hydrophobic drugs.79 As efficient carriers, RNA- and pDNA-loaded NLCs enable gene therapy and personalized medicine, modulating gene expression, and delivering therapeutic proteins. These lipid-based nanoparticles offer versatile platforms for nucleic-acid–based therapies to treat genetic diseases and cancers.
MD simulations offer valuable insights into the physicochemical properties and behavior of NLCs.74 For instance, the self-assembly behavior of mixed NLC systems containing Rhodamine, Miglyol 812, Tween 80, and Gelucire 44/14 was investigated by using MD simulations.128 The results demonstrated that raising the temperature to 358 K improved the stability of the NLCs, leading to enhanced component compaction. Furthermore, this study has shown that adding oil to the lipid formulation creates an imperfect core, effectively increasing the drug loading capacity of the NLCs.
NLCs offer advantages over other LNP formulations such as improved drug-loading capacity, controlled release, and enhanced stability for drug delivery.129,126 They are biocompatible and biodegradable, making them ideal drug carriers. NLCs have shown promise in increasing oral drug availability and have been studied in clinical trials for various pharmaceuticals, including COVID-19 mRNA vaccines, anticancer agents, antioxidants, and antiviral agents127,130−132 (Table 1). They also hold potential for gene therapy, chemotherapy, and applications in the food and cosmetic industry.133 However, challenges like low drug loading efficiency and particle stability may affect their drug-delivery effectiveness.129 Further research is required to tackle these challenges and unlock the full potential of NLCs in therapeutic and biomedical applications.
3.5. Lipid Polymer Hybrid Nanoparticles
Lipid polymer hybrid nanoparticles (LPNs) are another major type of lipid nanoparticle that combines the advantages of lipids and polymers for various biomedical applications.117 LPNs have polymer cores that contain therapeutic substances and lipid/lipid-PEG shells as a “stealth” coating for improved in vivo circulation.134 This unique structural composition offers optimal biocompatibility and physical stability, making them an ideal vehicle for drug delivery.117 LPNs have been successfully used to encapsulate various pharmaceuticals, including nucleic acids, for sustained release and improved stability. Furthermore, incorporating functional groups on the polymer surface facilitates the targeted drug delivery to specific cells or tissues.
Density functional theory (DFT) simulations, one of the computational modeling methods, were utilized to gain a better understanding of the interaction mechanism between the anticancer drug doxorubicin (DOX) and the polymers within LNPs.135 The study aimed to improve the therapeutic efficacy of doxorubicin (DOX) in lipid nanoparticles (LPNs). By analysis of the DOX-loaded LPNs, researchers found that polymers interacted strongly with DOX at various sites, forming stable complexes. The simulations indicated energetically favorable binding, suggesting a potential for enhancing oral bioavailability.
LPNs are gaining recognition as an advanced substitute for traditional liposomal and polymeric drug delivery systems due to their wide range of advantages, encompassing applications in combinatorial/active targeted drug deliveries, cancer gene therapy, vaccine development, and novel diagnostic imaging methods.136 Encouraging results from Phase I and II clinical trials demonstrate the potential of LPNs in delivering the anticancer drug Docetaxel, particularly for lung, pancreatic, and prostate cancers120−123 (Table 1). Nevertheless, LPNs encounter certain challenges, such as potential polymer component toxicity and difficulties in achieving consistent particle size and shape.18 Ongoing research endeavors aim to address these obstacles and further advance LPNs for use in diverse biomedical applications. Despite the challenges, LPNs exhibit great promise for drug delivery due to their distinctive properties and versatility in addressing various biomedical needs.
4. LNP Synthesis Methods
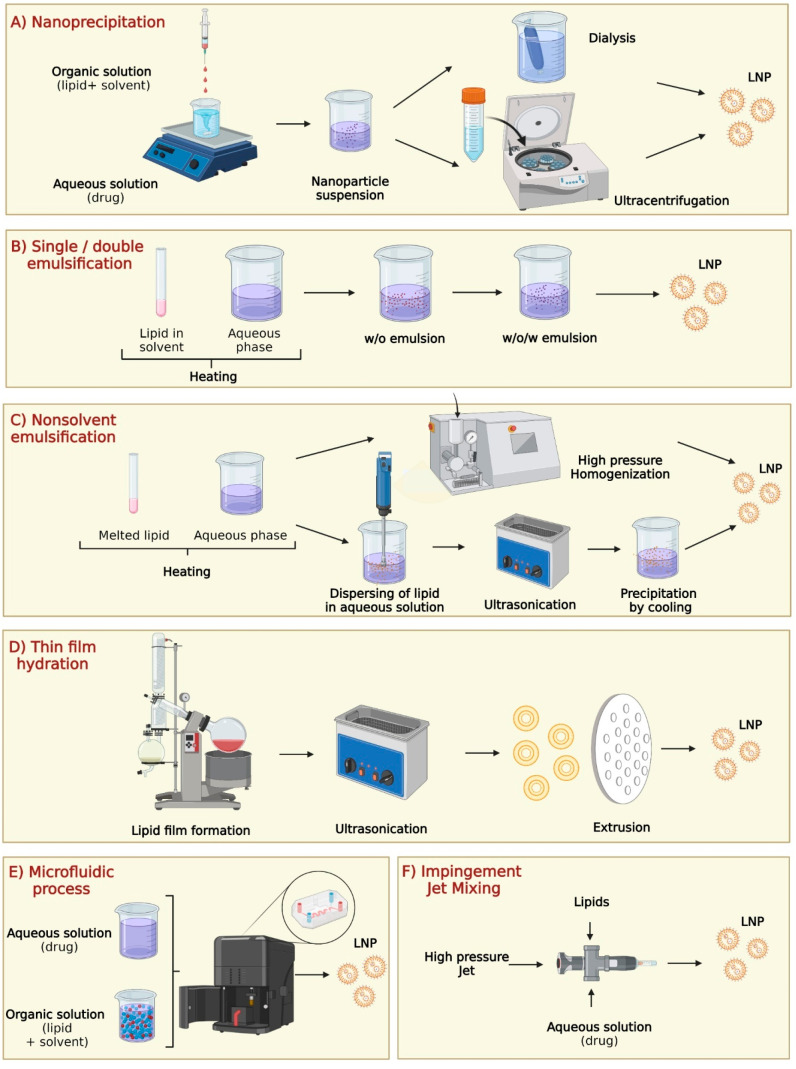
Nanoparticle synthesis typically involves two methods: bottom-up and top-down approaches. The bottom-up approach nucleates atomic-sized materials into nanoparticles using gas phase synthesis, block copolymer synthesis, Turkeyvich method, and microbial synthesis.135,136 The top-down approach physically dismantles bulk materials into nanosized fragments using milling, spark ablation, and laser ablation.72 For LNPs, the synthesis primarily follows a wet chemistry bottom-up approach due to their distinctive colloidal structure.137 This section provides an overview of the main LNP synthesis methods, including nanoprecipitation, single/double emulsification, nonsolvent emulsification, thin film hydration, microfluidic process, and impingement jet mixing technology137 (Figure 3).
Figure 3.
LNP synthesis processes in laboratory and industry environments: (A) Nanoprecipitation, (B) single/double emulsification, (C) nonsolvent emulsification, (D) thin film hydration, (E) microfluidic process, and (F) impingement jet mixer. Created with BioRender.com.
The choice of the LNP synthesis method plays a crucial role in determining their therapeutic applications, as it directly influences their physicochemical properties, drug loading efficiency, stability, and behavior in vivo. Each LNP synthesis method yields nanoparticles with distinct characteristics that directly impact their performance in various therapeutic applications.137
4.1. Nanoprecipitation
The nanoprecipitation method was first developed by Fessi et al. in 1989 and has been mainly used for encapsulating hydrophobic drugs (Figure 3A).21 This technique relies on a precipitation mechanism, wherein two miscible solvents, the organic phase and aqueous phase, are continuously mixed under moderate magnetic stirring to facilitate the spontaneous formation of LNPs. The organic phase consists of film-forming polymers, drug molecules, polymers, lipophilic surfactants, and organic solvents, while the aqueous phase contains water and stabilizer.134 The polymers utilized for LNP production can fall into two categories: nonbiodegradable, such as Eudragit, and biodegradable, such as polylactide (PLA), polylactide-co-glycolide (PLGA), and poly-ε-caprolactone (PCL).138−141
After particle formation, the organic solvent is removed from the formulation using methods like dialysis, ultracentrifugation, rotary evaporation, and freeze-drying.143 Dialysis, the most popular method for solvent removal, involves placing the LNP formulation in a dialysis bag or tubing with a semipermeable membrane, allowing the solvent to diffuse out into a buffer solution until desired solvent removal level is achieved.144 Ultracentrifugation uses high centrifugal forces to pellet LNPs based on size and density, leaving free lipids and unincorporated drugs in the supernatant.145 Rotary evaporation evaporates the solvent under reduced pressure and elevated temperatures, collecting the solvent separately and leaving behind a concentrated LNP suspension.146 Freeze-drying freezes the LNP suspension and removes the solvent through sublimation under vacuum, resulting in a dry, solid LNP powder.147 These methods are crucial for LNP formulation development, ensuring highly concentrated and purified LNPs for diverse biomedical applications.
The size and drug encapsulation efficiency of LNPs prepared by nanoprecipitation can be significantly affected by various parameters, such as the stirring rate, aqueous/organic phase ratio, and concentration of lipid/surfactant/drug.21 The formation of inhomogeneous and incomplete saturated lipid solutions may interfere with spontaneous nucleation during small particle formation, resulting in varied LNP sizes.21,142 Additionally, incomplete mixing of aqueous and organic solutions before precipitation can lead to unevenly small LNP sizes.143 Therefore, it is crucial to thoroughly characterize the size and surface morphology of the produced LNPs before any testing or application.
Incomplete mixing during nanoprecipitation is a critical challenge that contributes to batch-to-batch variation and compromises the overall quality of the LNPs.144 To overcome these issues, careful optimization of the mixing process is essential. By controlling crucial mixing parameters such as speed, duration, and solvent ratio, it is possible to achieve better homogenization of lipids and drug molecules.142 This fine-tuning ensures a more uniform distribution, resulting in consistent and efficient LNP formulations across different batches. Addressing these challenges in nanoprecipitation is vital to enhance the reproducibility and quality of LNPs, making them more suitable for large-scale production and expanding their potential in various therapeutic applications.144
4.2. Single/Double Emulsification
The single oil/water solvent emulsification method, invented by Gasko, is a commonly used technique for preparing LNPs carrying hydrophobic drugs (Figure 3B).145 It involves low melting lipids like stearic acid and Compritol 888 ATO, surfactants such as Epikuron 200 and Tween 80, emulsifiers like polysorbate 20 and polysorbate 600, and water.146 The solvent mixture is preheated, water is added, and then the mixture is emulsified to create oil-in-water emulsions. These emulsions are transferred to cold water with continuous stirring for LNP crystallization.
Another approach to synthesizing LNPs is the solvent-based emulsification method, which can be achieved through emulsion-solvent evaporation, solvent diffusion, solvent displacement, or solvent injection.147
In this method, lipids and poorly water-soluble drugs are dissolved in an organic solvent and emulsified with an aqueous solution to create oil-in-water emulsions. The emulsion is then evaporated under agitation to remove the organic solvent, followed by centrifugation. However, residual solvent toxicity can be a concern, especially if the lipid solubility in the solvent remains low.27
The double emulsification technique is developed for preparing hydrophilic-loaded LNPs. In this method, an aqueous solution is emulsified in melted lipid solvents to create a primary water-in-oil (w/o) emulsion. Next, the primary oil-in-water (o/w) emulsion is dispersed as droplets in another aqueous solution containing a hydrophilic emulsifier, resulting in the formation of a double water-in-oil-in-water (w/o/w) emulsion using either sonication or membrane emulsification.148 Typically, two surfactants are used in this process: one hydrophobic surfactant to stabilize the interface of the water-in-oil (w/o) internal emulsion and one hydrophilic surfactant for the external interface of the oil globules. Microemulsion methods can produce LNPs loaded with various drug compounds, such as Baclofen, Idarubicin, Ketoprofen, Nevirapine, and Tobramycin.2,149
Although the emulsification technique offers the advantage of low mechanical energy input, it also has several drawbacks, including a low dispersing degree, emulsion instability, lipid insolubility in organic solvents, and the need for additional solvent removal procedures.150,151 In line with LNPs prepared using nanoprecipitation and microfluidic methods, it is crucial to incorporate a solvent removal step in the synthesis process to mitigate the potential toxicity associated with any residual solvents in the produced LNPs.152
4.3. Nonsolvent Emulsification
Nonsolvent emulsification techniques offer a solvent-free approach to create oil-in-water emulsions using melted lipids as the liquid phase (Figure 3C).142 High-pressure homogenization and ultrasonication are commonly used methods for this purpose. In the homogenization process, melted lipids are mixed with drugs and a preheated aqueous phase containing surfactants. The mixture undergoes high-pressure homogenization for several cycles to achieve the desired nanoparticle size.153,154 Alternatively, ultrasonication involves homogenizing the aqueous phase with surfactants and melted lipids using high-speed stirring, followed by ultrasonication to break down large particles into smaller droplets.155
However, the nonsolvent emulsification technique involves complex procedures and multistep processes, leading to a potential batch-to-batch variation. Variations in homogenization/sonication time, lipid surfactant ratio, drug concentrations, and lipid/surfactant type can result in different particle sizes, polydispersion indices, zeta potential, and drug entrapment efficiency in the lipid nanoparticles, leading to variable product outcomes.156,157 To ensure consistent product quality, the careful optimization of these parameters is essential.
4.4. Thin Film Hydration
Thin film hydration is a widely used method in both laboratory and industrial settings for producing lipid nanoparticles (Figure 3D). In this process, lipids are dissolved and mixed in an organic solvent to achieve homogeneity, and then the solvent is removed through rotary evaporation, forming a thin lipid film on the flask’s sides.146 After hydrating the lipid suspension, it is passed through a filter with uniform pore sizes several times, resulting in uniform-sized lipid nanoparticles.146 Various parameters, such as applied pressure, temperatures, membrane pore size, extrusion force, and the number of cycles, can affect the mean size and polydispersity of the nanoparticles produced.2 The extrusion process is favored due to its lower energy requirements, reduced risk of contamination, and narrower particle size distribution achieved with consistent filters. Industry-utilized extruders like LIPEX and LiposoFast LF-50 have successfully produced liposomes with diverse payloads, including proteins, peptides, small molecules, and larger molecules.93
4.5. Microfluidic Process
The microfluidic process is widely acknowledged as a highly successful method for the industrial production of diverse lipid nanoparticles.158 This method is fundamentally based on nanoprecipitation due to the precisely controlled mixing of lipid and drug solutions in microchannels.159 The rapid mixing at the microscale in microfluidic devices enables more uniform and reproducible nanoparticle formation compared with traditional bulk mixing methods. Additionally, the small dimensions of microfluidic channels offer advantages in terms of heat and mass transfer, resulting in improved size control and homogeneity of LNPs.160 The microfluidic approach allows for precise tuning of formulation process parameters, such as flow rates, concentrations, and mixing ratios, to optimize LNP characteristics.159 By controlling these parameters, researchers can tailor the size, drug loading capacity, and stability of LNPs for specific therapeutic applications.
In a microfluidic device, lipid fluids are controlled in microchannels with dimensions in the tens of microns.96 There are two primary types of microfluidic devices used for producing LNPs: chip-based platforms, which involve the controlled mixing of organic and aqueous solvents within a specifically designed chip, and capillary-based platforms, where the organic solvent is introduced through a central tube surrounded by multiple tubes for rapid dilution with the exiting aqueous solution.161
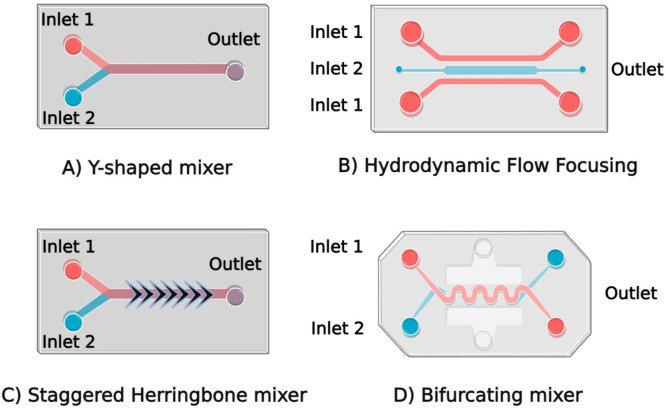
In the upcoming section, our focus will be on chip-based mixers, which utilize diverse micromixer designs to produce LNPs (Figure 4).
Figure 4.
Different designs of microchips used in microfluidic devices: (A) Y-shaped mixer, (B) hydrodynamic flow focusing, (C) staggered herringbone mixer, and (D) bifurcating mixer. Created with BioRender.com.
4.5.1. T- or Y-Mixer
Chip-based platforms use a variety of microchannel designs for LNP production. The flat microchannel, which contains T- and Y-shaped microchannels, is the earliest and simplest microchip to produce LNPs (Figure 4A). Upon introducing the lipid and buffer solutions at the inlets, LNPs form at the liquid interface through molecular diffusion-based organic solvent dilution.161,162 T- or Y-shaped mixing is a rapid mixing method requiring high flow rates (40 to 60 mL/min) to produce high volumes of LNPs (>100 μL).163 Although this flat microchannel method offers high throughput and straightforward device fabrication, it faces challenges in scaling down to the small volumes required for discovery experiments. Additionally, the limited control over LNP size, ranging from 30 to 250 nm, may not produce the ideal LNPs.161
To address the limitations in throughput volume and LNP size control, alternative mixer devices have been explored for LNP preparation.
4.5.2. Hydrodynamic Flow Focusing
Hydrodynamic flow focusing (HFF) is one of the most used micromixer designs (Figure 4B). HFF devices have 2D and 3D structures, where the former belongs to chip-based platforms and the latter is a capillary-based platform.144 For the 2D HFF, fluids are injected simultaneously via three inlets. The organic phase containing the precursor ingredients of the nanoparticles is introduced to the central stream, and the aqueous phase streams squeeze the central channel perpendicular and create a narrowly focused stream, generating rapid diffusion-based mixing.144,164 In a 2D HFF, the laminar flow condition develops interfacial force. Small-size LNPs (<150 nm) are produced by adjusting operating parameters which could influence the interface of the aqueous and organic phases.1,3 A comparison study demonstrated that an HFF device could generate small siRNA-LNPs with an average size of 38 nm. The 20% enhanced encapsulation efficiency with gene silencing in vitro has been shown compared to vortex mixing.6 However, the low throughput (<10 mL/h) limits the application of 2D HFF, and the lipid aggregation happening at the wall of the microfluidic channel affects the particle size control and results in channel blocking.4,165
To further investigate the HFF, a 3D HFF was developed by DeVoe’s group.166 This multicapillary-arrayed device contains seven small capillary arrays embedded in one larger capillary where the lipid stream inlet was in the middle, and an aqueous solution was injected into the outer channel. This 3D HFF can produce consistently uniformed liposomes with four-time increase in throughput compared to the conventional 2D HFF.166 However, the main drawback of this multicapillary arrayed 3D HFF device is the complicated manufacturing operation with costly equipment.167 Meanwhile, when forming the smallest LNPs using the HFF technique, the high flow rate ratio will dilute the sample concentration, which may affect the following in vivo experiment.163 Those issues limit the application of HFF; thus, this mixer is not extensively used as other microfluidic devices.
4.5.3. Staggered Herringbone Micromixer
Several passive mixers have been reported to enhance production efficiency by increasing the contact area between the aqueous and solvent phases.4 One such mixer is the staggered herringbone micromixer (SHM), featuring an asymmetric herringbone groove pattern in microchannel (Figure 4C).139 The herringbone structure disrupts the laminar flow, leading to chaotic advection and rapid, controlled mixing (<10 ms) to form homogeneous and small LNPs (30 nm) with high reproducibility.4,144,163 However, the low throughput of the SHM (<100 mL/h) limits its full clinical potential as the demand for LNPs in clinical applications grows, making it a significant bottleneck in scaling up LNP production for large-scale therapeutic use.165
We introduce the groundbreaking parallelized microfluidic device (PMD), a revolutionary system that ushers in a new era of scalable LNP production. From the early discovery phase with milliliter per hour rates to the clinically relevant milliliter per hour rates, the PMD offers an impressive 100-fold enhancement over single microfluidic channels, addressing the pressing need for efficient and versatile manufacturing processes in the field of LNP synthesis. With ladder geometry and SHM mixing channels (1×, 10×, and 128×), the PMD enables precise scalability.165 Additionally, individual flow resistors ensure uniform fluid distribution, enhancing formulation consistency.165 Validation studies confirm remarkable therapeutic potential, surpassing conventional bulk mixing methods in in vivo siRNA and mRNA delivery. The success of the SHM led to NanoAssemblr platforms, offering user-friendly and rapid solutions for scalable LNP production, advancing nanomedicine for clinical applications.168
4.5.4. Bifurcating Mixer
The NxGenTM mixer by NanoAssemblr is a groundbreaking advancement designed for the large-scale production of LNPs.169 It overcomes LNP scale-up reproducibility challenges, achieving over 25 times the throughput of the classic mixer without compromising identical particle formation conditions, ensuring highly reproducible outcomes across various particle types. Its innovative chip design features numerous bifurcating mixers, inducing chaotic flow and rapid mixing, enabling LNP production at exceptional rates of up to 200 mL/min while maintaining LNP encapsulation rate and polydispersity control (Figure 4D).
The NxGen mixer promises rapid and efficient large-scale LNP manufacturing. It is indispensable for researchers and pharmaceutical companies seeking scalable, high-throughput LNP production, advancing therapeutic developments, and personalized medicine. This mixer revolutionizes LNP formulation, bridging the gap from the laboratory to real-world clinical applications.4
4.6. Impingement Jet Mixer
Impingement jet mixing (IJM), also referred to as the tea stirrer, is an innovative microfluidic mixing technique for the small-scale production of mRNA-LNPs170 (Figure 3F). It is a device that creates a high-velocity stream of fluid being directed toward another stream of fluid, resulting in intense mixing and shearing forces that can effectively homogenize the two fluids.171 To produce LNPs, the IJM is first used to mix lipids with an aqueous solution containing the drug or therapeutic agent of interest. The high-pressure jet forces the lipids to form small droplets that are then stabilized by surfactants, resulting in the formation of LNPs. The size and properties of the resulting nanoparticles can be controlled by adjusting the processing parameters of the mixer such as the pressure and flow rate of the fluids.
Starting from the small size of the confined IJM, the IJM can be easily scaled up based on the original design, making it a valuable tool in pharmaceutical manufacturing, particularly during pandemics such as COVID-19. IJM systems have been widely used for mRNA-COVID-19 vaccine production by several companies, such as Knauer and Pfizer. At Knauer, the IJM system utilizes 400 pounds of pressure to combine a lipid solvent solution with an mRNA solution, effectively forcing the fluids to mix.172 Pfizer successfully replicated the quarter-sized mixers and implemented a parallelization of 100 static mixers, enabling continuous synthesis and significantly increasing vaccine productivity at the Kalamazoo site to 100 million doses per month.173 To automate the process, a computer system was implemented to control the flow rate and pressure. Overall, IJM is a powerful tool for producing uniform and stable LNPs for drug delivery applications.
4.7. Scaling-Up LNP Production by Microfluidic Devices
Microfluidic technologies can be utilized to achieve the scaled-up production of LNPs for industrial applications. To achieve this purpose, in microfluidic systems, strategies like pilling-up, numbering-up, or parallelization of microfluidic devices are employed.162
Pilling-up involves stacking multiple microfluidic devices in series, increasing throughput and production capacity, which enhances overall production efficiency.110 Numbering-up, on the other hand, entails running multiple identical microfluidic devices in parallel, multiplying the production rate and enabling higher LNP yields within the same time frame.110 Parallelization takes the integration of multiple microfluidic devices, optimized for specific LNP synthesis steps, into a single production platform, resulting in a streamlined workflow and improved overall LNP production.110 For example, a research group introduced iLiNP (invasive lipid nanoparticle production device), a microfluidic device comprising five-layered microchannels created by stacking glass-iLiNP devices and later parallelized (numbering-up) to achieve mass LNP production. The iLiNP system efficiently produces LNPs in the size range of 20 to 60 nm at a flow rate of 20–50 mL/min, demonstrating comparable performance to commercially available microfluidic systems like NanoAssemblr.111
By implementing these strategies, researchers and industry can overcome the limitations of single microfluidic devices and achieve significant scale-up improvements in LNP production, making them more viable for large-scale applications.
5. Main Challenges Associated with the Industrial Development of LNPs
LNPs have shown immense promise as therapeutic nanocarriers, creating high demand across diverse fields, especially in the pharmaceutical industry. Nevertheless, traditional manufacturing approaches pose significant challenges in scaling up nanomedicine production, limiting their clinical development.27 Furthermore, the substantial costs associated with commercial manufacturing act as a recognized barrier, hindering the smooth transition from bench research to clinical application.27
In this section, we will delve into the primary challenges associated with the industrial production of LNPs, encompassing aspects such as stability, sterilization, storage, regulatory compliance, and quality control of synthesized LNPs.
5.1. Stability and Sterilization
Stability is a crucial requirement in the industrial production of LNP formulations, and it can be influenced by lipid polymorphism, which refers to phase transformations.113,174 Different methods and lipid types used in LNPs can lead to polymorphism, potentially compromising their stability.175 For instance, exposure to radiation and high temperatures can trigger lipid polymorphism, resulting in LNP instability.27 Polymorphism has been observed in triglycerides and fatty acids prepared using the hot homogenization method.175
During preparation or storage, triglycerides in LNPs may convert from their α-form to the more stable β-form, leading to the formation of polymorphic crystalline aggregates and reduced amorphous zones in the carrier matrix, which can result in drug leakage.176 The kinetics of triglyceride polymorphic transitions depend on chain length, with longer-chain triglycerides crystallizing more slowly than shorter-chain ones.177 As a result, LNP formulations composed of long-chain triglycerides tend to be more stable than those containing short-chain triglycerides. Proper consideration of lipid polymorphism is vital for achieving stable LNP formulations during industrial production.
Sterilizing LNPs during industrial production poses a significant challenge due to potential destabilization caused by conventional methods.116 For instance, γ radiation commonly used for sterilization can lead to lipid oxidation and chain scission, affecting LNP stability and efficacy.178 Autoclaving may trigger phase transitions and thermal stress, further destabilizing LNPs.179 Filtration and aseptic processing may cause aggregation or deformation due to shear stress.180 To address this, alternative sterilization methods like UV irradiation, ethylene oxide sterilization, and mild sterile filtration have been explored.121 However, each method has limitations and requires careful optimization for each LNP formulation. Thus, selecting an appropriate sterilization method remains critical for ensuring the stability and efficacy of LNPs during industrial production.
5.2. Storage
Industrial production of LNPs poses significant challenges due to their inherent instability and susceptibility to physical and chemical changes during storage. These alterations can have adverse effects on drug encapsulation, release properties, and overall stability, leading to issues like aggregation, changes in particle size distribution, drug leakage, and decreased stability.27 Additionally, lipid oxidation during storage can impact particle surface charge, drug release properties, and stability, potentially resulting in the formation of toxic byproducts that can compromise therapeutic efficacy.122
Moreover, interactions with container materials, such as leaching of ions, absorption of surfactants, and changes in pH, can further influence LNP stability.181 Due to these issues, most LNP formulations have a relatively short shelf life of less than one year.182 Efforts to overcome these obstacles and extend the shelf life of LNPs are essential for successful commercialization and widespread clinical use of these promising nanocarriers.
To tackle these challenges, several strategies have been devised, encompassing lyophilization, incorporation of stabilizing agents like antioxidants or chelators to prevent oxidation and aggregation, utilization of excipients as buffers, osmolytes, or cryoprotectants, and specialized packaging materials to prevent container interactions.181,183 Freezing and lyophilization are commonly used techniques for long-term storage of lipid-based formulations.184,185 However, lyophilization can be costly and time-consuming, potentially leading to drug damage due to stresses from crystallization and vacuum dehydration, thereby compromising LNP stability unless cryoprotectants are included.186,187
Studies have shown that incorporating 5% (w/v) sucrose or trehalose (cryoprotectants) in LNP-mRNA formulations stored in liquid nitrogen can preserve mRNA in vivo efficacy for up to 3 months.187 Pfizer’s COVID-19 mRNA vaccines are stored in freezing conditions with added sucrose, and Moderna uses Tris-HCl buffer as a hydroxyl radical scavenger, providing additional stabilization for LNP-based mRNA vaccines.188,189 Implementing these strategies holds promise in extending the LNP shelf life and enhancing the stability of LNPs, thereby advancing their successful utilization in various therapeutic applications.
Existing LNP-formulated drugs often necessitate ultracold storage temperatures.190 For instance, during the COVID-19 pandemic, mRNA-LNP vaccines like Comirnaty (BNT162b2) and Spikevax (mRNA-1273) require storage at ultracold temperatures (−80 and −20 °C, respectively) to ensure their safety and efficacy as per regulatory guidelines.182 Consequently, the distribution and storage of these vaccines require advanced packaging technologies and specialized containers capable of withstanding ultralow temperatures and minimizing breakage during transportation.191
To address these challenges, Corning researchers have introduced Valor glass vials specifically designed for delivering LNP-based mRNA vaccines.192 Valor glass vials are made from aluminosilicate glass, providing superior strength and resistance to breakages, even in extremely cold conditions. These vials are produced using the ion exchange method, reducing cracks, breakage, and particulate contamination, while an inner coating diminishes friction and aesthetic flaws. These vials comply with USP Type I hydrolytic criteria and exhibit low extractable concentrations, ensuring a high product quality.
Moreover, SiO2Materials Science, a U.S.-based pharmaceutical company, has developed a novel container for LNP-mRNA vaccine storage. This SiO2 primary container combines a cyclic olefin polymer with nanolayer glass as the inner layer, achieved through plasma-enhanced chemical vapor deposition. This innovative SiO2 vial demonstrates exceptional resistance to physical, thermal, and chemical stress over its lifespan, eliminating concerns of breakage or leakage.193
In conclusion, optimizing the long-term storage of LNPs requires careful consideration and fine-tuning of storage conditions and packaging to ensure their enduring stability and effectiveness.190,194 Moreover, special attention should be devoted to shelf life during formulation design to facilitate the widespread use of LNP products, such as mRNA-LNP vaccines.184 While challenges related to LNP storage persist, ongoing research and innovation are essential to surmounting these hurdles and making LNP-formulated drugs more accessible and efficacious.
Conclusively, to improve the long-term storage of LNPs, careful consideration and optimization of storage conditions and packaging are required to ensure their stability and efficacy.80,180 Additionally, more attention should be given to shelf life in formulation design to facilitate large-scale use of LNP products such as mRNA-LNP vaccines.182,194 While challenges associated with LNP storage remain, continued research and innovation are necessary to overcome these obstacles and make LNP-formulated drugs more accessible and effective.
5.3. Regulatory Compliance
In addition to the challenges in research and development, it is anticipated that the regulatory approval process can pose an additional barrier for unfeasible LNP-based technologies, especially considering the diverse range of compounds that can be delivered by LNPs.195 Regulatory bodies such as the Food and Drug Administration (FDA) and European Medicines Agency (EMA) require LNPs to meet quality, safety, and efficacy standards before approval.196 During the preclinical development stage, cell and animal studies are conducted to evaluate the toxicological effects of the LNP formulation. If considered safe, then approval (via an FDA “Investigational New Drug” or EMA ‘European Investigational Medicinal Product Dossier’ form) is required to begin phase 1 clinical trial. This meticulous regulatory scrutiny ensures the safety and effectiveness of LNPs in therapeutic applications.
To ensure regulatory compliance, toxicology evaluations play a crucial role, particularly concerning LNPs, as their accumulation in healthy tissues can lead to cytotoxicity and genotoxicity (Table 2). This may be due to the cationic lipid components utilized in LNP formulations. For instance, first-generation monoalkyl cationic lipid, stearyl amine, has been shown to cause hemolysis and hemagglutination of human erythrocytes.81 Another commonly used cationic lipid, DOTAP, has the propensity to bind to serum proteins, lipoproteins, and the extracellular matrix, leading to aggregation or premature drug release.197,198 Similarly, LNPs containing YSK05, a pH-sensitive cationic lipid used for delivering short interfering RNA (siRNA), exhibited rapid interactions with cell surfaces, leading to concentration-dependent toxicity in human A375 and A375-SM melanoma cell lines.199 Addressing these toxicological concerns is vital for the safe and effective use of LNPs in therapeutic applications.
Table 2. Toxicity of LNP-Based Marketed Drugs.
| Marketed formulation | active drug | toxicity symptoms | ref |
|---|---|---|---|
| Doxil | doxorubicin | hand-foot syndrome, stomatitis, skin toxicity (facial swelling, itching), hypersensitivity reactions, mild myelosuppression and alopecia, dyspnea | (200−202) |
| Myocet | doxorubicin | neutropenia, mild cardiotoxic | (203) |
| Abelcet, Amphotec, AmBisome | amphotericin B | nephrotoxicity, hypersensitivity reactions such as rash, flushing, facial edema, bronchospasm, persistent fever and rigors, hypokalemia | (204−206) |
| Linhaliq/Pulmaquin | ciprofloxacin | dyspnea, bronchospasm, hemoptysis, cough, taste disorders. | (207) |
| Daunoxome | daunorubicin | hematological toxicity such as neutropenia, back pain | (208, 209) |
| Visudyne | verteporfin | back pain, chest pain, dyspnea, dizziness, rash | (210) |
| Arikace | amikacin | ototoxicity including deafness, dizziness, vertigo, dysphonia, pneumonitis, laryngitis | (211, 212) |
| Ambraxane | paclitaxel | neutropenia, hypersensitivity reactions, neuropathy, severe myelosuppression | (213) |
Following government approval for clinical use, LNP-based drugs undergo comprehensive testing in clinical trial phases 1–3.214 These trials involve evaluation in healthy individuals, patients with the target disease, and a larger population, with additional approvals needed between phases 2 and 3. Throughout each stage, the regulatory agency thoroughly assesses safety and efficacy data, potential side effects, dosing regimen, and overall safety profile to make informed decisions on authorizing the drug for commercial use. These rigorous evaluations ensure that LNP-based drugs meet the highest standards of safety and effectiveness before they become available for widespread clinical application.
Adhering to government regulatory standards is crucial to ensure patient safety and enable the therapeutic application of LNP-based drugs.195 In certain situations, approval can be expedited, as seen during the rapid authorization of LNP-formulated mRNA vaccines amidst the SARS-CoV-2 health emergency.215 Additionally, large pharmaceutical companies hold an advantage in the LNP field, leveraging prior regulatory approvals and successful R&D pipelines.216 However, the typical FDA and EMA approval process for new drugs takes around 6–9 months, considering the FDA’s requirement to approve or reject a drug within 10 months after phase 3 trial completion. Consequently, staggered approval of LNP-based drugs can be attributed to research and technical limitations.
A notable example is Patisiran (Onpattro), an FDA-approved medication for treating fatal hereditary polyneuropathy. Its R&D pipeline involved the design and screening of more than 300 ionizable lipids, evaluating suitable LNP size, encapsulation efficiency, surface charge, and injection site for targeted siRNA delivery. This exhaustive study showcases significant advancements in LNP research while emphasizing the considerable effort needed for successful regulatory approval.10,217
Ensuring regulatory compliance for LNPs is paramount to guarantee their safety and effectiveness in therapeutic applications. Manufacturers must adhere to established quality standards, conduct rigorous characterization studies and toxicology evaluations, and meet specific regulatory criteria for clinical trials before LNPs can be authorized for use.195 This comprehensive approach is essential to ensure that LNPs meet the necessary safety and efficacy standards, providing confidence in their therapeutic potential.
5.4. Quality Control
The significance of quality control in LNP industrial production cannot be overstated as it is pivotal to ensure the safety, efficacy, and consistent therapeutic performance of these products. Manufacturers must adhere to stringent quality control procedures throughout the entire production process to guarantee that the final LNP product aligns with the required specifications and standards. An essential aspect of quality control involves a thorough validation of the manufacturing process itself, verifying its reproducibility, consistency, and adherence to the required quality benchmarks. To achieve this, manufacturers should implement a robust quality management system, incorporating regular audits, inspections, and comprehensive documentation to ensure compliance with regulatory requirements.218
Characterizing LNPs through quality control is crucial, involving the assessment of physicochemical properties like particle size, polydispersity index (PDI), surface charge, morphology, and stability.4 These attributes termed critical quality attributes (CQAs) for liposome drug products, significantly impact the safety and performance of LNPs, necessitating stringent control and monitoring during production.219 Meeting regulatory requirements, LNPs should have a PDI value of ≤0.30.220 However, the conventional batch process often yields large, heterogeneous particles (>100 nm), necessitating additional steps like extrusion, sonication, and homogenization to reduce particle sizes, leading to challenges in quality control and batch-to-batch consistency. Variations in lipid and component batches can further compound inconsistencies in the final product, aggravated by production condition differences, such as temperature, pressure, and mixing parameters. This approach’s scalability to larger volumes poses challenges in quality control and increased variability.221,222
Alternatively, continuous processing, such as microfluidic mixing, offers better control over product quality and performance.223,224 The incorporation of turbidity probes and UV–vis spectrometers with predictive algorithms enables real-time monitoring and feedback, swiftly detecting and rectifying deviations from the desired specifications during production. The scalability achieved through pilot-scale mixing process scaling-out ensures enhanced reproducibility and facilitates scaled-up production.225
In conclusion, achieving superior quality control in industrial LNP production requires a comprehensive approach involving robust process validation and meticulous characterization. By adhering to required specifications and standards, manufacturers can confidently deliver safe, effective, and reliable LNP-based therapies to patients.
6. Future Prospects and Conclusion
This review has delved into the clinical potential of various types of LNPs and their production processes at both laboratory and industrial scales. To enable widespread clinical application, addressing challenges is crucial, such as developing efficient scaling-up methods such as continuous flow synthesis to increase yield and reduce costs.
Harmonizing regulatory policies for LNP use in clinical settings at a global level is essential. Consistency in regulations can foster the development and approval of new LNP-based therapies, especially for rare diseases that require international collaboration.226 The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines provide a framework for new drug development, and mutual recognition agreements between regulatory agencies facilitate the approval process for drugs approved in other countries.196
In conclusion, LNPs hold significant promise for improving drug delivery. Different types of LNPs and preparation methods offer distinct advantages and challenges, necessitating careful consideration when designing drug delivery systems. While clinical trials have demonstrated the effectiveness of LNPs, further improvements in their industrial development are needed to ensure safe and efficient drug delivery.
Acknowledgments
This work was financially supported by funding (GNT1181889) from the Australian National Health and Medical Research Council, fellowship award (2019/CDF1013) from Cancer Institute NSW, Australia.
Author Contributions
# M.M. and T.A.B. contributed equally to this work. Meenu Mehta conceptualization, writing original draft, writing-reviewing and editing; Thuy Anh Bui conceptualization, writing original draft, writing-reviewing and editing; Xinpu Yang writing original draft about Microfluidic devices, Yagiz Aksoy writing-reviewing and editing; Ewa M. Goldys reviewing and editing; Wei Deng funding acquisition, conceptualization, writing original draft, writing-reviewing and editing.
The authors declare no competing financial interest.
References
- Gurevich E. V.; Gurevich V. V. Beyond traditional pharmacology: new tools and approaches. Br. J. Pharmacol. 2015, 172 (13), 3229–3241. 10.1111/bph.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenchov R.; Bird R.; Curtze A. E.; Zhou Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15 (11), 16982–17015. 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- Mittal D.; Kaur G.; Singh P.; Yadav K.; Ali S. A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Frontiers in Nanotechnology 2020, 2, Review. 10.3389/fnano.2020.579954. [DOI] [Google Scholar]
- Shah S.; Dhawan V.; Holm R.; Nagarsenker M. S.; Perrie Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv Rev. 2020, 154–155, 102–122. 10.1016/j.addr.2020.07.002. [DOI] [PubMed] [Google Scholar]
- Beck H.; Harter M.; Hass B.; Schmeck C.; Baerfacker L. Small molecules and their impact in drug discovery: A perspective on the occasion of the 125th anniversary of the Bayer Chemical Research Laboratory. Drug Discov Today 2022, 27 (6), 1560–1574. 10.1016/j.drudis.2022.02.015. [DOI] [PubMed] [Google Scholar]
- Forbes N.; Hussain M. T.; Briuglia M. L.; Edwards D. P.; Horst J. H. T.; Szita N.; Perrie Y. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. Int. J. Pharm. 2019, 556, 68–81. 10.1016/j.ijpharm.2018.11.060. [DOI] [PubMed] [Google Scholar]
- Verma M.; Ozer I.; Xie W.; Gallagher R.; Teixeira A.; Choy M. The landscape for lipid-nanoparticle-based genomic medicines. Nat. Rev. Drug Discov 2023, 22, 349. 10.1038/d41573-023-00002-2. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Grainger D. W. Regulatory Considerations Specific to Liposome Drug Development as Complex Drug Products. Frontiers in Drug Delivery 2022, 2, 901281. 10.3389/fddev.2022.901281. [DOI] [Google Scholar]
- Wang Z.; Cui K.; Costabel U.; Zhang X. Nanotechnology-facilitated vaccine development during the coronavirus disease 2019 (COVID-19) pandemic. Exploration (Beijing) 2022, 2 (5), 20210082. 10.1002/EXP.20210082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinc A.; Maier M. A.; Manoharan M.; Fitzgerald K.; Jayaraman M.; Barros S.; Ansell S.; Du X.; Hope M. J.; Madden T. D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol 2019, 14 (12), 1084–1087. 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- Guo S.; Li K.; Hu B.; Li C.; Zhang M.; Hussain A.; Wang X.; Cheng Q.; Yang F.; Ge K.; et al. Membrane-destabilizing ionizable lipid empowered imaging-guided siRNA delivery and cancer treatment. Exploration (Beijing) 2021, 1 (1), 35–49. 10.1002/EXP.20210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G.; Kant A.; Kumar M.; Masram D. T. Synthesis, characterizations and kinetic study of metal organic framework nanocomposite excipient used as extended release delivery vehicle for an antibiotic drug. Inorg. Chim. Acta 2019, 496, 119036. 10.1016/j.ica.2019.119036. [DOI] [Google Scholar]
- Kumar G.; Chaudhary K.; Mogha N. K.; Kant A.; Masram D. T. Extended Release of Metronidazole Drug Using Chitosan/Graphene Oxide Bionanocomposite Beads as the Drug Carrier. ACS Omega 2021, 6 (31), 20433–20444. 10.1021/acsomega.1c02422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke S.; Braig S.; Preiß T.; Zimpel A.; Sicklinger J.; Bellomo C.; Rädler J. O.; Vollmar A. M.; Bein T. MOF nanoparticles coated by lipid bilayers and their uptake by cancer cells. Chem. Commun. (Camb) 2015, 51 (87), 15752–15755. 10.1039/C5CC06767G. [DOI] [PubMed] [Google Scholar]
- Róg T.; Girych M.; Bunker A. Mechanistic Understanding from Molecular Dynamics in Pharmaceutical Research 2: Lipid Membrane in Drug Design. Pharmaceuticals 2021, 14 (10), 1062. 10.3390/ph14101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout R. M.; Metwally A. A. Towards better modelling of drug-loading in solid lipid nanoparticles: Molecular dynamics, docking experiments and Gaussian Processes machine learning. Eur. J. Pharm. Biopharm. 2016, 108, 262–268. 10.1016/j.ejpb.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Cheng X.; Lee R. J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv Rev. 2016, 99 (Pt A), 129–137. 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- Puri A.; Loomis K.; Smith B.; Lee J. H.; Yavlovich A.; Heldman E.; Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev. Ther Drug Carrier Syst 2009, 26 (6), 523–580. 10.1615/CritRevTherDrugCarrierSyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Wang X.; Liu Y.; Yang G.; Falconer R. J.; Zhao C.-X. Lipid Nanoparticles for Drug Delivery. Advanced NanoBiomed Research 2022, 2 (2), 2100109. 10.1002/anbr.202100109. [DOI] [Google Scholar]
- Mora-Huertas C. E.; Fessi H.; Elaissari A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385 (1–2), 113–142. 10.1016/j.ijpharm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Martínez Rivas C. J.; Tarhini M.; Badri W.; Miladi K.; Greige-Gerges H.; Nazari Q. A.; Galindo Rodríguez S. A.; Román R.; Fessi H.; Elaissari A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532 (1), 66–81. 10.1016/j.ijpharm.2017.08.064. [DOI] [PubMed] [Google Scholar]
- Rasmussen M. K.; Pedersen J. N.; Marie R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11 (1), 2337. 10.1038/s41467-020-15889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. C.; Crist R. M.; Clogston J. D.; McNeil S. E. Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal Bioanal Chem. 2017, 409 (24), 5779–5787. 10.1007/s00216-017-0527-z. [DOI] [PubMed] [Google Scholar]
- Ruozi B.; Belletti D.; Tombesi A.; Tosi G.; Bondioli L.; Forni F.; Vandelli M. A. AFM, ESEM, TEM, and CLSM in liposomal characterization: a comparative study. Int. J. Nanomedicine 2011, 6, 557–563. 10.2147/IJN.S14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhigaltsev I. V.; Cullis P. R. Morphological Behavior of Liposomes and Lipid Nanoparticles. Langmuir 2023, 39 (9), 3185–3193. 10.1021/acs.langmuir.2c02794. [DOI] [PubMed] [Google Scholar]
- Knop K.; Hoogenboom R.; Fischer D.; Schubert U. S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem., Int. Ed. Engl. 2010, 49 (36), 6288–6308. 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- Battaglia L.; Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opin Drug Deliv 2012, 9 (5), 497–508. 10.1517/17425247.2012.673278. [DOI] [PubMed] [Google Scholar]
- Yu F.; Miao Y.; Wang M.; Liu Q.; Yuan L.; Geng R.; Qiu Q.; Ni C.; Kay M. Predicting nanoemulsion formulation and studying the synergism mechanism between surfactant and cosurfactant: A combined computational and experimental approach. Int. J. Pharm. 2022, 615, 121473. 10.1016/j.ijpharm.2022.121473. [DOI] [PubMed] [Google Scholar]
- Balouch M.; Storchmannova K.; Stepanek F.; Berka K. Computational Prodrug Design Methodology for Liposome Formulability Enhancement of Small-Molecule APIs. Mol. Pharmaceutics 2023, 20 (4), 2119–2127. 10.1021/acs.molpharmaceut.2c01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban V. V.; Khandelia H. Distribution of neutral lipids in the lipid droplet core. J. Phys. Chem. B 2014, 118 (38), 11145–11151. 10.1021/jp506693d. [DOI] [PubMed] [Google Scholar]
- Chaban V. V.; Khandelia H. Lipid structure in triolein lipid droplets. J. Phys. Chem. B 2014, 118 (35), 10335–10340. 10.1021/jp503223z. [DOI] [PubMed] [Google Scholar]
- Chan C.; Du S.; Dong Y.; Cheng X. Computational and Experimental Approaches to Investigate Lipid Nanoparticles as Drug and Gene Delivery Systems. Curr. Top Med. Chem. 2021, 21 (2), 92–114. 10.2174/1568026620666201126162945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwally A. A.; Hathout R. M. Computer-Assisted Drug Formulation Design: Novel Approach in Drug Delivery. Mol. Pharmaceutics 2015, 12 (8), 2800–2810. 10.1021/mp500740d. [DOI] [PubMed] [Google Scholar]
- Yadav D. K.; Kumar S.; Choi E. H.; Chaudhary S.; Kim M. H. Computational Modeling on Aquaporin-3 as Skin Cancer Target: A Virtual Screening Study. Front Chem. 2020, 8, 250. 10.3389/fchem.2020.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham A. D.; Standish M. M.; Watkins J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13 (1), 238–252. 10.1016/S0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- Wang H. F.; Ran R.; Liu Y.; Hui Y.; Zeng B.; Chen D.; Weitz D. A.; Zhao C. X. Tumor-Vasculature-on-a-Chip for Investigating Nanoparticle Extravasation and Tumor Accumulation. ACS Nano 2018, 12 (11), 11600–11609. 10.1021/acsnano.8b06846. [DOI] [PubMed] [Google Scholar]
- Has C.; Sunthar P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30 (4), 336–365. 10.1080/08982104.2019.1668010. [DOI] [PubMed] [Google Scholar]
- Harashima H.; Sakata K.; Funato K.; Kiwada H. Enhanced hepatic uptake of liposomes through complement activation depending on the size of liposomes. Pharm. Res. 1994, 11 (3), 402–406. 10.1023/A:1018965121222. [DOI] [PubMed] [Google Scholar]
- Parchekani J.; Allahverdi A.; Taghdir M.; Naderi-Manesh H. Design and simulation of the liposomal model by using a coarse-grained molecular dynamics approach towards drug delivery goals. Sci. Rep 2022, 12 (1), 2371. 10.1038/s41598-022-06380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaalem M.; Hadrioui N.; Derouiche A.; Ridouane H. Structure and dynamics of liposomes designed for drug delivery: coarse-grained molecular dynamics simulations to reveal the role of lipopolymer incorporation. RSC Adv. 2020, 10 (7), 3745–3755. 10.1039/C9RA08632C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambeck J. P.; Eriksson E. S.; Laaksonen A.; Lyubartsev A. P.; Eriksson L. A. Molecular Dynamics Studies of Liposomes as Carriers for Photosensitizing Drugs: Development, Validation, and Simulations with a Coarse-Grained Model. J. Chem. Theory Comput 2014, 10 (1), 5–13. 10.1021/ct400466m. [DOI] [PubMed] [Google Scholar]
- Dzieciuch M.; Rissanen S.; Szydlowska N.; Bunker A.; Kumorek M.; Jamroz D.; Vattulainen I.; Nowakowska M.; Rog T.; Kepczynski M. PEGylated Liposomes as Carriers of Hydrophobic Porphyrins. J. Phys. Chem. B 2015, 119 (22), 6646–6657. 10.1021/acs.jpcb.5b01351. [DOI] [PubMed] [Google Scholar]
- Lehtinen J.; Magarkar A.; Stepniewski M.; Hakola S.; Bergman M.; Rog T.; Yliperttula M.; Urtti A.; Bunker A. Analysis of cause of failure of new targeting peptide in PEGylated liposome: molecular modeling as rational design tool for nanomedicine. Eur. J. Pharm. Sci. 2012, 46 (3), 121–130. 10.1016/j.ejps.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Wagner A.; Vorauer-Uhl K. Liposome technology for industrial purposes. Journal of drug delivery 2011, 2011, 1. 10.1155/2011/591325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercombe L.; Veerati T.; Moheimani F.; Wu S. Y.; Sood A. K.; Hua S. Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharmacol 2015, 6, 286. 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino M. L.; Dosio F.; Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomedicine 2006, 1 (3), 297–315. [PMC free article] [PubMed] [Google Scholar]
- Lee Y.; Thompson D. H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip Rev. Nanomed Nanobiotechnol 2017, 9 (5), e1450. 10.1002/wnan.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail M.; Yang W.; Li Y.; Chai T.; Zhang D.; Du Q.; Muhammad P.; Hanif S.; Zheng M.; Shi B. Targeted liposomes for combined delivery of artesunate and Temozolomide to resistant glioblastoma. Biomaterials 2022, 287, 121608. 10.1016/j.biomaterials.2022.121608. [DOI] [PubMed] [Google Scholar]
- Barenholz Y.; Haran G.. Method of amphophilic drug loading into liposomes by pH gradient, US Patent 1993, 5 192 549.; Gabizon A.; Catane R.; Uziely B.; Kaufman B.; Safra T.; Cohen R.; Martin F.; Huang A.; Barenholz Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene glycol-coated liposomes. Cancer Res. 1994, 54, 987–992. [PubMed] [Google Scholar]
- Barenholz Y. C. Doxil®—The first FDA-approved nano-drug: Lessons learned. Journal of controlled release 2012, 160 (2), 117–134. 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Tadros T.; Izquierdo P.; Esquena J.; Solans C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Gupta A.Nanoemulsions. In Nanoparticles for Biomedical Applications, Chung E. J.; Leon L., Rinaldi C., Eds.; Elsevier, 2020; Ch. 21, pp 371–384. [Google Scholar]
- Aswathanarayan J. B.; Vittal R. R. Nanoemulsions and Their Potential Applications in Food Industry. Frontiers in Sustainable Food Systems 2019, 3, 95. 10.3389/fsufs.2019.00095. [DOI] [Google Scholar]
- Kralova I.; Sjöblom J. Surfactants Used in Food Industry: A Review. J. Dispersion Sci. Technol. 2009, 30 (9), 1363–1383. 10.1080/01932690902735561. [DOI] [Google Scholar]
- Ikeuchi-Takahashi Y.; Kobayashi A.; Ishihara C.; Matsubara T.; Matsubara H.; Onishi H. Influence of Polysorbate 60 on Formulation Properties and Bioavailability of Morin-Loaded Nanoemulsions with and without Low-Saponification-Degree Polyvinyl Alcohol. Biol. Pharm. Bull. 2018, 41 (5), 754–760. 10.1248/bpb.b17-00964. [DOI] [PubMed] [Google Scholar]
- Silva H. D.; Cerqueira M. A.; Vicente A. A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. Journal of Food Engineering 2015, 167, 89–98. 10.1016/j.jfoodeng.2015.07.037. [DOI] [Google Scholar]
- Wilson R. J.; Li Y.; Yang G.; Zhao C.-X. Nanoemulsions for drug delivery. Particuology 2022, 64, 85–97. 10.1016/j.partic.2021.05.009. [DOI] [Google Scholar]
- Ammar H. O.; Salama H. A.; Ghorab M.; Mahmoud A. A. Development of dorzolamide hydrochloride in situ gel nanoemulsion for ocular delivery. Drug Dev. Ind. Pharm. 2010, 36 (11), 1330–1339. 10.3109/03639041003801885. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Misra A.; Babbar A. K.; Mishra A. K.; Mishra P.; Pathak K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int. J. Pharm. 2008, 358 (1–2), 285–291. 10.1016/j.ijpharm.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Jaiswal M.; Dudhe R.; Sharma P. K. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech 2015, 5 (2), 123–127. 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormann K.; Zimmer A. Drug delivery and drug targeting with parenteral lipid nanoemulsions - A review. J. Controlled Release 2016, 223, 85–98. 10.1016/j.jconrel.2015.12.016. [DOI] [PubMed] [Google Scholar]
- Pirhadi S.; Amani A. Molecular dynamics simulation of self-assembly in a nanoemulsion system. Chemical Papers 2020, 74 (8), 2443–2448. 10.1007/s11696-020-01050-3. [DOI] [Google Scholar]
- Pyrhönen J.; Bansal K. K.; Bhadane R.; Wilén C.-E.; Salo-Ahen O. M. H.; Rosenholm J. M. Molecular Dynamics Prediction Verified by Experimental Evaluation of the Solubility of Different Drugs in Poly(decalactone) for the Fabrication of Polymeric Nanoemulsions. Advanced NanoBiomed Research 2022, 2 (1), 2100072. 10.1002/anbr.202100072. [DOI] [Google Scholar]
- Hong Z.; Xiao N.; Li L.; Xie X. Investigation of nanoemulsion interfacial properties: A mesoscopic simulation. Journal of Food Engineering 2020, 276, 109877. 10.1016/j.jfoodeng.2019.109877. [DOI] [Google Scholar]
- Glotzer S. C.; Paul W. Molecular and Mesoscale Simulation Methods for Polymer Materials. Annu. Rev. Mater. Res. 2002, 32 (1), 401–436. 10.1146/annurev.matsci.32.010802.112213. [DOI] [Google Scholar]
- Polchi A.; Magini A.; Mazuryk J.; Tancini B.; Gapinski J.; Patkowski A.; Giovagnoli S.; Emiliani C. Rapamycin Loaded Solid Lipid Nanoparticles as a New Tool to Deliver mTOR Inhibitors: Formulation and in Vitro Characterization. Nanomaterials (Basel) 2016, 6 (5), 87. 10.3390/nano6050087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R. M.; Mata J. P.; Bryant G.; Campo L.; Ife A.; Karpe A. V.; Jadhav S. R.; Eldridge D. S.; Palombo E. A.; Harding I. H. Structure Analysis of Solid Lipid Nanoparticles for Drug Delivery: A Combined USANS/SANS Study. Particle & Particle Systems Characterization 2019, 36 (1), 1800359. 10.1002/ppsc.201800359. [DOI] [Google Scholar]
- Duan Y.; Dhar A.; Patel C.; Khimani M.; Neogi S.; Sharma P.; Siva Kumar N.; Vekariya R. L. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10 (45), 26777–26791. 10.1039/D0RA03491F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geszke-Moritz M.; Moritz M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 982–994. 10.1016/j.msec.2016.05.119. [DOI] [PubMed] [Google Scholar]
- Qiu S.; Liang D.; Guo F.; Deng T.; Peng T.; Gao Y.; Zhang X.; Zhong H. Solid lipid nanoparticles modified with amphipathic chitosan derivatives for improved stability in the gastrointestinal tract. Journal of Drug Delivery Science and Technology 2018, 48, 288–299. 10.1016/j.jddst.2018.10.007. [DOI] [Google Scholar]
- Kuo Y. C.; Chung J. F. Physicochemical properties of nevirapine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2011, 83 (2), 299–306. 10.1016/j.colsurfb.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Venishetty V. K.; Komuravelli R.; Kuncha M.; Sistla R.; Diwan P. V. Increased brain uptake of docetaxel and ketoconazole loaded folate-grafted solid lipid nanoparticles. Nanomedicine 2013, 9 (1), 111–121. 10.1016/j.nano.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Kuo Y. C.; Liang C. T. Inhibition of human brain malignant glioblastoma cells using carmustine-loaded catanionic solid lipid nanoparticles with surface anti-epithelial growth factor receptor. Biomaterials 2011, 32 (12), 3340–3350. 10.1016/j.biomaterials.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Abdel-Mageed H. M.; Abd El Aziz A. E.; Mohamed S. A.; AbuelEzz N. Z. The tiny big world of solid lipid nanoparticles and nanostructured lipid carriers: an updated review. J. Microencapsul 2022, 39 (1), 72–94. 10.1080/02652048.2021.2021307. [DOI] [PubMed] [Google Scholar]
- Hathout R. M.; Metwally A. A. Towards better modelling of drug-loading in solid lipid nanoparticles: Molecular dynamics, docking experiments and Gaussian Processes machine learning. Eur. J. Pharm. Biopharm 2016, 108, 262–268. 10.1016/j.ejpb.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Pink D. L.; Loruthai O.; Ziolek R. M.; Wasutrasawat P.; Terry A. E.; Lawrence M. J.; Lorenz C. D. On the Structure of Solid Lipid Nanoparticles. Small 2019, 15 (45), e1903156 10.1002/smll.201903156. [DOI] [PubMed] [Google Scholar]
- Gastaldi L.; Battaglia L.; Peira E.; Chirio D.; Muntoni E.; Solazzi I.; Gallarate M.; Dosio F. Solid lipid nanoparticles as vehicles of drugs to the brain: current state of the art. Eur. J. Pharm. Biopharm 2014, 87 (3), 433–444. 10.1016/j.ejpb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Soares S.; Fonte P.; Costa A.; Andrade J.; Seabra V.; Ferreira D.; Reis S.; Sarmento B. Effect of freeze-drying, cryoprotectants and storage conditions on the stability of secondary structure of insulin-loaded solid lipid nanoparticles. Int. J. Pharm. 2013, 456 (2), 370–381. 10.1016/j.ijpharm.2013.08.076. [DOI] [PubMed] [Google Scholar]
- Mehnert W.; Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv Rev. 2001, 47 (2–3), 165–196. 10.1016/S0169-409X(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Shegokar R.; Singh K. K.; Muller R. H. Production & stability of stavudine solid lipid nanoparticles-from lab to industrial scale. Int. J. Pharm. 2011, 416 (2), 461–470. 10.1016/j.ijpharm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Farhood H.; Gao X.; Son K.; Yang Y. Y.; Lazo J. S.; Huang L.; Barsoum J.; Bottega R.; Epand R. M. Cationic liposomes for direct gene transfer in therapy of cancer and other diseases. Ann. N.Y. Acad. Sci. 1994, 716, 23–34. discussion 34–25 10.1111/j.1749-6632.1994.tb21701.x. [DOI] [PubMed] [Google Scholar]
- Araujo V. H. S.; Delello Di Filippo L.; Duarte J. L.; Spósito L.; Camargo B. A. F.; da Silva P. B.; Chorilli M. Exploiting solid lipid nanoparticles and nanostructured lipid carriers for drug delivery against cutaneous fungal infections. Crit Rev. Microbiol 2021, 47 (1), 79–90. 10.1080/1040841X.2020.1843399. [DOI] [PubMed] [Google Scholar]
- Hao J.; Wang F.; Wang X.; Zhang D.; Bi Y.; Gao Y.; Zhao X.; Zhang Q. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur. J. Pharm. Sci. 2012, 47 (2), 497–505. 10.1016/j.ejps.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Badawi N.; El-Say K.; Attia D.; El-Nabarawi M.; Elmazar M.; Teaima M. Development of Pomegranate Extract-Loaded Solid Lipid Nanoparticles: Quality by Design Approach to Screen the Variables Affecting the Quality Attributes and Characterization. ACS Omega 2020, 5 (34), 21712–21721. 10.1021/acsomega.0c02618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrosky-Zeichner L.; Marr K. A.; Rex J. H.; Cohen S. H. Amphotericin B: Time for a New ″Gold Standard″. Clin. Infect. Dis. 2003, 37 (3), 415. 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- FDA approves DaunoXome as first-line therapy for Kaposi’s sarcoma. Food and Drug Administration. J. Int. Assoc Physicians AIDS Care 1996, 2 ( (5), ), 50–51. [PubMed] [Google Scholar]
- Guo P.; Hsu T. M.; Zhao Y.; Martin C. R.; Zare R. N. Preparing amorphous hydrophobic drug nanoparticles by nanoporous membrane extrusion. Nanomedicine (Lond) 2013, 8 (3), 333–341. 10.2217/nnm.12.119. [DOI] [PubMed] [Google Scholar]
- FDA Fast Track Designation for Myocet for Metastatic Breast Cancer. Oncology Times 2010, 32 ( (3), ), 24. 10.1097/01.COT.0000368457.66658.a6 [DOI] [Google Scholar]
- FDA Approves Onivyde Combo Regimen for Advanced Pancreatic Cancer. Oncology Times 2015, 37 ( (22), ), 8. 10.1097/01.COT.0000475247.29686.b2 [DOI] [Google Scholar]
- Venkatakrishnan K.; Liu Y.; Noe D.; Mertz J.; Bargfrede M.; Marbury T.; Farbakhsh K.; Oliva C.; Milton A. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate hepatic impairment. Br. J. Clin. Pharmacol. 2014, 77 (6), 998–1010. 10.1111/bcp.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmarini C. M.; Mackey J. R.; Dumontet C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol 2002, 3 (7), 415–424. 10.1016/S1470-2045(02)00788-X. [DOI] [PubMed] [Google Scholar]
- O’Brien S.; Schiller G.; Lister J.; Damon L.; Goldberg S.; Aulitzky W.; Ben-Yehuda D.; Stock W.; Coutre S.; Douer D.; et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J. Clin Oncol 2013, 31 (6), 676–683. 10.1200/JCO.2012.46.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N.; Sachse A.; Bender J.; Schubert R.; Brandl M. Filter extrusion of liposomes using different devices: comparison of liposome size, encapsulation efficiency, and process characteristics. Int. J. Pharm. 2001, 223 (1–2), 55–68. 10.1016/S0378-5173(01)00721-9. [DOI] [PubMed] [Google Scholar]
- Gradishar W. J.; Tjulandin S.; Davidson N.; Shaw H.; Desai N.; Bhar P.; Hawkins M.; O’Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin Oncol 2005, 23 (31), 7794–7803. 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- Yardley D. A. nab-Paclitaxel mechanisms of action and delivery. J. Controlled Release 2013, 170 (3), 365–372. 10.1016/j.jconrel.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Ran R.; Sun Q.; Baby T.; Wibowo D.; Middelberg A. P.; Zhao C.-X. J. C. E. S. Multiphase microfluidic synthesis of micro-and nanostructures for pharmaceutical applications 2017, 169, 78–96. 10.1016/j.ces.2017.01.008. [DOI] [Google Scholar]
- Adedoyin A.; Bernardo J. F.; Swenson C. E.; Bolsack L. E.; Horwith G.; DeWit S.; Kelly E.; Klasterksy J.; Sculier J. P.; DeValeriola D.; et al. Pharmacokinetic profile of ABELCET (amphotericin B lipid complex injection): combined experience from phase I and phase II studies. Antimicrob. Agents Chemother. 1997, 41 (10), 2201–2208. 10.1128/AAC.41.10.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone N. R.; Bicanic T.; Salim R.; Hope W. Liposomal Amphotericin B (AmBisome((R))): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76 (4), 485–500. 10.1007/s40265-016-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons K. V.; Stevens D. A. Comparison of fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob. Agents Chemother. 1998, 42 (4), 899–902. 10.1128/AAC.42.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovier P. A. Epaxal: a virosomal vaccine to prevent hepatitis A infection. Expert Rev. Vaccines 2008, 7 (8), 1141–1150. 10.1586/14760584.7.8.1141. [DOI] [PubMed] [Google Scholar]
- Herzog C.; Hartmann K.; Kunzi V.; Kursteiner O.; Mischler R.; Lazar H.; Gluck R. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine 2009, 27 (33), 4381–4387. 10.1016/j.vaccine.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Cheng X.; Gao J.; Ding Y.; Lu Y.; Wei Q.; Cui D.; Fan J.; Li X.; Zhu E.; Lu Y.; et al. Multi-Functional Liposome: A Powerful Theranostic Nano-Platform Enhancing Photodynamic Therapy. Adv. Sci. (Weinh) 2021, 8 (16), e2100876 10.1002/advs.202100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M.; Doenicke A.; Nebauer A. E.; Hepting L. Propofol and etomidate-Lipuro for induction of general anesthesia. Hemodynamics, vascular compatibility, subjective findings and postoperative nausea. Anaesthesist 1996, 45 (11), 1082–1084. 10.1007/s001010050343. [DOI] [PubMed] [Google Scholar]
- Patel H. H.; P M.; Patel P. M.; Roth D. M.. General Anesthetics and Therapeutic Gases; McGraw Hill. [Google Scholar]
- Bakshi P.; Jiang Y.; Nakata T.; Akaki J.; Matsuoka N.; Banga A. K. Formulation Development and Characterization of Nanoemulsion-Based Formulation for Topical Delivery of Heparinoid. J. Pharm. Sci. 2018, 107 (11), 2883–2890. 10.1016/j.xphs.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Salim N.; Jose García-Celma M.; Escribano E.; Nolla J.; Llinàs M.; Basri M.; Solans C.; Esquena J.; Tadros T. F. Formation of Nanoemulsion Containing Ibuprofen by PIC Method for Topical Delivery. Materials Today: Proceedings 2018, 5, S172–S179. 10.1016/j.matpr.2018.08.062. [DOI] [Google Scholar]
- Stahelin H. F. The history of cyclosporin A (Sandimmune) revisited: another point of view. Experientia 1996, 52 (1), 5–13. 10.1007/BF01922409. [DOI] [PubMed] [Google Scholar]
- Dickman D. A. Process Chemistry Development of the HIV Protease Inhibitor Drug Kaletra: A Mixture of Ritonavir and Lopinavir 2022, 57. 10.1002/9781119847281.ch4. [DOI] [Google Scholar]
- Zhou Q.; Sun X.; Zeng L.; Liu J.; Zhang Z. A randomized multicenter phase II clinical trial of mitoxantrone-loaded nanoparticles in the treatment of 108 patients with unresected hepatocellular carcinoma. Nanomedicine 2009, 5 (4), 419–423. 10.1016/j.nano.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Holtze C. Large-scale droplet production in microfluidic devices—an industrial perspective. J. Phys. D: Appl. Phys. 2013, 46 (11), 114008. 10.1088/0022-3727/46/11/114008. [DOI] [Google Scholar]
- Maeki M.; Okada Y.; Uno S.; Sugiura K.; Suzuki Y.; Okuda K.; Sato Y.; Ando M.; Yamazaki H.; Takeuchi M. Mass production system for RNA-loaded lipid nanoparticles using piling up microfluidic devices. Applied Materials Today 2023, 31, 101754. 10.1016/j.apmt.2023.101754. [DOI] [Google Scholar]
- Carvajal-Vidal P.; Gonzalez-Pizarro R.; Araya C.; Espina M.; Halbaut L.; Gomez de Aranda I.; Garcia M. L.; Calpena A. C. Nanostructured lipid carriers loaded with Halobetasol propionate for topical treatment of inflammation: Development, characterization, biopharmaceutical behavior and therapeutic efficacy of gel dosage forms. Int. J. Pharm. 2020, 585, 119480. 10.1016/j.ijpharm.2020.119480. [DOI] [PubMed] [Google Scholar]
- Luzzati V.; Tardieu A.; Gulik-Krzywicki T. Polymorphism of lipids. Nature 1968, 217 (5133), 1028–1030. 10.1038/2171028a0. [DOI] [PubMed] [Google Scholar]
- Agrawal Y.; Petkar K. C.; Sawant K. K. Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int. J. Pharm. 2010, 401 (1–2), 93–102. 10.1016/j.ijpharm.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Charoenputtakun P.; Pamornpathomkul B.; Opanasopit P.; Rojanarata T.; Ngawhirunpat T. Terpene composited lipid nanoparticles for enhanced dermal delivery of all-trans-retinoic acids. Biol. Pharm. Bull. 2014, 37 (7), 1139–1148. 10.1248/bpb.b14-00015. [DOI] [PubMed] [Google Scholar]
- El-Salamouni N. S.; Farid R. M.; El-Kamel A. H.; El-Gamal S. S. Effect of sterilization on the physical stability of brimonidine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Int. J. Pharm. 2015, 496 (2), 976–983. 10.1016/j.ijpharm.2015.10.043. [DOI] [PubMed] [Google Scholar]
- Hadinoto K.; Sundaresan A.; Cheow W. S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85 (3, Part A), 427–443. 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Baden L. R.; El Sahly H. M.; Essink B.; Kotloff K.; Frey S.; Novak R.; Diemert D.; Spector S. A.; Rouphael N.; Creech C. B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J. Med. 2021, 384 (5), 403–416. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F. P.; Thomas S. J.; Kitchin N.; Absalon J.; Gurtman A.; Lockhart S.; Perez J. L.; Perez Marc G.; Moreira E. D.; Zerbini C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J. Med. 2020, 383 (27), 2603–2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. Y.; Kim K. P.; Jeong S. Y.; Park J.; Park J.; Jung J.; Chung H. K.; Lee S. W.; Seo M. H.; Lee J. S.; et al. Polymeric nanoparticle-docetaxel for the treatment of advanced solid tumors: phase I clinical trial and preclinical data from an orthotopic pancreatic cancer model. Oncotarget 2016, 7 (47), 77348–77357. 10.18632/oncotarget.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetten M. A.; Yah C. S.; Singh T.; Gulumian M. Challenges facing sterilization and depyrogenation of nanoparticles: effects on structural stability and biomedical applications. Nanomedicine 2014, 10 (7), 1391–1399. 10.1016/j.nano.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Packer M.; Gyawali D.; Yerabolu R.; Schariter J.; White P. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat. Commun. 2021, 12 (1), 6777. 10.1038/s41467-021-26926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autio K. A.; Dreicer R.; Anderson J.; Garcia J. A.; Alva A.; Hart L. L.; Milowsky M. I.; Posadas E. M.; Ryan C. J.; Graf R. P.; et al. Safety and Efficacy of BIND-014, a Docetaxel Nanoparticle Targeting Prostate-Specific Membrane Antigen for Patients With Metastatic Castration-Resistant Prostate Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2018, 4 (10), 1344–1351. 10.1001/jamaoncol.2018.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobovkina T.; Jacobson G. B.; Gonzalez-Gonzalez E.; Hickerson R. P.; Leake D.; Kaspar R. L.; Contag C. H.; Zare R. N. In vivo sustained release of siRNA from solid lipid nanoparticles. ACS Nano 2011, 5 (12), 9977–9983. 10.1021/nn203745n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi V. R.; Pawar P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. Journal of Drug Delivery Science and Technology 2019, 51, 255–267. 10.1016/j.jddst.2019.02.017. [DOI] [Google Scholar]
- Chauhan I.; Yasir M.; Verma M.; Singh A. P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10 (2), 150–165. 10.34172/apb.2020.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izza N.; Suga K.; Okamoto Y.; Watanabe N.; Bui T. T.; Wibisono Y.; Fadila C. R.; Umakoshi H. Systematic Characterization of Nanostructured Lipid Carriers from Cetyl Palmitate/Caprylic Triglyceride/Tween 80 Mixtures in an Aqueous Environment. Langmuir 2021, 37 (14), 4284–4293. 10.1021/acs.langmuir.1c00270. [DOI] [PubMed] [Google Scholar]
- Ortiz A. C.; Yanez O.; Salas-Huenuleo E.; Morales J. O. Development of a Nanostructured Lipid Carrier (NLC) by a Low-Energy Method, Comparison of Release Kinetics and Molecular Dynamics Simulation. Pharmaceutics 2021, 13 (4), 531. 10.3390/pharmaceutics13040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloqui A.; Solinis M. A.; Rodriguez-Gascon A.; Almeida A. J.; Preat V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12 (1), 143–161. 10.1016/j.nano.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Carvajal-Vidal P.; Fabrega M. J.; Espina M.; Calpena A. C.; Garcia M. L. Development of Halobetasol-loaded nanostructured lipid carrier for dermal administration: Optimization, physicochemical and biopharmaceutical behavior, and therapeutic efficacy. Nanomedicine 2019, 20, 102026. 10.1016/j.nano.2019.102026. [DOI] [PubMed] [Google Scholar]
- Cirri M.; Maestrini L.; Maestrelli F.; Mennini N.; Mura P.; Ghelardini C.; Di Cesare Mannelli L. Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Deliv 2018, 25 (1), 1910–1921. 10.1080/10717544.2018.1529209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.; Nie S.; Pan X.; Zhang R.; Fan Z.; Wang S. Quercetin-nanostructured lipid carriers: characteristics and anti-breast cancer activities in vitro. Colloids Surf. B Biointerfaces 2014, 113, 15–24. 10.1016/j.colsurfb.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Jaiswal P.; Gidwani B.; Vyas A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif Cells Nanomed Biotechnol 2016, 44 (1), 27–40. 10.3109/21691401.2014.909822. [DOI] [PubMed] [Google Scholar]
- Zhang L. I.; Zhang L. Lipid-Polymer Hybrid Nanoparticles: Synthesis, Characterization and Applications. Nano LIFE 2010, 01 (01n02), 163–173. 10.1142/S179398441000016X. [DOI] [Google Scholar]
- Shafique M.; Ur Rehman M.; Kamal Z.; Alzhrani R. M.; Alshehri S.; Alamri A. H.; Bakkari M. A.; Sabei F. Y.; Safhi A. Y.; Mohammed A. M.; et al. Formulation development of lipid polymer hybrid nanoparticles of doxorubicin and its in-vitro, in-vivo and computational evaluation. Front Pharmacol 2023, 14, 1025013. 10.3389/fphar.2023.1025013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadinoto K.; Sundaresan A.; Cheow W. S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur. J. Pharm. Biopharm 2013, 85 (3 Pt A), 427–443. 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Musielak E.; Feliczak-Guzik A.; Nowak I. Synthesis and Potential Applications of Lipid Nanoparticles in Medicine. Materials (Basel) 2022, 15 (2), 682. 10.3390/ma15020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazylińska U.; Lewińska A.; Lamch L.; Wilk K. A. Polymeric nanocapsules and nanospheres for encapsulation and long sustained release of hydrophobic cyanine-type photosensitizer. Colloids Surf., A 2014, 442, 42–49. 10.1016/j.colsurfa.2013.02.023. [DOI] [Google Scholar]
- Siqueira-Moura M. P.; Primo F. L.; Espreafico E. M.; Tedesco A. C. Development, characterization, and photocytotoxicity assessment on human melanoma of chloroaluminum phthalocyanine nanocapsules. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33 (3), 1744–1752. 10.1016/j.msec.2012.12.088. [DOI] [PubMed] [Google Scholar]
- Mazzarino L.; Travelet C.; Ortega-Murillo S.; Otsuka I.; Pignot-Paintrand I.; Lemos-Senna E.; Borsali R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012, 370 (1), 58–66. 10.1016/j.jcis.2011.12.063. [DOI] [PubMed] [Google Scholar]
- Katara R.; Majumdar D. K. Eudragit RL 100-based nanoparticulate system of aceclofenac for ocular delivery. Colloids Surf. B Biointerfaces 2013, 103, 455–462. 10.1016/j.colsurfb.2012.10.056. [DOI] [PubMed] [Google Scholar]
- Dong Y.; Ng W. K.; Shen S.; Kim S.; Tan R. B. Solid lipid nanoparticles: continuous and potential large-scale nanoprecipitation production in static mixers. Colloids Surf. B Biointerfaces 2012, 94, 68–72. 10.1016/j.colsurfb.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Riewe J.; Erfle P.; Melzig S.; Kwade A.; Dietzel A.; Bunjes H. Antisolvent precipitation of lipid nanoparticles in microfluidic systems - A comparative study. Int. J. Pharm. 2020, 579, 119167. 10.1016/j.ijpharm.2020.119167. [DOI] [PubMed] [Google Scholar]
- Streck S.; Neumann H.; Nielsen H. M.; Rades T.; McDowell A. Comparison of bulk and microfluidics methods for the formulation of poly-lactic-co-glycolic acid (PLGA) nanoparticles modified with cell-penetrating peptides of different architectures. International journal of pharmaceutics: X 2019, 1, 100030. 10.1016/j.ijpx.2019.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.; Eral H. B.; Hatton T. A.; Doyle P. S. Nanoemulsions: formation, properties and applications. Soft Matter 2016, 12 (11), 2826–2841. 10.1039/C5SM02958A. [DOI] [PubMed] [Google Scholar]
- Ganesan P.; Narayanasamy D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustainable Chemistry and Pharmacy 2017, 6, 37–56. 10.1016/j.scp.2017.07.002. [DOI] [Google Scholar]
- Battaglia L.; Trotta M.; Gallarate M.; Carlotti M. E.; Zara G. P.; Bargoni A. Solid lipid nanoparticles formed by solvent-in-water emulsion-diffusion technique: development and influence on insulin stability. J. Microencapsul 2007, 24 (7), 672. 10.1080/02652040701532981. [DOI] [PubMed] [Google Scholar]
- Vandergraaf S.; Schroen C.; Boom R. Preparation of double emulsions by membrane emulsification?a review. J. Membr. Sci. 2005, 251 (1–2), 7–15. 10.1016/j.memsci.2004.12.013. [DOI] [Google Scholar]
- Gibaud S.; Attivi D. Microemulsions for oral administration and their therapeutic applications. Expert Opin Drug Deliv 2012, 9 (8), 937–951. 10.1517/17425247.2012.694865. [DOI] [PubMed] [Google Scholar]
- Trotta M.; Debernardi F.; Caputo O. Preparation of solid lipid nanoparticles by a solvent emulsification-diffusion technique. Int. J. Pharm. 2003, 257 (1–2), 153–160. 10.1016/S0378-5173(03)00135-2. [DOI] [PubMed] [Google Scholar]
- Sjöström B.; Bergenståhl B. Preparation of submicron drug particles in lecithin-stabilized o/w emulsions I. Model studies of the precipitation of cholesteryl acetate. Int. J. Pharm. 1992, 88 (1–3), 53–62. 10.1016/0378-5173(92)90303-J. [DOI] [Google Scholar]
- Trotta M.; Gallarate M.; Pattarino F.; Morel S. Emulsions containing partially water-miscible solvents for the preparation of drug nanosuspensions. J. Controlled Release 2001, 76 (1–2), 119–128. 10.1016/S0168-3659(01)00432-1. [DOI] [PubMed] [Google Scholar]
- Gamal A.; Gad S.; Gardouh A. Formulation and pharmacokinetic evaluation of rifampicin solid lipid nanoparticles. J. Res. Pharmacy 2020, 24, 539. 10.35333/jrp.2020.202. [DOI] [Google Scholar]
- Roumi S.; Tabrizi M. H.; Eshaghi A.; Abbasi N. Teucrium polium extract-loaded solid lipid nanoparticles: A design and in vitro anticancer study. J. Food Biochem 2021, 45 (9), e13868 10.1111/jfbc.13868. [DOI] [PubMed] [Google Scholar]
- Subramanian P. Lipid-Based Nanocarrier System for the Effective Delivery of Nutraceuticals. Molecules 2021, 26 (18), 5510. 10.3390/molecules26185510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.; Ng W. K.; Kanaujia P.; Kim S.; Tan R. B. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: effects of process variables. Colloids Surf. B Biointerfaces 2011, 88 (1), 483–489. 10.1016/j.colsurfb.2011.07.036. [DOI] [PubMed] [Google Scholar]
- Khairnar S. V.; Pagare P.; Thakre A.; Nambiar A. R.; Junnuthula V.; Abraham M. C.; Kolimi P.; Nyavanandi D.; Dyawanapelly S. Review on the Scale-Up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14 (9), 1886. 10.3390/pharmaceutics14091886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S.; Anton N.; Vandamme T. F.; Serra C. A. Microfluidic nanoprecipitation systems for preparing pure drug or polymeric drug loaded nanoparticles: an overview. Expert Opin Drug Deliv 2016, 13 (10), 1447–1460. 10.1080/17425247.2016.1193151. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Yang G.; Hui Y.; Ranaweera S.; Zhao C. X. Microfluidic Nanoparticles for Drug Delivery. Small 2022, 18 (36), e2106580 10.1002/smll.202106580. [DOI] [PubMed] [Google Scholar]
- Pourabed A.; Younas T.; Liu C.; Shanbhag B. K.; He L.; Alan T. High throughput acoustic microfluidic mixer controls self-assembly of protein nanoparticles with tuneable sizes. J. Colloid Interface Sci. 2021, 585, 229–236. 10.1016/j.jcis.2020.11.070. [DOI] [PubMed] [Google Scholar]
- Maeki M.; Kimura N.; Sato Y.; Harashima H.; Tokeshi M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Delivery Rev. 2018, 128, 84–100. 10.1016/j.addr.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Maeki M.; Uno S.; Niwa A.; Okada Y.; Tokeshi M. Microfluidic technologies and devices for lipid nanoparticle-based RNA delivery. J. Controlled Release 2022, 344, 80–96. 10.1016/j.jconrel.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd S. J.; Issadore D.; Mitchell M. J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. 10.1016/j.biomaterials.2021.120826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baby T.; Liu Y.; Middelberg A. P.; Zhao C.-X. J. C. E. S. Fundamental studies on throughput capacities of hydrodynamic flow-focusing microfluidics for producing monodisperse polymer nanoparticles 2017, 169, 128–139. 10.1016/j.ces.2017.04.046. [DOI] [Google Scholar]
- Shepherd S. J.; Warzecha C. C.; Yadavali S.; El-Mayta R.; Alameh M. G.; Wang L. L.; Weissman D.; Wilson J. M.; Issadore D.; Mitchell M. J. Scalable mRNA and siRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device. Nano Lett. 2021, 21 (13), 5671–5680. 10.1021/acs.nanolett.1c01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood R. R.; DeVoe D. L.; Atencia J.; Vreeland W. N.; Omiatek D. M. A facile route to the synthesis of monodisperse nanoscale liposomes using 3D microfluidic hydrodynamic focusing in a concentric capillary array. Lab Chip 2014, 14 (14), 2403–2409. 10.1039/C4LC00334A. [DOI] [PubMed] [Google Scholar]
- Ahn G. Y.; Choi I.; Ryu T. K.; Ryu Y. H.; Oh D. H.; Kang H. W.; Kang M. H.; Choi S. W. Continuous production of lipid nanoparticles by multiple-splitting in microfluidic devices with chaotic microfibrous channels. Colloids Surf. B Biointerfaces 2023, 224, 113212. 10.1016/j.colsurfb.2023.113212. [DOI] [PubMed] [Google Scholar]
- Abstiens K.; Goepferich A. M. Microfluidic manufacturing improves polydispersity of multicomponent polymeric nanoparticles. Journal of Drug Delivery Science and Technology 2019, 49, 433–439. 10.1016/j.jddst.2018.12.009. [DOI] [Google Scholar]
- Precision Nanosystems . NxGen TM - A Disruptive Technology Enabling Transformative Medicine. https://www.precisionnanosystems.com/platform-technologies/nxgen.
- Hao Y.; Seo J. H.; Hu Y.; Mao H. Q.; Mittal R. Flow physics and mixing quality in a confined impinging jet mixer. AIP Adv. 2020, 10 (4), 045105. 10.1063/5.0002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J.; Chow S. F.; Zheng Y. Application of flash nanoprecipitation to fabricate poorly water-soluble drug nanoparticles. Acta Pharm. Sin B 2019, 9 (1), 4–18. 10.1016/j.apsb.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer. Impingement Jets Mixing Skids for high-flow production of nanoparticles (LNP, microemulsions, etc.), 2022. https://www.knauer.net/en/Systems-Solutions/LNP_lipid_nanoparticles/impingement-jets-mixing-skids-for-high-flow-production-of-nanoparticles (accessed 21 Mar 2023).
- Warne N.; Ruesch M.; Siwik P.; Mensah P.; Ludwig J.; Hripcsak M.; Godavarti R.; Prigodich A.; Dolsten M. Delivering 3 billion doses of Comirnaty in 2021. Nat. Biotechnol. 2023, 41 (2), 183–188. 10.1038/s41587-022-01643-1. [DOI] [PubMed] [Google Scholar]
- Siekmann B.; Westesen K. Thermoanalysis of the recrystallization process of melt-homogenized glyceride nanoparticles. Colloids Surf., B 1994, 3 (3), 159–175. 10.1016/0927-7765(94)80063-4. [DOI] [Google Scholar]
- Battaglia L.; Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opinion on Drug Delivery 2012, 9 (5), 497–508. 10.1517/17425247.2012.673278. [DOI] [PubMed] [Google Scholar]
- Souto E. B.; Müller R. H. Cosmetic features and applications of lipid nanoparticles (SLN, NLC). Int. J. Cosmet Sci. 2008, 30 (3), 157–165. 10.1111/j.1468-2494.2008.00433.x. [DOI] [PubMed] [Google Scholar]
- Bunjes H.; Westesen K.; Koch M. H. J. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int. J. Pharm. 1996, 129 (1), 159–173. 10.1016/0378-5173(95)04286-5. [DOI] [Google Scholar]
- Youshia J.; Kamel A. O.; El Shamy A.; Mansour S. Gamma sterilization and in vivo evaluation of cationic nanostructured lipid carriers as potential ocular delivery systems for antiglaucoma drugs. Eur. J. Pharm. Sci. 2021, 163, 105887. 10.1016/j.ejps.2021.105887. [DOI] [PubMed] [Google Scholar]
- Gokce E. H.; Sandri G.; Bonferoni M. C.; Rossi S.; Ferrari F.; Guneri T.; Caramella C. Cyclosporine A loaded SLNs: evaluation of cellular uptake and corneal cytotoxicity. Int. J. Pharm. 2008, 364 (1), 76–86. 10.1016/j.ijpharm.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Hou X.; Zaks T.; Langer R.; Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6 (12), 1078–1094. 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G. J.; Yoon B. K.; Sut T. N.; Yoo K. Y.; Lee S. H.; Jeon W. Y.; Jackman J. A.; Ariga K.; Cho N. J. Lipid coating technology: A potential solution to address the problem of sticky containers and vanishing drugs. View 2022, 3 (3), 20200078. 10.1002/VIW.20200078. [DOI] [Google Scholar]
- Schoenmaker L.; Witzigmann D.; Kulkarni J. A.; Verbeke R.; Kersten G.; Jiskoot W.; Crommelin D. J. A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubek Z. J.; Chen S.; Zaifman J.; Tam Y. Y. C.; Zou S. Lipid Nanoparticle and Liposome Reference Materials: Assessment of Size Homogeneity and Long-Term −70 degrees C and 4 degrees C Storage Stability. Langmuir 2023, 39 (7), 2509–2519. 10.1021/acs.langmuir.2c02657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L.; Daoussi R.; Vervaet C.; Remon J.-P.; De Beer T. Freeze-drying of live virus vaccines: a review. Vaccine 2015, 33 (42), 5507–5519. 10.1016/j.vaccine.2015.08.085. [DOI] [PubMed] [Google Scholar]
- Jakubek Z. J.; Chen S.; Zaifman J.; Tam Y. Y. C.; Zou S. Lipid Nanoparticle and Liposome Reference Materials: Assessment of Size Homogeneity and Long-Term −70 and 4 °C Storage Stability. Langmuir 2023, 39 (7), 2509–2519. 10.1021/acs.langmuir.2c02657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Chen J.; Xu Q. Current Developments and Challenges of mRNA Vaccines. Annu. Rev. Biomed Eng. 2022, 24, 85–109. 10.1146/annurev-bioeng-110220-031722. [DOI] [PubMed] [Google Scholar]
- Zhao P.; Hou X.; Yan J.; Du S.; Xue Y.; Li W.; Xiang G.; Dong Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioactive Materials 2020, 5 (2), 358–363. 10.1016/j.bioactmat.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Eygeris Y.; Gupta M.; Sahay G. Self-assembled mRNA vaccines. Adv. Drug Deliv Rev. 2021, 170, 83–112. 10.1016/j.addr.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-moderna-epar-product-information_en.pdf (accessed 15 April 2023).
- Kim B.; Hosn R. R.; Remba T.; Yun D.; Li N.; Abraham W.; Melo M. B.; Cortes M.; Li B.; Zhang Y.; et al. Optimization of storage conditions for lipid nanoparticle-formulated self-replicating RNA vaccines. J. Controlled Release 2023, 353, 241–253. 10.1016/j.jconrel.2022.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakanth D.; Singh S.; Maji P. K.; Lee Y. S.; Gaikwad K. K. Advanced packaging for distribution and storage of COVID-19 vaccines: a review. Environmental Chemistry Letters 2021, 19 (5), 3597–3608. 10.1007/s10311-021-01256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer D.A Revolutionary Glass Package Designed Specifically for Pharmaceutical Use. https://www.corning.com/worldwide/en/products/pharmaceutical-technologies/valor-glass/revolutionary-glass-package-valor-pharmaceutical.html (accessed 15 April 2023).
- Weikart C.; Langer R.. A New Hybrid Material for Packaging COVID-19 Vaccines and Other Biologics: Combining the Best of Glass and Plastic, October 28, 2020. https://www.americanpharmaceuticalreview.com/Featured-Articles/569163-A-New-Hybrid-Material-for-Packaging-COVID-19-Vaccines-and-Other-Biologics-Combining-the-Best-of-Glass-and-Plastic/ (accessed 15 April 2023).
- Ball R. L.; Bajaj P.; Whitehead K. A. Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization. Int. J. Nanomedicine 2017, 12, 305–315. 10.2147/IJN.S123062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes R.; Man E.; Thind J.; Yeung S.; Joy A.; Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomater Sci. 2020, 8 (17), 4653–4664. 10.1039/D0BM00558D. [DOI] [PubMed] [Google Scholar]
- Bawa R.; Audette G. F.; Reese B.. Handbook of Clinical Nanomedicine: Law, Business, Regulation, Safety, and Risk; Jenny Stanford Publishing, 2016. 10.1201/b19910 [DOI] [Google Scholar]
- Dokka S.; Toledo D.; Shi X.; Castranova V.; Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm. Res. 2000, 17 (5), 521–525. 10.1023/A:1007504613351. [DOI] [PubMed] [Google Scholar]
- Lv H.; Zhang S.; Wang B.; Cui S.; Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Controlled Release 2006, 114 (1), 100–109. 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- AlBaloul A. Y.; Sato Y.; Maishi N.; Hida K.; Harashima H. Two Modes of Toxicity of Lipid Nanoparticles Containing a pH-Sensitive Cationic Lipid on Human A375 and A375-SM Melanoma Cell Lines. BPB Reports 2019, 2 (4), 48–55. 10.1248/bpbreports.2.4_48. [DOI] [Google Scholar]
- Uziely B.; Jeffers S.; Isacson R.; Kutsch K.; Wei-Tsao D.; Yehoshua Z.; Libson E.; Muggia F. M.; Gabizon A. Liposomal doxorubicin: antitumor activity and unique toxicities during two complementary phase I studies. Journal of Clinical Oncology 1995, 13 (7), 1777–1785. 10.1200/JCO.1995.13.7.1777. [DOI] [PubMed] [Google Scholar]
- Skubitz K. M.; Skubitz A. P. Mechanism of transient dyspnea induced by pegylated-liposomal doxorubicin (Doxil). Anti-cancer drugs 1998, 9 (1), 45–50. 10.1097/00001813-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A.; Szebeni J.; Savay S.; Liebes L.; Rafique N.; Alving C.; Muggia F. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil®): possible role in hypersensitivity reactions. Annals of Oncology 2003, 14 (9), 1430–1437. 10.1093/annonc/mdg374. [DOI] [PubMed] [Google Scholar]
- Nagykálnai T. [Non-pegylated doxorubicin (Myocet®) as the less cardiotoxic alternative of free doxorubicin]. Magy Onkol 2010, 54 (4), 359–367. 10.1556/MOnkol.54.2010.4.10. [DOI] [PubMed] [Google Scholar]
- Wong-Beringer A.; Jacobs R. A.; Guglielmo B. J. Lipid Formulations of Amphotericin B: Clinical Efficacy and Toxicities. Clinical Infectious Diseases 1998, 27 (3), 603–618. 10.1086/514704. [DOI] [PubMed] [Google Scholar]
- Cook G.; Franklin I. M. Adverse drug reactions associated with the administration of amphotericin B lipid complex (Abelcet). Bone Marrow Transplantation 1999, 23 (12), 1325–1326. 10.1038/sj.bmt.1701824. [DOI] [PubMed] [Google Scholar]
- Walsh T. J.; Finberg R. W.; Arndt C.; Hiemenz J.; Schwartz C.; Bodensteiner D.; Pappas P.; Seibel N.; Greenberg R. N.; Dummer S.; et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. New England Journal of Medicine 1999, 340 (10), 764–771. 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- Linhaliq withdrawal assessment report; European Medical Agency, 2019. https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-linhaliq_en.pdf (accessed 28 July 2023).
- Money-Kyrle J. F.; Bates F.; Ready J.; Gazzard B. G.; Phillips R. H.; Boag F. C. Liposomal daunorubicin in advanced Kaposi’s sarcoma: a phase II study. Clin Oncol (R Coll Radiol) 1993, 5 (6), 367–371. 10.1016/S0936-6555(05)80088-3. [DOI] [PubMed] [Google Scholar]
- Cabriales S.; Bresnahan J.; Testa D.; Espina B. M.; Scadden D. T.; Ross M.; Gill P. S. Extravasation of liposomal daunorubicin in patients with AIDS-associated Kaposi’s sarcoma: a report of four cases. Oncol Nurs Forum 1998, 25 (1), 67–70. [PubMed] [Google Scholar]
- Bressler N. M. Photodynamic Therapy of Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration With Verteporfin: Two-Year Results of 2 Randomized Clinical Trials—TAP Report 2. Archives of Ophthalmology 2001, 119 (2), 198–207. [PubMed] [Google Scholar]
- Morita A.; Namkoong H.; Yagi K.; Asakura T.; Hosoya M.; Tanaka H.; Lee H.; Ogawa T.; Kusumoto T.; Azekawa S.; et al. Early-phase adverse effects and management of liposomal amikacin inhalation for refractory Mycobacterium avium complex lung disease in real-world settings. Infection and Drug Resistance 2022, Volume 15, 4001–4011. 10.2147/IDR.S373783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffelers R.; Storm G.; Bakker-Woudenberg I. Liposome-encapsulated aminoglycosides in pre-clinical and clinical studies. J. Antimicrob. Chemother. 2001, 48 (3), 333–344. 10.1093/jac/48.3.333. [DOI] [PubMed] [Google Scholar]
- ABRAXANE for Injectable Suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound); FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021660s031lbl.pdf (accessed 28 July 2023).
- Bawa R. Regulating nanomedicine - can the FDA handle it?. Curr. Drug Deliv 2011, 8 (3), 227–234. 10.2174/156720111795256156. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. A New Vaccine to Battle Covid-19. N Engl J. Med. 2021, 384 (5), 470–471. 10.1056/NEJMe2035557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Grainger D. W. Regulatory considerations specific to liposome drug development as complex drug products. Frontiers in Drug Delivery 2022, 2, 10. 10.3389/fddev.2022.901281. [DOI] [Google Scholar]
- Let’s talk about lipid nanoparticles. Nat. Rev. Mater. 2021, 6 ( (2), ), 99. 10.1038/s41578-021-00281-4 [DOI] [Google Scholar]
- Roces C. B.; Lou G.; Jain N.; Abraham S.; Thomas A.; Halbert G. W.; Perrie Y. Manufacturing Considerations for the Development of Lipid Nanoparticles Using Microfluidics. Pharmaceutics 2020, 12 (11), 1095. 10.3390/pharmaceutics12111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aftaab S. U.; Ali S. A.. Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation. Guidance for Industry; FDA, 2021. (acccessed 3 Apr 2023).
- Chen M.; Liu X.; Fahr A. Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: In vitro study with finite and infinite dosage application. Int. J. Pharm. 2011, 408 (1–2), 223–234. 10.1016/j.ijpharm.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Amrani S.; Tabrizian M. Characterization of Nanoscale Loaded Liposomes Produced by 2D Hydrodynamic Flow Focusing. ACS Biomater Sci. Eng. 2018, 4 (2), 502–513. 10.1021/acsbiomaterials.7b00572. [DOI] [PubMed] [Google Scholar]
- Roberts S. A.; Parikh N.; Blower R. J.; Agrawal N. SPIN: rapid synthesis, purification, and concentration of small drug-loaded liposomes. J. Liposome Res. 2018, 28 (4), 331–340. 10.1080/08982104.2017.1381115. [DOI] [PubMed] [Google Scholar]
- Kimura N.; Maeki M.; Sato Y.; Ishida A.; Tani H.; Harashima H.; Tokeshi M. Development of a Microfluidic-Based Post-Treatment Process for Size-Controlled Lipid Nanoparticles and Application to siRNA Delivery. ACS Appl. Mater. Interfaces 2020, 12 (30), 34011–34020. 10.1021/acsami.0c05489. [DOI] [PubMed] [Google Scholar]
- Smiatek J.; Jung A.; Bluhmki E. Towards a Digital Bioprocess Replica: Computational Approaches in Biopharmaceutical Development and Manufacturing. Trends Biotechnol 2020, 38 (10), 1141–1153. 10.1016/j.tibtech.2020.05.008. [DOI] [PubMed] [Google Scholar]
- Valencia P. M.; Farokhzad O. C.; Karnik R.; Langer R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol 2012, 7 (10), 623–629. 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scioli Montoto S.; Muraca G.; Ruiz M. E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front Mol. Biosci 2020, 7, 587997. 10.3389/fmolb.2020.587997. [DOI] [PMC free article] [PubMed] [Google Scholar]